A Novel Serum Glycobiomarker for Diagnosis and Prognosis of Cholangiocarcinoma Detected by Butea monosperma Agglutinin
Abstract
1. Introduction
2. Results
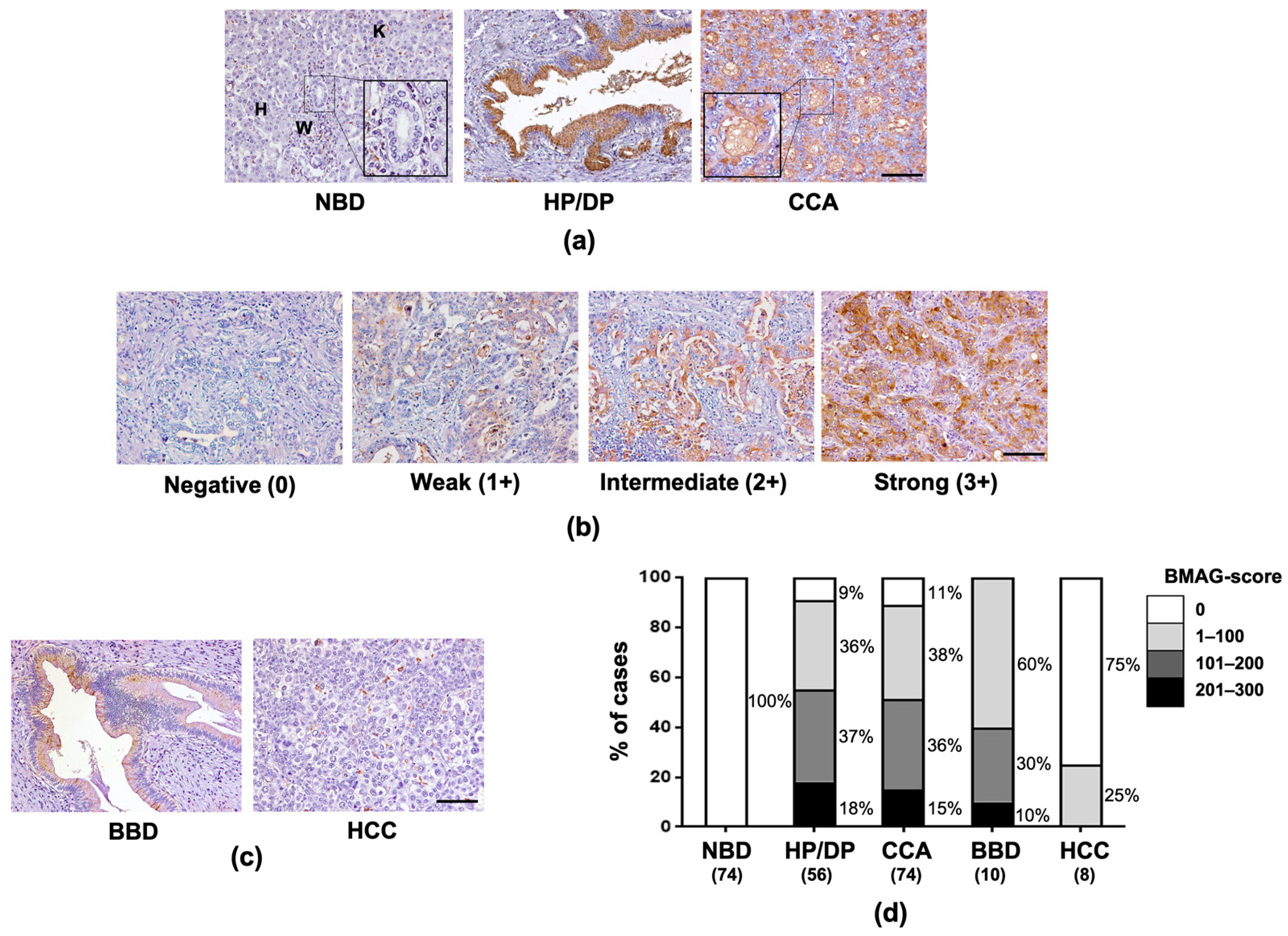
2.1. BMAG Was Significantly Increased in Pre-Neoplastic Bile Ducts and CCA Cells
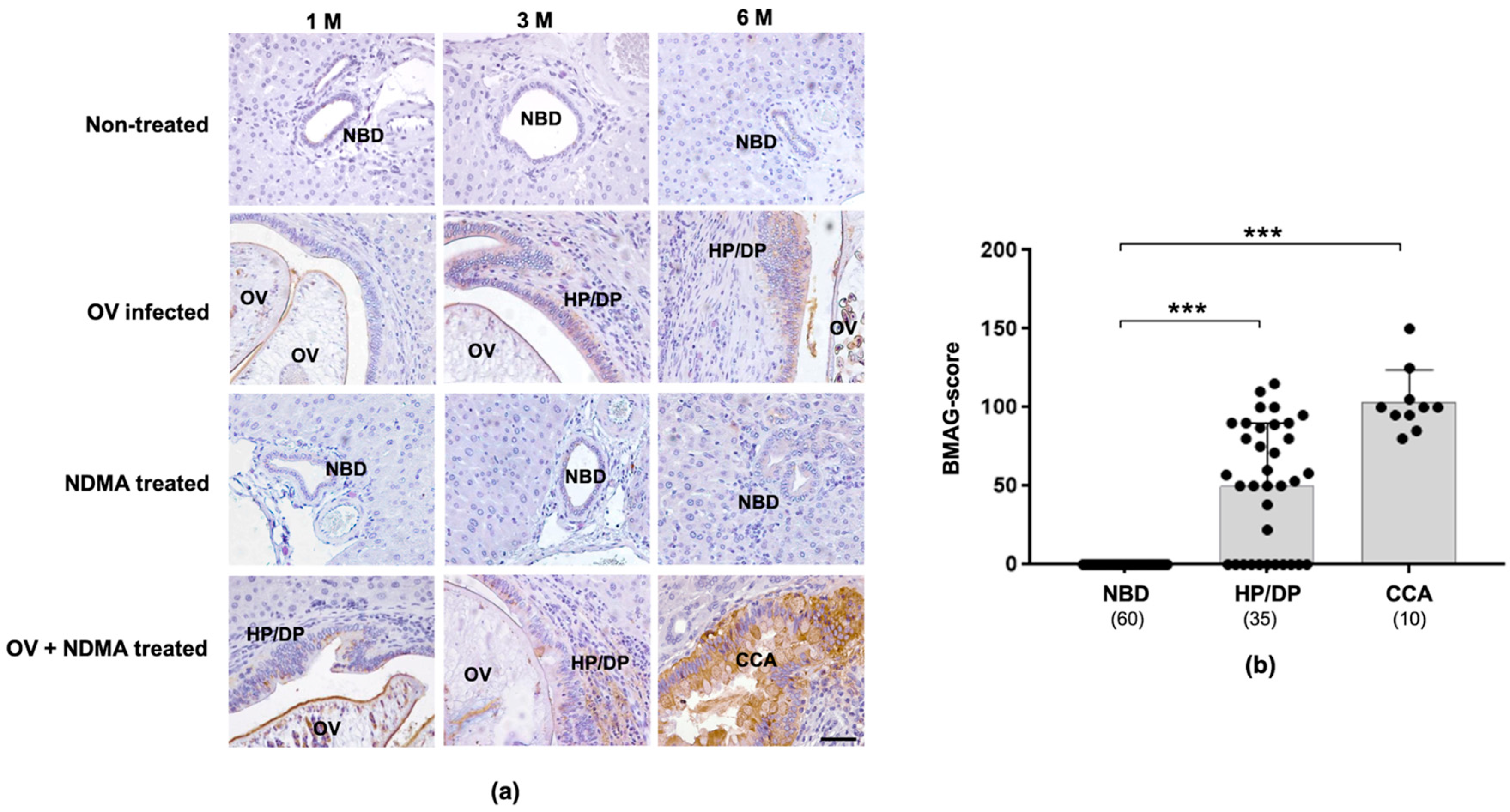
2.2. Expression of Tissue BMAG Was Associated With CCA Genesis in Hamster Model
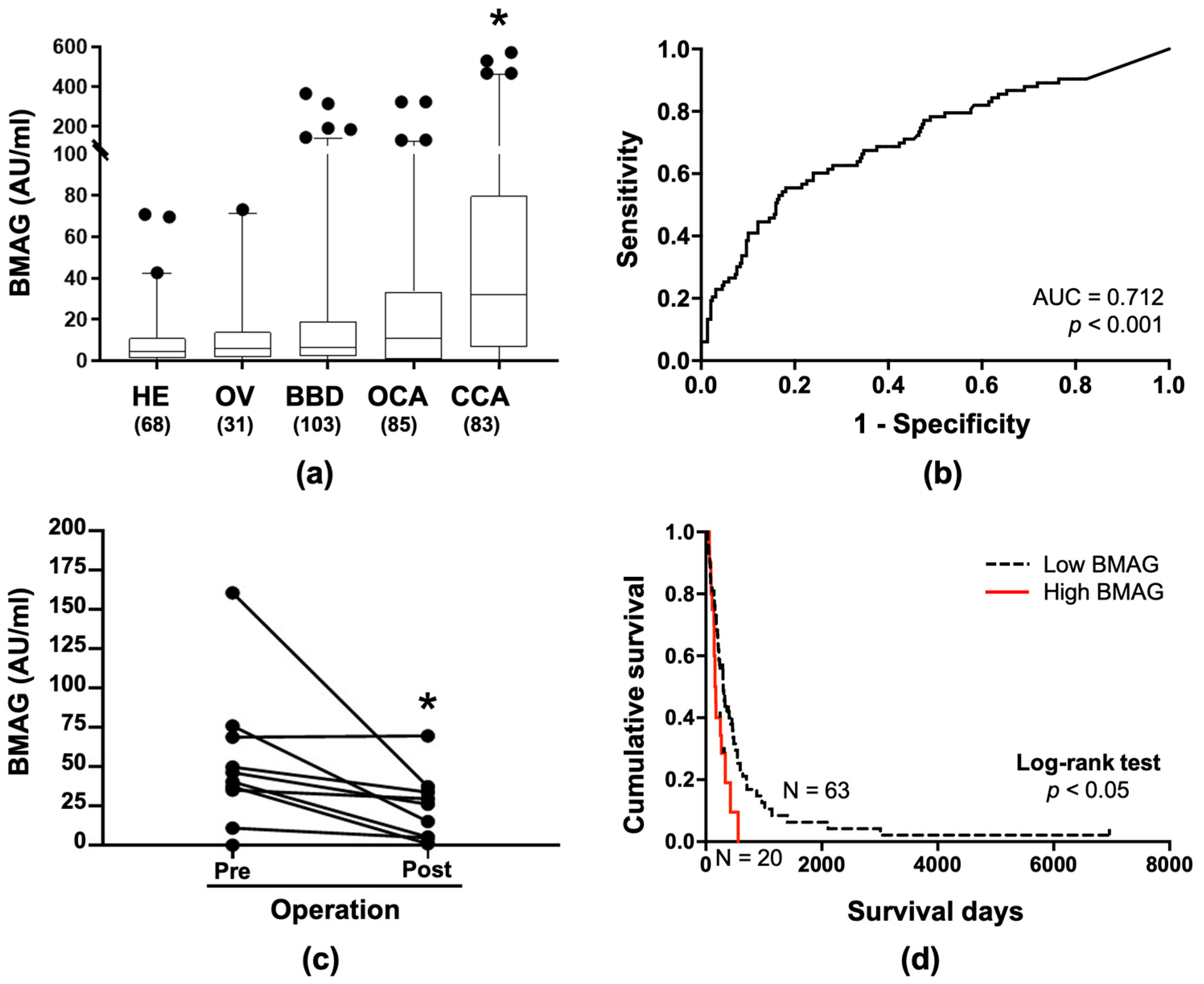
2.3. BMAG Was Detected in the Patients’ Sera and Can Be a Diagnostic Marker for CCA
2.4. Serum BMAG Was an Independent Poor Prognostic Indicator for CCA
3. Discussion
4. Materials and Methods
4.1. Patients’ Tissues and Sera
4.2. CCA Hamster Tissues
4.3. Purification and Biotinylation of Butea monosperma Agglutinin
4.4. BMA Histochemistry
4.5. Double BMA Sandwich Enzyme-Linked Lectin Assay
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sripa, B.; Pairojkul, C. Cholangiocarcinoma: Lessons from Thailand. Curr. Opin. Gastroenterol. 2008, 24, 349–356. [Google Scholar] [CrossRef]
- Matsuda, A.; Kuno, A.; Kawamoto, T.; Matsuzaki, H.; Irimura, T.; Ikehara, Y.; Zen, Y.; Nakanuma, Y.; Yamamoto, M.; Ohkohchi, N.; et al. Wisteria floribunda agglutinin-positive mucin 1 is a sensitive biliary marker for human cholangiocarcinoma. Hepatology 2010, 52, 174–182. [Google Scholar] [CrossRef]
- Indramanee, S.; Silsirivanit, A.; Pairojkul, C.; Wongkham, C.; Wongkham, S. Aberrant glycosylation in cholangiocarcinoma demonstrated by lectin-histochemistry. Asian Pac. J. Cancer Prev. 2012, 13 (Suppl.), 119–124. [Google Scholar]
- Juntermanns, B.; Radunz, S.; Heuer, M.; Hertel, S.; Reis, H.; Neuhaus, J.P.; Vernadakis, S.; Trarbach, T.; Paul, A.; Kaiser, G.M. Tumor markers as a diagnostic key for hilar cholangiocarcinoma. Eur. J. Med. Res. 2010, 15, 357–361. [Google Scholar] [CrossRef]
- Silsirivanit, A.; Araki, N.; Wongkham, C.; Pairojkul, C.; Narimatsu, Y.; Kuwahara, K.; Narimatsu, H.; Wongkham, S.; Sakaguchi, N. A novel serum carbohydrate marker on mucin 5AC: Values for diagnostic and prognostic indicators for cholangiocarcinoma. Cancer 2011, 117, 3393–3403. [Google Scholar] [CrossRef]
- Silsirivanit, A.; Araki, N.; Wongkham, C.; Vaeteewoottacharn, K.; Pairojkul, C.; Kuwahara, K.; Narimatsu, Y.; Sawaki, H.; Narimatsu, H.; Okada, S.; et al. CA-S27: A novel Lewis a associated carbohydrate epitope is diagnostic and prognostic for cholangiocarcinoma. Cancer Sci. 2013, 104, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Wattanavises, S.; Silsirivanit, A.; Sawanyawisuth, K.; Cha’on, U.; Waraasawapati, S.; Saentaweesuk, W.; Luang, S.; Chalermwat, C.; Wongkham, C.; Wongkham, S. Increase of MAL-II Binding Alpha2,3-Sialylated Glycan Is Associated with 5-FU Resistance and Short Survival of Cholangiocarcinoma Patients. Medicina (Kaunas) 2019, 55, 761. [Google Scholar] [CrossRef] [PubMed]
- Bamrungphon, W.; Prempracha, N.; Bunchu, N.; Rangdaeng, S.; Sandhu, T.; Srisukho, S.; Boonla, C.; Wongkham, S. A new mucin antibody/enzyme-linked lectin-sandwich assay of serum MUC5AC mucin for the diagnosis of cholangiocarcinoma. Cancer Lett. 2007, 247, 301–308. [Google Scholar] [CrossRef]
- Saentaweesuk, W.; Silsirivanit, A.; Vaeteewoottacharn, K.; Sawanyawisuth, K.; Pairojkul, C.; Cha’on, U.; Indramanee, S.; Pinlaor, S.; Boonmars, T.; Araki, N.; et al. Clinical significance of GalNAcylated glycans in cholangiocarcinoma: Values for diagnosis and prognosis. Clin. Chim. Acta 2018, 477, 66–71. [Google Scholar] [CrossRef]
- Detarya, M.; Sawanyawisuth, K.; Aphivatanasiri, C.; Chuangchaiya, S.; Saranaruk, P.; Sukprasert, L.; Silsirivanit, A.; Araki, N.; Wongkham, S.; Wongkham, C. The O-GalNAcylating enzyme GALNT5 mediates carcinogenesis and progression of cholangiocarcinoma via activation of AKT/ERK signaling. Glycobiology 2020, 30, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Wongkham, S.; Wongkham, C.; Trisonthi, C.; Boonsiri, P.; Simasathiansophon, S.; Atisook, K. Isolation and properties of a lectin from the seeds of Butea monosperma. Plant Sci. 1994, 103, 121–126. [Google Scholar] [CrossRef]
- Wongkham, S.; Taketa, K.; Liu, M.; Taga, H. Affinity electrophoretic determination of oligosaccharide specificity of Butea monosperma agglutinin. Electrophoresis 1996, 17, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Silsirivanit, A.; Sawanyawisuth, K.; Riggins, G.J.; Wongkham, C. Cancer biomarker discovery for cholangiocarcinoma: The high-throughput approaches. J. Hepatobiliary Pancreat. Sci. 2014, 21, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Rana, F.; Avijit, M. Review on Butea Monosperma. Int. J. Res. Pharm. Chem. 2012, 2, 1035–1039. [Google Scholar]
- Silsirivanit, A.; Matsuda, A.; Kuno, A.; Tsuruno, C.; Uenoyama, Y.; Seubwai, W.; Angata, K.; Teeravirote, K.; Wongkham, C.; Araki, N.; et al. Multi-serum glycobiomarkers improves the diagnosis and prognostic prediction of cholangiocarcinoma. Clin. Chim. Acta 2020, 510, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.F.; Campos, D.; Reis, C.A.; Gomes, C. Targeting Glycosylation: A New Road for Cancer Drug Discovery. Trends Cancer 2020, 6, 757–766. [Google Scholar] [CrossRef]
- Vajaria, B.N.; Patel, P.S. Glycosylation: A hallmark of cancer? Glycoconj. J. 2017, 34, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Silsirivanit, A. Chapter Five-Glycosylation markers in cancer. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Cambridge, MA, USA, 2019; Volume 89, pp. 189–213. [Google Scholar]
- Silsirivanit, A. Glycans: Potential therapeutic targets for cholangiocarcinoma and their therapeutic and diagnostic implications. Expert Opin. Ther. Targets 2021, 25, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Song Sc Fau-Sugii, S.; Sugii S Fau-Herp, A.; Herp, A. Differential binding properties of Gal/GalNAc specific lectins available for characterization of glycoreceptors. Indian J. Biochem. Biophys. 1997, 34, 61–71. [Google Scholar] [PubMed]
- Wu, A.M. Expression of binding properties of Gal/GalNAc reactive lectins by mammalian glycotopes (an updated report). Adv. Exp. Med. Biol. 2001, 491, 55–64. [Google Scholar] [PubMed]
- Teeravirote, K.; Luang, S.; Wongkham, S.; Boonsiri, P.; Silsirivanit, A. Suppression of in vitro metastatic ability of cholangiocarcinoma cells using lectin from Butea monosperma seeds. SMJ 2019, 34, 1–5. [Google Scholar]
- Indramanee, S.; Sawanyawisuth, K.; Silsirivanit, A.; Dana, P.; Phoomak, C.; Kariya, R.; Klinhom-On, N.; Sorin, S.; Wongkham, C.; Okada, S.; et al. Terminal fucose mediates progression of human cholangiocarcinoma through EGF/EGFR activation and the Akt/Erk signaling pathway. Sci. Rep. 2019, 9, 17266. [Google Scholar] [CrossRef] [PubMed]
- Prakobwong, S.; Yongvanit, P.; Hiraku, Y.; Pairojkul, C.; Sithithaworn, P.; Pinlaor, P.; Pinlaor, S. Involvement of MMP-9 in peribiliary fibrosis and cholangiocarcinogenesis via Rac1-dependent DNA damage in a hamster model. Int. J. Cancer 2010, 127, 2576–2587. [Google Scholar] [CrossRef] [PubMed]
- Fedchenko, N.; Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue-a review. Diagn. Pathol. 2014, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Seubwai, W.; Vaeteewoottacharn, K.; Sawanyawisuth, K.; Silsirivanit, A.; Kaewkong, W.; Muisuk, K.; Dana, P.; Phoomak, C.; Lert-Itthiporn, W.; et al. Functional and genetic characterization of three cell lines derived from a single tumor of an Opisthorchis viverrini-associated cholangiocarcinoma patient. Hum. Cell 2020, 33, 695–708. [Google Scholar] [CrossRef]
| Subjects | |||||
|---|---|---|---|---|---|
| HE | OV | BBD | OCA | CCA | |
| Number of cases | 68 | 31 | 103 | 85 | 83 |
| Level of BMAG (AU/mL) | |||||
| Mean ± SD | 9.4 ± 14.7 | 11.2 ± 17.5 | 25.0 ± 56.5 | 27.8 ± 54.2 | 82.5 ± 128.9 |
| p-value (t-Test) | |||||
| vs HE | - | 0.612 | 0.027 | 0.007 | <0.001 |
| vs OV | - | - | 0.182 | 0.096 | 0.002 |
| vs BBD | - | - | - | 0.730 | <0.001 |
| vs OCA | - | - | - | - | <0.001 |
| Variables | N | Serum-BMAG | p-Value | |
|---|---|---|---|---|
| <82.5 AU/mL | ≥82.5 AU/mL | |||
| Sex | 0.009 | |||
| Female | 26 | 15 | 11 | |
| Male | 57 | 48 | 9 | |
| Age | 0.920 | |||
| <56 years old | 34 | 27 | 7 | |
| ≥56 years old | 49 | 36 | 13 | |
| Tumor size | 0.515 | |||
| <7 cm | 45 | 35 | 10 | |
| ≥7 cm | 35 | 25 | 10 | |
| Histological types | 0.793 | |||
| Papillary type | 23 | 17 | 6 | |
| Non-papillary type | 60 | 46 | 14 | |
| Tumor stages | 0.855 | |||
| I–III | 16 | 13 | 3 | |
| IVA | 39 | 29 | 10 | |
| IVB | 28 | 21 | 7 | |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Sex | - | - | - | |||
| Female | 1 | |||||
| Male | 0.978 | 0.604–1.581 | 0.926 | |||
| Age | - | - | - | |||
| <56 years old | 1 | |||||
| ≥56 years old | 1.266 | 0.800–2.004 | 0.314 | |||
| Tumor size | - | - | - | |||
| <7 cm | 1 | |||||
| ≥7 cm | 1.312 | 0.837–2.055 | 0.237 | |||
| Histological types | - | - | - | |||
| Papillary type | 1 | |||||
| Non-papillary type | 1.353 | 0.834–2.212 | 0.219 | |||
| Tumor stages | ||||||
| I–III | 1 | 1 | ||||
| IVA | 1.295 | 0.709–2.367 | 0.391 | 1.224 | 0.667–2.245 | 0.533 |
| IVB | 2.198 | 1.157–4.174 | 0.016 | 2.068 | 1.084–3.945 | 0.009 |
| Serum-BMAG | ||||||
| BMAG < 82.5 AU/mL | 1 | 1 | ||||
| BMAG ≥ 82.5 AU/mL | 1.960 | 1.151–3.336 | 0.013 | 1.873 | 1.096–3.199 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teeravirote, K.; Luang, S.; Waraasawapati, S.; Boonsiri, P.; Wongkham, C.; Wongkham, S.; Silsirivanit, A. A Novel Serum Glycobiomarker for Diagnosis and Prognosis of Cholangiocarcinoma Detected by Butea monosperma Agglutinin. Molecules 2021, 26, 2782. https://doi.org/10.3390/molecules26092782
Teeravirote K, Luang S, Waraasawapati S, Boonsiri P, Wongkham C, Wongkham S, Silsirivanit A. A Novel Serum Glycobiomarker for Diagnosis and Prognosis of Cholangiocarcinoma Detected by Butea monosperma Agglutinin. Molecules. 2021; 26(9):2782. https://doi.org/10.3390/molecules26092782
Chicago/Turabian StyleTeeravirote, Karuntarat, Sukanya Luang, Sakda Waraasawapati, Patcharee Boonsiri, Chaisiri Wongkham, Sopit Wongkham, and Atit Silsirivanit. 2021. "A Novel Serum Glycobiomarker for Diagnosis and Prognosis of Cholangiocarcinoma Detected by Butea monosperma Agglutinin" Molecules 26, no. 9: 2782. https://doi.org/10.3390/molecules26092782
APA StyleTeeravirote, K., Luang, S., Waraasawapati, S., Boonsiri, P., Wongkham, C., Wongkham, S., & Silsirivanit, A. (2021). A Novel Serum Glycobiomarker for Diagnosis and Prognosis of Cholangiocarcinoma Detected by Butea monosperma Agglutinin. Molecules, 26(9), 2782. https://doi.org/10.3390/molecules26092782







