Serial Hydrolysis for the Simultaneous Analysis of Catecholamines and Steroids in the Urine of Patients with Alopecia Areata
Abstract
1. Introduction
2. Results
2.1. Optimization of the Dansyl Chloride Derivatization Procedure
2.2. Optimization of the Sample Preparation Procedure
2.3. Liquid Chromatography–Tandem Mass Spectrometry
2.4. Method Validation
2.5. Application
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of the Standard Solutions
3.3. Liquid Chromatography–Tandem Mass Spectrometry
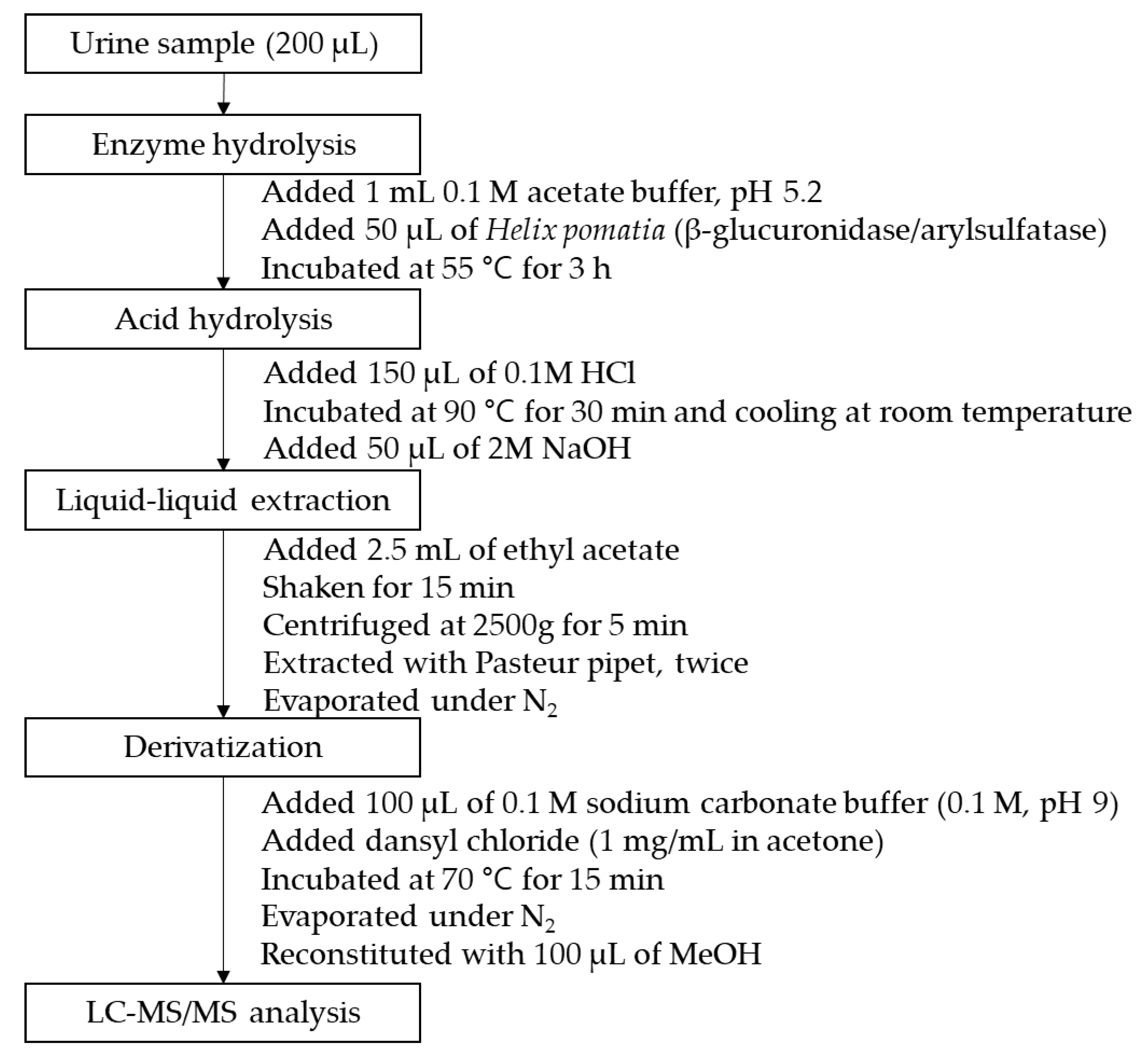
3.4. Sample Preparation
3.5. Sample Information
3.6. Validation
3.7. Creatinine Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wasserman, D.; Guzman-Sanchez, D.A.; Scott, K.; McMichael, A. Alopecia areata. Int. J. Dermatol. 2007, 46, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Hordinsky, M.K. Overview of alopecia areata. J. Investig. Dermatol. Symp. Proc. 2013, 16, S13–S15. [Google Scholar] [CrossRef]
- Liakopoulou, M.; Alifieraki, T.; Katideniou, A.; Kakourou, T.; Tselalidou, E.; Tsiantis, J.; Stratigos, J. Children with alopecia areata: Psychiatric symptomatology and life events. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 678–684. [Google Scholar] [CrossRef]
- Willemsen, R.; Vanderlinden, J.; Roseeuw, D.; Haentjens, P. Increased history of childhood and lifetime traumatic events among adults with alopecia areata. J. Am. Acad. Dermatol. 2009, 60, 388–393. [Google Scholar] [CrossRef]
- Pratt, C.H.; King, L.E., Jr.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia areata. Nat. Rev. Dis. Primers 2017, 3, 17011. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, M.M.; Urry, F.M.; Frank, E.L.; Roberts, W.L.; Shushan, B. Analysis of catecholamines in urine by positive-ion electrospray tandem mass spectrometry. Clin. Chem. 2002, 48, 323–331. [Google Scholar] [CrossRef]
- Lee, W.; Park, N.H.; Ahn, T.B.; Chung, B.C.; Hong, J. Profiling of a wide range of neurochemicals in human urine by very-high-performance liquid chromatography-tandem mass spectrometry combined with in situ selective derivatization. J. Chromatogr. A 2017, 1526, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Dimsdale, J.E.; Moss, J. Short-term catecholamine response to psychological stress. Psychosom. Med. 1980, 42, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Atienza, F.; Gurpegui, M. Environmental stress but not subjective distress in children or adolescents with alopecia areata. J. Psychosom. Res. 2011, 71, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.M.; Purdy, R.H. Neuroactive steroids. FASEB J. 1992, 6, 2311–2322. [Google Scholar] [CrossRef] [PubMed]
- Heiblum, R.; Arnon, E.; Gvaryahu, G.; Robinzon, B.; Snapir, N. Short-term stress increases testosterone secretion from testes in male domestic fowl. Gen. Comp. Endocrinol. 2000, 120, 55–66. [Google Scholar] [CrossRef]
- Wirth, M.M. Beyond the HPA Axis: Progesterone-Derived Neuroactive Steroids in Human Stress and Emotion. Front. Endocrinol. 2011, 2, 19. [Google Scholar] [CrossRef]
- Lee, Y.R.; Kim, H.; Lew, B.L.; Sim, W.Y.; Lee, J.; Oh, H.B.; Hong, J.; Chung, B.C. Sex-related differences in urinary immune-related metabolic profiling of alopecia areata patients. Metabolomics 2020, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- Peitzsch, M.; Pelzel, D.; Glockner, S.; Prejbisz, A.; Fassnacht, M.; Beuschlein, F.; Januszewicz, A.; Siegert, G.; Eisenhofer, G. Simultaneous liquid chromatography tandem mass spectrometric determination of urinary free metanephrines and catecholamines, with comparisons of free and deconjugated metabolites. Clin. Chim. Acta 2013, 418, 50–58. [Google Scholar] [CrossRef]
- Gomes, R.L.; Meredith, W.; Snape, C.E.; Sephton, M.A. Analysis of conjugated steroid androgens: Deconjugation, derivatisation and associated issues. J. Pharm. Biomed. Anal. 2009, 49, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Im, E.; Lew, B.L.; Lee, M.Y.; Lee, J.; Paeng, K.J.; Chung, B.C. Simultaneous determination of androgens and prostaglandins in human urine using ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2019, 1109, 45–53. [Google Scholar] [CrossRef]
- Lee, Y.R.; Lee, J.W.; Hong, J.; Chung, B.C. Simultaneous Determination of Polyamines and Steroids in Human Serum from Breast Cancer Patients Using Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2021, 26, 1153. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Q.; Li, N.; Ling, J.; Liu, R.; Wang, Y.; Sun, L.; Chen, X.H.; Bi, K. Simultaneous determination of catecholamines and their metabolites related to Alzheimer’s disease in human urine. J. Sep. Sci. 2011, 34, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Pesek, J.J.; Matyska, M.T.; Fischer, S.M.; Sana, T.R. Analysis of hydrophilic metabolites by high-performance liquid chromatography-mass spectrometry using a silica hydride-based stationary phase. J. Chromatogr. A 2008, 1204, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Komarneni, P.; Kandikere, V.; Boggavarapu, R.; Bhyrapuneni, G.; Benade, V.; Gorentla, S. A sensitive and selective quantification of catecholamine neurotransmitters in rat microdialysates by pre-column dansyl chloride derivatization using liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2013, 913, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.L.; Zhu, R.H.; Li, H.D. Determination of dansylated monoamine and amino acid neurotransmitters and their metabolites in human plasma by liquid chromatography-electrospray ionization tandem mass spectrometry. Anal. Biochem. 2010, 396, 103–111. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.G.; Yang, C.H.; Lee, S. Silica stationary phase-based on-line sample enrichment coupled with LC-MS/MS for the quantification of dopamine, serotonin and their metabolites in rat brain microdialysates. Anal. Chim. Acta 2016, 923, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Linnoila, M.; Karoum, F.; Miller, T.; Potter, W.Z. Reliability of urinary monoamine and metabolite output measurements in depressed patients. Am. J. Psychiatry 1983, 140, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Linnoila, M.; Oliver, J.; Adinoff, B.; Potter, W.Z. High correlations of norepinephrine, dopamine, and epinephrine and their major metabolite excretion rates. Arch. Gen. Psychiatry 1988, 45, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Linnoila, M.; Karoum, F.; Pickar, D. Urinary excretion of free tyramine and of norepinephrine and its metabolites in unipolar depressed patients. Biol. Psychiatry 1986, 21, 221–224. [Google Scholar] [CrossRef]
- Free, M.L.; Oei, T.P.; Appleton, C. Biological and psychological processes in recovery from depression during cognitive therapy. J. Behav. Ther. Exp. Psychiatry 1998, 29, 213–226. [Google Scholar] [CrossRef]
- Kook, P.H.; Boretti, F.S.; Hersberger, M.; Glaus, T.M.; Reusch, C.E. Urinary catecholamine and metanephrine to creatinine ratios in healthy dogs at home and in a hospital environment and in 2 dogs with pheochromocytoma. J. Vet. Intern. Med. 2007, 21, 388–393. [Google Scholar] [CrossRef]
- Naccarato, A.; Gionfriddo, E.; Sindona, G.; Tagarelli, A. Development of a simple and rapid solid phase microextraction-gas chromatography-triple quadrupole mass spectrometry method for the analysis of dopamine, serotonin and norepinephrine in human urine. Anal. Chim. Acta 2014, 810, 17–24. [Google Scholar] [CrossRef]
- Peterson, Z.D.; Collins, D.C.; Bowerbank, C.R.; Lee, M.L.; Graves, S.W. Determination of catecholamines and metanephrines in urine by capillary electrophoresis-electrospray ionization-time-of-flight mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002, 776, 221–229. [Google Scholar] [CrossRef]
- Moon, J.Y.; Kwon, W.; Suh, S.; Cheong, J.C.; In, M.K.; Chung, B.C.; Kim, J.Y.; Choi, M.H. Reference ranges for urinary levels of testosterone and epitestosterone, which may reveal gonadal function, in a Korean male population. J. Steroid Biochem. Mol. Biol. 2014, 140, 100–105. [Google Scholar] [CrossRef]
- Newman, M.; Pratt, S.M.; Curran, D.A.; Stanczyk, F.Z. Evaluating urinary estrogen and progesterone metabolites using dried filter paper samples and gas chromatography with tandem mass spectrometry (GC-MS/MS). BMC Chem. 2019, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- van de Merbel, N.C. Quantitative determination of endogenous compounds in biological samples using chromatographic techniques. TrAC Trends Anal. Chem. 2008, 27, 924–933. [Google Scholar] [CrossRef]

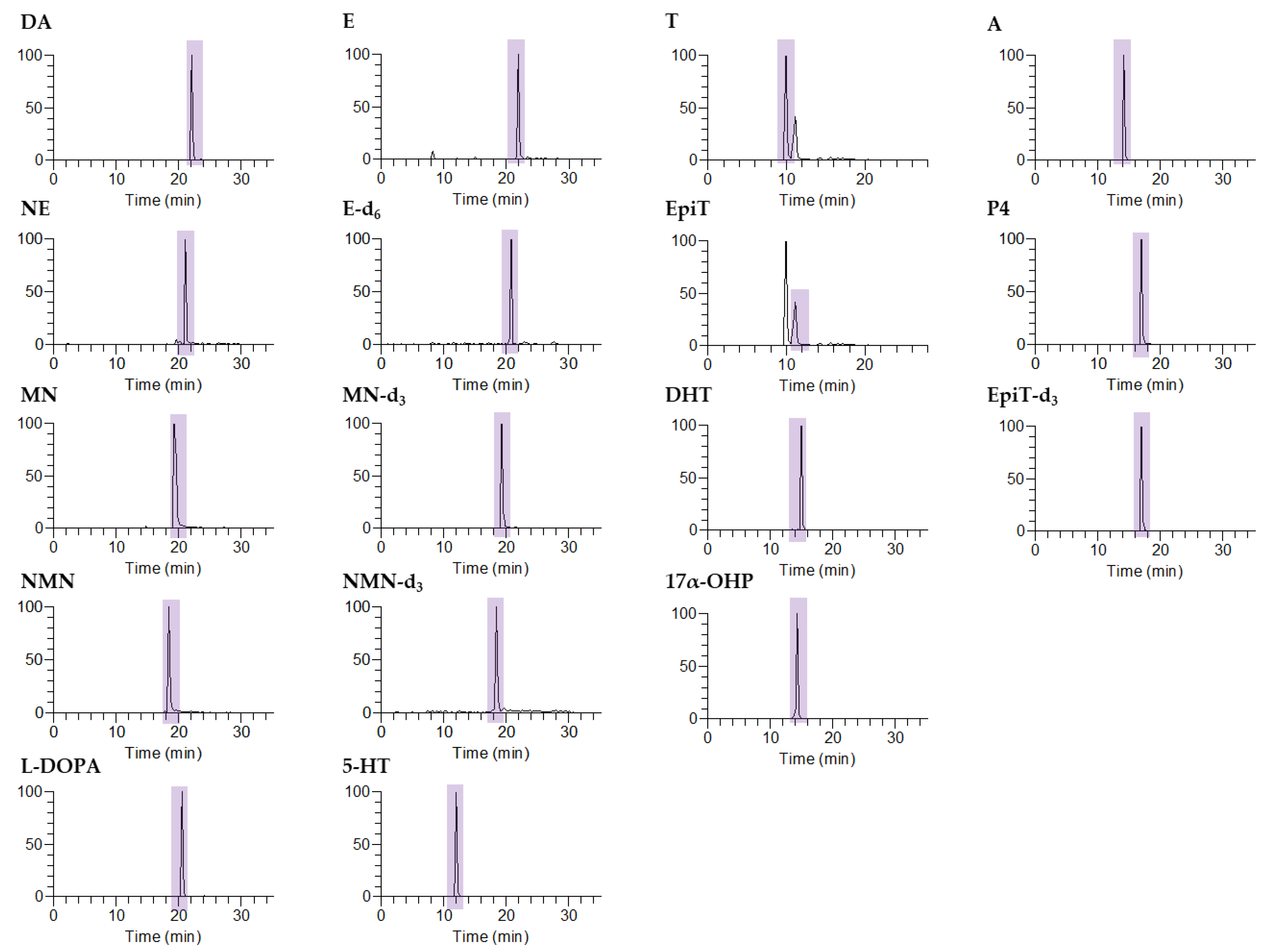
| Compound | Abbrev. | Precursor Ion (m/z) | Product Ion (m/z) | Normalized Collision Energy (%) | Retention Time (min) |
|---|---|---|---|---|---|
| Catecholamines | |||||
| Dopamine | DA | 853 | 619 | 39 | 22.1 |
| Norepinephrine | NE | 869 | 851 | 32 | 21 |
| Normetanephrine | NMN | 650 | 632 | 30 | 18.1 |
| Metanephrine | MN | 664 | 646 | 31 | 19.2 |
| L-3,4-dihydroxyphenylalanine | L-DOPA | 897 | 663 | 30 | 20.5 |
| Epinephrine | E | 883 | 865 | 27 | 21.9 |
| Serotonin | 5-HT | 410 | 160 | 25 | 11.9 |
| Steroids | |||||
| Testosterone | T | 289.2 | 271.3 | 56 | 13.4 |
| Dihydrotestosterone | DHT | 291.2 | 255.3 | 24 | 14.9 |
| Epitestosterone | EpiT | 289.2 | 271.3 | 26 | 14 |
| Androstenedione | A | 287.2 | 269.3 | 48 | 13.8 |
| Progesterone | P4 | 315.2 | 297.3 | 30 | 16.6 |
| 17α-Hydroxyprogesterone | 17α-OHP | 331.2 | 313.3 | 22 | 13.6 |
| Internal standards | |||||
| Metanephrine-d3 | MN-d3 | 667 | 649 | 27 | 19.2 |
| Normetanephrine-d3 | NMN-d3 | 653 | 635 | 25 | 18.1 |
| Epinephrine-d6 | E-d6 | 889 | 871 | 31 | 20.7 |
| Epitestosterone-d3 | EpiT-d3 | 292.2 | 256.4 | 35 | 14.6 |
| Analytes | Spiked Concentration (ng/mL) | Intra-Day Assay | Inter-Day Assay | ||
|---|---|---|---|---|---|
| Amount Found (Mean ± SD) | % CV | Amount Found (Mean ± SD) | % CV | ||
| DA | 10 | 10.88 ± 0.66 | 6 | 10.27 ± 1.72 | 16.7 |
| 50 | 51.39 ± 9.85 | 19.2 | 53.04 ± 6.8 | 12.8 | |
| 100 | 102.25 ± 8.4 | 8.2 | 89.92 ± 7.26 | 8.1 | |
| 500 | 520.75 ± 75.44 | 14.5 | 454.32 ± 70.47 | 15.5 | |
| 5000 | 5202.45 ± 122.27 | 2.4 | 4919.39 ± 393.18 | 8 | |
| NE | 10 | 8.43 ± 0.67 | 8 | 11.25 ± 0.7 | 6.2 |
| 50 | 49.03 ± 3.18 | 6.5 | 51.31 ± 5.17 | 10.1 | |
| 100 | 96.86 ± 14.78 | 15.3 | 100.83 ± 11.57 | 11.5 | |
| 500 | 503.66 ± 85.16 | 16.9 | 439.01 ± 32.12 | 7.3 | |
| 5000 | 5426.45 ± 69.68 | 1.3 | 4733.22 ± 532.58 | 11.3 | |
| MN | 10 | 10.53 ± 1.22 | 11.6 | 9.01 ± 0.49 | 5.5 |
| 50 | 53.01 ± 6.51 | 12.3 | 54.11 ± 5.73 | 10.6 | |
| 100 | 95.77 ± 17 | 17.8 | 95.6 ± 11.06 | 11.6 | |
| 500 | 508.78 ± 58.68 | 11.5 | 440.56 ± 54.75 | 12.4 | |
| 5000 | 4774.86 ± 103.34 | 2.2 | 4863 ± 103.34 | 2.1 | |
| NMN | 10 | 10.32 ± 0.38 | 3.7 | 10.24 ± 1.07 | 10.5 |
| 50 | 45.79 ± 3.14 | 6.9 | 51 ± 7.04 | 13.8 | |
| 100 | 99.69 ± 15.36 | 15.4 | 95.12 ± 8.21 | 8.6 | |
| 500 | 519.83 ± 75.53 | 14.5 | 399.37 ± 24.48 | 6.1 | |
| 5000 | 4652.22 ± 507.11 | 10.9 | 5265.81 ± 885.64 | 16.8 | |
| L-DOPA | 50 | 50.5 ± 8.18 | 16.2 | 58.1 ± 1.47 | 2.5 |
| 100 | 97 ± 3.71 | 3.8 | 103.71 ± 10.52 | 10.1 | |
| 500 | 532.9 ± 60.88 | 11.4 | 501.39 ± 59.99 | 12 | |
| 5000 | 5483.72 ± 232.77 | 4.2 | 4204.8 ± 346.51 | 8.2 | |
| E | 10 | 10.52 ± 1.32 | 12.5 | 8.51 ± 0.66 | 7.7 |
| 50 | 55.47 ± 8.85 | 16 | 46.82 ± 8.09 | 17.3 | |
| 100 | 91.65 ± 11.73 | 12.8 | 105.4 ± 20.52 | 19.5 | |
| 500 | 427.72 ± 20.21 | 4.7 | 436.41 ± 15.01 | 3.4 | |
| 5000 | 4767.63 ± 300.46 | 6.3 | 4961.5 ± 390.27 | 7.9 | |
| 5-HT | 10 | 9.37 ± 0.84 | 9 | 10.03 ± 1.29 | 12.8 |
| 50 | 43.56 ± 2.48 | 5.7 | 48.64 ± 4.73 | 9.7 | |
| 100 | 103.13 ± 12.04 | 11.7 | 98.7 ± 3.19 | 3.2 | |
| 500 | 523.62 ± 57.83 | 11 | 506.1 ± 80.12 | 15.8 | |
| 5000 | 5278.65 ± 345.51 | 6.6 | 5390.38 ± 723.93 | 13.4 | |
| T | 10 | 10.59 ± 1.18 | 11.1 | 10.41 ± 0.32 | 3.1 |
| 50 | 48.34 ± 5.61 | 11.6 | 52.53 ± 6.95 | 13.2 | |
| 100 | 104.83 ± 8.02 | 7.7 | 97.14 ± 5.03 | 5.2 | |
| 500 | 474.64 ± 31.72 | 6.7 | 473.68 ± 28.46 | 6 | |
| 5000 | 4185.64 ± 129.65 | 3.1 | 4643.86 ± 210.06 | 4.5 | |
| EpiT | 10 | 9.66 ± 0.35 | 3.6 | 11.56 ± 1.7 | 14.7 |
| 50 | 46.56 ± 6.46 | 13.9 | 52.8 ± 6.16 | 11.7 | |
| 100 | 99.27 ± 16.46 | 16.6 | 82.6 ± 13.4 | 16.2 | |
| 500 | 465.64 ± 59.24 | 12.7 | 504.23 ± 85.7 | 17 | |
| 5000 | 5738.34 ± 24.61 | 0.4 | 5116.56 ± 790.31 | 15.5 | |
| DHT | 10 | 9.79 ± 0.44 | 4.5 | 10.35 ± 0.23 | 2.3 |
| 50 | 47.93 ± 6.02 | 12.6 | 47.45 ± 6.83 | 14.4 | |
| 100 | 105.11 ± 6.83 | 6.5 | 101 ± 1.97 | 2 | |
| 500 | 505.37 ± 91.54 | 18.1 | 484.7 ± 32.76 | 6.8 | |
| 5000 | 5181.97 ± 1111.79 | 21.5 | 5655.49 ± 236.7 | 4.2 | |
| 17α-OHP | 10 | 10.25 ± 1.47 | 14.4 | 10.56 ± 1.04 | 9.9 |
| 50 | 51.04 ± 7.45 | 14.6 | 50.53 ± 2.93 | 5.8 | |
| 100 | 98.82 ± 6.47 | 6.6 | 86.37 ± 2.88 | 3.3 | |
| 500 | 426.97 ± 23.82 | 5.6 | 500.5 ± 72.76 | 14.5 | |
| 5000 | 5058.79 ± 960.5 | 19 | 4784.76 ± 1032.4 | 21.6 | |
| A | 10 | 10.12 ± 1.49 | 14.7 | 9.21 ± 0.7 | 7.7 |
| 50 | 54.6 ± 7.59 | 13.9 | 49.93 ± 8.19 | 16.4 | |
| 100 | 111.76 ± 3.53 | 3.2 | 112.89 ± 14.08 | 12.5 | |
| 500 | 528.46 ± 65.34 | 12.4 | 468.24 ± 51.44 | 11 | |
| 5000 | 5247.42 ± 558.88 | 10.7 | 4634.58 ± 475.97 | 10.3 | |
| P4 | 10 | 9.25 ± 0.77 | 8.3 | 9.49 ± 0.81 | 8.5 |
| 50 | 52.23 ± 9.67 | 18.5 | 58.33 ± 3.22 | 5.5 | |
| 100 | 103.54 ± 13.58 | 13.1 | 100.94 ± 17.48 | 17.3 | |
| 500 | 433.69 ± 40.93 | 9.4 | 413.79 ± 14.05 | 3.4 | |
| 5000 | 4717.45 ± 645.9 | 13.7 | 5097.21 ± 501.55 | 9.8 | |
| Normal Controls (n = 29) | Patients (n = 40) | p Value | |||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| DA | 202.23 ± 132.6 | 31.26–507.96 | 147.47 ± 216.85 | 8.28–903.06 | 0.302 |
| NE | 125.88 ± 210.98 | 8.73–1052.7 | 115.9 ± 222.37 | 1.29–1261.24 | 0.856 |
| MN | 48.88 ± 115.67 | 3.87–612.13 | 134.72 ± 183.23 | 5.45–720 | 0.036 |
| NMN | 27.99 ± 50.52 | 2.1–239.32 | 81.43 ± 109.72 | 1.49–444.94 | 0.053 |
| L-DOPA | 236.64 ± 299.21 | 14.36–1437.92 | 222.46 ± 625.27 | 1.89–3530.4 | 0.914 |
| E | 29.44 ± 35.7 | 5.33–181.96 | 36.47 ± 72.21 | 1.04–296.97 | 0.644 |
| 5-HT | 203.67 ± 259.88 | 6.51–1082.05 | 112.19 ± 156.61 | 2.57–574.75 | 0.317 |
| T | 42.16 ± 114.11 | 2.25–508.47 | 22.76 ± 43.8 | 1.94–243.06 | 0.397 |
| EpiT | 42.53 ± 76.91 | 4.04–410.4 | 24.69 ± 24.56 | 1.86–105.89 | 0.201 |
| DHT | 26.69 ± 41.71 | 2.06–171.75 | 15.75 ± 17.66 | 1.12–66.46 | 0.232 |
| 17α-OHP | 12.29 ± 15.66 | 1.16–58.77 | 7.48 ± 6.45 | 1.22–33.13 | 0.117 |
| A | 10.27 ± 19.88 | 1.65–88.2 | 5.54 ± 5.77 | 1.25–22.65 | 0.284 |
| P4 | 8.96 ± 8.28 | 1.75–31.17 | 13.42 ± 15.22 | 1.18–71.32 | 0.248 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-R.; Lew, B.-L.; Sim, W.-Y.; Hong, J.; Chung, B.-C. Serial Hydrolysis for the Simultaneous Analysis of Catecholamines and Steroids in the Urine of Patients with Alopecia Areata. Molecules 2021, 26, 2734. https://doi.org/10.3390/molecules26092734
Lee Y-R, Lew B-L, Sim W-Y, Hong J, Chung B-C. Serial Hydrolysis for the Simultaneous Analysis of Catecholamines and Steroids in the Urine of Patients with Alopecia Areata. Molecules. 2021; 26(9):2734. https://doi.org/10.3390/molecules26092734
Chicago/Turabian StyleLee, Yu-Ra, Bark-Lynn Lew, Woo-Young Sim, Jongki Hong, and Bong-Chul Chung. 2021. "Serial Hydrolysis for the Simultaneous Analysis of Catecholamines and Steroids in the Urine of Patients with Alopecia Areata" Molecules 26, no. 9: 2734. https://doi.org/10.3390/molecules26092734
APA StyleLee, Y.-R., Lew, B.-L., Sim, W.-Y., Hong, J., & Chung, B.-C. (2021). Serial Hydrolysis for the Simultaneous Analysis of Catecholamines and Steroids in the Urine of Patients with Alopecia Areata. Molecules, 26(9), 2734. https://doi.org/10.3390/molecules26092734






