Stabilization of Near Identical Hydrogen Bonded Octameric Water Clusters in Crystal Structures of Three Distinct Non-Charged Polyamide Macrocyclic Host Molecules
Abstract
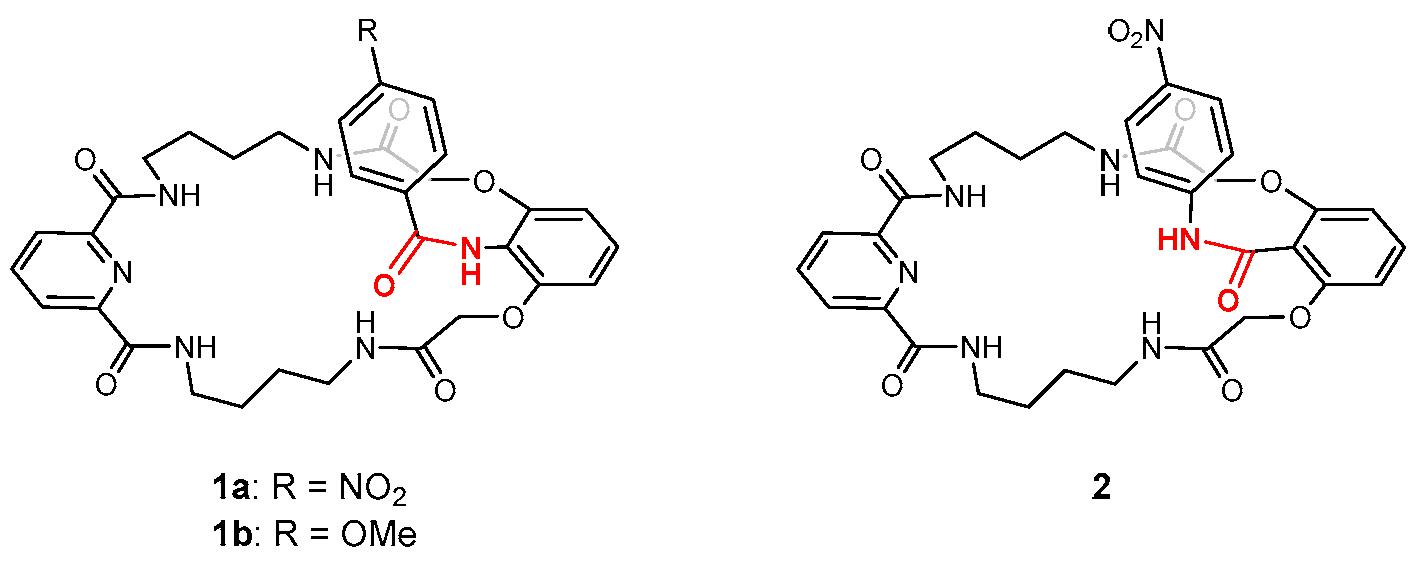
1. Introduction
2. Results and Discussion
2.1. Stabilization of Discrete Octameric Water Cluster
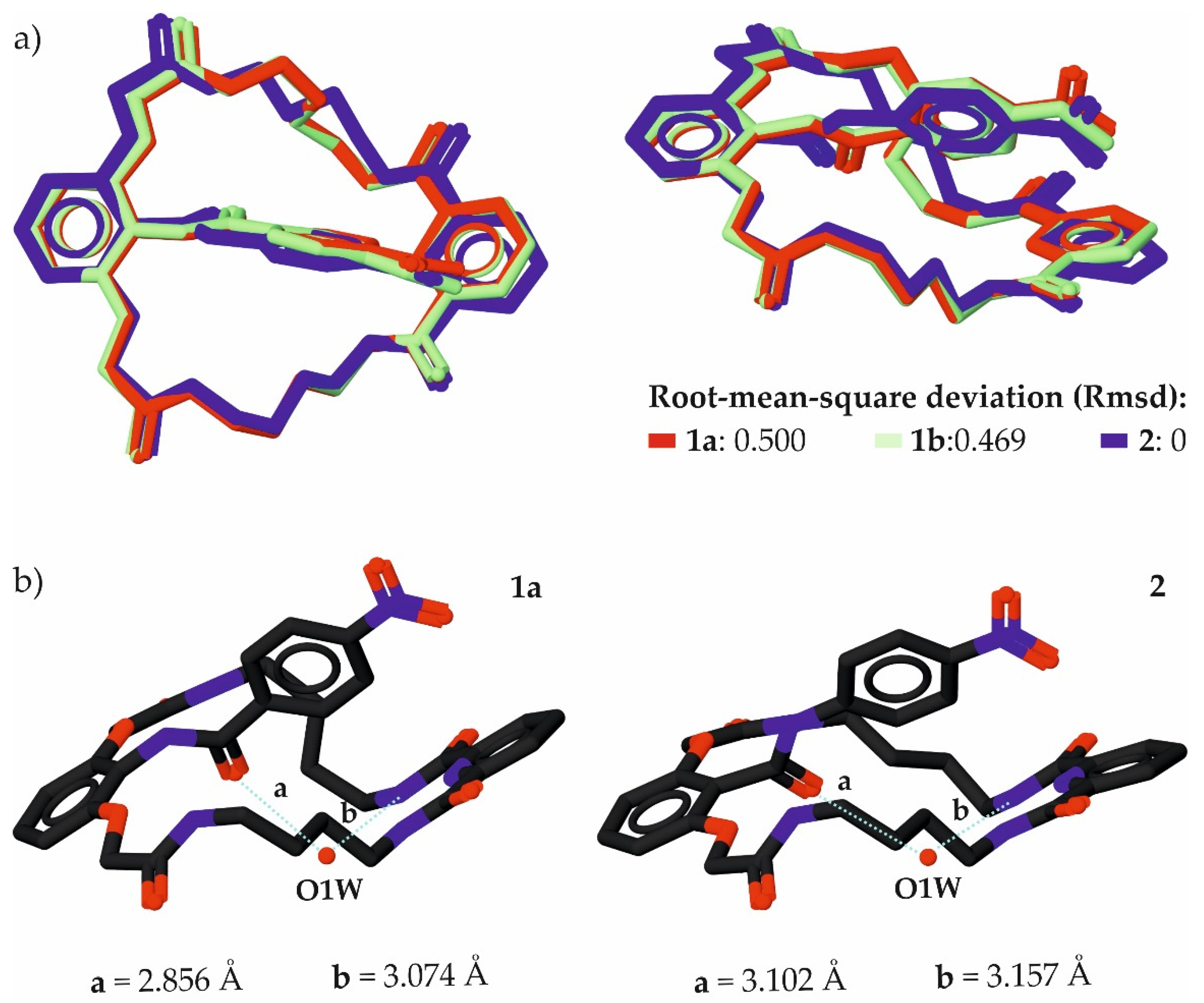
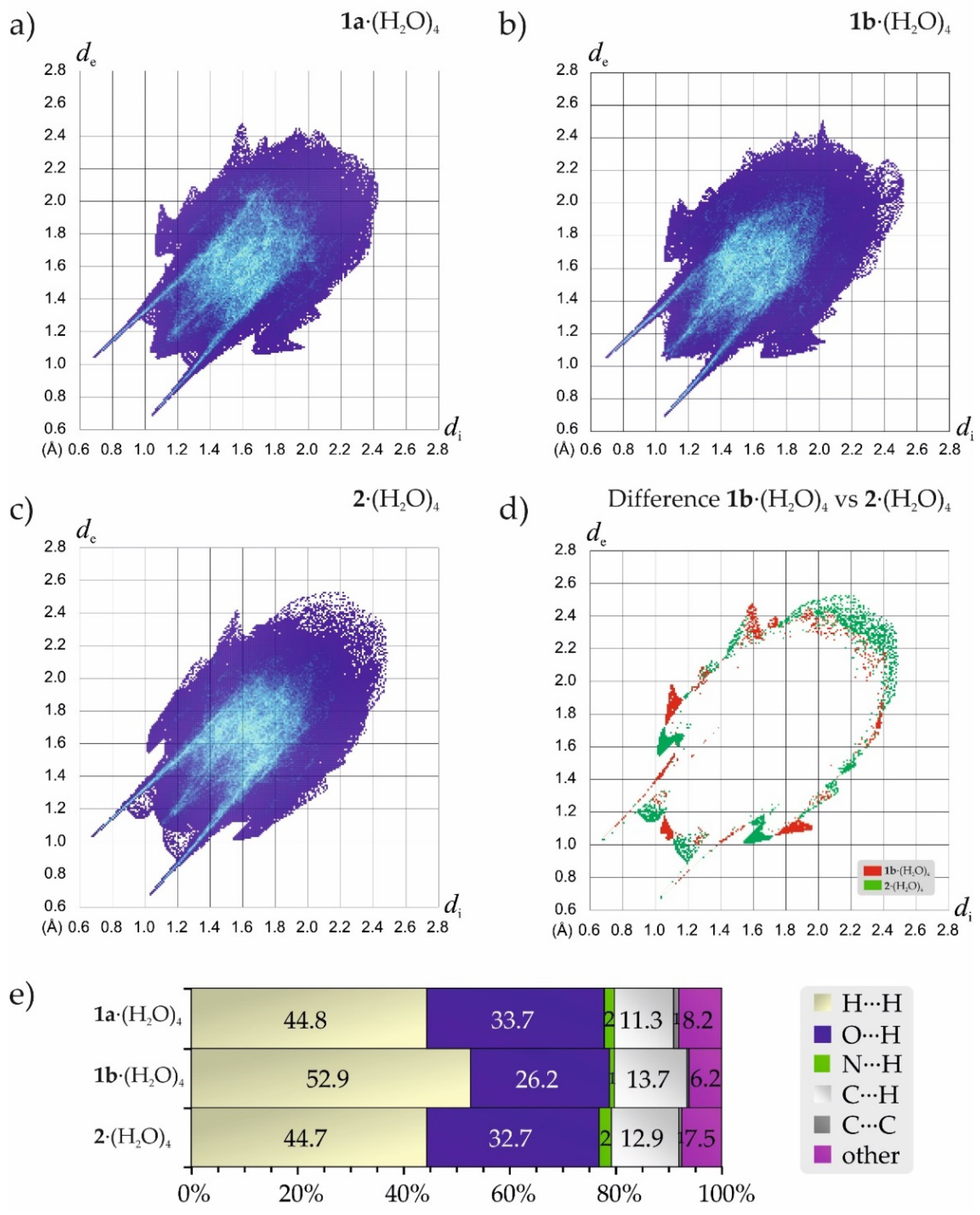
2.2. Comparative Analysis of Crystals
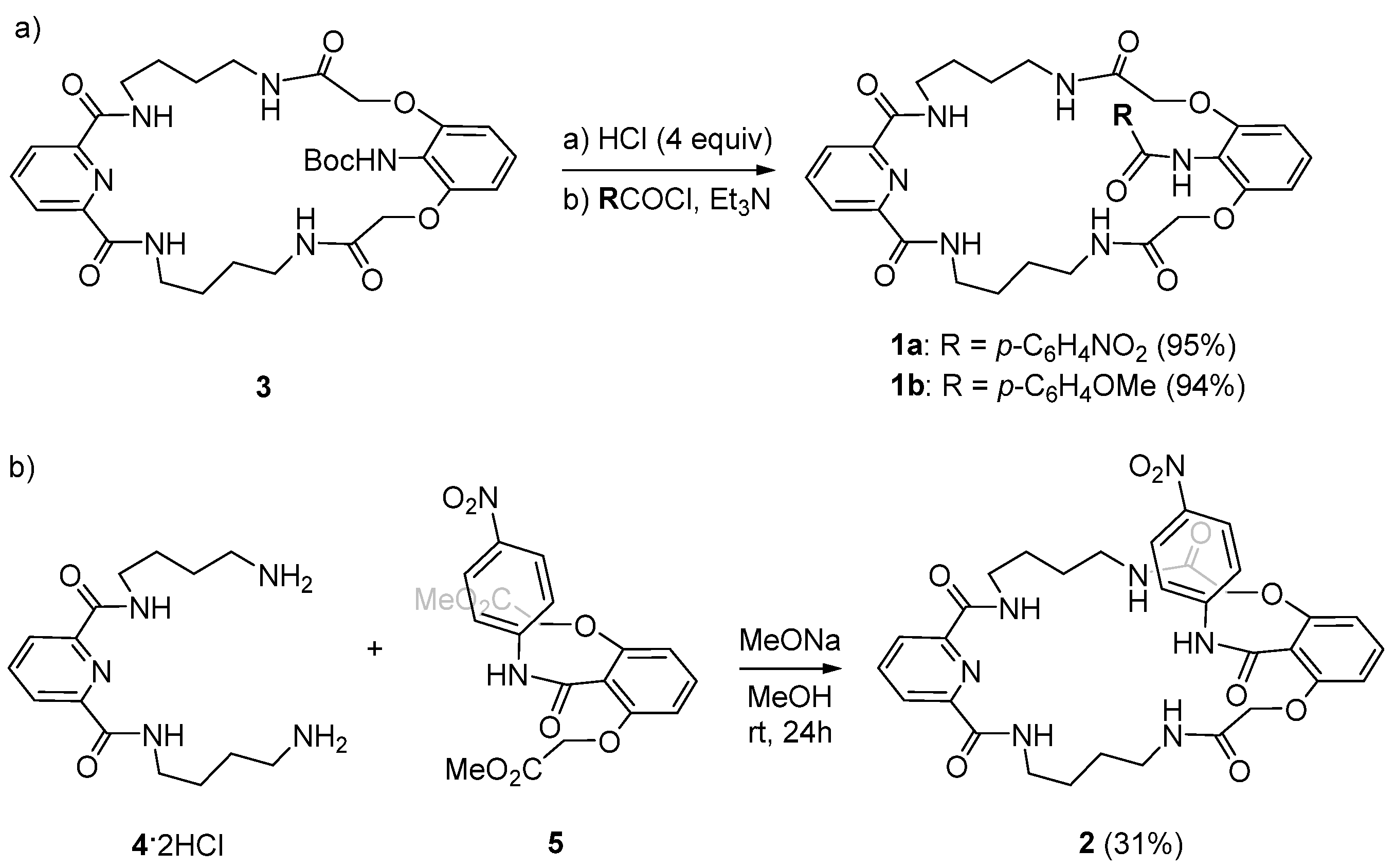
3. Materials and Methods
3.1. Reagents and General Methods
3.2. Synthesis of Molecular Host 2
3.3. Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Desiraju, G.R. Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef]
- Kálmán, A.; Párkányi, L.; Argay, G. Classification of the isostructurality of organic molecules in the crystalline state. Acta Cryst. 1993, B49, 1039–1049. [Google Scholar] [CrossRef]
- Buldakov, A.V.; Kinzhalov, M.A.; Kryukova, M.A.; Ivanov, D.M.; Novikov, A.S.; Smirnov, A.S.; Starova, G.L.; Bokach, N.A.; Kukushkin, V.Y. Isomorphous Series of PdII-Containing Halogen-Bond Donors Exhibiting Cl/Br/I Triple Halogen Isostruc-tural Exchange. Cryst. Growth Des. 2020, 20, 1975–1984. [Google Scholar] [CrossRef]
- Dey, D.; Chopra, D. Evaluation of the Role of Isostructurality in Fluorinated Phenyl Benzoates. Cryst. Growth. Des. 2017, 17, 5117–5128. [Google Scholar] [CrossRef]
- Thakuria, R.; Nath, N.K.; Roy, S.; Nangia, A. Polymorphism and isostructurality in sulfonylhydrazones. CrystEngComm 2014, 16, 4681–4690. [Google Scholar] [CrossRef]
- Kupka, A.; Schauerte, C.; Merz, K. Isostructural Crystallization Behavior of Dihydroanthracene and Dihydroacridine. Cryst. Growth. Des. 2014, 14, 2985–2989. [Google Scholar] [CrossRef]
- Patyk, R.; Podsiadło, M.; Katrusiak, A. CH⋯N Bonds and Dynamics in Isostructural Pyrimidine Polymorphs. Cryst. Growth. Des. 2015, 15, 4039–4044. [Google Scholar] [CrossRef]
- Kumar, M.; Balaji, P. CH…pi interactions in proteins: Prevalence, pattern of occurrence, residue propensities, location and contribution to protein stability. J. Mol. Model. 2014, 20, 2136. [Google Scholar] [CrossRef]
- Corpinot, K.; Guo, R.; Tocher, D.A.; Buanz, A.B.M.; Gaisford, S.; Price, S.L.; Bucar, D.-K. Are Oxygen and Sulfur Atoms Struc-turally Equivalent in Organic Crystals? Cryst. Growth. Des. 2017, 17, 827–833. [Google Scholar] [CrossRef]
- Dąbrowa, K.; Ceborska, M.; Jurczak, J. Solid-state entrapment of water clusters by 26-membered pentamide unclosed cryptands—Probing the para-substituent effect. Supramol. Chem. 2018, 30, 464–472. [Google Scholar] [CrossRef]
- Cincic, D.; Friscic, T.; Jones, W. A cocrystallisation-based strategy to construct isostructural solids. New J. Chem. 2008, 32, 1776–1781. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Saha, B.K. Isostructurality in the Guest Free Forms and in the Clathrates of 1,3,5-Triethyl-2,4,6-tris(4-halophenoxy)methylbenzenes. Cryst. Growth. Des. 2012, 12, 169–178. [Google Scholar] [CrossRef]
- Galcera, J.; Friscic, T.; Molinsa, E.; Jones, W. Isostructurality in three-component crystals achieved by the combination of persistent hydrogen bonding motifs and solvent inclusion. CrystEngComm 2013, 15, 1332–1338. [Google Scholar] [CrossRef]
- Cruz Cabeza, A.J.; Day, G.M.; Motherwell, W.D.S.; Jones, W. Prediction and Observation of Isostructurality Induced by Sol-vent Incorporation in Multicomponent Crystals. J. Am. Chem. Soc. 2006, 128, 14466–144667. [Google Scholar] [CrossRef]
- Chen, J.; Weijie Ji, W.; Zhu, B.; Guo, C.; Qi, M.; Ren, G. Crystal structure and physical stability of ginsenoside com-pound-K solvate. CrystEngComm 2019, 21, 7313–7321. [Google Scholar] [CrossRef]
- Putra, O.D.; Yonemochi, E.; Uekusa, H. Isostructural Multicomponent Gliclazide Crystals with Improved Solubility. Cryst. Growth. Des. 2016, 16, 6568–6573. [Google Scholar] [CrossRef]
- Bennion, J.C.; Vogt, L.; Tuckerman, M.E.; Matzger, A.J. Isostructural Cocrystals of 1,3,5-Trinitrobenzene Assembled by Halogen Bonding. Cryst. Growth. Des. 2016, 16, 4688–4693. [Google Scholar] [CrossRef]
- Galcera, J.; Friscic, T.; Hejczyk, K.E.; Fabian, L.; Clarke, S.M.; Day, G.M.; Molinsa, E.; Jones, W. Isostructural organic bi-nary-host frameworks with tuneable and diversely decorated in-clusion cavities. CrystEngComm 2012, 14, 7898–7906. [Google Scholar] [CrossRef]
- Clarke, H.D.; Hickey, M.B.; Moulton, B.; Perman, J.A.; Peterson, M.L.; Wojtas, Ł.; Almarsson, Ö.; Zaworotko, M.J. Crystal Engineering of Isostructural Quaternary Multicomponent Crystal Forms of Olanzapine. Cryst. Growth. Des. 2012, 12, 4194–4201. [Google Scholar] [CrossRef]
- Turunen, L.; Pan, F.; Beyeh, N.K.; Cetina, M.; Trant, J.F.; Ras, R.H.A.; Rissanen, K. Halogen-bonded solvates of tetra-haloethynyl cavitands. CrystEngComm 2017, 19, 5223–5229. [Google Scholar] [CrossRef]
- Danylyuk, O. Exploring cucurbit[6]uril–peptide interactions in the solid state: Crystal structure of cucurbit[6]uril complexes with glycyl-containing dipeptides. CrystEngComm 2017, 19, 3892–3897. [Google Scholar] [CrossRef]
- Dąbrowa, K.; Lindner, M.; Wasiłek, S.; Jurczak, J. Selective Recognition of Chloride by a 24-Membered Macrocyclic Host with a Hydrophobic Methylenepyrene Substituent. Eur. J. Org. Chem. 2020, 2020, 4528–4533. [Google Scholar] [CrossRef]
- Niedbała, P.; Majdecki, M.; Dąbrowa, K.; Jurczak, J. Selective Carboxylate Recognition Using Urea-Functionalized Unclosed Cryptands: Mild Synthesis and Complexation Studies. J. Org. Chem. 2020, 85, 5058–5064. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowa, K.; Pawlak, M.; Duszewski, P.; Jurczak, J. “Unclosed Cryptands”: A Point of Departure for Developing Potent Neutral Anion Receptors. Org. Lett. 2012, 14, 6298–6301. [Google Scholar] [CrossRef] [PubMed]
- Tyszka-Gumkowska, A.; Jurczak, J. A General Method for High-Pressure-Promoted Postfunctionalization of Unclosed Cryptands: Potential Phase-Transfer Catalysts. J. Org. Chem. 2020, 85, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, J.; Sobczuk, A.; Dabrowa, K.; Lindner, M.; Niedbała, P. An Indirect Synthetic Approach toward Conformationally Constrained 20-Membered Unclosed Cryptands via Late-Stage Installation of Intraannular Substituents. J. Org. Chem. 2018, 83, 13560–13567. [Google Scholar] [CrossRef] [PubMed]
- Dabrowa, K.; Niedbala, P.; Majdecki, M.; Duszewski, P.; Jurczak, J. A General Method for Synthesis of Unclosed Cryptands via H-Bond Templated Macrocyclization and Subsequent Mild Postfunctionalization. Org. Lett. 2015, 17, 4774–4777. [Google Scholar] [CrossRef]
- Kang, S.O.; Llinares, J.M.; Day, V.W.; Bowman-James, K. Cryptand-like anion receptors. Chem. Soc. Rev. 2010, 39, 3980–4003. [Google Scholar] [CrossRef] [PubMed]
- Graf, E.; Hosseini, M.W. Cryptands revisited: Design, synthesis, complexation behaviour and structural analysis of borocryptands. Coord. Chem. Rev. 1998, 1193–1209. [Google Scholar] [CrossRef]
- Alibrandi, G.; Amendola, V.; Bergamaschi, G.; Fabbrizzi, L.; Licchelli, M. Bistren cryptands and cryptates: Versatile receptors for anion inclusion and recognition in water. Org. Biomol. Chem. 2015, 13, 3510–3524. [Google Scholar] [CrossRef]
- Ziach, K.; Ceborska, M.; Jurczak, J. Toward dynamic combinatorial libraries of cryptands. Tetrahedron Lett. 2011, 52, 4452–4455. [Google Scholar] [CrossRef]
- Dietrich, B.; Lehn, J.M.; Sauvage, P. Diaza-polyoxa-macrocycles et macrobicycles. Tetrahedron Lett. 1969, 10, 2885–2888. [Google Scholar] [CrossRef]
- Dąbrowa, K.; Ceborska, M.; Jurczak, J. Trapping of Octameric Water Cluster by the Neutral Unclosed Cryptand Environment. Cryst. Growth Des. 2014, 14, 4906–4910. [Google Scholar] [CrossRef]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Water Dynamics in the Hydration Shells of Biomolecules. Chem. Rev. 2017, 117, 10694–10725. [Google Scholar] [CrossRef]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Perspective: Structure and ultrafast dynamics of biomolecular hydration shells. Struct. Dynam. 2017, 4, 044018. [Google Scholar] [CrossRef]
- Dąbrowa, K.; Ceborska, M.; Pawlak, M.; Jurczak, J. Comparative Structural Studies of Four Homologous Thioamidic Unclosed Crytpands: Self-Encapsulation of Lariat Arm, Odd–Even Effects, Anomalously Short S⋯S Chalcogen Bonding, and More. Cryst. Growth Des. 2017, 17, 701–710. [Google Scholar] [CrossRef]
- Ziach, K.; Dąbrowa, K.; Niedbała, P.; Kalisiak, J.; Jurczak, J. Exploration of structural motifs influencing solid-state confor-mation and packing of unclosed cryptands sharing the same 19-membered macrocyclic core. Tetrahedron 2016, 72, 8373–8381. [Google Scholar] [CrossRef]
- Thakur, S.; Frontera, A.; Chattopadhyay, S. A tetrameric uudd type water cluster encapsulated in a dinuclear vanadium(V) Schiff base complex and its role in the formation of supramolecular assemblies: A joint experimental and theoretical study. Inorg. Chim. Acta 2021, 515, 120057. [Google Scholar] [CrossRef]
- Massera, C.; Melegari, M.; Ugozzoli, F.; Dalcanale, E. Formation of tetrameric water clusters driven by a cavitand template. Chem. Commun. 2010, 46, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Fabelo, O.; Pasán, J.; Cañadillas-Delgado, L.; Delgado, F.S.; Labrador, A.; Lloret, F.; Julve, M.; Ruiz-Pérez, C. Well-resolved unusual alternating cyclic water tetramers embedded in a crystal host. CrystEngComm 2008, 10, 1743–1746. [Google Scholar] [CrossRef]
- Zuhayra, M.; Kampen, W.U.; Henze, E.; Soti, Z.; Zsolnai, L.; Huttner, G.; Oberdorfer, F. A Planar Water Tetramer with Tetrahedrally Coordinated Water Embedded in a Hydrogen Bonding Network of [Tc4(CO)12-(μ3-OH)4·4H2O]. J. Am. Chem. Soc. 2005, 128, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Long, L.-S.; Wu, Y.-R.; Huang, R.-B.; Zheng, L.-S. A Well-Resolved uudd Cyclic Water Tetramer in the Crystal Host of [Cu(adipate)(4,4-bipyridine)]·(H2O)2. Inorg. Chem. 2004, 43, 3798–3800. [Google Scholar] [CrossRef] [PubMed]
- Day, M.B.; Kirschner, K.N.; Shields, G.C. Global Search for Minimum Energy (H2O)n Clusters, n = 3−5. J. Phys. Chem. A 2005, 109, 6773–6778. [Google Scholar] [CrossRef] [PubMed]
- Agilent Technologies. CrysAlis PRO; Version 1.171.35.11; Agilent Technologies: Yarnton, Oxfordshire, UK, 2011. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refine-ment and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- International Tables for Crystallography; Wilson, A.J.C., Ed.; Kluwer: Dordrecht, The Netherlands, 1992; Volume C. [Google Scholar]
- Macrae, C.F.; Edgington, P.R.; McCabe, R.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Spartan’18 for Windows; Wavefunction Inc.: Irvinde, CA, USA, 2018.
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer 17; University of Western Australia: Perth, Australia, 2017; Available online: http://hirshfeldsurface.net (accessed on 20 January 2021).
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. Cryst. Eng. Comm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. Cryst. Eng. Commun. 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]

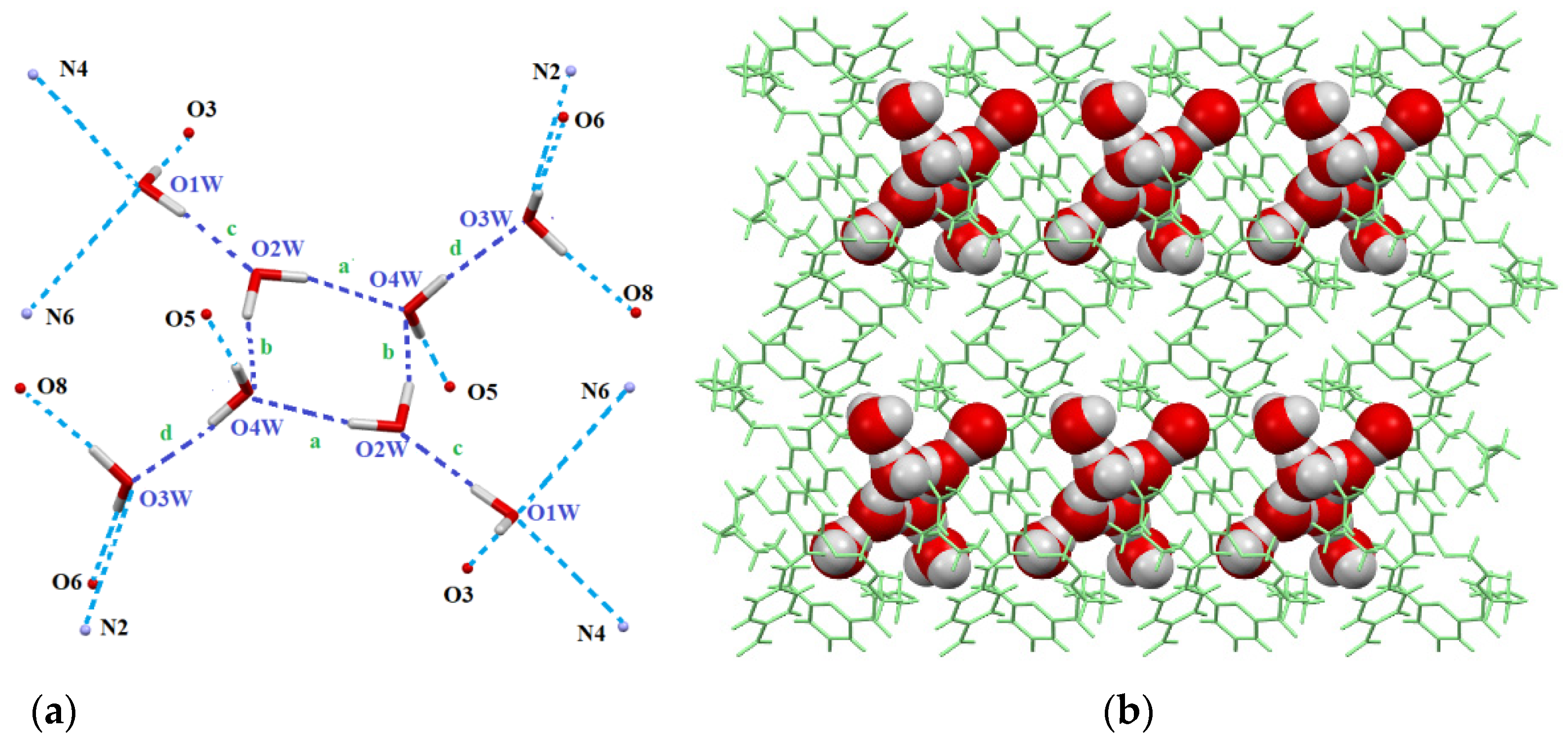


| Entry | Interaction | d(D⋯A) [Å] | d(H⋯A) [Å] | <(D-H⋯A) [o] |
|---|---|---|---|---|
| 1 | N2-H2⋯O3W [a] | 3.058 | 2.214 | 165.4 |
| 2 | N4-H4⋯O1W | 3.158 | 2.336 | 155.0 |
| 3 | N6-H6⋯O1W | 3.157 | 2.339 | 156.3 |
| 4 | N7-H7⋯O3 | 3.240 | 2.482 | 148.3 |
| 5 | O1W-H1V⋯O3 | 3.102 | 2.212 | 176.2 |
| 6 | O1W-H1V⋯O2W | 2.773 | 1.924 | 176.7 |
| 7 | O3W [b]-H3V [b]⋯O6 | 2.710 | 1.884 | 159.3 |
| 8 | O3W [c]-H3W [c]⋯O8 | 2.681 | 1.831 | 169.0 |
| 9 | O4W [d]-H4W [d]⋯O5 | 2.793 | 1.940 | 178.2 |
| 10 | O2W-H⋯O4W | 2.894 | 2.006 | 167.69 |
| 11 | O4W⋯H-O2W’ | 2.812 | 1.921 | 172.68 |
| 12 | O2W⋯H-O1W | 2.773 | 1.924 | 176.71 |
| 13 | O4W-H⋯O3W | 2.763 | 1.881 | 176.39 |
| Entry | Parameter [a] | 1a | 1b | 2 | Avg [b] |
|---|---|---|---|---|---|
| 1 | SG | P-1 (Triclinic) | - | ||
| 2 | a [Å] | 9.6293(4) | 9.7432(8) | 10.1059(3) | 0.1865 |
| 3 | b [Å] | 10.5860(4) | 10.5621(9) | 10.7645(3) | 0.0846 |
| 4 | c [Å] | 18.1620(7) | 17.9336(15) | 16.9216(5) | 0.5005 |
| 5 | α [o] | 100.749(3) | 95.803(3) | 92.533(2) | 6.630 |
| 6 | β [o] | 105.086(3) | 104.647(3) | 104.115(2) | 13.096 |
| 7 | γ [o] | 99.485(3) | 99.899(3) | 101.344(2) | 8.9362 |
| 8 | Z | 2 | - | ||
| 9 | Dx [g cm−3] | 1.398 | 1.373 | 1.399 | 0.011 |
| 10 | V [Å3] | 1742.95 | 1738.61 | 1743.38 | 2.02 |
| 11 | CPk [b] | 0.792 [33] | 0.813 | 0.805 | 0.803 |
| z | Parameter | 1a | 2 | Diff. [b] |
|---|---|---|---|---|
| 1 | a [Å] | 2.925 | 2.894 | −0.031 |
| 2 | b [Å] | 2.818 | 2.812 | −0.006 |
| 3 | c [Å] | 2.831 | 2.773 | −0.058 |
| 4 | d [Å] | 2.788 | 2.763 | −0.025 |
| 5 | <D-H⋯A (a) [o] | 167.73 | 167.69 | −0.04 |
| 6 | <D-H⋯A (b) [o] | 171.4 | 172.68 | 1.28 |
| 7 | <D-H⋯A (c) [o] | 171.64 | 176.61 | 4.97 |
| 8 | <D-H⋯A (d) [o] | 172.47 | 176.39 | 3.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowa, K.; Ceborska, M.; Jurczak, J. Stabilization of Near Identical Hydrogen Bonded Octameric Water Clusters in Crystal Structures of Three Distinct Non-Charged Polyamide Macrocyclic Host Molecules. Molecules 2021, 26, 2787. https://doi.org/10.3390/molecules26092787
Dąbrowa K, Ceborska M, Jurczak J. Stabilization of Near Identical Hydrogen Bonded Octameric Water Clusters in Crystal Structures of Three Distinct Non-Charged Polyamide Macrocyclic Host Molecules. Molecules. 2021; 26(9):2787. https://doi.org/10.3390/molecules26092787
Chicago/Turabian StyleDąbrowa, Kajetan, Magdalena Ceborska, and Janusz Jurczak. 2021. "Stabilization of Near Identical Hydrogen Bonded Octameric Water Clusters in Crystal Structures of Three Distinct Non-Charged Polyamide Macrocyclic Host Molecules" Molecules 26, no. 9: 2787. https://doi.org/10.3390/molecules26092787
APA StyleDąbrowa, K., Ceborska, M., & Jurczak, J. (2021). Stabilization of Near Identical Hydrogen Bonded Octameric Water Clusters in Crystal Structures of Three Distinct Non-Charged Polyamide Macrocyclic Host Molecules. Molecules, 26(9), 2787. https://doi.org/10.3390/molecules26092787







