The Anti-Leukemic Activity of Natural Compounds
Abstract
1. Introduction
2. Natural Compounds in Acute Myeloid Leukemia (AML)
3. Natural Compounds in Chronic Myeloid Leukemia (CML)
4. Natural Compounds in Acute Lymphoblastic Leukemia (ALL)
5. Natural Compounds in Chronic Lymphocytic Leukemia (CLL)
6. Clinical Trials and Synergic Activity with Conventional Anti-Leukemic Drugs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Talib, W.H.; Alsalahat, I.; Daoud, S.; Abutayeh, R.F.; Mahmod, A.I. Plant-derived natural products in cancer research: Extraction, mechanism of action, and drug formulation. Molecules 2020, 25, 5319. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Watts, J.; Nimer, S. Recent advances in the understanding and treatment of acute myeloid leukemia. F1000Research 2018, 7, 1196. [Google Scholar] [CrossRef]
- Rafiq, S.; Raza, M.H.; Younas, M.; Naeem, F.; Adeeb, R.; Iqbal, J.; Anwar, P.; Sajid, U.; Manzoor, H.M. Molecular targets of curcumin and future therapeutic role in leukemia. JBM 2018, 6, 33–50. [Google Scholar] [CrossRef]
- Naimi, A.; Entezari, A.; Hagh, M.F.; Hassanzadeh, A.; Saraei, R.; Solali, S. Quercetin sensitizes human myeloid leukemia KG-1 cells against TRAIL-induced apoptosis. J. Cell Physiol. 2019, 234, 13233–13241. [Google Scholar] [CrossRef] [PubMed]
- Kayser, S.; Levis, M.J. Advances in targeted therapy for acute myeloid leukaemia. Br. J. Haematol. 2018, 180, 484–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gu, Y.; Chen, B. Mechanisms of drug resistance in acute myeloid leukemia. Onco Targets Ther. 2019, 12, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Crossnohere, N.L.; Richardson, D.R.; Reinhart, C.; O’Donoghue, B.; Love, S.M.; Smith, B.D.; Bridges, J.F.P. Side effects from acute myeloid leukemia treatment: Results from a national survey. Curr. Med. Res. Opin. 2019, 35, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, L.; Shu, Q.; Li, D.; Wang, R. Leukemia stem cells promote chemoresistance by inducing downregulation of lumican in mesenchymal stem cells. Oncol. Lett. 2019, 18, 4317–4327. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Campbell, S.; Abdel-Rahman, F.; Whaley, S.; Stone, W.L. Cancer chemoprevention drug targets. Curr. Drug Targets 2003, 4, 45–54. [Google Scholar] [CrossRef]
- Raguz, S.; Yagüe, E. Resistance to chemotherapy: New treatments and novel insights into an old problem. Br. J. Cancer 2008, 99, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.J.; Zhang, L.; Zhang, L.; Wan, J.; Song, W.; Jiang, X.; Park, Y.D.; Si, Y.X. Metabolic responses and arginine kinase expression of juvenile cuttlefish (Sepia pharaonis) under salinity stress. Int. J. Biol. Macromol. 2018, 113, 881–888. [Google Scholar] [CrossRef]
- Avato, P.; Migoni, D.; Argentieri, M.; Fanizzi, F.P.; Tava, A. Activity of saponins from Medicago species against HeLa and MCF-7 cell lines and their capacity to potentiate cisplatin effect. Anti Cancer Agents Med. Chem. 2017, 17, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Vishwakarma, R.A.; Bharate, S.B. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur. J. Med. Chem. 2017, 138, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Oberley, T.D.; Oberley, L.W. Antioxidant enzyme level in cancer. Histol. Histopathol. 1997, 12, 525–535. [Google Scholar]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef]
- Chabner, B.A. Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 11th ed.; Brunton, L.L., Lazo, J.S., Parker, K.L., Eds.; McGraw-Hill: New York, NY, USA, 2006; pp. 1257–1262. [Google Scholar] [CrossRef]
- Guérritte, F. Anticancer Agents from Natural Products, 1st ed.; Cragg, G.M., Kingston, D.G.I., Newman, D.J., Eds.; CRC/Taylor & Francis Press: Boca Raton, FL, USA, 2005; pp. 123–135. [Google Scholar] [CrossRef]
- Lee, K.H. Anticancer Agents from Natural Products, 1st ed.; Cragg, G.M., Kingston, D.G.I., Newman, D.J., Eds.; CRC/Taylor & Francis Press: Boca Raton, FL, USA, 2005; pp. 71–87. [Google Scholar] [CrossRef]
- Hande, K.R. Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer. 1998, 34, 1514–1521. [Google Scholar] [CrossRef]
- Silalahi, J. Anticancer and health protective properties of citrus fruit components. Asia Pac. J. Clin. Nutr. 2002, 11, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Kellof, G.J. Perspective on cancer chemoprevention research and drug development. Adv. Cancer Res. 2000, 78, 199–334. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Fratantonio, D.; Hasan, M.M.; Sharifi, S.; Fathi, N.; Ullah, H.; Rastrelli, L. Apoptosis induced by luteolin in breast cancer: Mechanistic and therapeutic perspectives. Phytomedicine 2019, 59, 152883. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxid. Med. Cell Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [PubMed]
- Ornano, L.; Venditti, A.; Donno, Y.; Sanna, C.; Ballero, M.; Bianco, A. Phytochemical analysis of non-volatile fraction of Artemisia caerulescens subsp. densiflora (Viv.) (Asteraceae), an endemic species of La Maddalena Archipelago (Sardinia–Italy). Nat. Prod. Res. 2016, 30, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Maggi, F.; Vittori, S.; Papa, F.; Serrilli, A.M.; Di Cecco, M.; Bianco, A. Antioxidant and α-glucosidase inhibitory activities of Achillea tenorii. Pharm. Biol. 2015, 53, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Guarcini, L.; Bianco, A.; Rosselli, S.; Bruno, M.; Senatore, F. Phytochemical analysis of Achillea ligustica all. from Lipari Island (Aeolian islands). Nat. Prod. Res. 2016, 30, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; Del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Venditti, A.; Frezza, C.; Sciubba, F.; Serafini, M.; Bianco, A.; Cianfaglione, K.; Maggi, F. Volatile components, polar constituents and biological activity of tansy daisy (Tanacetum macrophyllum (Waldst. et Kit.) Schultz Bip. Ind. Crop. Prod. 2018, 118, 225–235. [Google Scholar] [CrossRef]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef]
- Russo, E.R.; Facincani, I.; Nakazato, K.C.; Coimbra, T.M.; Crevelin, E.J.; Pereira, A.M.S.; Carmona, F. Oral administration of powdered dried rhizomes of Curcuma longa L. (turmeric, Zingiberaceae) is effective in the treatment of doxorubicin-induced kidney injury in rats. Phytother. Res. 2018, 32, 2408–2416. [Google Scholar] [CrossRef]
- Arroo, R.R.J.; Alfa, H.H. Chemical properties of thymoquinone, a monoterpene isolated from the seeds of Nigella sativa Linn. Pharmacol Res. 2018, 133, 151. [Google Scholar] [CrossRef]
- Pang, J.; Shen, N.; Yan, F.; Zhao, N.; Dou, L.; Wu, L.C.; Seiler, C.L.; Yu, L.; Yang, K.; Bachanova, V.; et al. Thymoquinone exerts potent growth-suppressive activity on leukemia through DNA hypermethylation reversal in leukemia cells. Oncotarget 2017, 8, 34453–34467. [Google Scholar] [CrossRef]
- Tang, T.; Yin, L.; Yang, J.; Shan, G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur. J. Pharmacol. 2007, 567, 177–185. [Google Scholar] [CrossRef]
- Chen, Y.; Gan, D.; Huang, Q.; Luo, X.; Lin, D.; Hu, J. Emodin and its combination with cytarabine induce apoptosis in resistant acute myeloid leukemia cells in vitro and in vivo. Cell Physiol. Biochem. 2018, 48, 2061–2073. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Czyz, M. Parthenolide as cooperating agent for anti-cancer treatment of various malignancies. Pharmaceuticals 2020, 13, 194. [Google Scholar] [CrossRef]
- Shi, H.; Li, X.Y.; Chen, Y.; Zhang, X.; Wu, Y.; Wang, Z.X.; Chen, P.H.; Dai, H.Q.; Feng, J.; Chatterjee, S.; et al. Quercetin induces apoptosis via downregulation of vascular endothelial growth factor/Akt signaling pathway in acute myeloid leukemia cells. Front. Pharmacol. 2020, 11, 534171. [Google Scholar] [CrossRef]
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute myeloid leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Calgarotto, A.K.; Maso, V.; Junior, G.C.F.; Nowill, A.E.; Filho, P.L.; Vassallo, J.; Saad, S.T.O. Antitumor activities of quercetin and green tea in xenografts of human leukemia HL60 cells. Sci. Rep. 2018, 8, 3459. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.; Buechner, T.; Dombret, H.; Ebert, B.; Fenaux, P.; Larson, R.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.L.; Rossi, R.M.; Karnischky, L.; Li, X.; Peterson, D.R.; Howard, D.S.; Jordan, C.T. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005, 105, 4163–4169. [Google Scholar] [CrossRef] [PubMed]
- Kouhpeikar, H.; Butler, A.E.; Bamian, F.; Barreto, G.E.; Majeed, M.; Sahebkar, A. Curcumin as a therapeutic agent in leukemia. J. Cell Physiol. 2019, 234, 12404–12414. [Google Scholar] [CrossRef] [PubMed]
- Pesakhov, S.; Khanin, M.; Studzinski, G.P.; Danilenko, M. Distinct combinatorial effects of the plant polyphenols curcumin, carnosic acid, and silibinin on proliferation and apoptosis in acute myeloid leukemia cells. Nutr. Cancer 2010, 62, 811–824. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Jiang, L.; Lin, X.; Tseng, K.F.; Lu, Z.; Wang, X. Luteolin, a novel p90 ribosomal S6 kinase inhibitor, suppresses proliferation and migration in leukemia cells. Oncol. Lett. 2017, 13, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.W.; Kong, J.Y.; Han, S.Y. Flavonoids as receptor tyrosine kinase FLT3 inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 1768–1770. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Hsiao, M.; Chang, J.L.; Yang, S.F.; Tseng, T.H.; Cheng, C.W.; Chow, J.M.; Lin, K.H.; Lin, Y.W.; Liu, C.C.; et al. Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenograft. Arch. Toxicol. 2015, 89, 1103–1117. [Google Scholar] [CrossRef]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef]
- Ren, M.X.; Deng, X.H.; Ai, F.; Yuan, G.Y.; Song, H.Y. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro. Exp. Ther. Med. 2015, 10, 579–583. [Google Scholar] [CrossRef]
- Lee, W.J.; Chen, Y.R.; Tseng, T.H. Quercetin induces FasL-related apoptosis, in part, through promotion of histone H3 acetylation in human leukemia HL-60 cells. Oncol. Rep. 2011, 25, 583–591. [Google Scholar] [CrossRef]
- Avci, C.B.; Yilmaz, S.; Dogan, Z.O.; Saydam, G.; Dodurga, Y.; Ekiz, H.A.; Kartal, M.; Sahin, F.; Baran, Y.; Gunduz, C. Quercetin-induced apoptosis involves increased hTERT enzyme activity of leukemic cells. Hematology 2011, 16, 303–307. [Google Scholar] [CrossRef]
- Larocca, L.M.; Teofili, L.; Leone, G.; Sica, S.; Pierelli, L.; Menichella, G.; Scambia, G.; Benedetti Panici, P.; Ricci, R.; Piantelli, M.; et al. Antiproliferative activity of quercetin on normal bone marrow and leukaemic progenitors. Br. J. Haematol. 1991, 79, 562–566. [Google Scholar] [CrossRef]
- Yuan, B.; Okusumi, S.; Yoshino, Y.; Moriyama, C.; Tanaka, S.; Hirano, T.; Takagi, N.; Toyoda, H. Delphinidin induces cytotoxicity and potentiates cytocidal effect in combination with arsenite in an acute promyelocytic leukemia NB4 cell line. Oncol. Rep. 2015, 34, 431–438. [Google Scholar] [CrossRef]
- Raynal, N.J.; Momparler, L.; Charbonneau, M.; Momparler, R.L. Antileukemic activity of genistein, a major isoflavone present in soy products. J. Nat. Prod. 2008, 71, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Pajak, B.; Gajkowska, B.; Orzechowski, A. Molecular basis of parthenolide-dependent proapoptotic activity in cancer cells. Folia Histochem. Cytobiol. 2008, 46, 129–135. [Google Scholar] [CrossRef]
- Guzman, M.L.; Rossi, R.M.; Neelakantan, S.; Li, X.; Corbett, C.A.; Hassane, D.C.; Becker, M.W.; Bennett, J.M.; Sullivan, E.; Lachowicz, J.L.; et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood 2007, 110, 4427–4435. [Google Scholar] [CrossRef]
- Curry, E.A., 3rd; Murry, D.J.; Yoder, C.; Fife, K.; Armstrong, V.; Nakshatri, H.; O’Connell, M.; Sweeney, C.J. Phase I dose escalation trial of feverfew with standardized doses of parthenolide in patients with cancer. Investig. New Drugs. 2004, 22, 299–305. [Google Scholar] [CrossRef]
- Kolev, J.N.; O’Dwyer, K.M.; Jordan, C.T.; Fasan, R. Discovery of potent parthenolide-based antileukemic agents enabled by late-stage P450-mediated C-H functionalization. ACS Chem. Biol. 2014, 9, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castillo, M.; Villegas-Sepúlveda, N.; Meraz-Rios, M.A.; Hernández-Zavala, A.; Berumen, J.; Coleman, M.A.; Orozco, L.; Cordova, E.J. Curcumin differentially affects cell cycle and cell death in acute and chronic myeloid leukemia cells. Oncol. Lett. 2018, 15, 6777–6783. [Google Scholar] [CrossRef]
- Xia, L.; Chen, D.; Han, R.; Fang, Q.; Waxman, S.; Jing, Y. Boswellic acid acetate induces apoptosis through caspase-mediated pathways in myeloid leukemia cells. Mol. Cancer Ther. 2005, 4, 381–388. [Google Scholar] [CrossRef]
- Britschgi, A.; Simon, H.U.; Tobler, A.; Fey, M.F.; Tschan, M.P. Epigallocatechin-3-gallate induces cell death in acute myeloid leukaemia cells and supports all-trans retinoic acid-induced neutrophil differentiation via death-associated protein kinase 2. Br. J. Haematol. 2010, 149, 55–64. [Google Scholar] [CrossRef]
- Papież, M.A.; Bukowska-Straková, K.; Krzysciak, W.; Baran, J. (−)-Epicatechin enhances etoposide-induced antileukaemic effect in rats with acute myeloid leukaemia. Anticancer Res. 2012, 32, 2905–2913. [Google Scholar]
- Alvarez, M.C.; Maso, V.; Torello, C.O.; Ferro, K.P.; Saad, S.T.O. The polyphenol quercetin induces cell death in leukemia by targeting epigenetic regulators of pro-apoptotic genes. Clin. Epigenetics 2018, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Maso, V.; Calgarotto, A.K.; Franchi, G.C., Jr.; Nowill, A.E.; Filho, P.L.; Vassallo, J.; Saad, S.T. Multitarget effects of quercetin in leukemia. Cancer Prev. Res. 2014, 7, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Yoon, J.H.; Song, K.S. Chrysin inhibited stem cell factor (SCF)/c-Kit complex-induced cell proliferation in human myeloid leukemia cells. Biochem. Pharmacol. 2007, 74, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, K.; Lee, Y.M.; Lim, T.K.; Port, S.A.; Han, J.H.; Chen, C.S.; Lin, Q. Genistein exerts anti-leukemic effects on genetically different acute myeloid leukemia cell lines by inhibiting protein synthesis and cell proliferation while inducing apoptosis—Molecular insights from an iTRAQ™ quantitative proteomics study. Oncoscience 2015, 2, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Zhang, M.; Meng, H.; Xu, D.; Xie, Y. Gallic acid targets acute myeloid leukemia via Akt/mTOR-dependent mitochondrial respiration inhibition. Biomed. Pharmacother. 2018, 105, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.H.; Song, K.H.; Motomura, M.; Suzuki, I.; Gu, Y.H.; Kang, Y.J.; Moon, T.C.; Kim, C.H. Caffeic acid phenethyl ester induces mitochondria-mediated apoptosis in human myeloid leukemia U937 cells. Mol. Cell. Biochem. 2008, 310, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Papież, M.A.; Krzyściak, W.; Szade, K.; Bukowska-Straková, K.; Kozakowska, M.; Hajduk, K.; Bystrowska, B.; Dulak, J.; Jozkowicz, A. Curcumin enhances the cytogenotoxic effect of etoposide in leukemia cells through induction of reactive oxygen species. Drug Des. Devel. Ther. 2016, 10, 557–570. [Google Scholar] [CrossRef][Green Version]
- Peng, D.Y.; Song, H.; Liu, L.B. Resveratrol-downregulated phosphorylated liver kinase B1 is involved in senescence of acute myeloid leukemia stem cells. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2015, 35, 485–489. [Google Scholar] [CrossRef]
- Su, Y.C.; Li, S.C.; Wu, Y.C.; Wang, L.M.; Chao, K.S.; Liao, H.F. Resveratrol downregulates interleukin-6-stimulated sonic hedgehog signaling in human acute myeloid leukemia. Evid. Based. Complement. Alternat. Med. 2013, 2013, 547430. [Google Scholar] [CrossRef]
- Yaseen, A.; Chen, S.; Hock, S.; Rosato, R.; Dent, P.; Dai, Y.; Grant, S. Resveratrol sensitizes acute myelogenous leukemia cells to histone deacetylase inhibitors through reactive oxygen species-mediated activation of the extrinsic apoptotic pathway. Mol. Pharmacol. 2012, 82, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.C.; Chou, Y.E.; Tan, P.; Lee, W.J.; Yang, S.F.; Chow, J.M.; Chen, H.Y.; Lin, C.H.; Lee, L.M.; Chien, M.H. Pterostilbene simultaneously induced G0/G1-phase arrest and MAPK-mediated mitochondrial-derived apoptosis in human acute myeloid leukemia cell lines. PLoS ONE 2014, 9, e105342. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hui, H.; Li, Z.; Wang, H.M.; You, Q.D.; Lu, N. Gambogic acid induces growth inhibition and differentiation via upregulation of p21waf1/cip1 expression in acute myeloid leukemia cells. J. Asian Nat. Prod. Res. 2014, 16, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wang, H.; Hu, Z.; Huang, Y.; Yao, F.; Sun, S.; Wu, B. Quercetin inhibits proliferation and drug resistance in KB/VCR oral cancer cells and enhances its sensitivity to vincristine. Nutr. Cancer. 2015, 67, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.A.; Angka, L.; Rota, S.G.; Hanlon, T.; Mitchell, A.; Hurren, R.; Wang, X.M.; Gronda, M.; Boyaci, E.; Bojko, B.; et al. Targeting mitochondria with avocatin B induces selective leukemia cell death. Cancer Res. 2015, 75, 2478–2488. [Google Scholar] [CrossRef] [PubMed]
- Zahedpanah, M.; Shaiegan, M.; Ghaffari, S.H.; Nikbakht, M.; Nikugoftar, M.; Mohammadi, S. Parthenolide induces apoptosis in committed progenitor AML cell line U937 via reduction in osteopontin. Rep. Biochem. Mol. Biol. 2016, 4, 82–88. [Google Scholar] [PubMed]
- Chen, Y.; Li, J.; Hu, J.; Zheng, J.; Zheng, Z.; Liu, T.; Lin, Z.; Lin, M. Emodin enhances ATRA-induced differentiation and induces apoptosis in acute myeloid leukemia cells. Int. J. Oncol. 2014, 45, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Musalli, M.G.; Hassan, M.; Sheikh, R.A.; Kalantan, A.A.; Halwani, M.; Zeyadi, M.; Hosawi, S.; Alhosin, M. Thymoquinone induces cell proliferation inhibition and apoptosis in acute myeloid leukemia cells: Role of apoptosis-related WT1 and BCL2 genes. Eur. J. Cell Sci. 2019, 1, 2–9. [Google Scholar] [CrossRef]
- Ahmed, N.; Laverick, L.; Sammons, J.; Zhang, H.; Maslin, D.J.; Hassan, H.T. Ajoene, a garlic-derived natural compound, enhances chemotherapy-induced apoptosis in human myeloid leukaemia CD34-positive resistant cells. Anticancer Res. 2001, 21, 3519–3523. [Google Scholar]
- Bai, L.Y.; Weng, J.R.; Chiu, C.F.; Wu, C.Y.; Yeh, S.P.; Sargeant, A.M.; Lin, P.H.; Liao, Y.M. OSU-A9, an indole-3-carbinol derivative, induces cytotoxicity in acute myeloid leukemia through reactive oxygen species-mediated apoptosis. Biochem. Pharmacol. 2013, 86, 1430–1440. [Google Scholar] [CrossRef]
- Eden, R.E.; Coviello, J.M. Chronic Myelogenous Leukemia; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK531459/ (accessed on 3 March 2021).
- Rohrbacher, M.; Hasford, J. Epidemiology of chronic myeloid leukaemia (CML). Best Pract. Res. Clin. Haematol. 2009, 22, 295–302. [Google Scholar] [CrossRef]
- Medina, J.; Kantarjian, H.; Talpaz, M.; O’Brien, S.; Garcia-Manero, G.; Giles, F.; Rios, M.B.; Hayes, K.; Cortes, J. Chromosomal abnormalities in Philadelphia chromosome-negative metaphases appearing during imatinib mesylate therapy in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase. Cancer 2003, 98, 1905–1911. [Google Scholar] [CrossRef]
- Sillaber, C.; Mayerhofer, M.; Agis, H.; Sagaster, V.; Mannhalter, C.; Sperr, W.R.; Geissler, K.; Valent, P. Chronic myeloid leukemia: Pathophysiology, diagnostic parameters, and current treatment concepts. Wien Klin. Wochenschr. 2003, 115, 485–504. [Google Scholar] [CrossRef]
- Mencalha, A.L.; Correa, S.; Abdelhay, E. Role of calcium-dependent protein kinases in chronic myeloid leukemia: Combined effects of PKC and BCR-ABL signaling on cellular alterations during leukemia development. Onco Targets Ther. 2014, 7, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Pohnert, S.C.; Shelton, J.G.; Franklin, R.A.; Bertrand, F.E.; McCubrey, J.A. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia 2004, 18, 189–218. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, S.; Adan Gokbulut, A.; Cincin, B.; Ozdogu, H.; Boga, C.; Cakmakoglu, B.; Kozanoglu, I.; Baran, Y. Therapeutic potential of apigenin, a plant flavonoid, for imatinib-sensitive and resistant chronic myeloid leukemia cells. Nutr. Cancer. 2014, 66, 599–612. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zaric, M.; Mitrovic, M.; Nikolic, I.; Baskic, D.; Popovic, S.; Djurdjevic, P.; Milosavljevic, Z.; Zelen, I. Chrysin induces apoptosis in peripheral blood lymphocytes isolated from human chronic lymphocytic leukemia. Anticancer Agents Med. Chem. 2015, 15, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Hosseinzadeh, E.; Rezapour, S.; Vahedi, G.; Haghnavaz, N.; Marofi, F. Quercetin promotes cell cycle arrest and apoptosis and attenuates the proliferation of human chronic myeloid leukemia cell line-K562 through interaction with HSPs (70 and 90), MAT2A and FOXM1. Anticancer Agents Med. Chem. 2019, 19, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, Y.; Tao, B.; Zhang, Y. Effects of quercetin on hedgehog signaling in chronic myeloid leukemia KBM7 cells. Chin. J. Integr. Med. 2014, 20, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Mutlu Altundağ, E.; Yılmaz, A.M.; Koçtürk, S.; Taga, Y.; Yalçın, A.S. Synergistic induction of apoptosis by quercetin and curcumin in chronic myeloid leukemia (K562) cells. Nutr. Cancer. 2018, 70, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Carlo-Stella, C.; Dotti, G.; Mangoni, L.; Regazzi, E.; Garau, D.; Bonati, A.; Almici, C.; Sammarelli, G.; Savoldo, B.; Rizzo, M.T.; et al. Selection of myeloid progenitors lacking BCR/ABL mRNA in chronic myelogenous leukemia patients after in vitro treatment with the tyrosine kinase inhibitor genistein. Blood 1996, 88, 3091–3100. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, K.; Xu, Y.; Peng, H.; Wang, Z.; Liu, S.; Zhang, G. (−)-Epigallocatechin-3-gallate induces cell apoptosis in chronic myeloid leukaemia by regulating Bcr/Abl-mediated p38-MAPK/JNK and JAK2/STAT3/AKT signalling pathways. Clin. Exp. Pharmacol. Physiol. 2019, 46, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kumazoe, M.; Bae, J.; Yamada, S.; Takai, M.; Hidaka, S.; Yamashita, S.; Kim, Y.; Won, Y.; Murata, M.; et al. Green tea polyphenol epigallocatechin-O-gallate induces cell death by acid sphingomyelinase activation in chronic myeloid leukemia cells. Oncol. Rep. 2015, 34, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Feriotto, G.; Tagliati, F.; Giriolo, R.; Casciano, F.; Tabolacci, C.; Beninati, S.; Khan, M.T.H.; Mischiati, C. Caffeic acid enhances the anti-leukemic effect of imatinib on chronic myeloid leukemia cells and triggers apoptosis in cells sensitive and resistant to imatinib. Int. J. Mol. Sci. 2021, 22, 1644. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, G.; Biswas, T.; Roy, K.C.; Mandal, S.; Mandal, C.; Pal, B.C.; Bhattacharya, S.; Rakshit, S.; Bhattacharya, D.K.; Chaudhuri, U.; et al. Chlorogenic acid inhibits Bcr-Abl tyrosine kinase and triggers p38 mitogen-activated protein kinase-dependent apoptosis in chronic myelogenous leukemic cells. Blood 2004, 104, 2514–2522. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.G.; Zhong, L.; Liu, Y.L.; Shi, X.J.; Shi, L.Q.; Zeng, L.; Liu, B.Z. Emodin exerts an antiapoptotic effect on human chronic myelocytic leukemia K562 cell lines by targeting the PTEN/PI3K-AKT signaling pathway and deleting BCR-ABL. Integr. Cancer Ther. 2017, 16, 526–539. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, M.; Zhang, Q.; Xu, J.; Ouyang, J. Anticancer effect and apoptosis induction of gambogic acid in human leukemia cell line K562 in vitro. Med. Sci. Monit. 2015, 21, 1604–1610. [Google Scholar] [CrossRef]
- Shi, X.; Chen, X.; Li, X.; Lan, X.; Zhao, C.; Liu, S.; Huang, H.; Liu, N.; Liao, S.; Song, W.; et al. Gambogic acid induces apoptosis in imatinib-resistant chronic myeloid leukemia cells via inducing proteasome inhibition and caspase-dependent Bcr-Abl downregulation. Clin. Cancer Res. 2014, 20, 151–163. [Google Scholar] [CrossRef]
- Taverna, S.; Giallombardo, M.; Pucci, M.; Flugy, A.; Manno, M.; Raccosta, S.; Rolfo, C.; De Leo, G.; Alessandro, R. Curcumin inhibits in vitro and in vivo chronic myelogenous leukemia cells growth: A possible role for exosomal disposal of miR-21. Oncotarget 2015, 6, 21918–21933. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.P.; Xiong, M.; Xu, C.S.; Duan, L.N.; Dong, Y.Q.; Luo, Y.; Niu, T.H.; Lu, C.R. Resveratrol induces apoptosis of human chronic myelogenous leukemia cells in vitro through p38 and JNK-regulated H2AX phosphorylation. Acta Pharmacol. Sin. 2015, 36, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, J.; Gong, Z. Resveratrol induces apoptosis in K562 cells via the regulation of mitochondrial signaling pathways. Int. J. Clin. Exp. Med. 2015, 8, 16926–16933. [Google Scholar] [PubMed]
- Can, G.; Cakir, Z.; Kartal, M.; Gunduz, U.; Baran, Y. Apoptotic effects of resveratrol, a grape polyphenol, on imatinib-sensitive and resistant K562 chronic myeloid leukemia cells. Anticancer Res. 2012, 32, 2673–2678. [Google Scholar]
- Roy, M.; Sarkar, R.; Mukherjee, A.; Mukherjee, S. Inhibition of crosstalk between Bcr-Abl and PKC signaling by PEITC, augments imatinib sensitivity in chronic myelogenous leukemia cells. Chem. Biol. Interact. 2015, 242, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, S.; Wang, J.; Fang, Q.; Chai, Q. Phenethyl isothiocyanate inhibits growth of human chronic myeloid leukemia K562 cells via reactive oxygen species generation and caspases. Mol. Med. Rep. 2014, 10, 543–549. [Google Scholar] [CrossRef]
- Safa, M.; Jafari, L.; Alikarami, F.; Manafi Shabestari, R.; Kazemi, A. Indole-3-carbinol induces apoptosis of chronic myelogenous leukemia cells through suppression of STAT5 and Akt signaling pathways. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef]
- Chun-Guang, W.; Jun-Qing, Y.; Bei-Zhong, L.; Dan-Ting, J.; Chong, W.; Liang, Z.; Dan, Z.; Yan, W. Anti-tumor activity of emodin against human chronic myelocytic leukemia K562 cell lines in vitro and in vivo. Eur. J. Pharmacol. 2010, 627, 33–41. [Google Scholar] [CrossRef]
- Ozkan, T.; Hekmatshoar, Y.; Pamuk, H.; Ozcan, M.; Yaman, G.; Yagiz, G.C.; Akdemir, C.; Sunguroglu, A. Cytotoxic effect of 6-Shogaol in Imatinib sensitive and resistant K562 cells. Mol. Biol. Rep. 2021, 48, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Flores-Lopez, G.; Moreno-Lorenzana, D.; Ayala-Sanchez, M.; Aviles-Vazquez, S.; Torres-Martinez, H.; Crooks, P.A.; Guzman, M.L.; Mayani, H.; Chávez-González, A. Parthenolide and DMAPT induce cell death in primitive CML cells through reactive oxygen species. J. Cell. Mol. Med. 2018, 22, 4899–4912. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Du, J.; Kong, D.; Yang, G.; Zhou, Q.; You, F.; Lin, Y.; Wang, Y. Gambogic acid inhibits proliferation and induces apoptosis of human acute T-cell leukemia cells by inducing autophagy and downregulating β-catenin signaling pathway: Mechanisms underlying the effect of Gambogic acid on T-ALL cells. Oncol. Rep. 2020, 44, 1747–1757. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, P.; Ferrando, A. The molecular basis of T cell acute lymphoblastic leukemia. J. Clin. Investig. 2012, 122, 3398–3406. [Google Scholar] [CrossRef]
- Goldberg, J.M.; Silverman, L.B.; Levy, D.E.; Dalton, V.K.; Gelber, R.D.; Lehmann, L.; Cohen, H.J.; Sallan, S.E.; Asselin, B.L. Childhood T-cell acute lymphoblastic leukemia: The Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J. Clin. Oncol. 2003, 21, 3616–3622. [Google Scholar] [CrossRef] [PubMed]
- Zi, C.T.; Gao, Y.S.; Yang, L.; Feng, S.Y.; Huang, Y.; Sun, L.; Jin, Y.; Xu, F.Q.; Dong, F.W.; Li, Y.; et al. Design, synthesis, and biological evaluation of novel biotinylated podophyllotoxin derivatives as potential antitumor agents. Front. Chem. 2019, 7, 434. [Google Scholar] [CrossRef]
- Popović, D.; Đukić, D.; Katić, V.; Jović, Z.; Jović, M.; Lalić, J.; Golubović, I.; Stojanović, S.; Ulrih, N.P.; Stanković, M.; et al. Antioxidant and proapoptotic effects of anthocyanins from bilberry extract in rats exposed to hepatotoxic effects of carbon tetrachloride. Life Sci. 2016, 157, 168–177. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Sharif, T.; Auger, C.; Abbas, M.; Fuhrmann, G.; Schini-Kerth, V.B. Anthocyanin-rich bilberry extract induces apoptosis in acute lymphoblastic leukemia cells via redox-sensitive epigenetic modifications. J. Funct. Foods 2018, 44, 227–234. [Google Scholar] [CrossRef]
- Sorrenti, V.; Di Giacomo, C.; Acquaviva, R.; Bognanno, M.; Grilli, E.; D’Orazio, N.; Galvano, F. Dimethylarginine dimethylaminohydrolase/nitric oxide synthase pathway in liver and kidney: Protective effect of cyanidin 3-O-β-D-glucoside on ochratoxin-A toxicity. Toxins 2012, 4, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Hautzinger, A.; Rossmann, A.; Holzhauser, S.; Popovic, D.; Hertrampf, A.; Oesterle, D.; Spiller, C.; Boll, M.; Wenzel, U. Potential role of P-gp for flavone-induced diminished apoptosis and increased adenoma size in the small intestine of APC(min/+) mice. Cancer Investig. 2011, 29, 396–404. [Google Scholar] [CrossRef]
- Feng, R.; Ni, H.M.; Wang, S.Y.; Tourkova, I.L.; Shurin, M.R.; Harada, H.; Yin, X.M. Cyanidin-3-rutinoside, a natural polyphenol antioxidant, selectively kills leukemic cells by induction of oxidative stress. J. Biol. Chem. 2007, 282, 13468–13476. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Sharif, T.; Kayali, A.; Abbas, M.; Dandache, I.; Etienne-Selloum, N.; Kevers, C.; Pincemail, J.; Auger, C.; Chabert, P.; et al. Delphinidin-3-O-glucoside and delphinidin-3-O-rutinoside mediate the redox-sensitive caspase 3-related pro-apoptotic effect of blackcurrant juice on leukaemia Jurkat cells. J. Funct. Foods 2015, 17, 847–856. [Google Scholar] [CrossRef]
- Shi, Y.; Su, X.; Cui, H.; Yu, L.; Du, H.; Han, Y. Combination of quercetin and Adriamycin effectively suppresses the growth of refractory acute leukemia. Oncol. Lett. 2019, 18, 153–160. [Google Scholar] [CrossRef]
- Goto, H.; Yanagimachi, M.; Goto, S.; Takeuchi, M.; Kato, H.; Yokosuka, T.; Kajiwara, R.; Yokota, S. Methylated chrysin reduced cell proliferation, but antagonized cytotoxicity of other anticancer drugs in acute lymphoblastic leukemia. Anticancer Drugs 2012, 23, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Pirbaluti, M.; Pourgheysari, B.; Shirzad, H.; Sourani, Z.; Beshkar, P. The inhibitory effect of epigallocatechin gallate on the viability of T lymphoblastic leukemia cells is associated with increase of caspase-3 level and Fas expression. Indian J. Hematol. Blood Transfus. 2018, 34, 253–260. [Google Scholar] [CrossRef]
- Kuttikrishnan, S.; Siveen, K.S.; Prabhu, K.S.; Khan, A.Q.; Ahmed, E.I.; Akhtar, S.; Ali, T.A.; Merhi, M.; Dermime, S.; Steinhoff, M.; et al. Curcumin induces apoptotic cell death via inhibition of PI3-kinase/AKT pathway in B-precursor acute lymphoblastic leukemia. Front. Oncol. 2019, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- William, B.M.; Goodrich, A.; Peng, C.; Li, S. Curcumin inhibits proliferation and induces apoptosis of leukemic cells expressing wild-type or T315I-BCR-ABL and prolongs survival of mice with acute lymphoblastic leukemia. Hematology 2008, 13, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Liu, Y.; Li, Q.; Guo, X.; Gu, L.; Ma, Z.G.; Zhu, Y.P. Resveratrol induces apoptosis and autophagy in T-cell acute lymphoblastic leukemia cells by inhibiting Akt/mTOR and activating p38-MAPK. Biomed. Environ. Sci. 2013, 26, 902–911. [Google Scholar] [CrossRef]
- Ramezani, G.; Pourgheysari, B.; Shirzad, H.; Sourani, Z. Pterostilbene increases Fas expression in T-lymphoblastic leukemia cell lines. Res. Pharm. Sci. 2019, 14, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sourani, Z.; Pourgheysari, B.; Rafieian-Kopaei, M.; Shirzad, H.; Shirzad, M. The effect of gallic acid on Jurkat cell line. J. HerbMed Pharmacol. 2015, 4, 129–132. [Google Scholar]
- Diamanti, P.; Cocs, C.V.; Moppett, J.P.; Blair, A. Parthenolide eliminates leukemia-initiating cell populations and improves survival in xenografts of childhood acute lymphoblastic leukemia. Blood 2013, 121, 1384–1393. [Google Scholar] [CrossRef]
- Soltani, A.; Pourgheysari, B.; Shirzad, H.; Sourani, Z. Antiproliferative and apoptosis-inducing activities of thymoquinone in lymphoblastic leukemia cell line. Indian J. Hematol. Blood Transfus. 2017, 33, 516–524. [Google Scholar] [CrossRef]
- Salim, L.Z.; Mohan, S.; Othman, R.; Abdelwahab, S.I.; Kamalidehghan, B.; Sheikh, B.Y.; Ibrahim, M.Y. Thymoquinone induces mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in vitro. Molecules 2013, 18, 11219–11240. [Google Scholar] [CrossRef]
- Safa, M.; Tavasoli, B.; Manafi, R.; Kiani, F.; Kashiri, M.; Ebrahimi, S.; Kazemi, A. Indole-3-carbinol suppresses NF-κB activity and stimulates the p53 pathway in pre-B acute lymphoblastic leukemia cells. Tumour Biol. 2015, 36, 3919–3930. [Google Scholar] [CrossRef]
- Hallek, M. Chronic lymphocytic leukemia: 2013 update on diagnosis, risk stratification and treatment. Am. J. Hematol. 2013, 88, 803–816. [Google Scholar] [CrossRef]
- Stephens, J.M.; Gramegna, P.; Laskin, B.; Botteman, M.F.; Pashos, C.L. Chronic lymphocytic leukemia: Economic burden and quality of life: Literature review. Am. J. Ther. 2005, 12, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Mukkamalla, S.K.R.; Taneja, A.; Malipeddi, D.; Master, S.R. Chronic Lymphocytic Leukemia; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://pubmed.ncbi.nlm.nih.gov/29261864/ (accessed on 2 March 2021).
- Golombick, T.; Diamond, T.H.; Manoharan, A.; Ramakrishna, R. B-cell disorders and curcumin. Integr. Cancer Ther. 2017, 16, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Rozman, C.; Montserrat, E. Chronic lymphocytic leukemia. N. Engl. J. Med. 1995, 333, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Stilgenbauer, S.; Flinn, I.W. Chronic lymphocytic leukemia. Hematol. Am. Soc. Hematol. Educ. Program. 2004, 163–183. [Google Scholar] [CrossRef] [PubMed]
- Gokbulut, A.A.; Apohan, E.; Baran, Y. Resveratrol and quercetin-induced apoptosis of human 232B4 chronic lymphocytic leukemia cells by activation of caspase-3 and cell cycle arrest. Hematology 2013, 18, 144–150. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; Gandhi, V. Bcl-2 antagonists: A proof of concept for CLL therapy. Invest. New Drugs. 2013, 31, 1384–1394. [Google Scholar] [CrossRef]
- Hamblin, T. Natural products and the treatment of leukemia. Leuk. Res. 2006, 30, 649–650. [Google Scholar] [CrossRef] [PubMed]
- Alhosin, M.; León-González, A.J.; Dandache, I.; Lelay, A.; Rashid, S.K.; Kevers, C.; Pincemail, J.; Fornecker, L.M.; Mauvieux, L.; Herbrecht, R.; et al. Bilberry extract (Antho 50) selectively induces redox-sensitive caspase 3-related apoptosis in chronic lymphocytic leukemia cells by targeting the Bcl-2/Bad pathway. Sci. Rep. 2015, 5, 8996. [Google Scholar] [CrossRef] [PubMed]
- Sak, K.; Kasemaa, K.; Everaus, H. Potentiation of luteolin cytotoxicity by flavonols fisetin and quercetin in human chronic lymphocytic leukemia cell lines. Food Funct. 2016, 7, 3815–3824. [Google Scholar] [CrossRef]
- Hashemi, M.; Nouri Long, M.; Entezari, M.; Nafisi, S.; Nowroozii, H. Anti-mutagenic and pro-apoptotic effects of apigenin on human chronic lymphocytic leukemia cells. Acta Med. Iran. 2010, 48, 283–288. [Google Scholar] [PubMed]
- Lee, Y.K.; Bone, N.D.; Strege, A.K.; Shanafelt, T.D.; Jelinek, D.F.; Kay, N.E. VEGF receptor phosphorylation status and apoptosis is modulated by a green tea component, epigallocatechin-3-gallate (EGCG), in B-cell chronic lymphocytic leukemia. Blood 2004, 104, 788–794. [Google Scholar] [CrossRef]
- Salimi, A.; Roudkenar, M.H.; Seydi, E.; Sadeghi, L.; Mohseni, A.; Pirahmadi, N.; Pourahmad, J. Chrysin as an anti-cancer agent exerts selective toxicity by directly inhibiting mitochondrial complex II and V in CLL B-lymphocytes. Cancer Investig. 2017, 35, 174–186. [Google Scholar] [CrossRef]
- Roman, V.; Billard, C.; Kern, C.; Ferry-Dumazet, H.; Izard, J.C.; Mohammad, R.; Mossalayi, D.M.; Kolb, J.P. Analysis of resveratrol-induced apoptosis in human B-cell chronic leukaemia. Br. J. Haematol. 2002, 117, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Golombick, T.; Diamond, T.; Manoharan, A.; Ramakrishna, R. The effect of curcumin (as meriva) on absolute lymphocyte count (ALC), NK cells and T cell populations in patients with stage 0/1 chronic lymphocytic leukemia. J. Cancer Ther. 2015, 06, 566–571. [Google Scholar] [CrossRef]
- Hayun, R.; Okun, E.; Berrebi, A.; Shvidel, L.; Bassous, L.; Sredni, B.; Nir, U. Rapamycin and curcumin induce apoptosis in primary resting B chronic lymphocytic leukemia cells. Leuk. Lymphoma 2009, 50, 625–632. [Google Scholar] [CrossRef]
- Loisel, S.; Le Ster, K.; Meyer, M.; Berthou, C.; Youinou, P.; Kolb, J.P.; Billard, C. Therapeutic activity of two xanthones in a xenograft murine model of human chronic lymphocytic leukemia. J. Hematol. Oncol. 2010, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, L.B.; Tu, Y.; Hu, H.; Huang, Y.R.; Sun, W. Emodin ameliorates cisplatin-induced apoptosis of rat renal tubular cells in vitro by activating autophagy. Acta Pharmacol. Sin. 2016, 37, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Steele, A.J.; Jones, D.T.; Ganeshaguru, K.; Duke, V.M.; Yogashangary, B.C.; North, J.M.; Lowdell, M.W.; Kottaridis, P.D.; Mehta, A.B.; Prentice, A.G.; et al. The sesquiterpene lactone parthenolide induces selective apoptosis of B-chronic lymphocytic leukemia cells in vitro. Leukemia 2006, 20, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Marín, G.; Mansilla, E. Parthenolide has apoptotic and cytotoxic selective effect on B-chronic lymphocytic leukemia cells. J. Appl. Biomed. 2006, 4, 135–139. [Google Scholar] [CrossRef]
- Arditti, F.D.; Rabinkov, A.; Miron, T.; Reisner, Y.; Berrebi, A.; Wilchek, M.; Mirelman, D. Apoptotic killing of B-chronic lymphocytic leukemia tumor cells by allicin generated in situ using a rituximab-alliinase conjugate. Mol. Cancer Ther. 2005, 4, 325–331. [Google Scholar]
- Perez-Chacon, G.; Martinez-Laperche, C.; Rebolleda, N.; Somovilla-Crespo, B.; Muñoz-Calleja, C.; Buño, I.; Zapata, J.M. Indole-3-carbinol synergizes with and restores fludarabine sensitivity in chronic lymphocytic leukemia cells irrespective of p53 activity and treatment resistances. Clin. Cancer Res. 2016, 22, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Call, T.G.; Zent, C.S.; LaPlant, B.; Bowen, D.A.; Roos, M.; Secreto, C.R.; Ghosh, A.K.; Kabat, B.F.; Lee, M.J.; et al. Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J. Clin. Oncol. 2009, 27, 3808–3814. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Call, T.G.; Zent, C.S.; Leis, J.F.; LaPlant, B.; Bowen, D.A.; Roos, M.; Laumann, K.; Ghosh, A.K.; Lesnick, C.; et al. Phase 2 trial of daily, oral Polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer 2013, 119, 363–370. [Google Scholar] [CrossRef] [PubMed]
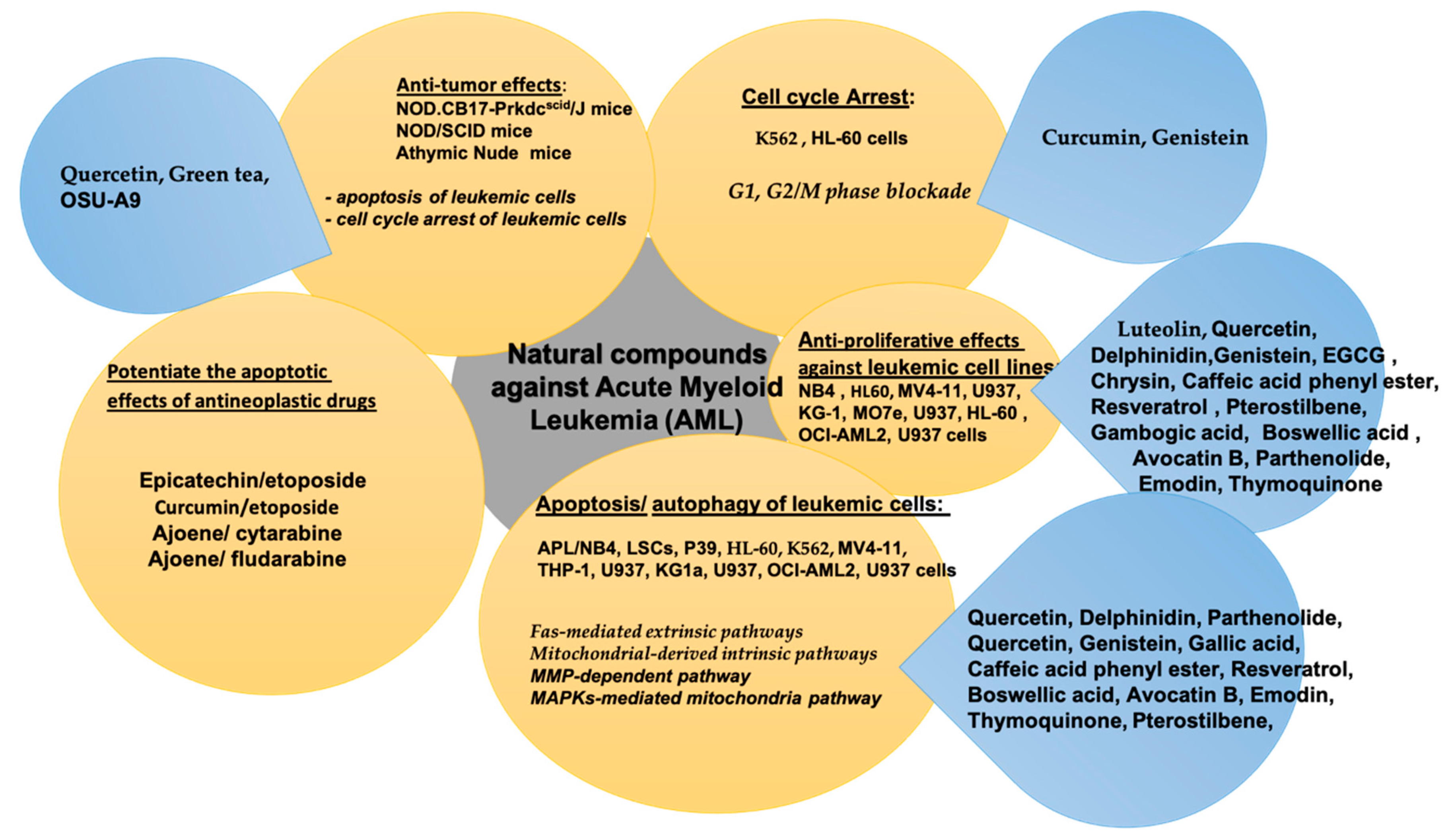
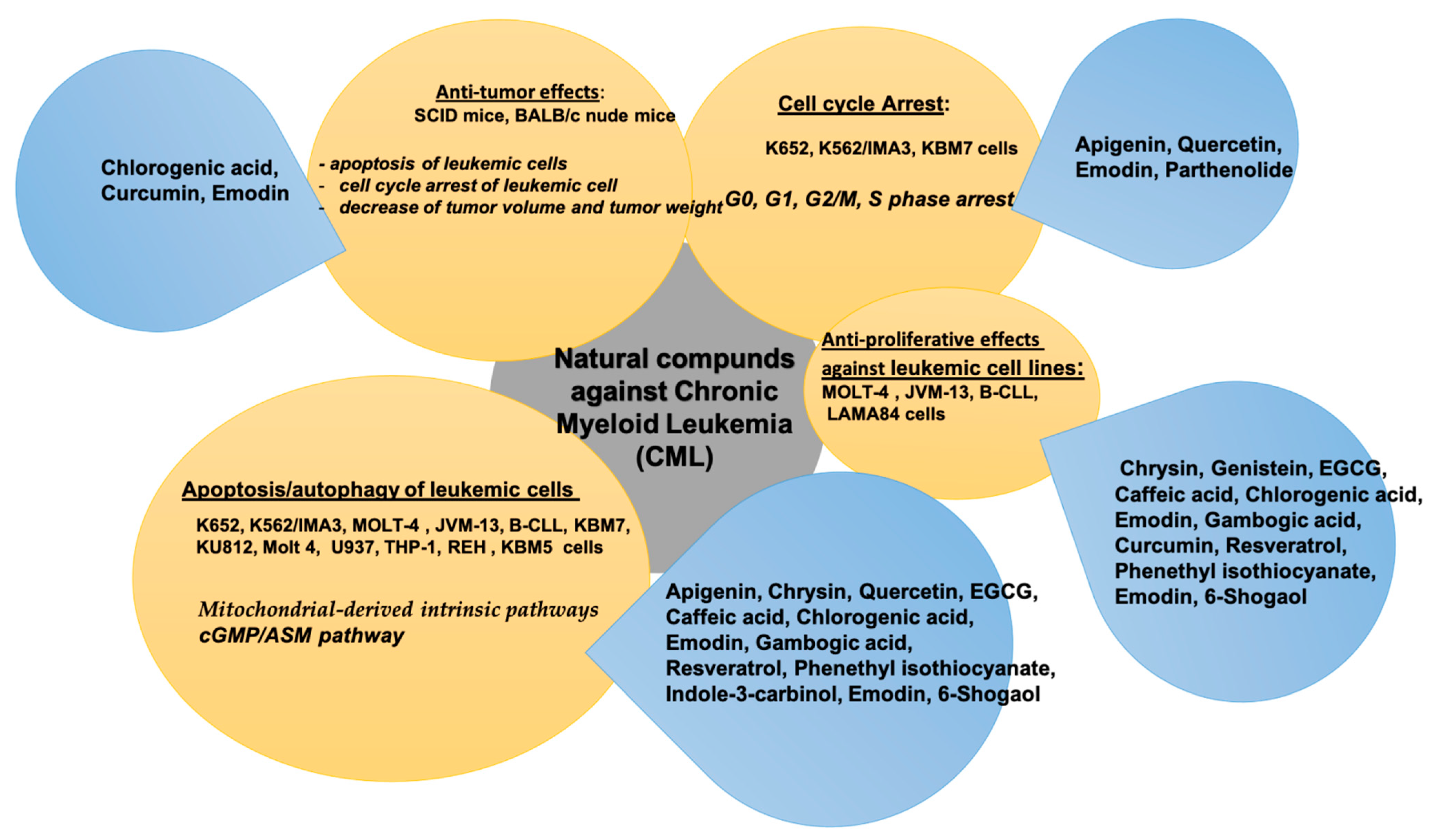
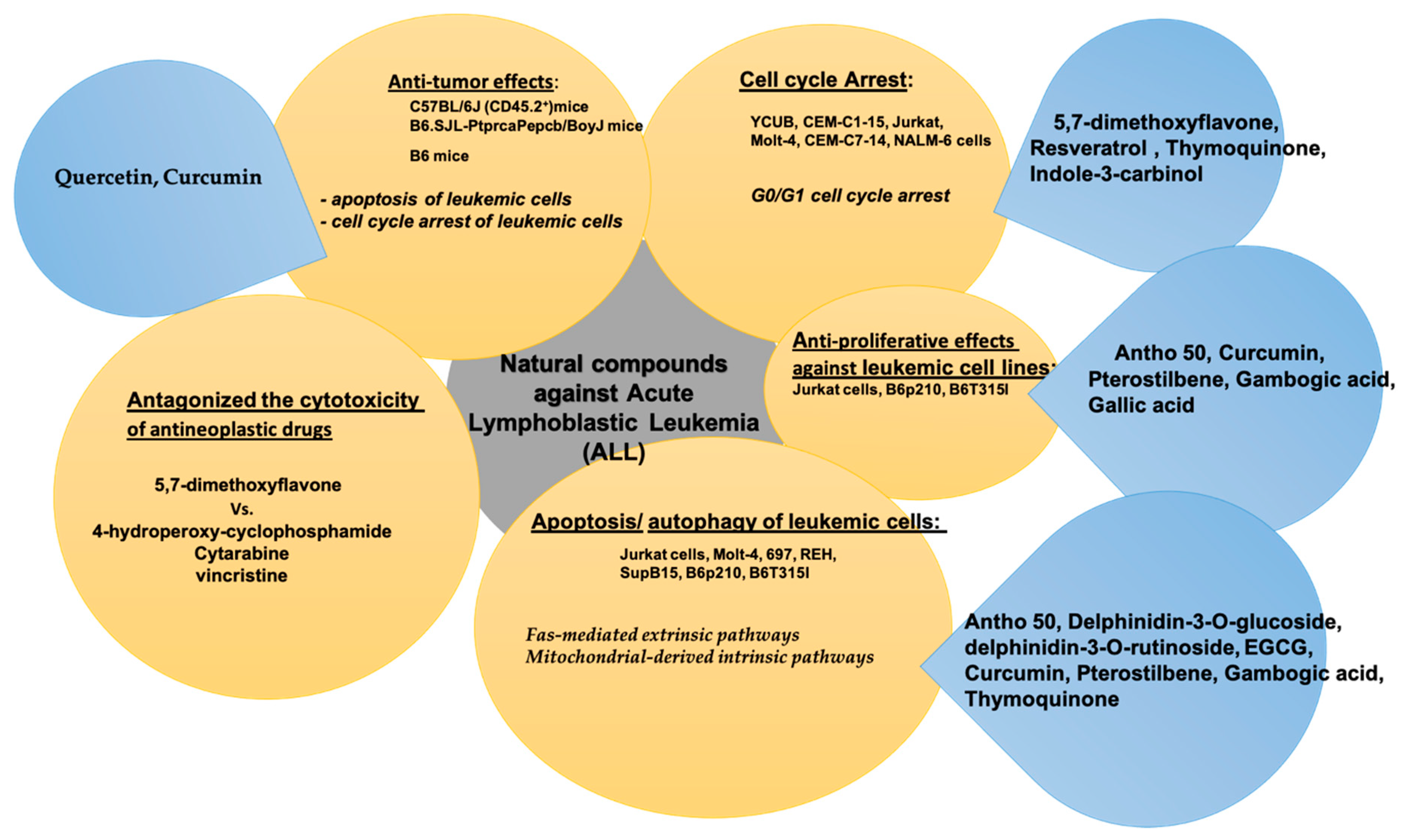
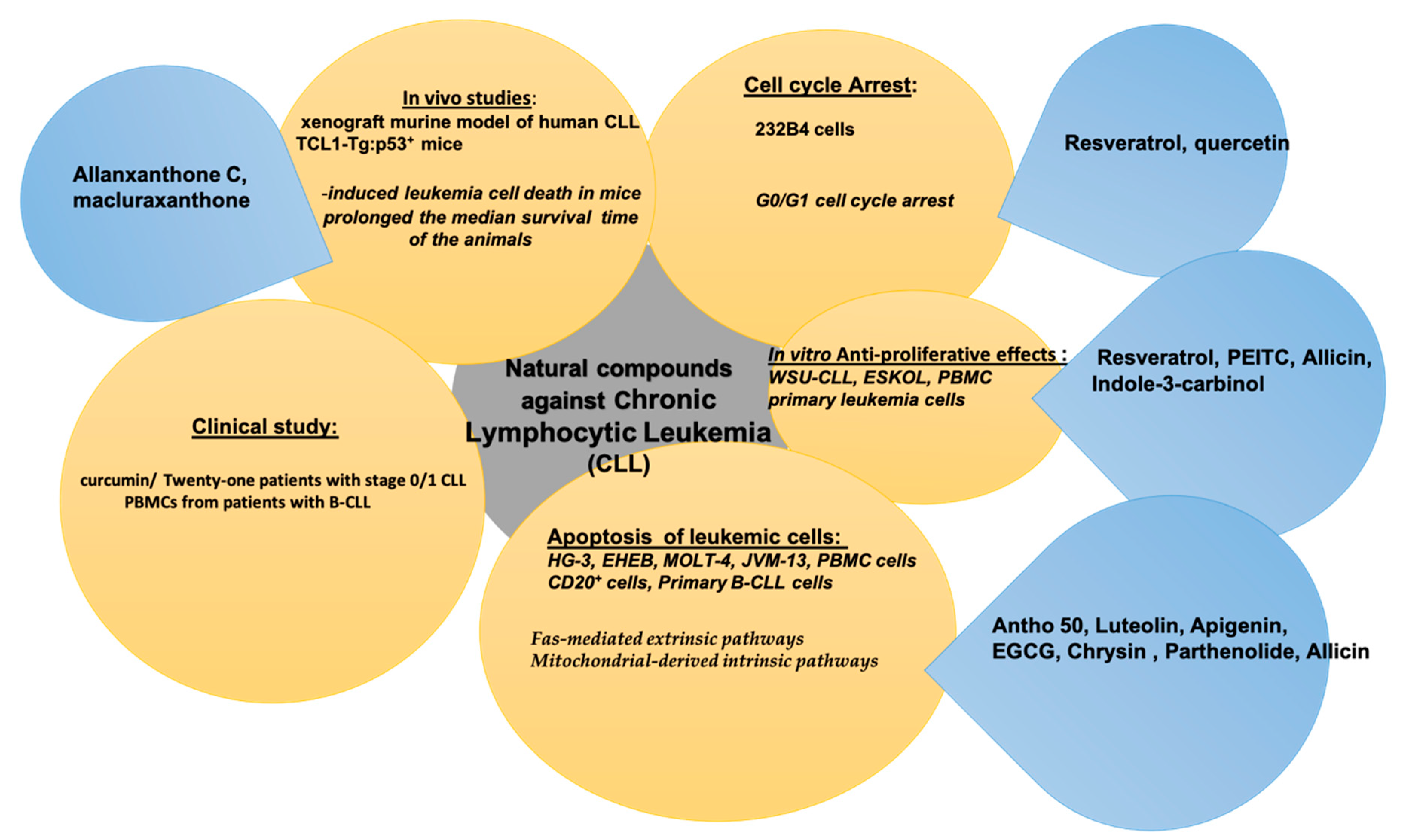
| Bioactive Compound | In Vitro/In Vivo/Clinical Study | Cancer Cell Line and Animal Model | Bioactive Effect | References |
|---|---|---|---|---|
| Luteolin | In vitro | MOLM-13 and Kasumi-1 cells | -inhibited leukemic cell proliferation and induced apoptosis by inhibition of the RSK1 pathways -triggered RSK-dependent antileukemic responses with dephosphorylation of Bad or KIBRA | [46] |
| EGCG | In vitro | NB4 and HL60 cells | -induced cell death in myeloid leukemic cells -↑ DAPK2 levels in AML cells -EGCG/ATRA cotreatment of myeloid leukemic cells enhanced neutrophil differentiation | [62] |
| (−)-Epicatechin | In vivo | Brown Norway rats | ↑ the in vivo apoptotic effect of etoposide ↑ the oxidative stress induced by etoposide in leukemic rats | [63] |
| Quercetin | In vitro | MV4-11 and HL-60 cells | -promoted AML cell death -induced caspase-dependent apoptosis in AML cells -induced apoptosis via mitochondrial pathway -suppressed VEGFR2 and PI3K/Akt signaling pathway | [39] |
| Quercetin | In vitro | HL60 and U937 cells | -down-regulated DNMTs and STAT3 -induced H3 and H4 global acetylation -enriched H3ac and H4ac in the promoter region of the apoptosis pathway genes and increased their transcription levels ↓ the protein expression of class I HDACs in leukemia cells -caused proteasome-mediated protein degradation of HADCs in leukemia cells -down-regulated DNMTs and HADCs at the protein levels, in xenograft models | [64] |
| Quercetin | In vitro | human myeloid leukemia KG-1 cells | -cytotoxicity effect against KG-1 cells -augmented the TRAIL-induced cell death in KG-1 cells ↑ mRNA expression levels of DR genes in acute myeloid KG-1 cells ↓ mRNA expression of apoptosis inhibitor genes in the acute myeloid KG-1 cells ↓ mRNA expression of NF-κB (p65 subunit) gene in the acute myeloid KG-1 cells | [5] |
| Quercetin | In vitro | P39 cells | -induced apoptosis in P39 leukemia cells ↓ Bcl-2, Bcl-xL, Mcl-1 down-regulation ↓ Bax -induced mitochondrial translocation, triggering cytochrome c release and caspases activation | [65] |
| In vivo | NOD.CB17-Prkdcscid/J mice | -induced the expression of FasL protein ↑ cell arrest in G1 phase of the cell cycle ↓ in CDK2, CDK6, cyclin D, cyclin E, and cyclin A proteins ↓ Rb phosphorylation ↑ p21 and p27 expression -induced autophagosome formation in P39 cell line ↓ tumor volume in P39 xenografts in vivo | ||
| Quercetin and green tea | In vivo | NOD/SCID mice | ↓ tumor growth in HL-60 xenografts accompanied by decreased expression of anti-apoptotic proteins, Bcl-2, BCL-xL, and Mcl-1 and increased expression of Bax, a pro-apoptotic protein -induced apoptosis of leukemic cells -induced activation of caspase-3 -induced cell cycle arrest of leukemic cells -mediated G1 phase cell cycle arrest in HL-60 xenografts -induced conversion of LC3-I to LC3-II ↑ autophagy in leukemic cells | [41] |
| Chrysin | In vitro | MO7e cells | -inhibited SCF/c-Kit complex-induced cell proliferation in human myeloid leukemia cells -inhibited SCF-induced phosphorylation of c-Kit -inhibited cell proliferation in MO7e cells by blocking c-Kit phosphorylation | [66] |
| Genistein | In vitro | MV4-11 and HL-60 cells | -arrested the mTOR pathway leading to down-regulation of protein synthesis -induced cell death via apoptosis -regulatory effects on the cell cycle of the two cell lines, with the induction of G2⁄M phase arrest in HL-60 cells but not in MV4-11 cells | [67] |
| Gallic acid | In vitro | THP-1 and MV411 cells | -induced caspase-dependent apoptosis of AML cell lines, primary MNC and CD34 stem/progenitors isolated from AML patients via caspase-dependent pathway -enhanced cytarabine and daunorubicin efficacy in vitro cell culture system and in vivo xenograft model -inhibited mitochondrial respiration in AML cells, leading to decreased ATP production and oxidative stress -acted on AML cells via Akt/mTOR-dependent inhibition of mitochondrial respiration | [68] |
| Caffeic acid phenyl ester (CAPE) | In vitro | U937 cells | ↓ cell viability of U937 cells -induced the mitochondria-mediated apoptosis-release of cytochrome C, reduction of Bcl-2 expression, increase of Bax expression, activation/cleavage of caspase-3, and activation/cleavage of PARP | [69] |
| Curcumin | In vitro | HL-60 cells | -potentiated the cytotoxic effect of etoposide -intensified apoptosis and phosphorylation of the histone H2AX induced by this cytostatic drug in leukemic HL-60 cells -curcumin modified the cytotoxic action of etoposide in HL-60 cells through intensification of ROS production -enhanced the antileukemic activity of etoposide in BNML rats and induced apoptosis of BNML cells more efficiently than etoposide alone, but this treatment protected nonleukemic B-cells from apoptosis | [70] |
| In vivo | Brown Norway rats with acute myeloid leukemia (BNML) | |||
| Resveratrol | In vitro | CD34+ CD38− KG1a cells | ↓ pLKB1 in CD34+ CD38− KG1a cells ↑ the expression of SIRT1 in CD34+ CD38− KG1a cells -induced senescence and apoptosis of CD34+CD38− KG1a cells | [71] |
| Resveratrol | In vitro | HL-60 cells | ↓ CSC-related Shh expression, Gli-1 nuclear translocation, and cell viability in IL-6-treated HL-60 cells -had synergistic effect with Shh inhibitor cyclopamine on inhibiting cell growth | [72] |
| Resveratrol | In vitro | U937 and MV-4-11 cells | -interacted synergistically with HDACIs in human myeloid leukemia cells -coadministration with HDACIs led to enhanced DNA damage, mitochondrial injury, and caspase-3, caspase-9, and caspase-8 activation -blocked HDACI-mediated RelA acetylation and NF-κB activation -induced S-phase accumulation and sensitized leukemia cells to HDACIs | [73] |
| Pterostilbene | In vitro | MV4-11 HL-60, U937, and THP-1 AML cells | -suppressed cell proliferation in various AML cell lines -induced G0/G1-phase arrest when expressions of cyclin D3 and CDK2/6 were inhibited -induced cell apoptosis occurred through activation of caspases-8/-9/-3, and a MMP-dependent pathway -treatment of HL-60 cells with PTER induced sustained activation of ERK1/2 and JNK1/2, and inhibition of both MAPKs by their specific inhibitors significantly abolished the PTER-induced activation of caspases-8/-9/-3 -PTER-induced cell growth inhibition was only partially reversed by the caspase-3-specific inhibitor, Z-DEVE-FMK -promoted disruption of LMP and release activated cathepsin B -induced HL-60 cell death via MAPKs-mediated mitochondria apoptosis pathway | [74] |
| Gambogic acid | In vitro | U937 and HL-60 cells | -had cytotoxic effect on AML cells -inhibited cell growth and promoted differentiation in U937 and HL-60 cells ↑ the expression of p21waf1/cip1 in the two cell lines | [75] |
| 3-O-acetyl-11-keto-β-boswellic acid (AKBA) | In vitro | HL-60 cells | -inhibited dose-dependent proliferation of HL-60 and apoptosis rate of HL-60 cells -changed the cell cycle by increasing of G(1) phase and decreasing of S phase -anti-proliferation and apoptosis-inducing effects on HL-60 cells | [76] |
| Boswellic acid acetate | In vitro | NB4, SKNO-1, nK562, U937, ML-1, and HL-60 cells | -inhibited cell growth and induced cell toxicity of myeloid leukemia cell lines -induced apoptosis through a p53-independent pathway by activation of caspase-8 induced proteolysis of Bid ↓ mitochondrial membrane potential without production of hydrogen peroxide ↑ the levels of DR4 and DR5 mRNA in apoptotic cells | [61] |
| Avocatin B | In vitro | OCI-AML2 cells | ↓ human primary AML cell viability without effect on normal peripheral blood stem cells -selectively toxic toward leukemia progenitor and stem cells -induced mitochondria-mediated apoptosis -inhibited fatty acid oxidation and ↓ NADPH levels, resulting in ROS-dependent leukemia cell death | [77] |
| Parthenolide | In vitro | U937 cells | -inhibited growth of U937 cells -induced apoptosis in U937 cells ↓ the CD38+ population of U937 cells ↓ osteopontin gene expression in U937 cells | [78] |
| Parthenolide | In vitro | AML cells, bcCML cells, normal bone marrow, and umbilical cord blood cells | -induced apoptosis in primary human AML cells and bcCML cells sparing normal hematopoietic cells -targeted preferentially leukemic but not normal progenitor and stem cell activity | [43] |
| In vivo | Nonobese diabetic/severe NOD/SCID mice | -the molecular mechanism of PTL mediated apoptosis is associated with inhibition of NF-κB, proapoptotic activation of p53, and increased ROS -the activity of PTL triggers LSC-specific apoptosis | ||
| Emodin | In vitro | AML HL-60/ADR cells | -induced growth inhibition and apoptotic effects in resistant HL-60/ADR cells in vitro as well as in the HL-60/H3 xenograft models in vivo ↑ chemosensitivity of AML cells to Ara-C, inhibited leukemic cell growth, and improved survival in mouse xenograft model of AML | [37] |
| In vivo | BALB/C-nude mice | |||
| Emodin | In vitro | NB4, MR2 and primary AML cells | -inhibited cell proliferation in NB4 cells, MR2 cells, and primary AML cells -enhanced differentiation induction of ATRA in retinoid-responsive NB4 cells as well as in retinoid-resistant MR2 cells -induced cell apoptosis in NB4 cells, MR2 cells, and primary AML cells -the apoptotic induction in AML cells was associated with the activation of caspase cascades involving caspase-9, caspase-3, and PARP cleavage -induced the activation of the caspase-dependent pathway -induced the degradation of RARα protein in NB4 and MR2 cells -inhibited activation of the PI3K/Akt signaling pathway in AML cells -inhibited p-Akt at Ser473 as efficiently as mTOR at Ser2448 -suppressed the phosphoration of mTOR downstream targets, 4E-BP1 and p70S6K | [79] |
| Thymoquinone | In vitro | HL-60 cells | ↓ HL-60 cell viability -induced apoptosis in HL-60 cells ↓ the expression of WT1 and BCL2 genes | [80] |
| Ajoene | In vitro | KG1 cells | ↓ bcl-2-expression ↑ the inhibitory effect of the two chemotherapeutic drugs, cytarabine and fludarabine, on Bcl-2-expression in KGI cells -the two drugs, cytarabine and fludarabine, ↑ the activated caspase-3 level in KGI myeloid leukemia cells -ajoene enhanced the activation of caspase-3 in both cytarabine- and fludarabine-treated KGI cells | [81] |
| OSU-A9 | In vitro | HL-60 and THP-1 cells and primary leukemia cells from AML patients | -induced cytotoxicity in AML cell lines and primary leukemia cells from AML patients ↓ cyclin A and cyclin B1 in AML cell lines -induced apoptosis, caspase activation, and PARP cleavage in AML cell lines -induced autophagy but not autophagic cell death in AML cell lines -OSU-A9-mediated cytotoxicity and hypophosphorylation of Akt were dependent on the generation of ROS -suppressed the growth of THP-1 xenograft tumors and prolonged the survival of tumor-bearing athymic nude mice | [82] |
| In vivo | athymic nude mice |
| Bioactive Compound | In Vitro/In Vivo/Clinical Study | Cancer Cell Line and Animal Model | Bioactive Effect | References |
|---|---|---|---|---|
| Apigenin | In vitro | K652 and K562/IMA3 cells | -induced cytotoxic and apoptotic effects in K562 and K562/IMA3 cells -induced loss of mitochondrial membrane potential in both K562 and K562/IMA3 cells ↑ caspase-3 activity in both K562 and K562/IMA3 Cells -arrested cell cycle progression in G2/M phase in K562 cells -induced S phase arrest in K562/IMA3 cells -regulated a set of genes in K652 and K562/IMA3 cells | [89] |
| Chrysin | In vitro | MOLT-4 and JVM-13 cell lines, B-CLL cells derived from 28 patients and PBMC from 16 healthy subjects | ↓ the viability of of leukemic cells -induced apoptosis of peripheral blood lymphocytes isolated from human CLL patients via mitochondrial pathway -induced the activation of proapoptotic Bax ↓ the expression of antiapoptotic Bcl-2 protein -released cytochrome c from mitochondria into cytosol -activated caspase-3, subsequently leading to the activation of apoptosis of B-CLL cells | [90] |
| Quercetin | In vitro | K-562 cells | -induced apoptosis in K-562 cells -abrogated K-562 cells proliferation ↓ genes expression of HSP70, Bcl-X(L), and FOXM1 -improved Bax, caspase-3, and caspase-8 expression | [91] |
| Quercetin | In vitro | KBM7 cells | -inhibited KBM7 cell proliferation -induced cell apoptosis -blocked cell cycle at G1 phase ↓ the mRNA and protein expression of Smoothened and Glioma1 (Gli1) ↓ Bcl-2 and cyclin D1 ↑ p53 and caspase-3 expression -inhibited Hh signaling and its downstream targets in the KBM7 cells | [92] |
| Quercetin and curcumin | K562 cells | -induced changes in several genes in 10 different pathways related to cell proliferation, apoptosis, cell cycle, inflammation, hypoxia, and oxidative stress ↓ CDKN1B, AKT1, IFN-γ ↑ BTG2, CDKN1A, FAS | [93] | |
| Genistein | In vitro | CML and CFU-Mix BFU-E and CFU-GM hematopoietic progenitors | -suppressed colony formation -suppressed progenitor cell growth ↓ marrow BCR/ABL+ progenitors -exerted a strong antiproliferative effect on CFU-Mix, BFU-E, and CFU-GM ↓ the percentage of leukemic LTC-IC -induced apoptosis of CML mononuclear and CD34+ | [94] |
| EGCG | In vitro | K562, K562R, KCL-22, BaF3/p210 and BaF3/p210T315I cell lines | -inhibited the proliferation of CML cell lines and primary CML cells ↓ the mitochondrial membrane permeability of CML cell lines -induced the apoptosis of CML cells through caspase-independent and AIF-mediated cell death pathways -suppressed the expression of Bcr/Abl and phospho-Bcr/Abl in CML cell -regulated Bcr/Abl downstream JAK2/STAT3/AKT and p38-MAPK/JNK signaling pathways in CML | [95] |
| EGCG | In vitro | KU812 cells | -induced ASM activation and lipid raft clustering in CML cells -induced phosphorylation of protein kinase Cδ at Ser664 -induced cell death via the cGMP/ASM pathway in CML cells | [96] |
| Caffeic acid | In vitro | K562 cells | -induced mitochondrial membrane depolarization, genomic DNA fragmentation, and phosphatidylserine exposure, hallmarks of apoptosis ↓ cell proliferation -↑ expression of two cell cycle repressor genes, CDKN1A and CHES1 | [97] |
| Chlorogenic acid | In vitro | K562, Molt 4, U937, THP-1, REH cell lines | -induced apoptosis of several Bcr-Abl–positive CML cell lines and primary cells from CML patients in vitro -destroyed Bcr-Abl–positive K562 cells in vivo -no effect on the growth and viability of Bcr-Abl–negative lymphocytic and myeloid cell lines and primary CML cells -↓ viability of Bcr-Abl–positive cells in vitro and in vivo -induced apoptosis of Bcr-Abl–positive cells -inhibited autophosphorylation of p210Bcr-Abl fusion protein -modulated MAP kinase pathways in K562 cells | [98] |
| In vivo | Nude female mice | |||
| Emodin | In vitro | K562 cells | -inhibited the growth of K562 cells harboring BCR-ABL in vitro and in vivo -induced apoptosis by inhibition of PETN/PI3K/Akt level and deletion of BCR-ABL | [99] |
| Gambogic acid | In vitro | K562 cells | -inhibited the viability of K562 cells -induced the accumulation of autophagic vacuoles and up-regulation of two autophagy-related proteins (Beclin 1 and LC3) ↓ mRNA levels of BCR/ABL fusion genes and SQSTM1/sequestosome 1 (p62) protein levels -induced cell death through autophagy and apoptosis pathways in CML K562 cells | [100] |
| Gambogic acid | In vitro | KBM5, KBM5-T315I, and K562 cells | -induced apoptosis and cell proliferation inhibition in CML cells -induced caspase activation in CML cells -inhibited the proteasome function in CML cells -down-regulated Bcr-Abl protein and inhibited its downstream signaling -inhibited the growth of imatinib-resistant Bcr-Abl-T315I xenografts in nude mice | [101] |
| Curcumin | In vitro | K562 and LAMA84 cells | ↓ miR-21 levels in CML cells -induced PTEN expression in CML cells ↓ AKT phosphorylation and VEGF expression and release ↓ CML cells migration ↓ Bcr-Abl expression in CML cells through the cellular increase of miR-196b -curcumin-treated mice developed smaller tumors | [102] |
| In vivo | SCID mice | |||
| Resveratrol | In vitro | K562 cells | -induced apoptosis and phosphorylation of H2AX at Ser139 -stimulated p38 and JNK activation in K562 cells during apoptosis -p38 and JNK regulated resveratrol-induced H2AX phosphorylation in K562 cells ↓ phosphorylation of histone H3 at Ser10 | [103] |
| Resveratrol | In vitro | K562 cells | ↓ cell viability and triggered cell apoptosis in K562 cells ↑ Bax/Bcl-2 ratio and release of cytochrome c into the cytosol -induced the activation of caspase-3 ↑ cleaved PARP | [104] |
| Resveratrol | In vitro | K562 and K562/IMA-3 cells | -inhibited cell growth ↑ in loss of mitochondrial membrane potential ↑ caspase-3 activity -induced apoptosis in K562 and K562/IMA-3 cells | [105] |
| Phenethyl isothiocyanate (PEITC) | In vitro | K-562, KU812 cells | ↑ cytotoxic efficacy of IM PEITC in combination with IM down-regulated the expression of p210bcr/abl in chronic myelogenous leukemia cell lines (K-562) -inhibited the expressions of PKCα, PKCβII, and PKCζ (both phosphorylated and total form) ↓ expression of Raf1 and ERK1/2, two important target proteins in PKC signaling cascade ↓ expression of Raf1 and ERK1/2 through Bcr-Abl and PKC inhibition | [106] |
| PEITC | In vitro | K562 cells | -induced cell death through the induction of ROS stress and oxidative damage -suppressed cell growth and caused apoptosis by promoting Fas and Fas ligand expression, increasing ROS generation and by the successive release of cytochrome c as well as the activation of caspase-9 and caspase-3 | [107] |
| Indole-3-carbinol | In vitro | K562 cells | -promoted mitochondrial apoptosis of CML-derived K562 cells, as evidenced by the activation of caspases and PARP cleavage ↓ the cellular levels of phospho-Akt and phospho-signal transducer and activator of transcription 5 -activated the p38 mitogen-activated protein kinase ↓ expression of human telomerase and c-Myc | [108] |
| Emodin | In vitro | K562 cells | -inhibited K562 cell viability in vitro -caused K562 cell morphological changes in vitro -induced K562 cell division cycle arrest at G0/G1 phase in vitro -induced K562 cell apoptosis in vitro and in vivo ↓ Bcl-2 ↑ Bax -induced the activation of caspase-3, -8, and -9 in vitro and in vivo ↓ the tumor volume and tumor weight in nude mice | [109] |
| In vivo | BALB/c nude mice | |||
| 6-Shogaol | In vitro | K562S and K562R cells | -inhibited cell viability, induced apoptosis in both K562S and K562R ↑ pro-apoptotic Bax gene and ↓ anti-apoptotic BCL-2 gene expression levels significantly in both treated K562S and K562R cells ↑ MDR-1 mRNA expression level in K562S and K562R cells ↓ MRP-1 mRNA expression level in K562S cells | [110] |
| Parthenolide and DMAPT | In vitro | K562, Meg-01, and KCL-22, HL-60 cells | ↓ viability of CML bulk and progenitor cells -induced cell death in CML cells ↑ ROS levels in CML cells -inhibited NF-κB activation in CML cells -inhibited cell proliferation and arrested cell cycle of CML cells in G0 and G2 phases, correlated with down-regulation of cyclin D1 and cyclin A | [111] |
| Bioactive Compound | In Vitro/In Vivo/Clinical Study | Cancer Cell Line and Animal Model | Bioactive Effect | References |
|---|---|---|---|---|
| Quercetin | In vivo | C57BL/6J (CD45.2+) and B6.SJL-PtprcaPepcb/BoyJ mice | -enhanced the cytotoxicity of Adriamycin to leukemic cells -improved the survival of mice with T-ALL -enhanced the SOD activity and reduced the MDA content in the heart | [122] |
| Antho 50 | In vitro | Jurkat cells | -induced apoptosis in Jurkat cells ↑ ROS formation ↑ tumor suppressor p73 and cell cycle regulator p21 expression levels -cleaved caspase-3 expression levels ↓ expression levels of p-Akt, survivin, PcG proteins, HDACs, DNMT1, and UHRF1 | [117] |
| Delphinidin-3-O-glucoside and delphinidin-3-O-rutinoside | In vitro | Jurkat and Molt-4 cell lines | -induced proapoptotic response in Jurkat cells | [121] |
| DMF | In vitro | YCUB series | -induced G0/G1 cell cycle arrest ↓ the expression of phosphorylated retinoblastoma-associated protein 1 ↑ induced apoptosis in ALL cell lines ↓ the intracellular levels of glutathione -antagonized the cytotoxicity of 4-hydroperoxy-cyclophosphamide, cytarabine, vincristine, and L-asparaginase in all tested ALL cells | [123] |
| EGCG | In vitro | Jurkat cells | -decreased viability of cells -induced apoptosis of lymphoblastic leukemia cells -enhanced Fas expression in Jurkat cells -increased caspase-3 positive cells | [124] |
| Curcumin | In vitro | 697, REH, RS4;11, and SupB15 cells | -suppressed the viability in B-Pre-ALL cell lines -induced apoptosis in B-Pre-ALL cell lines via activation of caspase-8 and truncation of BID protein ↑ the ratio of Bax/Bcl-2 -induced the dephosphorylation of the constitutive phosphorylated AKT/PKB ↓ the expression of cIAP1, and XIAP ↑ ROS | [125] |
| Curcumin | In vitro | B6p210 and B6T315I cells | -inhibited proliferation -induced apoptosis ↓ NF-κB levels ↑ p53 levels ↓ c-Abl levels in cells expressing the wild, but not the mutant, BCR-ABL oncogene -improved survival in diseased mice and ↓ WBC and GFP cell counts | [126] |
| In vivo | B6 mice | |||
| Resveratrol | In vitro | GC-resistant CEM-C1-15, Jurkat, Molt-4, and GC-sensitive CEM-C7-14 cells | -inhibited the proliferation and induced apoptosis and autophagy in T-ALL cells -induced cell cycle arrest at G0/G1 phase via up regulating CDK inhibitors p21 and p27 and down-regulating cyclin A and cyclin D1 ↓ the expression of antiapoptotic proteins (Mcl-1 and Bcl-2) ↑ the expression of proapoptotic proteins (Bax, Bim, and Bad) | [127] |
| Pterostilbene | In vitro | Jurkat and Molt-4 cells | ↓ cell viability with different extent in two ALL cell lines -induced apoptosis in lymphoblastic cells ↑ Fas expression both in mRNA and surface levels that results in apoptosis signal transduction improvement, which sensitized cells to apoptosis by immune effector cells | [128] |
| Gambogic acid | In vitro | Jurkat and Molt-4 cells | -inhibited proliferation, induced apoptosis, and activated autophagy in T-ALL cell lines -antileukemic effect against peripheral blood lymphocyte cells in patients with ALL -inhibited phospho-GSK3β S9 protein levels to inactivate Wnt signaling -suppressed β-catenin protein levels | [112] |
| Gallic acid | In vitro | Jurkat cells | ↓ cell viability | [129] |
| Parthenolide | In vitro | B- and T-ALL cells | -effective against bulk B- and T-ALL cells -prevented engraftment of multiple LIC populations in NOD/LtSz-scld IL-2Rγc-null mice -restoration of normal murine hemopoiesis | [130] |
| In vivo | NOD/LtSz-scld IL-2Rγc-null mice | |||
| Thymoquinone | In vitro | Jurkat cells | ↓ cell viability of Jurkat cells -induced apoptosis in Jurkat lymphoblastic cell line -combination with doxorubicine lead to a synergistic cytotoxicity | [131] |
| Thymoquinone | In vitro | CEMss cells | -induced apoptosis in CEMss cells ↑ in chromatin condensation in the cell nucleus ↑ number of cellular DNA breaks in treated cells ↑ apoptosis with cell death-transducing signals by a down-regulation of Bcl-2 and up-regulation of Bax ↑ generation of cellular ROS, HSP70, and activation of caspases -3 and -8 -the mitochondrial apoptosis was associated with the S phase cell cycle arrest | [132] |
| Indole-3-carbinol | In vitro | NALM-6 cells | -induced cell-growth inhibition, G1 cell-cycle arrest, and apoptosis in NALM-6 cells ↑ the expression of p53, p21, and Bax proteins -induced p53 accumulation and expression of pro-apoptotic p53 target genes ↑ PUMA, NOXA, and Apaf-1 -suppressed NF-κB activation and inhibited the protein expression of NF-κB-regulated antiapoptotic (IAP1, Bcl-xL, Bcl-2, XIAP) and proliferative (c-Myc) gene products -repressed antiapoptotic NF-κB target genes -potentiated doxorubicin-induced apoptosis through caspase activation and PARP cleavage -inhibited doxorubicin-induced NF-κB activation in NALM-6 cells | [133] |
| Bioactive Compound | In Vitro/In Vivo/Clinical Study | Cancer Cell Line and Animal Model | Bioactive Effect | References |
|---|---|---|---|---|
| Antho 50 | In vitro | -induced apoptosis in B CLL cells -induced an early caspase-3 activation and UHRF1 down-regulation in B CLL cells independently of the status of tumor suppressor genes p53 and p73 ↓ Bcl-2 associated with Bad dephosphorylation -induced PEG-catalase-sensitive formation of ROS in B CLL cells | [144] | |
| Luteolin | In vitro | HG-3 and EHEB cells | -↑ the apoptotic cell population in both CLL cells lines by increasing the activities of caspase-3 and -9 and triggering the intrinsic apoptotic pathway | [145] |
| Apigenin | In vitro | Eheb cells | -induced apoptosis in human lymphoma B cells in vitro -prevented the reverted mutations | [146] |
| EGCG | In vitro | CLL B cells | -induced CLL B-cell apoptosis -suppressed Bcl-2, XIAP, and Mcl-1 -down-regulated the phosphorylation of VEGF-R1 and VEGF-R2 | [147] |
| Chrysin | In vitro | CLL and healthy B-lymphocytes | ↑ cytotoxicity, intracellular ROS, mitochondrial membrane potential collapse, ADP/ATP ratio, caspase-3 activation and apoptosis -inhibited complex II and ATPases in cancerous mitochondria -promoted apoptosis in CLL B-lymphocytes by selectively targeting of mitochondria | [148] |
| Chrysin | In vitro | MOLT-4 and JVM-13 cell lines, B-CLL cells derived from 28 patients | -induced the activation of proapoptotic Bax ↓ the expression of antiapoptotic Bcl-2 protein -released cytochrome c from mitochondria into cytosol -activated caspase-3 -induced apoptosis of peripheral blood lymphocytes isolated from human CLL patients | [90] |
| Resveratrol | In vitro | WSU-CLL and ESKOL cells | -inhibited proliferation in leukemic B-cell lines -induced apoptosis in the two cell lines as well as in B-CLL patients’ cells, as evidenced by the increase in annexin V binding, caspase activation, DNA fragmentation, and decrease of the mitochondrial transmembrane potential -inhibited in situ NO release in WSU-CLL, ESKOL, and B-CLL patients’ cells -down-regulation of the two anti-apoptotic proteins iNOS and Bcl-2 | [149] |
| In vitro | leukemic lymphocytes from patients with B-CLL | |||
| Resveratrol and quercetin | In vitro | human 232B4 CLL cells | ↓ proliferation of human 232B4 CLL cells -induced apoptosis in 232B4 CLL cells through induction of caspase-3 activity -inhibited cell cycle progression -arrested cell cycle mainly in G0/G1 | [140] |
| Curcumin | Clinical study | Twenty-one patients with stage 0/1 CLL | ↓ ALC at four patients (20%) ↓ in ALC was accompanied by an ↑ in CD4, CD8, and NK cells | [150] |
| Curcumin and rapamycin | PBMCs from patients with B-CLL | -induced apoptosis in B-CLL cells obtained from patients with CLL ↑ caspase-9, -3, and -7 activity ↓ anti-apoptotic Bcl-2 levels, ↑ the pro-apoptotic protein Bax | [151] | |
| Allanxanthone C and macluraxanthone | In vivo | xenograft murine model of human CLL | -prolongation of the survival in mice injected with the two xanthones | [152] |
| PEITC | In vitro | Primary leukemia cells | -killed CLL cells with 17p-deletion -cytotoxic effect against p53-/-leukemia cells from mice in vitro and in vivo ↑ ROS accumulation and GSH depletion in p53-deficient CLL cells ↓ Mcl-1 protein in CLL cells -induced leukemia cell death in mice -prolonged the median survival time of the animals | [153] |
| In vivo | TCL1-Tg:p53+ mice | |||
| Parthenolide | In vitro | cells isolated from CLL patients | -induced apoptosis in CLL cells -activated the mitochondrial pathway of apoptosis -induced a proapoptotic Bax conformational change, release of mitochondrial cytochrome c, and caspase activation ↓ nuclear levels of the antiapoptotic transcription factor NF-κB and diminished phosphorylation of its negative regulator IκB | [154] |
| Parthenolide | In vitro | PBMCs from B-CLL patients | -displayed potent cytotoxic and apoptotic effects on B-CLL cells in vitro ↓ in the cell viability of B-CLL cells | [155] |
| Allicin | In vitro | PBMC cells CD20+ cells | -induced in vitro apoptosis -killed the CD20+ tumor B cells via apoptosis -exhibited tumoricidal effect in vivo | [156] |
| In vivo | BALB/c mice | |||
| Indole-3-carbinol | In vitro | PBMCs cells hMSC-TERT cells | -induced cytotoxicity in CLL cells but not in normal lymphocytes ↓ XIAP and cIAP1/2 and induced caspase 9-dependent apoptosis of CLL cells -sinergic activity with fludarabine in CLL cells and overcame stroma-mediated drug-resistance -mechanism of cell death involved p53-dependent and independent apoptosis -sinergic activity with F-ara-A in all types of CLL cells and restored F-ara-A sensitivity in fludarabine-resistant CLL cells | [157] |
| In vivo | C57bl/6 mice |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotoraci, C.; Ciceu, A.; Sasu, A.; Miutescu, E.; Hermenean, A. The Anti-Leukemic Activity of Natural Compounds. Molecules 2021, 26, 2709. https://doi.org/10.3390/molecules26092709
Cotoraci C, Ciceu A, Sasu A, Miutescu E, Hermenean A. The Anti-Leukemic Activity of Natural Compounds. Molecules. 2021; 26(9):2709. https://doi.org/10.3390/molecules26092709
Chicago/Turabian StyleCotoraci, Coralia, Alina Ciceu, Alciona Sasu, Eftimie Miutescu, and Anca Hermenean. 2021. "The Anti-Leukemic Activity of Natural Compounds" Molecules 26, no. 9: 2709. https://doi.org/10.3390/molecules26092709
APA StyleCotoraci, C., Ciceu, A., Sasu, A., Miutescu, E., & Hermenean, A. (2021). The Anti-Leukemic Activity of Natural Compounds. Molecules, 26(9), 2709. https://doi.org/10.3390/molecules26092709








