Quality Evaluation of Light- and Dark-Colored Hungarian Honeys, Focusing on Botanical Origin, Antioxidant Capacity and Mineral Content
Abstract
1. Introduction
2. Results and Discussion
2.1. Pollen Study, Sensory Characteristics and Color of Honey Samples
2.2. Total Antioxidant Capacities of Honeys
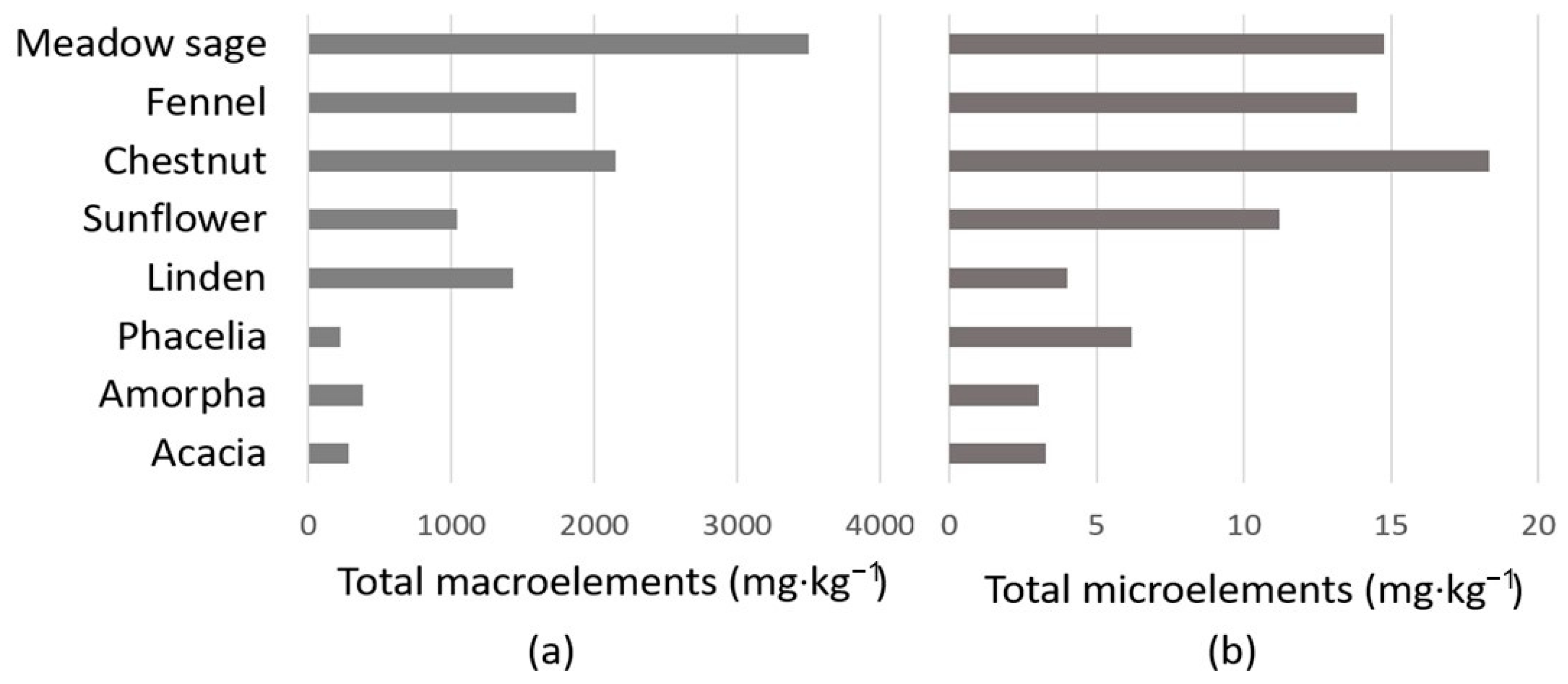
2.3. Multielement Analysis of Honeys
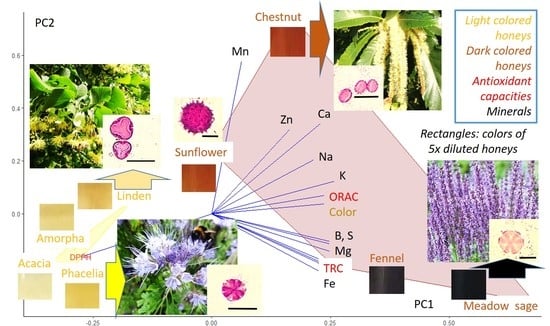
2.4. Correlation Analysis
3. Materials and Methods
3.1. Samples
3.2. Melissopalynological Analysis
3.3. Determination of Color Intensity (ABS450)
3.4. Total Reducing Capacity (TRC)
3.5. Antiradical Power (DPPH)
3.6. Oxygen Radical Absorbance Capacity (ORAC)
3.7. Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES)
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kıvrak, Ş.; Kıvrak, İ. Assessment of phenolic profile of Turkish honeys. Int. J. Food Prop. 2017, 20, 864–876. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, P.R. Floral markers in honey of various botanical and geographic origins: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Bridi, R.; Montenegro, G. The Value of Chilean Honey: Floral Origin Related to Their Antioxidant and Antibacterial Activities; Honey Analysis; Arnaut, V., Ed.; Intechopen: Rijeka, Croatia, 2017; pp. 63–78. [Google Scholar]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Gül, A.; Pehlivan, T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J. Biol. Sci. 2018, 25, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [PubMed]
- Gośliński, M.; Nowak, D.; K\lębukowska, L. Antioxidant properties and antimicrobial activity of manuka honey versus Polish honeys. J. Food Sci. Technol. 2020, 57, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Halagarda, M.; Groth, S.; Popek, S.; Rohn, S.; Pedan, V. Antioxidant activity and phenolic profile of selected organic and conventional honeys from Poland. Antioxidants 2020, 9, 44. [Google Scholar] [CrossRef]
- Stagos, D.; Soulitsiotis, N.; Tsadila, C.; Papaeconomou, S.; Arvanitis, C.; Ntontos, A.; Karkanta, F.; Adamou-Androulaki, S.; Petrotos, K.; Spandidos, D.A. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018, 42, 726–734. [Google Scholar] [CrossRef]
- Srećković, N.Z.; Mihailović, V.B.; Katanić-Stanković, J.S. Physico-chemical, antioxidant and antimicrobial properties of three different types of honey from Central Serbia. Kragujev. J. Sci. 2019, 53–68. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitability for Romanian honey authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Melissopalynology, antioxidant activity and multielement analysis of two types of early spring honeys from Hungary. Food Biosci. 2020, 35, 100587. [Google Scholar] [CrossRef]
- Gorjanović, S.Ž.; Alvarez-Suarez, J.M.; Novaković, M.M.; Pastor, F.T.; Pezo, L.; Battino, M.; Sužnjević, D.Ž. Comparative analysis of antioxidant activity of honey of different floral sources using recently developed polarographic and various spectrophotometric assays. J. Food Compos. Anal. 2013, 30, 13–18. [Google Scholar] [CrossRef]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef]
- Kuś, P.M.; Congiu, F.; Teper, D.; Sroka, Z.; Jerković, I.; Tuberoso, C.I.G. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT Food Sci. Technol. 2014, 55, 124–130. [Google Scholar] [CrossRef]
- Salonen, A.; Virjamo, V.; Tammela, P.; Fauch, L.; Julkunen-Tiitto, R. Screening bioactivity and bioactive constituents of Nordic unifloral honeys. Food Chem. 2017, 237, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive components and antioxidant and antibacterial activities of different varieties of honey: A screening prior to clinical application. J. Agric. Food Chem. 2018, 67, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Lewoyehu, M.; Amare, M. Comparative assessment on selected physicochemical parameters and antioxidant and antimicrobial activities of honey samples from selected districts of the Amhara and Tigray regions, Ethiopia. Int. J. Food Sci. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Solayman, M.; Islam, M.A.; Paul, S.; Ali, Y.; Khalil, M.I.; Alam, N.; Gan, S.H. Physicochemical properties, minerals, trace elements, and heavy metals in honey of different origins: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Kılıç Altun, S.; Dinç, H.; Paksoy, N.; Temamoğulları, F.K.; Savrunlu, M. Analyses of mineral content and heavy metal of honey samples from south and east region of Turkey by using ICP-MS. Int. J. Anal. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Sager, M. The honey as a bioindicator of the environment. Ecol. Chem. Eng. S 2017, 24, 583–594. [Google Scholar] [CrossRef]
- Conti, M.E.; Canepari, S.; Finoia, M.G.; Mele, G.; Astolfi, M.L. Characterization of Italian multifloral honeys on the basis of their mineral content and some typical quality parameters. J. Food Compos. Anal. 2018, 74, 102–113. [Google Scholar] [CrossRef]
- Czipa, N.; Andrási, D.; Kovács, B. Determination of essential and toxic elements in Hungarian honeys. Food Chem. 2015, 175, 536–542. [Google Scholar] [CrossRef]
- Sajtos, Z.; Herman, P.; Harangi, S.; Baranyai, E. Elemental analysis of Hungarian honey samples and bee products by MP-AES method. Microchem. J. 2019, 149, 103968. [Google Scholar] [CrossRef]
- Bogdanov, S.; Haldimann, M.; Luginbühl, W.; Gallmann, P. Minerals in honey: Environmental, geographical and botanical aspects. J. Apic. Res. 2007, 46, 269–275. [Google Scholar] [CrossRef]
- Madejczyk, M.; Baralkiewicz, D. Characterization of Polish rape and honeydew honey according to their mineral contents using ICP-MS and F-AAS/AES. Anal. Chim. Acta 2008, 617, 11–17. [Google Scholar] [CrossRef]
- Mohammed, F.; Abdulwali, N.; Guillaume, D.; Bchitou, R. Element content of Yemeni honeys as a long-time marker to ascertain honey botanical origin and quality. LWT 2018, 88, 43–46. [Google Scholar] [CrossRef]
- Nayik, G.A.; Nanda, V. Physico-chemical, enzymatic, mineral and colour characterization of three different varieties of honeys from Kashmir valley of India with a multivariate approach. Pol. J. Food Nutr. Sci. 2015, 65, 101–108. [Google Scholar] [CrossRef]
- Nayik, G.A.; Suhag, Y.; Majid, I.; Nanda, V. Discrimination of high altitude Indian honey by chemometric approach according to their antioxidant properties and macro minerals. J. Saudi Soc. Agric. Sci. 2018, 17, 200–207. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Tezcan, F.; Erim, F.B.; Yildiz, O.; Sahin, H.; Can, Z.; Kolayli, S. Characterization of Anatolian honeys based on minerals, bioactive components and principal component analysis. LWT-Food Sci. Technol. 2016, 68, 273–279. [Google Scholar] [CrossRef]
- Perna, A.; Simonetti, A.; Intaglietta, I.; Sofo, A.; Gambacorta, E. Metal content of southern Italy honey of different botanical origins and its correlation with polyphenol content and antioxidant activity. Int. J. Food Sci. Technol. 2012, 47, 1909–1917. [Google Scholar] [CrossRef]
- Escuredo, O.; Rodríguez-Flores, M.S.; Rojo-Martínez, S.; Seijo, M.C. Contribution to the chromatic characterization of unifloral honeys from Galicia (NW Spain). Foods 2019, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterization and classification of Thymus capitatus (L.) honey according to geographical origin based on volatile compounds, physicochemical parameters and chemometrics. Food Res. Int. 2014, 55, 363–372. [Google Scholar] [CrossRef]
- Kenjerić, D.; Mandić, M.L.; Primorac, L.; Čačić, F. Flavonoid pattern of sage (Salvia officinalis L.) unifloral honey. Food Chem. 2008, 110, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Manzanares, A.B.; García, Z.H.; Galdón, B.R.; Rodríguez-Rodríguez, E.M.; Romero, C.D. Physicochemical characteristics and pollen spectrum of monofloral honeys from Tenerife, Spain. Food Chem. 2017, 228, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Oddo, L.P.; Piro, R.; Bruneau, É.; Guyot-Declerck, C.; Ivanov, T.; Piskulová, J.; Flamini, C.; Lheritier, J.; Morlot, M.; Russmann, H. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, S38–S81. [Google Scholar] [CrossRef]
- Sari, E.; Ayyildiz, N. Biological activities and some physicochemical properties of sunflower honeys collected from the Thrace region of Turkey. Pak. J. Biol. Sci. PJBS 2012, 15, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Terrab, A.; González-Miret, L.; Heredia, F.J. Colour characterisation of thyme and avocado honeys by diffuse reflectance spectrophotometry and spectroradiometry. Eur. Food Res. Technol. 2004, 218, 488–492. [Google Scholar] [CrossRef]
- Cimpoiu, C.; Hosu, A.; Miclaus, V.; Puscas, A. Determination of the floral origin of some Romanian honeys on the basis of physical and biochemical properties. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2013, 100, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Flanjak, I.; Kenjerić, D.; Bubalo, D.; Primorac, L. Characterisation of selected Croatian honey types based on the combination of antioxidant capacity, quality parameters, and chemometrics. Eur. Food Res. Technol. 2016, 242, 467–475. [Google Scholar] [CrossRef]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R.M. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Pontis, J.A.; Costa, L.A.M.A.; Silva, S.J.R.D.; Flach, A. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci. Technol. 2014, 34, 69–73. [Google Scholar] [CrossRef]
- Czipa, N.; Phillips, C.J.; Kovács, B. Composition of acacia honeys following processing, storage and adulteration. J. Food Sci. Technol. 2019, 56, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Kek, S.P.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Total phenolic contents and colour intensity of Malaysian honeys from the Apis spp. and Trigona spp. bees. Agric. Agric. Sci. Procedia 2014, 2, 150–155. [Google Scholar] [CrossRef]
- Bogdanov, S. Honey as nutrient and functional food. Proteins 2012, 1100, 1400–2700. [Google Scholar]
- Lachman, J.; Orsák, M.; Hejtmánková, A.; Kovářová, E. Evaluation of antioxidant activity and total phenolics of selected Czech honeys. LWT-Food Sci. Technol. 2010, 43, 52–58. [Google Scholar] [CrossRef]
- Baltrušaitytė, V.; Venskutonis, P.R.; Čeksterytė, V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem. 2007, 101, 502–514. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.-H.; Engeseth, N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002, 50, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, M. Különleges Fajtamezek Botanikai Eredetének és illó Komponenseinek Összefüggése. Ph.D. Thesis, Budapesti Corvinus Egyetem, Budapest, Hungary, 2009. [Google Scholar]
- Bilandžić, N.; Gačić, M.; DJokić, M.; Sedak, M.; Šipušić, D.I.; Končurat, A.; Gajger, I.T. Major and trace elements levels in multifloral and unifloral honeys in Croatia. J. Food Compos. Anal. 2014, 33, 132–138. [Google Scholar] [CrossRef]
- Terrab, A.; Recamales, A.F.; Gonzalez-Miret, M.L.; Heredia, F.J. Contribution to the study of avocado honeys by their mineral contents using inductively coupled plasma optical emission spectrometry. Food Chem. 2005, 92, 305–309. [Google Scholar] [CrossRef]
- Ritter, L. Sources, pathways, and relative risks of contaminants in surface water and groundwater: A perspective prepared for the Walkerton inquiry. J. Toxicol. Environ. Health A 2002, 65, 1–142. [Google Scholar]
- Afroz, R.; Tanvir, E.M.; Zheng, W.; Little, P.J. Molecular pharmacology of honey. Clin. Exp. Pharm. 2016, 6, 1–13. [Google Scholar]
- Pisani, A.; Protano, G.; Riccobono, F. Minor and trace elements in different honey types produced in Siena County (Italy). Food Chem. 2008, 107, 1553–1560. [Google Scholar] [CrossRef]
- Islam, M.N.; Khalil, M.I.; Islam, M.A.; Gan, S.H. Toxic compounds in honey. J. Appl. Toxicol. 2014, 34, 733–742. [Google Scholar] [CrossRef]
- Sowa, P.; Grabek-Lejko, D.; Wesołowska, M.; Swacha, S.; Dżugan, M. Hydrogen peroxide-dependent antibacterial action of Melilotus albus honey. Lett. Appl. Microbiol. 2017, 65, 82–89. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sulaiman, S.A.; Boukraa, L. Antioxidant properties of honey and its role in preventing health disorder. Open Nutraceuticals J. 2010, 3. [Google Scholar] [CrossRef]
- Von Der Ohe, W.; Oddo, L.P.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Kőszegi, T.; Sali, N.; Raknić, M.; Horváth-Szalai, Z.; Csepregi, R.; Končić, M.Z.; Papp, N.; Poór, M. A novel luminol-based enhanced chemiluminescence antioxidant capacity microplate assay for use in different biological matrices. J. Pharmacol. Toxicol. Methods 2017, 88, 153–159. [Google Scholar] [CrossRef]
- Patay, É.B.; Sali, N.; Kőszegi, T.; Csepregi, R.; Balázs, V.L.; Németh, T.S.; Németh, T.; Papp, N. Antioxidant potential, tannin and polyphenol contents of seed and pericarp of three Coffea species. Asian Pac. J. Trop. Med. 2016, 9, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Horikoshi, M.; Tang, Y.; Dickey, A.; Grenié, M.; Thompson, R.; Selzer, L.; Strbenac, D.; Voronin, K. Data Visualization Tools for Statistical Analysis Results. 2016. Available online: https://CRAN.R-project.org/package=ggfortify (accessed on 7 April 2021).


| Nr. | Honey Type Plant Name | Dominant Pollen (%) | Sensory Characteristics (Color, Odor and Consistency) | ABS450 (mAU) |
|---|---|---|---|---|
| 1 | Acacia Robinia pseudoacacia | Robinia pseudoacacia (45.27%) | Pale, yellowish green, weak odor, liquid, viscous | 136 ± 4 |
| 2 | Amorpha Amorpha fruticosa | Amorpha fruticosa (74.77%) | Pale yellow, weak odor, liquid, viscous | 191 ± 5 |
| 3 | Phacelia Phacelia tanacetifolia | Phacelia tanacetifolia (74.07%) | Light beige, moderately intense odor, fine granulated, semisolid | 247 ± 11 |
| 4 | Linden Tilia spp. | Tilia spp. (45.89%) | Light amber, strong odor, fine granulated, semisolid | 285 ± 8 |
| 5 | Sunflower Helianthus annuus | Helianthus annuus (47.44%) | Bright yellow, moderately intense odor, coarsely granulated, solid | 719 ± 5 |
| 6 | Chestnut Castanea sativa | Castanea sativa (90.81%) | Dark amber with reddish tone, strong odor, liquid, viscous | 920 ± 10 |
| 7 | Fennel Foeniculum vulgare | Asteraceae (21.97%) Apiaceae (19.23%) | Almost black, strong, caramel odor, liquid, viscous | 1087 ± 33 |
| 8 | Meadow sage Salvia pratensis | Lamiaceae (24.63%) | Black, strong, sage-like odor, liquid, viscous | 1459 ± 52 |
| Nr. | Honey Types | TRC (mg·GAE·kg−1) | DPPH (IC50mg·mL−1) | ORAC (µmol·TE·g−1) |
|---|---|---|---|---|
| 1 | Acacia | 60.08 ± 6.24 a | 61.76 ± 2.85 a | 19.81 ± 1.72 a |
| 2 | Amorpha | 80.81 ± 15.81 b | 55.48 ± 1.86 a | 14.78 ± 1.16 b |
| 3 | Phacelia | 91.67 ± 19.03 b | 55.78 ± 1.95 a | 13.79 ± 0.58 b |
| 4 | Linden | 119.14 ± 13.80c | 35.86 ± 0.62 b | 71.68 ± 5.43 c |
| 5 | Sunflower | 230.25 ± 8.35 d | 26.62 ± 0.49 c | 34.32 ± 3.57 d |
| 6 | Chestnut | 232.82 ± 24.97 d | 17.37 ± 0.57 d | 75.20 ± 4.71 c |
| 7 | Fennel | 468.00 ± 73.16 e | 12.28 ± 0.25 e | 61.33 ± 5.83 e |
| 8 | Meadow sage | 1116.15 ± 83.84 f | 5.47 ± 0.02 f | 114.89 ± 10.43 f |
| Total | ||||
| Light-colored honeys (nr. 1–4) | 87.92 ± 25.67 a | 52.22 ± 10.35 a | 30.84 ± 25.01 a | |
| Dark-colored honeys (nr. 5–8) | 511.81 ± 371.2 b | 15.43 ± 8.07 b | 71.54 ± 29.77 b | |
| Nr. | Honey Types | K (mg·kg−1) | Ca (mg·kg−1) | P (mg·kg−1) | S (mg·kg−1) | Mg (mg·kg−1) | Na (mg·kg−1) |
|---|---|---|---|---|---|---|---|
| 1. | Acacia | 226.56 ± 17.42 a | 12.39 ± 1.44 a | 24.92 ± 1.62 a | 7.07 ± 0.35 a | 5.24 ± 0.28 a | 5.99 ± 0.16 a |
| 2. | Amorpha | 282.47 ± 19.22 b | 16.61 ± 2.50 a,b | 42.25 ± 3.65 b | 13.11 ± 1.44 b | 8.38 ± 0.29 b | 14.25 ± 2.05 b |
| 3. | Phacelia | 145.62 ± 4.23 c | 19.88 ± 3.11 b | 33.68 ± 2.46 c | 13.04 ± 0.88 b | 6.04 ± 0.33 c | 3.56 ± 3.09 a |
| 4. | Linden | 1278.08 ± 18.97 d | 67.85 ± 8.01 c | 41.52 ± 4.46 b,c | 15.89 ± 4.46 c | 16.51 ± 0.30 d | 9.29 ± 1.03 c |
| 5. | Sunflower | 758.95 ± 18.69 e | 126.37 ± 14.93 d,e | 76.25 ± 8.22 d | 26.53 ± 8.22 d | 33.26 ± 1.28 e | 13.23 ± 1.70 b |
| 6. | Chestnut | 1815.79 ± 20.69 f | 153.01 ± 12.60 d | 79.04 ± 5.41 d | 35.55 ± 5.41 d,e | 45.38 ± 17.32 e | 20.94 ± 0.80 d |
| 7. | Fennel | 1373.99 ± 41.36 g | 103.46 ± 14.64 e | 251.85 ± 28.47 e | 44.91 ± 28.47 e | 86.82 ± 3.29 f | 9.14 ± 0.31 c |
| 8. | Meadow sage | 2523.02 ± 28.45 h | 135.60 ± 21.16 d | 549.66 ± 54.64 f | 96.68 ± 54.64 f | 167.12 ± 2.08 g | 25.08 ± 2.76 e |
| Nr. | Honey Types | B (mg·kg−1) | Cu (mg·kg−1) | Fe (mg·kg−1) | Mn (mg·kg−1) | Zn (mg·kg−1) |
|---|---|---|---|---|---|---|
| 1. | Acacia | 2.99 ± 0.21a | <0.10 | <0.05 | 0.12 ± 0.02 a | 0.15 ± 0.08 a |
| 2. | Amorpha | 2.58 ± 0.41a | <0.10 | <0.05 | 0.13 ± 0.01 a | 0.31 ± 0.04 a |
| 3. | Phacelia | 4.10 ± 0.52 b | <0.10 | 0.91 ± 0.54 a | <0.10 | 1.17 ± 0.25 b |
| 4. | Linden | 2.70 ± 0.09 a | 0.12 ± 0.02 a | <0.05 | 1.01 ± 0.03 b | 0.15 ± 0.08 a |
| 5. | Sunflower | 4.90 ± 0.61 b,c | 0.23 ± 0.02 b | 0.75 ± 0.09 a | 0.45 ± 0.01c | 4.87 ± 0.15 c |
| 6. | Chestnut | 4.51 ± 0.44 b | 0.34 ± 0.20 a,b | 1.16 ± 0.85 a,b | 8.45 ± 2.81d | 3.88 ± 1.23 c,d |
| 7. | Fennel | 6.46 ± 0.97 c | 0.82 ± 0.03 c | 3.07 ± 0.47 c | 0.27 ± 0.03 e | 3.21 ± 0.18 d |
| 8. | Meadow sage | 7.56 ± 0.74 c | 1.67 ± 0.03 d | 2.35 ± 0.26 b,c | 0.56 ± 0.01f | 2.63 ± 0.20 e |
| Variable | Color | TRC | DPPH | ORAC |
|---|---|---|---|---|
| TRC | 0.886 ** | |||
| DPPH | 0.947 ** | 0.763 ** | ||
| ORAC | 0.825 ** | 0.817 ** | 0.865 ** | |
| K | 0.879 ** | 0.816 ** | 0.908 ** | 0.983 ** |
| Ca | 0.845 ** | 0.607 ** | 0.914 ** | 0.754 ** |
| P | 0.860 ** | 0.968 ** | 0.731 ** | 0.783 ** |
| S | 0.920 ** | 0.966 ** | 0.817 ** | 0.844 ** |
| Mg | 0.929 ** | 0.980 ** | 0.825 ** | 0.843 ** |
| Na | 0.713 ** | 0.679 ** | 0.682 ** | 0.716 ** |
| B | 0.908 ** | 0.836 ** | 0.801 ** | 0.652 ** |
| Cu | 0.900 ** | 0.979 ** | 0.789 ** | 0.824 ** |
| Fe | 0.841 ** | 0.715 ** | 0.765 ** | 0.576 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodó, A.; Radványi, L.; Kőszegi, T.; Csepregi, R.; Nagy, D.U.; Farkas, Á.; Kocsis, M. Quality Evaluation of Light- and Dark-Colored Hungarian Honeys, Focusing on Botanical Origin, Antioxidant Capacity and Mineral Content. Molecules 2021, 26, 2825. https://doi.org/10.3390/molecules26092825
Bodó A, Radványi L, Kőszegi T, Csepregi R, Nagy DU, Farkas Á, Kocsis M. Quality Evaluation of Light- and Dark-Colored Hungarian Honeys, Focusing on Botanical Origin, Antioxidant Capacity and Mineral Content. Molecules. 2021; 26(9):2825. https://doi.org/10.3390/molecules26092825
Chicago/Turabian StyleBodó, Alexandra, Lilla Radványi, Tamás Kőszegi, Rita Csepregi, Dávid U. Nagy, Ágnes Farkas, and Marianna Kocsis. 2021. "Quality Evaluation of Light- and Dark-Colored Hungarian Honeys, Focusing on Botanical Origin, Antioxidant Capacity and Mineral Content" Molecules 26, no. 9: 2825. https://doi.org/10.3390/molecules26092825
APA StyleBodó, A., Radványi, L., Kőszegi, T., Csepregi, R., Nagy, D. U., Farkas, Á., & Kocsis, M. (2021). Quality Evaluation of Light- and Dark-Colored Hungarian Honeys, Focusing on Botanical Origin, Antioxidant Capacity and Mineral Content. Molecules, 26(9), 2825. https://doi.org/10.3390/molecules26092825