The Use of Yeast Mixed Cultures for Deacidification and Improvement of the Composition of Cold Climate Grape Wines
Abstract
1. Introduction
2. Results and Discussion
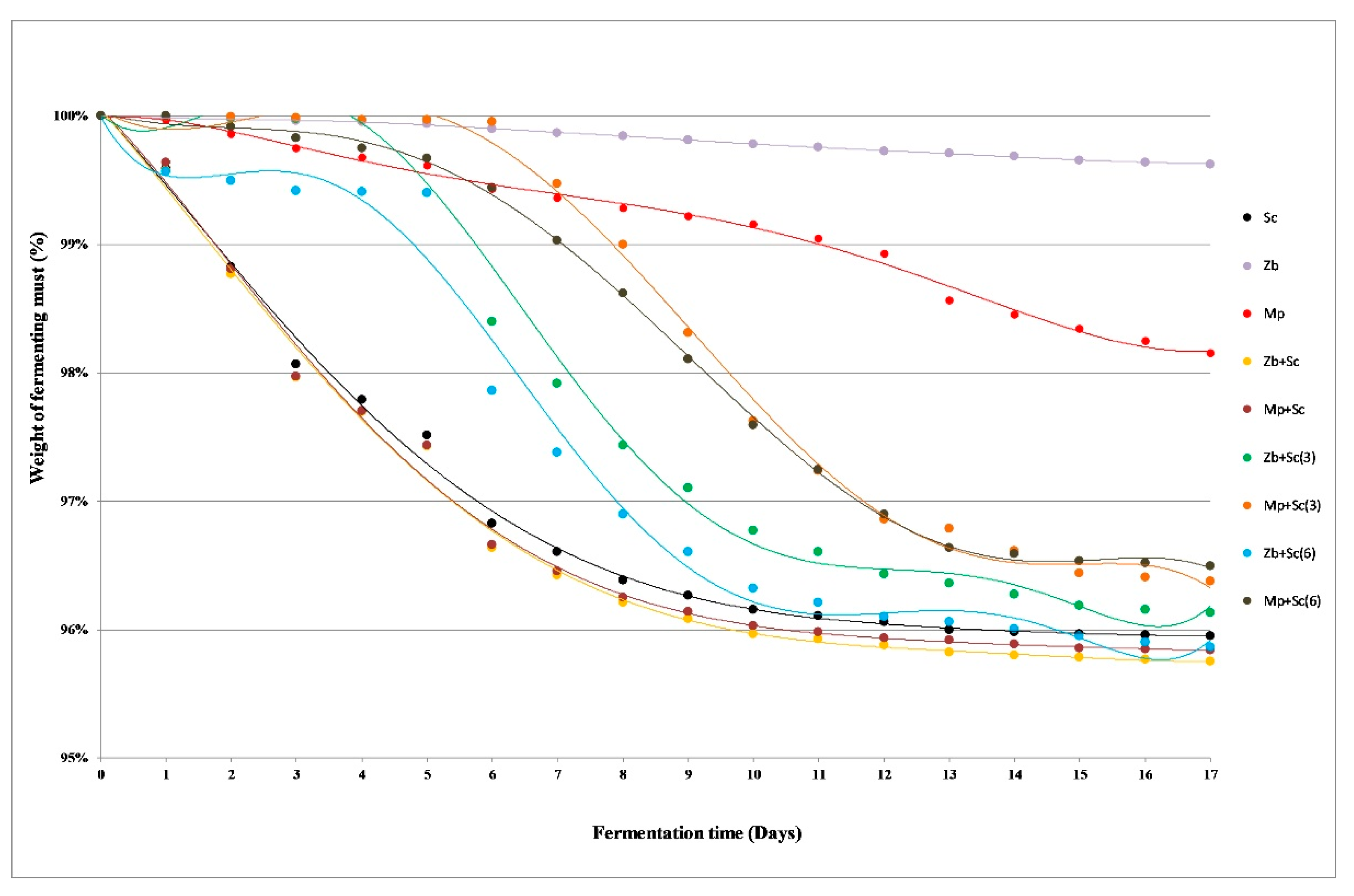
2.1. The Dynamics of the Fermentation Process
2.2. Oenological Parameters
2.3. Total Acidity and Organic Acids
2.4. Volatile Compounds
3. Materials and Methods
3.1. Materials
3.1.1. Inoculum Preparation
3.1.2. Grape Musts Fermentation
3.2. Analytical Methods
3.2.1. Determination of Fermentation Dynamics
3.2.2. Determination of Biomass Growth Yield
3.2.3. Determination of Ethyl Alcohol Content, Real Extract, Total Acidity and Volatile Acidity
3.2.4. Determination of Nitrogen Compounds
3.2.5. Determination of Volatile Compounds (SPME-GC-MS)
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Redzepovic, S.; Orlic, S.; Majdak, A.; Kozina, B.; Volschenk, H.; Viljoen-Bloom, M. Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int. J. Food Microbiol. 2003, 83, 49–61. [Google Scholar] [CrossRef]
- Soyer, Y.; Koca, N.; Karadeniz, F. Organic acid profile of Turkish white grapes and grape juices. J. Food Compos. Anal. 2003, 16, 629–636. [Google Scholar] [CrossRef]
- Coloretti, F.; Zambonelli, C.; Castellari, L.; Tini, V.; Rainieri, S. The effect of DL-malic acid on the metabolism of L-malic acid during wine alcoholic fermentation. Food Technol. Biotechnol. 2002, 40, 317–320. [Google Scholar]
- Jackson, R.S. Wine Science: Principles and Applications; Academic Press: Cambridge, MA, USA, 2008; ISBN 0080568742. [Google Scholar]
- Kunicka-Styczynska, A. Glucose, L-Malic acid and pH effect on fermentation products in biological deacidification. Czech. J. Food Sci. 2009, 27, 319–322. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Fleet, G.H. Wine Microbiology and Biotechnology; CRC Press: Boca Raton, FL, USA, 1993; ISBN 0415278503. [Google Scholar]
- Volschenk, H.; Van Vuuren, H.J.J.; Viljoen-Bloom, M. Malic acid in wine: Origin, function and metabolism during vinification. S. Afr. J. Enol. Vitic. 2006, 27, 123–136. [Google Scholar] [CrossRef]
- Zoecklein, B.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 1475769679. [Google Scholar]
- le Roux, S.I. The microbiology of South African wine making. Part VIII. The microflora of healthy and Botrytis cinerea infected grapes. Phytophylactica 1973, 5, 51–54. [Google Scholar]
- Rankine, B.C. Influence of yeast strain and malo-lactic fermentation on composition and quality of table wines. Am. J. Enol. Vitic. 1972, 23, 152–158. [Google Scholar]
- Balmaseda, A.; Bordons, A.; Reguant, C.; Bautista-Gallego, J. Non-Saccharomyces in wine: Effect upon Oenococcus oeni and malolactic fermentation. Front. Microbiol. 2018, 9, 534. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M. Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef] [PubMed]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Conchillo, L.B.; Ruiz, J.; Navascués, E.; Marquina, D.; Santos, A. Selection and use of pectinolytic yeasts for improving clarification and phenolic extraction in winemaking. Int. J. Food Microbiol. 2016, 223, 1–8. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of Non-Saccharomyces Yeasts for the Reduction of Alcohol Content in Wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Ortega, N.; Martín-Santamaría, M.; Acedo, A.; Marquina, D.; Pascual, O.; Rozès, N.; Zamora, F.; Santos, A.; Belda, I. Occurrence and enological properties of two new non-conventional yeasts (Nakazawaea ishiwadae and Lodderomyces elongisporus) in wine fermentations. Int. J. Food Microbiol. 2019, 305, 1–9. [Google Scholar] [CrossRef]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Reduction of volatile acidity of wines by selected yeast strains. Appl. Microbiol. Biotechnol. 2008, 80, 881. [Google Scholar] [CrossRef]
- Thornton, R.J.; Rodriguez, S.B. Deacidification of red and white wines by a mutant of Schizosaccharomyces malidevorans under commercial winemaking conditions. Food Microbiol. 1996, 13, 475–482. [Google Scholar] [CrossRef]
- Tang, K.; Li, Q. Biochemistry of wine and beer fermentation. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 281–304. [Google Scholar]
- Fernandes, L.; Côrte-Real, M.; Loureiro, V.; Loureiro-Dias, M.C.; Leao, C. Glucose respiration and fermentation in Zygosaccharomyces bailii and Saccharomyces cerevisiae express different sensitivity patterns to ethanol and acetic acid. Lett. Appl. Microbiol. 1997, 25, 249–253. [Google Scholar] [CrossRef]
- Thomas, D.S.; Davenport, R.R. Zygosaccharomyces bailii—A profile of characteristics and spoilage activities. Food Microbiol. 1985, 2, 157–169. [Google Scholar] [CrossRef]
- Pawlikowska, E.; Gregiel, D. Niekonwencjonalne drożdże Metschnikowia pulcherrima i ich zastosowanie w biotechnologii. Postępy Mikrobiol. 2017, 56, 4. [Google Scholar]
- Pitt, J.I.; Miller, M.W. The parasexual cycle in yeasts of the genus Metschnikowia. Mycologia 1970, 62, 462–473. [Google Scholar] [CrossRef]
- Fugelsang, K.; Edwards, C. Wine Microbiology; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Spayd, S.E.; Andersen-Bagge, J. Free amino acid composition of grape juice from 12 Vitis vinifera cultivars in Washington. Am. J. Enol. Vitic. 1996, 47, 389–402. [Google Scholar]
- Xu, Y.; Zhi, Y.; Wu, Q.; Du, R. Zygosaccharomyces bailii is a potential producer of various flavor compounds in Chinese Maotai-flavor liquor fermentation. Front. Microbiol. 2017, 8, 2609. [Google Scholar] [CrossRef]
- Sadoudi, M.; Rousseaux, S.; David, V.; Alexandre, H.; Tourdot-Maréchal, R. Metschnikowia pulcherrima influences the expression of genes involved in PDH bypass and glyceropyruvic fermentation in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 1137. [Google Scholar] [CrossRef] [PubMed]
- OIV. Compendium of International Methods of Wine and Must Analysis. In Organisation Internationale de la Vigne et du Vin; OIV: Paris, France, 2012. [Google Scholar]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons: New York, NY, USA, 2006; Volume 1, ISBN 0470010355. [Google Scholar]
- Volschenk, H.; Van Vuuren, H.J.J.; Viljoen–Bloom, M. Malo-ethanolic fermentation in Saccharomyces and Schizosaccharomyces. Curr. Genet. 2003, 43, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, M.; Pretorius, I.S. Microbial spoilage and preservation of wine: Using weapons for nature’s own arsenal. S. Afr. J. Enol. Vitic. 2000, 21, 74–96. [Google Scholar] [CrossRef]
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Capece, A. Function of yeast species and strains in wine flavour. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef]
- Mendoza, L.M.; de Nadra, M.C.M.; Farías, M.E. Kinetics and metabolic behavior of a composite culture of Kloeckera apiculata and Saccharomyces cerevisiae wine related strains. Biotechnol. Lett. 2007, 29, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Zott, K.; Thibon, C.; Bely, M.; Lonvaud-Funel, A.; Dubourdieu, D.; Masneuf-Pomarede, I. The grape must non-Saccharomyces microbial community: Impact on volatile thiol release. Int. J. Food Microbiol. 2011, 151, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Harding, J. The Oxford Companion to Wine; American Chemical Society: Oxford, UK, 2015; ISBN 0198705387. [Google Scholar]
- Dequin, S.; Baptista, E.; Barre, P. Acidification of grape musts by Saccharomyces cerevisiae wine yeast strains genetically engineered to produce lactic acid. Am. J. Enol. Vitic. 1999, 50, 45–50. [Google Scholar]
- Vonach, R.; Lendl, B.; Kellner, R. High-performance liquid chromatography with real-time Fourier-transform infrared detection for the determination of carbohydrates, alcohols and organic acids in wines. J. Chromatogr. A 1998, 824, 159–167. [Google Scholar] [CrossRef]
- Henick-Kling, T.; Edinger, W.; Daniel, P.; Monk, P. Selective effects of sulfur dioxide and yeast starter culture addition on indigenous yeast populations and sensory characteristics of wine. J. Appl. Microbiol. 1998, 84, 865–876. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Gąstol, M.; Wanat, A.; Krośniak, M.; Jancik, M.; Zagrodzki, P. Wine of cool-climate areas in South Poland. S. Afr. J. Enol. Vitic. 2014, 35, 1–9. [Google Scholar] [CrossRef]
- Muñoz-Robredo, P.; Robledo, P.; Manríquez, D.; Molina, R.; Defilippi, B.G. Characterization of sugars and organic acids in commercial varieties of table grapes. Chil. J. Agric. Res. 2011, 71, 452. [Google Scholar] [CrossRef]
- Kordis-Krapez, M.; Abram, V.; Kac, M.; Ferjancic, S. Determination of organic acids in white wines by RP-HPLC. Food Technol. Biotechnol. 2001, 39, 93–100. [Google Scholar]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Polo, M.C. Wine Chemistry and Biochemistry, 1st ed.; Springer: New York, NY, USA, 2009; Volume 735, ISBN 978-0-387-74118-5. [Google Scholar]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Mendes-Faia, A. The role of yeasts and lactic acid bacteria on the metabolism of organic acids during winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; Del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of non-Saccharomyces yeasts to wine freshness. A review. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.A.; Curtin, C.; Varela, C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015, 205, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Hutkins, R.W. Microbiology and Technology of Fermented Foods; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 22, ISBN 047027624X. [Google Scholar]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii–Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Rantsiou, K.; Dolci, P.; Giacosa, S.; Torchio, F.; Tofalo, R.; Torriani, S.; Suzzi, G.; Rolle, L.; Cocolin, L. Candida zemplinina can reduce acetic acid produced by Saccharomyces cerevisiae in sweet wine fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Y. The influence of yeast strains on the volatile flavour compounds of Chinese rice wine. J. Inst. Brew. 2010, 116, 190–196. [Google Scholar] [CrossRef]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma-a review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality-a review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- Romano, P.; Suzzi, G. Higher alcohol and acetoin production by Zygosaccharomyces wine yeasts. J. Appl. Bacteriol. 1993, 75, 541–545. [Google Scholar] [CrossRef]
- Zironi, R.; Romano, P.; Suzzi, G.; Battistutta, F.; Comi, G. Volatile metabolites produced in wine by mixed and sequential cultures of Hanseniaspora guilliermondii or Kloeckera apiculata and Saccharomyces cerevisiae. Biotechnol. Lett. 1993, 15, 235–238. [Google Scholar] [CrossRef]
- Tominac, V.P.; Ganić, K.K.; KomeS, D.; Gracin, L.; Banović, M.; Marić, V. Influence of media composition and temperature on volatile aroma production by various wine yeast strains. Czech J. Food Sci. 2008, 26, 376–382. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aromatic compound production and fermentative behaviour within different non-Saccharomyces species and clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Ivit, N.N.; Kemp, B. The impact of non-Saccharomyces yeast on traditional method sparkling Wine. Fermentation 2018, 4, 73. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Satora, P.; Skoneczny, S.; Klimczak, K. Physicochemical characterization of wines produced using indigenous yeasts from cold climate grapes. Eur. Food Res. Technol. 2021, 201–209. [Google Scholar] [CrossRef]
- Clemente-Jimenez, J.M.; Mingorance-Cazorla, L.; Martínez-Rodríguez, S.; Las Heras-Vázquez, F.J.; Rodríguez-Vico, F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 2004, 21, 149–155. [Google Scholar] [CrossRef]
- Elias, R.J.; Laurie, V.F.; Ebeler, S.E.; Wong, J.W.; Waterhouse, A.L. Analysis of selected carbonyl oxidation products in wine by liquid chromatography with diode array detection. Anal. Chim. Acta 2008, 626, 104–110. [Google Scholar] [CrossRef]
- Li, E.; Mira de Orduña, R. Acetaldehyde kinetics of enological yeast during alcoholic fermentation in grape must. J. Ind. Microbiol. Biotechnol. 2017, 44, 229–236. [Google Scholar] [CrossRef]
- Mateos, J.A.R.; Pérez-Nevado, F.; Fernández, M.R. Influence of Saccharomyces cerevisiae yeast strain on the major volatile compounds of wine. Enzyme Microb. Technol. 2006, 40, 151–157. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in wine biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Satora, P.; Skoneczny, S.; Skotniczny, M. Yeasts associated with the spontaneously fermented grape musts obtained from cool climate white grape varieties. J. Food Nutr. Res. 2019, 58, 295–306. [Google Scholar]
- Cioch-Skoneczny, M.; Satora, P.; Skotniczny, M.; Skoneczny, S. Quantitative and qualitative composition of yeast microbiota in spontaneously fermented grape musts obtained from cool climate grape varieties Rondo and Regent. FEMS Yeast Res. 2018, 18. [Google Scholar] [CrossRef]
- Cioch-Skoneczny, M.; Satora, P.; Skoneczny, S.; Skotniczny, M. Biodiversity of yeasts isolated during spontaneous fermentation of cool climate grape musts. Arch. Microbiol. 2021, 203, 153–162. [Google Scholar] [CrossRef] [PubMed]
| Grape Must | Total Acidity * [g/L] | FAN [mg/L] | Citric Acid [g/L] | Tartaric Acid [g/L] | l-Malic Acid [g/L] | Succinic Acid [g/L] | Lactic Acid [g/L] | Acetic Acid [g/L] |
|---|---|---|---|---|---|---|---|---|
| 10.00 ** (±0.00) | 106 (±5.6) | 0.06 (±0.00) | 2.54 (±0.21) | 3.89 (±0.20) | 0.94 (±0.07) | 0.00 (±0.00) | 0.00 (±0.00) |
| Yeast/Parameters of Wine | Biomass [g/L] | Total Acidity * [g/L] | Volatile Acidity ** [g/L] | Ethanol [v/v] | Extract [g/L] | FAN [mg/L] |
|---|---|---|---|---|---|---|
| S. cerevisiae MH020215 (Sc) | 0.88 a (±0.11) | 9.29 abc (±0.37) | 0.19 a (±0.05) | 9.89 a (±0.12) | 23.2 a (±0.00) | 14.6 ab (±2.3) |
| Z. bailli 749 (Zb) | 1.16 a (±0.24) | 9.00 bc (±0.1) | 0.25 ab (±0.05) | 4.25 c (±2.57) | 117 c (±4.4) | 83.6 d (±3.5) |
| M. pulcherrima MG970690 (Mp) | 1.17 a (±0.21) | 10.1 a (±0.67) | 0.30 c (±0.07) | 6.82 b (±2.5) | 73.4 b (±4.4) | 77.2 d (±2.5) |
| Z. bailli 749 + S. cerevisiae MH020215 (Zb+Sc) | 1.02 a (±0.12) | 9.62 abc (±0.43) | 0.16 a (±0.03) | 9.73 a (±0.17) | 24.1 a (±1.5) | 16.4 abc (±3.5) |
| M. pulcherrima MG970690 + S. cerevisiae MH020215 (Mp+Sc) | 1.15 a (±0.22) | 10.1 a (±0.68) | 0.16 a (±0.02) | 9.79 a (±0.33) | 24.1 a (±1.5) | 13.0 a (±0.8) |
| Z. bailli 749 + S. cerevisiae MH020215 (Zb+Sc(3)) | 1.03 a (±0.18) | 9.29 abc (±0.5) | 0.21 a (±0.08) | 9.37 a (±0.75) | 22.3 a (±1.5) | 13.7 ab (±0.1) |
| M. pulcherrima MG970690 + S. cerevisiae MH020215 (Mp+Sc(3)) | 0.85 a (±0.17) | 10.1 a (±0.61) | 0.18 a (±0.03) | 9.10 ab (±0.21) | 20.6 a (±0.00) | 20.8 bc (±2.7) |
| Z. bailli 749 + S. cerevisiae MH020215 (Zb+Sc(6)) | 1.00 a (±0.26) | 8.77 b (±0.12) | 0.18 a (±0.03) | 8.66 ab (±0.4) | 23.2 a (±0.00) | 35.9 e (±2.6) |
| M. pulcherrima MG970690 + S. cerevisiae MH020215 (Mp+Sc(6)) | 1.71 b (±0.71) | 9.89 ac (±0.57) | 0.16 a (±0.02) | 8.63 ab (±0.26) | 25.8 a (±0.00) | 23.2 c (±2.7) |
| Yeast/Parameters of Wine | Citric Acid [g/L] | Tartaric Acid [g/L] | l-Malic Acid [g/L] | Succinic Acid [g/L] | Lactic Acid [g/L] | Acetic Acid [g/L] |
|---|---|---|---|---|---|---|
| S. cerevisiae MH020215 (Sc) | 0.07 a (±0.00) | 2.60 (±0.55) | 3.20 abc (±0.39) | 1.62 ab (±0.15) | 0.00 a (±0.00) | 0.02 a (±0.02) |
| Z. bailli 749 (Zb) | 0.08 a (±0.01) | 2.25 (±0.54) | 3.62 c (±0.12) | 0.83 e (±0.05) | 2.30 c (±0.33) | 0.06 ab (±0.03) |
| M. pulcherrima MG970690 (Mp) | 0.13 b (±0.06) | 2.23 (±0.31) | 3.57 bc (±0.33) | 1.16 d (±0.29) | 1.61 b (±0.04) | 0.09 b (±0.05) |
| Z. bailli 749 + S. cerevisiae MH020215 (Zb+Sc) | 0.07 a (±0.01) | 2.33 (±0.38) | 3.36 abc (±0.06) | 1.71 b (±0.02) | 0.00 a (±0.00) | 0.00 a (±0.00) |
| M. pulcherrima MG970690 + S. cerevisiae MH020215 (Mp+Sc) | 0.06 a (±0.01) | 2.12 (±0.03) | 3.26 abc (±0.30) | 1.67 ab (±0.06) | 0.00 a (±0.00) | 0.02 a (±0.02) |
| Z. bailli 749 + S. cerevisiae MH020215 (Zb+Sc(3)) | 0.07 a (±0.01) | 2.10 (±0.26) | 3.06 ab (±0.49) | 1.47 abc (±0.26) | 0.00 a (±0.00) | 0.04 ab (±0.04) |
| M. pulcherrima MG970690 + S. cerevisiae MH020215 (Mp+Sc(3)) | 0.07 a (±0.00) | 2.20 (±0.54) | 3.00 a (±0.20) | 1.32 cd (±0.12) | 0.00 a (±0.00) | 0.02 a (±0.00) |
| Z. bailli 749 + S. cerevisiae MH020215 (Zb+Sc(6)) | 0.07 a (±0.00) | 2.86 (±0.13) | 3.24 abc (±0.19) | 1.42 acd (±0.03) | 0.00 a (±0.00) | 0.02 a (±0.01) |
| M. pulcherrima MG970690 + S. cerevisiae MH020215 (Mp+Sc(6)) | 0.06 a (±0.00) | 2.17 (±0.72) | 3.06 ab (±0.04) | 1.49 abc (±0.06) | 0.00 a (±0.00) | 0.01 a (±0.00) |
| Compound | LRI 2 | [µg/L] |
|---|---|---|
| Hexanal | 800 | 39.3 (±4.89) |
| 2-Hexenal 3 | 853 | 22.3 (±3.50) |
| 3-Hexen-1-ol | 858 | 82.3 (±6.81) |
| 2-Hexen-1-ol | 872 | 7.41 (±1.14) |
| 1-Hexanol | 880 | 714 (±26.41) |
| 2-Heptenal 3 | 958 | 37.4 (±6.73) |
| 1-Octen-3-one | 988 | 16.5 (±2.30) |
| 1-Octen-3-ol | 999 | 26.3 (±4.05) |
| 2-Pentylfuran 3 | 1010 | 5.01 (±1.30) |
| 2-Ethyl-1-hexanol 3 | 1020 | 43.3 (±6.06) |
| 2-Octenal 3 | 1049 | 8.79 (±0.84) |
| 2-Octen-1-ol 3 | 1066 | 7.97 (±0.30) |
| Nonanal | 1102 | 42.6 (±3.45) |
| 1-Nonanol | 1156 | 57.3 (±8.60) |
| Decanal | 1182 | 45.2 (±6.33) |
| 2-[(2-Ethylhexyl)oxy]-ethanol 3 | 1226 | 282 (±25.06) |
| 2-Decenal 3 | 1250 | 1.91 (±0.27) |
| 1-Decanol | 1272 | 208 (±17.32) |
| 2-Undecenal 3 | 1350 | 3.99 (±0.57) |
| 1-Undecanol | 1374 | 244 (±27.32) |
| β-Damascenone | 1384 | 24.5 (±8.59) |
| 2-Dodecanol 3 | 1417 | 5.62 (±0.80) |
| Geranylacetone | 1453 | 32.6 (±1.95) |
| trans-β-Ionone | 1485 | 0.63 (±0.13) |
| 2,4-Di-tert-butylphenol 3 | 1490 | 7.44 (±0.10) |
| Compound [µg/L] | LRI2 | S. cerevisiae MH020215 | Z. bailli 749 | M. pulcherrima MG970690 | Z. bailli 749 + S. cerevisiae MH020215 | M. pulcherrima MG970690 + S. cerevisiae MH020215 | Z. bailli 749+ S. cerevisiae MH020215 (3) | M. pulcherrima MG970690+ S. cerevisiae MH020215 (3) | Z. bailli 749 + S. cerevisiae MH020215 (6) | M. pulcherrima MG970690 + S. cerevisiae MH020215 (6) |
|---|---|---|---|---|---|---|---|---|---|---|
| Esters | ||||||||||
| Ethyl acetate | 614 | 33921 a | 8753 e | 36483 ab | 65972 c | 41459 ab | 46897 ab | 133551 f | 51376 bc | 34026 a |
| Ethyl propionate | 714 | 5269 c | 190 b | 196 b | 5116 c | 7150 d | 2424 a | 7513 d | 2888 a | 2266 a |
| Isobutyl acetate | 771 | 16.3 a | 18.2 a | 191.4 c | 17.7 a | 34.9 a | 33.6 a | 97.5 b | 44.9 a | 41.4 a |
| Ethyl pyroracemate 3 | 785 | 228 b | 24 a | 197 b | 645 e | 0 a | 419 c | 1543 g | 544 d | 897 f |
| Ethyl butanoate | 789 | 153 c | 0 a | 11 a | 135 bc | 141 bc | 97 bd | 258 e | 76 d | 27 a |
| 2-Hydroxyethyl propionate 3 | 798 | 99 a | 0 b | 1 b | 96 a | 468 c | 135 a | 338 d | 102 a | 520 c |
| Ethyl 2-ethyl-3-methylbutanoate 3 | 847 | 12.9 b | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a |
| Ethyl 3-methylbutanoate | 849 | 11.9 c | 0.0 a | 0.0 a | 6.7 b | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a |
| Isoamyl acetate | 884 | 1415 b | 85 a | 137 a | 1596 b | 300 a | 673 c | 280 a | 270 a | 110 a |
| 2-Methylbutyl acetate | 886 | 0.0 a | 12.4 b | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 65.7 c |
| Ethyl hexanoate | 986 | 257.8 de | 7.7 a | 3.2 a | 295.6 e | 230.7 d | 174.1 c | 467.9 f | 147 bc | 116.9 b |
| Diethyl succinate | 1149 | 953.2 ab | 0.0 c | 16.6 c | 1707.6 d | 1450.7 bd | 774.8 a | 2864 e | 1174.4 ab | 3667.0 f |
| Ethyl octanoate | 1180 | 536.7 de | 8.0 a | 0.0 a | 661.8 f | 559.3 ef | 396.2 bc | 1058.4 g | 438.4 cd | 292.9 b |
| 2-Phenylethyl acetate | 1228 | 17.7 a | 5.0 a | 38.1 ab | 54.2 bc | 40.0 ab | 201.2 e | 84.2 cd | 398.3 f | 33431.6 g |
| Propyl nonanoate 3 | 1390 | 1.01 b | 1.28 c | 0.98 b | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.00 a |
| Ethyl decanoate | 1397 | 36.6 e | 2.1 c | 1.6 c | 95.8 ab | 124.9 b | 167.9 d | 87.7 a | 199.2 d | 96.6 ab |
| Ethyl isopentyl succinate 3 | 1430 | 2.0 a | 0.0 a | 0.0 a | 9.5 ac | 21.2 b | 24.9 b | 17.9 bc | 35.8 d | 85.4 e |
| Ethyl dodecanoate | 1581 | 67.4 b | 1.4 b | 1.2 b | 229.7 ac | 269.4 ac | 193.6 a | 194.2 a | 581.9 d | 322.5 c |
| Isopentyl decanoate 3 | 1653 | 3.0 a | 0.0 a | 0.0 a | 9.8 b | 16.4 c | 12.9 bc | 11.5 bc | 25.8 d | 25.4 d |
| Ethyl tetradecanoate | 1790 | 17.3 ac | 0.9 a | 4.3 a | 49.2 abc | 67.1 bc | 137.2 d | 74.4 b | 391.6 f | 83.0 b |
| Ethyl E-11-hexadecenoate 3 | 1974 | 0.7 a | 0.0 a | 0.0 a | 5.1 a | 10.8 ab | 41.2 b | 7.0 ab | 21.0 ab | 10.8 ab |
| Ethyl hexadecanoate | 1990 | 69.8 a | 2.6 a | 0.0 a | 209.2 a | 455.2 c | 1120.7 d | 802.3 b | 1369.3 e | 661.2 b |
| Methyl linoleate 3 | 2092 | 1.8 a | 0.0 a | 0.0 a | 9.6 b | 11.8 bc | 21.1 d | 14.2 c | 43.3 f | 31.6 e |
| Ethyl octadecanoate | 2189 | 1.8 ab | 0.0 a | 0.0 a | 4.4 b | 9.9 e | 30.8 d | 15.8 c | 34.2 d | 15.5 c |
| Alcohols | ||||||||||
| 2-Methyl-1-propanol | 617 | 26200 b | 70261 a | 182815 d | 53733 ab | 71599 a | 79316 a | 224949 e | 145161 c | 326167 f |
| 3-Methyl-1-butanol | 734 | 18954 ab | 4215 a | 28835 ab | 43757 b | 158869 d | 119238 c | 118715 c | 112660 c | 181879 d |
| 2-Methyl-1-butanol | 740 | 20717a | 4871 c | 10249 c | 33176 b | 53604 d | 28710 ab | 52710 d | 22126 a | 27322 ab |
| Isohexanol 3 | 838 | 20.4 ab | 0.0 c | 0.0 c | 15.2 a | 25.9 b | 35.5 d | 78.0 e | 14.4 a | 21.6 ab |
| 3-Methylpentanol 3 | 843 | 132 ad | 0 c | 0 c | 153 ab | 198 b | 409 e | 88 d | 143 a | 146 a |
| 3-Hexen-1-ol | 858 | 21.4 ab | 40.4 a | 43.0 a | 0.0 b | 23.6 ab | 37.2 a | 92.5 c | 25.2 ab | 39.0 a |
| 1-Hexanol | 880 | 431 a | 407 a | 786 bc | 531 ab | 714 b | 654 ab | 1498 d | 609 ab | 968 c |
| 2-Ethyl-1-hexanol 3 | 1020 | 33.2 ab | 59.7 c | 46.6 ac | 37.0 ab | 44.7 a | 43.2 ab | 112.0 d | 29.9 b | 47.1 ac |
| Phenylethanol | 1114 | 9248 a | 1745 b | 2074 b | 15516 d | 24082 c | 11098 a | 31932 e | 11071 a | 27422 c |
| 1-Nonanol | 1156 | 16.8 b | 60.9 d | 46.3 c | 24.6 b | 0.0 a | 0.0 a | 0.0 a | 0.0 a | 0.0 a |
| 2-[(2-Ethylhexyl)oxy]-ethanol 3 | 1226 | 4.3 a | 38.5 c | 7.3 ab | 19.0 ab | 23.3 bc | 80.7d | 117.7 f | 98.0 e | 94.7 de |
| 1-Decanol | 1272 | 2.8 a | 33.8 de | 5.7 a | 26.6 cd | 12.7 ac | 60.4 bf | 44.7 be | 75.3 f | 57.4 b |
| 1-Undecanol | 1374 | 1.2 b | 20.0 c | 4.0 b | 11.3 bc | 16.4 c | 55.0 a | 51.7 a | 57.0 a | 55.6 a |
| 2-Dodecanol 3 | 1417 | 2.50 c | 1.55 b | 5.94 d | 1.06 b | 0.00 a | 0.00 a | 0.00 a | 0.00 a | 0.00 a |
| 1-Dodecanol | 1448 | 0.4 ab | 2.8 a | 2.4 ab | 0.0 b | 3.0 a | 15.3 d | 13.0 cd | 11.9 c | 12.1 c |
| 2,4-Di-tert-butylphenol 3 | 1490 | 2.0 a | 6.1 a | 6.5 a | 5.1 a | 7.6 a | 30.2 b | 29.9 b | 28.6 b | 14.4 c |
| Acetals | ||||||||||
| Acetaldehyde | 725 | 1227 ab | 40 a | 132 a | 2448 b | 2070 ab | 1671 ab | 13696 e | 7226 c | 11068 d |
| Nonanal | 1102 | 25.2 a | 23.4 a | 25.7 a | 25.9 a | 53.0 b | 50.1 b | 0.0 c | 30.5 a | 68.3 d |
| Decanal | 1182 | 13.3 ab | 20.8 ce | 16.9 bc | 25.8 f | 11.6 ad | 7.5 d | 22.8 ef | 16.1 abc | 31.5 g |
| Carboxylic acids | ||||||||||
| Hexanoic acid | 982 | 154 b | 0 a | 0 a | 0 a | 239 b | 484 c | 1802 e | 750 d | 572 c |
| Octanoic acid | 1160 | 610 d | 0 a | 5 a | 347 c | 883 b | 1983 e | 5292 g | 2965 f | 927 b |
| n-Decanoic acid | 1371 | 0 a | 0 a | 0 a | 0 a | 25 b | 314 c | 96 b | 444 d | 0 a |
| Terpenes | ||||||||||
| α-Terpineol | 1171 | 13.8 abc | 10.1 bc | 14.6 ab | 16.1 ab | 18.2 a | 18.3 a | 30.9 d | 14.8 ab | 8.1 c |
| Damascenone | 1384 | 5.0 a | 9.2 ab | 6.1 a | 13.0 ab | 16.4 b | 45.5 c | 44.6 c | 53.5 e | 32.9 d |
| 2,6-di-tert-Butyl-p-benzoquinone 3 | 1459 | 0.46 a | 0.78 ab | 0.85 ab | 1.66 ab | 2.62 b | 8.88 d | 5.94 c | 8.10 d | 4.97 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioch-Skoneczny, M.; Grabowski, M.; Satora, P.; Skoneczny, S.; Klimczak, K. The Use of Yeast Mixed Cultures for Deacidification and Improvement of the Composition of Cold Climate Grape Wines. Molecules 2021, 26, 2628. https://doi.org/10.3390/molecules26092628
Cioch-Skoneczny M, Grabowski M, Satora P, Skoneczny S, Klimczak K. The Use of Yeast Mixed Cultures for Deacidification and Improvement of the Composition of Cold Climate Grape Wines. Molecules. 2021; 26(9):2628. https://doi.org/10.3390/molecules26092628
Chicago/Turabian StyleCioch-Skoneczny, Monika, Michał Grabowski, Paweł Satora, Szymon Skoneczny, and Krystian Klimczak. 2021. "The Use of Yeast Mixed Cultures for Deacidification and Improvement of the Composition of Cold Climate Grape Wines" Molecules 26, no. 9: 2628. https://doi.org/10.3390/molecules26092628
APA StyleCioch-Skoneczny, M., Grabowski, M., Satora, P., Skoneczny, S., & Klimczak, K. (2021). The Use of Yeast Mixed Cultures for Deacidification and Improvement of the Composition of Cold Climate Grape Wines. Molecules, 26(9), 2628. https://doi.org/10.3390/molecules26092628






