Evaluation of Quality, Antioxidant Capacity, and Digestibility of Chickpea (Cicer arietinum L. cv Blanoro) Stored under N2 and CO2 Atmospheres
Abstract
:1. Introduction
2. Results and Discussions
2.1. Proximal Analysis
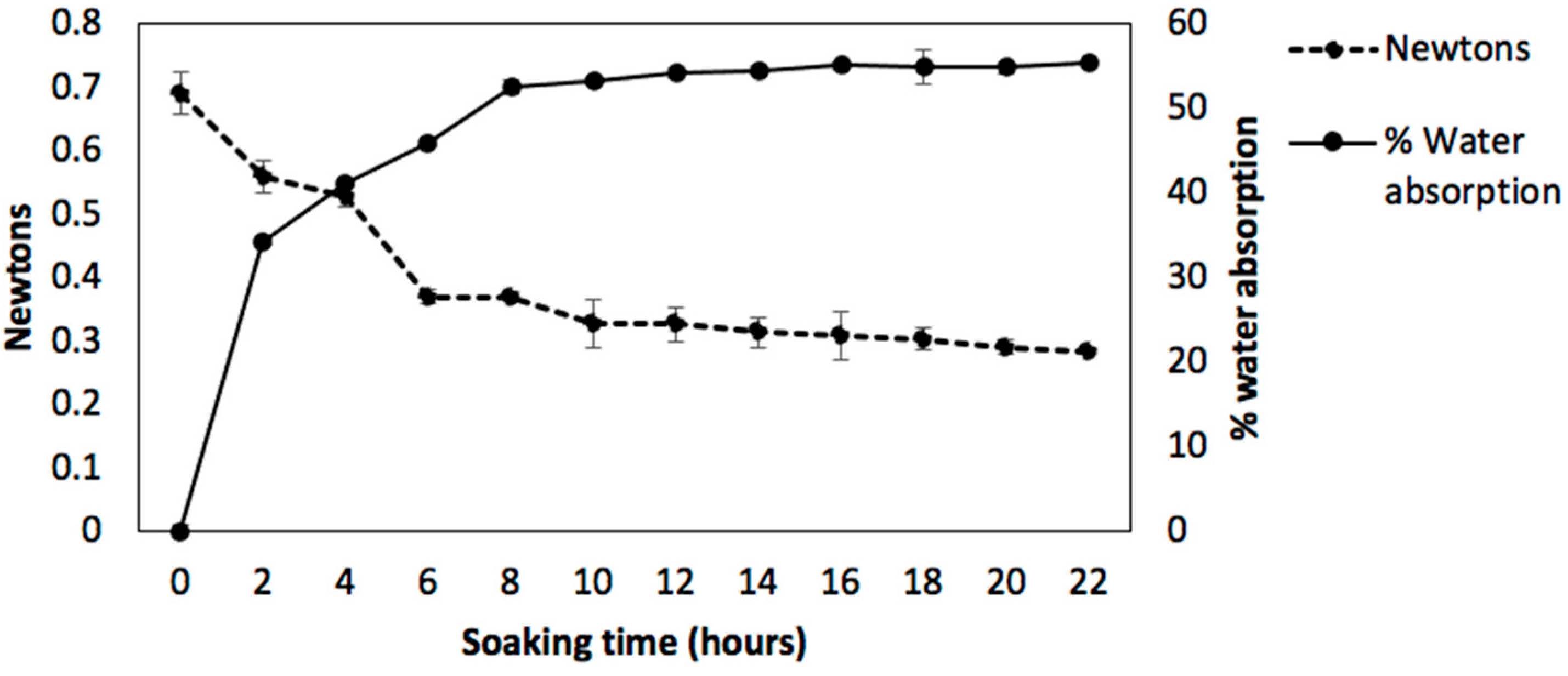
2.2. Technological Quality
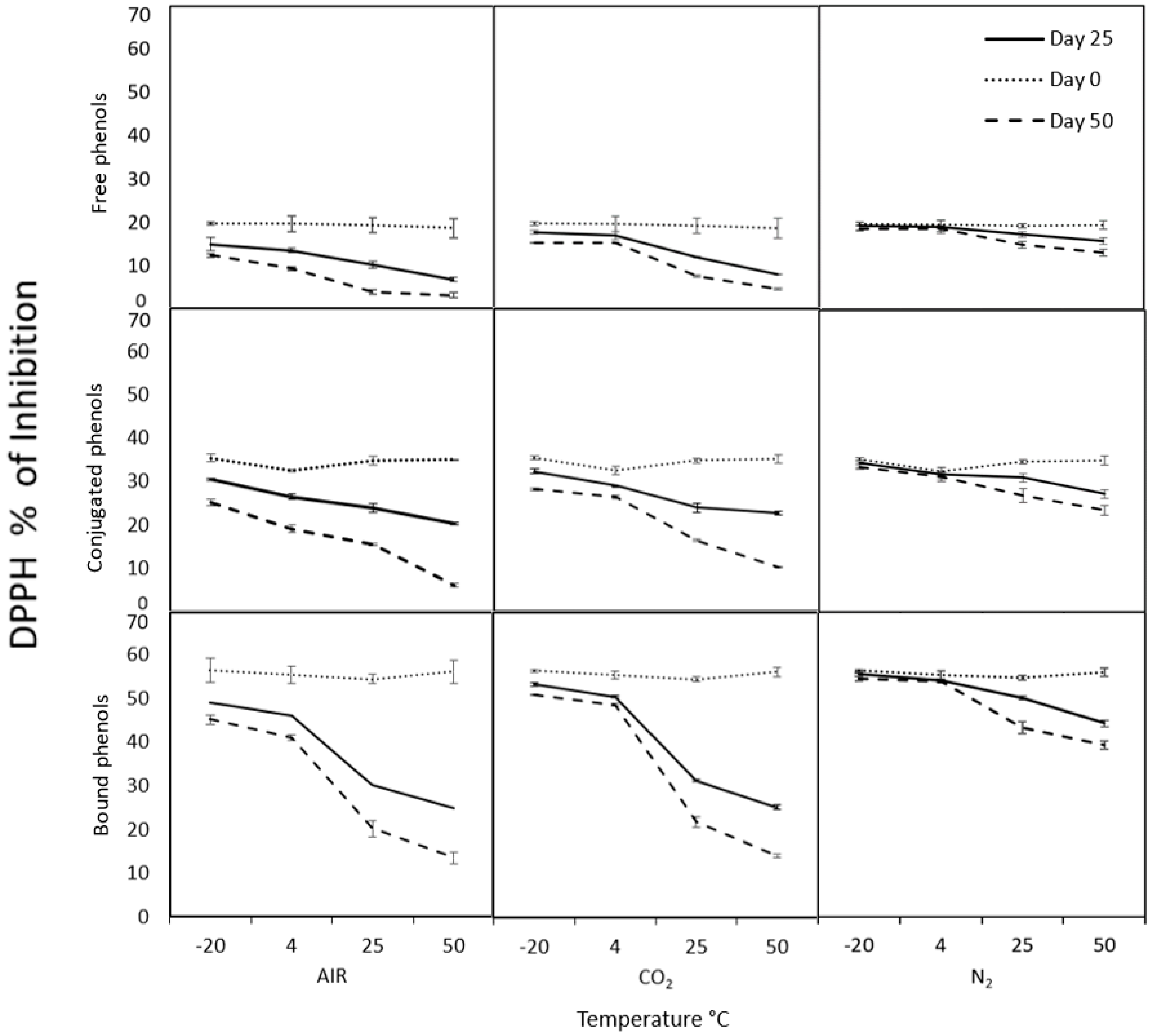
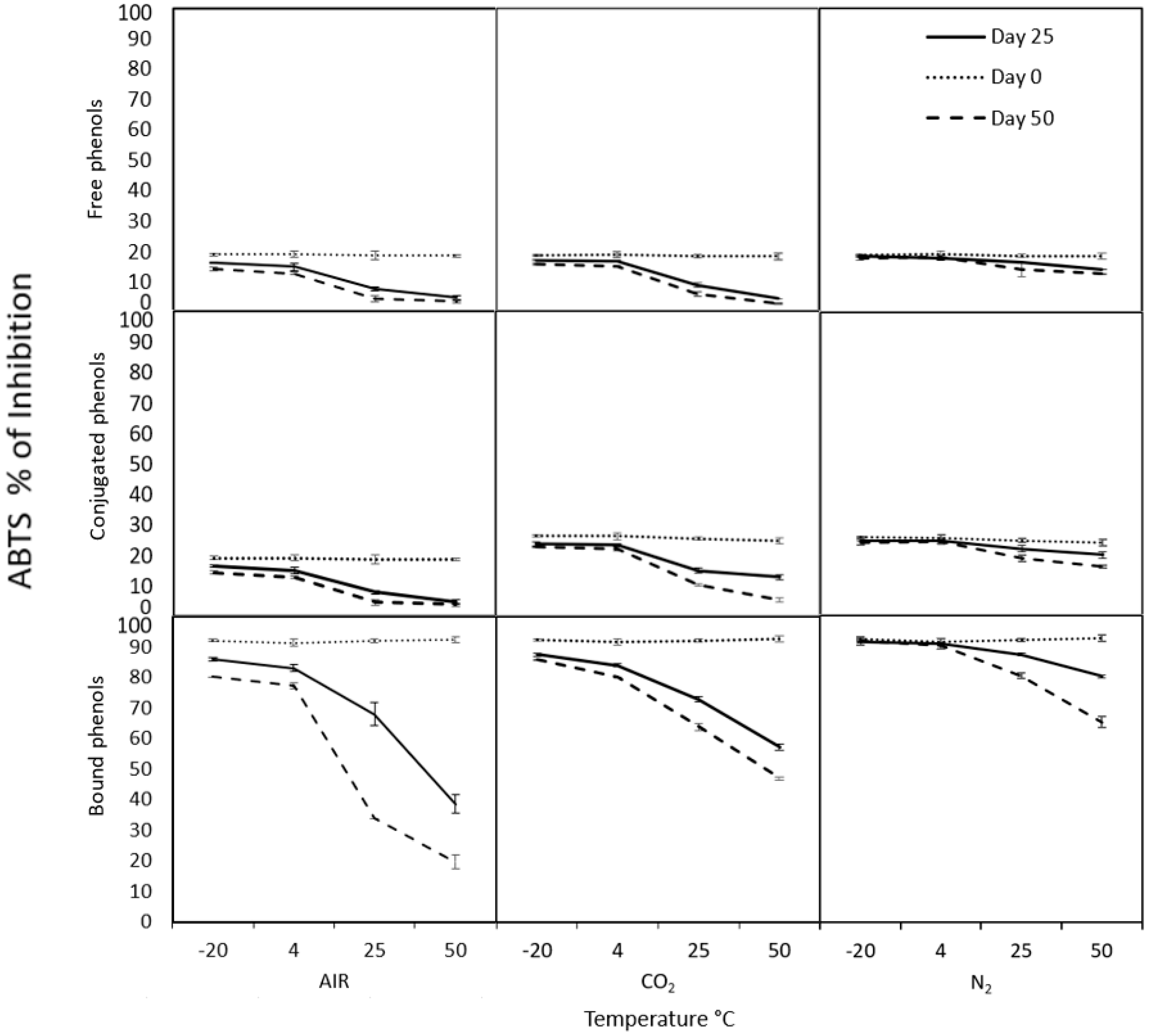
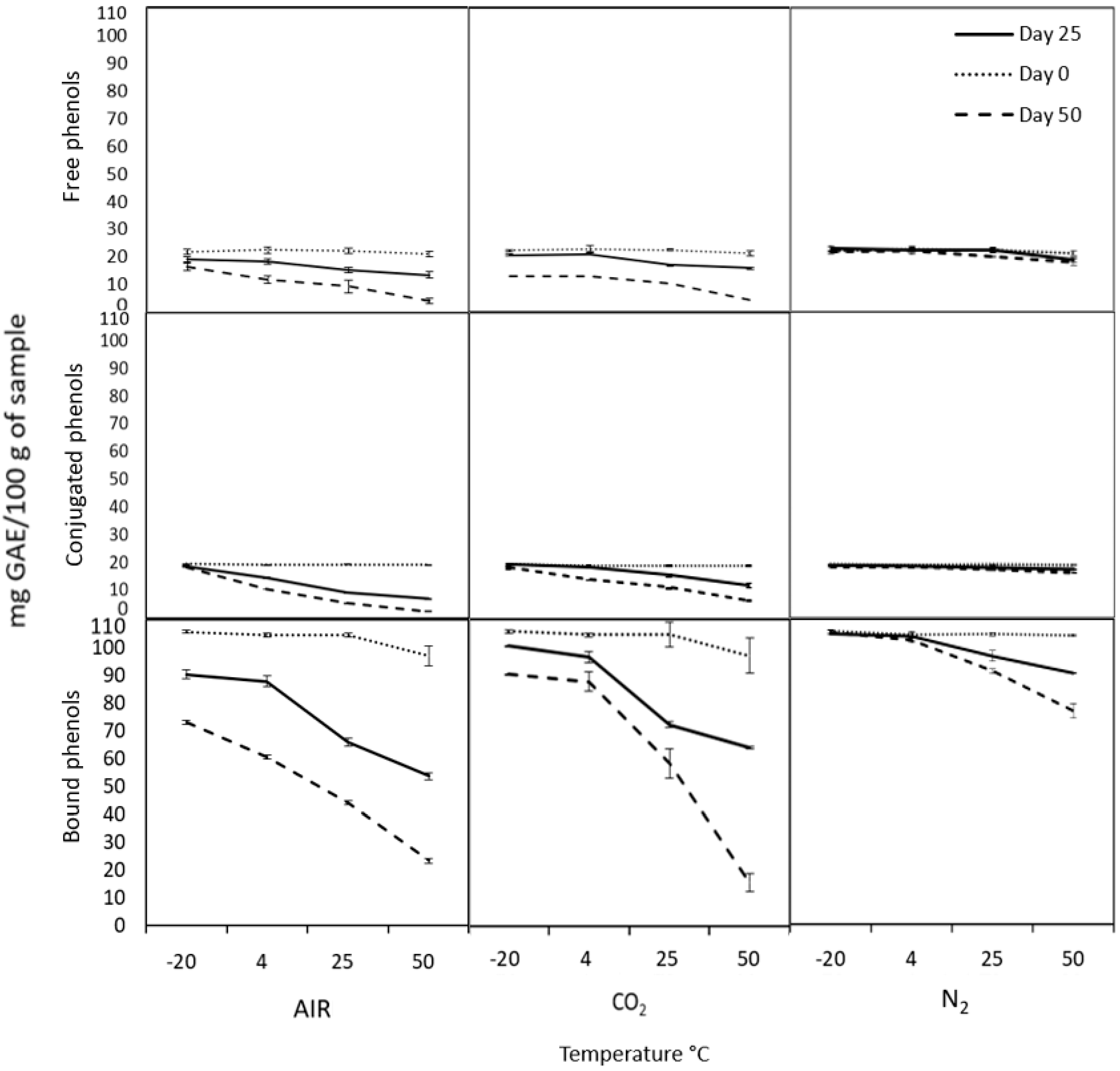
2.3. Modified Atmospheres Test
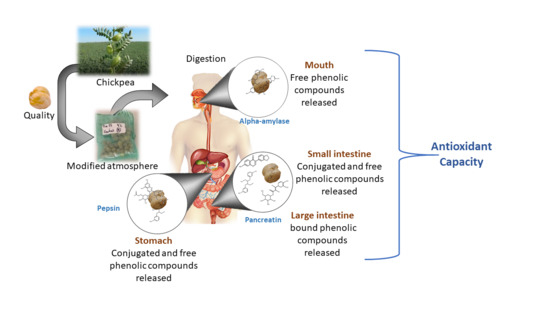
2.4. In Vitro Gastrointestinal Digestion
2.5. Identification of Phenolic Compounds by UPLC-MS
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Preparation of Sample
3.4. Proximal Analysis
3.5. Technological Quality
3.5.1. Water Absorption
3.5.2. Texture
3.5.3. Color
3.6. Controlled Atmosphere Conditions
3.7. In Vitro Digestibility Test
3.8. Extraction of Free, Conjugated and Bound Phenolic Compounds
3.9. Determination of Antioxidant Capacity
3.9.1. DPPH
3.9.2. ABTS
3.9.3. FRAP
3.9.4. AAPH
3.9.5. Total Phenolic Content
3.10. Identification of Phenolic Compounds by UPLC-MS
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- SIAP. Altas Agroalimentario. Servicio de Información Agroalimentaria y Pesquera. 2018. Available online: https://nube.siap.gob.mx/gobmx_publicaciones_siap/pag/2018/Atlas-Agroalimentario-2018 (accessed on 11 February 2021).
- Perez-Perez, L.M.; García-Borbón, L.L.; González-Vega, R.I.; Rodríguez-Figueroa, J.C.; Rosas-Burgos, E.C.; Huerta-Ocampo, J.Á.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Rueda-Puente, E.O.; et al. Liberación de compuestos fenólicos ligados en el garbanzo (Cicer arietinum L.) utilizando microbiota humana intestinal. BIOtecnia 2018, 20, 146–154. [Google Scholar] [CrossRef]
- Herrera-Flores, T.S.; Delgado-Alvarado, A.; Ramírez-Carrasco, P.; Licea de Anda, E.M.; Moreno-Contreras, M.G.; Machu-ca-Chavez, C.P. Estudio de la composición proximal de variedades de garbanzo (Cicer arietinum L.) Costa 2004 y Blanoro. Cienc. Tecnol. Agropec. 2014, 2, 9–15. [Google Scholar]
- Ferreira, C.D.; Bubolz, V.K.; da Silva, J.; Dittgen, C.L.; Ziegler, V.; Raphaelli, C.D.O.; de Oliveira, M. Changes in the chemical composition and bioactive compounds of chickpea (Cicer arietinum L.) fortified by germination. LWT 2019, 111, 363–369. [Google Scholar] [CrossRef]
- He, Y.; Shim, Y.Y.; Mustafa, R.; Meda, V.; Reaney, M.J. Chickpea Cultivar Selection to Produce Aquafaba with Superior Emulsion Properties. Foods 2019, 8, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summo, C.; De Angelis, D.; Ricciardi, L.; Caponio, F.; Lotti, C.; Pavan, S.; Pasqualone, A. Data on the chemical composition, bioactive compounds, fatty acid composition, physico-chemical and functional properties of a global chickpea collection. Data Brief 2019, 27, 104612. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Niño-Medina, G.; Muy-Range, l.D.; Garza-Juárez, A.; Vázquez-Rodríguez, J.A.; Méndez-Zamora, G.; Urías-Orona, V. Composición nutricional, compuestos fenólicos y capacidad antioxidante de cascarilla de garbanzo (Cicer arietinum L.). Arch. Latinoam. Nutr. 2017, 67, 68–73. [Google Scholar]
- Barbosa, C.; Alves, M.R.; Rocha, S.; Oliveira, M.B.P. Modified atmosphere packaging of precooked vegetables: Effect on physicochemical properties and sensory quality. Food Chem. 2016, 194, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Iturralde-García, R.D.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J.; Riudavets, J.; Del Toro-Sánchez, C.L.; Rueda-Puente, E.O.; Martínez-Cruz, O.; Wong-Corral, F.J. Effect of controlled atmospheres on the insect Callosobruchus maculatus Fab. in stored chickpea. J. Stored Prod. Res. 2016, 69, 78–85. [Google Scholar] [CrossRef]
- Kumar, S.; Gautam, S. A combination process to ensure microbiological safety, extend storage life and reduce anti-nutritional factors in legume sprouts. Food Biosci. 2019, 27, 18–29. [Google Scholar] [CrossRef]
- Park, J.-J.; Lee, J.-J.; Olawuyi, I.F.; Lee, W. Effect of argon- and nitrogen-based modified atmosphere packaging on shiitake mushroom quality. Korean J. Food Preserv. 2019, 26, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Zhang, M.; Devahastin, S.; Guo, Z. Effects of pressurized argon and nitrogen treatments in combination with modified atmosphere on quality characteristics of fresh-cut potatoes. Postharvest Biol. Technol. 2019, 149, 159–165. [Google Scholar] [CrossRef]
- Sauceda, A.E.Q.; Palafox, H.; Sánchez, R.M.R.; Aguilar, G.A.G. Interacción de compuestos fenólicos y fibra dietaria: Capacidad antioxidante y biodisponibilidad. BIOtecnia 2011, 13, 3–11. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Arispuro, D.M.; Cuevas-Rodríguez, E.O.; Milán-Carrillo, J.; León-López, L.; Gutiérrez-Dorado, R.; Reyes-Moreno, C. Optimal germination condition impacts on the antioxidant activity and phenolic acids profile in pigmented desi chickpea (Cicer arietinum L.) seeds. J. Food Sci. Technol. 2017, 55, 638–647. [Google Scholar] [CrossRef]
- Sun, Y.; Deng, Z.; Liu, R.; Zhang, H.; Zhu, H.; Jiang, L.; Tsao, R. A comprehensive profiling of free, conjugated and bound phenolics and lipophilic antioxidants in red and green lentil processing by-products. Food Chem. 2020, 325, 126925. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Mercado, G.; de la Rosa, L.A.; Alvarez-Parrilla, E. Effect of pectin on the interactions among phenolic compounds determined by antioxidant capacity. J. Mol. Struct. 2020, 1199, 126967. [Google Scholar] [CrossRef]
- Zhu, L.; Li, W.; Deng, Z.; Li, H.; Zhang, B. The Composition and Antioxidant Activity of Bound Phenolics in Three Legumes, and Their Metabolism and Bioaccessibility of Gastrointestinal Tract. Foods 2020, 9, 1816. [Google Scholar] [CrossRef]
- Alminger, M.; Aura, A.-M.; Bohn, T.; Dufour, C.; El, S.; Gomes, A.; Karakaya, S.; Martínez-Cuesta, M.; McDougall, G.; Requena, T.; et al. In Vitro Models for Studying Secondary Plant Metabolite Digestion and Bioaccessibility. Compr. Rev. Food Sci. Food Saf. 2014, 13, 413–436. [Google Scholar] [CrossRef] [Green Version]
- Melini, V.; Melini, F.; Acquistucci, R. Phenolic Compounds and Bioaccessibility Thereof in Functional Pasta. Antioxidants 2020, 9, 343. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T.; Villaró, S.; Bobo, G.; Simó, J.; Aguiló-Aguayo, I. Bioaccessibility and antioxidant activity of phenolic compounds in cooked pulses. Int. J. Food Sci. Technol. 2019, 54, 1816–1823. [Google Scholar] [CrossRef] [Green Version]
- Koehnlein, E.A.; Koehnlein, É.M.; Corrêa, R.C.G.; Nishida, V.S.; Correa, V.G.; Bracht, A.; Peralta, R.M. Analysis of a whole diet in terms of phenolic content and antioxidant capacity: Effects of a simulated gastrointestinal digestion. Int. J. Food Sci. Nutr. 2016, 67, 614–623. [Google Scholar] [CrossRef]
- Mtolo, M.; Gerrano, A.; Mellem, J. Effect of simulated gastrointestinal digestion on the phenolic compound content andin vitroantioxidant capacity of processed Cowpea (V. unguiculata) cultivars. CyTA-J. Food 2017, 15, 391–399. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Laurent, C.; Besançon, P.; Caporiccio, B. Flavonoids from a grape seed extract interact with digestive secretions and intestinal cells as assessed in an in vitro digestion/Caco-2 cell culture model. Food Chem. 2007, 100, 1704–1712. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Navarro-Coves, S.; Pérez-Álvarez, J.A.; Fernández-López, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of polyphenolic profile stability and changes in the antioxidant potential of maqui berry (Aristotelia chilensis (Molina) Stuntz) during in vitro gastrointestinal digestion. Ind. Crop. Prod. 2016, 94, 774–782. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Álvarez, J.A.P.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef]
- De Santiago, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.-P. Digestibility of (Poly)phenols and Antioxidant Activity in Raw and Cooked Cactus Cladodes (Opuntia ficus-indica). J. Agric. Food Chem. 2018, 66, 5832–5844. [Google Scholar] [CrossRef]
- Aguilera, Y.; Benitez, V.; Mollá, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Influence of Dehydration Process in Castellano Chickpea: Changes in Bioactive Carbohydrates and Functional Properties. Plant Foods Hum. Nutr. 2011, 66, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Heiras-Palazuelos, M.J.; Ochoa-Lugo, M.I.; Gutiérrez-Dorado, R.; Lopez-Valenzuela, J.A.; Mora-Rochín, S.; Milán-Carrillo, J.; Garzón-Tiznado, J.A.; Reyes-Moreno, C. Technological properties, antioxidant activity and total phenolic and flavonoid content of pigmented chickpea (Cicer arietinum L.) cultivars. Int. J. Food Sci. Nutr. 2012, 64, 69–76. [Google Scholar] [CrossRef]
- Singh, K.B.; Williams, P.C.; Nakkoul, H. Influence of growing season, location and planting time on some quality parameters of kabuli chickpea. J. Sci. Food Agric. 1990, 53, 429–441. [Google Scholar] [CrossRef]
- Patanè, C.; Iacoponi, E.; Raccuia, S.A. Physico-chemical characteristics, water absorption, soaking and cooking properties of some Sicilian populations of chickpea (Cicer arietinum L.). Int. J. Food Sci. Nutr. 2004, 55, 547–554. [Google Scholar] [CrossRef]
- Ouazib, M.; Dura, A.; Zaidi, F.; Rosell, C. Effect of Partial Substitution of Wheat Flour by Processed (Germinated, Toasted, Cooked) Chickpea on Bread Quality. Int. J. Agric. Sci. Technol. 2016, 4, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Marconi, E.; Ruggeri, S.; Cappelloni, M.; Leonardi, D.; Carnovale, E. Physicochemical, nutritional, and microstructural characteristics of chickpeas (Cicer arietinum L.) and common beans (Phaseolus vulgaris L.) following microwave cooking. J. Agric. Food Chem. 2000, 48, 5986–5994. [Google Scholar] [CrossRef] [PubMed]
- Pujolà, M.; Farreras, A.; Casañas, F. Protein and starch content of raw, soaked and cooked beans (Phaseolus vulgaris L.). Food Chem. 2007, 102, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Johnny, S.; Razavi, S.M.A.; Khodaei, D. Hydration kinetics and physical properties of split chickpea as affected by soaking temperature and time. J. Food Sci. Technol. 2015, 52, 8377–8382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Baranzini, A.; Falcón, V.M.; Barrón, H.J.; Silveira, G.M.; Alfaro, R.R. Evaluación de color del garbanzo (Cicerarie-tinum L.) por métodos instrumentales y sensoriales. Rev. Mex. Agroneg. 2014, 27, 323–335. [Google Scholar]
- Favela-Gomez, M.A.; García-Armenta, E.; Reyes-Moreno, C.; Tiznado-Garzón, J.A.; Perales-Sanchez, J.X.K.; Caro-Corrales, J.J.; Gutierrez-Dorado, E. Modelling of water absorption in chickpea (Cicer arietinum L.) sedes grown in Mexico’s Northwest. Rev. Mex. Ing. Quim 2017, 16, 179–191. [Google Scholar] [CrossRef]
- Reyes-Moreno, C.; Milán-Carrillo, J.; Rouzaud-Sandez, J.; Garzón-Tiznado, R.; Mora-Escobedo, R. Descascarilla-do/Suavización/Extrusión (Dse): Alternativa Tecnológica Para Mejorar La Calidad Nutricional Del Garbanzo (Cicer arietinum L.). Agrociencia 2002, 36, 181–189. [Google Scholar]
- Kaur, M.; Singh, N.; Sodhi, N.S. Physicochemical, cooking, textural and roasting characteristics of chickpea (Cicer arietinum L.) cultivars. J. Food Eng. 2005, 69, 511–517. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, L.M.; Armenta-Villegas, L.; Santacruz-Ortega, H.; Gutiérrez-Lomelí, M.; Aguilar, J.A.; Reynoso-Marin, F.J.; Robles-García, M.A.; Robles-Zepeda, R.E.; Ruiz-Cruz, S.; Del-Toro-Sánchez, C.L. Characterization of Anemopsis californica essential oil–β-cyclodextrin inclusion complex as antioxidant prolonged-release system. Chem. Pap. 2017, 71, 1331–1342. [Google Scholar] [CrossRef]
- Von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the Antioxidant Activity of Aspalathin with That of Other Plant Phenols of Rooibos Tea (Aspalathus linearis), α-Tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Yokozawa, T.; Chen, C.P.; Dong, E.; Tanaka, T.; Nonaka, G.-I.; Nishioka, I. Study on the Inhibitory Effect of Tannins and Flavonoids against the 1,1-Diphenyl-2-picrylhydrazyl Radical. Biochem. Pharmacol. 1998, 56, 213–222. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- FAO. Production Data. 2017. Available online: http://www.fao.org/faostat/en/?#data/QC (accessed on 24 February 2021).
- Andrés-Lacueva, C.; Medina-Remón, A.; Llorach, R.; Urpi-Sarda, M.; Khan, N.; Chiva-Blanch, G.; Zamora-Ros, R.; Rotches-Ribalta, M.; Lamuela-Raventos, R.M. Phenolic Compounds: Chemistry and Occurrence in Fruits and Vegetables. In Fruit and Vegetable Phytochemicals; Blackwell Publishing: Ames, IA, USA, 2010; pp. 53–88. [Google Scholar]
- González-Vega, R.I.; Cárdenas-López, J.L.; López-Elías, J.A.; Ruiz-Cruz, S.; Reyes-Díaz, A.; Perez-Perez, L.M.; Cinco-Moroyoqui, F.J.; Robles-Zepeda, R.E.; Borboa-Flores, J.; Del-Toro-Sánchez, C.L. Optimization of growing conditions for pigments production from microalga Navicula incerta using response surface methodology and its antioxidant capacity. Saudi J. Biol. Sci. 2020, 28, 1401–1416. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J.-D. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yang, Q.; Lin, L.; Sun, B.; Wang, Y. Intracellular antioxidant activities of selected cereal phenolic extracts and mechanisms underlying the protective effects of adlay phenolic extracts on H2O2 -induced oxidative stress in human erythrocytes. J. Funct. Foods 2017, 31, 160–171. [Google Scholar] [CrossRef]
- Phenol-Explorer 3.6. 2021. Available online: http://phenol-explorer.eu/ (accessed on 15 January 2021).
- Prasad, K. Antioxidant activity of secoisolariciresinol diglucoside-derived metabolites, secoisolariciresinol, Enterodiol, and enterolactone. Int. J. Angiol. 2000, 9, 220–225. [Google Scholar] [CrossRef]
- McCann, S.E.; Freudenheim, J.L.; Marshall, J.R.; Graham, S. Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J. Nutr. 2003, 133, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Horn-Ross, P.L.; John, E.M.; Canchola, A.J.; Stewart, S.L.; Lee, M.M. Phytoestrogen intake and endometrial cancer risk. J. Natl. Cancer Inst. 2003, 95, 1158–1164. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.-K.; Jeon, Y.-D.; Jin, J.-S. Apoptotic effect of enterodiol, the final metabolite of edible lignans, in colorectal cancer cells. J. Sci. Food Agric. 2018, 99, 2411–2419. [Google Scholar] [CrossRef]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2019, 275, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Jin, Z.; Ohm, J.-B.; Schwarz, P.; Rao, J.; Chen, B. Effect of germination time on antioxidative activity and composition of yellow pea soluble free and polar soluble bound phenolic compounds. Food Funct. 2019, 10, 6840–6850. [Google Scholar] [CrossRef] [PubMed]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef]
- Ortega-Moo, C.; Garza, J.; Vargas, R. The substituent effect on the antioxidant capacity of catechols and resorcinols. Theor. Chem. Accounts 2016, 135. [Google Scholar] [CrossRef]
- Moser, S.; Aragon, I.; Furrer, A.; Van Klinken, J.-W.; Kaczmarczyk, M.; Lee, B.-H.; George, J.; Hamaker, B.R.; Mattes, R.; Ferruzzi, M.G. Potato phenolics impact starch digestion and glucose transport in model systems but translation to phenolic rich potato chips results in only modest modification of glycemic response in humans. Nutr. Res. 2018, 52, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [Green Version]
- Velickovic, T.D.C.; Stanic-Vucinic, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2017, 17, 82–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giusti, F.; Capuano, E.; Sagratini, G.; Pellegrini, N. A comprehensive investigation of the behaviour of phenolic compounds in legumes during domestic cooking and in vitro digestion. Food Chem. 2019, 285, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Segev, A.; Badani, H.; Galili, L.; Hovav, R.; Kapulnik, Y.; Shomer, I.; Galili, S.; Aharon, S.; Hana, B.; Liel, G.; et al. Effects of Baking, Roasting and Frying on Total Polyphenols and Antioxidant Activity in Colored Chickpea Seeds. Food Nutr. Sci. 2012, 3, 369–376. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; The Association of Official Analytical Chemists: Arlington, TX, USA, 1997. [Google Scholar]
- Romero-Baranzini, A.L.; Barrón-Hoyos, J.M.; Falcón-Villa, M.R.; Anduaga-Cota, R. Evaluación de la calidad comercial y tecnológica de variedades y líneas avanzadas de garbanzo (Cicer Arientinum L.) Cosechadas en el noroeste de México. BIOtecnia 2014, 16, 7–14. [Google Scholar] [CrossRef]
- Ponce-García, N.; Ramírez-Wong, B.; Torres-Chávez, P.I.; Figueroa-Cárdenas, J.D.D.; Serna-Saldívar, S.O.; Cortez-Rocha, M.O. Effect of Moisture Content on the Viscoelastic Properties of Individual Wheat Kernels Evaluated by the Uniaxial Compression Test Under Small Strain. Cereal Chem. J. 2013, 90, 558–563. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Ramírez-Wong, B.; Torres-Chávez, P.I.; López-Cervantes, J.; Figueroa-Cárdenas, J.D.D.; Barrón-Hoyos, J.M.; Morales-Rosas, I.; Ponce-García, N.; Gutiérrez-Dorado, R. Obtaining Ready-to-Eat Blue Corn Expanded Snacks with Anthocyanins Using an Extrusion Process and Response Surface Methodology. Molecules 2014, 19, 21066–21084. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Zafrilla, P.; Tomás-Barberán, F.A. An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Eur. Food Res. Technol. 2002, 214, 155–159. [Google Scholar] [CrossRef]
- Cabrera-Soto, M.L.; Salinas-Moreno, Y.; Velázquez-Cardenas, A.; Espinoza-Trujillo, E. Contenido de fenoles solubles e inso-lubles en las estructuras de grano de maíz y su relación con propiedades físicas. Agrociencia 2009, 43, 827–839. [Google Scholar]
- Molyneux, P. The use of the stable radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Jin, Y.; Liu, G.; Zhu, N.; Gui, M.; Yu, A.; Li, X. Flavonoids from the leaves of Actinidia kolomikta. Chem. Nat. Compd. 2010, 46, 205–208. [Google Scholar] [CrossRef]
- Eldeen, I.; Seow, E.-M.; Abdullah, R.; Sulaiman, S. In vitro antibacterial, antioxidant, total phenolic contents and anti-HIV-1 reverse transcriptase activities of extracts of seven Phyllanthus sp. S. Afr. J. Bot. 2011, 77, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ruiz, K.L.; Ruiz-Cruz, S.; Cira-Chávez, L.A.; Gassos-Ortega, L.E.; Ornelas-Paz, J.D.J.; Del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; López-Mata, M.A.; Rodríguez-Félix, F. Evaluation of Antioxidant Capacity, Protective Effect on Human Erythrocytes and Phenolic Compound Identification in Two Varieties of Plum Fruit (Spondias spp.) by UPLC-MS. Molecules 2018, 23, 3200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| COMPOSITION (%) | RAW | COOKED |
|---|---|---|
| Carbohydrate | 64.40 a ± 0.5 | 68.32 a ± 0.34 |
| * Moisture | 5.96 a ± 0.10 | 47.28 b ± 0.07 |
| Ash | 2.8 a ± 0.47 | 1.75 b ± 0.04 |
| Protein | 19.16 a ± 0.51 | 18.38 b ± 1.98 |
| Fat | 6.23 a ± 0.07 | 5.35 b ± 0.08 |
| Fiber | 7.41 a ± 0.97 | 7.2 a ± 0.82 |
| Sample | L* | a* | b* |
|---|---|---|---|
| Raw | 60.44 ± 3.5 a | 6.98 ± 0.4 a | 17.74 ± 1.25 a |
| Cooked | 55.45 ± 3.81 b | 8.63 ± 0.62 b | 22.4 ± 1.9 b |
| COMPOSITION (%) | DAY 0 | DAY 50 |
|---|---|---|
| Carbohydrate | 68.32 a ± 0.34 | 67.29 a ± 0.65 |
| Ash | 1.75 a ± 0.04 | 2.05 a ± 0.03 |
| Protein | 18.38 a ± 1.98 | 17.18 a ± 2.06 |
| Fat | 5.35 a ± 0.08 | 5.45 a ± 0.02 |
| Fiber | 7.20 a ± 0.82 | 7.30 a ± 0.60 |
| Antioxidant Technique/Phenolic Fraction | Raw | Cooked | Mouth * | Stomach * | Small Intestine * | |
|---|---|---|---|---|---|---|
| (Alpha-Amylase) | (Pepsin) | (Pancreatin) Inside Membrane | (Portal Vein) Outside Nembrane | |||
| DPPH ** | ||||||
| Free | 16.8 aA ± 1.5 | 8.2 bA ± 0.5 | 0.6 cA ± 0.1 | 3.7 deA ± 1.2 | 4.9 eA ± 0.4 | 3.0 dA ± 0.4 |
| Conjugated | 17.3 aA ± 0.7 | 9.7 bA ± 0.3 | 0.17 cB ± 0.0 | 2.5 dA ± 0.0 | 6.7 eA ± 1.0 | 5.0 eB ± 1.0 |
| Bound | 35.1 aB ± 3.7 | 26.8 bB ± 1.2 | 0.0 cB ± 0.0 | 9.8 dB ± 1.7 | 16.2 eB ± 0.5 | 0.0 cC ± 0.0 |
| ABTS ** | ||||||
| Free | 15.6 aA ± 1.4 | 8.7 bA ± 0.2 | 1.9 cA ± 0.6 | 3.2 cA ± 1.1 | 8.3 cA ± 0.1 | 6.7 bA ± 0.1 |
| Conjugated | 19.6 aA ± 4.3 | 9.3 bA ± 0.0 | 2.3 cB ± 0.1 | 6.9 bcA ± 2.4 | 8.3 bcA ± 0.9 | 5.3 bcAB ± 1.2 |
| Bound | 41.8 aB ± 2.1 | 21.7 bB ± 0.2 | 0.75 cC ± 0.0 | 8.1 dA ± 2.1 | 11.1 dB ± 0.1 | 2.6 cB ± 0.1 |
| FRAP ** | ||||||
| Free | 18.7 aA ± 0.2 | 7.2 bA ± 0.1 | 4.5 cA ± 0.2 | 3.3 dA ± 0.4 | 6.3 eA ± 0.1 | 5.1 cA ± 0.3 |
| Conjugated | 66.2 aB ± 0.2 | 41.0 bB ± 3.1 | 3.4 cB ± 0.3 | 11.7 dB ± 0.1 | 32.0 eB ± 0.2 | 28.2 fB ± 0.5 |
| Bound | 71.2 aC ± 0.1 | 30.2 bC ± 0.2 | 3.6 cB ± 0.1 | 6.8 dC ± 1.1 | 13.5 eC ± 0.1 | 3.9 cC ± 0.1 |
| AAPH ** | ||||||
| Free | 11.3 aA ± 1.3 | 6.1bA ± 1.7 | 0.5 cA ± 0.0 | 2.6 cdA ± 0.7 | 5.1 dbA ± 0.7 | 4.6 dbA ± 1.5 |
| Conjugated | 67.7 aB ± 4.0 | 31.9 bB ± 1.6 | 3.8 cB ± 0.4 | 11.0 dB ± 0.4 | 30.6 bB ± 0.7 | 19.0 eB ± 0.4 |
| Bound | 76.8 aC ± 1.6 | 43.0 bC ± 0.7 | 2.5 cC ± 0.4 | 7.4 dC ± 0.7 | 9.7 eA ± 0.7 | 0.0 fC ± 0.0 |
| Phenol Compounds *** | ||||||
| Free | 57.5 aA ± 5.0 | 23.7 bA ± 1.0 | 3.0 eA ± 0.1 | 5.5 dA ± 0.3 | 7.7 cA ± 0.6 | 7.1 cA ± 0.1 |
| Conjugated | 27.2 aB ± 0.1 | 19.4 bA ± 0.0 | 1.8 cB ± 0.1 | 5.5 dA ± 0.3 | 9.0 eA ± 1.1 | 8.8 eA ± 0.1 |
| Bound | 146.0 aC ± 4.0 | 106.6 bB ± 4.0 | 4.2 cC ± 0.5 | 13.9 dB ± 2.6 | 39.3 eB ± 0.6 | 1.4 fB ± 0.1 |
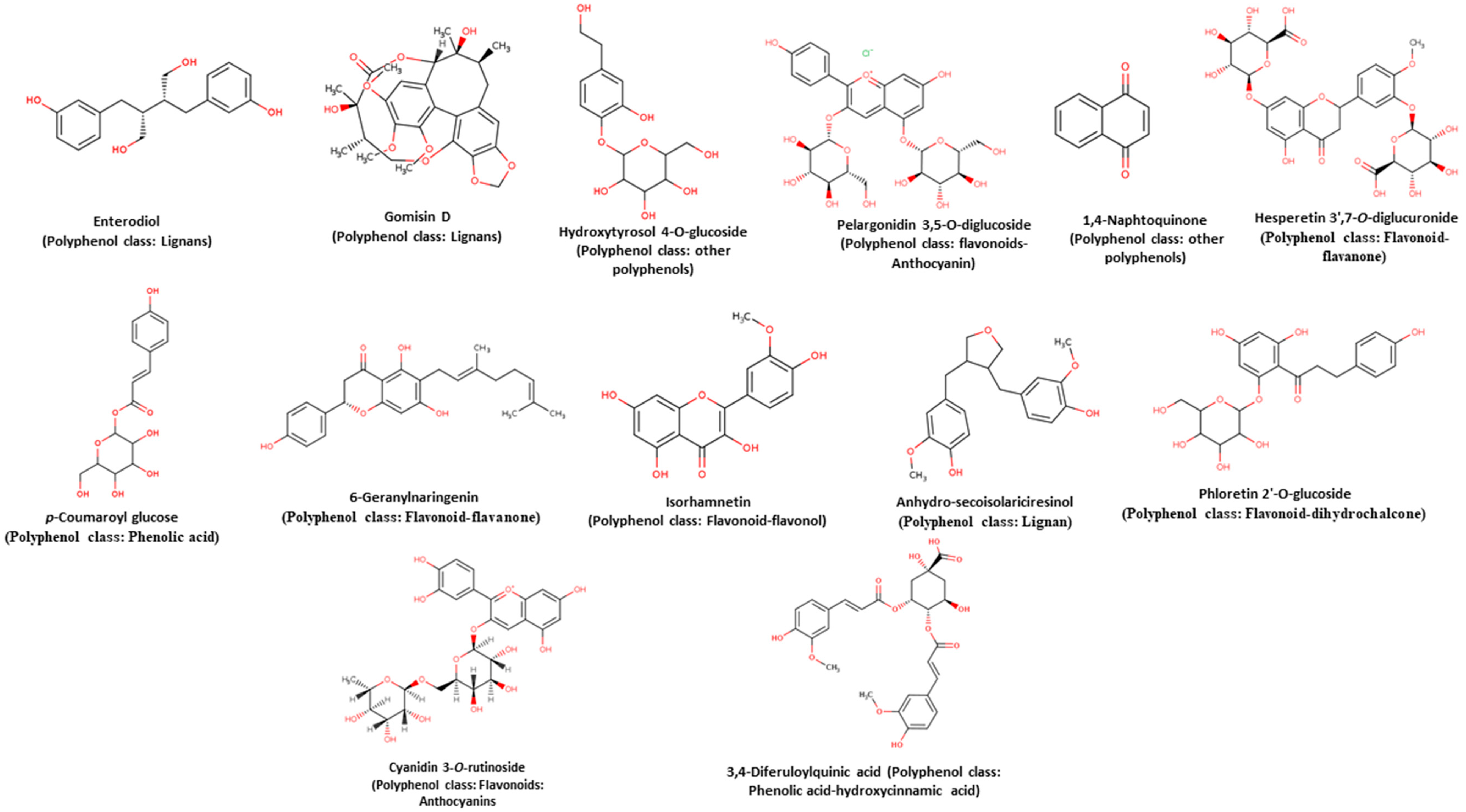
| Sample/Phenolic Compounds | No. | RT (min) | % of Area | Compound * | Observed m/z | Exp.a Mass (Da) | Theor.b Mass (Da) | Mass Delta (Da) | Molecular Formula |
|---|---|---|---|---|---|---|---|---|---|
| Raw chickpea/Free | 1 | 0.63 | 6.40 | Gomisin D | 529.33 | 530.33 | 530.21 | 0.12 | C28H34O10 |
| 2 | 0.99 | 46.40 | Enterodiol | 301.33 | 302.33 | 302.15 | 0.18 | C18H22O4 | |
| 3 | 9.71 | 3.08 | Hydroxytyrosol 4-O-glucoside | 315.18 | 316.18 | 316.12 | 0.06 | C14H20O8 | |
| 4 | 9.91 | 8.67 | Pelargonidin 3,5-O-diglucoside | 629.35 | 630.35 | 630.13 | 0.22 | C27H31ClO15 | |
| 5 | 10.06 | 3.54 | 1,4-Naphtoquinone | 157.18 | 158.18 | 158.04 | 0.14 | C10H6O2 | |
| 6 | 11.43 | 2.90 | p-Coumaroyl glucose | 325.19 | 326.19 | 326.10 | 0.09 | C15H18O8 | |
| Raw chickpea/Conjugated | 1 | 0.55 | 7.46 | 6-Geranylnaringenin | 407.27 | 408.27 | 408.19 | 0.08 | C25H28O5 |
| 2 | 0.63 | 9.72 | Gomisin D | 529.33 | 530.33 | 530.21 | 0.12 | C28H34O10 | |
| 3 | 0.99 | 57.12 | Enterodiol | 301.33 | 302.33 | 302.15 | 0.18 | C18H22O4 | |
| 4 | 9.71 | 1.55 | Isorhamnetin | 315.11 | 316.11 | 316.05 | 0.06 | C16H12O7 | |
| 5 | 9.97 | 1.95 | Pelargonidin 3,5-O-diglucoside | 629.48 | 630.48 | 630.13 | 0.35 | C27H31ClO15 | |
| Raw chickpea/Bound | 1 | 0.56 | 4.50 | Hydroxytyrosol 4-O-glucoside | 315.31 | 316.31 | 316.11 | 0.20 | C14H20O8 |
| 2 | 0.64 | 6.75 | Gomisin D | 529.33 | 530.33 | 530.21 | 0.12 | C28H34O10 | |
| 3 | 0.99 | 55.29 | Enterodiol | 301.33 | 302.33 | 302.15 | 0.18 | C18H22O4 | |
| 4 | 1.57 | 12.43 | Anhydro-secoisolariciresinol | 343.38 | 344.38 | 344.16 | 0.22 | C20H24O5 | |
| 5 | 9.72 | 1.67 | Phloretin 2′-O-glucoside | 434.96 | 435.96 | 436.13 | −0.17 | C21H24O10 | |
| Cooked chickpea/Free | 1 | 0.55 | 5.62 | Hydroxytyrosol 4-O-glucoside | 315.31 | 316.31 | 316.11 | 0.20 | C14H20O8 |
| 2 | 0.63 | 10.33 | Gomisin D | 529.33 | 530.33 | 530.21 | 0.12 | C28H34O10 | |
| 3 | 0.99 | 59.15 | Enterodiol | 301.20 | 302.20 | 302.15 | 0.05 | C18H22O4 | |
| 4 | 3.39 | 5.00 | Hesperetin 3′,7-O-diglucuronide | 653.27 | 654.27 | 654.14 | 0.13 | C28H30O18 | |
| 5 | 9.72 | 4.42 | Hydroxytyrosol 4-O-glucoside | 315.05 | 316.05 | 316.12 | −0.07 | C14H20O8 | |
| 6 | 9.94 | 2.38 | Pelargonidin 3,5-O-diglucoside | 629.55 | 630.55 | 630.13 | 0.42 | C27H31ClO15 | |
| Cooked chickpea/Conjugated | 1 | 0.55 | 7.73 | 6-Geranylnarin-genin | 407.40 | 408.40 | 408.19 | 0.21 | C25H28O5 |
| 2 | 0.63 | 10.09 | Gomisin D | 529.46 | 530.46 | 530.21 | 0.25 | C28H34O10 | |
| 3 | 0.99 | 67.42 | Enterodiol | 301.20 | 302.20 | 302.15 | 0.05 | C18H22O4 | |
| Cooked chickpea/Bound | 1 | 0.52 | 3.87 | Hydroxytyrosol 4-O-glucoside | 315.37 | 316.37 | 316.11 | 0.26 | C14H20O8 |
| 2 | 0.62 | 5.81 | Gomisin D | 529.39 | 530.39 | 530.21 | 0.18 | C28H34O10 | |
| 3 | 0.99 | 55.62 | Enterodiol | 301.40 | 302.40 | 302.15 | 0.25 | C18H22O4 | |
| 4 | 1.56 | 12.78 | Anhydro-secoisolariciresinol | 343.38 | 344.38 | 344.16 | 0.22 | C20H24O5 | |
| 5 | 9.71 | 6.61 | 3,4-Diferuloylquinic acid | 543.43 | 544.43 | 544.16 | 0.27 | C27H28O12 |
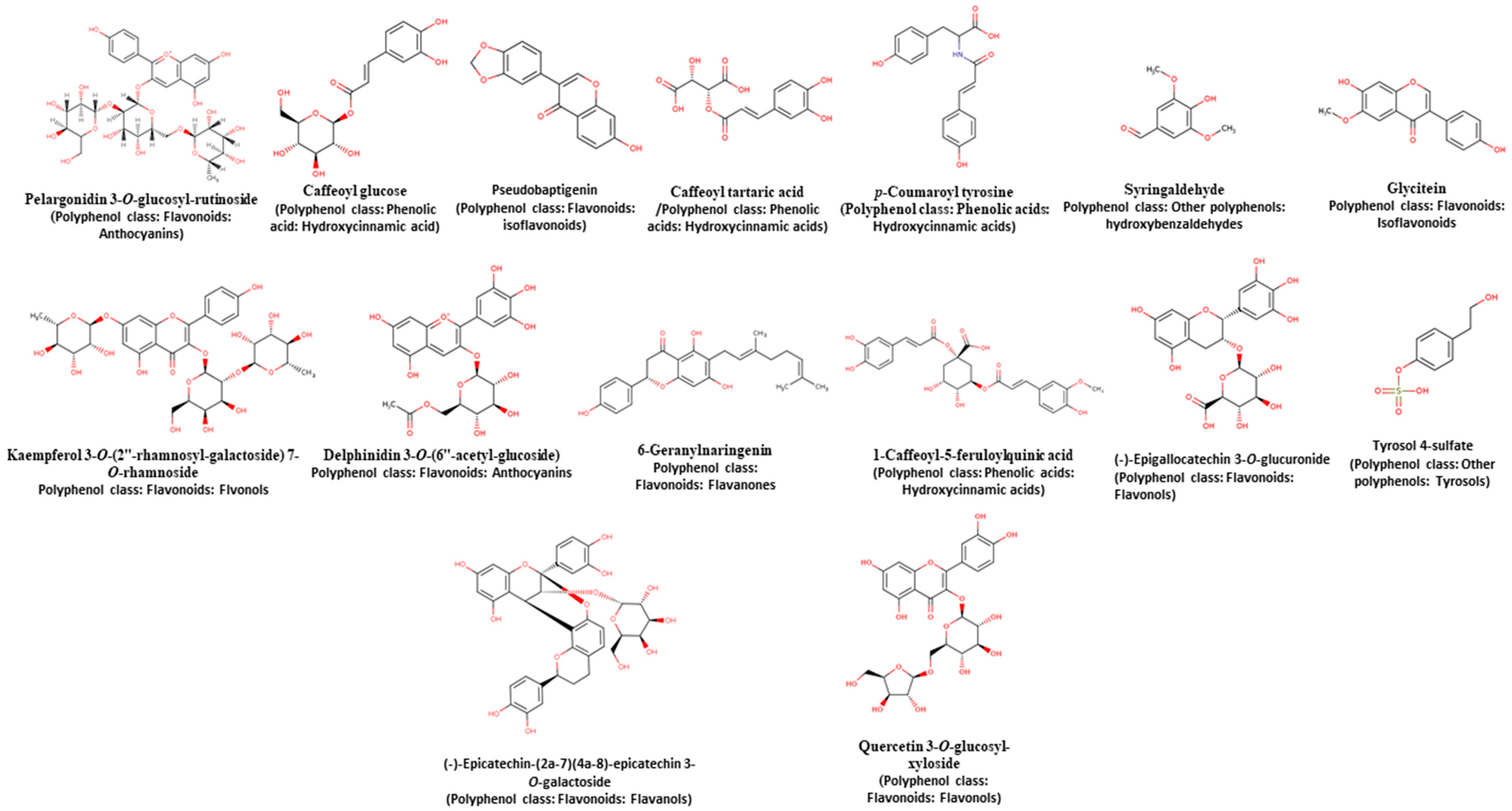
| Simulated Gastrointestinal Tract Section/Phenolic Compounds | No. | RT (min) | % of Area | Compound * | Observed m/z | Exp.a Mass (Da) | Theor.b Mass (Da) | Mass Delta (Da) | Molecular Formula |
|---|---|---|---|---|---|---|---|---|---|
| Mouth/Free | 1 | 9.92 | 7.93 | Caffeoyl tartaric acid | 311.08 | 312.08 | 312.04 | 0.04 | C13H12O9 |
| 2 | 10.36 | 1.87 | p-Coumaroyl tyrosine | 326.23 | 327.23 | 327.11 | 0.12 | C18H17NO5 | |
| Mouth/Conjugated | Not identified | ||||||||
| Mouth/Bound | 1 | 11.45 | 13.27 | Syringaldehyde | 181.15 | 182.15 | 182.06 | 0.09 | C9H10O4 |
| 2 | 11.79 | 9.82 | Glycitein | 283.40 | 284.40 | 284.07 | 0.33 | C16H12O5 | |
| Stomach/Free | 1 | 8.18 | 12.36 | Kaempferol 3-O-(2″-rhamnosyl-galactoside) 7-O-rhamnoside | 738.93 | 739.93 | 740.21 | −0.28 | C33H40O19 |
| 2 | 10.00 | 25.43 | Delphinidin 3-O-(6″-acetyl-glucoside) | 506.52 | 507.52 | 507.11 | 0.41 | C23H23O13 | |
| Stomach/Conjugated | 1 | 1.8 | 1.84 | 6-Geranylnaringenin | 407.40 | 408.40 | 408.19 | 0.21 | C25H28O5 |
| 2 | 9.91 | 6.89 | 1-Caffeoyl-5-feruloylquinic acid | 529.46 | 530.46 | 530.14 | 0.32 | C26H26O12 | |
| Stomach/Bound | 1 | 9.43 | 5.44 | (-)-Epigallocatechin 3-O-glucuronide | 481.10 | 482.10 | 482.10 | 0.00 | C21H22O13 |
| 2 | 9.99 | 5.39 | Delphinidin 3-O-(6″-acetyl-glucoside) | 506.52 | 507.52 | 507.11 | 0.41 | C23H23O13 | |
| 3 | 10.3 | 9.54 | Cyanidin 3-O-rutinoside | 594.26 | 595.26 | 595.16 | 0.10 | C27H31O15 | |
| 4 | 10.47 | 3.76 | Quercetin 3-O-glucosyl-xyloside | 594.65 | 595.65 | 596.13 | −0.48 | C26H28O16 | |
| Intestine/Free | 1 | 10.3 | 3.93 | Tyrosol 4-sulfate | 216.97 | 217.97 | 218.02 | −0.05 | C8H10O5S |
| 2 | 10.5 | 2.67 | (-)-Epicatechin-(2a-7)(4a-8)-epicatechin 3-O-galactoside | 705.13 (18) | 706.13 | 706.19 | −0.06 | C36H34O15 | |
| Intestine/Conjugated | 1 | 8.2 | 10.90 | Pelargonidin 3-O-glucosyl-rutinoside | 739.91 | 740.91 | 741.22 | −0.31 | C33H41O19 |
| Intestine/Bound | 1 | 1.85 | 6.82 | Caffeoyl glucose | 341.43 | 342.43 | 342.09 | 0.34 | C15H18O9 |
| 2 | 4.06 | 1.98 | Pseudobaptigenin | 281.38 | 282.38 | 282.05 | 0.33 | C16H10O5 | |
| 3 | 9.98 | 8.40 | Delphinidin 3-O-(6″-acetyl-glucoside) | 506.39 | 507.39 | 507.11 | 0.28 | C23H23O13 | |
| 4 | 10.3 | 6.25 | Tyrosol 4-sulfate | 217.04 | 218.04 | 218.02 | 0.02 | C8H10O5S | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Perez, L.M.; Huerta-Ocampo, J.Á.; Ruiz-Cruz, S.; Cinco-Moroyoqui, F.J.; Wong-Corral, F.J.; Rascón-Valenzuela, L.A.; Robles-García, M.A.; González-Vega, R.I.; Rosas-Burgos, E.C.; Corella-Madueño, M.A.G.; et al. Evaluation of Quality, Antioxidant Capacity, and Digestibility of Chickpea (Cicer arietinum L. cv Blanoro) Stored under N2 and CO2 Atmospheres. Molecules 2021, 26, 2773. https://doi.org/10.3390/molecules26092773
Perez-Perez LM, Huerta-Ocampo JÁ, Ruiz-Cruz S, Cinco-Moroyoqui FJ, Wong-Corral FJ, Rascón-Valenzuela LA, Robles-García MA, González-Vega RI, Rosas-Burgos EC, Corella-Madueño MAG, et al. Evaluation of Quality, Antioxidant Capacity, and Digestibility of Chickpea (Cicer arietinum L. cv Blanoro) Stored under N2 and CO2 Atmospheres. Molecules. 2021; 26(9):2773. https://doi.org/10.3390/molecules26092773
Chicago/Turabian StylePerez-Perez, Liliana Maribel, José Ángel Huerta-Ocampo, Saúl Ruiz-Cruz, Francisco Javier Cinco-Moroyoqui, Francisco Javier Wong-Corral, Luisa Alondra Rascón-Valenzuela, Miguel Angel Robles-García, Ricardo Iván González-Vega, Ema Carina Rosas-Burgos, María Alba Guadalupe Corella-Madueño, and et al. 2021. "Evaluation of Quality, Antioxidant Capacity, and Digestibility of Chickpea (Cicer arietinum L. cv Blanoro) Stored under N2 and CO2 Atmospheres" Molecules 26, no. 9: 2773. https://doi.org/10.3390/molecules26092773
APA StylePerez-Perez, L. M., Huerta-Ocampo, J. Á., Ruiz-Cruz, S., Cinco-Moroyoqui, F. J., Wong-Corral, F. J., Rascón-Valenzuela, L. A., Robles-García, M. A., González-Vega, R. I., Rosas-Burgos, E. C., Corella-Madueño, M. A. G., & Del-Toro-Sánchez, C. L. (2021). Evaluation of Quality, Antioxidant Capacity, and Digestibility of Chickpea (Cicer arietinum L. cv Blanoro) Stored under N2 and CO2 Atmospheres. Molecules, 26(9), 2773. https://doi.org/10.3390/molecules26092773