Oenothein B in Eucalyptus Leaf Extract Suppresses Fructose Absorption in Caco-2 Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Inhibitory Activities of ELE on Fructose Absorption
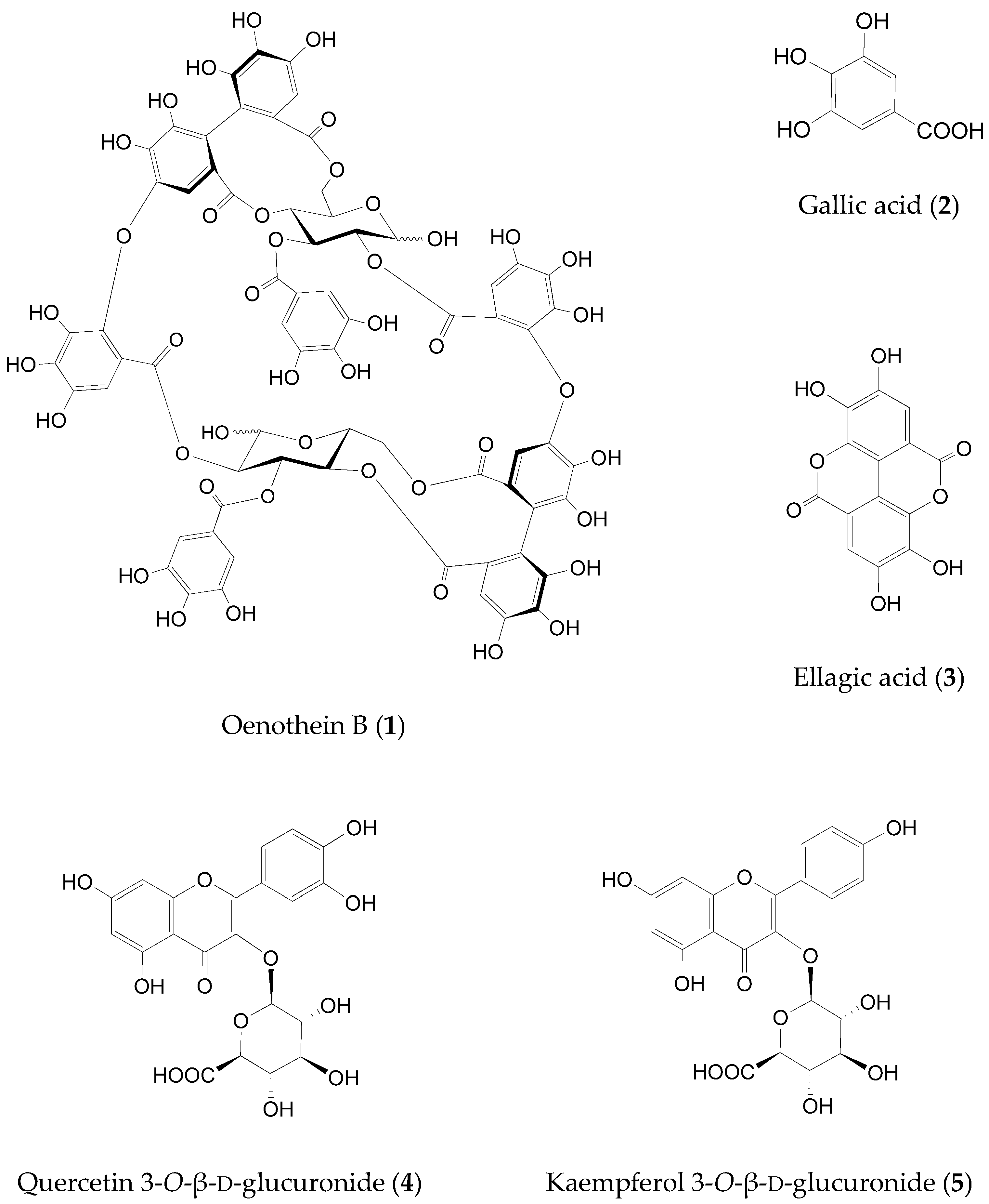
2.2. Identification of the Fructose Absorption Inhibitor in ELE
3. Materials and Methods
3.1. Materials
3.2. Cells
3.3. Measurement of Fructose and Glucose Absorption Inhibitory Activity
3.4. Treatment of ELE with PVPP
3.5. Measurement of Total Polyphenol Content
3.6. HPLC-DAD-ESI-MS Analysis of ELE
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Sugar: World Markets and Trade. Available online: https://www.fas.usda.gov/data/sugar-world-markets-and-trade (accessed on 15 November 2021).
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M. Toxic AGEs (TAGE) theory: A new concept for preventing the development of diseases related to lifestyle. Diabetol. Metab. Syndr. 2020, 12, 105. [Google Scholar] [CrossRef] [PubMed]
- Amani, S.; Fatima, S. Glycation with fructose: The bitter side of nature’s own sweetener. Curr. Diabetes Rev. 2020, 16, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, weight gain, and the insulin resistance syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef] [PubMed]
- ter Horst, K.W.; Schene, M.R.; Holman, R.; Romijn, J.A.; Serlie, M.J. Effect of fructose consumption on insulin sensitivity in nondiabetic subjects: A systematic review and meta-analysis of diet-intervention trials. Am. J. Clin. Nutr. 2016, 104, 1562–1576. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Rosato, V.; Masarone, M.; Torre, P.; Dallio, M.; Romeo, M.; Persico, M. The role of fructose in non-alcoholic steatohepatitis: Old relationship and new insights. Nutrients 2021, 13, 1314. [Google Scholar] [CrossRef] [PubMed]
- Barone, S.; Fussell, S.L.; Singh, A.K.; Lucas, F.; Xu, J.; Kim, C.; Wu, X.; Yu, Y.; Amla, H.; Seidler, U.; et al. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J. Biol. Chem. 2009, 284, 5056–5066. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Li, Y.; Pan, A.; De Koning, L.; Schernhammer, E.; Willett, W.C.; Hu, F.B. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation 2019, 139, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Calls on Countries to Reduce Sugars Intake Among Adults and Children. Available online: https://www.who.int/mediacentre/news/releases/2015/sugar-guideline/en/ (accessed on 15 November 2021).
- Ishimoto, T.; Lanaspa, M.A.; Le, M.T.; Garcia, G.E.; Diggle, C.P.; Maclean, P.S.; Jackman, M.R.; Asipu, A.; Roncal-Jimenez, C.A.; Kosugi, T.; et al. Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 4320–4325. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, R.P.; Choe, J.Y.; Patel, C.R. Intestinal absorption of fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.M.; Noworolski, S.M.; Erkin-Cakmak, A.; Korn, N.J.; Wen, M.J.; Tai, V.W.; Jones, G.M.; Palii, S.P.; Velasco-Alin, M.; Pan, K.; et al. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology 2017, 153, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Lustig, R.H.; Mulligan, K.; Noworolski, S.M.; Tai, V.W.; Wen, M.J.; Erkin-Cakmak, A.; Gugliucci, A.; Schwarz, J.M. Isocaloric fructose restriction and metabolic improvement in children with obesity and metabolic syndrome. Obesity 2016, 24, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Simons, N.; Veeraiah, P.; Simons, P.I.H.G.; Schaper, N.C.; Kooi, M.E.; Schrauwen-Hinderling, V.B.; Feskens, E.J.M.; van der Ploeg, E.M.C.L.; Van den Eynde, M.D.G.; Schalkwijk, C.G.; et al. Effects of fructose restriction on liver steatosis (FRUITLESS); a double-blind randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Coello, S.; Carrillo-Fernández, L.; Gobierno-Hernández, J.; Méndez-Abad, M.; Borges-Álamo, C.; García-Dopico, J.A.; Aguirre-Jaime, A.; León, A.C. Decreased consumption of added fructose reduces waist circumference and blood glucose concentration in patients with overweight and obesity. The DISFRUTE Study: A randomised trial in primary care. Nutrients 2020, 12, 1149. [Google Scholar] [CrossRef] [PubMed]
- Hajeski, N.J. National Geographic Complete Guide to Herbs and Spices: Remedies, Seasonings, and Ingredients to Improve Your Health and Enhance Your Life; National Geographic Society: Washington, DC, USA, 2016. [Google Scholar]
- Gray, A.M.; Flatt, P.R. Antihyperglycemic actions of Eucalyptus globulus (Eucalyptus) are associated with pancreatic and extra-pancreatic effects in mice. J. Nutr. 1998, 128, 2319–2323. [Google Scholar] [CrossRef] [PubMed]
- Amakura, Y.; Umino, Y.; Tsuji, S.; Ito, H.; Hatano, T.; Yoshida, T.; Tonogai, Y. Constituents and their antioxidative effects in eucalyptus leaf extract used as a natural food additive. Food Chem. 2002, 77, 47–56. [Google Scholar] [CrossRef]
- Amakura, Y.; Yoshimura, M.; Sugimoto, N.; Yamazaki, T.; Yoshida, T. Marker constituents of the natural antioxidant Eucalyptus leaf extract for the evaluation of food additives. Biosci. Biotechnol. Biochem. 2009, 73, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Inagaki, Y.; Tanaka, M.; Ojima, M.; Kataoka, K.; Kuboniwa, M.; Nishida, N.; Shimizu, K.; Osawa, K.; Shizukuishi, S. Effect of eucalyptus extract chewing gum on periodontal health: A double-masked, randomized trial. J. Periodontol. 2008, 79, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, T.; Nakagawa, Y.; Nakagawa, M.; Kawai, Y.; Nagaoka, E.; Konno, T.; Ishikawa, S.; Inoue, M.; Hasebe, K.; Tamura, S. Efficacy of new skin care hand cream when used by nurses. Jpn. J. Pharm. Health Care Sci. 2006, 32, 1275–1279. [Google Scholar] [CrossRef][Green Version]
- Inoue, S. Report of the MHLW-Grant-in-Aid Research Project: Hazard-Based Peer-Review Studies on the Safety Surveillance of Existing Food Additives; Ministry of Health, Labor and Welfare of Japan: Tokyo, Japan, 2007; pp. 14–15. Available online: http://www.mhlw.go.jp/shingi/2007/07/dl/s0704-7k.pdf (accessed on 15 November 2021).
- Sugimoto, K.; Nakagawa, K.; Fujiwara, S.; Sakano, K.; Ebihara, S. Safety assessment of eucalyptus leaf extract oral consumption for 4 weeks in human subjects: A pilot study. Jpn. J. Complement. Alternat. Med. 2020, 17, 23–31. [Google Scholar] [CrossRef]
- Sugimoto, K.; Suzuki, J.; Nakagawa, K.; Hayashi, S.; Fujita, T.; Yamaji, R.; Inui, H.; Nakano, Y. Eucalyptus leaf extract inhibits intestinal fructose absorption, and suppresses adiposity due to dietary sucrose in rats. Br. J. Nutr. 2005, 93, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Kawasaki, T.; Tomoda, M.; Nakagawa, K.; Hayashi, S.; Inui, H.; Kajimoto, T.; Yamanouchi, T. Lowering of postprandial hyperfructosemia in humans by eucalyptus leaf extract: A randomized, double-blind, placebo-controlled crossover study. Food Sci. Technol. Res. 2010, 16, 509–512. [Google Scholar] [CrossRef]
- Gonçalves, A.S.; Andrade, N.; Martel, F. Intestinal fructose absorption: Modulation and relation to human diseases. Pharma Nutr. 2020, 14, 100235. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Schreck, K.; Melzig, M.F. Intestinal saturated long-chain fatty acid, glucose and fructose transporters and their inhibition by natural plant extracts in Caco-2 cells. Molecules 2018, 23, 2544. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.; Araújo, J.R.; Correia-Branco, A.; Carletti, J.V.; Martel, F. Effect of dietary polyphenols on fructose uptake by human intestinal epithelial (Caco-2) cells. J. Funct. Foods 2017, 36, 429–439. [Google Scholar] [CrossRef]
- Concha, I.I.; Velásquez, F.V.; Martínez, J.M.; Angulo, C.; Droppelmann, A.; Reyes, A.M.; Slebe, J.C.; Vera, J.C.; Golde, D.W. Human erythrocytes express GLUT5 and transport fructose. Blood 1997, 89, 4190–4195. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Satsu, H.; Makino, T. Inhibitory effect of bofutsushosan (fang feng tong sheng san) on glucose transporter 5 function in vitro. J. Nat. Med. 2018, 72, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Gauer, J.S.; Tumova, S.; Lippiat, J.D.; Kerimi, A.; Williamson, G. Differential patterns of inhibition of the sugar transporters GLUT2, GLUT5 and GLUT7 by flavonoids. Biochem. Pharmacol. 2018, 152, 11–20. [Google Scholar] [CrossRef] [PubMed]
- George Thompson, A.M.; Iancu, C.V.; Nguyen, T.T.; Kim, D.; Choe, J.Y. Inhibition of human GLUT1 and GLUT5 by plant carbohydrate products; insights into transport specificity. Sci. Rep. 2015, 5, 12804. [Google Scholar] [CrossRef] [PubMed]
- Kerimi, A.; Gauer, J.S.; Crabbe, S.; Cheah, J.W.; Lau, J.; Walsh, R.; Cancalon, P.F.; Williamson, G. Effect of the flavonoid hesperidin on glucose and fructose transport, sucrase activity and glycaemic response to orange juice in a crossover tri-al on healthy volunteers. Br. J. Nutr. 2019, 121, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lim, Y.; Kwon, O. Selected phytochemicals and culinary plant extracts inhibit fructose uptake in Caco-2 cells. Molecules 2015, 20, 17393–17404. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, G.; Martel, F. The effect of dietary polyphenols on intestinal absorption of glucose and fructose: Relation with obesity and type 2 diabetes. Food Rev. Int. 2019, 35, 390–406. [Google Scholar] [CrossRef]
- Satsu, H.; Awara, S.; Unno, T.; Shimizu, M. Suppressive effect of nobiletin and epicatechin gallate on fructose uptake in human intestinal epithelial Caco-2 cells. Biosci. Biotechnol. Biochem. 2018, 82, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Slavic, K.; Derbyshire, E.T.; Naftalin, R.J.; Krishna, S.; Staines, H.M. Comparison of effects of green tea catechins on apicomplexan hexose transporters and mammalian orthologues. Mol. Biochem. Parasitol. 2009, 168, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Zakłos-Szyda, M.; Pietrzyk, N.; Kowalska-Baron, A.; Nowak, A.; Chałaśkiewicz, K.; Ratajewski, M.; Budryn, G.; Koziołkiewicz, M. Phenolics-rich extracts of dietary plants as regulators of fructose uptake in Caco-2 cells via GLUT5 involvement. Molecules 2021, 26, 4745. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S. Quantification of Tannins in Tree and Shrub Foliage; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; p. 5. [Google Scholar]
- Yoshida, T.; Yoshimura, M.; Amakura, Y. Chemical and biological significance of oenothein B and related ellagitannin oligomers with macrocyclic structure. Molecules 2018, 23, 552. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Wada, S.; Yang, S.; Gosis, B.; Zeng, X.; Zhang, Z.; Shen, Y.; Lee, G.; Arany, Z.; Rabinowitz, J.D. The small intestine shields the liver from fructose-induced steatosis. Nat. Metab. 2020, 2, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Baert, N.; Karonen, M.; Salminen, J.P. Isolation, characterisation and quantification of the main oligomeric macrocyclic ellagitannins in Epilobium angustifolium by ultra-high performance chromatography with diode array detection and electrospray tandem mass spectrometry. J. Chromatogr. A 2015, 1419, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.H.; Blazics, B.; Kéry, A. Polyphenol composition and antioxidant capacity of Epilobium species. J. Pharm. Biomed. Anal. 2009, 49, 26–31. [Google Scholar] [CrossRef] [PubMed]
- John, H.; Walden, M.; Schäfer, S.; Genz, S.; Forssmann, W.G. Analytical procedures for quantification of peptides in pharmaceutical research by liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2004, 378, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Inui, H.; Yamanouchi, T. Chapter 27 Assays of fructose in experimental nutrition. In Dietary Sugars: Chemistry, Analysis, Function and Effects; Preedy, V.R., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2012; pp. 464–483. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Arapitsas, P. Hydrolyzable tannin analysis in food. Food Chem. 2012, 135, 1708–1717. [Google Scholar] [CrossRef] [PubMed]


| Sample | Total Polyphenols (%) | Dose (mg/mL) | Inhibition 1 (%) |
|---|---|---|---|
| ELE | 30 | 1 | 65 |
| PVPP-treated ELE | 5 | 0.26 | <20 |
| Sample | Dose (μg/mL) | Inhibition 1 (%) |
|---|---|---|
| Oenothein B (1) | 5 | 63 |
| Gallic acid (2) | 50 | <20 |
| Ellagic acid (3) | 50 | <30 |
| Quercetin 3-O-β-d-glucuronide (4) | 50 | <30 |
| Kaempferol 3-O-β-d-glucuronide (5) | 50 | <30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugimoto, K.; Amako, M.; Takeuchi, H.; Nakagawa, K.; Yoshimura, M.; Amakura, Y.; Fujita, T.; Takenaka, S.; Inui, H. Oenothein B in Eucalyptus Leaf Extract Suppresses Fructose Absorption in Caco-2 Cells. Molecules 2022, 27, 122. https://doi.org/10.3390/molecules27010122
Sugimoto K, Amako M, Takeuchi H, Nakagawa K, Yoshimura M, Amakura Y, Fujita T, Takenaka S, Inui H. Oenothein B in Eucalyptus Leaf Extract Suppresses Fructose Absorption in Caco-2 Cells. Molecules. 2022; 27(1):122. https://doi.org/10.3390/molecules27010122
Chicago/Turabian StyleSugimoto, Keiichiro, Midori Amako, Hiroaki Takeuchi, Kazuya Nakagawa, Morio Yoshimura, Yoshiaki Amakura, Tomoyuki Fujita, Shigeo Takenaka, and Hiroshi Inui. 2022. "Oenothein B in Eucalyptus Leaf Extract Suppresses Fructose Absorption in Caco-2 Cells" Molecules 27, no. 1: 122. https://doi.org/10.3390/molecules27010122
APA StyleSugimoto, K., Amako, M., Takeuchi, H., Nakagawa, K., Yoshimura, M., Amakura, Y., Fujita, T., Takenaka, S., & Inui, H. (2022). Oenothein B in Eucalyptus Leaf Extract Suppresses Fructose Absorption in Caco-2 Cells. Molecules, 27(1), 122. https://doi.org/10.3390/molecules27010122






