A Glossary for Chemical Approaches towards Unlocking the Trove of Metabolic Treasures in Actinomycetes
Abstract
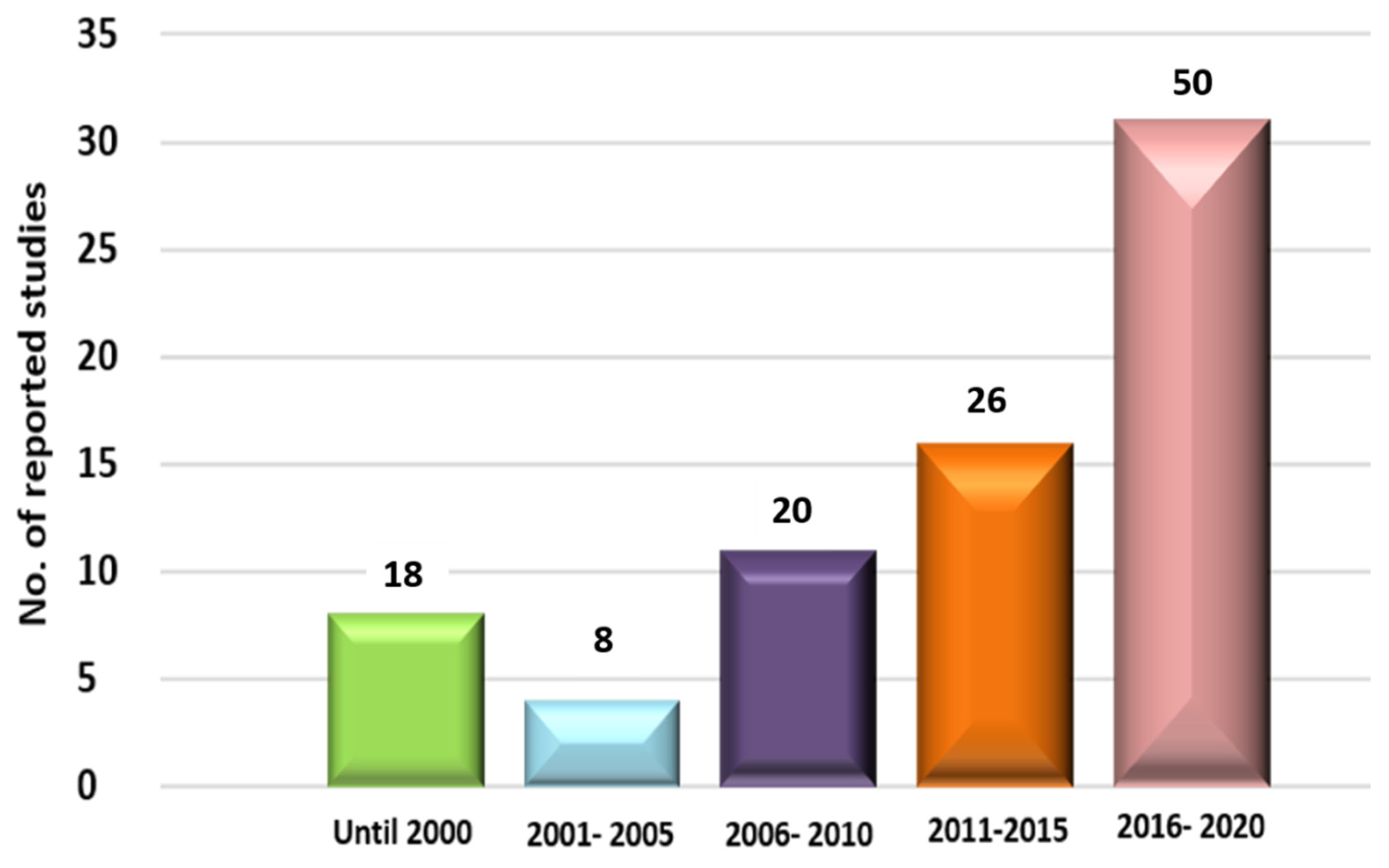
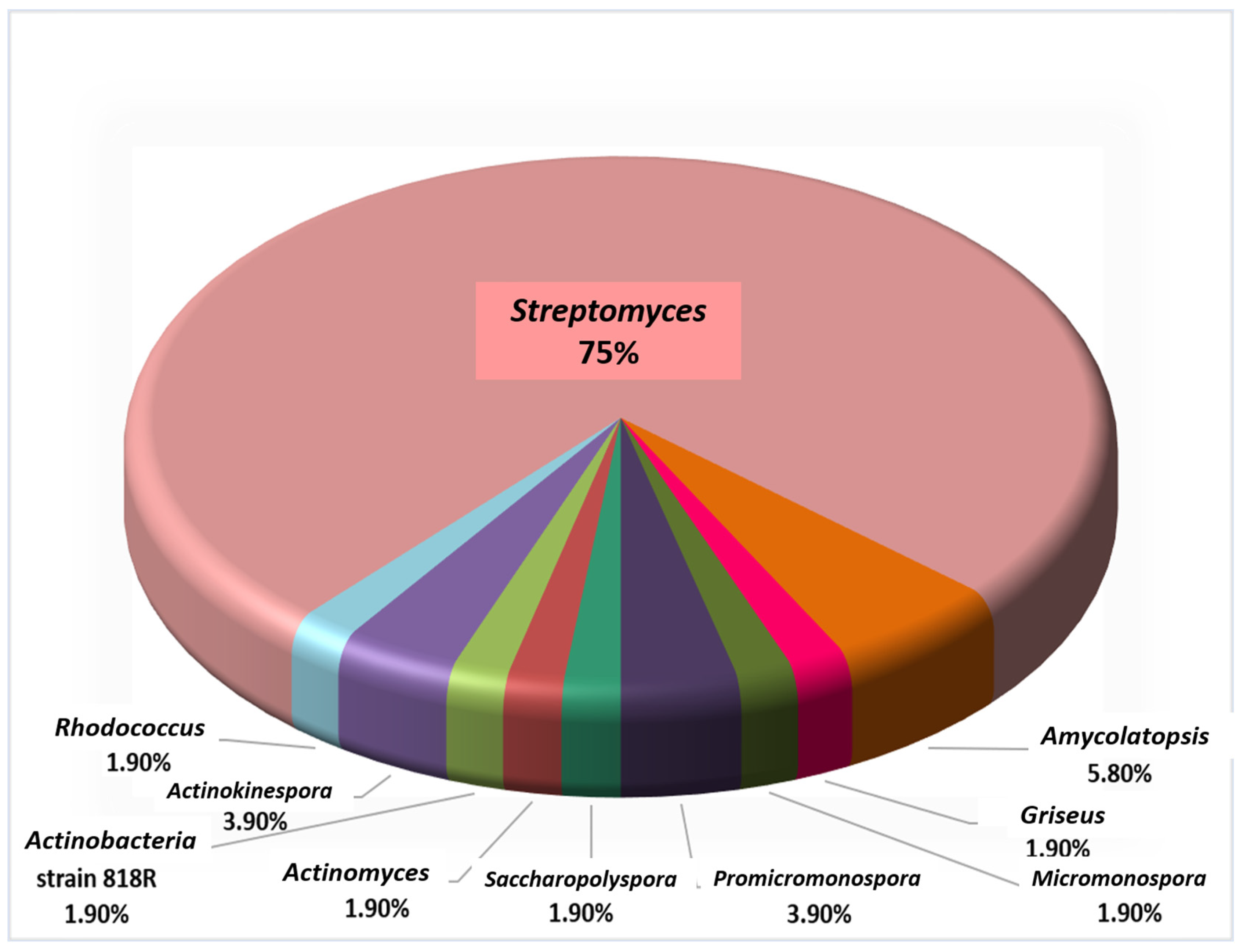
:1. Introduction
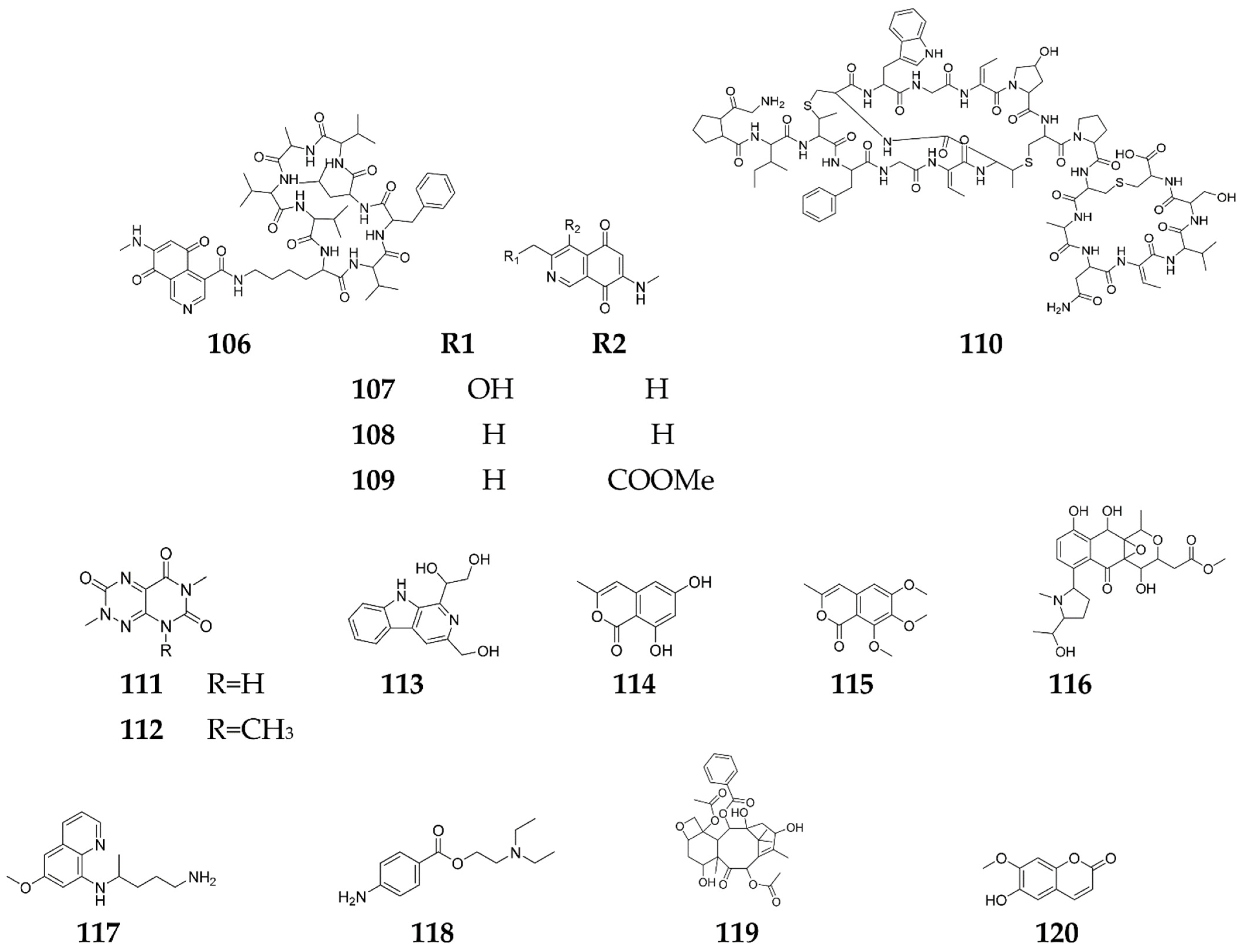
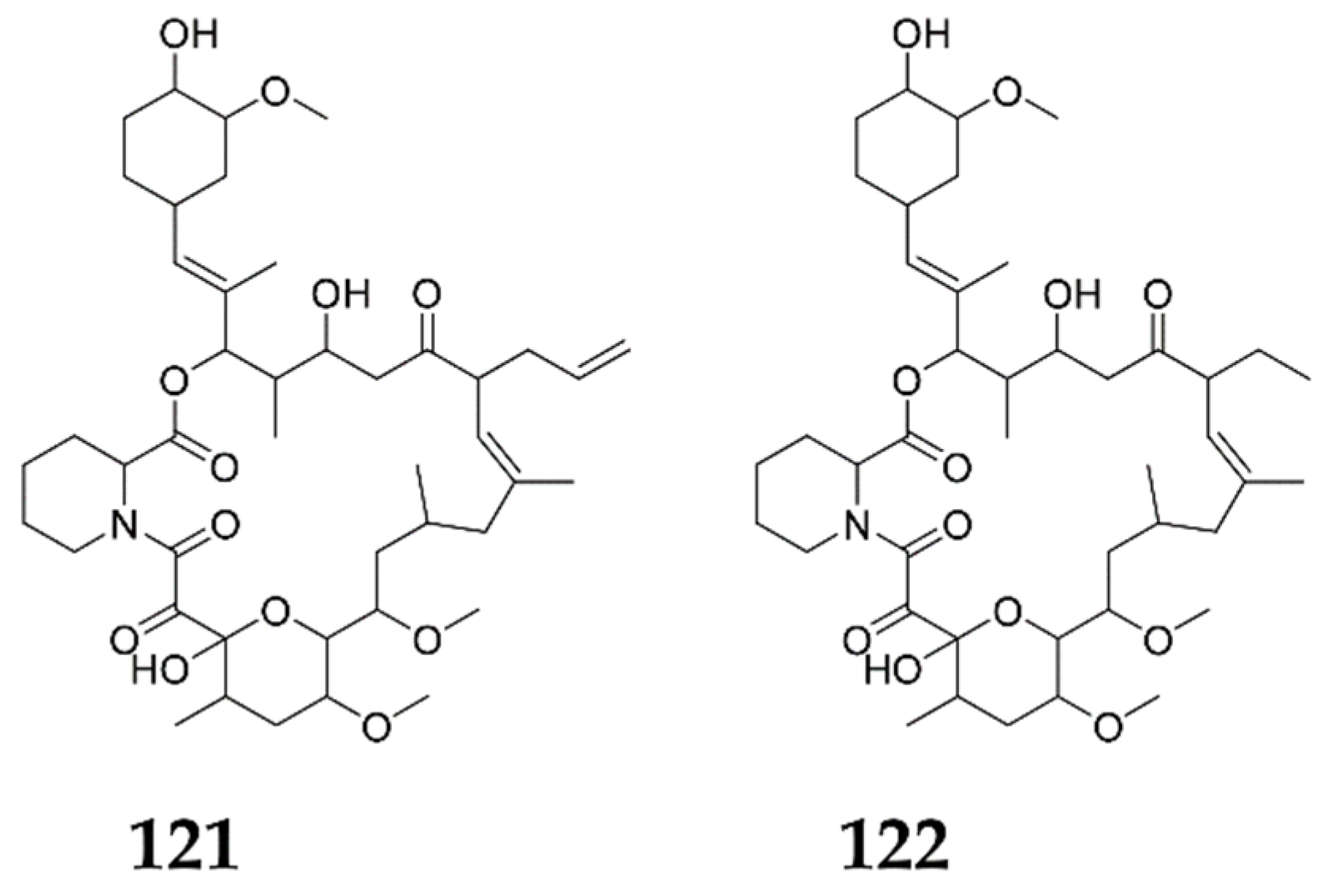
2. Specific Chemical Elicitors
2.1. Antibiotics and Their Biosynthetic Intermediates
2.1.1. Antibiotics at Sub-Inhibitory Concentrations
2.1.2. Antibiotics as Autoregulators of Their Own Biosynthesis
2.1.3. Antibiotics as Autoregulators of Other Biosynthetic Pathways
2.1.4. Antibiotics as Cross-Regulators of Other Antibiotics Biosyntheses
2.2. Antibiotic Remodeling Compounds (ARCs)
2.3. Histone Deacetylase Inhibitors (HDACIs)
2.4. Hormone-like Signaling Molecules (Autoregulators)
2.5. Gamma-Butyrolactones (GBLs)
2.6. Aromatic Furans
2.7. γ-Butenolides
2.8. PI Factor
2.9. N-Methylphenylalanyl-dehydrobutyrine Diketopiperazine (MDD)
2.10. Metabolic Signals: GlcNAc and Siderophores
2.11. Rare Earth Elements (REEs)
2.12. Heavy Metals
2.13. Organic Solvents
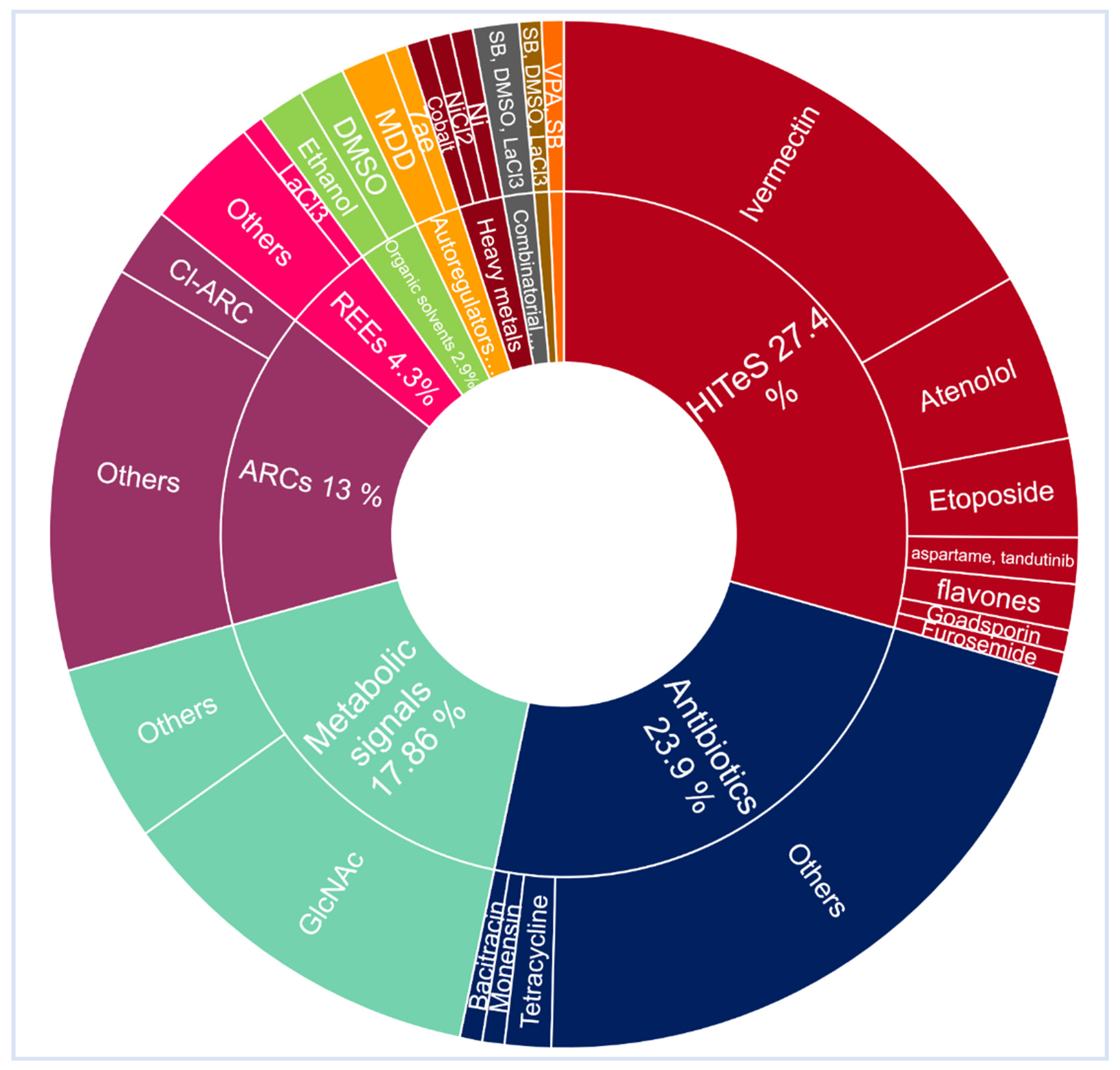
3. Recent Approaches in Activating Silent BGCs
3.1. HITES Approach
3.2. Cumulative Effect of More than One Chemical Elicitor
3.3. Combinatorial Engineering Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakker, M.G.; Schlatter, D.C.; Otto-Hanson, L.; Kinkel, L.L. Diffuse symbioses: Roles of plant–plant, plant–microbe and microbe–microbe interactions in structuring the soil microbiome. Mol. Ecol. 2014, 23, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Albohy, A.; Khalil, A.; Ibrahim, A.H.; Ahmed, H.A.; El-Hossary, E.M.; Bringmann, G.; Abdelmohsen, U.R. Bioactivity potential of marine natural products from scleractinia-associated microbes and in silico anti-SARS-CoV-2 evaluation. Mar. Drugs 2020, 18, 645. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, X.; Nie, J.; Niu, G. Regulation of Antibiotic Production by Signaling Molecules in Streptomyces. Front. Microbiol. 2019, 10, 2927. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.-S. Recent Advances of Actinomycetes. Biomolecules 2021, 11, 134. [Google Scholar] [CrossRef]
- De Simeis, D.; Serra, S. Actinomycetes: A Never-Ending Source of Bioactive Compounds—An Overview on Antibiotics Production. Antibiotics 2021, 10, 483. [Google Scholar] [CrossRef] [PubMed]
- Albohy, A.; Zahran, E.M.; Abdelmohsen, U.R.; Salem, M.A.; Al-Warhi, T.; Al-Sanea, M.M.; Abelyan, N.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A. Multitarget in silico studies of Ocimum menthiifolium, family Lamiaceae against SARS-CoV-2 supported by molecular dynamics simulation. J. Biomol. Struct. Dyn. 2020, 1–11. [Google Scholar] [CrossRef]
- Pye, C.R.; Bertin, M.J.; Lokey, R.S.; Gerwick, W.H.; Linington, R.G. Retrospective analysis of natural products provides insights for future discovery trends. Proc. Natl. Acad. Sci. USA 2017, 114, 5601–5606. [Google Scholar] [CrossRef] [Green Version]
- Jensen, P.R.; Fenical, W. Marine bacterial diversity as a resource for novel microbial products. J. Ind. Microbiol. 1996, 17, 346–351. [Google Scholar] [CrossRef]
- Escalante-Réndiz, D.; de-la-Rosa-García, S.; Tapia-Tussell, R.; Martín, J.; Reyes, F.; Vicente, F.; Gamboa-Angulo, M. Molecular Identification of Selected Streptomyces Strains Isolated from Mexican Tropical Soils and their Anti-Candida Activity. Int. J. Environ. Res. Public Health 2019, 16, 1913. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, N.; Hwang, S.; Kim, W.; Lee, Y.; Cho, S.; Palsson, B.O.; Cho, B.-K. Discovery of novel secondary metabolites encoded in actinomycete genomes through coculture. J. Ind. Microbiol. Biotechnol. 2021, 48, kuaa001. [Google Scholar] [CrossRef]
- Zarins-Tutt, J.S.; Barberi, T.T.; Gao, H.; Mearns-Spragg, A.; Zhang, L.; Newman, D.J.; Goss, R.J.M. Prospecting for new bacterial metabolites: A glossary of approaches for inducing, activating and upregulating the biosynthesis of bacterial cryptic or silent natural products. Nat. Prod. Rep. 2016, 33, 54–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lima Procópio, R.E.; da Silva, I.R.; Martins, M.K.; de Azevedo, J.L.; de Araújo, J.M. Antibiotics produced by Streptomyces. Braz. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D. Extending the “one strain many compounds” (OSMAC) principle to marine microorganisms. Mar. Drugs 2018, 16, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medema, M.H.; Fischbach, M.A. Computational approaches to natural product discovery. Nat. Chem. Biol. 2015, 11, 639. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Grkovic, T.; Balasubramanian, S.; Kamel, M.S.; Quinn, R.J.; Hentschel, U. Elicitation of secondary metabolism in actinomycetes. Biotechnol. Adv. 2015, 33, 798–811. [Google Scholar] [CrossRef] [Green Version]
- Gamaleldin, N.M.; Bakeer, W.; Sayed, A.M.; Shamikh, Y.I.; El-Gendy, A.O.; Hassan, H.M.; Horn, H.; Abdelmohsen, U.R.; Hozzein, W.N. Exploration of chemical diversity and antitrypanosomal activity of some red sea-derived actinomycetes using the OSMAC approach supported by LC-MS-based metabolomics and molecular modelling. Antibiotics 2020, 9, 629. [Google Scholar] [CrossRef]
- Dashti, Y.; Grkovic, T.; Abdelmohsen, U.R.; Hentschel, U.; Quinn, R.J. Production of induced secondary metabolites by a co-culture of sponge-associated actinomycetes, Actinokineospora sp. EG49 and Nocardiopsis sp. RV163. Mar. Drugs 2014, 12, 3046–3059. [Google Scholar] [CrossRef]
- Tyurin, A.P.; Alferova, V.A.; Korshun, V.A. Chemical elicitors of antibiotic biosynthesis in actinomycetes. Microorganisms 2018, 6, 52. [Google Scholar] [CrossRef] [Green Version]
- Romero, D.; Traxler, M.F.; López, D.; Kolter, R. Antibiotics as signal molecules. Chem. Rev. 2011, 111, 5492–5505. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, S.; Onaka, H.; Abe, I. Activation of silent biosynthetic pathways and discovery of novel secondary metabolites in actinomycetes by co-culture with mycolic acid-containing bacteria. J. Ind. Microbiol. Biotechnol. 2019, 46, 363–374. [Google Scholar] [CrossRef]
- Zhu, H.; Sandiford, S.K.; van Wezel, G.P. Triggers and cues that activate antibiotic production by actinomycetes. J. Ind. Microbiol. Biotechnol. 2014, 41, 371–386. [Google Scholar] [CrossRef]
- Goh, E.-B.; Yim, G.; Tsui, W.; McClure, J.; Surette, M.G.; Davies, J. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc. Natl. Acad. Sci. USA 2002, 99, 17025–17030. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, J.; Yuan, J.; Jiang, L.; Jiang, X.; Yang, B.; Zhao, G.; Liu, B.; Huang, D. Generation of Streptomyces hygroscopicus cell factories with enhanced ascomycin production by combined elicitation and pathway-engineering strategies. Biotechnol. Bioeng. 2019, 116, 3382–3395. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; He, L.; Niu, G. Regulation of antibiotic biosynthesis in actinomycetes: Perspectives and challenges. Synth. Syst. Biotechnol. 2018, 3, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Mitova, M.I.; Lang, G.; Wiese, J.; Imhoff, J.F. Subinhibitory Concentrations of Antibiotics Induce Phenazine Production in a Marine Streptomyces sp. J. Nat. Prod. 2008, 71, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Amano, S.-I.; Sakurai, T.; Endo, K.; Takano, H.; Beppu, T.; Furihata, K.; Sakuda, S.; Ueda, K. A cryptic antibiotic triggered by monensin. J. Antibiot. 2011, 64, 703. [Google Scholar] [CrossRef]
- Imai, Y.; Sato, S.; Tanaka, Y.; Ochi, K.; Hosaka, T. Lincomycin at subinhibitory concentrations potentiates secondary metabolite production by Streptomyces spp. Appl. Environ. Microbiol. 2015, 81, 3869–3879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Moreno, M.A.; Caballero, J.; Hopwood, D.A.; Malpartida, F. The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA gene of streptomyces. Cell 1991, 66, 769–780. [Google Scholar] [CrossRef]
- Tanaka, Y.; Izawa, M.; Hiraga, Y.; Misaki, Y.; Watanabe, T.; Ochi, K. Metabolic perturbation to enhance polyketide and nonribosomal peptide antibiotic production using triclosan and ribosome-targeting drugs. Appl. Microbiol. Biotechnol. 2017, 101, 4417–4431. [Google Scholar] [CrossRef]
- Koba, M.; Konopa, J. Actinomycin D and its mechanisms of action. Postepy Hig. I Med. Dosw. 2005, 59, 290–298. [Google Scholar]
- Hosaka, T.; Ohnishi-Kameyama, M.; Muramatsu, H.; Murakami, K.; Tsurumi, Y.; Kodani, S.; Yoshida, M.; Fujie, A.; Ochi, K. Antibacterial discovery in actinomycetes strains with mutations in RNA polymerase or ribosomal protein S12. Nat. Biotechnol. 2009, 27, 462–464. [Google Scholar] [CrossRef]
- Liu, X.F.; Xiang, L.; Zhou, Q.; Carralot, J.-P.; Prunotto, M.; Niederfellner, G.; Pastan, I. Actinomycin D enhances killing of cancer cells by immunotoxin RG7787 through activation of the extrinsic pathway of apoptosis. Proc. Natl. Acad. Sci. USA 2016, 113, 10666–10671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Jia, Y.; Xie, Y.; Zhang, C.; Ma, J.; Sun, C.; Ju, J. Identification of the Actinomycin D Biosynthetic Pathway from Marine-Derived Streptomyces costaricanus SCSIO ZS0073. Mar. Drugs 2019, 17, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choong, M.L.; Yang, H.; Lee, M.A.; Lane, D.P. Specific activation of the p53 pathway by low dose actinomycin D: A new route to p53 based cyclotherapy. Cell Cycle 2009, 8, 2810–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rill, R.L.; Hecker, K.H. Sequence-specific actinomycin D binding to single-stranded DNA inhibits HIV reverse transcriptase and other polymerases. Biochemistry 1996, 35, 3525–3533. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, G.; Ding, X. Morphology engineering of Streptomyces coelicolor M145 by sub-inhibitory concentrations of antibiotics. Sci. Rep. 2017, 7, 13226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankovic, N.; Senerovic, L.; Ilic-Tomic, T.; Vasiljevic, B.; Nikodinovic-Runic, J. Properties and applications of undecylprodigiosin and other bacterial prodigiosins. Appl. Microbiol. Biotechnol. 2014, 98, 3841–3858. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, G.; Zou, Z.; Fan, K.; Yang, K.; Tan, H. JadR*-mediated feed-forward regulation of cofactor supply in jadomycin biosynthesis. Mol. Microbiol. 2013, 90, 884–897. [Google Scholar] [CrossRef]
- Wang, L.; Tian, X.; Wang, J.; Yang, H.; Fan, K.; Xu, G.; Yang, K.; Tan, H. Autoregulation of antibiotic biosynthesis by binding of the end product to an atypical response regulator. Proc. Natl. Acad. Sci. USA 2009, 106, 8617–8622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, J.; Tian, Z.; Xu, Y.; Zhang, J.; Liu, W.; Tan, H. Coordinative modulation of chlorothricin biosynthesis by binding of the glycosylated intermediates and end product to a responsive regulator ChlF1. J. Biol. Chem. 2016, 291, 5406–5417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, Y.; Niu, G.; Guo, H.; Qiu, Y.; Lin, Z.; Liu, W.; Tan, H. NosP-regulated nosiheptide production responds to both peptidyl and small-molecule ligands derived from the precursor peptide. Cell Chem. Biol. 2018, 25, 143–153.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosoda, K.; Koyama, N.; Kanamoto, A.; Tomoda, H. Discovery of Nosiheptide, Griseoviridin, and Etamycin as Potent Anti-Mycobacterial Agents against Mycobacterium avium Complex. Molecules 2019, 24, 1495. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Yang, R.; Zhao, R.-X.; Han, J.-T.; Jia, W.-J.; Li, D.-Y.; Wang, Y.; Zhang, N.; Wu, Y.; Zhang, L.-Q. Transcriptional regulator PhlH modulates 2, 4-diacetylphloroglucinol biosynthesis in response to the biosynthetic intermediate and end product. Appl. Environ. Microbiol. 2017, 83, e01419-17. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Sun, D.; Liu, W.; Chen, Z.; Li, J.; Wen, Y. AvaR2, a pseudo γ-butyrolactone receptor homologue from Streptomyces avermitilis, is a pleiotropic repressor of avermectin and avenolide biosynthesis and cell growth. Mol. Microbiol. 2016, 102, 562–578. [Google Scholar] [CrossRef]
- Jansson, R.K.; Dybas, R.A. Avermectins: Biochemical Mode of Action, Biological Activity and Agricultural Importance. In Insecticides with Novel Modes of Action: Mechanisms and Application; Ishaaya, I., Degheele, D., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 152–170. [Google Scholar]
- Huang, J.; Shi, J.; Molle, V.; Sohlberg, B.; Weaver, D.; Bibb, M.J.; Karoonuthaisiri, N.; Lih, C.J.; Kao, C.M.; Buttner, M.J. Cross-regulation among disparate antibiotic biosynthetic pathways of Streptomyces coelicolor. Mol. Microbiol. 2005, 58, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhang, J.; Cai, X.; Yang, F.; Zhu, W.; Zhang, W.; Cai, M.; Yu, Z.; Li, X.; Wu, T. Bilobalide protects astrocytes from oxygen and glucose deprivation-induced oxidative injury by upregulating manganese superoxide dismutase. Phytother. Res. 2019, 33, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Yin, M.; Wu, S.; Han, X.; Ji, K.; Wen, M.; Lu, T. GdmRIII, a TetR family transcriptional regulator, controls geldanamycin and elaiophylin biosynthesis in Streptomyces autolyticus CGMCC0516. Sci. Rep. 2017, 7, 4803. [Google Scholar] [CrossRef]
- Ochel, H.-J.; Eichhorn, K.; Gademann, G. Geldanamycin: The prototype of a class of antitumor drugs targeting the heat shock protein 90 family of molecular chaperones. Cell Stress Chaperones 2001, 6, 105. [Google Scholar] [CrossRef] [Green Version]
- Ochi, K.; Okamoto, S. A Magic Bullet for Antibiotic Discovery. Chem. Biol. 2012, 19, 932–934. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Craney, A.; Pimentel-Elardo, S.M.; Nodwell, J.R. A Synthetic, Species-Specific Activator of Secondary Metabolism and Sporulation in Streptomyces coelicolor. ChemBioChem 2013, 14, 83–91. [Google Scholar] [CrossRef]
- Craney, A.; Ozimok, C.; Pimentel-Elardo, S.M.; Capretta, A.; Nodwell, J.R. Chemical perturbation of secondary metabolism demonstrates important links to primary metabolism. Chem. Biol. 2012, 19, 1020–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olano, C.; Lombo, F.; Mendez, C.; Salas, J.A. Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metab. Eng. 2008, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Matsumoto, D.; Kawaide, H.; Natsume, M. Physiological role of germicidins in spore germination and hyphal elongation in Streptomyces coelicolor A3 (2). J. Antibiot. 2011, 64, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, A.; Dorgerloh, M.; Deeg, M.; Hagenmaier, H. The structures of novel insecticidal macrolides: Bafilomycins D and E, and oxohygrolidin. Agric. Biol. Chem. 1985, 49, 2509–2511. [Google Scholar]
- Popović-Djordjević, J.B.; Klaus, A.S.; Žižak, Ž.S.; Matić, I.Z.; Drakulić, B.J. Antiproliferative and antibacterial activity of some glutarimide derivatives. J. Enzym. Inhib. Med. Chem. 2016, 31, 915–923. [Google Scholar] [CrossRef]
- Pimentel-Elardo, S.M.; Sørensen, D.; Ho, L.; Ziko, M.; Bueler, S.A.; Lu, S.; Tao, J.; Moser, A.; Lee, R.; Agard, D. Activity-independent discovery of secondary metabolites using chemical elicitation and cheminformatic inference. ACS Chem. Biol. 2015, 10, 2616–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.T.; Laatsch, H.; von Tiedemann, A. Inhibitory Effects of Macrotetrolides from Streptomyces spp. On Zoosporogenesis and Motility of Peronosporomycete Zoospores Are Likely Linked with Enhanced ATPase Activity in Mitochondria. Front. Microbiol. 2016, 7, 1824. [Google Scholar] [CrossRef]
- Lewandowski, S.L.; Janardhan, H.P.; Trivedi, C.M. Histone deacetylase 3 coordinates deacetylase-independent epigenetic silencing of transforming growth factor-β1 (TGF-β1) to orchestrate second heart field development. J. Biol. Chem. 2015, 290, 27067–27089. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; D’Mello, S.R. Neuroprotection by histone deacetylase-7 (HDAC7) occurs by inhibition of c-jun expression through a deacetylase-independent mechanism. J. Biol. Chem. 2011, 286, 4819–4828. [Google Scholar] [CrossRef] [Green Version]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhong, Q. Histone deacetylase inhibitors and cell death. Cell. Mol. Life Sci. 2014, 71, 3885–3901. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, W.-G. Targeting histone deacetylases for cancer therapy: From molecular mechanisms to clinical implications. Int. J. Biol. Sci. 2014, 10, 757. [Google Scholar] [CrossRef] [Green Version]
- Mohammadipanah, F.; Kermani, F.; Salimi, F. Awakening the Secondary Metabolite Pathways of Promicromonospora kermanensis Using Physicochemical and Biological Elicitors. Appl. Biochem. Biotechnol. 2020, 192, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y. Microbial hormones and microbial chemical ecology. Compr. Nat. Prod. Chem. 1998, 8, 377–413. [Google Scholar]
- Ohnishi, Y.; Kameyama, S.; Onaka, H.; Horinouchi, S. The A-factor regulatory cascade leading to streptomycin biosynthesis in Streptomyces griseus: Identification of a target gene of the A-factor receptor. Mol. Microbiol. 1999, 34, 102–111. [Google Scholar] [CrossRef]
- Niu, G.; Chater, K.F.; Tian, Y.; Zhang, J.; Tan, H. Specialised metabolites regulating antibiotic biosynthesis in Streptomyces spp. FEMS Microbiol. Rev. 2016, 40, 554–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GrÅfe, U.; Schade, W.; Eritt, I.; Fleck, W.; Radics, L. A new inducer of anthracycline biosynthesis from Streptomyces viridochromogenes. J. Antibiot. 1982, 35, 1722–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawachi, R.; Akashi, T.; Kamitani, Y.; Sy, A.; Wangchaisoonthorn, U.; Nihira, T.; Yamada, Y. Identification of an AfsA homologue (BarX) from Streptomyces virginiae as a pleiotropic regulator controlling autoregulator biosynthesis, virginiamycin biosynthesis and virginiamycin M1 resistance. Mol. Microbiol. 2000, 36, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Novakova, R.; Rehakova, A.; Kutas, P.; Feckova, L.; Kormanec, J. The role of two SARP family transcriptional regulators in regulation of the auricin gene cluster in Streptomyces aureofaciens CCM 3239. Microbiology 2011, 157, 1629–1639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alia, D.; Eggle, D.; Nieselt, K.; Hu, W.S.; Breitling, R.; Takano, E. Deletion of the signalling molecule synthase ScbA has pleiotropic effects on secondary metabolite biosynthesis, morphological differentiation and primary metabolism in Streptomyces coelicolor A3 (2). Microb. Biotechnol. 2011, 4, 239–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawfike, A.; Attia, E.Z.; Desoukey, S.Y.; Hajjar, D.; Makki, A.A.; Schupp, P.J.; Edrada-Ebel, R.; Abdelmohsen, U.R. New bioactive metabolites from the elicited marine sponge-derived bacterium Actinokineospora spheciospongiae sp. nov. AMB Express 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, S.; Beppu, T. A-factor as a microbial hormone that controls cellular differentiation and secondary metabolism in Streptomyces griseus. Mol. Microbiol. 1994, 12, 859–864. [Google Scholar] [CrossRef]
- Corre, C.; Song, L.; O’Rourke, S.; Chater, K.F.; Challis, G.L. 2-Alkyl-4-hydroxymethylfuran-3-carboxylic acids, antibiotic production inducers discovered by Streptomyces coelicolor genome mining. Proc. Natl. Acad. Sci. USA 2008, 105, 17510–17515. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Wang, Y.; Chu, J. A preliminary study on the impact of exogenous A-Factor analogue 1,4-butyrolactone on stimulating bitespiramycin biosynthesis. Bioprocess Biosyst. Eng. 2019, 42, 1903–1913. [Google Scholar] [CrossRef]
- Kondo, K.; Higuchi, Y.; Sakuda, S.; Nihira, T.; Yamada, Y. New virginiae butanolides from Streptomyces virginiae. J. Antibiot. 1989, 42, 1873–1876. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.K.; Morikawa, M.; Shimizu, H.; Shioya, S.; Suga, K.I.; Nihira, T.; Yamada, Y. Maximum virginiamycin production by optimization of cultivation conditions in batch culture with autoregulator addition. Biotechnol. Bioeng. 1996, 49, 437–444. [Google Scholar] [CrossRef]
- Hashimoto, K.; Nihira, T.; Sakuda, S.; Yamada, Y. IM-2, a butyrolactone autoregulator, induces production of several nucleoside antibiotics in Streptomyces sp. FRI-5. J. Ferment. Bioeng. 1992, 73, 449–455. [Google Scholar] [CrossRef]
- Yoon, V.; Nodwell, J.R. Activating secondary metabolism with stress and chemicals. J. Ind. Microbiol. Biotechnol. 2014, 41, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Kitani, S.; Miyamoto, K.T.; Takamatsu, S.; Herawati, E.; Iguchi, H.; Nishitomi, K.; Uchida, M.; Nagamitsu, T.; Omura, S.; Ikeda, H. Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis. Proc. Natl. Acad. Sci. USA 2011, 108, 16410–16415. [Google Scholar] [CrossRef] [Green Version]
- Arakawa, K. Genetic and biochemical analysis of the antibiotic biosynthetic gene clusters on the Streptomyces linear plasmid. Biosci. Biotechnol. Biochem. 2014, 78, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yim, G.; Wang, H.H.; Davies, J. Antibiotics as signalling molecules. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1195–1200. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, A.T.; Abou El-Magd, R.M.; Xiao, J.; Lewis, C.W.; Tilli, T.M.; Arakawa, K.; Nindita, Y.; Chan, G.; Sun, L.; Glover, M.; et al. Antitumor Activity of Lankacidin Group Antibiotics Is Due to Microtubule Stabilization via a Paclitaxel-like Mechanism. J. Med. Chem. 2016, 59, 9532–9540. [Google Scholar] [CrossRef]
- Arakawa, K.; Tsuda, N.; Taniguchi, A.; Kinashi, H. The butenolide signaling molecules SRB1 and SRB2 induce lankacidin and lankamycin production in Streptomyces rochei. ChemBioChem 2012, 13, 1447–1457. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-H.; Kim, T.-W.; Park, S.-H.; Lee, K.; Park, H.-Y.; Song, E.; Joo, H.-S.; Kim, Y.-G.; Hahn, J.-S.; Kim, B.-G. Cell-free Escherichia coli-based system to screen for quorum-sensing molecules interacting with quorum receptor proteins of Streptomyces coelicolor. Appl. Environ. Microbiol. 2009, 75, 6367–6372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidda, J.D.; Poon, V.; Song, L.; Wang, W.; Yang, K.; Corre, C. Overproduction and identification of butyrolactones SCB1–8 in the antibiotic production superhost Streptomyces M1152. Org. Biomol. Chem. 2016, 14, 6390–6393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recio, E.; Colinas, Á.; Rumbero, Á.; Aparicio, J.F.; Martín, J.F. PI factor, a novel type quorum-sensing inducer elicits pimaricin production in Streptomyces natalensis. J. Biol. Chem. 2004, 279, 41586–41593. [Google Scholar] [CrossRef] [Green Version]
- Terra, L.; Abreu, P.A.; Teixeira, V.L.; Paixão, I.C.P.; Pereira, R.; Leal, B.; Lourenço, A.L.; Rampelotto, P.H.; Castro, H.C. Mycoses and Antifungals: Reviewing the basis of a current problem that still is a biotechnological target for marine products. Front. Mar. Sci. 2014, 1, 354–379. [Google Scholar] [CrossRef] [Green Version]
- Matselyukh, B.; Mohammadipanah, F.; Laatsch, H.; Rohr, J.; Efremenkova, O.; Khilya, V. N-methylphenylalanyl-dehydrobutyrine diketopiperazine, an A-factor mimic that restores antibiotic biosynthesis and morphogenesis in Streptomyces globisporus 1912-B2 and Streptomyces griseus 1439. J. Antibiot. 2015, 68, 9–14. [Google Scholar] [CrossRef]
- Chater, K.F.; Biro, S.; Lee, K.J.; Palmer, T.; Schrempf, H. The complex extracellular biology of Streptomyces. FEMS Microbiol. Rev. 2010, 34, 171–198. [Google Scholar] [CrossRef] [Green Version]
- Nothaft, H.; Dresel, D.; Willimek, A.; Mahr, K.; Niederweis, M.; Titgemeyer, F. The phosphotransferase system of Streptomyces coelicolor is biased for N-acetylglucosamine metabolism. J. Bacteriol. 2003, 185, 7019–7023. [Google Scholar] [CrossRef] [Green Version]
- Dashti, Y.; Grkovic, T.; Abdelmohsen, U.R.; Hentschel, U.; Quinn, R.J. Actinomycete Metabolome Induction/Suppression with N-Acetylglucosamine. J. Nat. Prod. 2017, 80, 828–836. [Google Scholar] [CrossRef]
- Meena, K.R.; Sharma, A.; Kanwar, S.S. Antitumoral and Antimicrobial Activity of Surfactin Extracted from Bacillus subtilis KLP2015. Int. J. Pept. Res. Ther. 2020, 26, 423–433. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Ayoub, A.T.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Metabolic profiling, histopathological anti-ulcer study, molecular docking and molecular dynamics of ursolic acid isolated from Ocimum forskolei Benth.(family Lamiaceae). S. Afr. J. Bot. 2020, 131, 311–319. [Google Scholar] [CrossRef]
- Kunimoto, S.; Lu, J.; Esumi, H.; Yamazaki, Y.; Kinoshita, N.; Honma, Y.; Hamada, M.; Ohsono, M.; Ishizuka, M.; Takeuchi, T. Kigamicins, novel antitumor antibiotics. I. Taxonomy, isolation, physico-chemical properties and biological activities. J. Antibiot. 2003, 56, 1004–1011. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, K.; Oikawa, H.; Ogawa, H.-O.; Hosono, K.; Shinmachi, F.; Takano, H.; Sakuda, S.; Beppu, T.; Ueda, K. Desferrioxamine E produced by Streptomyces griseus stimulates growth and development of Streptomyces tanashiensis. Microbiology 2005, 151, 2899–2905. [Google Scholar] [CrossRef]
- Ueda, K.; Kawai, S.; Ogawa, H.-O.; Kiyama, A.; Kubota, T.; Kawanobe, H.; Beppu, T. Wide distribution of interspecific stimulatory events on antibiotic production and sporulation among Streptomyces species. J. Antibiot. 2000, 53, 979–982. [Google Scholar] [CrossRef]
- Tam, T.F.; Leung-Toung, R.; Li, W.; Wang, Y.; Karimian, K.; Spino, M. Iron chelator research: Past, present, and future. Curr. Med. Chem. 2003, 10, 983–995. [Google Scholar] [CrossRef]
- Ochi, K.; Tanaka, Y.; Tojo, S. Activating the expression of bacterial cryptic genes by rpoB mutations in RNA polymerase or by rare earth elements. J. Ind. Microbiol. Biotechnol. 2014, 41, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Wang, G.; Okamoto, S.; Ochi, K. The rare earth, scandium, causes antibiotic overproduction in Streptomyces spp. FEMS Microbiol. Lett. 2007, 274, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Inaoka, T.; Ochi, K. Scandium stimulates the production of amylase and bacilysin in Bacillus subtilis. Appl. Environ. Microbiol. 2011, 77, 8181–8183. [Google Scholar] [CrossRef] [Green Version]
- Ochi, K.; Hosaka, T. New strategies for drug discovery: Activation of silent or weakly expressed microbial gene clusters. Appl. Microbiol. Biotechnol. 2013, 97, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Pan, C.; Auckloo, B.N.; Chen, X.; Chen, C.-T.A.; Wang, K.; Wu, X.; Ye, Y.; Wu, B. Stress-driven discovery of a cryptic antibiotic produced by Streptomyces sp. WU20 from Kueishantao hydrothermal vent with an integrated metabolomics strategy. Appl. Microbiol. Biotechnol. 2017, 101, 1395–1408. [Google Scholar] [CrossRef]
- Hassan, S.S.; Shah, S.A.A.; Pan, C.; Fu, L.; Cao, X.; Shi, Y.; Wu, X.; Wang, K.; Wu, B. Production of an antibiotic enterocin from a marine actinobacteria strain H1003 by metal-stress technique with enhanced enrichment using response surface methodology. Pak. J. Pharm. Sci. 2017, 30, 313–324. [Google Scholar]
- Haferburg, G.; Groth, I.; Möllmann, U.; Kothe, E.; Sattler, I. Arousing sleeping genes: Shifts in secondary metabolism of metal tolerant actinobacteria under conditions of heavy metal stress. Biometals 2009, 22, 225. [Google Scholar] [CrossRef] [PubMed]
- So, A.G.; Davie, E.W. Effects of amino acids, s-RNA, and ethanol on coding ambiguity with polyuridylic acid. Biochemistry 1965, 4, 1973–1979. [Google Scholar] [CrossRef]
- Chen, G.; LI, X.; Waters, B.; Davies, J. Enhanced production of microbial metabolites in the presence of dimethyl sulfoxide. J. Antibiot. 2000, 53, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettit, R.K. Small-molecule elicitation of microbial secondary metabolites. Microb. Biotechnol. 2011, 4, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Seyedsayamdost, M.R. High-throughput platform for the discovery of elicitors of silent bacterial gene clusters. Proc. Natl. Acad. Sci. USA 2014, 111, 7266–7271. [Google Scholar] [CrossRef] [Green Version]
- Ninomiya, A.; Katsuyama, Y.; Kuranaga, T.; Miyazaki, M.; Nogi, Y.; Okada, S.; Wakimoto, T.; Ohnishi, Y.; Matsunaga, S.; Takada, K. Biosynthetic gene cluster for surugamide A encompasses an unrelated decapeptide, surugamide F. ChemBioChem 2016, 17, 1709–1712. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, W.; Gong, J.; Cheng, Y. Pre-conditioning with tanshinone IIA attenuates the ischemia/reperfusion injury caused by liver grafts via regulation of HMGB1 in rat Kupffer cells. Biomed. Pharmacother. 2017, 89, 1392–1400. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R. Toward a global picture of bacterial secondary metabolism. J. Ind. Microbiol. Biotechnol. 2019, 46, 301–311. [Google Scholar] [CrossRef]
- Takada, K.; Ninomiya, A.; Naruse, M.; Sun, Y.; Miyazaki, M.; Nogi, Y.; Okada, S.; Matsunaga, S. Surugamides a–e, cyclic octapeptides with four d-amino acid residues, from a marine Streptomyces sp.: Lc–ms-aided inspection of partial hydrolysates for the distinction of d-and l-amino acid residues in the sequence. J. Org. Chem. 2013, 78, 6746–6750. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ren, D.; Xu, H.; Liu, W.; Liu, T.; Li, L.; Li, J.; Li, Y.; Wen, A. Antioxidant and pro-angiogenic effects of corilagin in rat cerebral ischemia via Nrf2 activation. Oncotarget 2017, 8, 114816. [Google Scholar] [CrossRef] [Green Version]
- Kuang, S.; Liu, G.; Cao, R.; Zhang, L.; Yu, Q.; Sun, C. Mansouramycin C kills cancer cells through reactive oxygen species production mediated by opening of mitochondrial permeability transition pore. Oncotarget 2017, 8, 104057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Nazari, B.; Moon, K.; Bushin, L.B.; Seyedsayamdost, M.R. Discovery of a Cryptic Antifungal Compound from Streptomyces albus J1074 Using High-Throughput Elicitor Screens. J. Am. Chem. Soc. 2017, 139, 9203–9212. [Google Scholar] [CrossRef] [Green Version]
- Moon, K.; Xu, F.; Zhang, C.; Seyedsayamdost, M.R. Bioactivity-HiTES unveils cryptic antibiotics encoded in actinomycete bacteria. ACS Chem. Biol. 2019, 14, 767–774. [Google Scholar] [CrossRef]
- Haydon, D.J.; Guest, J.R. A new family of bacterial regulatory proteins. FEMS Microbiol. Lett. 1991, 79, 291–296. [Google Scholar] [CrossRef]
- Moon, K.; Xu, F.; Seyedsayamdost, M.R. Cebulantin, a Cryptic Lanthipeptide Antibiotic Uncovered Using Bioactivity-Coupled HiTES. Angew. Chem. 2019, 131, 6034–6038. [Google Scholar] [CrossRef]
- Taylor, E.C. Design and Synthesis of Inhibitors of Folate Dependent Enzymes as Antitumor Agents. In Chemistry and Biology of Pteridines and Folates; Springer: Berlin/Heidelberg, Germany, 1993; pp. 387–408. [Google Scholar]
- Camarda, G.; Jirawatcharadech, P.; Priestley, R.S.; Saif, A.; March, S.; Wong, M.H.L.; Leung, S.; Miller, A.B.; Baker, D.A.; Alano, P.; et al. Antimalarial activity of primaquine operates via a two-step biochemical relay. Nat. Commun. 2019, 10, 3226. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.M.; Rosenkranz, H.S. Antimicrobial activity of local anesthetics: Lidocaine and procaine. J. Infect. Dis. 1970, 121, 597–607. [Google Scholar] [CrossRef]
- Onaka, H. Novel antibiotic screening methods to awaken silent or cryptic secondary metabolic pathways in actinomycetes. J. Antibiot. 2017, 70, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.A.; Burckart, G.J.; Venkataramanan, R. Tacrolimus: A new immunosuppressive agent. Am. J. Health Pharm. 1995, 52, 1521–1535. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, D.; Liang, S. Identification and metabolomic analysis of chemical elicitors for tacrolimus accumulation in Streptomyces tsukubaensis. Appl. Microbiol. Biotechnol. 2018, 102, 7541–7553. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Hassan, H.A.; Abdelmohsen, U.R.; Zahran, E.M. A Glossary for Chemical Approaches towards Unlocking the Trove of Metabolic Treasures in Actinomycetes. Molecules 2022, 27, 142. https://doi.org/10.3390/molecules27010142
Zhang J, Hassan HA, Abdelmohsen UR, Zahran EM. A Glossary for Chemical Approaches towards Unlocking the Trove of Metabolic Treasures in Actinomycetes. Molecules. 2022; 27(1):142. https://doi.org/10.3390/molecules27010142
Chicago/Turabian StyleZhang, Jianye, Heba Ali Hassan, Usama Ramadan Abdelmohsen, and Eman Maher Zahran. 2022. "A Glossary for Chemical Approaches towards Unlocking the Trove of Metabolic Treasures in Actinomycetes" Molecules 27, no. 1: 142. https://doi.org/10.3390/molecules27010142
APA StyleZhang, J., Hassan, H. A., Abdelmohsen, U. R., & Zahran, E. M. (2022). A Glossary for Chemical Approaches towards Unlocking the Trove of Metabolic Treasures in Actinomycetes. Molecules, 27(1), 142. https://doi.org/10.3390/molecules27010142







