Established Beta Amyloid Pathology Is Unaffected by TREM2 Elevation in Reactive Microglia in an Alzheimer’s Disease Mouse Model
Abstract
1. Introduction
2. Results
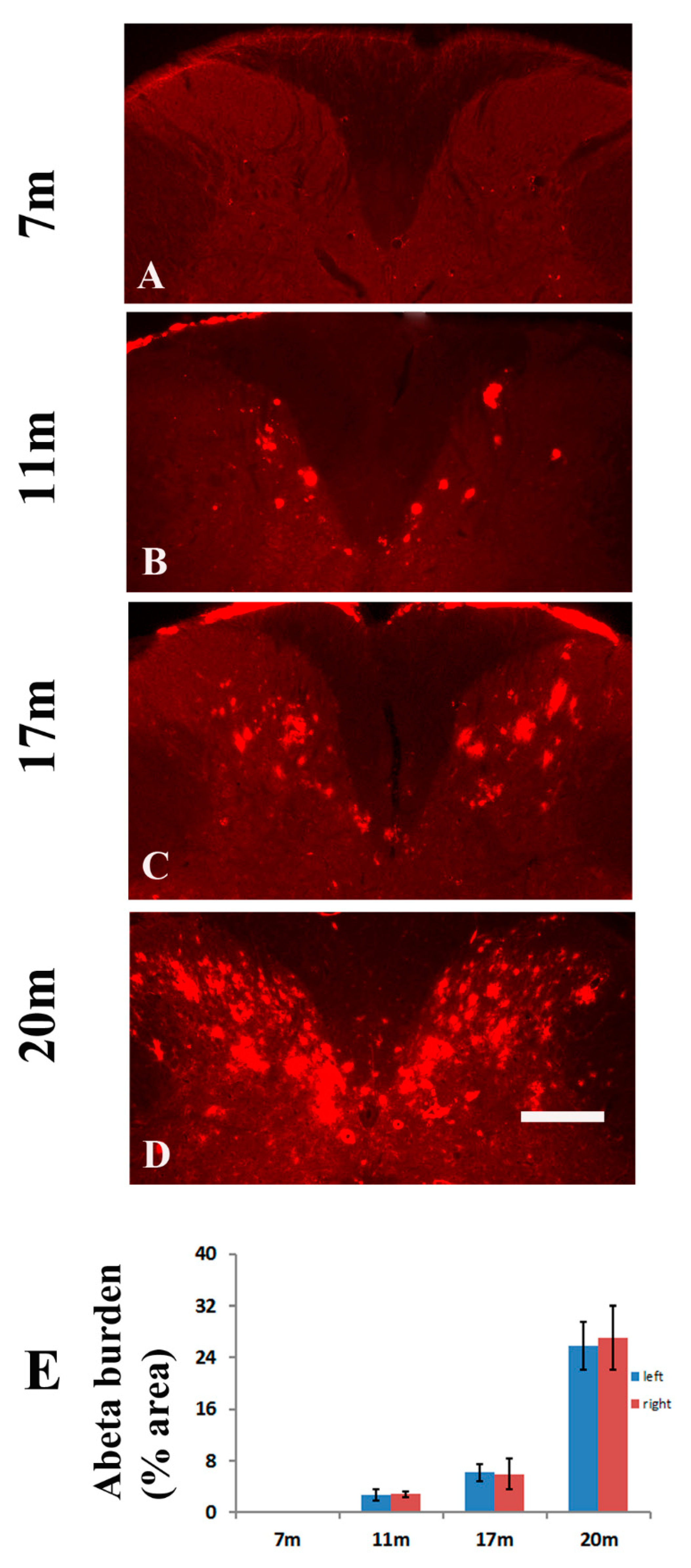
2.1. Age-Dependent Amyloid Burden in the Spinal Cord of TgCRND8 Mice
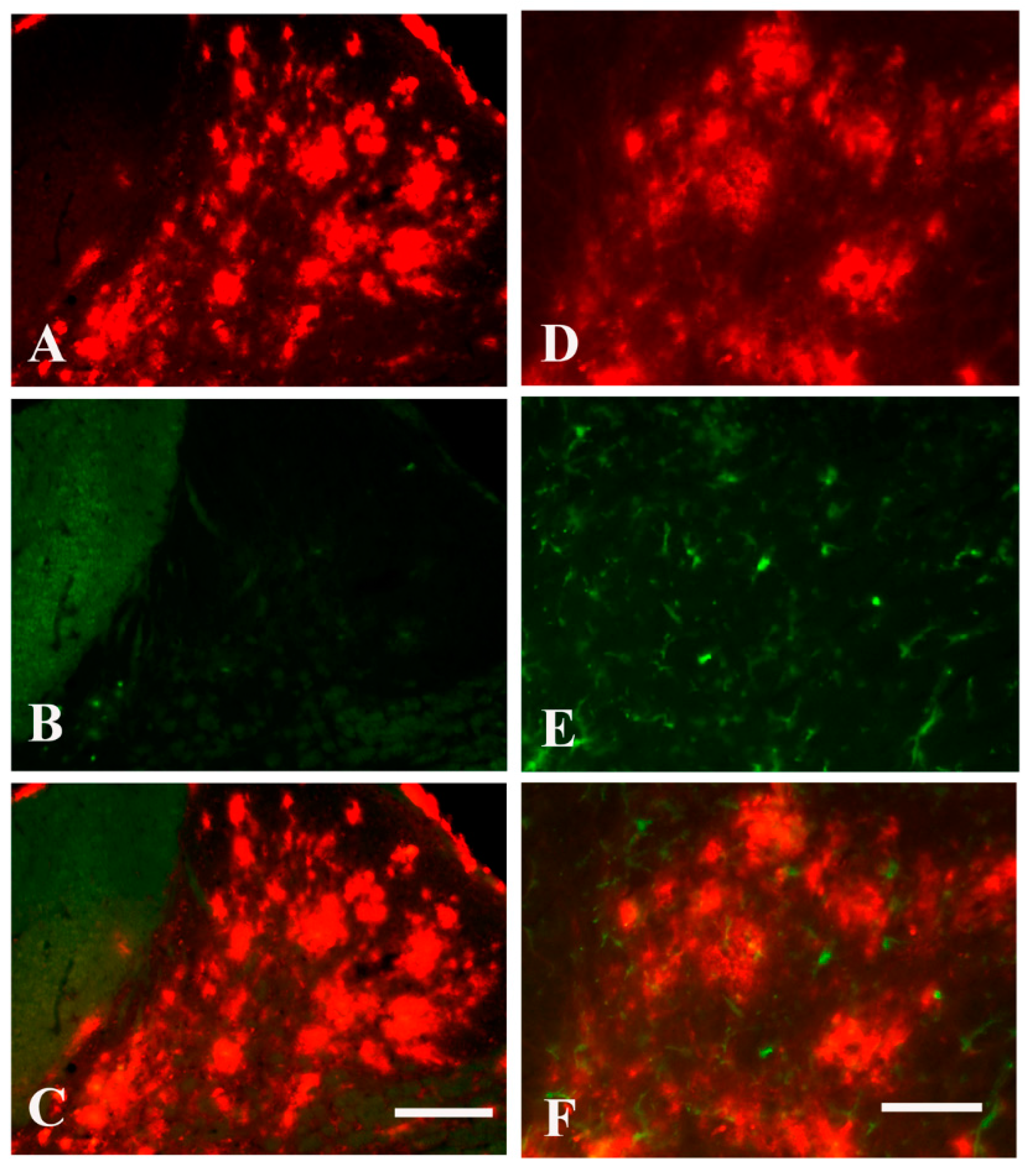
2.2. Brachial Plexus Nerve Ligation Led to Microglia Activation in the Dorsal Horn of Spinal Cord in Tgcrnd8 Mice
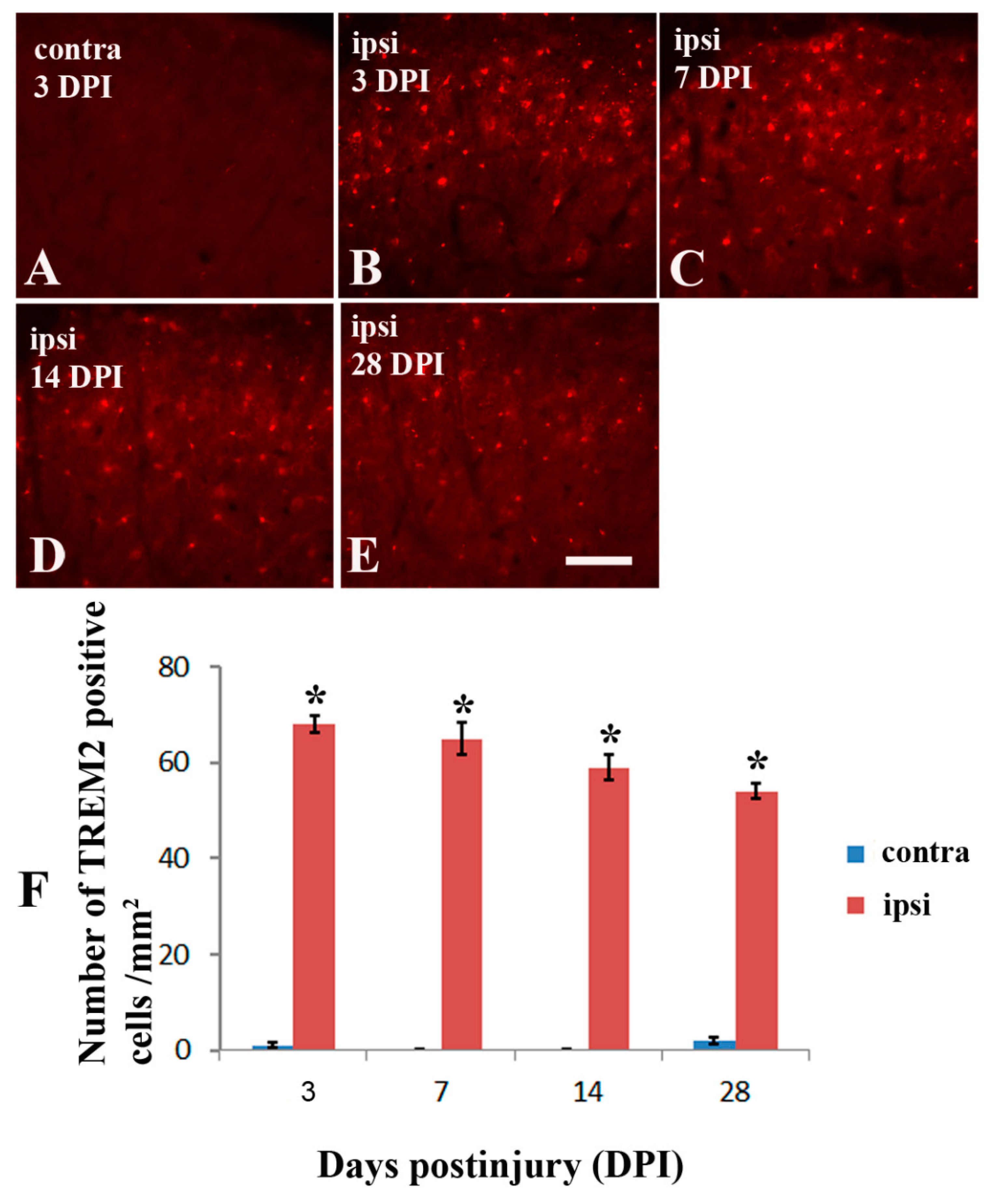
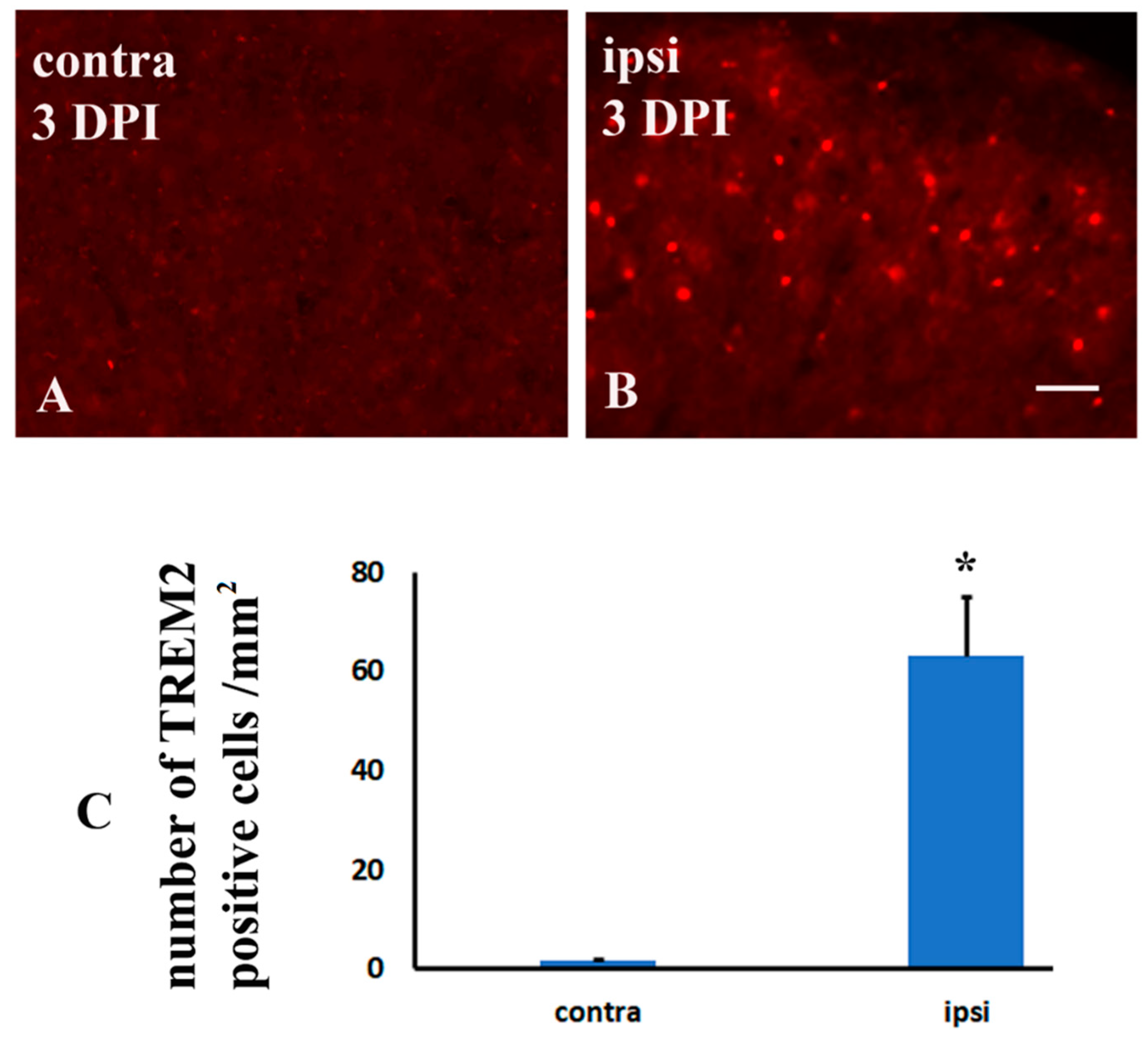
2.3. Brachial Plexus Nerve Ligation Induced TREM2 in Microglia in the Dorsal Horn of Spinal Cord in Tgcrnd8 Mice
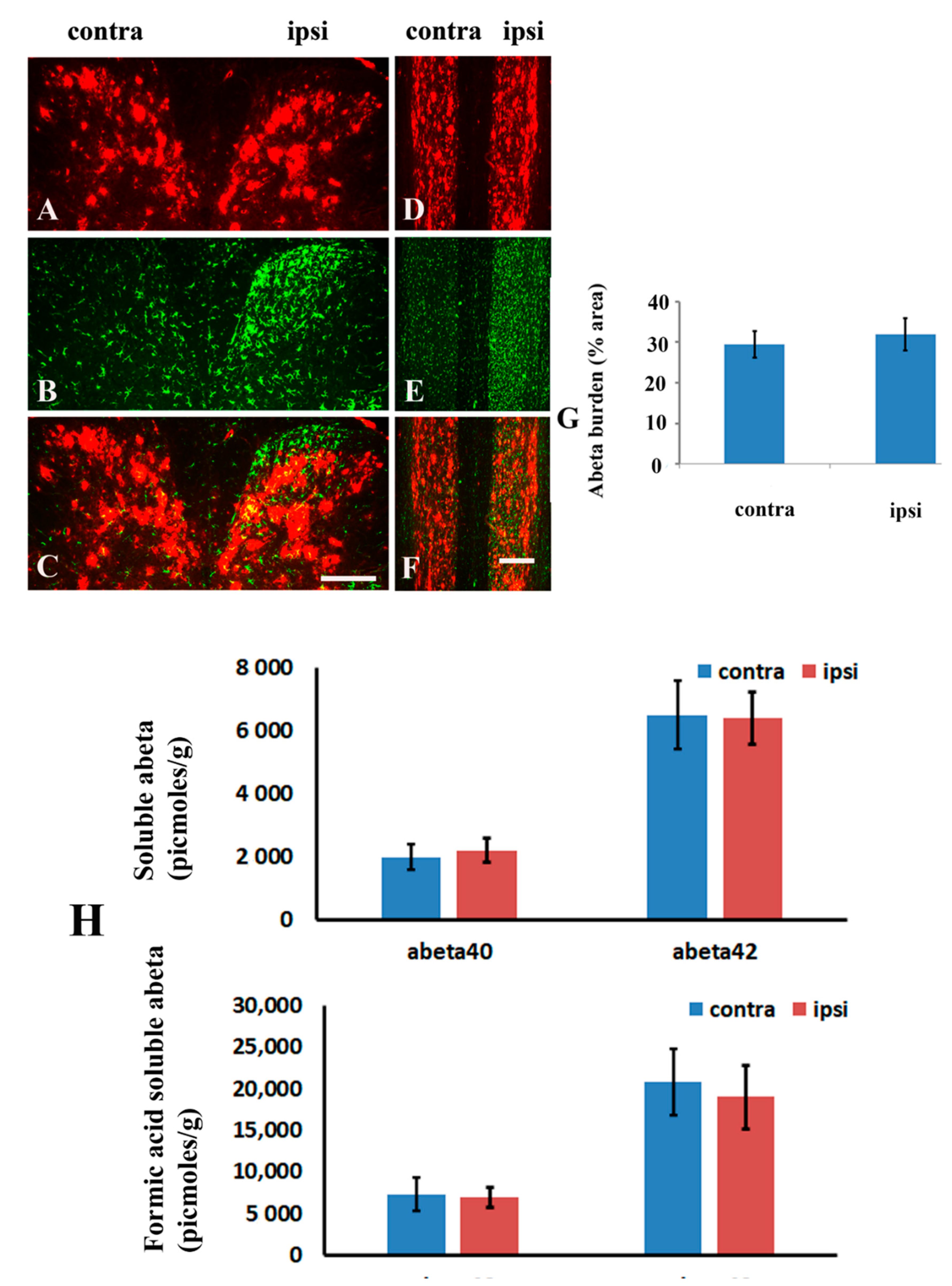
2.4. Brachial Plexus Nerve Ligation Did Not Affect Amyloid Burden in Spinal Cord
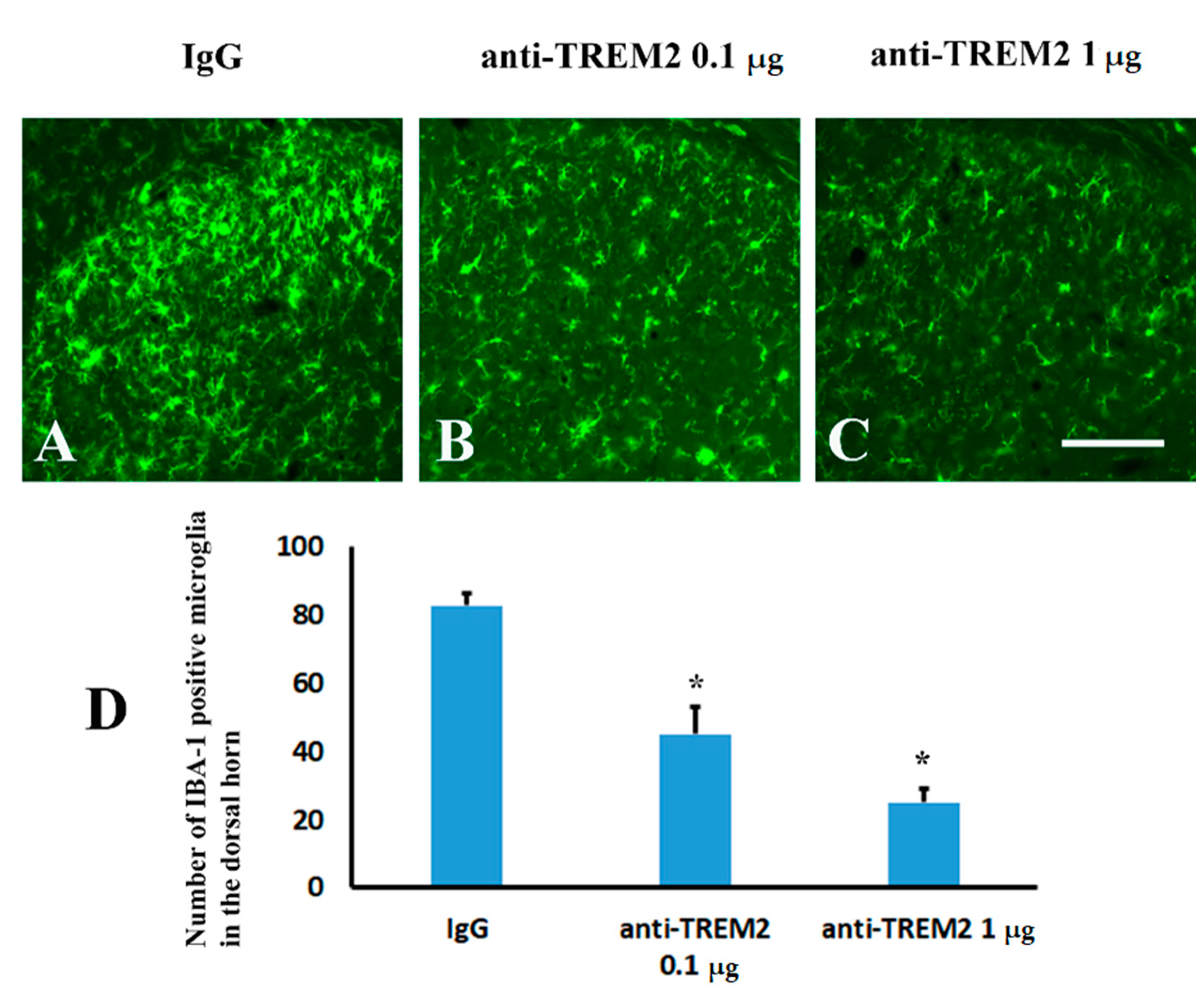
2.5. Blockage of Spinal TREM2 Did Not Attenuate Aβ Plaques in the Spinal Cord Following Peripheral Nerve Ligation
3. Discussion
3.1. TREM2 Elevation Did Not Affect Aβ Plaque Deposition in Aged Tgcrnd8 Mice
3.2. Microglia Activation Did Not Affect Aβ Plaque Deposition in Aged Tgcrnd8 Mice
4. Materials and Methods
4.1. Animal Model
4.2. Spinal Nerve Injury Model and Surgical Procedures
4.3. Intrathecal Injection of TREM2 Neutralizing Antibody
4.4. Perfusion and Tissue Processing
4.5. Immunohistochemical Analysis
4.6. Assessment of Aβ Levels
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.; Hasan, A.; Tiwari, S.; Pandey, L.M. Therapeutic Advancement in Alzheimer Disease: New Hopes on the Horizon? CNS Neurol. Disord. Drug Targets 2018, 17, 571–589. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Gratuze, M.; Leyns, C.E.G.; Holtzman, D.M. New insights into the role of TREM2 in Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 1–16. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, C.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.; Younkin, S.; et al. Alzheimer Genetic Analysis, TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef]
- Guerreiro, R.J.; Lohmann, E.; Bras, J.M.; Gibbs, J.R.; Rohrer, J.D.; Gurunlian, N.; Dursun, B.; Bilgic, B.; Hanagasi, H.; Gurvit, H.; et al. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal demen-tia-like syndrome without bone involvement. JAMA Neurol. 2013, 70, 78–84. [Google Scholar] [CrossRef]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 associated with the risk of Alz-heimer’s disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef]
- Wolfe, C.M.; Fitz, N.F.; Nam, K.N.; Lefterov, I.; Koldamova, R. The Role of APOE and TREM2 in Alzheimers Disease—Current Understanding and Perspectives. Int. J. Mol. Sci. 2018, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.D.; Daggett, A.; Gu, X.; Jiang, L.L.; Langfelder, P.; Li, X.; Wang, N.; Zhao, Y.; Park, C.S.; Cooper, Y.; et al. Elevated TREM2 Gene Dosage Reprograms Microglia Responsivity and Ameliorates Patho-logical Phenotypes in Alzheimer’s Disease Models. Neuron 2018, 97, 1032–1048.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, X.; Li, X.; Jiang, L.L.; Gui, X.; Liu, Y.; Sun, Y.; Zhu, B.; Pina-Crespo, J.C.; Zhang, M.; et al. TREM2 Is a Receptor for beta-Amyloid that Mediates Microglial Function. Neuron 2018, 97, 1023–1031.e7. [Google Scholar] [CrossRef] [PubMed]
- Parhizkar, S.; Arzberger, T.; Brendel, M.; Kleinberger, G.; Deussing, M.; Focke, C.; Nuscher, B.; Xiong, M.; Ghasemigharagoz, A.; Katzmarski, N.; et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat. Neurosci. 2019, 22, 191–204. [Google Scholar] [CrossRef]
- Wang, Y.; Cella, M.; Mallinson, K.; Ulrich, J.D.; Young, K.L.; Robinette, M.L.; Gilfillan, S.; Krishnan, G.M.; Sudhakar, S.; Zinselmeyer, B.H.; et al. TREM2 Lipid Sensing Sustains the Microglial Response in an Alzheimer’s Disease Model. Cell 2015, 160, 1061–1071. [Google Scholar] [CrossRef]
- Ulrich, J.D.; Finn, M.B.; Wang, Y.; Shen, A.; Mahan, T.E.; Jiang, H.; Stewart, F.R.; Piccio, L.; Colonna, M.; Holtzman, D.M. Altered microglial response to Abeta plaques in APPPS1-21 mice heterozygous for TREM2. Mol. Neurodegener. 2014, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ulland, T.K.; Ulrich, J.D.; Song, W.; Tzaferis, J.A.; Hole, J.T.; Yuan, P.; Mahan, T.E.; Shi, Y.; Gilfillan, S.; et al. TREM2-mediated early microglial response limits dif-fusion and toxicity of amyloid plaques. J. Exp. Med. 2016, 213, 667–675. [Google Scholar] [CrossRef]
- Jay, T.R.; Miller, C.M.; Cheng, P.J.; Graham, L.C.; BeMiller, S.; Broihier, M.L.; Xu, G.; Margevicius, D.; Karlo, J.C.; Sousa, G.L.; et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 2015, 212, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Udeochu, J.; Sayed, F.A.; Gan, L. TREM2 and Amyloid Beta: A Love-Hate Relationship. Neuron 2018, 97, 991–993. [Google Scholar] [CrossRef]
- Yuan, Q.; Su, H.; Zhang, Y.; Chau, W.H.; Ng, C.T.; Song, Y.Q.; Huang, J.D.; Wu, W.; Lin, Z.X. Amyloid pathology in spinal cord of the transgenic Alzheimer’s disease mice is correlated to the corticospinal tract pathway. J. Alzheimer’s Dis. 2013, 35, 675–685. [Google Scholar] [CrossRef]
- Yuan, Q.; Su, H.; Zhang, Y.; Chau, W.H.; Ng, C.T.; Wu, W.; Lin, Z.-X. Existence of different types of senile plaques between brain and spinal cord of TgCRND8 mice. Neurochem. Int. 2013, 62, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Yang, J.; Wu, W.; Lin, Z.X. Motor deficits are independent of axonopathy in an Alzheimer’s disease mouse model of TgCRND8 mice. Oncotarget 2017, 8, 97900–97912. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, M.; Konishi, H.; Sayo, A.; Takai, T.; Kiyama, H. TREM2/DAP12 Signal Elicits Proinflammatory Response in Microglia and Exacerbates Neuropathic Pain. J. Neurosci. 2016, 36, 11138–11150. [Google Scholar] [CrossRef] [PubMed]
- Chishti, M.A.; Yang, D.-S.; Janus, C.; Phinney, A.L.; Horne, P.; Pearson, J.; Strome, R.; Zuker, N.; Loukides, J.; French, J.; et al. Early-onset Amyloid Deposition and Cognitive Deficits in Transgenic Mice Expressing a Double Mutant Form of Amyloid Precursor Protein 695. J. Biol. Chem. 2001, 276, 21562–21570. [Google Scholar] [CrossRef]
- Inoue, K.; Tsuda, M. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci. 2018, 19, 138–152. [Google Scholar] [CrossRef]
- Tozaki-Saitoh, H.; Masuda, J.; Kawada, R.; Kojima, C.; Yoneda, S.; Masuda, T.; Inoue, K.; Tsuda, M. Transcription factor MafB contributes to the activation of spinal microglia underlying neuropathic pain development. Glia 2019, 67, 729–740. [Google Scholar] [CrossRef]
- Tsuda, M. Microglia in the CNS and Neuropathic Pain. Adv. Exp. Med. Biol. 2018, 1099, 77–91. [Google Scholar]
- Tsuda, M. Modulation of Pain and Itch by Spinal Glia. Neurosci. Bull. 2017, 34, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Y.; Zhou, Y.; Cui, W.Q.; Hu, X.M.; Du, L.X.; Mi, W.L.; Chu, Y.X.; Wu, G.C.; Wang, Y.Q.; Mao-Ying, Q.L. Triggering receptor expressed on myeloid cells 2 (TREM2) dependent microglial activation promotes cisplatin-induced peripheral neu-ropathy in mice. Brain Behav. Immun. 2018, 68, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Tyzack, G.E.; Sitnikov, S.; Barson, D.; Adams-Carr, K.L.; Lau, N.K.; Kwok, J.C.; Zhao, C.; Franklin, R.J.M.; Karadottir, R.T.; Fawcett, J.W.; et al. Astrocyte response to motor neuron injury promotes structural synaptic plasticity via STAT3-regulated TSP-1 expression. Nat. Commun. 2014, 5, 4294. [Google Scholar] [CrossRef]
- Jiang, T.; Wan, Y.; Zhang, Y.-D.; Zhou, J.-S.; Gao, Q.; Zhu, X.-C.; Shi, J.-Q.; Lu, H.; Tan, L.; Yu, J.-T. TREM2 Overexpression has No Improvement on Neuropathology and Cognitive Impairment in Aging APPswe/PS1dE9 Mice. Mol. Neurobiol. 2017, 54, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Kim, S.K.; Jin, S.M.; Hwang, E.M.; Kim, Y.S.; Huh, K.; Mook-Jung, I. IFN-gamma-induced BACE1 expression is mediated by activation of JAK2 and ERK1/2 signaling pathways and direct binding of STAT1 to BACE1 promoter in astrocytes. Glia 2007, 55, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Sastre, M.; Dumitrescu-Ozimek, L.; Dewachter, I.; Walter, J.; Klockgether, T.; Van Leuven, F. Focal glial acti-vation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J. Neuroinflammation 2005, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Ringheim, G.; Szczepanik, A.M.; Petko, W.; Burgher, K.L.; Zu Zhu, S.; Chao, C.C. Enhancement of beta-amyloid precursor protein transcription and expression by the soluble interleukin-6 receptor/interleukin-6 complex. Mol. Brain Res. 1998, 55, 35–44. [Google Scholar] [CrossRef]
- Sastre, M.; Klockgether, T.; Heneka, M.T. Contribution of inflammatory processes to Alzheimer’s disease: Molecular mechanisms. Int. J. Dev. Neurosci. 2006, 24, 167–176. [Google Scholar] [CrossRef]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Micro-glia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature 2017, 552, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Shaftel, S.S.; Carlson, T.J.; Olschowka, J.A.; Kyrkanides, S.; Matousek, S.B.; O’Banion, M.K. Chronic interleukin-1beta ex-pression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J. Neurosci. 2007, 27, 9301–9309. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, J.; Toft, M.; Hickman, S.E.; Means, T.K.; Terada, K.; Geula, C.; Luster, A.D. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007, 13, 432–438. [Google Scholar] [CrossRef]
- Wilcock, D.M.; DiCarlo, G.; Henderson, D.; Jackson, J.; Clarke, K.; Ugen, K.E.; Gordon, M.N.; Morgan, D. Intracranially ad-ministered anti-Abeta antibodies reduce beta-amyloid deposition by mechanisms both independent of and associated with microglial activation. J. Neurosci. 2003, 23, 3745–3751. [Google Scholar] [CrossRef]
- Wilcock, D.M.; Munireddy, S.K.; Rosenthal, A.; Ugen, K.E.; Gordon, M.N.; Morgan, D. Microglial activation facilitates Abeta plaque removal following intracranial anti-Abeta antibody administration. Neurobiol. Dis. 2004, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Pluvinage, J.V.; Haney, M.S.; Smith, B.A.H.; Sun, J.; Iram, T.; Bonanno, L.; Li, L.; Lee, D.P.; Morgens, D.W.; Yang, A.C.; et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature 2019, 568, 187–192. [Google Scholar] [CrossRef]
- Yuan, Q.; Su, H.; Chau, W.H.; Toa Ng, C.; Huang, J.D.; Wu, W.; Lin, Z.X. Behavioral stress fails to accelerate the onset and progression of plaque pathology in the brain of a mouse model of Alzheimer’s disease. PLoS ONE 2013, 8, e53480. [Google Scholar] [CrossRef][Green Version]
- Yuan, Q.; Su, H.; Guo, J.; Tsang, K.Y.; Cheah, K.S.; Chiu, K.; Yang, J.; Wong, W.-M.; So, K.-F.; Huang, J.-D.; et al. Decreased c-Jun expression correlates with impaired spinal motoneuron regeneration in aged mice following sciatic nerve crush. Exp. Gerontol. 2012, 47, 329–336. [Google Scholar] [CrossRef]
- Yuan, Q.; Su, H.; Guo, J.; Wu, W.; Lin, Z.-X. Induction of phosphorylated c-Jun in neonatal spinal motoneurons after axonal injury is coincident with both motoneuron death and regeneration. J. Anat. 2014, 224, 575–582. [Google Scholar] [CrossRef]
- Yuan, Q.; Su, H.; Wu, W.; Lin, Z.X. P75 and phosphorylated c-Jun are differentially regulated in spinal motoneurons following axotomy in rats. Neural Regen. Res. 2012, 7, 2005–2011. [Google Scholar] [PubMed]
- Narita, M.; Yoshida, T.; Nakajima, M.; Narita, M.; Miyatake, M.; Takagi, T.; Yajima, Y.; Suzuki, T. Direct evidence for spinal cord microglia in the development of a neuropathic pain-like state in mice. J. Neurochem. 2006, 97, 1337–1348. [Google Scholar] [CrossRef] [PubMed]



| Antibodies | Species | Working Dilution | Vendor or Producer |
|---|---|---|---|
| Bam-10 | Mouse IgG | 1:3000 | Sigma |
| IBA-1 | Rabbit IgG | 1:3000 | Wako |
| TREM2 | Sheep IgG | 1:1000 | R & D systems |
| Goat anti-rabbit 488 | 1:800 | Molecular probes | |
| Goat anti-mouse 568 Donkey anti-sheep 568 | 1:800 1:800 | Molecular probes Molecular probes | |
| thioflavin S ELISA kits for Aβ40 and Aβ42 | 2% | Sigma Wako |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Q.; Liu, X.; Zhang, Y.; Xian, Y.-F.; Zou, J.; Zhang, X.; Huang, P.; Song, Y.-Q.; Lin, Z.-X. Established Beta Amyloid Pathology Is Unaffected by TREM2 Elevation in Reactive Microglia in an Alzheimer’s Disease Mouse Model. Molecules 2021, 26, 2685. https://doi.org/10.3390/molecules26092685
Yuan Q, Liu X, Zhang Y, Xian Y-F, Zou J, Zhang X, Huang P, Song Y-Q, Lin Z-X. Established Beta Amyloid Pathology Is Unaffected by TREM2 Elevation in Reactive Microglia in an Alzheimer’s Disease Mouse Model. Molecules. 2021; 26(9):2685. https://doi.org/10.3390/molecules26092685
Chicago/Turabian StyleYuan, Qiuju, Xiaodong Liu, Yi Zhang, Yan-Fang Xian, Juntao Zou, Xie Zhang, Pengyun Huang, You-Qiang Song, and Zhi-Xiu Lin. 2021. "Established Beta Amyloid Pathology Is Unaffected by TREM2 Elevation in Reactive Microglia in an Alzheimer’s Disease Mouse Model" Molecules 26, no. 9: 2685. https://doi.org/10.3390/molecules26092685
APA StyleYuan, Q., Liu, X., Zhang, Y., Xian, Y.-F., Zou, J., Zhang, X., Huang, P., Song, Y.-Q., & Lin, Z.-X. (2021). Established Beta Amyloid Pathology Is Unaffected by TREM2 Elevation in Reactive Microglia in an Alzheimer’s Disease Mouse Model. Molecules, 26(9), 2685. https://doi.org/10.3390/molecules26092685







