Recent Advances in Aggregation-Induced Emission Active Materials for Sensing of Biologically Important Molecules and Drug Delivery System
Abstract
:1. Introduction
2. Aggregation Induced Emission (AIE) Active Molecules for Sensing Application
3. AIE Active Molecules for Biological Cell Imaging
4. AIE Molecules for Drug Delivery Systems
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elahi, N.; Kamali, M.; Baghersad, M.H.; Amini, B. A Fluorescence Nano-Biosensors Immobilization on Iron (MNPs) and Gold (AuNPs) Nanoparticles for Detection of Shigella spp. Mater. Sci. Eng. C 2019, 105, 110113. [Google Scholar] [CrossRef]
- Reineck, P.; Francis, A.; Orth, A.; Lau, D.W.M.; Nixon-Luke, R.D.V.; Rastogi, I.; Razali, W.A.W.; Cordina, N.M.; Parker, L.M.; Sreenivasan, V.K.A.; et al. Brightness and Photostability of Emerging Red and Near-IR Fluorescent Nanomaterials for Bioimaging. Adv. Opt. Mater. 2016, 4, 1549–1557. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, C.J.; Liu, B. A Photoactivatable AIE Polymer for Light-Controlled Gene Delivery: Concurrent Endo/Lysosomal Escape and DNA Unpacking. Angew. Chem. Int. Ed. 2015, 54, 11419–11423. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.H.; Wei, Q.; Sun, D.W. Carbon Dots: Principles and Their Applications in Food Quality and Safety Detection. Crit. Rev. Food Sci. Nutr. 2018, 58, 2466–2475. [Google Scholar] [CrossRef] [PubMed]
- Lange, L.; Meyer, A.S. Potentials and Possible Safety Issues of Using Biorefinery Products in Food Value Chains. Trends Food Sci. Technol. 2019, 84, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Mattarozzi, M.; Bianchi, F.; Maffini, M.; Vescovi, F.; Catellani, D.; Suman, M.; Careri, M. ESEM-EDS-Based Analytical Approach to Assess Nanoparticles for Food Safety and Environmental Control. Talanta 2019, 196, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Sun, D.W.; Pu, H.; Wei, Q. Development of Nanozymes for Food Quality and Safety Detection: Principles and Recent Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1496–1513. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yang, S.; De Ruyck, K.; Beloglazova, N.; Eremin, S.A.; De Saeger, S.; Zhang, S.; Shen, J.; Wang, Z. Fluorescence Polarization Assays for Chemical Contaminants in Food and Environmental Analyses. TrAC Trends Anal. Chem. 2019, 114, 293–313. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. AIE Luminogens: Emission Brightened by Aggregation. Mater. Today 2015, 18, 365–377. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, W.; Zhou, T.; Peng, Q.; Zhai, D.; Li, H.; Yuan, W.Z.; Zhang, Y.; Tang, B.Z. Conjugation-Induced Rigidity in Twisting Molecules: Filling the Gap between Aggregation-Caused Quenching and Aggregation-Induced Emission. Adv. Mater. 2015, 27, 4496–4501. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Phenomenon, Mechanism and Applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; et al. Aggregation-Induced Emission of 1-Methyl-1,2,3,4,5-Pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Yang, M.; Xiao, H.; Wang, L.; Chen, Z.; Liu, S.; Li, J.; Li, S.; James, T.D. “Irregular” Aggregation-Induced Emission Luminogens. Coord. Chem. Rev. 2020, 418, 213358. [Google Scholar] [CrossRef]
- Gu, M.; Zeng, Z.; Xing, M.; Xiong, Y.; Deng, Z.; Chen, S.; Wang, L. The Biological Applications of Two Aggregation-Induced Emission Luminogens. Biotechnol. J. 2019, 14, e1900212. [Google Scholar] [CrossRef]
- Cai, X.; Liu, B. Aggregation-Induced Emission: Recent Advances in Materials and Biomedical Applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886. [Google Scholar] [CrossRef]
- Ma, J.; Sengupta, M.K.; Yuan, D.; Dasgupta, P.K. Speciation and Detection of Arsenic in Aqueous Samples: A Review of Recent Progress in Non-Atomic Spectrometric Methods. Anal. Chim. Acta 2014, 831, 1–23. [Google Scholar] [CrossRef]
- Hong, Y. Aggregation-Induced Emission-Fluorophores and Applications. Methods Appl. Fluoresc. 2016, 4, 22003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, G.; Kwok, R.T.K.; Tang, B.Z.; Liu, B. Functionality and Versatility of Aggregation-Induced Emission Luminogens. Appl. Phys. Rev. 2017, 4, 021307. [Google Scholar] [CrossRef]
- Niu, G.; Zhang, R.; Shi, X.; Park, H.; Xie, S.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE Luminogens as Fluorescent Bioprobes. TrAC Trends Anal. Chem. 2020, 123, 115769. [Google Scholar] [CrossRef]
- Leung, N.L.C.; Xie, N.; Yuan, W.; Liu, Y.; Wu, Q.; Peng, Q.; Miao, Q.; Lam, J.W.Y.; Tang, B.Z. Restriction of Intramolecular Motions: The General Mechanism behind Aggregation-Induced Emission. Chem. A Eur. J. 2014, 20, 15349–15353. [Google Scholar] [CrossRef]
- Yang, J.; Chi, Z.; Zhu, W.; Tang, B.Z.; Li, Z. Aggregation-Induced Emission: A Coming-of-Age Ceremony at the Age of Eighteen. Sci. China Chem. 2019, 62, 1090–1098. [Google Scholar] [CrossRef]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Tetraphenylethylene-Based AIE-Active Probes for Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 12189–12216. [Google Scholar] [CrossRef]
- He, T.; Wang, H.; Chen, Z.; Liu, S.; Li, J.; Li, S. Natural Quercetin AIEgen Composite Film with Antibacterial and Antioxidant Properties for in Situ Sensing of Al3+ Residues in Food, Detecting Food Spoilage, and Extending Food Storage Times. ACS Appl. Bio Mater. 2018, 1, 636–642. [Google Scholar] [CrossRef]
- Khan, I.M.; Niazi, S.; Iqbal Khan, M.K.; Pasha, I.; Mohsin, A.; Haider, J.; Iqbal, M.W.; Rehman, A.; Yue, L.; Wang, Z. Recent Advances and Perspectives of Aggregation-Induced Emission as an Emerging Platform for Detection and Bioimaging. TrAC Trends Anal. Chem. 2019, 119, 115637. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Al Kobaisi, M.; Jadhav, R.W.; Morajakar, P.P.; Jones, L.A.; George, S. Naphthalene Diimides: Perspectives and Promise. Chem. Soc. Rev. 2021, 50, 9845–9998. [Google Scholar] [CrossRef]
- Mente, A.; O’donnell, M.; Yusuf, S. Sodium Intake and Health: What Should We Recommend Based on the Current Evidence? Nutrients 2021, 13, 3232. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Gritter, M.; Rotmans, J.I.; Hoorn, E.J. Role of Dietary K+ in Natriuresis, Blood Pressure Reduction, Cardiovascular Protection, and Renoprotection. Hypertension 2019, 73, 15–23. [Google Scholar] [CrossRef]
- Xiong, M.; Zhu, H.; Rong, Q.; Yang, C.; Qiu, L.; Zhang, X.B.; Tan, W. A Membrane-Anchored Fluorescent Probe for Detecting K+ in the Cell Microenvironment. Chem. Commun. 2016, 52, 4679–4682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Shao, Y.; Peng, J.; Huang, C.; Liu, H.; Zhang, L. Molecular Rotor-Based Fluorescent Probe for Selective Recognition of Hybrid G-Quadruplex and as a K+ Sensor. Anal. Chem. 2014, 86, 1622–1631. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Liu, T.; Zhang, G.; Liu, S. Highly Sensitive and Selective Fluorometric Off-on K+ Probe Constructed via Host-Guest Molecular Recognition and Aggregation-Induced Emission. J. Mater. Chem. 2012, 22, 8622–8628. [Google Scholar] [CrossRef]
- Lu, D.; He, L.; Wang, Y.; Xiong, M.; Hu, M.; Liang, H.; Huan, S.; Zhang, X.B.; Tan, W. Tetraphenylethene Derivative Modified DNA Oligonucleotide for in Situ Potassium Ion Detection and Imaging in Living Cells. Talanta 2017, 167, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, H.; Takagi, M.; Takenaka, S. A Novel Potassium Sensing in Aqueous Media with a Synthetic Oligonucleotide Derivative. Fluorescence Resonance Energy Transfer Associated with Guanine Quartet-Potassium Ion Complex Formation. J. Am. Chem. Soc. 2002, 124, 14286–14287. [Google Scholar] [CrossRef]
- Breitwieser, G.E. Extracellular Calcium as an Integrator of Tissue Function. Int. J. Biochem. Cell Biol. 2008, 40, 1467–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akkaya, E.U.; Turkyilmaz, S. A Squaraine-Based near IR Fluorescent Chemosensor for Calcium. Tetrahedron Lett. 1997, 38, 4513–4516. [Google Scholar] [CrossRef]
- Gao, M.; Li, Y.; Chen, X.; Li, S.; Ren, L.; Tang, B.Z. Aggregation-Induced Emission Probe for Light-Up and in Situ Detection of Calcium Ions at High Concentration. ACS Appl. Mater. Interfaces 2018, 10, 14410–14417. [Google Scholar] [CrossRef]
- Wang, P.; Jia, K.; Zhou, X.; Guan, X.; Wang, L.; Tian, Y.; Wu, C.; Liu, X. Ca2+ Induced Crosslinking of AIE-Active Polyarylene Ether Nitrile into Fluorescent Polymeric Nanoparticles for Cellular Bioimaging. Macromol. Rapid Commun. 2017, 38, 1700360. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Z.; Wang, S.; She, M.; Zhang, Z.; Cai, W.; Liu, P.; Li, J. Water Soluble Chemosensor for Ca2+ Based on Aggregation-Induced Emission Characteristics and Its Fluorescence Imaging in Living Cells. Dye Pigment. 2018, 150, 112–120. [Google Scholar] [CrossRef]
- Ishiwari, F.; Hasebe, H.; Matsumura, S.; Hajjaj, F.; Horii-Hayashi, N.; Nishi, M.; Someya, T.; Fukushima, T. Bioinspired Design of a Polymer Gel Sensor for the Realization of Extracellular Ca2+ Imaging. Sci. Rep. 2016, 6, 24275. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, G.; Zhang, D.; Xue, L.; Jiang, H. Aqueous Fluorescence “Turn-on” Sensor for Zn2+ with a Tetraphenylethylene Compound. Org. Lett. 2011, 13, 6378–6381. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, L.; Huang, J.; Zhao, J.; Yan, Y. Multifunctional Metallo-Organic Vesicles Displaying Aggregation-Induced Emission: Two-Photon Cell-Imaging, Drug Delivery, and Specific Detection of Zinc Ion. ACS Appl. Nano Mater. 2018, 1, 1819–1827. [Google Scholar] [CrossRef]
- Tang, A.; Yin, Y.; Chen, Z.; Fan, C.; Liu, G.; Pu, S. A Multifunctional Aggregation-Induced Emission (AIE)-Active Fluorescent Chemosensor for Detection of Zn2+ and Hg2+. Tetrahedron 2019, 75, 130489. [Google Scholar] [CrossRef]
- Maity, S.; Shyamal, M.; Maity, R.; Mudi, N.; Hazra, P.; Giri, P.K.; Samanta, S.S.; Pyne, S.; Misra, A. An Antipyrine Based Fluorescent Probe for Distinct Detection of Al3+ and Zn2+ and Its AIEE Behaviour. Photochem. Photobiol. Sci. 2020, 19, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Diana, R.; Panunzi, B. Zinc (Ii) and Aiegens: The “Clip Approach” for a Novel Fluorophore Family: A Review. Molecules 2021, 26, 4176. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, Y.; Nie, J.; Wei, J.; Miao, B.; Zhao, Y.; Zhang, L.; Ni, Z. Multifunctional AIE-ESIPT Dual Mechanism Tetraphenylethene-Based Schiff Base for Inkless Rewritable Paper and a Colorimetric/Fluorescent Dual-Channel Zn2+ sensor. Mater. Chem. Front. 2021, 5, 347–354. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Zhang, L.; Fang, G.; Liu, J.; Wang, S. Sensing and Intracellular Imaging of Zn2+ Based on Affinity Peptide Using an Aggregation Induced Emission Fluorescence “Switch-on” Probe. Sens. Actuators B Chem. 2018, 271, 289–299. [Google Scholar] [CrossRef]
- FN, C.; MF, M. Factors Affecting Water Pollution: A Review. J. Ecosyst. Ecography 2017, 7, 5–8. [Google Scholar] [CrossRef]
- Vongdala, N.; Tran, H.D.; Xuan, T.D.; Teschke, R.; Khanh, T.D. Heavy Metal Accumulation in Water, Soil, and Plants of Municipal Solid Waste Landfill in Vientiane, Laos. Int. J. Environ. Res. Public Health 2019, 16, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebenstein, A. The Consequences of Industrialization: Evidence From. Rev. Econ. Stat. 2012, 94, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Moss, B. Water Pollution by Agriculture. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Chua, M.H.; Zhou, H.; Zhu, Q.; Tang, B.Z.; Xu, J.W. Recent Advances in Cation Sensing Using Aggregation-Induced Emission. Mater. Chem. Front. 2021, 5, 659–708. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Li, H.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular Materials Based on AIE Luminogens (AIEgens): Construction and Applications. Chem. Soc. Rev. 2020, 49, 1144–1172. [Google Scholar] [CrossRef] [PubMed]
- Baglan, M.; Atılgan, S. Selective and Sensitive “Turn-on” Fluorescent Sensing of Arsenite Based on Cysteine Fused Tetraphenylethene with AIE Characteristics in Aqueous Media. Chem. Commun. 2013, 49, 5325–5327. [Google Scholar] [CrossRef]
- Wen, X.; Yan, L.; Fan, Z. A Novel AIE Active NIR Fluorophore Based Triphenylamine for Sensing of Hg2+ and CN− and Its Multiple Application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118664. [Google Scholar] [CrossRef]
- Ma, J.; Xiao, Y.; Zhang, C.; Zhang, M.; Wang, Q.; Zheng, W.; Zhang, S. Preparation a Novel Pyrene-Based AIE-Active Ratiometric “Turn-on” Fluorescent Probe for Highly Selective and Sensitive Detection of Hg2+. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 259, 114582. [Google Scholar] [CrossRef]
- Zalmi, G.A.; Gawade, V.K.; Nadimetla, D.N.; Bhosale, S.V. Aggregation Induced Emissive Luminogens for Sensing of Toxic Elements. ChemistryOpen 2021, 10, 681–696. [Google Scholar] [CrossRef]
- Nadimetla, D.N.; Bhosale, S.V. Tetraphenylethylene AIEgen Bearing Thiophenylbipyridine Receptor for Selective Detection of Copper(Ii) Ion. New J. Chem. 2021, 45, 7614–7621. [Google Scholar] [CrossRef]
- Jiang, S.; Qiu, J.; Chen, S.; Guo, H.; Yang, F. Double-Detecting Fluorescent Sensor for ATP Based on Cu2+ and Zn2+ Response of Hydrazono-Bis-Tetraphenylethylene. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117568. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, W.; Yu, L.; Wang, Y. Click Synthesis of a Novel Triazole Bridged AIE Active Cyclodextrin Probe for Specific Detection of Cd2+. Chem. Commun. 2015, 51, 4298–4301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalmi, G.A.; Nadimetla, D.N.; Kotharkar, P.; Puyad, A.L.; Kowshik, M.; Bhosale, S.V. Aggregation-Induced Emission-Based Material for Selective and Sensitive Recognition of Cyanide Anions in Solution and Biological Assays. ACS Omega 2021, 6, 16704–16713. [Google Scholar] [CrossRef]
- Nhien, P.Q.; Chou, W.L.; Cuc, T.T.K.; Khang, T.M.; Wu, C.H.; Thirumalaivasan, N.; Hue, B.T.B.; Wu, J.I.; Wu, S.P.; Lin, H.C. Multi-Stimuli Responsive FRET Processes of Bifluorophoric AIEgens in an Amphiphilic Copolymer and Its Application to Cyanide Detection in Aqueous Media. ACS Appl. Mater. Interfaces 2020, 12, 10959–10972. [Google Scholar] [CrossRef]
- Nadimetla, D.N.; Zalmi, G.A.; Bhosale, S.V. An AIE-Active Tetraphenylethylene-Based Cyclic Urea as a Selective and Efficient Optical and Colorimetric Chemosensor for Fluoride Ions. ChemistrySelect 2020, 5, 8566–8571. [Google Scholar] [CrossRef]
- Anuradha; Latham, K.; Bhosale, S.V. Selective Detection of Nitrite Ion by an AIE-Active Tetraphenylethene Dye through a Reduction Step in Aqueous Media. RSC Adv. 2016, 6, 45009–45013. [Google Scholar] [CrossRef]
- Li, K.; Li, Z.; Liu, D.; Chen, M.; Wang, S.C.; Chan, Y.T.; Wang, P. Tetraphenylethylene(TPE)-Containing Metal-Organic Nanobelt and Its “Turn-on” Fluorescence for Sulfide (S2−). Inorg. Chem. 2020, 59, 6640–6645. [Google Scholar] [CrossRef]
- Chen, M.; Xiang, S.; Lv, P.; Qi, C.; Feng, H.T.; Tang, B.Z. A Novel Fluorescence Tool for Monitoring Agricultural Industry Chain Based on AIEgens. Chem. Res. Chinese Univ. 2021, 37, 38–51. [Google Scholar] [CrossRef]
- Zhou, H.; Chua, M.H.; Tang, B.Z.; Xu, J. Aggregation-Induced Emission (AIE)-Active Polymers for Explosive Detection. Polym. Chem. 2019, 10, 3822–3840. [Google Scholar] [CrossRef]
- Hou, X.G.; Wu, Y.; Cao, H.T.; Sun, H.Z.; Li, H.B.; Shan, G.G.; Su, Z.M. A Cationic Iridium(Iii) Complex with Aggregation-Induced Emission (AIE) Properties for Highly Selective Detection of Explosives. Chem. Commun. 2014, 50, 6031–6034. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.L.; Rananaware, A.; Rix, C.; Bhosale, S.V.; Latham, K. Highly Fluorescent Metal-Organic Framework for the Sensing of Volatile Organic Compounds. Cryst. Growth Des. 2016, 16, 3067–3071. [Google Scholar] [CrossRef]
- Chang, J.; Li, H.; Hou, T.; Duan, W.; Li, F. Paper-Based Fluorescent Sensor via Aggregation Induced Emission Fluorogen for Facile and Sensitive Visual Detection of Hydrogen Peroxide and Glucose. Biosens. Bioelectron. 2018, 104, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Chen, S.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Water-Soluble Tetraphenylethene Derivatives as Fluorescent “Light-up” Probes for Nucleic Acid Detection and Their Applications in Cell Imaging. Chem. Asian J. 2013, 8, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xiong, L.H.; Zhao, Z.; Wang, Z.; Luo, L.; Lam, J.W.Y.; Kwok, R.T.K.; Tang, B.Z. AIE-Based Theranostic Systems for Detection and Killing of Pathogens. Theranostics 2019, 9, 3223–3248. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Wang, D.; Wang, Z.; Wang, X.; Tian, C.; Luan, F.; Fu, X. Functionalized Copper Nanoclusters-Based Fluorescent Probe with Aggregation-Induced Emission Property for Selective Detection of Sulfide Ions in Food Additives. J. Agric. Food Chem. 2020, 68, 11301–11308. [Google Scholar] [CrossRef]
- Lu, Q.; Wu, C.J.; Liu, Z.; Niu, G.; Yu, X. Fluorescent AIE-Active Materials for Two-Photon Bioimaging Applications. Front. Chem. 2020, 8, 617463. [Google Scholar] [CrossRef]
- Li, K.; Qin, W.; Ding, D.; Tomczak, N.; Geng, J.; Liu, R.; Liu, J.; Zhang, X.; Liu, H.; Liu, B.; et al. Photostable Fluorescent Organic Dots with Aggregation-Induced Emission (AIE Dots) for Noninvasive Long-Term Cell Tracing. Sci. Rep. 2013, 3, srep01150. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Chen, C.; Ding, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens: Union Is Strength, Gathering Illuminates Healthcare. Adv. Healthc. Mater. 2018, 7, e1800477. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, S.; Lam, J.W.Y.; Qin, W.; Kwok, R.T.K.; Xie, N.; Hu, Q.; Tang, B.Z. Long-Term Fluorescent Cellular Tracing by the Aggregates of AIE Bioconjugates. J. Am. Chem. Soc. 2013, 135, 8238–8245. [Google Scholar] [CrossRef]
- Qin, W.; Alifu, N.; Cai, Y.; Lam, J.W.Y.; He, X.; Su, H.; Zhang, P.; Qian, J.; Tang, B.Z. Synthesis of an Efficient Far-Red/near-Infrared Luminogen with AIE Characteristics for: In Vivo Bioimaging Applications. Chem. Commun. 2019, 55, 5615–5618. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Chen, J.; Lin, G.; Li, S.; Wang, L.; Qin, A.; Zhao, Z.; Ren, L.; Wang, Y.; Tang, B.Z. Long-Term Tracking of the Osteogenic Differentiation of Mouse BMSCs by Aggregation-Induced Emission Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 17878–17884. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, P.; Gao, M.; Zeng, F.; Qin, A.; Wu, S.; Tang, B.Z. Ratiometric Detection and Imaging of Endogenous Hypochlorite in Live Cells and: In Vivo Achieved by Using an Aggregation Induced Emission (AIE)-Based Nanoprobe. Chem. Commun. 2016, 52, 7288–7291. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Qi, C.; Cheng, C.; Yang, Z.; Cao, H.; Yang, Z.; Tong, J.; Yao, X.; Lei, Z. AIE-Active Tetraphenylethylene Cross-Linked N-Isopropylacrylamide Polymer: A Long-Term Fluorescent Cellular Tracker. ACS Appl. Mater. Interfaces 2016, 8, 8341–8348. [Google Scholar] [CrossRef]
- Wan, Q.; Jiang, R.; Guo, L.; Yu, S.; Liu, M.; Tian, J.; Liu, G.; Deng, F.; Zhang, X.; Wei, Y. Novel Strategy toward AIE-Active Fluorescent Polymeric Nanoparticles from Polysaccharides: Preparation and Cell Imaging. ACS Sustain. Chem. Eng. 2017, 5, 9955–9964. [Google Scholar] [CrossRef]
- Gao, M.; Su, H.; Li, S.; Lin, Y.; Ling, X.; Qin, A.; Tang, B.Z. An Easily Accessible Aggregation-Induced Emission Probe for Lipid Droplet-Specific Imaging and Movement Tracking. Chem. Commun. 2017, 53, 921–924. [Google Scholar] [CrossRef]
- Gao, M.; Su, H.; Lin, Y.; Ling, X.; Li, S.; Qin, A.; Tang, B.Z. Photoactivatable Aggregation-Induced Emission Probes for Lipid Droplets-Specific Live Cell Imaging. Chem. Sci. 2017, 8, 1763–1768. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Qin, W.; Xu, B.; Qian, J.; Tang, B.Z. AIE Nanoparticles with High Stimulated Emission Depletion Efficiency and Photobleaching Resistance for Long-Term Super-Resolution Bioimaging. Adv. Mater. 2017, 29, 1703643. [Google Scholar] [CrossRef]
- Xu, D.; Liu, M.; Zou, H.; Huang, Q.; Huang, H.; Tian, J.; Jiang, R.; Wen, Y.; Zhang, X.; Wei, Y. Fabrication of AIE-Active Fluorescent Organic Nanoparticles through One-Pot Supramolecular Polymerization and Their Biological Imaging. J. Taiwan Inst. Chem. Eng. 2017, 78, 455–461. [Google Scholar] [CrossRef]
- Liow, S.S.; Zhou, H.; Sugiarto, S.; Guo, S.; Chalasani, M.L.S.; Verma, N.K.; Xu, J.; Loh, X.J. Highly Efficient Supramolecular Aggregation-Induced Emission-Active Pseudorotaxane Luminogen for Functional Bioimaging. Biomacromolecules 2017, 18, 886–897. [Google Scholar] [CrossRef]
- Guan, X.; Lu, B.; Jin, Q.; Li, Z.; Wang, L.; Wang, K.; Lai, S.; Lei, Z. AIE-Active Fluorescent Nonconjugated Polymer Dots for Dual-Alternating-Color Live Cell Imaging. Ind. Eng. Chem. Res. 2018, 57, 14889–14898. [Google Scholar] [CrossRef]
- Zhuang, W.; Yang, L.; Li, G.; Wang, Y. Redox and Ph Dual-Responsive Polymeric Micelle with Aggregation-Induced Emission Feature for Cellular Imaging and Chemotherapy. Trans. Annu. Meet. Soc. Biomater. Annu. Int. Biomater. Symp. 2019, 40, 557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Y.; Chen, Y.; Zhao, E.; Hong, Y.; Chen, S.; Lam, J.W.Y.; Chen, Y.; Hou, J.; Tang, B.Z. Amphiphilic Tetraphenylethene-Based Pyridinium Salt for Selective Cell-Membrane Imaging and Room-Light-Induced Special Reactive Oxygen Species Generation. ACS Appl. Mater. Interfaces 2019, 11, 10567–10577. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, Y.; He, X.; Liu, H.; Zhao, Z.; Kwok, R.T.K.; Elsegood, M.R.J.; Lam, J.W.Y.; Tang, B.Z. A Substitution-Dependent Light-Up Fluorescence Probe for Selectively Detecting Fe3+ Ions and Its Cell Imaging Application. Adv. Funct. Mater. 2018, 28, 1802833. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Liu, Y.Y.; Li, Y.; Zhuang, J.B.; Cui, R.R.; Gong, Q.; Zhao, N.; Tang, B.Z. Fine Tuning of Emission Behavior, Self-Assembly, Anion Sensing, and Mitochondria Targeting of Pyridinium-Functionalized Tetraphenylethene by Alkyl Chain Engineering. ACS Appl. Mater. Interfaces 2018, 10, 24249–24257. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhou, T.; Li, B.; Kwok, R.T.K.; Shen, J.; Qin, A.; Tang, B.Z. Selective Viable Cell Discrimination by a Conjugated Polymer Featuring Aggregation-Induced Emission Characteristic. Biomaterials 2020, 230, 119658. [Google Scholar] [CrossRef]
- Lin, M.; Huang, J.; Zeng, F.; Wu, S. A Fluorescent Probe with Aggregation-Induced Emission for Detecting Alkaline Phosphatase and Cell Imaging. Chem. Asian J. 2019, 14, 802–808. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, C.; Han, X.; Liu, S.H.; Tan, Y.; Yin, J. Fluorophore-Labeling Tetraphenylethene Dyes Ranging from Visible to Near-Infrared Region: AIE Behavior, Performance in Solid State, and Bioimaging in Living Cells. J. Org. Chem. 2019, 84, 14498–14507. [Google Scholar] [CrossRef]
- Wang, J.; Hang, Y.; Hua, J. Mannose-Functionalized Diketopyrrolopyrrole as AIE-Active Probes for Lectin Detection and Cancer Cell Imaging. Sens. Actuators B Chem. 2019, 282, 232–242. [Google Scholar] [CrossRef]
- Yan, L.; Wen, X.; Fan, Z. A Large-Stokes-Shift Fluorescent Probe for Zn2+ Based on AIE, and Application in Live Cell Imaging. Anal. Bioanal. Chem. 2020, 412, 1453–1463. [Google Scholar] [CrossRef]
- Wang, X.; Ding, G.; Duan, Y.; Zhu, Y.; Zhu, G.; Wang, M.; Li, X.; Zhang, Y.; Qin, X.; Hung, C.H. A Novel Triphenylamine-Based Bis-Schiff Bases Fluorophores with AIE-Activity as the Hydrazine Fluorescence Turn-off Probes and Cell Imaging in Live Cells. Talanta 2020, 217, 121029. [Google Scholar] [CrossRef] [PubMed]
- Tarai, A.; Huang, M.; Das, P.; Pan, W.; Zhang, J.; Gu, Z.; Yan, W.; Qu, J.; Yang, Z. ICT and AIE Characteristics Two Cyano-Functionalized Probes and Their Photophysical Properties, DFT Calculations, Cytotoxicity, and Cell Imaging Applications. Molecules 2020, 25, 585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Xie, G.; Tao, Y.; Zhu, H.; Zhang, Y.; Chi, Z.; Liu, S.; Xu, J. Preparation of AIE Active and Thermoresponsive Poly(1-Ethenyl-4-(1,2,2-Triphenylethenyl)-Benzene-b-N-Isopropylacrylamide) Micelles for Cell Imaging. Dye Pigment. 2021, 184, 108776. [Google Scholar] [CrossRef]
- Sayed, S.M.; Jia, H.R.; Jiang, Y.W.; Zhu, Y.X.; Ma, L.; Yin, F.; Hussain, I.; Khan, A.; Ma, Q.; Wu, F.G.; et al. Photostable AIE Probes for Wash-Free, Ultrafast, and High-Quality Plasma Membrane Staining. J. Mater. Chem. B 2021, 9, 4303–4308. [Google Scholar] [CrossRef] [PubMed]
- Dineshkumar, S.; Raj, A.; Srivastava, A.; Mukherjee, S.; Pasha, S.S.; Kachwal, V.; Fageria, L.; Chowdhury, R.; Laskar, I.R. Facile Incorporation of “Aggregation-Induced Emission”—Active Conjugated Polymer into Mesoporous Silica Hollow Nanospheres: Synthesis, Characterization, Photophysical Studies, and Application in Bioimaging. ACS Appl. Mater. Interfaces 2019, 11, 31270–31282. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A Boon to Drug Delivery, Therapeutics, Diagnostics and Imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-Responsive Nanocarriers for Drug Delivery, Tumor Imaging, Therapy and Theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in Cellular Drug Delivery. Bioorganic Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Liu, L.; Guo, K.; Lu, J.; Venkatraman, S.S.; Luo, D.; Ng, K.C.; Ling, E.A.; Moochhala, S.; Yang, Y.Y. Biologically Active Core/Shell Nanoparticles Self-Assembled from Cholesterol-Terminated PEG-TAT for Drug Delivery across the Blood-Brain Barrier. Biomaterials 2008, 29, 1509–1517. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N. Nano and Microparticles as Controlled Drug Delivery Devices. J. Pharm. Pharm. Sci. 2000, 3, 234–258. [Google Scholar]
- Wang, T.; Bhosale, S.; Bhosale, S.; Li, G.; Fuhrhop, J.H. Hydrophobic and Hydrophilic Yoctowells. Acc. Chem. Res. 2006, 39, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.; Li, G.; Li, F.; Wang, T.; Ludwig, R.; Emmler, T.; Buntkowsky, G.; Fuhrhop, J.H. Counting of Labelled Tyrosine Molecules in Hydrophobic Yoctolitre Wells Filled with Water. Chem. Commun. 2005, 3559–3561. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.V.; Langford, S.J. The Development of Yoctowells as a Basis for Modeling Biological Systems. Org. Biomol. Chem. 2007, 5, 3733–3744. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.V.; Bhosale, S.V. Yoctowells as a Simple Model System for the Encapsulation and Controlled Release of Bioactive Molecules. Sci. Rep. 2013, 3, srep01982. [Google Scholar] [CrossRef]
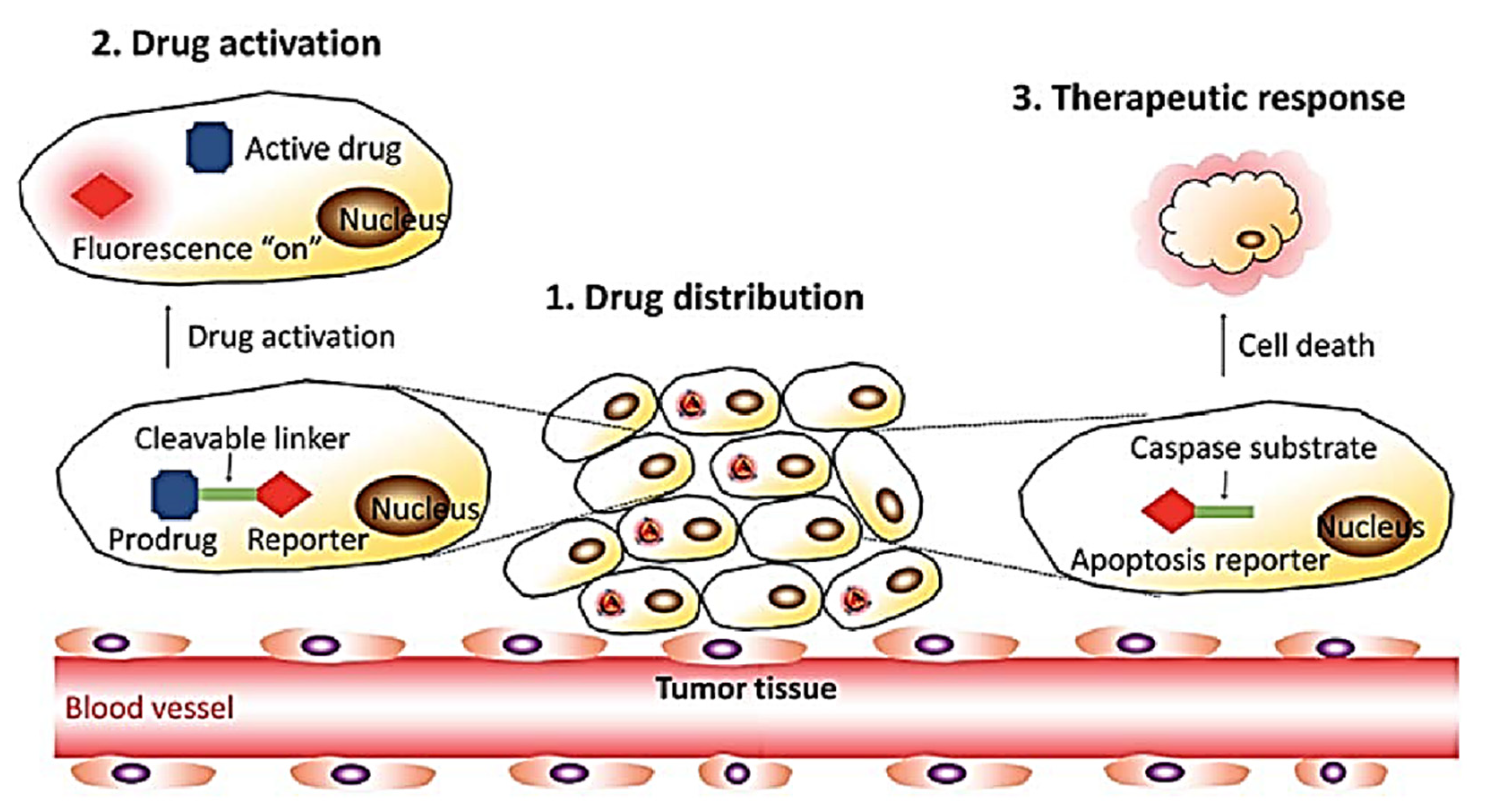
- Qian, Y.; Wang, Y.; Jia, F.; Wang, Z.; Yue, C.; Zhang, W.; Hu, Z.; Wang, W. Tumor-Microenvironment Controlled Nanomicelles with AIE Property for Boosting Cancer Therapy and Apoptosis Monitoring. Biomaterials 2019, 188, 96–106. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent Developments in Functionalized Polymer Nanoparticles for Efficient Drug Delivery System. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Bera, K.; Maiti, S.; Maity, M.; Mandal, C.; Maiti, N.C. Porphyrin-Gold Nanomaterial for Efficient Drug Delivery to Cancerous Cells. ACS Omega 2018, 3, 4602–4619. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, F.; Shao, Q.; Min, Y.; Costa, M.; Yeow, E.K.L.; Xing, B. Enzyme-Responsive Cell-Penetrating Peptide Conjugated Mesoporous Silica Quantum Dot Nanocarriers for Controlled Release of Nucleus-Targeted Drug Molecules and Real-Time Intracellular Fluorescence Imaging of Tumor Cells. Adv. Healthc. Mater. 2014, 3, 1230–1239. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. PH-Responsive Mesoporous Silica and Carbon Nanoparticles for Drug Delivery. Bioengineering 2017, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Achazi, K.; Jia, Y.; Wei, Q.; Haag, R.; Li, J. Complex Assembly of Polymer Conjugated Mesoporous Silica Nanoparticles for Intracellular PH-Responsive Drug Delivery. Langmuir 2016, 32, 12453–12460. [Google Scholar] [CrossRef]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Zhang, L.; Shi, J. Nuclear Targeting via TAT on Mesoporous NPs. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bi, A.; Zeng, W.; Cheng, Z. Upconversion Nanocomposites for Photo-Based Cancer Theranostics. J. Mater. Chem. B 2016, 4, 5331–5348. [Google Scholar] [CrossRef] [PubMed]
- Agemy, L.; Friedmann-Morvinski, D.; Kotamraju, V.R.; Roth, L.; Sugahara, K.N.; Girard, O.M.; Mattrey, R.F.; Verma, I.M.; Ruoslahti, E. Targeted Nanoparticle Enhanced Proapoptotic Peptide as Potential Therapy for Glioblastoma. Proc. Natl. Acad. Sci. USA 2011, 108, 17450–17455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, A.; Saraf, S.; Saraf, S. Cyclodextrin Based Novel Drug Delivery Systems. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 23–42. [Google Scholar] [CrossRef]
- Huang, H.; Liu, M.; Chen, J.; Mao, L.; Wan, Q.; Wen, Y.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. Fabrication of β Cyclodextrin Containing AIE-Active Polymeric Composites through Formation of Dynamic Phenylboronic Borate and Their Theranostic Applications. Cellulose 2019, 26, 8829–8841. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, J.; Liang, X.J. Aggregation-Induced Emission (AIE) Fluorophores as Imaging Tools to Trace the Biological Fate of Nano-Based Drug Delivery Systems. Adv. Drug Deliv. Rev. 2019, 143, 161–176. [Google Scholar] [CrossRef]
- Guo, M.; Song, H.; Li, K.; Ma, M.; Liu, Y.; Fu, Q.; He, Z. A New Approach to Developing Diagnostics and Therapeutics: Aggregation-Induced Emission-Based Fluorescence “Turn-On”. Med. Res. Rev. 2020, 40, 27–53. [Google Scholar] [CrossRef]
- Zhu, C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: A Trailblazing Journey to the Field of Biomedicine. ACS Appl. Bio-Mater. 2018, 1, 1768–1786. [Google Scholar] [CrossRef]
- He, X.; Xiong, L.H.; Huang, Y.; Zhao, Z.; Wang, Z.; Lam, J.W.Y.; Kwok, R.T.K.; Tang, B.Z. AIE-Based Energy Transfer Systems for Biosensing, Imaging, and Therapeutics. TrAC Trends Anal. Chem. 2020, 122, 115743. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, B. Visualization of Drug Delivery Processes Using AIEgens. Chem. Sci. 2017, 8, 2537–2546. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Liang, Z.; Chen, J.; Yu, J.; Xu, R. AIE Luminogen Bridged Hollow Hydroxyapatite Nanocapsules for Drug Delivery. Dalt. Trans. 2013, 42, 9877–9883. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Zhao, Y.; Dai, L.; Zhang, X.; Hao, X.; Zhang, C.; Huo, S.; Liu, J.; Liu, C.; Kumar, A.; et al. Spatiotemporal Drug Release Visualized through a Drug Delivery System with Tunable Aggregation-Induced Emission. Adv. Mater. 2014, 26, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jin, S.; Li, S.; Xue, X.; Liu, J.; Huang, Y.; Jiang, Y.; Chen, W.Q.; Zou, G.; Liang, X.J. Imaging Intracellular Anticancer Drug Delivery by Self-Assembly Micelles with Aggregation-Induced Emission (AIE Micelles). ACS Appl. Mater. Interfaces 2014, 6, 5212–5220. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Xie, Z. Tetraphenylethylene-Based Fluorescent Coordination Polymers for Drug Delivery. J. Mater. Chem. B 2016, 4, 4263–4266. [Google Scholar] [CrossRef] [PubMed]
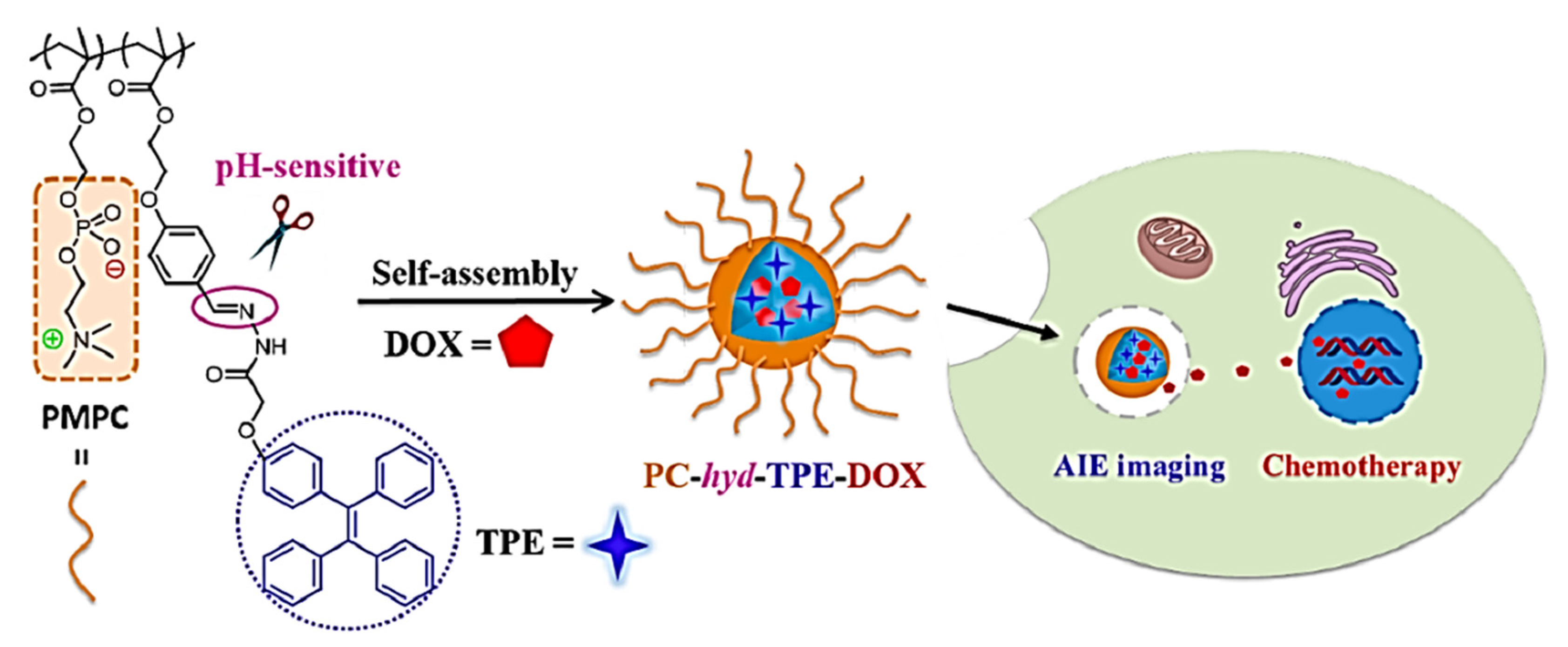
- Chen, Y.; Han, H.; Tong, H.; Chen, T.; Wang, H.; Ji, J.; Jin, Q. Zwitterionic Phosphorylcholine-TPE Conjugate for PH-Responsive Drug Delivery and AIE Active Imaging. ACS Appl. Mater. Interfaces 2016, 8, 21185–21192. [Google Scholar] [CrossRef] [PubMed]
- Liow, S.S.; Dou, Q.; Kai, D.; Li, Z.; Sugiarto, S.; Yu, C.Y.Y.; Kwok, R.T.K.; Chen, X.; Wu, Y.L.; Ong, S.T.; et al. Long-Term Real-Time In Vivo Drug Release Monitoring with AIE Thermogelling Polymer. Small 2017, 13, 1603404. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Qin, A.; Jin, Q.; Tang, B.Z.; Ji, J. Theranostic Hyaluronic Acid Prodrug Micelles with Aggregation-Induced Emission Characteristics for Targeted Drug Delivery. Sci. China Chem. 2016, 59, 1609–1615. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Zhuang, Y.; Gao, P.; Yang, F.; Shen, H.; Guo, H.; Wu, D. Fabrication of PH-Responsive Nanoparticles with an AIE Feature for Imaging Intracellular Drug Delivery. Biomacromolecules 2016, 17, 2920–2929. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Sun, C.; Wu, Z.; Xu, L.; Gao, W.; Cao, J.; Li, L.; He, B. Fabrication of Polymeric Micelles with Aggregation-Induced Emission and Forster Resonance Energy Transfer for Anticancer Drug Delivery. Bioconjug. Chem. 2017, 28, 1944–1954. [Google Scholar] [CrossRef]
- Liu, X.; Wan, Q.; Zhao, Z.; Liu, J.; Zhang, Z.; Deng, F.; Liu, M.; Wen, Y.; Zhang, X. Microwave-Assisted Diels-Alder Reaction for Rapid Synthesis of Luminescent Nanodiamond with AIE-Active Dyes and Their Biomedical Applications. Mater. Chem. Phys. 2017, 197, 256–265. [Google Scholar] [CrossRef]
- Su, X.; Ma, B.; Hu, J.; Yu, T.; Zhuang, W.; Yang, L.; Li, G.; Wang, Y. Dual-Responsive Doxorubicin-Conjugated Polymeric Micelles with Aggregation-Induced Emission Active Bioimaging and Charge Conversion for Cancer Therapy. Bioconjug. Chem. 2018, 29, 4050–4061. [Google Scholar] [CrossRef] [PubMed]
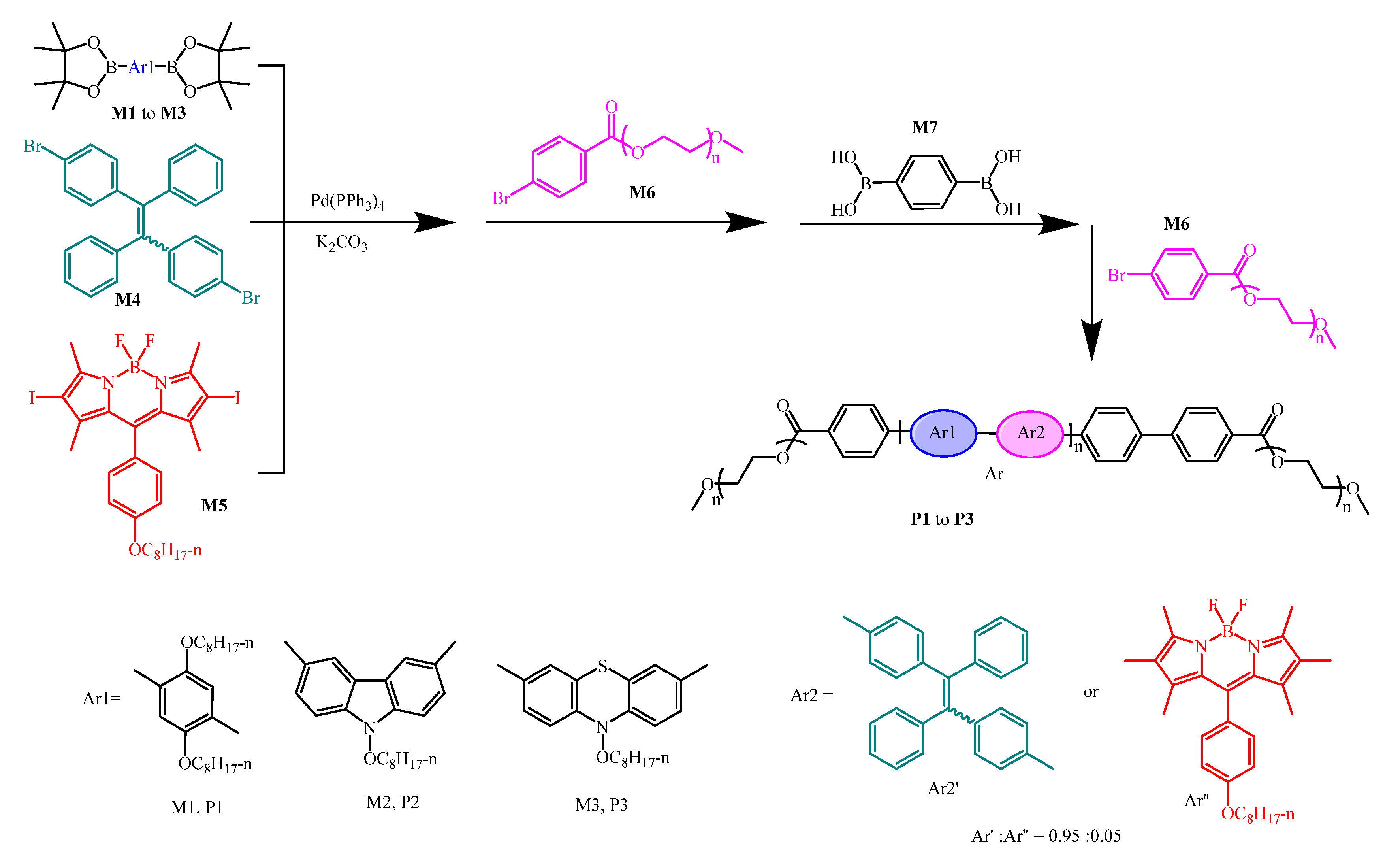
- Wang, Z.; Wang, C.; Fang, Y.; Yuan, H.; Quan, Y.; Cheng, Y. Color-Tunable AIE-Active Conjugated Polymer Nanoparticles as Drug Carriers for Self-Indicating Cancer Therapy: Via Intramolecular FRET Mechanism. Polym. Chem. 2018, 9, 3205–3214. [Google Scholar] [CrossRef]
- Gao, X.; Cao, J.; Song, Y.; Shu, X.; Liu, J.; Sun, J.Z.; Liu, B.; Tang, B.Z. A Unimolecular Theranostic System with H2O2-Specific Response and AIE-Activity for Doxorubicin Releasing and Real-Time Tracking in Living Cells. RSC Adv. 2018, 8, 10975–10979. [Google Scholar] [CrossRef] [Green Version]
- Shamsipur, M.; Molaabasi, F.; Sarparast, M.; Roshani, E.; Vaezi, Z.; Alipour, M.; Molaei, K.; Naderi-Manesh, H.; Hosseinkhani, S. Photoluminescence Mechanisms of Dual-Emission Fluorescent Silver Nanoclusters Fabricated by Human Hemoglobin Template: From Oxidation- and Aggregation-Induced Emission Enhancement to Targeted Drug Delivery and Cell Imaging. ACS Sustain. Chem. Eng. 2018, 6, 11123–11137. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Zhao, X.; Yu, F.; Quan, Y.; Cheng, Y.; Yuan, H. DOX Loaded Aggregation-Induced Emission Active Polymeric Nanoparticles as a Fluorescence Resonance Energy Transfer Traceable Drug Delivery System for Self-Indicating Cancer Therapy. Acta Biomater. 2019, 85, 218–228. [Google Scholar] [CrossRef]
- Wu, P.; Wang, X.; Wang, Z.; Ma, W.; Guo, J.; Chen, J.; Yu, Z.; Li, J.; Zhou, D. Light-Activatable Prodrug and AIEgen Copolymer Nanoparticle for Dual-Drug Monitoring and Combination Therapy. ACS Appl. Mater. Interfaces 2019, 11, 18691–18700. [Google Scholar] [CrossRef]
- Dong, Z.; Bi, Y.; Cui, H.; Wang, Y.; Wang, C.; Li, Y.; Jin, H.; Wang, C. AIE Supramolecular Assembly with FRET Effect for Visualizing Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 23840–23847. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Gan, Q.; Cao, Y.; Yuan, H.; Hua, D. Donor-Acceptor-Type Conjugated Polymer-Based Multicolored Drug Carriers with Tunable Aggregation-Induced Emission Behavior for Self-Illuminating Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 41853–41861. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, H.T.; Chen, X.Y.; Gao, P.F.; Hu, C.H. A Multifunctional AIEgen with High Cell-Penetrating Ability for Intracellular Fluorescence Assays, Imaging and Drug Delivery. Mater. Chem. Front. 2019, 3, 1151–1158. [Google Scholar] [CrossRef]
- Ma, B.; Zhuang, W.; He, H.; Su, X.; Yu, T.; Hu, J.; Yang, L.; Li, G.; Wang, Y. Two-Photon AIE Probe Conjugated Theranostic Nanoparticles for Tumor Bioimaging and PH-Sensitive Drug Delivery. Nano Res. 2019, 12, 1703–1712. [Google Scholar] [CrossRef]
- Ma, W.; Bi, J.; Wu, H.; Zhang, G. An Amphiphilic Micromolecule Self-Assembles into Vesicles for Visualized and Targeted Drug Delivery. ACS Med. Chem. Lett. 2020, 11, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yu, S.; Long, W.; Huang, H.; Wen, Y.; Deng, F.; Liu, M.; Xu, W.; Zhang, X.; Wei, Y. The Utilization of Multifunctional Organic Dye with Aggregation-Induced Emission Feature to Fabricate Luminescent Mesoporous Silica Nanoparticles Based Polymeric Composites for Controlled Drug Delivery. Microporous Mesoporous Mater. 2020, 308, 110520. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; He, Z.; Fang, F.; Wang, C.; Zeng, K.; Gao, S.; Meng, F.; Luo, L.; Tang, B.Z. Multicationic AIEgens for Unimolecular Photodynamic Theranostics and Two-Photon Fluorescence Bioimaging. Mater. Chem. Front. 2020, 4, 1623–1633. [Google Scholar] [CrossRef]
- Zhao, J.; Pan, X.; Zhu, J.; Zhu, X. Novel AIEgen-Functionalized Diselenide-Crosslinked Polymer Gels as Fluorescent Probes and Drug Release Carriers. Polymers 2020, 12, 551. [Google Scholar] [CrossRef] [Green Version]
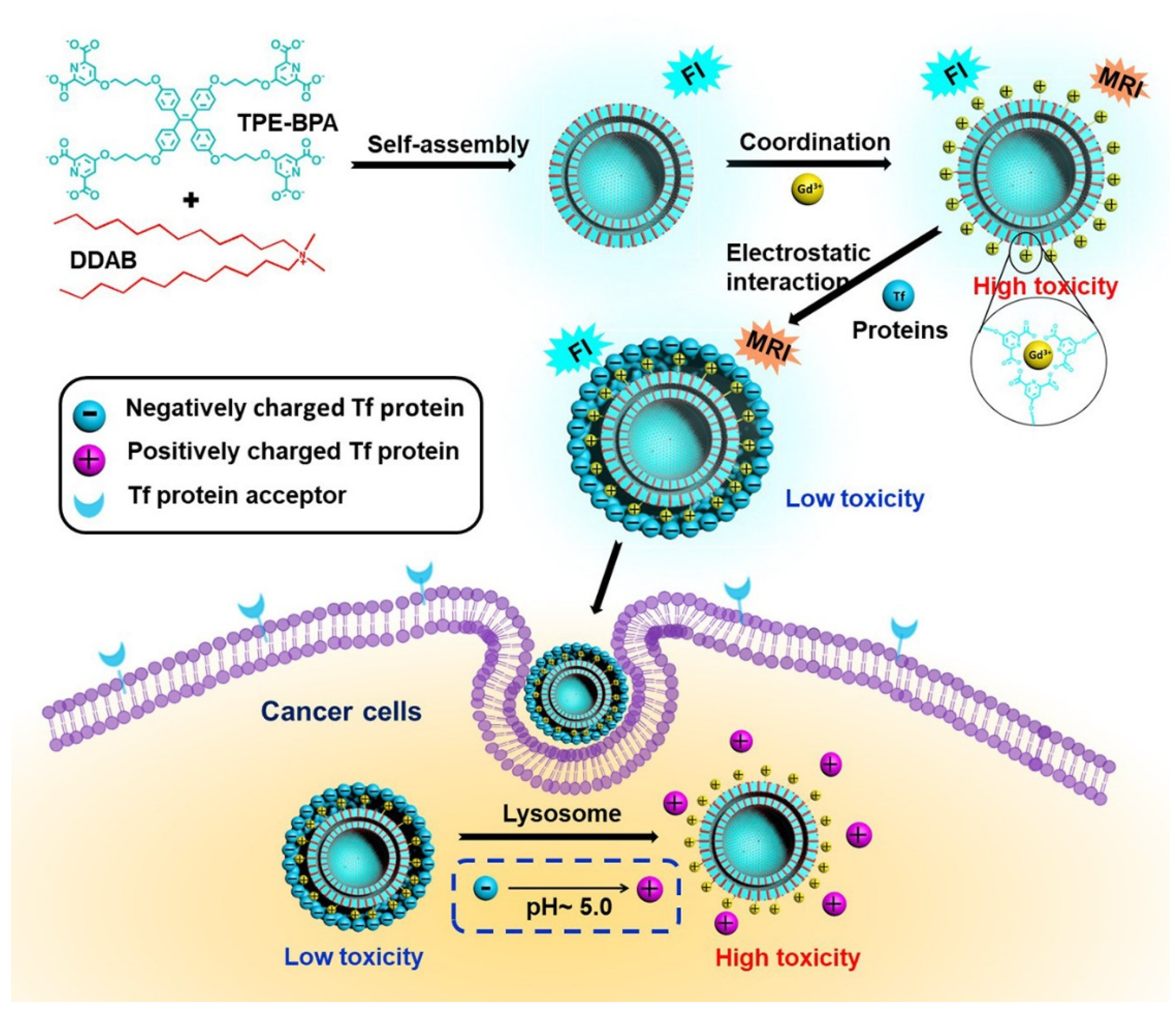
- Li, J.; Lee, M.M.S.; Li, H.; Tong, C.; Huang, J.; Yan, Y.; Wang, D.; Tang, B.Z. Programmed Self-Assembly of Protein-Coated AIE-Featured Nanoparticles with Dual Imaging and Targeted Therapy to Cancer Cells. ACS Appl. Mater. Interfaces 2020, 12, 29641–29649. [Google Scholar] [CrossRef]
- Sharath Kumar, K.S.; Girish, Y.R.; Ashrafizadeh, M.; Mirzaei, S.; Rakesh, K.P.; Hossein Gholami, M.; Zabolian, A.; Hushmandi, K.; Orive, G.; Kadumudi, F.B.; et al. AIE-Featured Tetraphenylethylene Nanoarchitectures in Biomedical Application: Bioimaging, Drug Delivery and Disease Treatment. Coord. Chem. Rev. 2021, 447, 214135. [Google Scholar] [CrossRef]
- Yan, K.; Feng, Y.; Gao, K.; Shi, X.; Zhao, X. Fabrication of Hyaluronic Acid-Based Micelles with Glutathione-Responsiveness for Targeted Anticancer Drug Delivery. J. Colloid Interface Sci. 2022, 606, 1586–1596. [Google Scholar] [CrossRef]
- Sun, C.; Lu, J.; Wang, J.; Hao, P.; Li, C.; Qi, L.; Yang, L.; He, B.; Zhong, Z.; Hao, N. Redox-Sensitive Polymeric Micelles with Aggregation-Induced Emission for Bioimaging and Delivery of Anticancer Drugs. J. Nanobiotech. 2021, 19. [Google Scholar] [CrossRef]
- Shi, H.; Liang, N.; Liu, J.; Li, S.; Gong, X.; Yan, P.; Sun, S. AIE-Active Polymeric Micelles Based on Modified Chitosan for Bioimaging-Guided Targeted Delivery and Controlled Release of Paclitaxel. Carbohydr. Polym. 2021, 269, 118327. [Google Scholar] [CrossRef]
- Gu, P.; Chen, B.; Zhai, T.; Li, Q.; Zuo, X.; Wang, L.; Qin, A.; Zhou, Y.; Shen, J. Immunostimulatory AIE Dots for Live-Cell Imaging and Drug Delivery. ACS Appl. Mater. Interfaces 2021, 13, 19660–19667. [Google Scholar] [CrossRef]
- Liu, D.; Du, J.; Qi, S.; Li, M.; Wang, J.; Liu, M.; Du, X.; Wang, X.; Ren, B.; Wu, D.; et al. Supramolecular Nanoparticles Constructed from Pillar[5]Arene-Based Host-Guest Complexation with Enhanced Aggregation-Induced Emission for Imaging-Guided Drug Delivery. Mater. Chem. Front. 2021, 5, 1418–1427. [Google Scholar] [CrossRef]
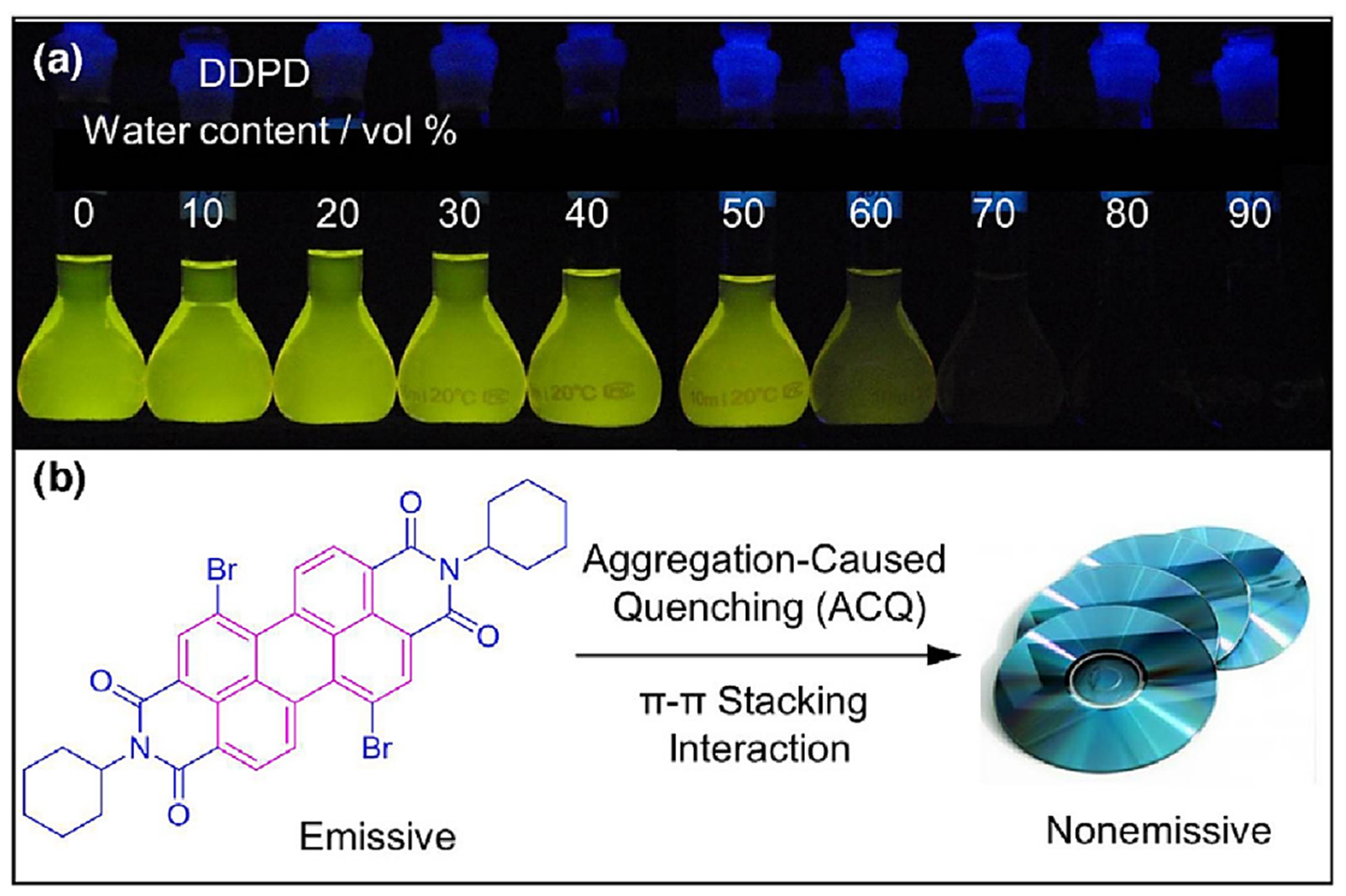


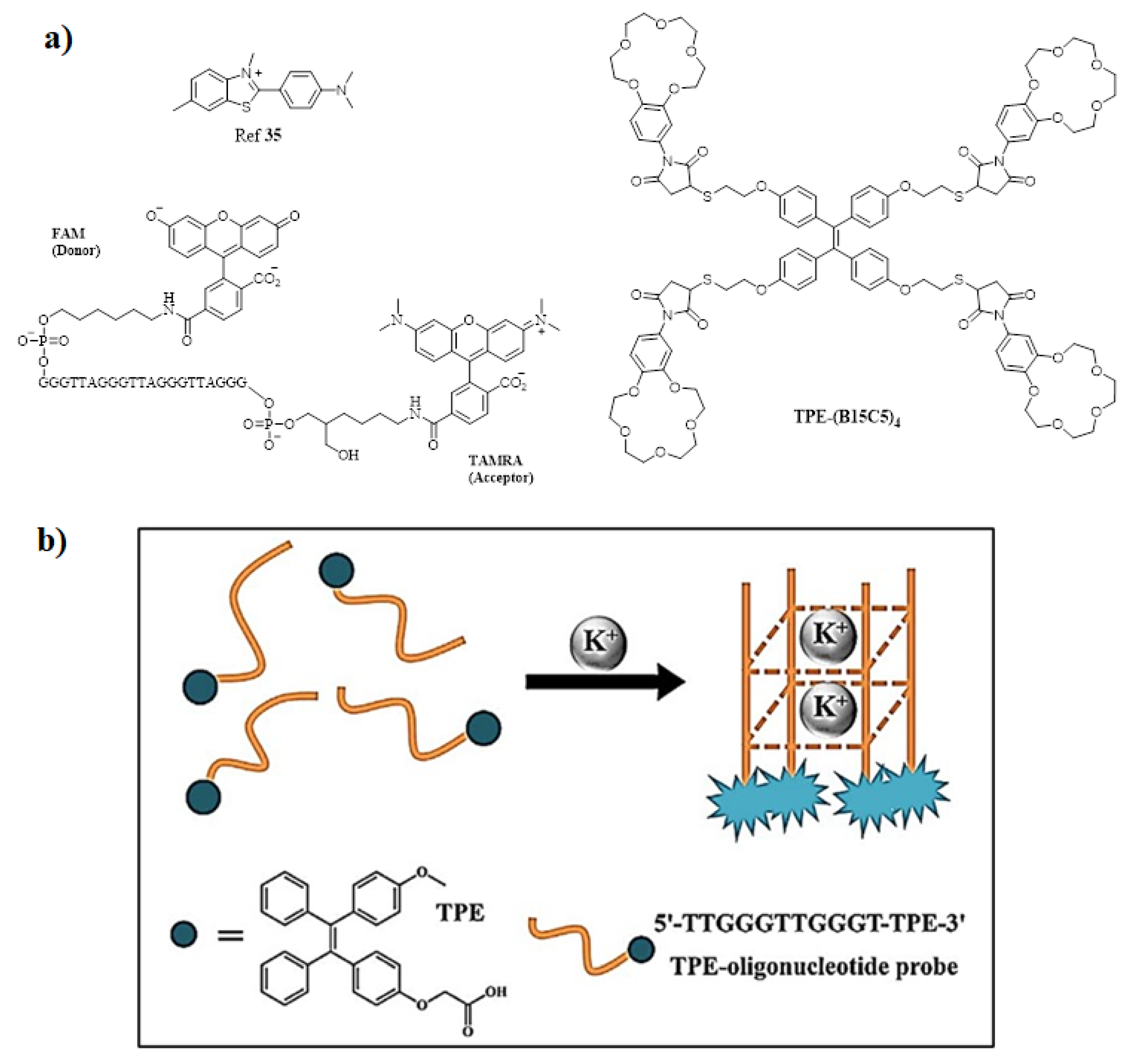
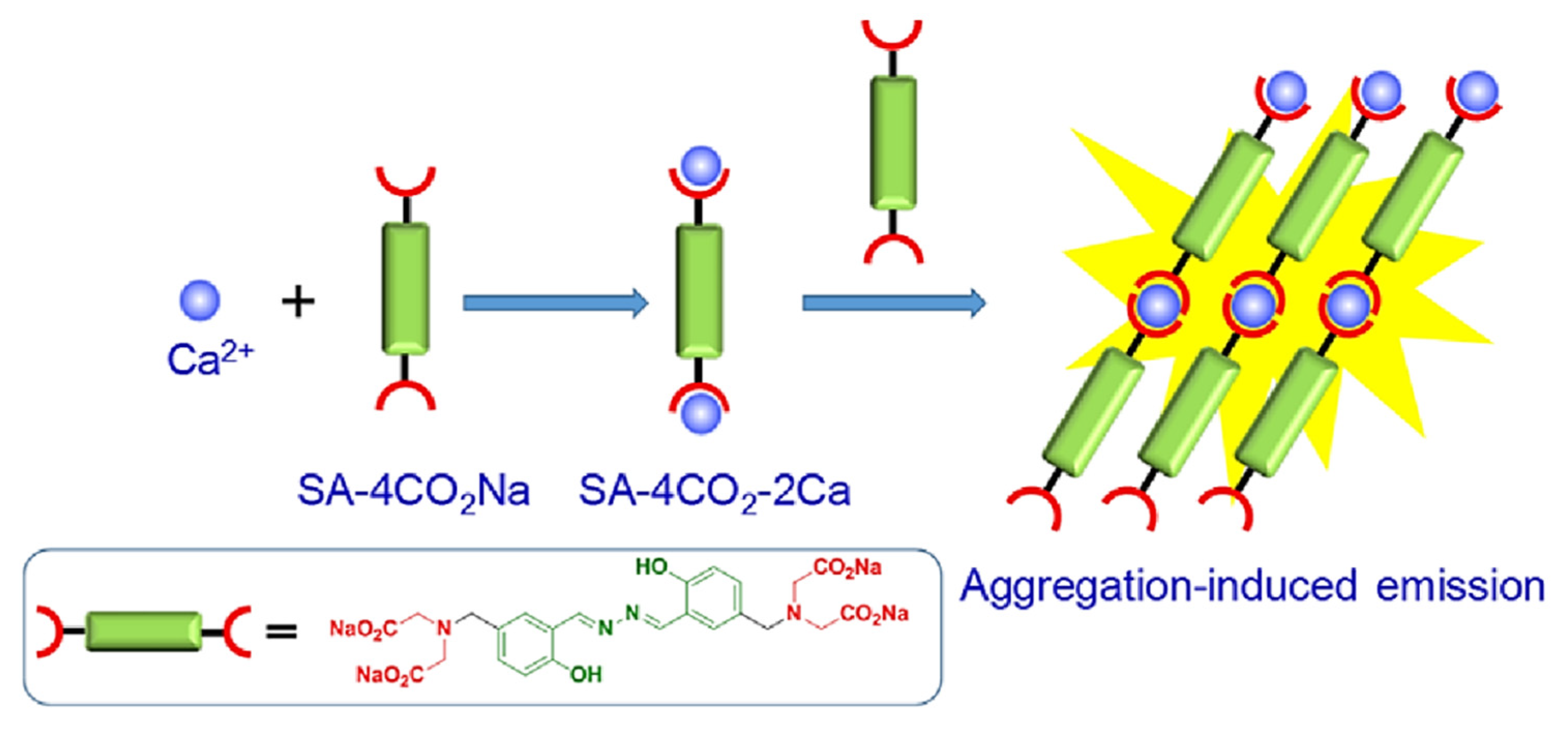

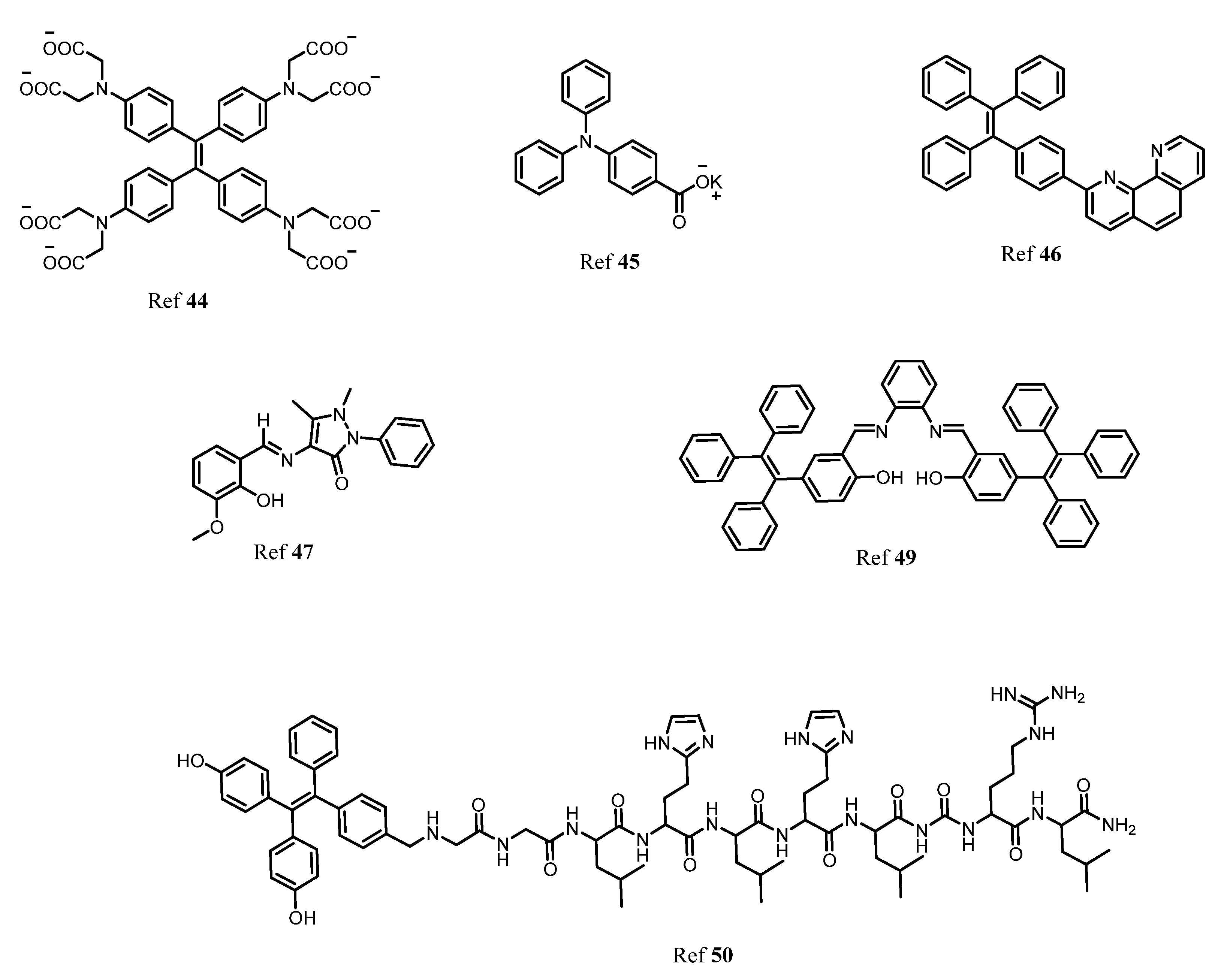




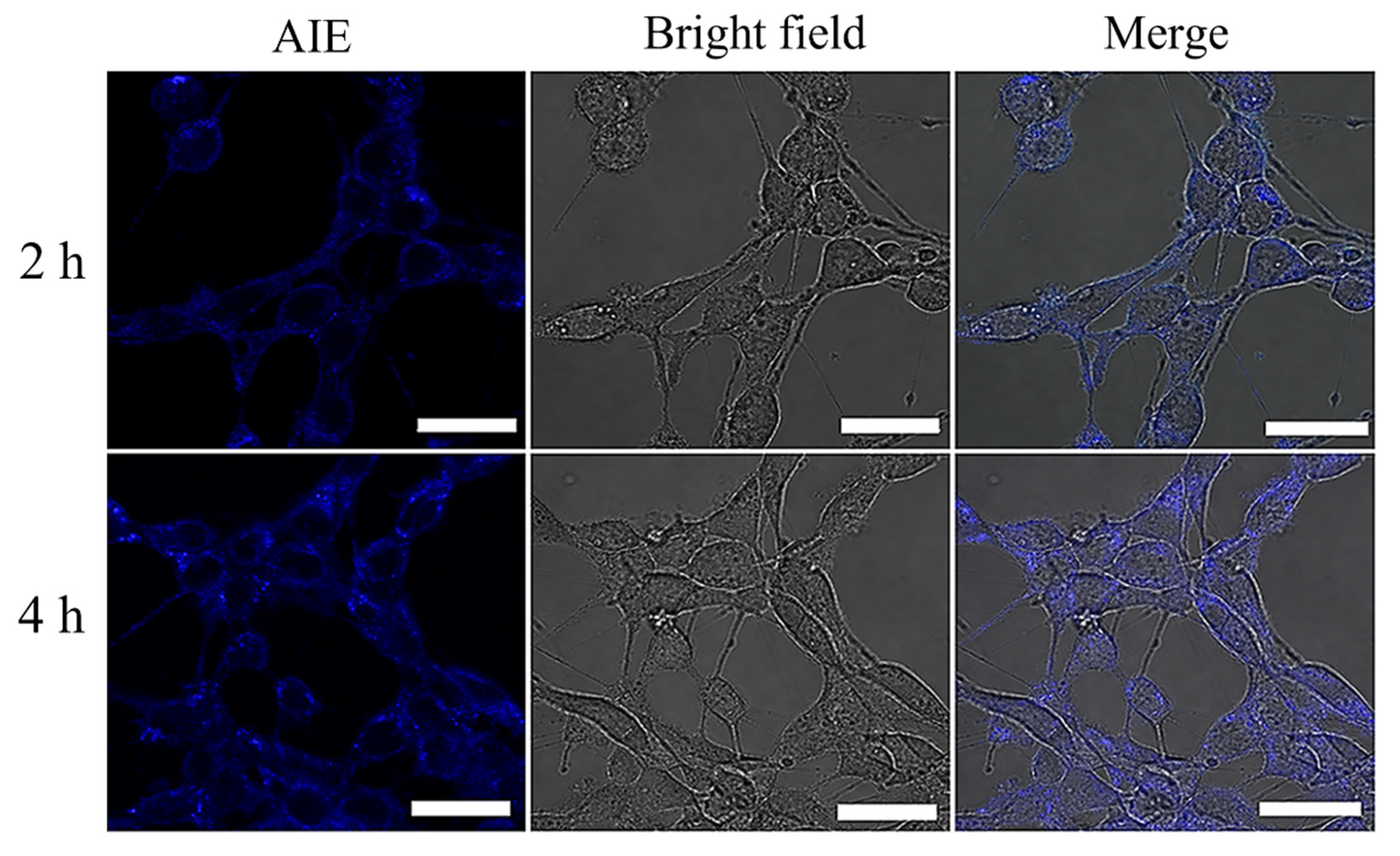
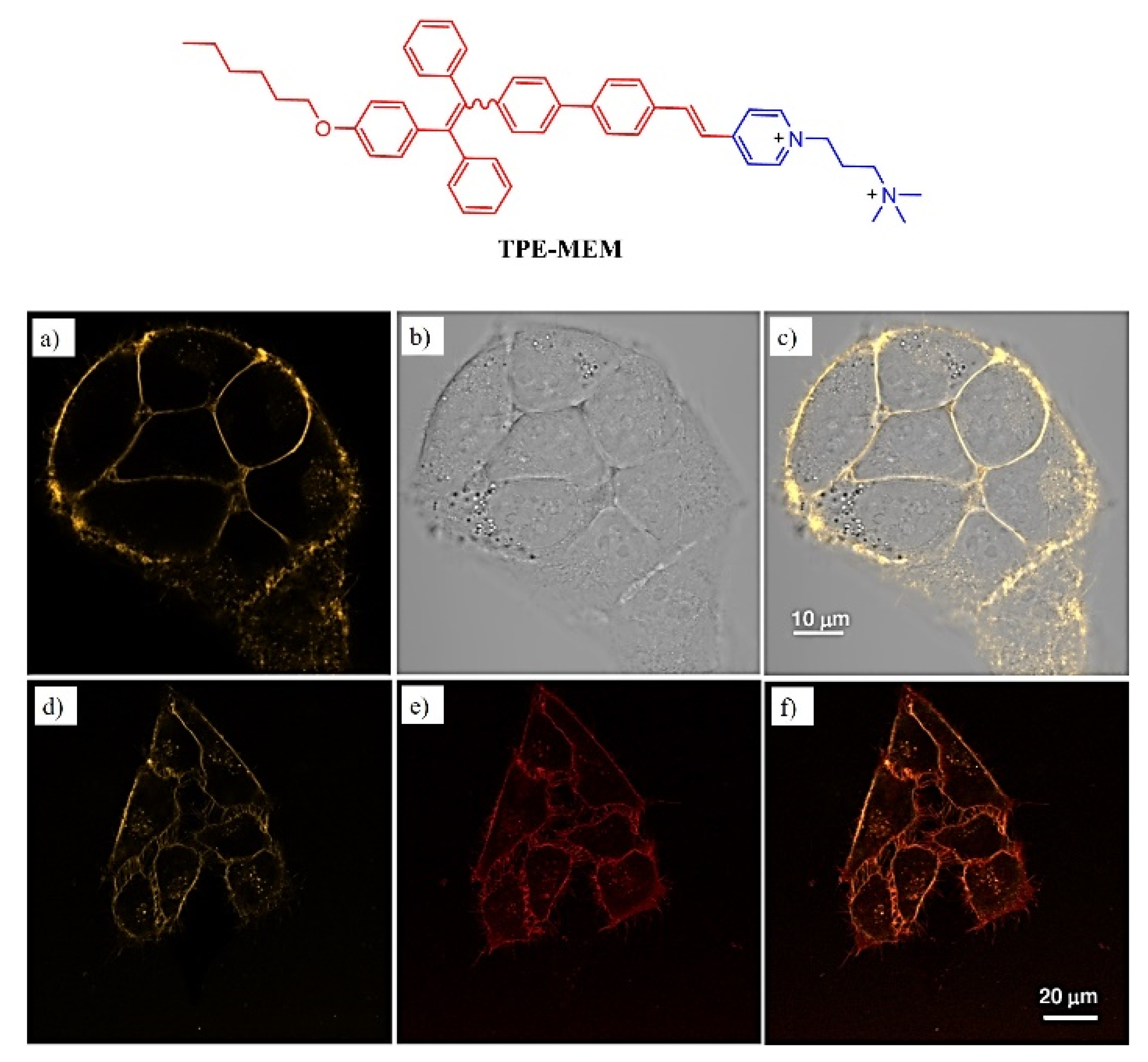

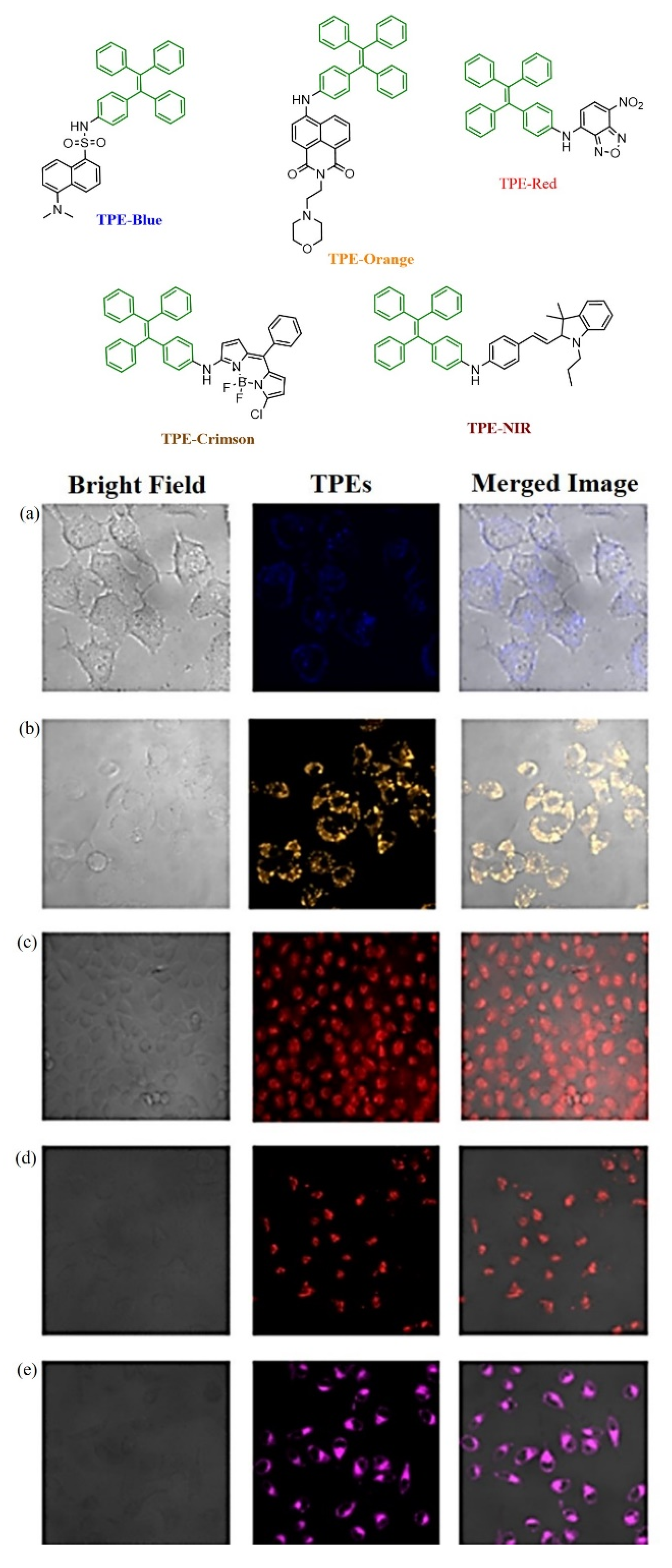
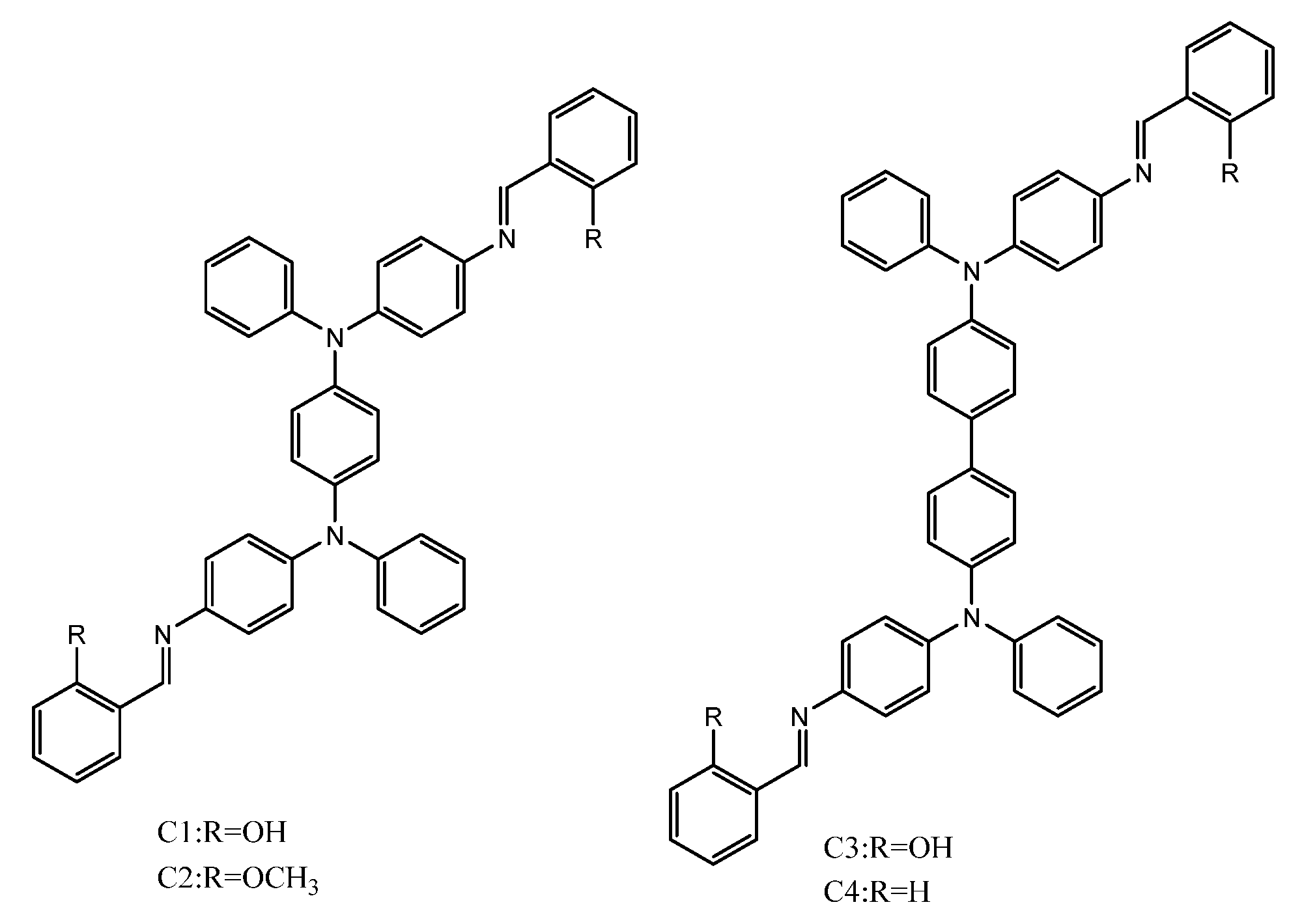






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zalmi, G.A.; Jadhav, R.W.; Mirgane, H.A.; Bhosale, S.V. Recent Advances in Aggregation-Induced Emission Active Materials for Sensing of Biologically Important Molecules and Drug Delivery System. Molecules 2022, 27, 150. https://doi.org/10.3390/molecules27010150
Zalmi GA, Jadhav RW, Mirgane HA, Bhosale SV. Recent Advances in Aggregation-Induced Emission Active Materials for Sensing of Biologically Important Molecules and Drug Delivery System. Molecules. 2022; 27(1):150. https://doi.org/10.3390/molecules27010150
Chicago/Turabian StyleZalmi, Geeta A., Ratan W. Jadhav, Harshad A. Mirgane, and Sheshanath V. Bhosale. 2022. "Recent Advances in Aggregation-Induced Emission Active Materials for Sensing of Biologically Important Molecules and Drug Delivery System" Molecules 27, no. 1: 150. https://doi.org/10.3390/molecules27010150
APA StyleZalmi, G. A., Jadhav, R. W., Mirgane, H. A., & Bhosale, S. V. (2022). Recent Advances in Aggregation-Induced Emission Active Materials for Sensing of Biologically Important Molecules and Drug Delivery System. Molecules, 27(1), 150. https://doi.org/10.3390/molecules27010150






