Determination of Polycyclic Aromatic Hydrocarbons and Their Methylated Derivatives in Sewage Sludge from Northeastern China: Occurrence, Profiles and Toxicity Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chemicals and Reagents
2.3. Sample Pre-Treatment and Instrumental Analysis
2.4. Quality Assurance/Quality Control (QA/QC)
2.5. Data Analysis
2.5.1. Positive Matrix Factorization and Statistical Analysis
2.5.2. Health Risk Assessment
2.5.3. Fluxes Calculations of PAHs and Me-PAHs
3. Results and Discussion
3.1. Occurrence of PAHs and Me-PAHs in Sludge
3.2. Composition Profile
3.3. PAHs and Me-PAHs in Sludge Worldwide
3.4. The PAHs and Me-PAHs Loads from Sewage Sludge Discharged from 10 WWTPs
3.5. Source Appointment
3.5.1. Principal Component Analysis
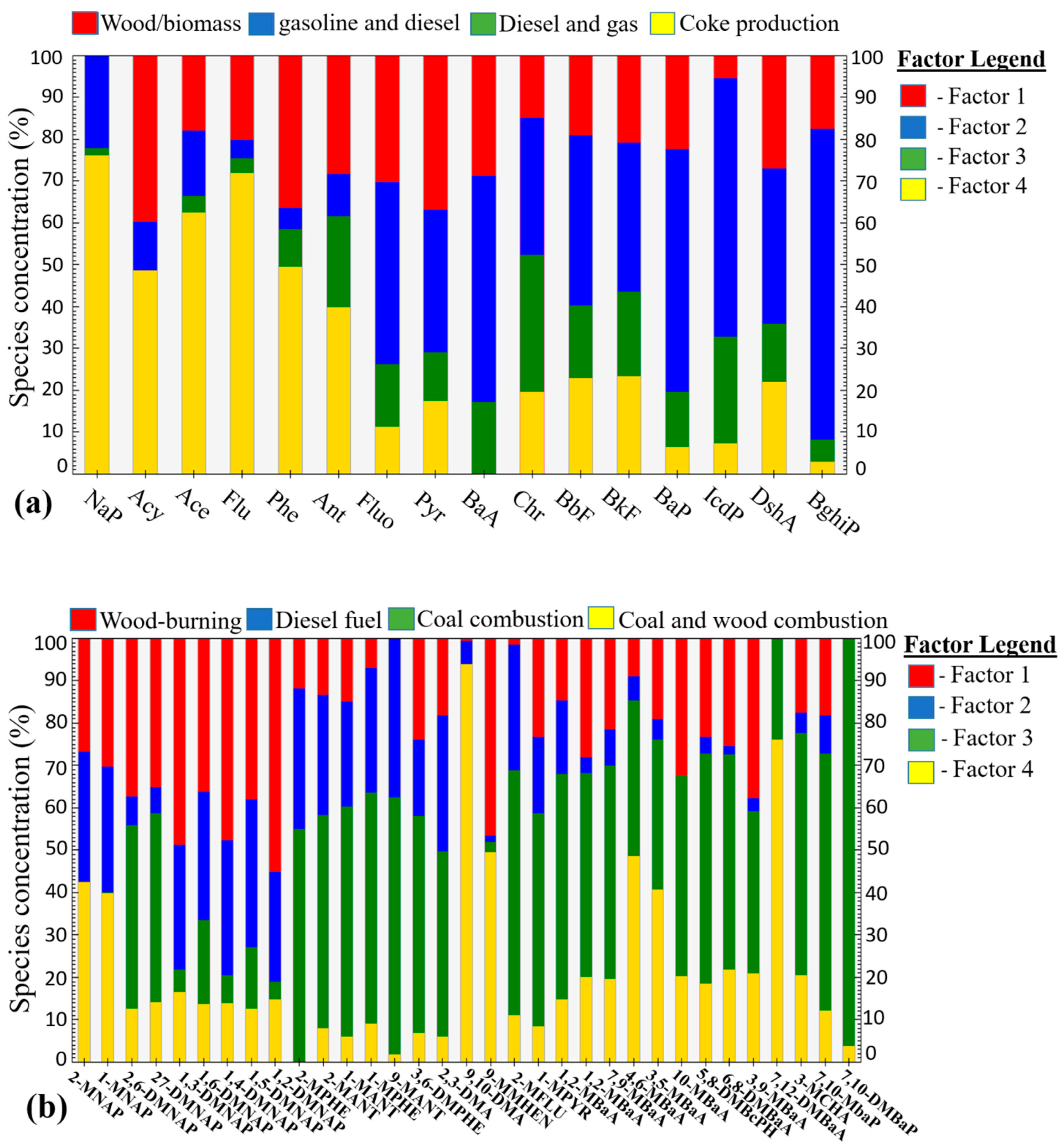
3.5.2. Positive matrix Factorization
3.5.3. Source Apportionment by Diagnostic Ratios
3.6. Toxicity Evaluation and Risk Assessment in Sewage Sludge
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hong, W.-J.; Jia, H.; Li, Y.-F.; Sun, Y.; Liu, X.; Wang, L. Polycyclic aromatic hydrocarbons (PAHs) and alkylated PAHs in the coastal seawater, surface sediment and oyster from Dalian, Northeast China. Ecotoxicol. Environ. Saf. 2016, 128, 11–20. [Google Scholar] [CrossRef]
- Bertrand, O.; Mondamert, L.; Grosbois, C.; Dhivert, E.; Bourrain, X.; Labanowski, J.; Desmet, M. Storage and source of polycyclic aromatic hydrocarbons in sediments downstream of a major coal district in France. Environ. Pollut. 2015, 207, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.B.; Chow, K.L.; Cheng, Z.; Mo, W.Y.; Chan, Y.H.; Lam, J.C.W.; Lau, F.T.K.; Fung, W.C.; Wong, M.H. Profiles and removal efficiency of polycyclic aromatic hydrocarbons by two different types of sewage treatment plants in Hong Kong. J. Environ. Sci. 2017, 53, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Wołejko, E.; Wydro, U.; Jabłońska-Trypuć, A.; Butarewicz, A.; Łoboda, T. The effect of sewage sludge fertilization on the concentration of PAHs in urban soils. Environ. Pollut. 2018, 232, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Tuyen, L.H.; Tue, N.M.; Takahashi, S.; Suzuki, G.; Viet, P.H.; Subramanian, A.; Bulbule, K.A.; Parthasarathy, P.; Ramanathan, A.; Tanabe, S. Methylated and unsubstituted polycyclic aromatic hydrocarbons in street dust from Vietnam and India: Occurrence, distribution and in vitro toxicity evaluation. Environ. Pollut. 2014, 194, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Cao, W.; Liu, B.; Bai, Y.; Qi, W.; Zhao, X.; Qu, J. Impact of upgrading wastewater treatment plant on the removal of typical methyl, oxygenated, chlorinated and parent polycyclic aromatic hydrocarbons. Sci. Total Environ. 2017, 603–604, 140–147. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Cao, S.-X.; Zhang, D.-L.; Gao, S.-T.; Yu, Z.-Q.; Li, H.-R.; Sheng, G.-Y.; Fu, J.-M. Levels and distribution of synthetic musks and polycyclic aromatic hydrocarbons in sludge collected from Guangdong Province. J. Environ. Sci. Heal. Part. A 2012, 47, 389–397. [Google Scholar] [CrossRef]
- Verlicchi, P.; Zambello, E. Pharmaceuticals and personal care products in untreated and treated sewage sludge: Occurrence and environmental risk in the case of application on soil—A critical review. Sci. Total Environ. 2015, 538, 750–767. [Google Scholar] [CrossRef]
- Cai, Q.-Y.; Mo, C.-H.; Wu, Q.-T.; Zeng, Q.-Y.; Katsoyiannis, A. Occurrence of organic contaminants in sewage sludges from eleven wastewater treatment plants, China. Chemosphere 2007, 68, 1751–1762. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, T.; Li, X.; Yao, J.; Liu, W.; Chang, S.; Chen, Y. The fate and enhanced removal of polycyclic aromatic hydrocarbons in wastewater and sludge treatment system: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1425–1475. [Google Scholar] [CrossRef]
- Chen, C.-F.; Yun-Ru, R.; Yee, C.L.; Shu-Ling, H.; Mei-Ling, T.; Pei-Pei, S.; Ravi, K.; Chiu-Wen, C. Determination of Polycyclic Aromatic Hydrocarbons in Sludge from Water and Wastewater Treatment Plants by GC-MS. Int. J. Environ. Res. Public Health 2019, 16, 2604. [Google Scholar] [CrossRef] [PubMed]
- Skowrońska, M.; Bielińska, E.J.; Szymański, K.; Futa, B.; Antonkiewicz, J.; Kołodziej, B. An integrated assessment of the long-term impact of municipal sewage sludge on the chemical and biological properties of soil. Catena 2020, 189, 104484. [Google Scholar] [CrossRef]
- Stevens, J.L.; Northcott, G.L.; Stern, G.A.; Tomy, G.T.; Jones, K.C. PAHs, PCBs, PCNs, Organochlorine Pesticides, Synthetic Musks, and Polychlorinatedn-Alkanes in U.K. Sewage Sludge: Survey Results and Implications. Environ. Sci. Technol. 2003, 37, 462–467. [Google Scholar] [CrossRef]
- Khadhar, S.; Higashi, T.; Hamdi, H.; Matsuyama, S.; Charef, A. Distribution of 16 EPA-priority polycyclic aromatic hydrocarbons (PAHs) in sludges collected from nine Tunisian wastewater treatment plants. J. Hazard. Mater. 2010, 183, 98–102. [Google Scholar] [CrossRef]
- Hu, Y.; Xia, Y.; Di Maio, F.; Yu, F.; Yu, W. Investigation of polycyclic aromatic hydrocarbons (PAHs) formed in three-phase products from the pyrolysis of various wastewater sewage sludge. J. Hazard. Mater. 2020, 389, 122045. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, Z.; Liang, J.; Kuo, D.T.F.; Chen, S.; Hu, X.; Deng, M.; Zhang, H.; Lu, Y. Assessing pollution and risk of polycyclic aromatic hydrocarbons in sewage sludge from wastewater treatment plants in China’s top coal-producing region. Environ. Monit. Assess. 2019, 191, 102. [Google Scholar] [CrossRef]
- Guo, Y.; Rene, E.R.; Wang, J.; Ma, W. Biodegradation of polyaromatic hydrocarbons and the influence of environmental factors during the co-composting of sewage sludge and green forest waste. Bioresour. Technol. 2020, 297, 122434. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tian, L.; Liu, Z.; He, J.; Fu, H.; Huang, Q.; Xue, H.; Huang, Z. Distribution and toxicity of polycyclic aromatic hydrocarbons during CaO-assisted hydrothermal carbonization of sewage sludge. Waste Manag. 2021, 120, 616–625. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, N.; Shen, Y.; Yuan, H. Towards efficient elimination of polycyclic aromatic hydrocarbons (PAHs) from waste activated sludge by ozonation. Environ. Res. 2021, 195, 110783. [Google Scholar] [CrossRef]
- Agudelo-Castañeda, D.M.; Teixeira, E.C. Seasonal changes, identification and source apportionment of PAH in PM 1.0. Atmos. Environ. 2014, 96, 186–200. [Google Scholar] [CrossRef]
- Balgobin, A.; Singh, N.R. Source apportionment and seasonal cancer risk of polycyclic aromatic hydrocarbons of sediments in a multi-use coastal environment containing a Ramsar wetland, for a Caribbean island. Sci. Total Environ. 2019, 664, 474–486. [Google Scholar] [CrossRef]
- Zhao, L.; Hou, H.; Shangguan, Y.; Cheng, B.; Xu, Y.; Zhao, R.; Zhang, Y.; Hua, X.; Huo, X.; Zhao, X. Occurrence, sources, and potential human health risks of polycyclic aromatic hydrocarbons in agricultural soils of the coal production area surrounding Xinzhou, China. Ecotoxicol. Environ. Saf. 2014, 108, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.O.; Song, W.-W.; Ma, Y.-L.; Liu, L.-Y.; Ma, W.-L.; Li, W.-L.; Li, Y.-F.; Wang, F.-Y.; Qi, M.-Y.; Lv, N.; et al. Distribution patterns, infiltration and health risk assessment of PM2.5-bound PAHs in indoor and outdoor air in cold zone. Chemosphere 2016, 155, 70–85. [Google Scholar] [CrossRef]
- Ma, W.-L.; Liu, L.-Y.; Qi, H.; Zhang, Z.-F.; Song, W.-W.; Shen, J.-M.; Chen, Z.-L.; Ren, N.-Q.; Grabuski, J.; Li, Y.-F. Polycyclic aromatic hydrocarbons in water, sediment and soil of the Songhua River Basin, China. Environ. Monit. Assess. 2013, 185, 8399–8409. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.; Wu, Q.; Kuo, D.T.F.; Chen, S.; Hu, X.; Deng, M.; Zhang, H.; Luo, M. Removal Efficiency and Risk Assessment of Polycyclic Aromatic Hydrocarbons in a Typical Municipal Wastewater Treatment Facility in Guangzhou, China. Int. J. Environ. Res. Public Health 2017, 14, 861. [Google Scholar] [CrossRef]
- Miki, S.; Uno, S.; Ito, K.; Koyama, J.; Tanaka, H. Distributions of polycyclic aromatic hydrocarbons and alkylated polycyclic aromatic hydrocarbons in Osaka Bay, Japan. Mar. Pollut. Bull. 2014, 85, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Moško, J.; Pohořelý, M.; Cajthaml, T.; Jeremiáš, M.; Robles-Aguilar, A.A.; Skoblia, S.; Beňo, Z.; Innemanová, P.; Linhartová, L.; Michalíková, K.; et al. Effect of pyrolysis temperature on removal of organic pollutants present in anaerobically stabilized sewage sludge. Chemosphere 2021, 265, 129082. [Google Scholar] [CrossRef]
- Liu, C.; Li, K.; Yu, L.; Xu, Y.; Huang, B.; Wu, J.; Wang, Z. POPs and their ecological risk in sewage sludge of waste water treatment plants in Beijing, China. Stoch. Environ. Res. Risk Assess. 2013, 27, 1575–1584. [Google Scholar] [CrossRef]
- Ju, J.-H.; Lee, I.-S.; Sim, W.-J.; Eun, H.; Oh, J.-E. Analysis and evaluation of chlorinated persistent organic compounds and PAHs in sludge in Korea. Chemosphere 2009, 74, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.-A.; Lin, M.-Q.; Shen, L.-Z.; Zhang, J.-H.; Wang, J.-Y.; Wang, Y.-J.; Yang, Z.-Y.; Liu, J.-Y. Levels, composition profiles and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in sludge from ten textile dyeing plants. Environ. Res. 2014, 132, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Jia, L.; Li, B.; Yuan, A.; Kong, L.; Qi, H.; Ma, W.; Zhang, A.; Wu, Y. The occurrence and fate of PAHs over multiple years in a wastewater treatment plant of Harbin, Northeast China. Sci. Total Environ. 2018, 624, 491–498. [Google Scholar] [CrossRef]
- Zeng, X.; Lin, Z.; Gui, H.; Shao, W.; Sheng, G.; Fu, J.; Yu, Z. Occurrence and distribution of polycyclic aromatic carbons in sludges from wastewater treatment plants in Guangdong, China. Environ. Monit. Assess. 2009, 169, 89–100. [Google Scholar] [CrossRef]
- Sánchez-Brunete, C.; Miguel, E.; Tadeo, J.L. Analysis of 27 polycyclic aromatic hydrocarbons by matrix solid-phase dispersion and isotope dilution gas chromatography-mass spectrometry in sewage sludge from the Spanish area of Madrid. J. Chromatogr. A 2007, 1148, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Mailler, R.; Gasperi, J.; Patureau, D.; Vulliet, E.; Delgenes, N.; Danel, A.; Deshayes, S.; Eudes, V.; Guerin, S.; Moilleron, R.; et al. Fate of emerging and priority micropollutants during the sewage sludge treatment: Case study of Paris conurbation. Part 1: Contamination of the different types of sewage sludge. Waste Manag. 2017, 59, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.-Q.; Wang, D.-F. Immobilization of Heavy Metals in Sewage Sludge during Land Application Process in China: A Review. Sustain. 2017, 9, 2020. [Google Scholar] [CrossRef]
- Blanchard, M.; Teil, M.J.; Ollivon, D.; Legenti, L.; Chevreuil, M. Polycyclic aromatic hydrocarbons and polychlorobiphenyls in wastewaters and sewage sludges from the Paris area (France). Environ. Res. 2004, 95, 184–197. [Google Scholar] [CrossRef]
- Aydin, Y.M.; Kara, M.; Dumanoglu, Y.; Odabasi, M.; Elbir, T. Source apportionment of polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in ambient air of an industrial region in Turkey. Atmos. Environ. 2014, 97, 271–285. [Google Scholar] [CrossRef]
- Anh, H.Q.; Minh, T.B.; Tran, T.M.; Takahashi, S. Road dust contamination by polycyclic aromatic hydrocarbons and their methylated derivatives in northern Vietnam: Concentrations, profiles, emission sources, and risk assessment. Environ. Pollut. 2019, 254, 113073. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Alam, M.S.; Harrison, R.M. Source apportionment of polycyclic aromatic hydrocarbons in urban air using positive matrix factorization and spatial distribution analysis. Atmos. Environ. 2013, 79, 271–285. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, L.; Xue, N.; Li, F.; Li, Y.; Vogt, R.D.; Cong, X.; Yan, Y.; Liu, B. Source apportionment of polycyclic aromatic hydrocarbons in soils of Huanghuai Plain, China: Comparison of three receptor models. Sci. Total Environ. 2013, 443, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Qishlaqi, A.; Beiramali, F. Potential sources and health risk assessment of polycyclic aromatic hydrocarbons in street dusts of Karaj urban area, northern Iran. J. Environ. Heal. Sci. Eng. 2019, 17, 1029–1044. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, Y.-S.; Wang, J.-Z.; Peng, S.-C.; Chen, T.-H.; Yin, D.-Q. Identification and determination of the contribution of iron–steel manufacturing industry to sediment-associated polycyclic aromatic hydrocarbons (PAHs) in a large shallow lake of eastern China. Environ. Sci. Pollut. Res. 2016, 23, 22037–22046. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Duan, Y.; Yang, Y.; Wang, X.; Tao, S. Source apportionment of polycyclic aromatic hydrocarbons in surface soil in Tianjin, China. Environ. Pollut. 2007, 147, 303–310. [Google Scholar] [CrossRef] [PubMed]



| Compounds | W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 | W10 |
|---|---|---|---|---|---|---|---|---|---|---|
| ∑PAHs | 2040 | 2790 | 1950 | 1150 | 4410 | 625 | 1410 | 1340 | 2610 | 1940 |
| ∑Me-PAHs | 106 | 252 | 224 | 132 | 425 | 228 | 147 | 116 | 90.0 | 335 |
| ∑PAHs-carc | 460 | 705 | 775 | 369 | 1710 | 213 | 419 | 386 | 610 | 396 |
| ∑LMW PAHs | 984 | 1250 | 547 | 469 | 1080 | 199 | 542 | 656 | 1450 | 1200 |
| ∑HMW PAHs | 1060 | 1540 | 1400 | 686 | 3330 | 426 | 873 | 693 | 1160 | 743 |
| ∑LMW/HMW | 0.92 | 0.81 | 0.38 | 0.68 | 0.32 | 0.46 | 0.62 | 0.94 | 1.25 | 1.61 |
| ∑LMW Me-PAHs | 77.9 | 181 | 127 | 76.7 | 323 | 127 | 98.5 | 81.7 | 65.3 | 203 |
| ∑HMW Me-PAHs | 28.9 | 70.4 | 96.9 | 54.8 | 102 | 102 | 48.7 | 34.6 | 24.6 | 132 |
| ∑LMW/HMW | 2.69 | 2.57 | 1.31 | 1.40 | 3.17 | 1.23 | 2.02 | 2.36 | 2.64 | 1.53 |
| Locations | No. of WWTPs | Sludge Types | Concentration (ng/g) | NO. of PAHs | Ref. |
|---|---|---|---|---|---|
| Harbin, Northeast (China) | 4 | Domestic/industrial | 8200 | 16 PAHs | [32] |
| Guangzhou, (China) | 10 | Domestic/industrial | 6386 | 16 PAHs | [31] |
| Taiwan (China) | 4 | Domestic | 750 | 16 PAHs | [11] |
| Hong Kong, (China) | 11 | Domestic/industrial | 30,000 | 16 PAHs | [9] |
| Beijing, (China) | 12 | Domestic/industrial | 1551 | 15 PAHs | [29] |
| Paris, (France) | 3 | Domestic/industrial | 2518 | 13 PAHs | [35] |
| Guangdong, (China) | 6 | Domestic/industrial | 3467 | 15 PAHs | [33] |
| Korea | 6 | Domestic/industrial | 10,400 | 16 PAHs | [30] |
| Guangdong, (China) | 19 | Domestic/industrial | 1276 | 15 PAHs | [7] |
| Spanish Madrid | 19 | Domestic/industrial | 5118 | 27 PAHs | [34] |
| Tunisian, Northern | 9 | Domestic/industrial | 11,216 | 16 PAHs | [14] |
| Heilongjiang (China) | 10 | Domestic/industrial | 2030 | 16 PAHs | This study |
| Heilongjiang (China) | 10 | Domestic/industrial | 202 | 33 Me-PAHs | This study |
| PAHs | TEF | W1 | W2 | W3 | W4 | W5 | W6 | W7 | W8 | W9 | W10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NaP | 0.001 | 0.29 | 0.27 | 0.22 | 0.09 | 0.16 | 0.009 | 0.21 | 0.19 | 0.64 | 0.37 |
| Acy | 0.001 | 0.05 | 0.04 | 0.02 | 0.01 | 0.02 | 0.005 | 0.01 | 0.01 | 0.03 | 0.02 |
| Ace | 0.001 | 0.03 | 0.04 | 0.02 | 0.01 | 0.03 | 0.008 | 0.01 | 0.02 | 0.05 | 0.04 |
| Flu | 0.001 | 0.13 | 0.15 | 0.06 | 0.04 | 0.09 | 0.03 | 0.04 | 0.08 | 0.19 | 0.19 |
| Phe | 0.001 | 0.43 | 0.67 | 0.18 | 0.27 | 0.67 | 0.12 | 0.22 | 0.30 | 0.48 | 0.52 |
| Ant | 0.01 | 0.36 | 0.61 | 0.24 | 0.21 | 0.85 | 0.14 | 0.13 | 0.37 | 0.48 | 0.29 |
| Fluo | 0.001 | 0.23 | 0.33 | 0.20 | 0.10 | 0.71 | 0.07 | 0.14 | 0.12 | 0.21 | 0.17 |
| Pyr | 0.001 | 0.26 | 0.35 | 0.20 | 0.12 | 0.57 | 0.07 | 0.19 | 0.11 | 0.19 | 0.15 |
| BaA | 0.1 | 6.31 | 9.69 | 4.10 | 3.14 | 23.0 | 1.89 | 4.57 | 3.04 | 6.28 | 3.08 |
| Chr | 0.01 | 0.83 | 1.05 | 1.47 | 0.57 | 3.54 | 0.33 | 0.60 | 1.18 | 1.07 | 1.04 |
| BbF | 0.1 | 11.5 | 17.6 | 22.1 | 11.4 | 38.5 | 5.31 | 11.2 | 9.64 | 17.2 | 13.0 |
| BkF | 0.1 | 3.54 | 4.26 | 4.29 | 2.87 | 9.96 | 1.22 | 2.92 | 2.74 | 3.94 | 3.24 |
| BaP | 1 | 69.3 | 98.0 | 96.7 | 44.2 | 225 | 27.4 | 67.0 | 28.0 | 78.1 | 31.8 |
| IcdP | 0.1 | 7.93 | 15.1 | 17.8 | 7.83 | 35.2 | 5.71 | 8.76 | 7.16 | 12.3 | 4.47 |
| DahA | 1 | 13.9 | 36 | 47.4 | 13.7 | 63.9 | 10.4 | 16.9 | 13.9 | 26.7 | 21.5 |
| BghiP | 0.01 | 1.05 | 1.43 | 2.15 | 0.87 | 3.25 | 0.61 | 1.18 | 0.71 | 1.39 | 0.18 |
| Mean | 0.15 | 7.26 | 11.6 | 12.3 | 5.34 | 25.3 | 3.34 | 7.13 | 4.23 | 9.34 | 5.01 |
| Min | 0.001 | 0.03 | 0.04 | 0.02 | 0.01 | 0.02 | 0.005 | 0.01 | 0.01 | 0.03 | 0.02 |
| Max | 1 | 69.3 | 98.0 | 96.7 | 44.2 | 225 | 27.4 | 67.0 | 28.0 | 78.1 | 31.8 |
| ∑ PAHscarc | 2.42 | 113 | 181 | 194 | 83.8 | 399 | 52.3 | 112 | 66.7 | 145 | 78.2 |
| ∑ 16 PAHs | 2.43 | 116 | 185 | 197 | 85.5 | 406 | 53.4 | 114 | 67.7 | 149 | 80.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, R.; Zhang, Z.-F.; Kan, Z.; Jiang, C.; Liu, L.-Y.; Ma, W.-L.; Song, W.-W.; Nikolaev, A.; Li, Y.-F. Determination of Polycyclic Aromatic Hydrocarbons and Their Methylated Derivatives in Sewage Sludge from Northeastern China: Occurrence, Profiles and Toxicity Evaluation. Molecules 2021, 26, 2739. https://doi.org/10.3390/molecules26092739
Mohammed R, Zhang Z-F, Kan Z, Jiang C, Liu L-Y, Ma W-L, Song W-W, Nikolaev A, Li Y-F. Determination of Polycyclic Aromatic Hydrocarbons and Their Methylated Derivatives in Sewage Sludge from Northeastern China: Occurrence, Profiles and Toxicity Evaluation. Molecules. 2021; 26(9):2739. https://doi.org/10.3390/molecules26092739
Chicago/Turabian StyleMohammed, Rashid, Zi-Feng Zhang, Ze Kan, Chao Jiang, Li-Yan Liu, Wan-Li Ma, Wei-Wei Song, Anatoly Nikolaev, and Yi-Fan Li. 2021. "Determination of Polycyclic Aromatic Hydrocarbons and Their Methylated Derivatives in Sewage Sludge from Northeastern China: Occurrence, Profiles and Toxicity Evaluation" Molecules 26, no. 9: 2739. https://doi.org/10.3390/molecules26092739
APA StyleMohammed, R., Zhang, Z.-F., Kan, Z., Jiang, C., Liu, L.-Y., Ma, W.-L., Song, W.-W., Nikolaev, A., & Li, Y.-F. (2021). Determination of Polycyclic Aromatic Hydrocarbons and Their Methylated Derivatives in Sewage Sludge from Northeastern China: Occurrence, Profiles and Toxicity Evaluation. Molecules, 26(9), 2739. https://doi.org/10.3390/molecules26092739








