Nitrogenous Compounds from the Antarctic Fungus Pseudogymnoascus sp. HSX2#-11
Abstract
1. Introduction
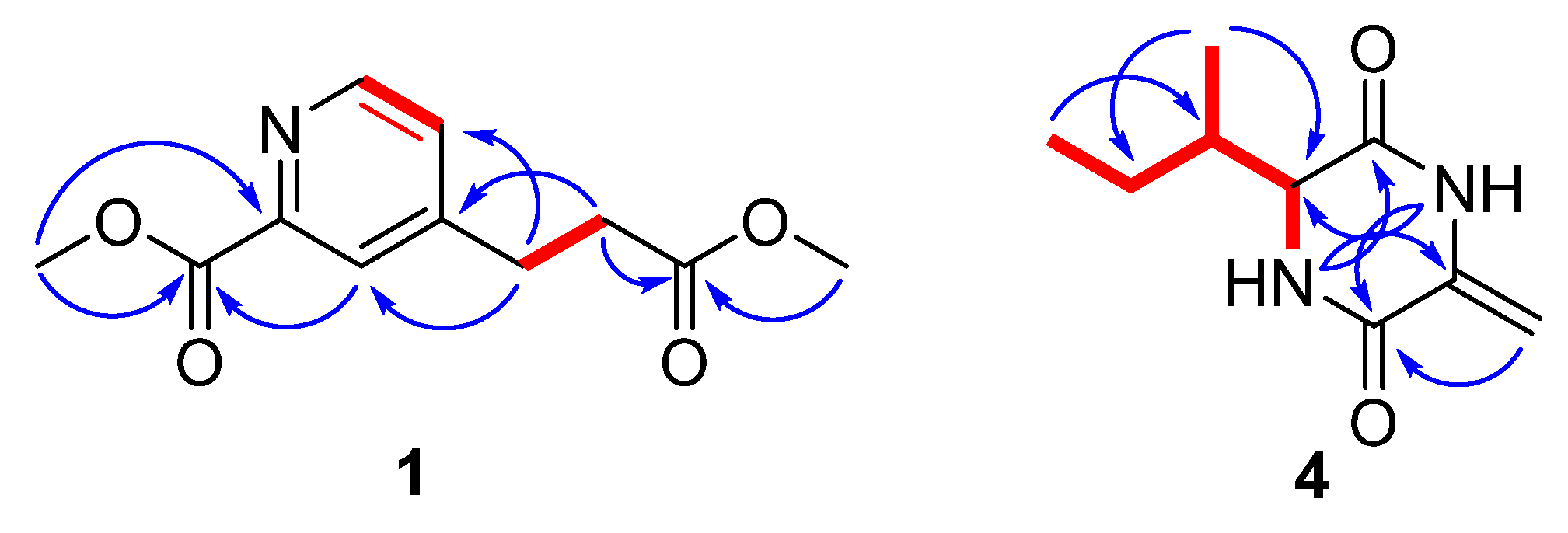
2. Results
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Materials
3.3. Extraction and Isolation
3.4. Antibacterial and Cytotoxic Activity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Elissawy, A.; Dehkordi, E.S.; Mehdinezhad, N.; Ashour, M.; Pour, P.M. Cytotoxic Alkaloids Derived from Marine Sponges: A Comprehensive Review. Biomolecules 2021, 11, 258. [Google Scholar] [CrossRef]
- Lai, Y.-W.; Wang, S.-W.; Hu, Y.-Y.; Hwang, T.-L.; Cheng, M.-J.; Chen, I.-S.; Sung, P.-J.; Chen, J.-J. Anti-inflammatory alkaloids from the root bark of Hernandia nymphaeifolia. Phytochemistry 2020, 173, 112326. [Google Scholar] [CrossRef]
- Ballard, E.; Yucel, R.; Melchers, W.J.G.; Brown, A.J.P.; Verweij, P.E.; Warris, A. Antifungal Activity of Antimicrobial Peptides and Proteins against Aspergillus fumigatus. J. Fungi 2020, 6, 65. [Google Scholar] [CrossRef]
- Sittmann, J.; Bae, M.; Mevers, E.; Li, M.; Quinn, A.; Sriram, G.; Clardy, J.; Liu, Z. Bacterial diketopiperazines stimulate diatom growth and lipid accumulation. Plant Physiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-N.; Chen, N.-H.; Tang, Q.; Chen, S.; Zhan, Z.-C.; Zhang, Y.-B.; Wang, G.-C.; Li, Y.-L.; Ye, W.-C. β-Carboline Alkaloids from the Seeds of Peganum harmala and Their Anti-HSV-2 Virus Activities. Org. Lett. 2020, 22, 7310–7314. [Google Scholar] [CrossRef]
- Hou, X.-M.; Liang, T.-M.; Guo, Z.-Y.; Wang, C.-Y.; Shao, C.-L. Discovery, absolute assignments, and total synthesis of asperversiamides A–C and their potent activity against Mycobacterium marinum. Chem. Commun. 2018, 55, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Cong, B.; Yin, X.; Deng, A.; Shen, J.; Tian, Y.; Wang, S.; Yang, H. Diversity of Cultivable Microbes From Soil of the Fildes Peninsula, Antarctica, and Their Potential Application. Front. Microbiol. 2020, 11, 570836. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Lee, H.; Ko, W.; Kim, D.-C.; Kim, K.-W.; Kwon, H.C.; Guo, Y.; Sohn, J.H.; Yim, J.H.; Kim, Y.-C.; et al. Chemical constituents isolated from Antarctic marine-derived Aspergillus sp. SF-5976 and their anti-inflammatory effects in LPS-stimulated RAW 264.7 and BV2 cells. Tetrahedron 2017, 73, 3905–3912. [Google Scholar] [CrossRef]
- Rusman, Y.; Held, B.W.; Blanchette, R.A.; He, Y.; Salomon, C.E. Cadopherone and colomitide polyketides from Cadophora wood-rot fungi associated with historic expedition huts in Antarctica. Phytochemistry 2018, 148, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Sun, Z.; Peng, J.; Zhu, M.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Secondary Metabolites Produced by Combined Culture of Penicillium crustosum and a Xylaria sp. J. Nat. Prod. 2019, 82, 2013–2017. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Z.; Ren, Z.; Yu, L.; Zhou, H.; Han, Y.; Shah, M.; Che, Q.; Zhang, G.; Li, D.; et al. Antibacterial Cyclic Tripeptides from Antarctica-Sponge-Derived Fungus Aspergillus insulicola HDN151418. Mar. Drugs 2020, 18, 532. [Google Scholar] [CrossRef]
- Figueroa, L.; Jiménez, C.; Rodríguez, J.; Areche, C.; Chávez, R.; Henríquez, M.; De La Cruz, M.; Díaz, C.; Segade, Y.; Vaca, I. 3-Nitroasterric Acid Derivatives from an Antarctic Sponge-Derived Pseudogymnoascus sp. Fungus. J. Nat. Prod. 2015, 78, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Yu, Y.-Y.; Dai, J.-J.; Zhang, Y.-T.; Hu, W.-P.; Zheng, L.; Shi, D.-Y. New polyketides from the Antarctic fungus Pseudogymnoascus sp. HSX2#-11. Mar. Drugs 2021, 19, 168–176. [Google Scholar] [PubMed]
- Bell, R.; Carmeli, S.; Sar, N. Vibrindole A, a Metabolite of the Marine Bacterium, Vibrio parahaemolyticus, Isolated from the Toxic Mucus of the Boxfish Ostracion cubicus. J. Nat. Prod. 1994, 57, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-J.; Gao, S.-S.; Li, C.-S.; Li, X.-M.; Wang, B.-G. Chemical Constituents of a Marine-Derived Endophytic Fungus Penicillium commune G2M. Molevules 2010, 15, 3270–3275. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.T.; Chhabra, S.R.; De Nys, R.; Stead, P.; Bainton, N.J.; Hill, P.J.; Manefield, M.; Kumar, N.; Labatte, M.; England, D.; et al. Quorum-sensing cross talk: Isolation and chemical characterization of cyclic dipeptides from Pseudomonas aeruginosa and other Gram-negative bacteria. Mol. Microbiol. 2002, 33, 1254–1266. [Google Scholar] [CrossRef]
- Ebata, M.; Miyazaki, K.; Otsuka, H. Studies on siomycin. II. The composition and degradation products of siomycin A. J. Antibiot. 1969, 22, 423–433. [Google Scholar] [CrossRef][Green Version]
- Park, S.Y.; Shim, S.H. Characterization of metabolites from cultures of Cellulosimicrobium cellulans. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 481–484. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Mori, Y.; Oda, A.; Okuno, Y.; Kiso, Y.; Hayashi, Y. Acid catalyzed monodehydro-2,5-diketopiperazine formation from N-α-ketoacyl amino acid amides. Tetrahedron 2009, 65, 3688–3694. [Google Scholar] [CrossRef]
- Al-Mourabit, A.; Laville, R.; Nguyen, T.B.; Moriou, C.; Petek, S.; Debitus, C. Marine Natural Occurring 2,5-Diketopiperazines: Isolation, Synthesis and Optical Properties. Heterocycles 2015, 90, 1351. [Google Scholar] [CrossRef]
- Stocking, E.M.; Sanz-Cervera, J.F.; Unkefer, C.J.; Williams, R.M. Studies on the biosynthesis of paraherquamide. Construction of the amino acid framework. Tetrahedron 2001, 57, 5303–5320. [Google Scholar] [CrossRef]
- Kimura, Y.; Tani, K.; Kojima, A.; Sotoma, G.; Okada, K.; Shimada, A. Cyclo-(l-tryptophyl-l-phenylalanyl), a plant growth regulator produced by the fungus Penicillium sp. Phytochemistry 1996, 41, 665–669. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial Cannabinoids from Cannabis sativa: A Structure−Activity Study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
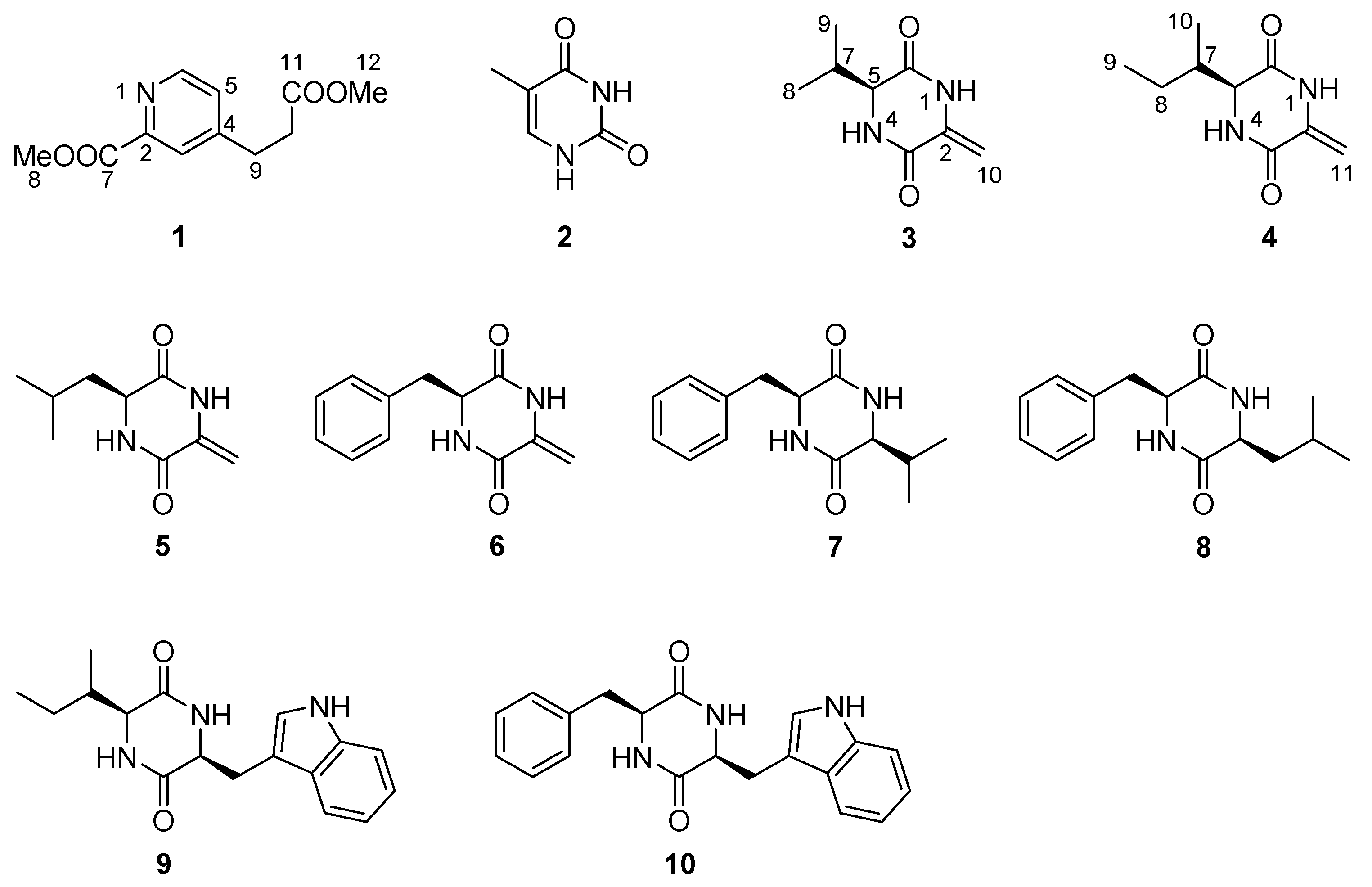
| No. | 1 | 4 | ||||
|---|---|---|---|---|---|---|
| Pos | δCa Type | δHa Multiple (Hz) | δCb Type | δHb Multiple (Hz) | δCb Type | δHb Multiple (Hz) |
| 1 | - | - | - | - | - | 10.53, s |
| 2 | 147.6, C | - | 147.5, C | - | 134.7, C | - |
| 3 | 125.4, CH | 8.00, br s | 124.9, CH | 7.94, dd (1.7, 0.8) | 158.6, C | - |
| 4 | 151.8, C | - | 151.1, C | - | - | 8.34, br s |
| 5 | 127.4, CH | 7.37, br s | 127.2, CH | 7.52, dd (5.0, 1.7) | 59.6, CH | 3.91, t (2.8) |
| 6 | 149.6, CH | 8.66, br s | 149.7, CH | 8.59, dd (5.0, 0.8) | 165.4, C | - |
| 7 | 165.4, C | - | 165.3, C | - | 40.7, CH | 1.85–1.80, m |
| 8 | 53.1, CH3 | 3.98, s | 52.4, CH3 | 3.87, s | 24.1, CH2 | 1.41–1.33, m |
| - | - | - | - | - | 1.20–1.12, m | |
| 9 | 30.1, CH2 | 3.03, t (7.5) | 29.2, CH2 | 2.95, t (7.5) | 11.7, CH3 | 0.85, t (7.4) |
| 10 | 34.0, CH2 | 2.69, t (7.5) | 33.2, CH2 | 2.73, t (7.5) | 14.8, CH3 | 0.88, d (7.0) |
| 11 | 172.4, C | - | 172.3, C | - | 98.8, CH2 | 5.17, br s |
| - | - | - | - | - | 4.76, br s | |
| 12 | 52.0, CH3 | 3.66, s | 51.4, CH3 | 3.58, s | - | - |
| Compounds | Natural [α] | Literature |
|---|---|---|
| 3 | −10.5 (c 0.18) | [α −95.7 (c 0.4) [17] |
| 4 | −19.3 (c 0.18) | - |
| 5 | −78.1 (c 0.18) | [α −163 (0.01) [18] |
| 6 | −41.3 (c 0.18) | [α −40.0 (1.08) a [19] |
| 7 | −25.3 (c 0.18) | [α −11.4 (1.0) a [20] |
| 8 | +18.9 (c 0.14) | [α +30.0 (0.3) [20] |
| 9 | −16.5 (c 0.18) | [α −31.5 (0.0065) [21] |
| 10 | −144.2 (c 0.14) | [α −254.9 (1.0) [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Zheng, L.; Li, X.-Q.; Dai, J.-J.; Zhang, Y.-T.; Yu, Y.-Y.; Hu, W.-P.; Shi, D.-Y. Nitrogenous Compounds from the Antarctic Fungus Pseudogymnoascus sp. HSX2#-11. Molecules 2021, 26, 2636. https://doi.org/10.3390/molecules26092636
Shi T, Zheng L, Li X-Q, Dai J-J, Zhang Y-T, Yu Y-Y, Hu W-P, Shi D-Y. Nitrogenous Compounds from the Antarctic Fungus Pseudogymnoascus sp. HSX2#-11. Molecules. 2021; 26(9):2636. https://doi.org/10.3390/molecules26092636
Chicago/Turabian StyleShi, Ting, Li Zheng, Xiang-Qian Li, Jia-Jia Dai, Yi-Ting Zhang, Yan-Yan Yu, Wen-Peng Hu, and Da-Yong Shi. 2021. "Nitrogenous Compounds from the Antarctic Fungus Pseudogymnoascus sp. HSX2#-11" Molecules 26, no. 9: 2636. https://doi.org/10.3390/molecules26092636
APA StyleShi, T., Zheng, L., Li, X.-Q., Dai, J.-J., Zhang, Y.-T., Yu, Y.-Y., Hu, W.-P., & Shi, D.-Y. (2021). Nitrogenous Compounds from the Antarctic Fungus Pseudogymnoascus sp. HSX2#-11. Molecules, 26(9), 2636. https://doi.org/10.3390/molecules26092636








