In Vitro Evaluation of the Anti-Diabetic Potential of Aqueous Acetone Helichrysum petiolare Extract (AAHPE) with Molecular Docking Relevance in Diabetes Mellitus
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Collection of Plant Material
2.3. Plant Extraction
2.4. Determination of the Enzymatic Inhibitory Activity of AAHPE
2.4.1. α-Amylase Inhibitory Assay
2.4.2. α-Glucosidase Inhibitory Assay
2.5. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis of AAHPE
2.6. In Vitro Studies
2.6.1. Cell Line and Culture Condition
2.6.2. Cell Cytotoxicity by MTT Assay of AAHPE
2.6.3. NBDG Glucose Uptake Assay
2.7. In Silico Drug-Likeness Analysis and ADMET Profiling
2.8. Molecular Docking
2.8.1. Protein Preparation
2.8.2. Ligand Preparation
2.8.3. Docking Protocol
2.8.4. Docking Method
2.9. Statistical Analysis
3. Results
3.1. Screening of the Flavonoid’s Compound of AAHPE Using LC-MS Analysis
3.2. The Effect of ACHPE on HepG2 Cell Viability
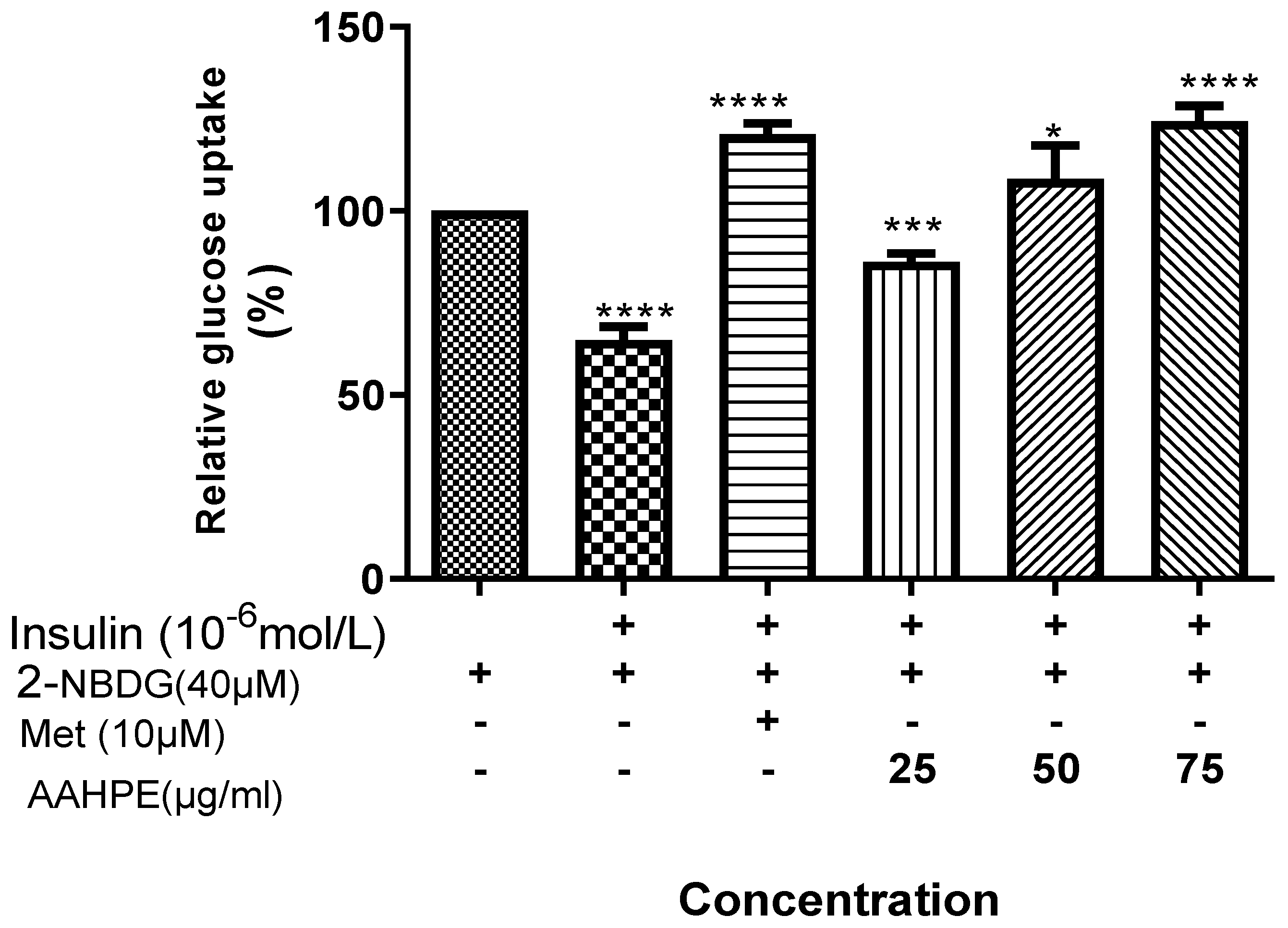
3.3. The Effect of AAHPE on Glucose Uptake
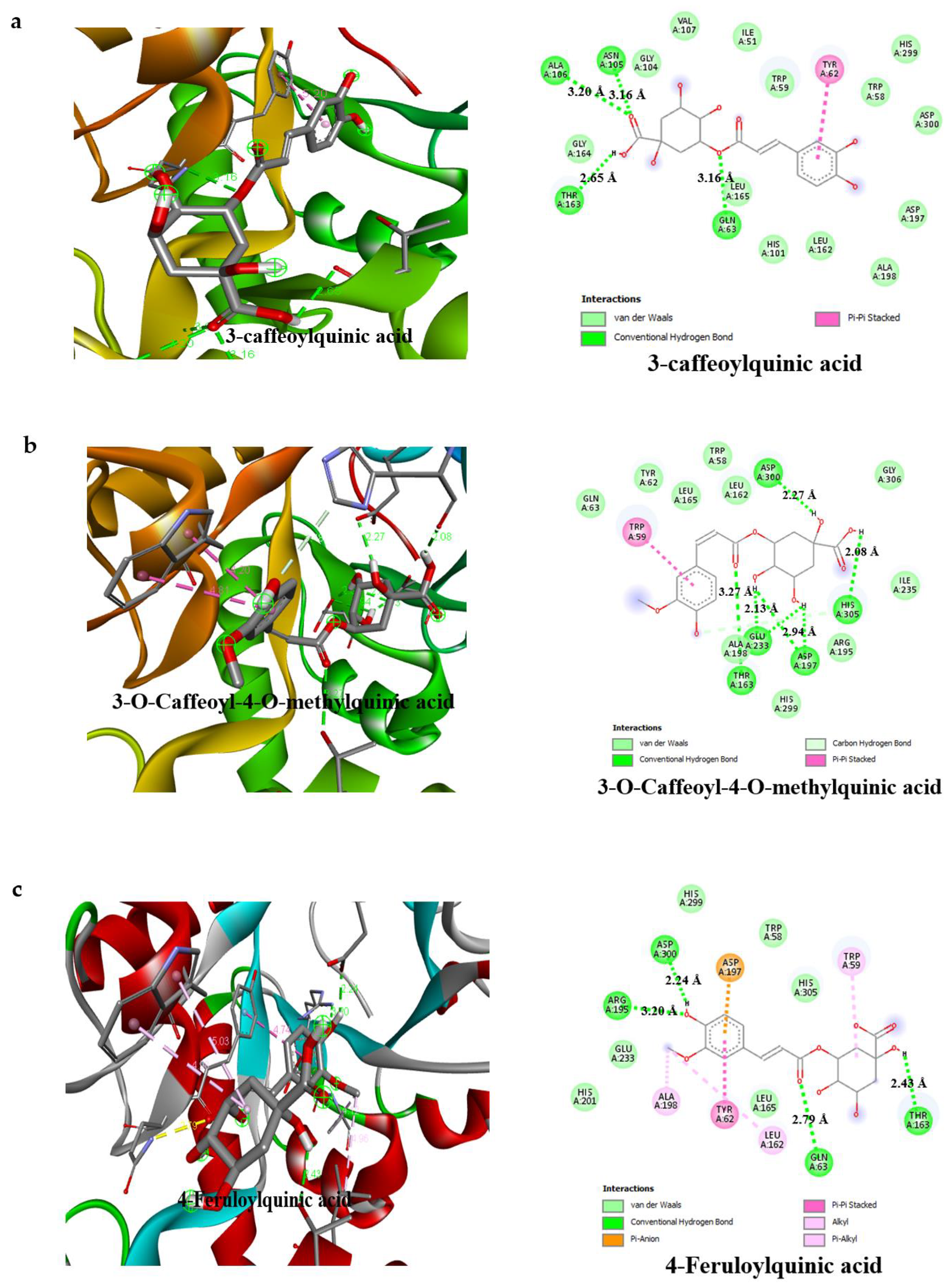
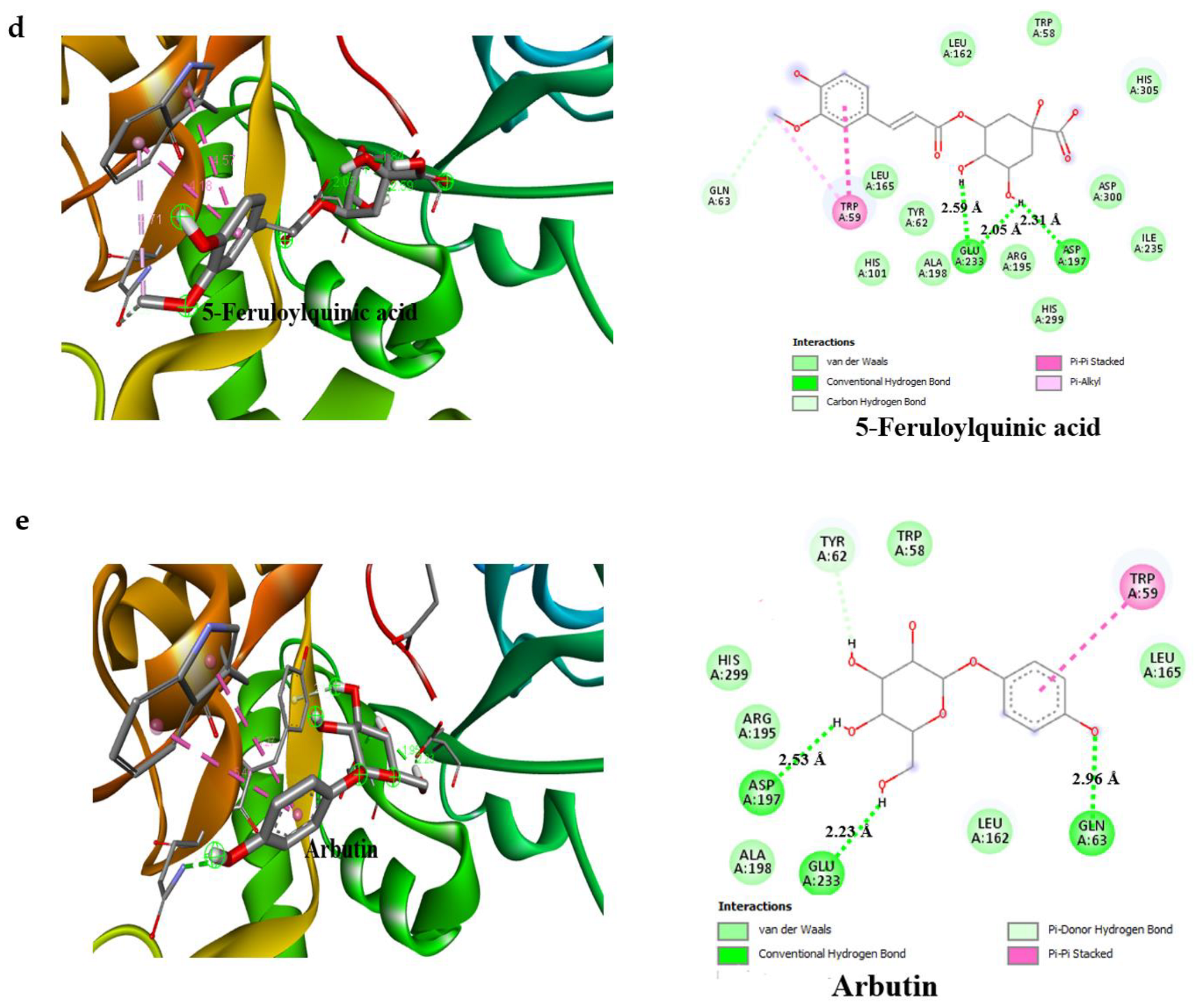
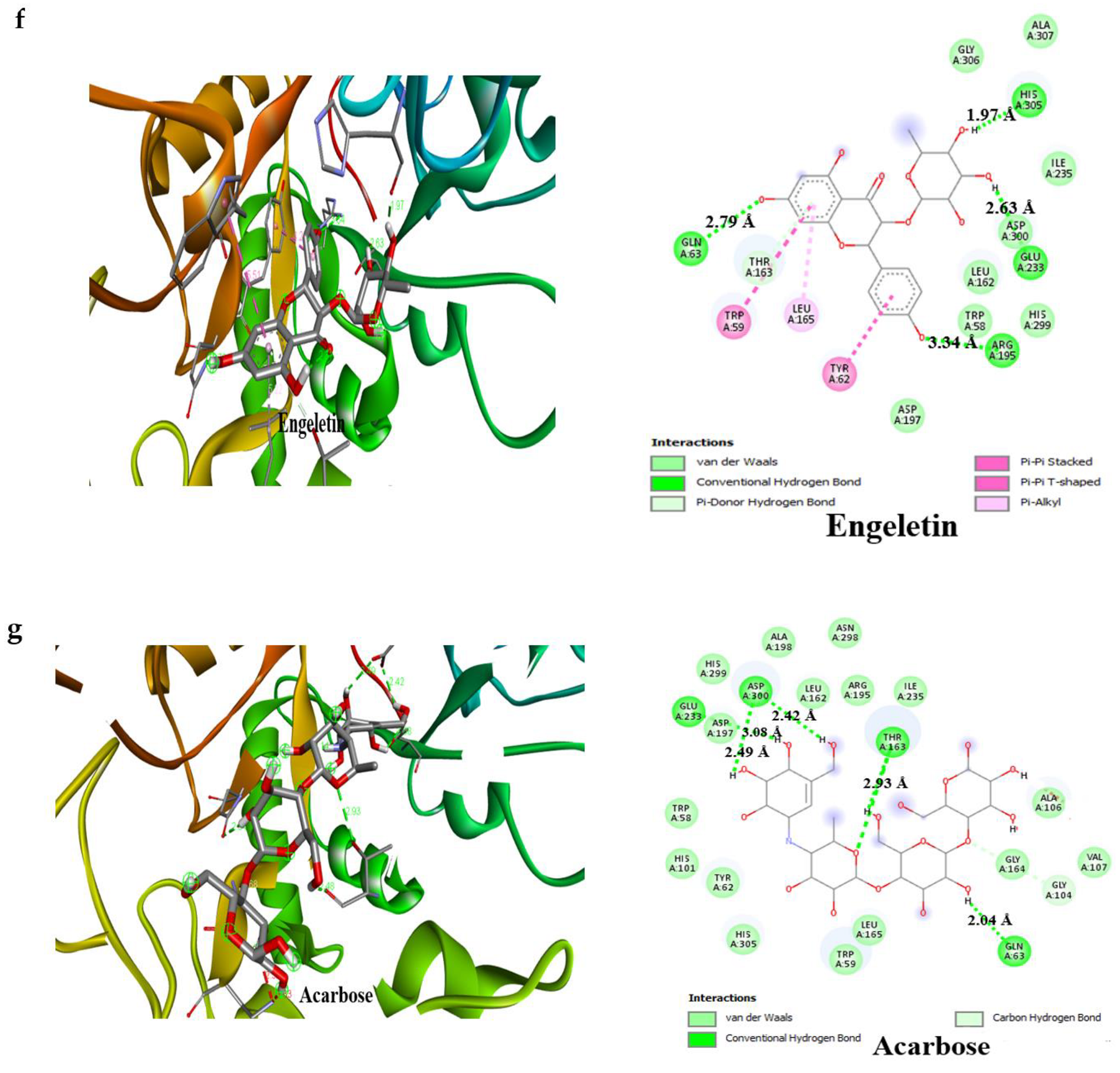
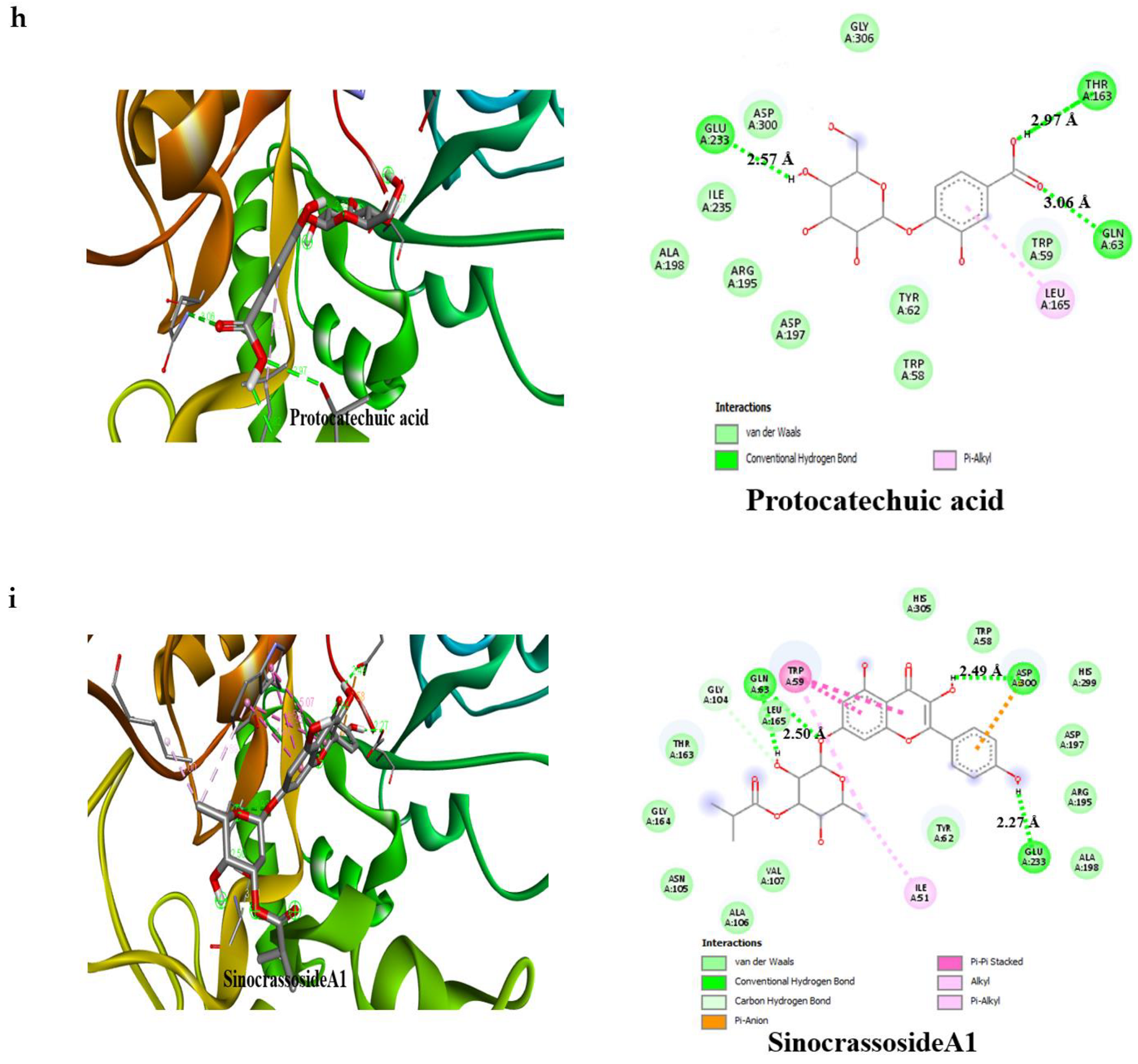
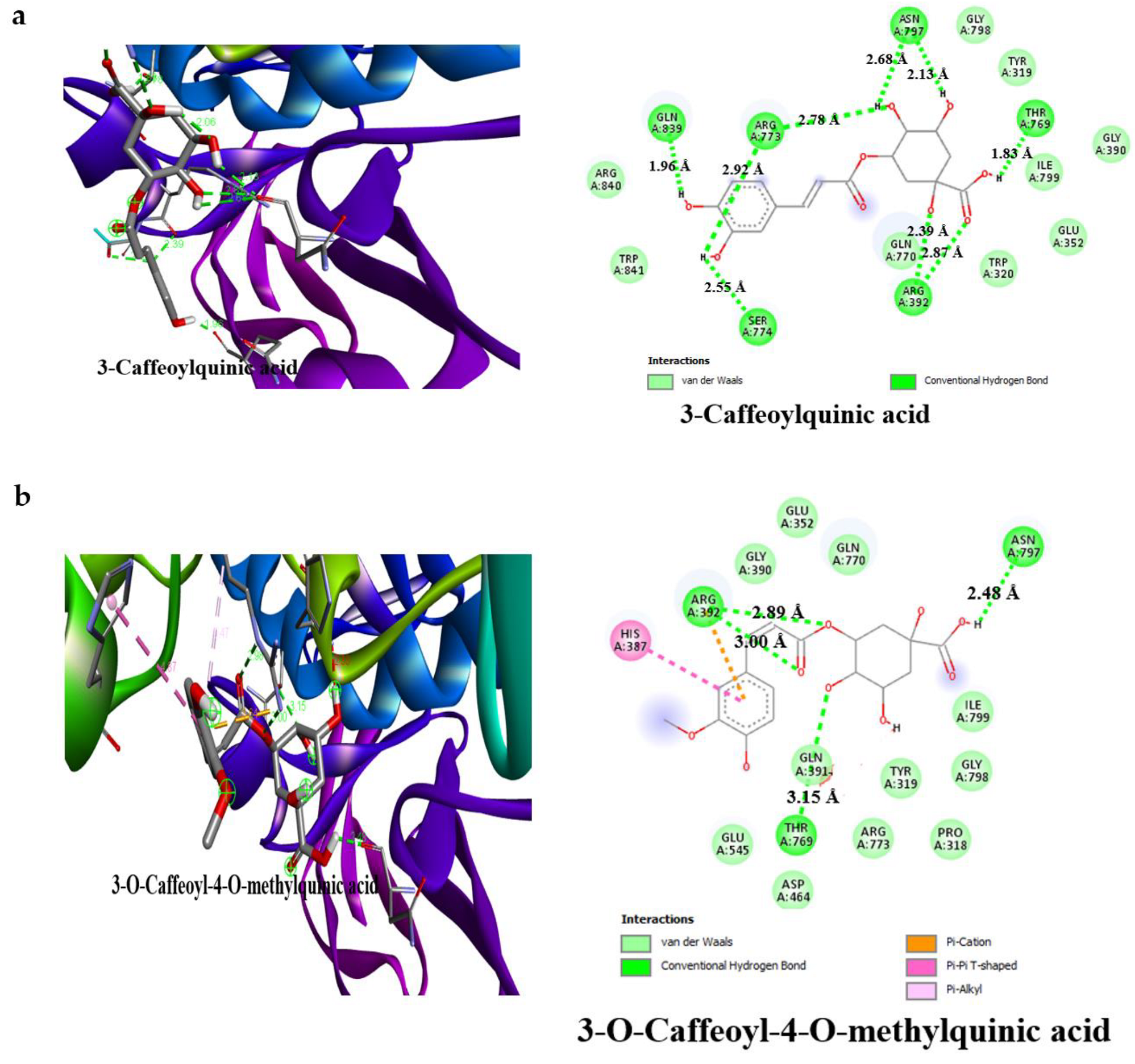
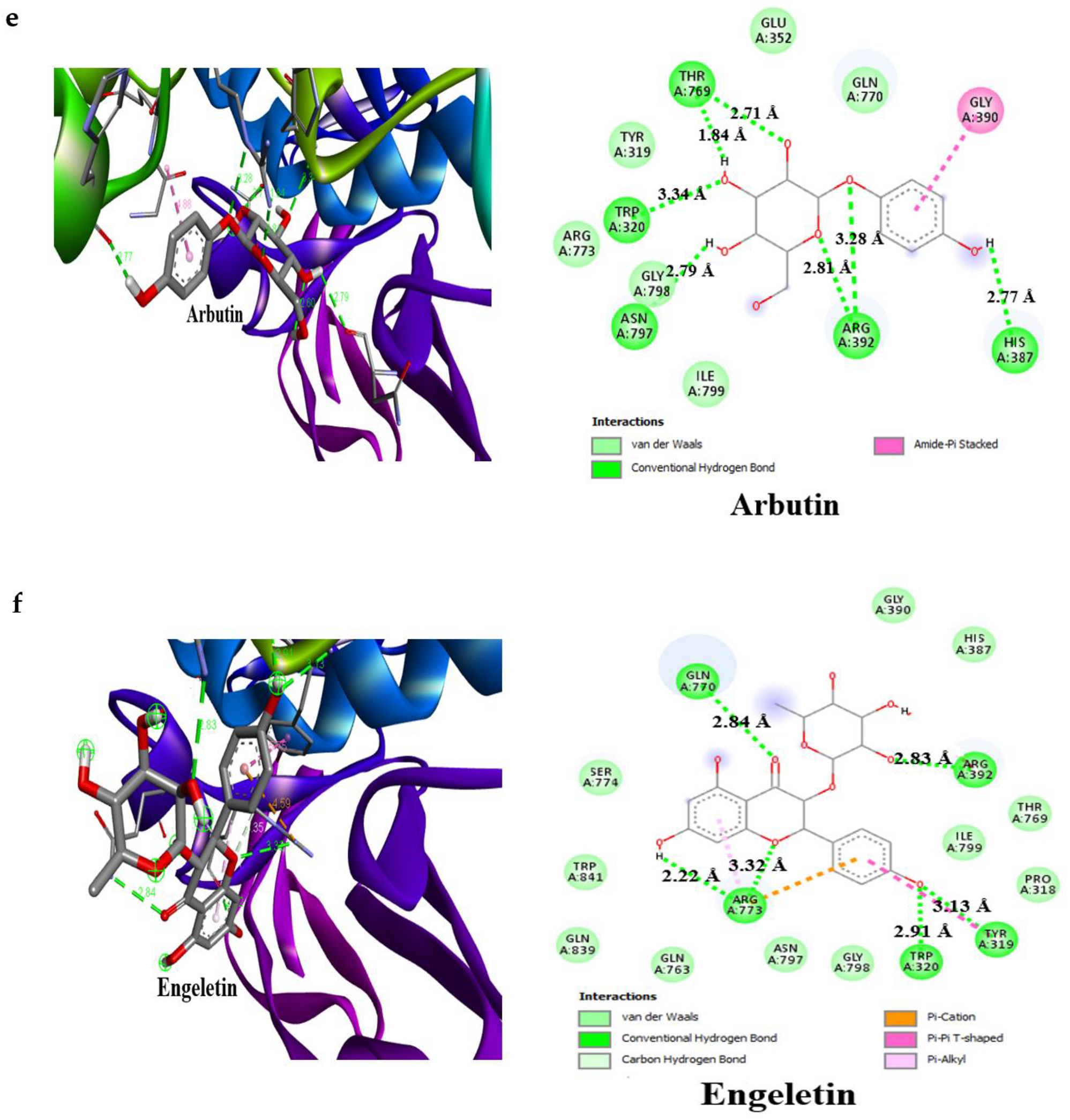
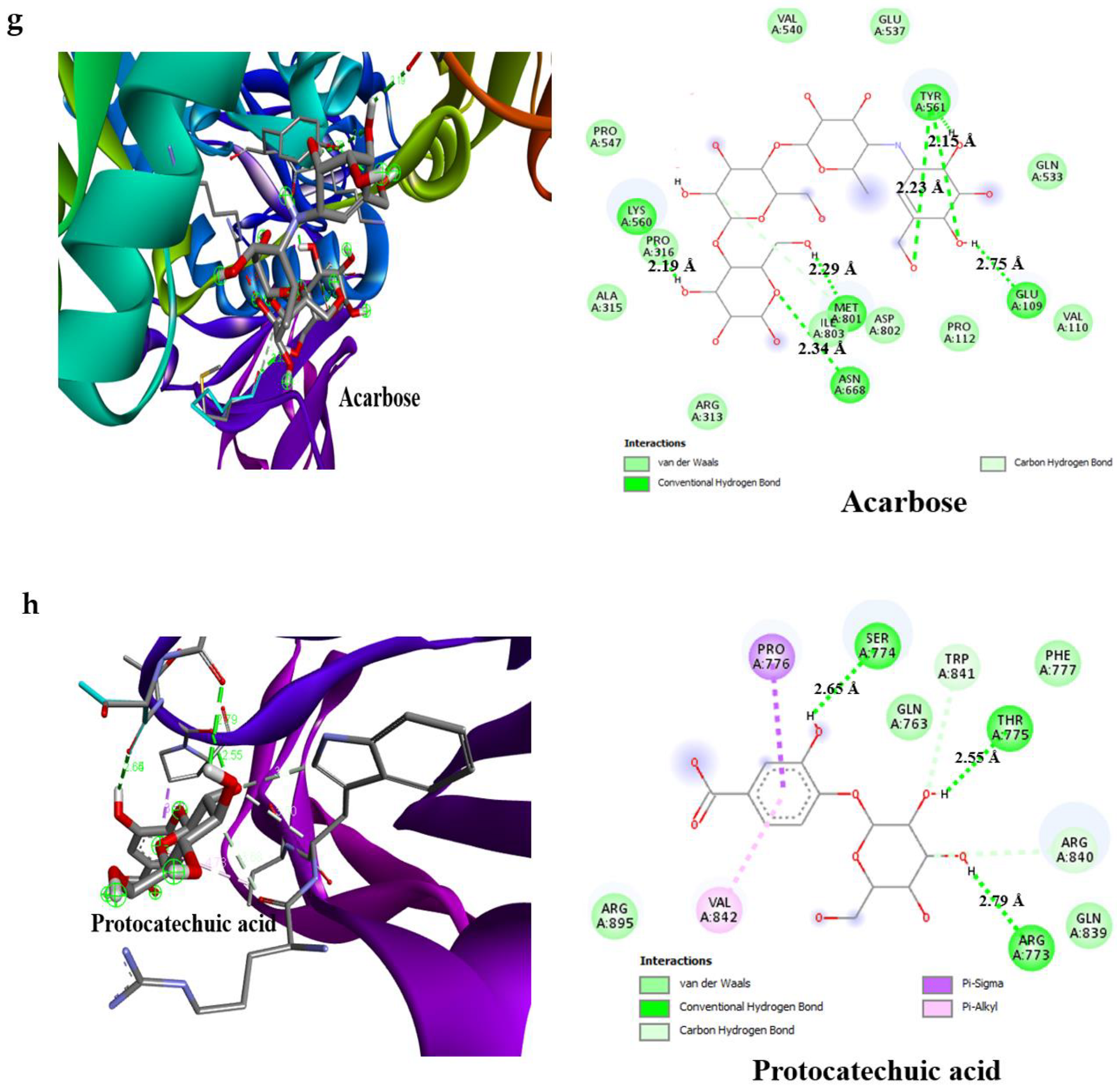
3.4. Result of Molecular Docking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Khafajy, D.A.; Majeed, M.J.; Al-Azzawi, O.F.; Khaleel, A.I. Role of CoQ10 and IGFBP-1 in Obese Male Patients with Diabetic Mellitus Type II. Indian J. Forensic Med. Toxicol. 2021, 15, 2205. [Google Scholar]
- Hu, Y.; Hou, Z.; Liu, D.; Yang, X. Tartary buckwheat flavonoids protect hepatic cells against high glucose-induced oxidative stress and insulin resistance via MAPK signaling pathways. Food Funct. 2016, 7, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Vanessa Fiorentino, T.; Prioletta, A.; Zuo, P.; Folli, F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr. Pharm. Des. 2013, 19, 5695–5703. [Google Scholar] [CrossRef] [PubMed]
- Adrar, N.S.; Madani, K.; Adrar, S. Impact of the inhibition of proteins activities and the chemical aspect of polyphenols-proteins interactions. PharmaNutrition 2019, 7, 100142. [Google Scholar] [CrossRef]
- Wang, S.; Noh, S.K.; Koo, S.I. Green tea catechins inhibit pancreatic phospholipase A2 and intestinal absorption of lipids in ovariectomized rats. J. Nutr. Biochem. 2006, 17, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Sugihara, N.; Kishimoto, Y.; Saita, E.; Taguchi, C.; Kobayashi, M.; Ichitani, M.; Ukawa, Y.; Sagesaka, Y.M.; Suzuki, E.; Kondo, K. Green tea catechins prevent low-density lipoprotein oxidation via their accumulation in low-density lipoprotein particles in humans. Nutr. Res. 2016, 36, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Warren, F.J.; Gidley, M.J. Soluble polysaccharides reduce binding and inhibitory activity of tea polyphenols against porcine pancreatic α-amylase. Food Hydrocoll. 2018, 79, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Karas, D.; Ulrichová, J.; Valentová, K. Galloylation of polyphenols alters their biological activity. Food Chem. Toxicol. 2017, 105, 223–240. [Google Scholar] [CrossRef]
- Omar, S.H. Biophenols pharmacology against the amyloidogenic activity in Alzheimer’s disease. Biomed. Pharmacother. 2017, 89, 396–413. [Google Scholar] [CrossRef]
- Miltonprabu, S.; Tomczyk, M.; Skalicka-Woźniak, K.; Rastrelli, L.; Daglia, M.; Nabavi, S.F.; Alavian, S.M.; Nabavi, S.M. Hepatoprotective effect of quercetin: From chemistry to medicine. Food Chem. Toxicol. 2017, 108, 365–374. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Plundrich, N.; Schneider, M.; Campbell, C.; Lila, M.A. Protein-polyphenol particles for delivering structural and health functionality. Food Hydrocoll. 2017, 72, 163–173. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Ahmed, D.; Kumar, V.; Sharma, M.; Verma, A. Target guided isolation, in-vitro antidiabetic, antioxidant activity and molecular docking studies of some flavonoids from Albizzia Lebbeck Benth. bark. BMC Complementary Altern. Med. 2014, 14, 155. [Google Scholar] [CrossRef] [Green Version]
- Dai, T.; Chen, J.; McClements, D.J.; Li, T.; Liu, C. Investigation the interaction between procyanidin dimer and α-glucosidase: Spectroscopic analyses and molecular docking simulation. Int. J. Biol. Macromol. 2019, 130, 315–322. [Google Scholar] [CrossRef]
- Sun, L.; Miao, M. Dietary polyphenols modulate starch digestion and glycaemic level: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 541–555. [Google Scholar] [CrossRef]
- Stratton, C.F.; Newman, D.J.; Tan, D.S. Cheminformatic comparison of approved drugs from natural product versus synthetic origins. Bioorganic Med. Chem. Lett. 2015, 25, 4802–4807. [Google Scholar] [CrossRef] [Green Version]
- Segneanu, A.E.; Velciov, S.M.; Olariu, S.; Cziple, F.; Damian, D.; Grozescu, I. Bioactive Molecules Profile from Natural Compounds. In Amino Acid—New Insights and Roles in Plant and Animal; Asao, T., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 209–228. [Google Scholar]
- Akinyede, K.A.; Cupido, C.N.; Hughes, G.D.; Oguntibeju, O.O.; Ekpo, O.E. Medicinal Properties and In Vitro Biological Activities of Selected Helichrysum Species from South Africa: A Review. Plants 2021, 10, 1566. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Sagdic, O.; Hamzaoglu, E. Compositions, antioxidant and antimicrobial activities of Helichrysum (Asteraceae) species collected from Turkey. Food Chem. 2010, 119, 114–122. [Google Scholar] [CrossRef]
- Lourens, A.; Viljoen, A.M.; Van Heerden, F. South African Helichrysum species: A review of the traditional uses, biological activity and phytochemistry. J. Ethnopharmacol. 2008, 119, 630–652. [Google Scholar] [CrossRef]
- Süzgeç-Selçuk, S.; Birteksöz, A. Flavonoids of Helichrysum chasmolycicum and its antioxidant and antimicrobial activities. S. Afr. J. Bot. 2011, 77, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Serabele, K.; Chen, W.; Tankeu, S.; Combrinck, S.; Veale, C.G.; van Vuuren, S.; Chaudhary, S.K.; Viljoen, A. Comparative chemical profiling and antimicrobial activity of two interchangeably used ‘Imphepho’species (Helichrysum odoratissimum and Helichrysum petiolare). S. Afr. J. Bot. 2021, 137, 117–132. [Google Scholar] [CrossRef]
- Lourens, A.; Van Vuuren, S.; Viljoen, A.; Davids, H.; Van Heerden, F. Antimicrobial activity and in vitro cytotoxicity of selected South African Helichrysum species. S. Afr. J. Bot. 2011, 77, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Odeyemi, S.; Bradley, G. Medicinal plants used for the traditional management of diabetes in the Eastern Cape, South Africa: Pharmacology and toxicology. Molecules 2018, 23, 2759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildirim, B.A.; Kordali, S.; Kapakin, K.A.T.; Yildirim, F.; Senocak, E.A.; Altun, S. Effect of Helichrysum plicatum DC. subsp. plicatum ethanol extract on gentamicin-induced nephrotoxicity in rats. J. Zhejiang Univ.-Sci. B 2017, 18, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Aslan, M.; Orhan, D.D.; Orhan, N.; Sezik, E.; Yeşilada, E. A study of antidiabetic and antioxidant effects of Helichrysum graveolens capitulums in streptozotocin-induced diabetic rats. J. Med. Food 2007, 10, 396–400. [Google Scholar] [CrossRef]
- Nasr, A.; Zhou, X.; Liu, T.; Yang, J.; Zhu, G.-P. Acetone-water mixture is a competent solvent to extract phenolics and antioxidants from four organs of Eucalyptus camaldulensis. Turk. J. Biochem. 2019, 44, 231–239. [Google Scholar] [CrossRef]
- Ali, H.; Houghton, P.; Soumyanath, A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef]
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-Glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J. Agric. Food Chem. 2001, 49, 1948–1951. [Google Scholar] [CrossRef]
- Stander, M.A.; Van Wyk, B.-E.; Taylor, M.J.; Long, H.S. Analysis of phenolic compounds in rooibos tea (Aspalathus linearis) with a comparison of flavonoid-based compounds in natural populations of plants from different regions. J. Agric. Food Chem. 2017, 65, 10270–10281. [Google Scholar] [CrossRef]
- Oselusi, S.O.; Christoffels, A.; Egieyeh, S.A. Cheminformatic Characterization of Natural Antimicrobial Products for the Development of New Lead Compounds. Molecules 2021, 26, 3970. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar]
- Bolton, E.E.; Wang, Y.; Thiessen, P.A.; Bryant, S.H. PubChem: Integrated Platform of Small Molecules and Biological Activities. In Annual Reports in Computational Chemistry; Elsevier: Amsterdam, The Netherlands, 2008; Volume 4, pp. 217–241. [Google Scholar]
- Williams, A.J. Public chemical compound databases. Curr. Opin. Drug Discov. Dev. 2008, 11, 393. [Google Scholar]
- Oyewusi, H.A.; Huyop, F.; Wahab, R.A. Molecular docking and molecular dynamics simulation of Bacillus thuringiensis dehalogenase against haloacids, haloacetates and chlorpyrifos. J. Biomol. Struct. Dyn. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Oyewusi, H.A.; Huyop, F.; Wahab, R.A.; Hamid, A.A.A. In silico assessment of dehalogenase from Bacillus thuringiensis H2 in relation to its salinity-stability and pollutants degradation. J. Biomol. Struct. Dyn. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Kidane, Y.; Bokrezion, T.; Mebrahtu, J.; Mehari, M.; Gebreab, Y.B.; Fessehaye, N.; Achila, O.O. In vitro inhibition of-amylase and-glucosidase by extracts from Psiadia punctulata and Meriandra bengalensis. Evid.-Based Complement. Altern. Med. 2018, 2018, 2164345. [Google Scholar] [CrossRef] [Green Version]
- Atchan Nwakiban, A.P.; Cicolari, S.; Piazza, S.; Gelmini, F.; Sangiovanni, E.; Martinelli, G.; Bossi, L.; Carpentier-Maguire, E.; Deutou Tchamgoue, A.; Agbor, G.A. Oxidative stress modulation by cameroonian spice extracts in hepg2 cells: Involvement of nrf2 and improvement of glucose uptake. Metabolites 2020, 10, 182. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Oselusi, S.O.; Egieyeh, S.A.; Christoffels, A. Cheminformatic Profiling and Hit Prioritization of Natural Products with Activities against Methicillin-Resistant Staphylococcus aureus (MRSA). Molecules 2021, 26, 3674. [Google Scholar] [CrossRef]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The application of in silico drug-likeness predictions in pharmaceutical research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef]
- Kirchmair, J.; Göller, A.H.; Lang, D.; Kunze, J.; Testa, B.; Wilson, I.D.; Glen, R.C.; Schneider, G. Predicting drug metabolism: Experiment and/or computation? Nat. Rev. Drug Discov. 2015, 14, 387–404. [Google Scholar] [CrossRef] [Green Version]
- Patil, R.; Das, S.; Stanley, A.; Yadav, L.; Sudhakar, A.; Varma, A.K. Optimized hydrophobic interactions and hydrogen bonding at the target-ligand interface leads the pathways of drug-designing. PLoS ONE 2010, 5, e12029. [Google Scholar] [CrossRef]
- Verma, M.; Gupta, S.J.; Chaudhary, A.; Garg, V.K. Protein tyrosine phosphatase 1B inhibitors as antidiabetic agents—A brief review. Bioorg. Chem. 2017, 70, 267–283. [Google Scholar] [CrossRef]
- Wang, L.-J.; Jiang, B.; Wu, N.; Wang, S.-Y.; Shi, D.-Y. Natural and semisynthetic protein tyrosine phosphatase 1B (PTP1B) inhibitors as anti-diabetic agents. RSC Adv. 2015, 5, 48822–48834. [Google Scholar] [CrossRef]
- Ali, M.Y.; Jannat, S.; Jung, H.A.; Choi, J.S. Insulin–Mimetic Dihydroxanthyletin-Type Coumarins from Angelica decursiva with Protein Tyrosine Phosphatase 1B and α-Glucosidase Inhibitory Activities and Docking Studies of Their Molecular Mechanisms. Antioxidants 2021, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.M. Carbohydrate digestion and absorption: Role of the small intestine. N. Engl. J. Med. 1975, 292, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Brogi, S.; Ramalho, T.C.; Kuca, K.; Medina-Franco, J.L.; Valko, M. In silico Methods for Drug Design and Discovery. Front. Chem. 2020, 8, 612. [Google Scholar] [CrossRef]
- Sun, H.; Scott, D.O. Structure-based drug metabolism predictions for drug design. Chem. Biol. Drug Des. 2010, 75, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Blundell, T.L.; Sibanda, B.L.; Montalvão, R.W.; Brewerton, S.; Chelliah, V.; Worth, C.L.; Harmer, N.J.; Davies, O.; Burke, D. Structural biology and bioinformatics in drug design: Opportunities and challenges for target identification and lead discovery. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 413–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jhong, C.H.; Riyaphan, J.; Lin, S.H.; Chia, Y.C.; Weng, C.F. S creening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. Biofactors 2015, 41, 242–251. [Google Scholar] [CrossRef]
- Aladejana, A.E.; Bradley, G.; Afolayan, A.J. In vitro evaluation of the anti-diabetic potential of Helichrysum petiolare Hilliard & BL Burtt using HepG2 (C3A) and L6 cell lines. F1000Research 2020, 9, 1240. [Google Scholar]
- Fernandez, E.; Ross, C.; Liang, H.; Javors, M.; Tardif, S.; Salmon, A.B. Evaluation of the pharmacokinetics of metformin and acarbose in the common marmoset. Pathobiol. Aging Age-Relat. Dis. 2019, 9, 1657756. [Google Scholar] [CrossRef] [Green Version]
- Zonoubi, A.; Prashantha, C.N.; Perumal, D.V.; Mafibaniasadi, Z. In silico Analysis of Active Constituents of Silymarin as Alpha-Glucosidase Enzyme Inhibitors in Type 2 Diabetes Mellitus. Asian J. Pharm. Clin. Res. 2019, 12, 225–229. [Google Scholar] [CrossRef]
- Oyinloye, B.E.; Adekiya, T.A.; Aruleba, R.T.; Ojo, O.A.; Ajiboye, B.O. Structure-based docking studies of GLUT4 towards exploring selected phytochemicals from Solanum xanthocarpum as a therapeutic target for the treatment of cancer. Curr. Drug Discov. Technol. 2019, 16, 406–416. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhou, H.; Li, B.; Mazurenko, I.K. Inhibitory Mechanism of Engeletin Against α-Glucosidase. Natural Product Communications 2021, 16, 1934578X20986723. [Google Scholar] [CrossRef]
- Yousefi, F.; Mahjoub, S.; Pouramir, M.; Khadir, F. Hypoglycemic activity of Pyrus biossieriana Buhse leaf extract and arbutin: Inhibitory effects on alpha amylase and alpha glucosidase. Casp. J. Intern. Med. 2013, 4, 763. [Google Scholar]
- Floris, S.; Fais, A.; Medda, R.; Pintus, F.; Piras, A.; Kumar, A.; Kuś, P.M.; Westermark, G.T.; Era, B. Washingtonia filifera seed extracts inhibit the islet amyloid polypeptide fibrils formations and α-amylase and α-glucosidase activity. J. Enzym. Inhib. Med. Chem. 2021, 36, 517–524. [Google Scholar] [CrossRef]
- Blakaj, D.M.; McConnell, K.J.; Beveridge, D.L.; Baranger, A.M. Molecular dynamics and thermodynamics of protein−RNA interactions: Mutation of a conserved aromatic residue modifies stacking interactions and structural adaptation in the U1A−stem loop 2 RNA complex. J. Am. Chem. Soc. 2001, 123, 2548–2551. [Google Scholar] [CrossRef]
- Pecsi, I.; Leveles, I.; Harmat, V.; Vertessy, B.G.; Toth, J. Aromatic stacking between nucleobase and enzyme promotes phosphate ester hydrolysis in dUTPase. Nucleic Acids Res. 2010, 38, 7179–7186. [Google Scholar] [CrossRef] [Green Version]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: Structural requirements for inhibiting human α-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef]
- Bano, S.; Khan, A.-u.; Asghar, F.; Usman, M.; Badshah, A.; Ali, S. Computational and pharmacological evaluation of Ferrocene-based acyl ureas and homoleptic cadmium carboxylate derivatives for anti-diabetic potential. Front. Pharmacol. 2018, 8, 1001. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.S.; Khan, A.-u.; Kury, L.T.A.; Shah, F.A. Computational and Pharmacological Evaluation of Carveol for Antidiabetic Potential. Front. Pharmacol. 2020, 11, 919. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageid, A.D.; Abou-Salem, M.E.S.; Salaam, N.M.H.A.; El-Garhy, H.A.S. The potential effect of garlic extract and curcumin nanoparticles against complication accompanied with experimentally induced diabetes in rats. Phytomedicine 2018, 43, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Safitri, A.; Sari, D.R.T.; Fatchiyah, F.; Roosdiana, A. Modeling of Aqueous Root Extract Compounds of Ruellia tuberosa L. for Alpha-Glucosidase Inhibition Through in Silico Study. Makara J. Sci. 2021, 25, 8. [Google Scholar]




| IC50 | AAHPE (µg/mL) | Acarbose (µg/mL) |
|---|---|---|
| α-amylase | 46.50 ± 6.17 | 0.32 ± 0.16 |
| α-glucosidase | 37.81 ± 5.15 | 5.38 ± 2.76 |
| Compounds | LogPo/w (MLOGP) | LogSw (ESOL) | MW (g/mol) | HBA | HBD | Reactivity (40–130) | tPSA | Solubility (mg/mL) | Rotatable Bonds (RoB) | n Violation |
|---|---|---|---|---|---|---|---|---|---|---|
| 3-caffeoylquinic acid | 0.96 | −1.62 | 354.31 | 9 | 6 | 83.50 | 164.75 | 8.50 | 5 | 1 |
| 3-O-Caffeoyl-4-O-methylquinic acid | 1.47 | −1.84 | 368.34 | 9 | 5 | 87.97 | 153.75 | 5.38 | 6 | 0 |
| 4-Feruloyl quinic acid/5-Feruloylquinic acid | 1.47 | −1.84 | 368.34 | 9 | 5 | 87.97 | 153.75 | 5.38 | 6 | 0 |
| Arbutin | 1.07 | −0.71 | 272.25 | 7 | 5 | 62.61 | 119.61 | 5.27 | 3 | 0 |
| Engeletin | 1.77 | −3.09 | 434.49 | 10 | 6 | 103.95 | 166.14 | 3.55 | 3 | 1 |
| Metformin | 0.34 | 0.29 | 129.16 | 2 | 3 | 36.93 | 91.49 | 2.53 | 2 | 0 |
| Protocatechuic acid | 1.09 | −1.89 | 154.12 | 4 | 3 | 37.45 | 77.76 | 1.99 | 1 | 0 |
| SinocrassosideA1 | 3.36 | −4.09 | 316.43 | 3 | 0 | 92.90 | 38.83 | 2.58 | 0 | 0 |
| Acarbose | 8.56 | −2.13 | 645.60 | 19 | 14 | 136.69 | 321.17 | 2.32 | 9 | 3 |
| Class | Properties | 3-Caffeoylquinic Acid | 3-O-Caffeoyl-4-O-Methylquinic Acid | 4-/5-Feruloyl Quinic Acid | SinocrassosideA1 | Engeletin | Metformin | Protocatechuic Acid | Acarbose | Arbutin |
|---|---|---|---|---|---|---|---|---|---|---|
| Absorption | Caco-2 permeability (˃−5.15 cm/s) | −6.58 | −6.331 | −6.331 | −4.56 | −6.581 | −5.502 | −5.107 | −0.8955 | −5.954 |
| Pgp-inhibitor | No | No | No | No | No | No | No | Yes | No | |
| Pgp-substrate | No | Yes | No | Yes | Yes | No | No | No | No | |
| HIA (≥30%: high, <30%: low) | Low | High | High | High | High | High | Low | Low | High | |
| Bioavailability score | 0.11 | 0.11 | 0.11 | 0.55 | 0.55 | 0.55 | 0.56 | 0.11 | 0.55 | |
| GI absorption | Low | Low | Low | High | Low | High | High | Low | High | |
| Skin permeation (Log Kp) (cm/s) | −8.76 | −8.62 | −8.62 | −5.65 | −8.42 | −7.99 | −6.39 | −5.16 | −8.92 | |
| Distribution | PPB (90%) | 41.961% | 41.1349% | 41.1349% | 97.16.5% | 42.5742% | 3.9577% | 42.6641% | 21.138% | 36.0499% |
| BBB | No | No | No | Yes | No | No | No | No | No | |
| Metabolism | CYP1A2 inhibitor | No | No | No | No | No | No | No | No | No |
| CYP1A2 substrate | No | No | No | No | No | No | No | Yes | No | |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No | No | |
| CYP3A4 substrate | Weakly | Weakly | Weakly | Yes | Weakly | No | Yes | Yes | Weakly | |
| CYP2C9 inhibitor | No | No | No | Yes | No | No | No | No | No | |
| CYP2C9 substrate | No | No | No | No | No | No | No | No | No | |
| CYP2C19 inhibitor | No | No | No | Yes | No | No | No | No | No | |
| CYP2C19 substrate | No | No | No | No | No | No | No | No | No | |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | Yes | No | |
| CYP2D6 substrate | No | No | No | No | No | Yes | No | Weakly | No | |
| Excretion | T1/2 (˃8 h: high; 3 h < Cl < 8 h: moderate; <3 h: low) | 0.442 | 0.565 | 0.565 | 1.587 | 1.213 | 1.838 | 0.318 | 1.32 | 0.713 |
| Clearance rate (˃15 mL/min/kg: high; 5mL/min/kg < Cl < ˃15 mL/min/kg: moderate; <5 mL/ min/kg: low) | 1.196 | 1.174 | 1.174 | 1.569 | 1.033 | 0.911 | 1.601 | 0.503 | 1.526 | |
| Toxicity | hERG I/II | No/No | No/No | No/No | No/No | No/No | Yes/Yes | No/No | Ambiguous | No/No |
| AMES toxicity | No | No | No | No | No | No | No | No | No | |
| H-HT (Human hepatotoxicity) | No | No | No | No | No | No | No | No | No | |
| Skin sensitization | No | No | No | No | No | Yes | No | No | No | |
| Max. tolerated dose (human) (log mg/kg/day) | −0.134 | 1.285 | 1.285 | −0.078 | 0.306 | 0.902 | 0.787 | 0.484 | 0.485 |
| Compounds | Binding Affinity (Kcal/mol)α-Amylase | No of H-Bonds | H-Bonds Residues with H-Bonds Length (Å) | Binding Affinity (Kcal/mol)α-glucosidase | No of H-Bonds | H-Bonds Residues with H-Bonds Length (Å) |
|---|---|---|---|---|---|---|
| 3-caffeoylquinic acid | −7.2 | 4 | Ala106 (3.20 Å), Asn105 (3.16 Å), Thr163 (2.65 Å), Gln63 (3.16 Å) | −7.8 | 6 | Gln839 (1.96 Å), Ser774 (2.55 Å), Asn797 (2.68 Å), Thr769 (1.83 Å), Arg773 (2.39 Å) Arg392 (2.78 Å) |
| 3-O-Caffeoyl-4-O-methylquinic acid | −7.4 | 5 | Asp300 (2.27 Å), Thr163 (3.27 Å), Glu233 (2.13 Å), Asp197 (2.94 Å), His305 (2.08 Å) | −7.6 | 3 | Asn797 (2.48 Å), Thr769 (3.15 Å), Arg392 (2.89 Å) |
| 4-Feruloylquinic acid | −7.7 | 4 | Arg195 (3.20 Å), Gln63 (2.70 Å), Thr163 (2.79 Å), Asp300 (2.24 Å) | −7.3 | 4 | Gln839 (2.70 Å), Trp841 (3.25 Å), Thr769 (2.62 Å), Arg392 (2.98 Å) |
| 5-Feruloylquinic acid | −7.7 | 2 | Glu233 (2.59 Å), Asp197 (2.31 Å) | −7.3 | 4 | Gly390 (2.50 Å), Ser774 (2.98 Å), Trp841 (2.88 Å), Arg392 (2.98 Å) |
| Arbutin | −7.0 | 3 | Glu233 (2.53 Å), Asp197 (2.23 Å), Gln63 (2.96 Å) | −6.8 | 5 | Thr769 (2.71 Å), Trp320 (3.34 Å), Asn797 (2.79 Å), Arg392 (3.28 Å), His387 (2.77 Å) |
| Engeletin | −8.5 | 4 | Glu233 (2.63 Å), Arg195 (3.34 Å), Gln63 (2.79 Å), His305 (1.97 Å) | −8.4 | 5 | Arg392 (2.83 Å), Tyr319 (3.13 Å), Trp320 (2.91 Å), Arg773 (3.32 Å), Gln770(2.84 Å) |
| Acarbose | −6.1 | 4 | Glu233 (3.08 Å) Asp300 (2.49 Å), Thr163 (2.93 Å), Gln63 (2.04 Å) | –6.3 | 4 | Glu109 (2.75 Å), Lys560 (2.19 Å), Thr561 (2.29 Å), Met801 (2.29 Å) |
| Protocatechuic acid | −7.2 | 3 | Glu233 (2.97 Å), Gln63 (3.06 Å), Thr163 (2.57 Å) | −6.6 | 3 | Thr775 (2.65 Å), Ser774 (2.55 Å), Arg773 (2.79 Å) |
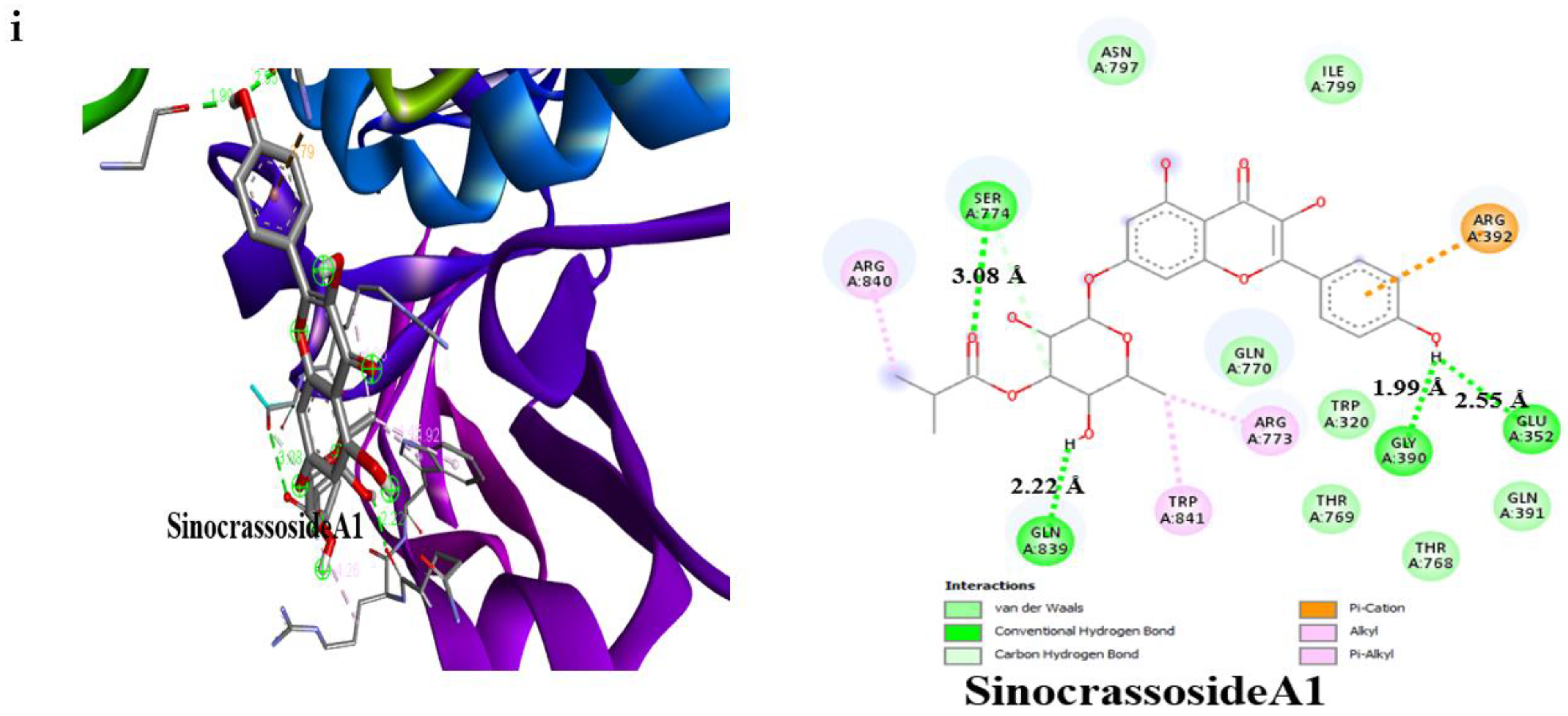
| SinocrassosideA1 | −9.6 | 3 | Gln63 (2.50 Å), Asp300 (2.49 Å), Glu233 (2.27 Å) | −9.0 | 4 | Gln839 (2.22 Å), Ser774 (3.08 Å), Glu352 (2.55 Å), Gly390 (1.99 Å) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinyede, K.A.; Oyewusi, H.A.; Hughes, G.D.; Ekpo, O.E.; Oguntibeju, O.O. In Vitro Evaluation of the Anti-Diabetic Potential of Aqueous Acetone Helichrysum petiolare Extract (AAHPE) with Molecular Docking Relevance in Diabetes Mellitus. Molecules 2022, 27, 155. https://doi.org/10.3390/molecules27010155
Akinyede KA, Oyewusi HA, Hughes GD, Ekpo OE, Oguntibeju OO. In Vitro Evaluation of the Anti-Diabetic Potential of Aqueous Acetone Helichrysum petiolare Extract (AAHPE) with Molecular Docking Relevance in Diabetes Mellitus. Molecules. 2022; 27(1):155. https://doi.org/10.3390/molecules27010155
Chicago/Turabian StyleAkinyede, Kolajo Adedamola, Habeebat Adekilekun Oyewusi, Gail Denise Hughes, Okobi Eko Ekpo, and Oluwafemi Omoniyi Oguntibeju. 2022. "In Vitro Evaluation of the Anti-Diabetic Potential of Aqueous Acetone Helichrysum petiolare Extract (AAHPE) with Molecular Docking Relevance in Diabetes Mellitus" Molecules 27, no. 1: 155. https://doi.org/10.3390/molecules27010155
APA StyleAkinyede, K. A., Oyewusi, H. A., Hughes, G. D., Ekpo, O. E., & Oguntibeju, O. O. (2022). In Vitro Evaluation of the Anti-Diabetic Potential of Aqueous Acetone Helichrysum petiolare Extract (AAHPE) with Molecular Docking Relevance in Diabetes Mellitus. Molecules, 27(1), 155. https://doi.org/10.3390/molecules27010155






