Astaxanthin for the Food Industry
Abstract
1. Introduction
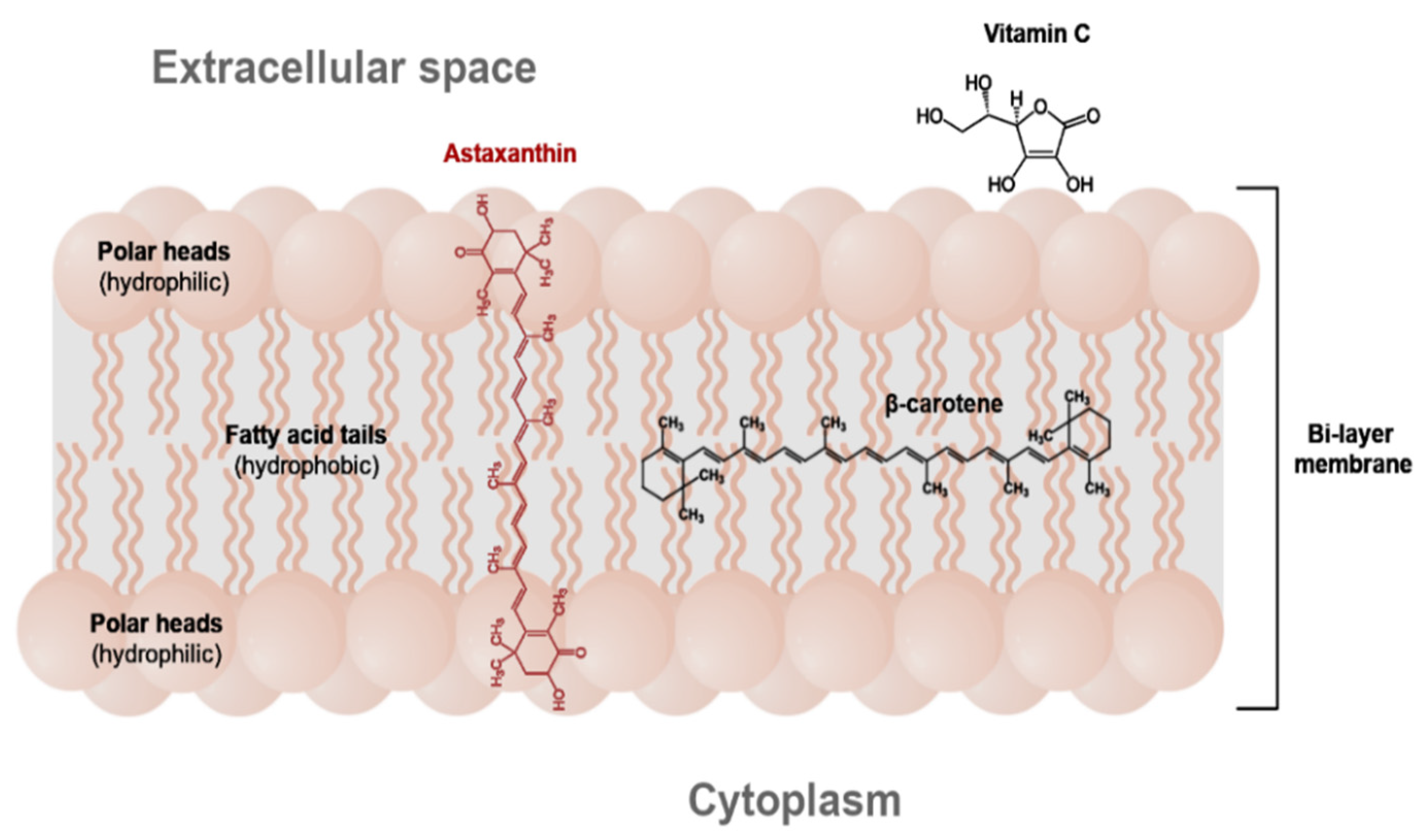
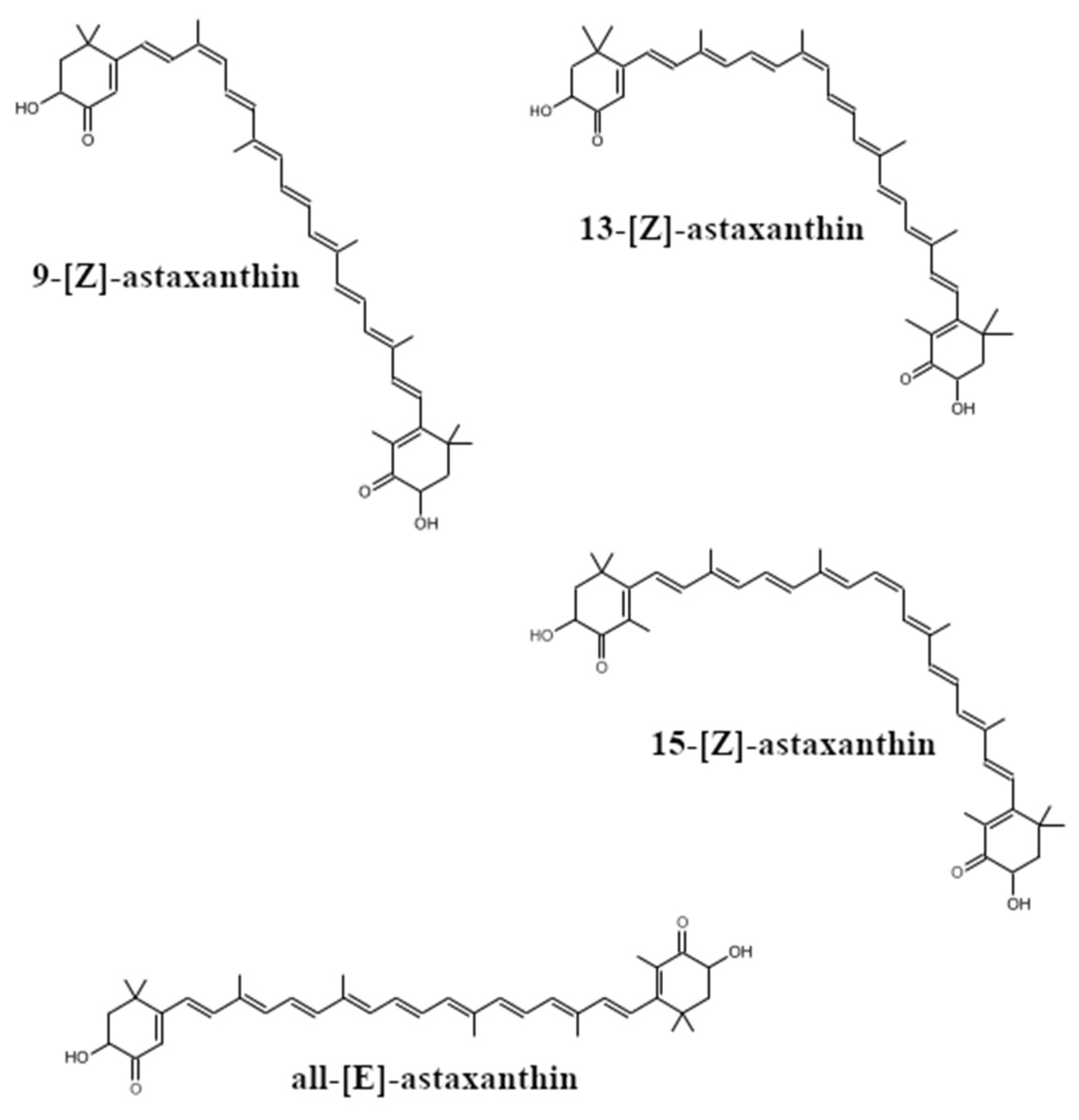
2. The Occurrence, Structure and Industrial Potential of Astaxanthin
3. Commercial Sources of Astaxanthin
3.1. Chemical Synthesis
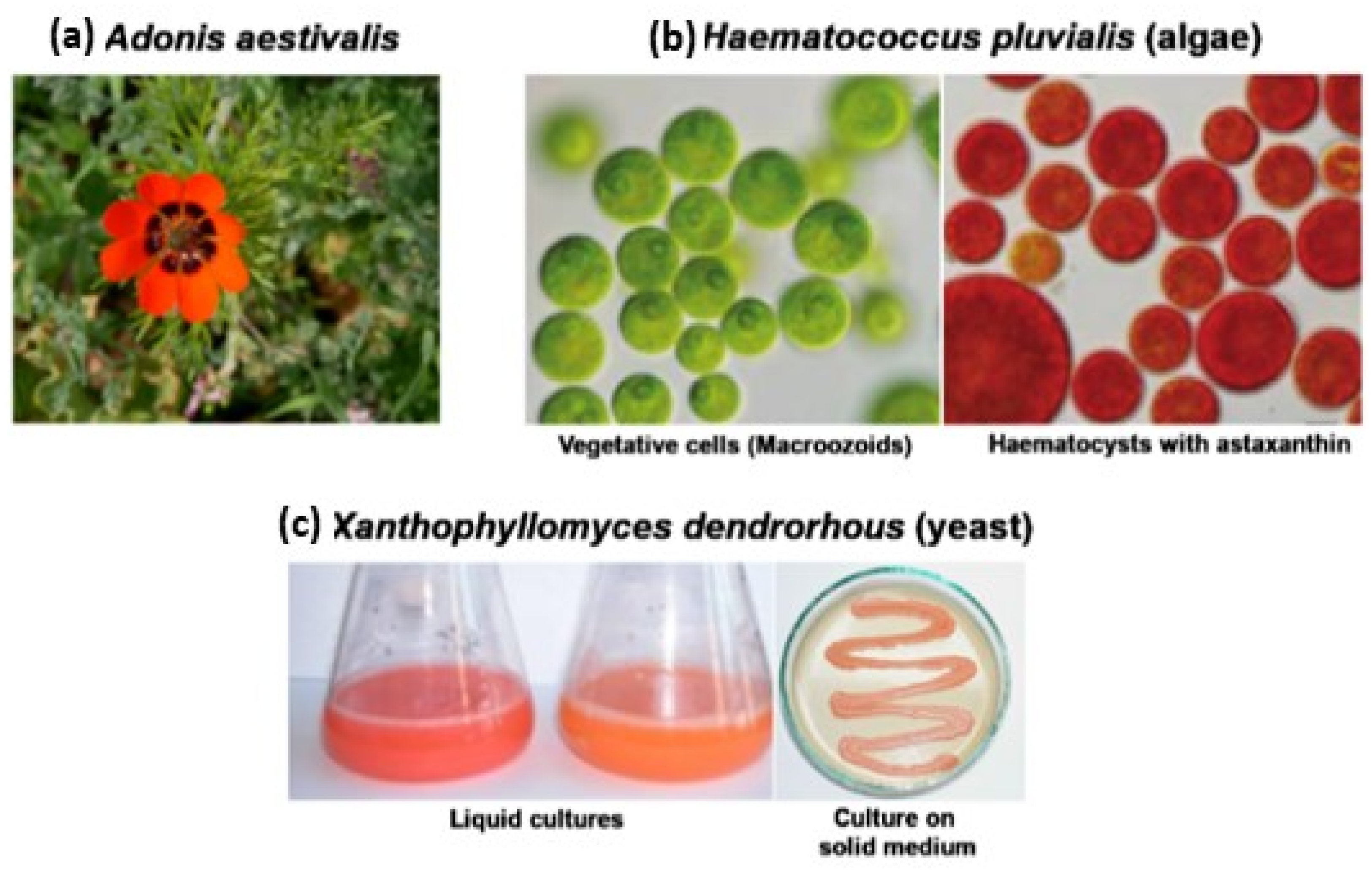
3.2. Natural Systems as a Source of Astaxanthin
3.2.1. Astaxanthin from Plant Systems
3.2.2. Microbiological Synthesis of Astaxanthin
3.2.3. Crustacean Byproducts
4. Methods of Astaxanthin Stabilization and Improvement of Its Bioavailability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Olson, J.A. Biological Actions of Carotenoids. J. Nutr. 1989, 119, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.T.; Cysewski, G.R. Commercial Potential for Haematococcus Microalgae as a Natural Source of Astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Hossain, A.K.M.M.; Brennan, M.A.; Mason, S.L.; Guo, X.; Zeng, X.A.; Brennan, C.S. The Effect of Astaxanthin-Rich Microalgae “Haematococcus Pluvialis” and Wholemeal Flours Incorporation in Improving the Physical and Functional Properties of Cookies. Foods 2017, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Akiba, Y.; Sato, K.; Takahashi, K.; Matsushita, K.; Komiyama, H.; Tsunekawa, H.; Nagao, H. Meat Color Modification in Broiler Chickens by Feeding Yeast Phaffia Rhodozyma Containing High Concentrations of Astaxanthin. J. Appl. Poult. Res. 2001, 10, 154–161. [Google Scholar] [CrossRef]
- Anarjan, N.; Tan, C.P. Chemical Stability of Astaxanthin Nanodispersions in Orange Juice and Skimmed Milk as Model Food Systems. Food Chem. 2013, 139, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Cerezal Mezquita, P.; Espinosa Álvarez, C.; Palma Ramírez, J.; Bugueño Muñoz, W.; Salinas Fuentes, F.; Ruiz-Domínguez, M.D. Isotonic Beverage Pigmented with Water-Dispersible Emulsion from Astaxanthin Oleoresin. Molecules 2020, 25, 841. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as Feed Supplement in Aquatic Animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Astaxanthin Market Size, Share & Trends Analysis Report By Source, By Product (Dried Algae Meal, Oil, Softgel), By Application (Nutraceutical, Cosmetics, Aquaculture and Animal Feed), And Segment Forecasts, 2020–2027. In Market Analysis Report; Grand View Research: San Francisco, CA, USA, 2020; p. 76. Available online: https://www.grandviewresearch.com/industry-analysis/global-astaxanthin-market (accessed on 15 February 2020).
- Breithaupt, D.E. Modern Application of Xanthophylls in Animal Feeding—A Review. Trends Food Sci. Technol. 2007, 18, 501–506. [Google Scholar] [CrossRef]
- Johnson, E.A.; Lewis, M.J. Astaxanthin Formation by the Yeast Phaffia Rhodozyma. J. Gen. Microbiol. 1979, 115, 173–183. [Google Scholar] [CrossRef]
- Jacobson, G.K.; Jolly, S.O.; Sedmak, J.J.; Skatrud, T.J.; Wasilewski, J.M. Astaxanthin Over-Producing Strains of Phaffia Rhodozyma, Methods for Their Cultivation and Their Use in Animal Feeds 2000. U.S. Patent No. 6,015,684. Available online: https://patentimages.storage.googleapis.com/f5/54/57/1e152be0823090/US6015684.pdf (accessed on 18 January 2000).
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus Astaxanthin: Applications for Human Health and Nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Lorenz, R.T. A Technical Review of Haematococcus Algae. In NatuRoseTM Tech. Bull.; Cyanotech Corporation: Kailua-Kona, HI, USA, 1999; Volume 60, pp. 1–12. [Google Scholar]
- Yamashita, E. Let Astaxanthin Be Thy Medicine. PharmaNutrition 2015, 3, 115–122. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient Radical Trapping at the Surface and inside the Phospholipid Membrane Is Responsible for Highly Potent Antiperoxidative Activity of the Carotenoid Astaxanthin. Biochim. Biophys. Acta Biomembr. 2001, 1512, 251–258. [Google Scholar] [CrossRef]
- Santocono, M.; Zurria, M.; Berrettini, M.; Fedeli, D.; Falcioni, G. Influence of Astaxanthin, Zeaxanthin and Lutein on DNA Damage and Repair in UVA-Irradiated Cells. J. Photochem. Photobiol. B Biol. 2006, 85, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Osawa, T. Cis Astaxanthin and Especially 9-Cis Astaxanthin Exhibits a Higher Antioxidant Activity in Vitro Compared to the All-Trans Isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as Singlet Oxygen Quenchers in Marine Organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Kao, C.-J.; Huang, H.-Y.; Huang, S.-Y.; Chen, C.-Y.; Lin, Y.-S.; Wen, Z.-H.; Wang, H.-M.D. Astaxanthin Reduces MMP Expressions, Suppresses Cancer Cell Migrations, and Triggers Apoptotic Caspases of In Vitro and In Vivo Models in Melanoma. J. Funct. Foods 2017, 31, 20–31. [Google Scholar] [CrossRef]
- Kavitha, K.; Kowshik, J.; Kishore, T.K.K.; Baba, A.B.; Nagini, S. Astaxanthin Inhibits NF-ΚB and Wnt/β-Catenin Signaling Pathways via Inactivation of Erk/MAPK and PI3K/Akt to Induce Intrinsic Apoptosis in a Hamster Model of Oral Cancer. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4433–4444. [Google Scholar] [CrossRef]
- Che, H.; Li, Q.; Zhang, T.; Wang, D.; Yang, L.; Xu, J.; Yanagita, T.; Xue, C.; Chang, Y.; Wang, Y. Effects of Astaxanthin and Docosahexaenoic-Acid-Acylated Astaxanthin on Alzheimer’s Disease in APP/PS1 Double-Transgenic Mice. J. Agric. Food Chem. 2018, 66, 4948–4957. [Google Scholar] [CrossRef]
- Grimmig, B.; Daly, L.; Subbarayan, M.; Hudson, C.; Williamson, R.; Nash, K.; Bickford, P.C. Astaxanthin Is Neuroprotective in an Aged Mouse Model of Parkinson’s Disease. Oncotarget 2018, 9, 10388–10401. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef] [PubMed]
- Capelli, B.; Keily, S.; Cysewski, G.R. The medical research of astaxanthin eye health. In The Medical Research of Astaxanthin; Cyanotech Corporation: Kailua-Kona, HI, USA, 2010; pp. 75–100. [Google Scholar]
- Li, H.; Li, J.; Hou, C.; Li, J.; Peng, H.; Wang, Q. The Effect of Astaxanthin on Inflammation in Hyperosmolarity of Experimental Dry Eye Model in Vitro and in Vivo. Exp. Eye Res. 2020, 197, 108113. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, H.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of Low-Density Lipoprotein Oxidation by Astaxanthin. J. Atheroscler. Thromb. 2000, 7, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Mimoun-Benarroch, M.; Lallement, J.; Rhazi, L.; Boroch, C.; Hugot, C.; Niamba, C.-N.; Younes, H.; Depeint, F. Free Form Astaxanthin from Yeast Phaffia Rhodozyma Fermentation Reduces Plasmatic Triglycerides in a Pre-Obesity Diet-Induced Dyslipidaemia Mouse Model. J. Food Compos. Anal. 2018, 65, 11–15. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A Potential Therapeutic Agent in Cardiovascular Disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef]
- Wang, X.; Willén, R.; Wadström, T. Astaxanthin-Rich Algal Meal and Vitamin C Inhibit Helicobacter Pylori Infection in BALB/cA Mice. Antimicrob. Agents Chemother. 2000, 44, 2452–2457. [Google Scholar] [CrossRef]
- Kaneko, M.; Kishimoto, Y.; Suzuki, R.; Kawai, Y.; Tateya, I.; Hirano, S. Protective Effect of Astaxanthin on Vocal Fold Injury and Inflammation Due to Vocal Loading: A Clinical Trial. J. Voice 2017, 31, 352–358. [Google Scholar] [CrossRef]
- Zhou, L.; Gao, M.; Xiao, Z.; Zhang, J.; Li, X.; Wang, A. Protective Effect of Astaxanthin against Multiple Organ Injury in a Rat Model of Sepsis. J. Surg. Res. 2015, 195, 559–567. [Google Scholar] [CrossRef]
- Park, J.; Chyun, J.; Kim, Y.; Line, L.L.; Chew, B.P. Astaxanthin Decreased Oxidative Stress and Inflammation and Enhanced Immune Response in Humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef]
- Yasui, Y.; Hosokawa, M.; Mikami, N.; Miyashita, K.; Tanaka, T. Dietary Astaxanthin Inhibits Colitis and Colitis-Associated Colon Carcinogenesis in Mice via Modulation of the Inflammatory Cytokines. Chem. Biol. Interact. 2011, 193, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic Benefits of Astaxanthin on Humans Subjects. Acta Biochim. Pol. 2012, 59. [Google Scholar] [CrossRef]
- Scientific Opinion on the Safety of Astaxanthin-Rich Ingredients (AstaREAL A1010 and AstaREAL L10) as Novel Food Ingredients. EFSA J. 2014, 12, 3757. [CrossRef]
- Buesen, R.; Schulte, S.; Strauss, V.; Treumann, S.; Becker, M.; Gröters, S.; Carvalho, S.; van Ravenzwaay, B. Safety Assessment of [3S, 3′S]-Astaxanthin—Subchronic Toxicity Study in Rats. Food Chem. Toxicol. 2015, 81, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Beutner, S.; Bloedorn, B.; Frixel, S.; Hernández Blanco, I.; Hoffmann, T.; Martin, H.-D.; Mayer, B.; Noack, P.; Ruck, C.; Schmidt, M.; et al. Quantitative Assessment of Antioxidant Properties of Natural Colorants and Phytochemicals: Carotenoids, Flavonoids, Phenols and Indigoids. The Role of β-Carotene in Antioxidant Functions: Antioxidant Properties of Natural Colorants and Phytochemicals. J. Sci. Food Agric. 2001, 81, 559–568. [Google Scholar] [CrossRef]
- Jackson, H.; Braun, C.L.; Ernst, H. The Chemistry of Novel Xanthophyll Carotenoids. Am. J. Cardiol. 2008, 101, S50–S57. [Google Scholar] [CrossRef] [PubMed]
- Snoeijs, P.; Häubner, N. Astaxanthin Dynamics in Baltic Sea Mesozooplankton Communities. J. Sea Res. 2014, 85, 131–143. [Google Scholar] [CrossRef]
- Chayen, N.E.; Cianci, M.; Grossmann, J.G.; Habash, J.; Helliwell, J.R.; Nneji, G.A.; Raftery, J.; Rizkallah, P.J.; Zagalsky, P.F. Unravelling the Structural Chemistry of the Colouration Mechanism in Lobster Shell. Acta Crystallogr. D Biol. Crystallogr. 2003, 59, 2072–2082. [Google Scholar] [CrossRef]
- Storebakken, T.; Sørensen, M.; Bjerkeng, B.; Hiu, S. Utilization of Astaxanthin from Red Yeast, Xanthophyllomyces Dendrorhous, in Rainbow Trout, Oncorhynchus Mykiss: Effects of Enzymatic Cell Wall Disruption and Feed Extrusion Temperature. Aquaculture 2004, 236, 391–403. [Google Scholar] [CrossRef]
- Fang, N.; Wang, C.; Liu, X.; Zhao, X.; Liu, Y.; Liu, X.; Du, Y.; Zhang, Z.; Zhang, H. De Novo Synthesis of Astaxanthin: From Organisms to Genes. Trends Food Sci. Technol. 2019, 92, 162–171. [Google Scholar] [CrossRef]
- Renstrøm, B.; Berger, H.; Liaaen-Jensen, S. Esterified, Optical Pure (3S, 3′S)-Astaxanthin from Flowers of Adonis Annua. Biochem. Syst. Ecol. 1981, 9, 249–250. [Google Scholar] [CrossRef]
- Maoka, T.; Etoh, T.; Kishimoto, S.; Sakata, S. Carotenoids and Their Fatty Acid Esters in the Petals of Adonis Aestivalis. J. Oleo Sci. 2011, 60, 47–52. [Google Scholar] [CrossRef]
- Hernández-Becerra, J.A.; Ochoa-Flores, A.A.; Soto-Rodriguez, I.; Rodriguez-Estrada, M.T.; García, H.S. Effect of Cooking Conditions on Cholesterol Oxidation and Astaxanthin in Dried Salted Shrimp: Oxysterols in Cooked Shrimp. Eur. J. Lipid Sci. Technol. 2014, 116, 872–884. [Google Scholar] [CrossRef]
- Niamnuy, C.; Devahastin, S.; Soponronnarit, S.; Vijaya Raghavan, G.S. Kinetics of Astaxanthin Degradation and Color Changes of Dried Shrimp during Storage. J. Food Eng. 2008, 87, 591–600. [Google Scholar] [CrossRef]
- Liñán-Cabello, M.A.; Paniagua-Michel, J.; Hopkins, P.M. Bioactive Roles of Carotenoids and Retinoids in Crustaceans: Carotenoids and Retinoids in Crustaceans. Acquac. Nutr. 2002, 8, 299–309. [Google Scholar] [CrossRef]
- Tsushlma, M.; Kawakami, T.; Mine, M.; Matsuno, T. The Role of Carotenoids in the Development of the Sea Urchin Pseudocentrotus Depressus. Invertebr. Reprod. Dev. 1997, 32, 149–153. [Google Scholar] [CrossRef]
- Haijima, Y.; Karino, K. Algal-Diet Enhances Sexual Ornament, Growth And Reproduction in the Guppy. Behaviour 2004, 141, 585–601. [Google Scholar] [CrossRef]
- Ahmadi, M.R.; Bazyar, A.A.; Safi, S.; Ytrestøyl, T.; Bjerkeng, B. Effects of Dietary Astaxanthin Supplementation on Reproductive Characteristics of Rainbow Trout (Oncorhynchus Mykis). J. Appl. Ichthyol. 2006, 22, 388–394. [Google Scholar] [CrossRef]
- Dansou, D.M.; Wang, H.; Nugroho, R.D.; He, W.; Zhao, Q.; Zhang, J. Assessment of Response to Moderate and High Dose Supplementation of Astaxanthin in Laying Hens. Animals 2021, 11, 1138. [Google Scholar] [CrossRef] [PubMed]
- Ausich, R.L. Commercial Opportunities for Carotenoid Production by Biotechnology. Pure Appl. Chem. 1997, 69, 2169–2174. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Sáiz, M.; de la Fuente, J.L.; Barredo, J.L. Xanthophyllomyces Dendrorhous for the Industrial Production of Astaxanthin. Appl. Microbiol. Biotechnol. 2010, 88, 645–658. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial Astaxanthin Production Derived by Green Alga Haematococcus Pluvialis: A Microalgae Process Model and a Techno-Economic Assessment All through Production Line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Calo, P.; Velazquez, J.B.; Sieiro, C.; Blanco, P.; Longo, E.; Villa, T.G. Analysis of Astaxanthin and Other Carotenoids from Several Phaffia RhodozymaMutants. J. Agric. Food Chem. 1995, 43, 1396–1399. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F. Kinetics for the Reversible Isomerization Reaction of Trans-Astaxanthin. Food Chem. 2001, 73, 131–137. [Google Scholar] [CrossRef]
- Widmer, E.; Zell, R.; Broger, E.A.; Crameri, Y.; Wagner, H.P.; Dinkel, J.; Schlageter, M.; Lukáč, T. Technische Verfahren Zur Synthese von Carotinoiden Und Verwandten Verbindungen Aus 6-Oxo-Isophoron. II. Ein Neues Konzept Für Die Synthese von (3RS, 3′RS )-Astaxanthin. Helv. Chim. Acta 1981, 64, 2436–2446. [Google Scholar] [CrossRef]
- Nguyen, K.D. Astaxanthin: Comperative Case of Synthetic vs. Natural Production. TRACE Faculty Publications and Other Works—Chemical and Biomolecular Engineering. 2013. Available online: http://trace.tennessee.edu/utk_chembiopubs/94 (accessed on 8 May 2013).
- Fang, T.J.; Chiou, T.-Y. Batch Cultivation and Astaxanthin Production by a Mutant of the Red Yeast Phaffia Rhodozyma NCHU-FS501. J. Ind. Microbiol. 1996, 16, 175–181. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2015/1415 of 20 August 2015 Concerning the Authorisation of Astaxanthin as a Feed Additive for Fish, Crustaceans and Ornamental Fish. OJEU 2015, L 220, 7–10. Available online: https://eur-lex.europa.eu/eli/reg_impl/2015/1415/oj (accessed on 21 August 2015).
- Commission Regulation (EC) No 393/2008 of 30 April 2008 Concerning the Authorisation of Astaxanthin Dimethyldisuccinate as a Feed Additive. OJEU 2008, L 117, 20–21. Available online: http://data.europa.eu/eli/reg/2008/393/oj (accessed on 1 May 2008).
- Commission Implementing Regulation (EU) 2020/998 of 9 July 2020 Concerning the Renewal of the Authorisation of Astaxanthin-Dimethyldisuccinate as a Feed Additive for Fish and Crustaceans and Repealing Regulation (EC) No 393/2008. OJEU 2020, L 221, 96–98. Available online: https://eur-lex.europa.eu/eli/reg_impl/2020/998/oj (accessed on 10 July 2020).
- Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the Addition of Vitamins and Minerals and of Certain Other Substances to Foods. OJEU 2006, L 404, 26–38. Available online: http://data.europa.eu/eli/reg/2006/1925/oj (accessed on 30 December 2006).
- Villalobos-Castillejos, F.; Cerezal-Mezquita, P.; Hernández-De Jesús, M.L.; Barragán-Huerta, B.E. Production and Stability of Water-Dispersible Astaxanthin Oleoresin from Phaffia Rhodozyma. Int. J. Food Sci. Technol. 2013, 48, 1243–1251. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F.; Liu, X.; Li, X.-Z. Carotenoid Composition in the Green Microalga Chlorococcum. Food Chem. 2002, 76, 319–325. [Google Scholar] [CrossRef]
- Bhosale, P.; Bernstein, P.S. Microbial Xanthophylls. Appl. Microbiol. Biotechnol. 2005, 68, 445–455. [Google Scholar] [CrossRef]
- Cunningham, F.X.; Gantt, E. A Portfolio of Plasmids for Identification and Analysis of Carotenoid Pathway Enzymes: Adonis Aestivalis as a Case Study. Photosynth. Res. 2007, 92, 245–259. [Google Scholar] [CrossRef]
- Gwak, Y.; Hwang, Y.; Wang, B.; Kim, M.; Jeong, J.; Lee, C.-G.; Hu, Q.; Han, D.; Jin, E. Comparative Analyses of Lipidomes and Transcriptomes Reveal a Concerted Action of Multiple Defensive Systems against Photooxidative Stress in Haematococcus Pluvialis. J. Exp. Bot. 2014, 65, 4317–4334. [Google Scholar] [CrossRef]
- Mawson, R. Astaxanthin from Flowers of the Genus Adonis Patent US5453565A. 1995. Available online: https://patents.google.com/patent/US5453565A/en (accessed on 26 September 1995).
- Cunningham, F.X. Carotenoid Ketolase Genes and Gene Products, Production of Ketocarotenoids and Methods of Modifying Carotenoids Using the Genes Patent US6551807B1. 2003. Available online: https://patents.google.com/patent/US6551807 (accessed on 22 April 2003).
- Cunningham, F.X.; Gantt, E. A Study in Scarlet: Enzymes of Ketocarotenoid Biosynthesis in the Flowers of Adonis Aestivalis: Adonis β-Ring Oxygenases. Plant J. 2005, 41, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.X.; Chase, C. Biochemical Route to Astaxanthin Patent US20070157339A1. 2007. Available online: https://patents.google.com/patent/US20070157339 (accessed on 5 July 2007).
- Huang, J.-C.; Zhong, Y.-J.; Liu, J.; Sandmann, G.; Chen, F. Metabolic Engineering of Tomato for High-Yield Production of Astaxanthin. Metab. Eng. 2013, 17, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Flachmann, R.; Klebsattel, M.; Luck, T.; Pfeiffer, A.-M.; Sauer, M.; Schopfer, C.R.; Voeste, D. Use of Astaxanthin-Containing Plants or Parts of Plants of the Genus Tagetes Patent AU2003264062B2. 2008. Available online: https://patents.google.com/patent/AU2003264062B2/en (accessed on 3 January 2008).
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial Pigments as Natural Color Sources: Current Trends and Future Perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678. [Google Scholar] [CrossRef]
- Ranjbar, R.; Inoue, R.; Katsuda, T.; Yamaji, H.; Katoh, S. High Efficiency Production of Astaxanthin in an Airlift Photobioreactor. J. Biosci. Bioeng. 2008, 106, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Meng, C.; Zhang, X.; Xu, D.; Miao, X.; Wang, Y.; Yang, L.; Lv, H.; Chen, L.; Ye, N. Induction of Salicylic Acid (SA) on Transcriptional Expression of Eight Carotenoid Genes and Astaxanthin Accumulation in Haematococcus Pluvialis. Enzym. Microb. Technol. 2012, 51, 225–230. [Google Scholar] [CrossRef]
- Wen, Z.; Liu, Z.; Hou, Y.; Liu, C.; Gao, F.; Zheng, Y.; Chen, F. Ethanol Induced Astaxanthin Accumulation and Transcriptional Expression of Carotenogenic Genes in Haematococcus Pluvialis. Enzym. Microb. Technol. 2015, 78, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Chidambara Murthy, K.N.; Ravishankar, G.A. Microorganisms and Microalgae as Sources of Pigments for Food Use: A Scientific Oddity or an Industrial Reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods. OJEU 2017, L 351, 72–201. Available online: https://eur-lex.europa.eu/eli/reg_impl/2017/2470/oj (accessed on 30 December 2017).
- Flen, B.; Christensen, I.; Larsen, R.; Johansen, S.R.; Johnson, E.A. Astaxanthin-Producing Yeast Cells, Methods for Their Preparation and Their Use Patent EP0367765B2. 2004. Available online: https://patentimages.storage.googleapis.com/b8/b4/a0/a6cae586a0456c/EP0367765B2.pdf (accessed on 28 April 2004).
- White, D.A.; Page, G.I.; Swaile, J.; Moody, A.J.; Davies, S.J. Effect of Esterification on the Absorption of Astaxanthin in Rainbow Trout, Oncorhynchus Mykiss (Walbaum): Absorption of Astaxanthin by Rainbow Trout. Aquac. Res. 2002, 33, 343–350. [Google Scholar] [CrossRef]
- An, G.-H.; Schuman, D.B.; Johnson, E.A. Isolation of Phaffia Rhodozyma Mutants with Increased Astaxanthin Content. Appl. Environ. Microbiol. 1989, 55, 116–124. [Google Scholar] [CrossRef]
- Stachowiak, B. Astaxanthin Synthesis by Xanthophyllomyces Dendrorhous DSM 5626 and Its Astaxanthin Overproducing Mutants on Xylose Media under Diferent Illumination. Acta Sci. Pol. Technol. Aliment. 2014, 13, 279–288. [Google Scholar] [CrossRef][Green Version]
- Schmidt, I.; Schewe, H.; Gassel, S.; Jin, C.; Buckingham, J.; Hümbelin, M.; Sandmann, G.; Schrader, J. Biotechnological Production of Astaxanthin with Phaffia Rhodozyma/Xanthophyllomyces Dendrorhous. Appl. Microbiol. Biotechnol. 2011, 89, 555–571. [Google Scholar] [CrossRef]
- Gassel, S.; Breitenbach, J.; Sandmann, G. Genetic Engineering of the Complete Carotenoid Pathway towards Enhanced Astaxanthin Formation in Xanthophyllomyces Dendrorhous Starting from a High-Yield Mutant. Appl. Microbiol. Biotechnol. 2014, 98, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhou, X.-R.; Moncalian, G.; Su, L.; Chen, W.-C.; Zhu, H.-Z.; Chen, D.; Gong, Y.-M.; Huang, F.-H.; Deng, Q.-C. Reprogramming Microorganisms for the Biosynthesis of Astaxanthin via Metabolic Engineering. Prog. Lipid Res. 2021, 81, 101083. [Google Scholar] [CrossRef]
- Mezzomo, N.; Maestri, B.; dos Santos, R.L.; Maraschin, M.; Ferreira, S.R.S. Pink Shrimp (P. Brasiliensis and P. Paulensis) Residue: Influence of Extraction Method on Carotenoid Concentration. Talanta 2011, 85, 1383–1391. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Martinez-Correa, H.A.; Paviani, L.C.; Cabral, F.A. Supercritical CO2 Extraction of Lipids and Astaxanthin from Brazilian Redspotted Shrimp Waste (Farfantepenaeus Paulensis). J. Supercrit. Fluids 2011, 56, 164–173. [Google Scholar] [CrossRef]
- Pu, J.; Bechtel, P.J.; Sathivel, S. Extraction of Shrimp Astaxanthin with Flaxseed Oil: Effects on Lipid Oxidation and Astaxanthin Degradation Rates. Biosyst. Eng. 2010, 107, 364–371. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Mahendrakar, N.S. Process Optimization for Extraction of Carotenoids from Shrimp Waste with Vegetable Oils. Bioresour. Technol. 2005, 96, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lu, W.; Lv, M.; Wang, Y.; Ding, R.; Wang, L. Extraction and Purification of Astaxanthin from Shrimp Shells and the Effects of Different Treatments on Its Content. Rev. Bras. Farm. 2019, 29, 24–29. [Google Scholar] [CrossRef]
- Durante, M.; Lenucci, M.S.; Marrese, P.P.; Rizzi, V.; De Caroli, M.; Piro, G.; Fini, P.; Russo, G.L.; Mita, G. α-Cyclodextrin Encapsulation of Supercritical CO2 Extracted Oleoresins from Different Plant Matrices: A Stability Study. Food Chem. 2016, 199, 684–693. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N.; Mahendrakar, N.S. Recovery of Carotenoids from Shrimp Waste in Organic Solvents. Waste Manag. 2006, 26, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, R.; Guo, Z.; Li, C.; Li, P. The Preparation and Stability of the Inclusion Complex of Astaxanthin with β-Cyclodextrin. Food Chem. 2007, 101, 1580–1584. [Google Scholar] [CrossRef]
- Tan, C.; Nakajima, M. β-Carotene Nanodispersions: Preparation, Characterization and Stability Evaluation. Food Chem. 2005, 92, 661–671. [Google Scholar] [CrossRef]
- Hix, L.M.; Frey, D.A.; McLaws, M.D.; Østerlie, M.; Lockwood, S.F.; Bertram, J.S. Inhibition of Chemically-Induced Neoplastic Transformation by a Novel Tetrasodium Diphosphate Astaxanthin Derivative. Carcinogenesis 2005, 26, 1634–1641. [Google Scholar] [CrossRef]
- Lockwood, S.F.; Gross, G.J. Disodium Disuccinate Astaxanthin (CardaxTM): Antioxidant and Antiinflammatory Cardioprotection. Cardiovasc. Drug Rev. 2006, 23, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, S.F.; O’Malley, S.; Mosher, G.L. Improved Aqueous Solubility of Crystalline Astaxanthin (3,3′-dihydroxy-β, Β-carotene-4,4′-dione) by Captisol® (Sulfobutyl Ether Β-Cyclodextrin). J. Pharm. Sci. 2003, 92, 922–926. [Google Scholar] [CrossRef]
- Anarjan, N.; Mirhosseini, H.; Baharin, B.S.; Tan, C.P. Effect of Processing Conditions on Physicochemical Properties of Sodium Caseinate-Stabilized Astaxanthin Nanodispersions. LWT 2011, 44, 1658–1665. [Google Scholar] [CrossRef]
- Shen, Q.; Quek, S.Y. Microencapsulation of Astaxanthin with Blends of Milk Protein and Fiber by Spray Drying. J. Food Eng. 2014, 123, 165–171. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of Its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Durante, M.; Milano, F.; Caroli, M.D.; Giotta, L.; Piro, G.; Mita, G.; Frigione, M.; Lenucci, M.S. Tomato Oil Encapsulation by α-, β-, and γ-Cyclodextrins: A Comparative Study on the Formation of Supramolecular Structures, Antioxidant Activity, and Carotenoid Stability. Foods 2020, 9, 1553. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage Stability and Antioxidant Activity of Complex of Astaxanthin with Hydroxypropyl-β-Cyclodextrin. Carbohydr. Polym. 2013, 91, 385–389. [Google Scholar] [CrossRef]
- Lancrajan, I.; Diehl, H.A.; Socaciu, C.; Engelke, M.; Zorn-Kruppa, M. Carotenoid Incorporation into Natural Membranes from Artificial Carriers: Liposomes and β-Cyclodextrins. Chem. Phys. Lipids 2001, 112, 1–10. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Ax, K.; Schubert, H. Stability of Lycopene Emulsions in Food Systems. J. Food Sci. 2003, 68, 2730–2734. [Google Scholar] [CrossRef]
- Dos Santos, P.P.; de Aguiar Andrade, L.; Flôres, S.H.; de Oliveira Rios, A. Nanoencapsulation of Carotenoids: A Focus on Different Delivery Systems and Evaluation Parameters. J. Food Sci. Technol. 2018, 55, 3851–3860. [Google Scholar] [CrossRef]
- Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial. OJEU 2011, L 275, 38–40. Available online: http://data.europa.eu/eli/reco/2011/696/oj (accessed on 20 October 2011).
- Yuan, J.-P.; Chen, F. Isomerization of Trans-Astaxanthin to Cis-Isomers in Organic Solvents. J. Agric. Food Chem. 1999, 47, 3656–3660. [Google Scholar] [CrossRef] [PubMed]
- Tachaprutinun, A.; Udomsup, T.; Luadthong, C.; Wanichwecharungruang, S. Preventing the Thermal Degradation of Astaxanthin through Nanoencapsulation. Int. J. Pharm. 2009, 374, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Anarjan, N.; Tan, C.P. Effects of Storage Temperature, Atmosphere and Light on Chemical Stability of Astaxanthin Nanodispersions. J. Am. Oil Chem. Soc. 2013, 90, 1223–1227. [Google Scholar] [CrossRef]
- Mezquita, P.C.; Huerta, B.E.B.; Ramírez, J.C.P.; Hinojosa, C.P.O. Milks Pigmentation with Astaxanthin and Determination of Colour Stability during Short Period Cold Storage. J. Food Sci. Technol. 2015, 52, 1634–1641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Stability of Astaxanthin-loaded Nanostructured Lipid Carriers in Beverage Systems. J. Sci. Food Agric. 2018, 98, 511–518. [Google Scholar] [CrossRef] [PubMed]
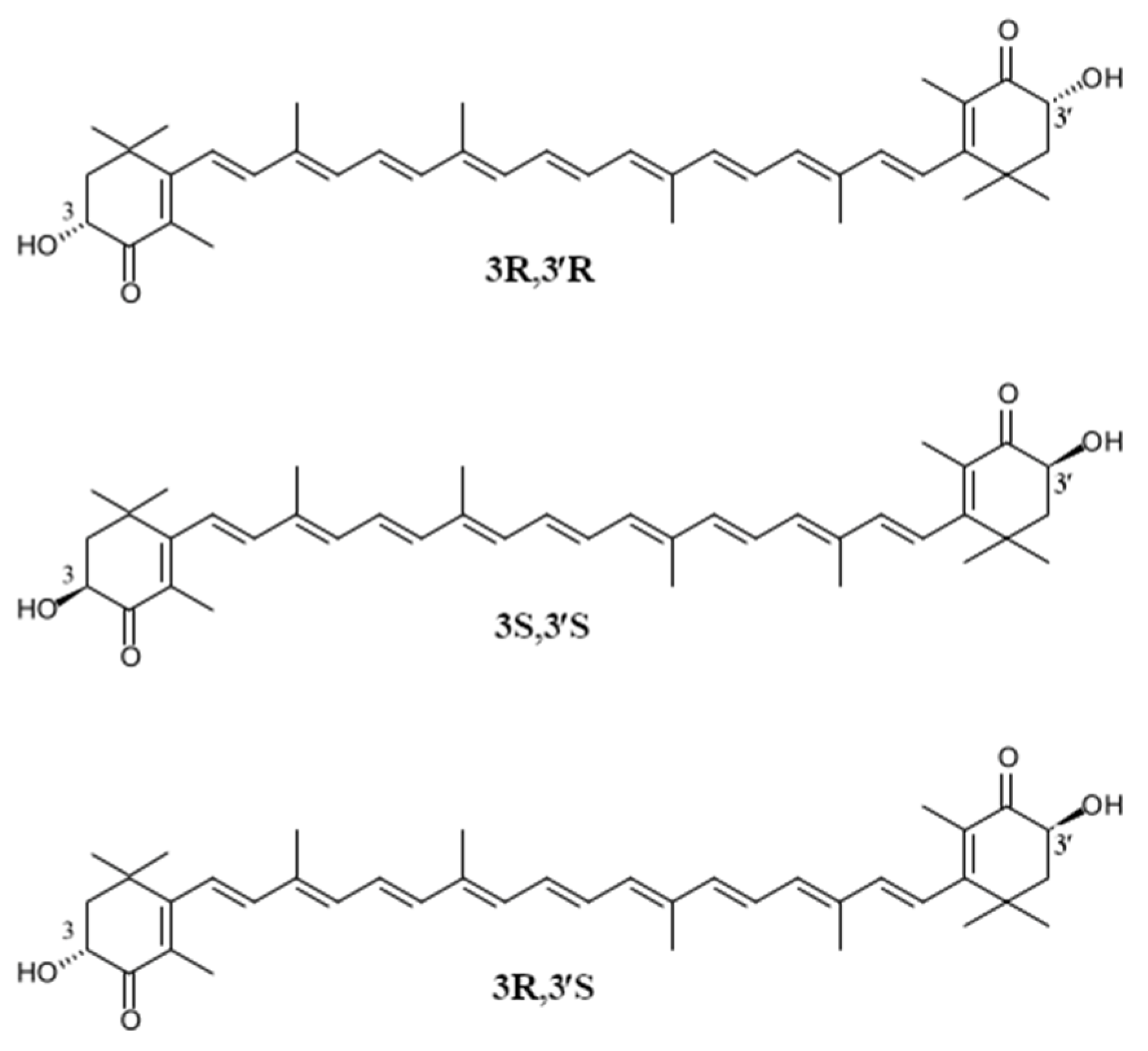
| Astaxanthin Source | Configurational Isomer [%] | References | ||
|---|---|---|---|---|
| 3S, 3′S | 3R, 3′R | Meso Form | ||
| Xanthophyllomyces dendrorhous (yeast) | - | 100 | - | [42] |
| Hematococcus pluvialis (algae) | 100 | - | - | [43] |
| Petels of Adonis spp. | 100 | - | - | [44,45] |
| Crustacyanine (lobster) | 33 | 39 | 28 | [39] |
| Pandalus borealis (shrimp) | 12–25 | - | 50–53 | [39] |
| Atlantic/Pacific salmon | 78–85 | 12–17 | 2–6 | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. https://doi.org/10.3390/molecules26092666
Stachowiak B, Szulc P. Astaxanthin for the Food Industry. Molecules. 2021; 26(9):2666. https://doi.org/10.3390/molecules26092666
Chicago/Turabian StyleStachowiak, Barbara, and Piotr Szulc. 2021. "Astaxanthin for the Food Industry" Molecules 26, no. 9: 2666. https://doi.org/10.3390/molecules26092666
APA StyleStachowiak, B., & Szulc, P. (2021). Astaxanthin for the Food Industry. Molecules, 26(9), 2666. https://doi.org/10.3390/molecules26092666







