A Review on Coordination Properties of Al(III) and Fe(III) toward Natural Antioxidant Molecules: Experimental and Theoretical Insights
Abstract
1. Introduction
1.1. Role of Al(III) and Fe(III) Ions
1.2. Choice of the Ligands
1.2.1. Hydroxycinnamic Acid Derivatives
1.2.2. Coumarin Derivatives
1.2.3. Flavonoids
1.2.4. Curcumin
2. Measurement of the Stability of Metal–Ligand Complexes in Aqueous Solution
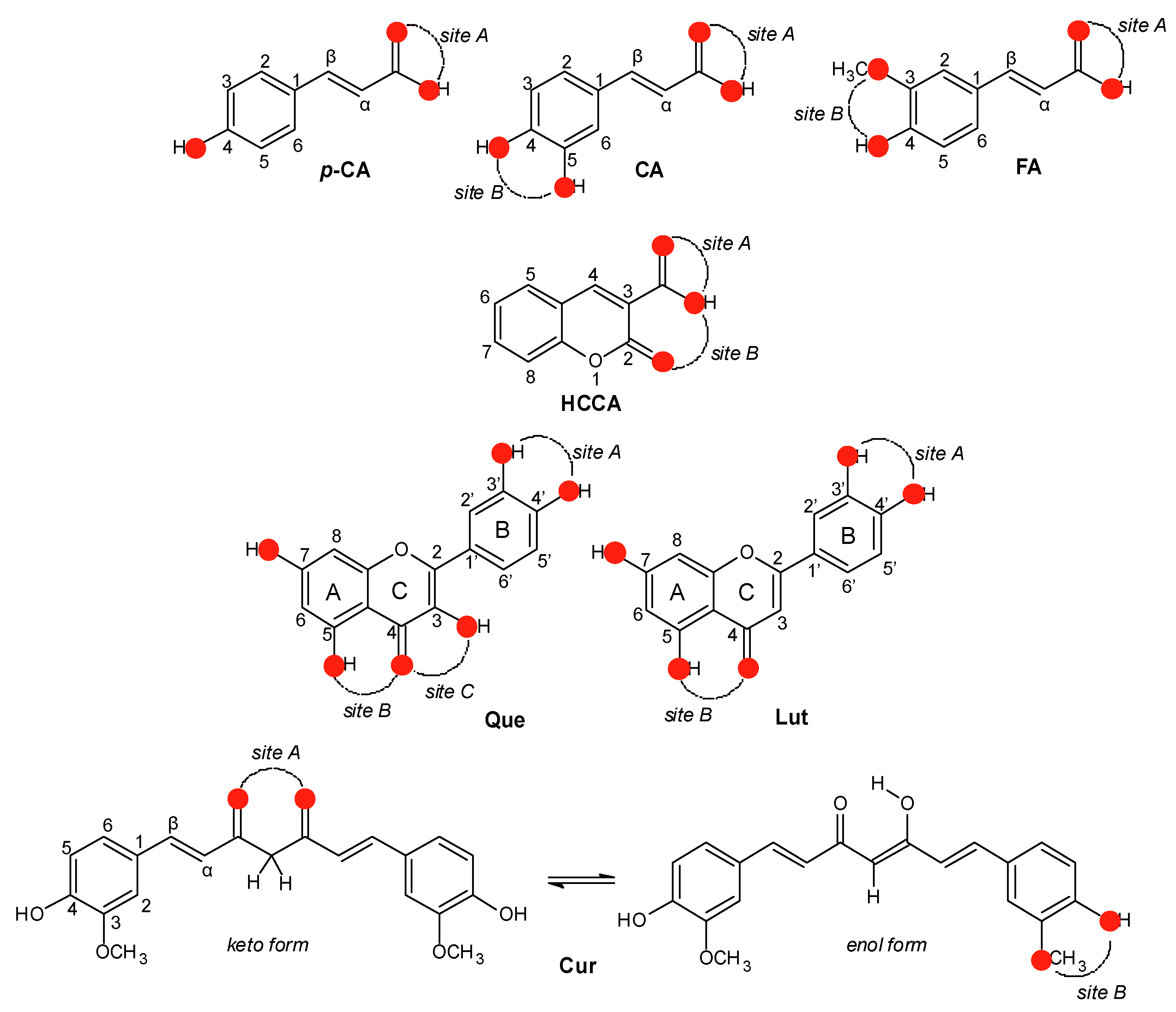
3. Binding Sites and Complexes Formation
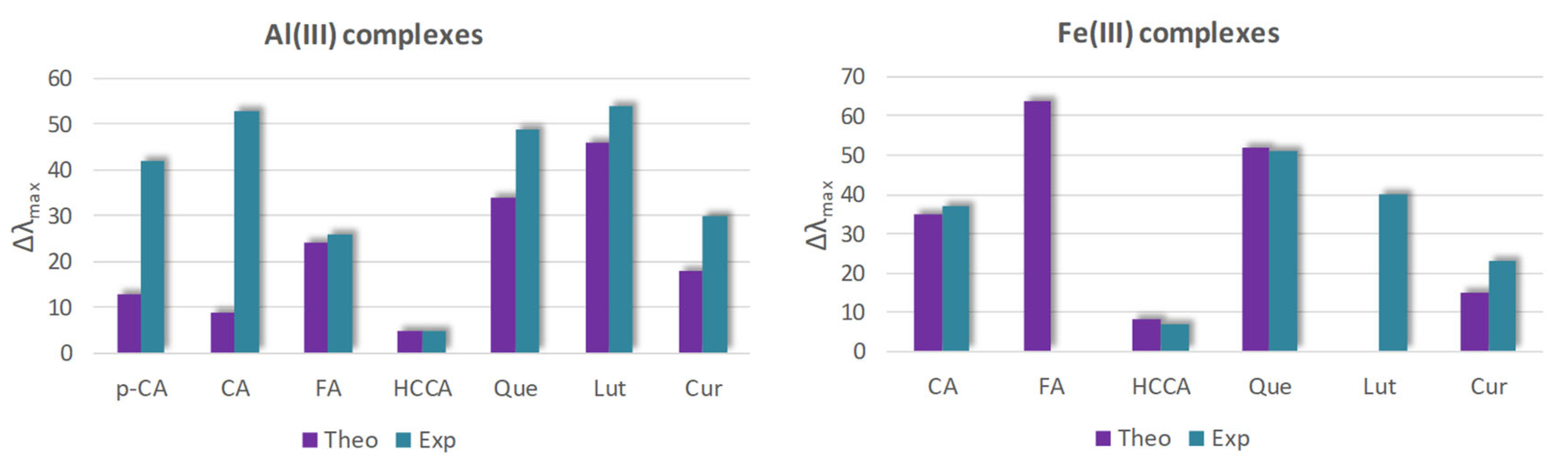
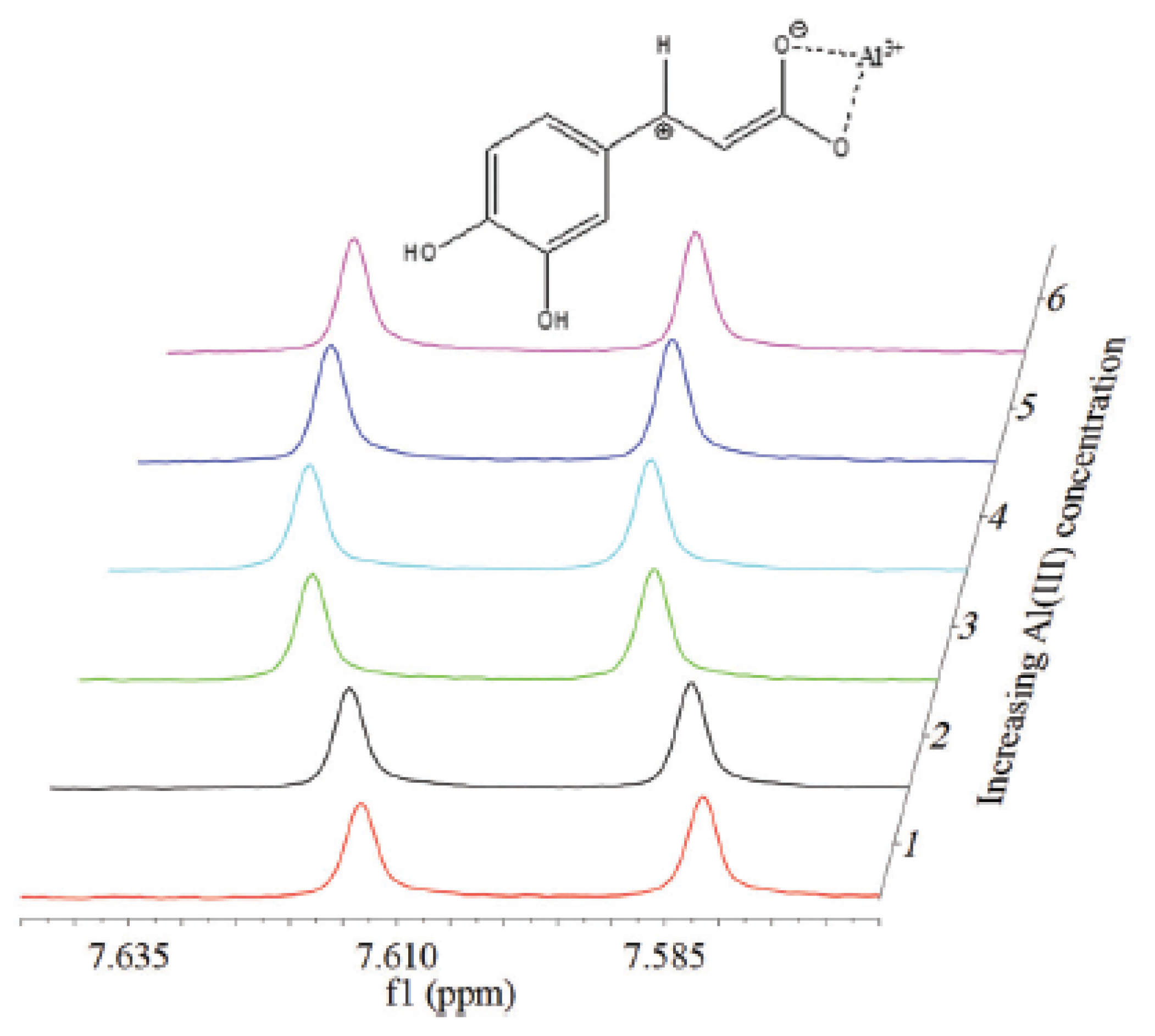
4. Complex Characterization by Spectroscopic and Spectrometric Techniques
5. Discussion
5.1. Hydroxycinnamic Acid Derivatives
5.2. Coumarin Derivatives
5.3. Flavonoids
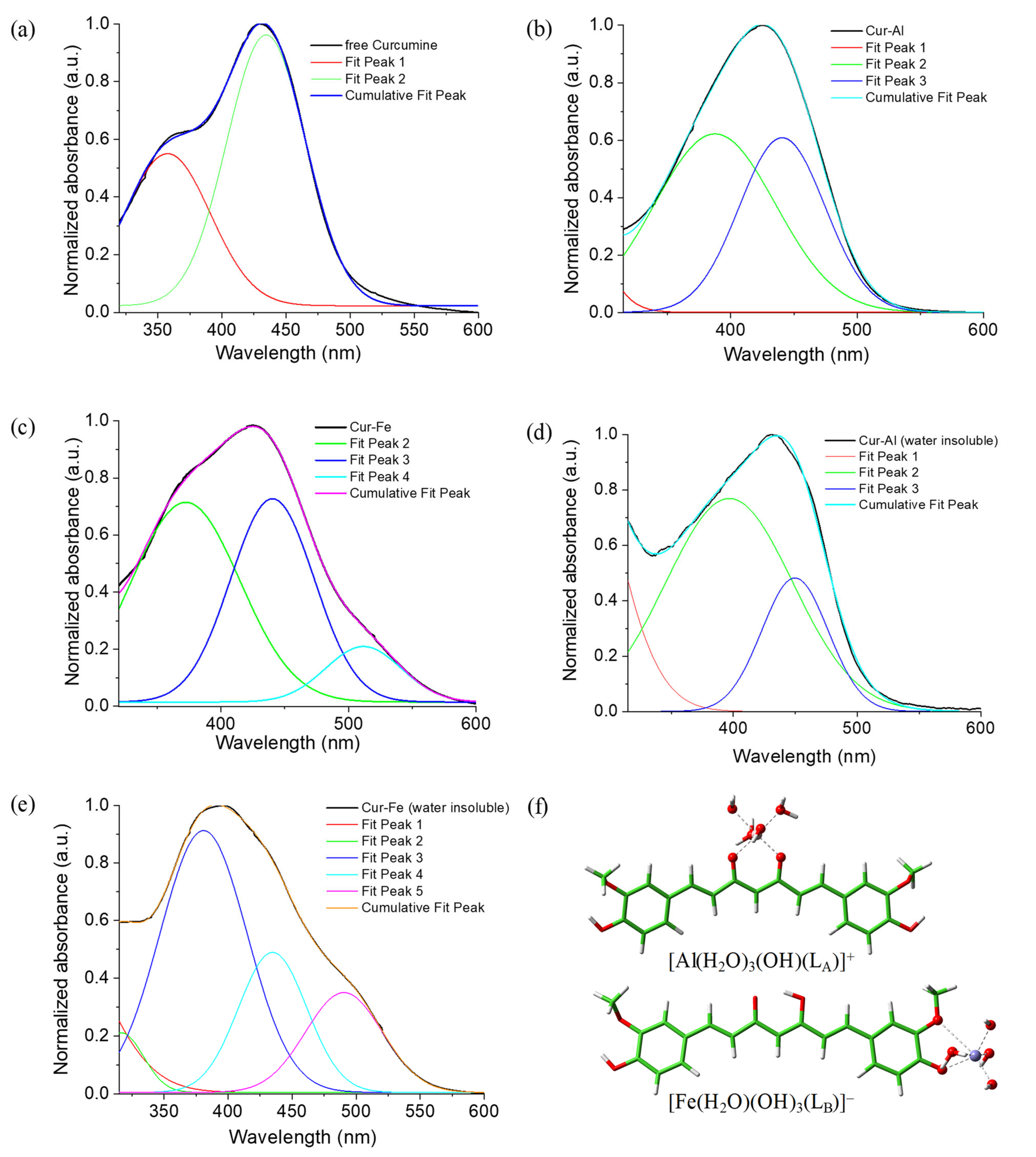
5.4. Curcumin
6. Conclusions
- -
- hydroxycinnamic acids (pCA, CA, and FA) are able to form more stable complexes with Al(III) than with Fe(III) coordinating the metal ion through the carboxylate site in all cases.
- -
- coumarin-3-carboxylic acid, similarly, prefers to bind Al(III) rather than Fe(III), forming 1:1 and 1:2 M:L stoichiometric ratio complexes, respectively. Consequently, octahedral complexes with Al(III), involving both carboxylate and lactone moieties, and tetrahedral complex with Fe(III) in a η1 ligand’s coordination were analyzed.
- -
- flavonoids (Que and Lut) formed 1:1 and 1:2 M:L complexes with both metal ions, though they showed a more intricate behavior, as more than one coordination mode was found plausible with both metal ions, making the identification of the preferred coordination site and thus the most probable complex in a water environment, especially in the case of Que, difficult.
- -
- Curcumin discriminates well between the two metal ions since it prefers to coordinate Al(III) through the diketo site while the Fe(III) results most probably bound to the guaiacol site.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crisponi, G.; Nurchi, V.M.; Bertolasi, V.; Remelli, M.; Faa, G. Chelating agents for human diseases related to aluminium overload. Coord. Chem. Rev. 2012, 256, 89–104. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, M. Studies on Transition Metal-Quercetin Complexes Using Electrospray Ionization Tandem Mass Spectrometry. Molecules 2015, 20, 8583–8594. [Google Scholar] [CrossRef] [PubMed]
- Nurchi, V.M.; Crespo-Alonso, M.; Toso, L.; Lachowicz, J.I.; Crisponi, G. Chelation Therapy for Metal Intoxication: Comments from a Thermodynamic Viewpoint. Mini-Rev. Med. Chem. 2013, 13, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Arias Arias, F.E.; Beneduci, A.; Chidichimo, F.; Furia, E.; Straface, S. Study of the adsorption of mercury (II) on lignocellulosic materials under static and dynamic conditions. Chemosphere 2017, 180, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Hofer, T.; Jørgensen, T.O.; Olsen, R.L. Comparison of food antioxidants and iron chelators in two cellular free radical assays: Strong protection by Luteolin. J. Agric. Food Chem. 2014, 62, 8402–8410. [Google Scholar] [CrossRef]
- Crisponi, G.; Dean, A.; Di Marco, V.; Lachowicz, J.I.; Nurchi, V.M.; Remelli, M.; Tapparo, A. Different approaches to the study of chelating agents for iron and aluminium overload pathologies. Anal. Bioanal. Chem. 2013, 405, 585–601. [Google Scholar] [CrossRef]
- Drüeke, T.B. Intestinal absorption of aluminium in renal failure. Nephrol. Dial. Transplant. 2002, 17, 13–16. [Google Scholar] [CrossRef][Green Version]
- Exley, C. Aluminum Should Now Be Considered a Primary Etiological Factor in Alzheimer’s Disease. J. Alzheimer’s Dis. Reports 2017, 1, 23–25. [Google Scholar] [CrossRef]
- Munoz, D.G. Is exposure to aluminum a risk factor for the development of Alzheimer disease?—No. Arch. Neurol. 1998, 55, 737–739. [Google Scholar] [CrossRef]
- Lidsky, T.I. Is the Aluminum Hypothesis Dead? J. Occup. Env. Med. 2014, 56, S73–S79. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- León-Carmona, J.R.; Alvarez-Idaboy, J.R.; Galano, A. On the peroxyl scavenging activity of hydroxycinnamic acid derivatives: Mechanisms, kinetics, and importance of the acid-base equilibrium. Phys. Chem. Chem. Phys. 2012, 14, 12534–12543. [Google Scholar] [CrossRef] [PubMed]
- Beneduci, A.; Furia, E.; Russo, N.; Marino, T. Complexation behaviour of caffeic, ferulic and p-coumaric acids towards aluminium cations: A combined experimental and theoretical approach. New J. Chem. 2017, 41, 5182–5190. [Google Scholar] [CrossRef]
- Furia, E.; Beneduci, A.; Russo, N.; Marino, T. Structural characterization of aluminium(III) and iron(III) complexes of coumarinic acid in aqueous solutions from combined experimental and theoretical investigations. New J. Chem. 2018, 42, 11006–11012. [Google Scholar] [CrossRef]
- Jabeen, E.; Janjua, N.K.; Ahmed, S.; Murtaza, I.; Ali, T.; Hameed, S. Radical scavenging propensity of Cu2+, Fe3+ complexes of flavonoids and in-vivo radical scavenging by Fe3+-primuletin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 432–438. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In Vitro Antioxidant versus Metal Ion Chelating Properties of Flavonoids: A Structure-Activity Investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Flavonoid-Metal Ion Complexes: A Novel Class of Therapeutic Agents. Med. Res. Rev. 2014, 34, 677–702. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Kostyuk, V.A.; Potapovich, A.I.; Kostyuk, T.V.; Cherian, M.G. Metal complexes of dietary flavonoids: Evaluation of radical scavenger properties and protective activity against oxidative stress in vivo. Cell. Mol. Biol. 2007, 53, 62–69. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Laganà, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Spoerlein, C.; Mahal, K.; Schmidt, H.; Schobert, R. Effects of chrysin, apigenin, genistein and their homoleptic copper(II) complexes on the growth and metastatic potential of cancer cells. J. Inorg. Biochem. 2013, 127, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Chebotarev, A.N.; Snigur, D.V. Study of the acid-base properties of quercetin in aqueous solutions by color measurements. J. Anal. Chem. 2015, 70, 55–59. [Google Scholar] [CrossRef]
- Jabeen, E.; Janjua, N.K.; Ahmed, S.; Murtaza, I.; Ali, T.; Masood, N.; Rizvi, A.S.; Murtaza, G. DFT predictions, synthesis, stoichiometric structures and anti-diabetic activity of Cu (II) and Fe (III) complexes of quercetin, morin, and primuletin. J. Mol. Struct. 2017, 1150, 459–468. [Google Scholar] [CrossRef]
- Escudero, L.B.; Fusari, C.M.; Altamirano, J.C.; Camargo, A.B.; Wuilloud, R.G. Stability of Iron-Quercetin Complexes in Synthetic Wine under In Vitro Digestion Conditions. J. Food Sci. 2014, 79, C1933–C1938. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Zhang, C.; Korshin, G.V.; Kuznetsov, A.M.; Yan, M. Experimental and quantum-chemical study of differential absorbance spectra of environmentally relevant species: A study of quercetin deprotonation and its interactions with copper (II) ions. Sci. Total Environ. 2019, 679, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem. 2014, 146, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Xu, X.; Xia, L.; Xia, C.; Tang, J.; Ouyang, Z. Quercetin-Iron Complex: Synthesis, Characterization, Antioxidant, DNA Binding, DNA Cleavage, and Antibacterial Activity Studies. J. Fluoresc. 2016, 26, 2023–2031. [Google Scholar] [CrossRef]
- Normaya, E.; Fazli, M.; Norazmi Ahmad, M.; Ku Bulat, K.H. COSMO-RS and DFT studies on development and optimization of quercetin as a chemosensor for Fe 3+ recognition in aqueous medium. J. Mol. Struct. 2019, 1184, 538–545. [Google Scholar] [CrossRef]
- Furia, E.; Marino, T.; Russo, N. Insights into the coordination mode of quercetin with the Al(III) ion from a combined experimental and theoretical study. Dalt. Trans. 2014, 43, 7269–7274. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E. Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal hydroxyflavonol complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 757–771. [Google Scholar] [CrossRef] [PubMed]
- De Castilho, T.S.; Matias, T.B.; Nicolini, K.P.; Nicolini, J. Study of interaction between metal ions and quercetin. Food Sci. Hum. Wellness 2018, 7, 215–219. [Google Scholar] [CrossRef]
- Corrente, G.A.; Malacaria, L.; Beneduci, A.; Furia, E.; Marino, T.; Mazzone, G. Experimental and theoretical study on the coordination properties of quercetin towards aluminum(III), iron(III) and copper(II) in aqueous solution. J. Mol. Liq. 2021, 325, 115171. [Google Scholar] [CrossRef]
- Çıkla Yılmaz, D.; Pekin, M. Potentiometric and Chromatographic Study of Cu(II) and Al(III) Complexes of Quercetin. Marmara Pharm. J. 2017, 21, 330–337. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, L.; Zhao, H.; Liu, X.; Gao, L.; Cheng, N.; Cao, W. Complexation of luteolin with lead (II): Spectroscopy characterization and theoretical researches. J. Inorg. Biochem. 2019, 193, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Xiong, Y.; Li, Z.; Peng, L.; Guo, Q.; Li, X.; Deng, X. ESI-TOF MS analysis and DNA cleavage activity of complexes formed by luteolin and five metal ions in hot water. Inorg. Nano-Metal Chem. 2020, 50, 1181–1188. [Google Scholar] [CrossRef]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, Epigallocatechin Gallate, Curcumin, and Resveratrol: From Dietary Sources to Human MicroRNA Modulation. Molecules 2019, 25, 63. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.J.; Lee, K.W. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J. Neurochem. 2010, 112, 1415–1430. [Google Scholar] [CrossRef]
- Banerjee, S.; Chakravarty, A.R. Metal Complexes of Curcumin for Cellular Imaging, Targeting, and Photoinduced Anticancer Activity. Acc. Chem. Res. 2015, 48, 2075–2083. [Google Scholar] [CrossRef]
- Sen, S.; Sharma, H.; Singh, N. Curcumin enhances Vinorelbine mediated apoptosis in NSCLC cells by the mitochondrial pathway. Biochem. Biophys. Res. Commun. 2005, 331, 1245–1252. [Google Scholar] [CrossRef]
- Khopde, S.M.; Priyadarsini, K.I.; Venkatesan, P.; Rao, M.N.A. Free radical scavenging ability and antioxidant efficiency of curcumin and its substituted analogue. Biophys. Chem. 1999, 80, 85–91. [Google Scholar] [CrossRef]
- Barik, A.; Mishra, B.; Shen, L.; Mohan, H.; Kadam, R.M.; Dutta, S.; Zhang, H.Y.; Priyadarsini, K.I. Evaluation of a new copper(II)-curcumin complex as superoxide dismutase mimic and its free radical reactions. Free Radic. Biol. Med. 2005, 39, 811–822. [Google Scholar] [CrossRef]
- Dutta, S.; Murugkar, A.; Gandhe, N.; Padhye, S. Enhanced antioxidant activities of metal conjugates of Curcumin derivatives. Met. Based. Drugs 2001, 8, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Beneduci, A.; Corrente, G.A.; Marino, T.; Aiello, D.; Bartella, L.; Di Donna, L.; Napoli, A.; Russo, N.; Romeo, I.; Furia, E. Insight on the chelation of aluminum(III) and iron(III) by curcumin in aqueous solution. J. Mol. Liq. 2019, 296, 111805. [Google Scholar] [CrossRef]
- Cornard, J.P.; Lapouge, C. Absorption spectra of caffeic acid, caffeate and their 1:1 complex with AI(III): Density functional theory and time-dependent density functional theory investigations. J. Phys. Chem. A 2006, 110, 7159–7166. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, G. On the Inhibition of Hydroxyl Radical Formation by Hydroxycinnamic Acids: The Case of Caffeic Acid as a Promising Chelating Ligand of a Ferrous Ion. J. Phys. Chem. A 2019, 123, 9560–9566. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron Chelation by the Powerful Antioxidant Flavonoid Quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef] [PubMed]
- Villuendas-Rey, Y.; Alvarez-Idaboy, J.R.; Galano, A. Assessing the Protective Activity of a Recently Discovered Phenolic Compound against Oxidative Stress Using Computational Chemistry. J. Chem. Inf. Model. 2015, 55, 2552–2561. [Google Scholar] [CrossRef]
- Truong, D.H.; Nhung, N.T.A.; Dao, D.Q. Iron ions chelation-based antioxidant potential vs. pro-oxidant risk of ferulic acid: A DFT study in aqueous phase. Comput. Theor. Chem. 2020, 1185, 112905. [Google Scholar] [CrossRef]
- Amat, A.; Clementi, C.; Miliani, C.; Romani, A.; Sgamellotti, A.; Fantacci, S. Complexation of apigenin and luteolin in weld lake: A DFT/TDDFT investigation. Phys. Chem. Chem. Phys. 2010, 12, 6672–6684. [Google Scholar] [CrossRef] [PubMed]
- Primikyri, A.; Mazzone, G.; Lekka, C.; Tzakos, A.G.; Russo, N.; Gerothanassis, I.P. Understanding zinc(II) chelation with quercetin and luteolin: A combined NMR and theoretical study. J. Phys. Chem. B 2015, 119, 83–95. [Google Scholar] [CrossRef]
- Dimitrić Marković, J.M.; Marković, Z.S.; Brdarić, T.P.; Pavelkić, V.M.; Jadranin, M.B. Iron complexes of dietary flavonoids: Combined spectroscopic and mechanistic study of their free radical scavenging activity. Food Chem. 2011, 129, 1567–1577. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, A. Kinetics of complex formation of Fe(III) with caffeic acid: Experimental and theoretical study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 148–153. [Google Scholar] [CrossRef]
- Favaro, G.; Clementi, C.; Romani, A.; Vickackaite, V. Acidichromism and ionochromism of luteolin and apigenin, the main components of the naturally occurring yellow weld: A spectrophotometric and fluorimetric study. J. Fluoresc. 2007, 17, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.H.; Shi, X.Y.; Li, X.; Li, F.F.; Zhang, Q.Q.; Jiang, S.X.; Cui, J.Z.; Gao, H.L. Spectroscopic and electrochemical studies on the evaluation of the radical scavenging activities of luteolin by chelating iron. RSC Adv. 2014, 4, 25227–25233. [Google Scholar] [CrossRef]
- Ahmedova, A.; Paradowska, K.; Wawer, I. 1H, 13C MAS NMR and DFT GIAO study of quercetin and its complex with Al(III) in solid state. J. Inorg. Biochem. 2012, 110, 27–35. [Google Scholar] [CrossRef]
- Rygula, A.; Wrobel, T.P.; Szklarzewicz, J.; Baranska, M. Raman and UV-vis spectroscopy studies on luteolin-Al(III) complexes. Vib. Spectrosc. 2013, 64, 21–26. [Google Scholar] [CrossRef]
- Sun, J.; Wu, Y.; Xiao, D.; Lin, X.; Li, H. Spectrofluorimetric determination of aluminum ions via complexation with luteolin in absolute ethanol. Luminescence 2014, 29, 456–461. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, L.; Zhang, S.; Sun, P.C.; Ding, C.F.; Chu, Y.Q.; Zhou, P. Interaction of curcumin with Al(III) and its complex structures based on experiments and theoretical calculations. J. Mol. Struct. 2011, 1004, 163–173. [Google Scholar] [CrossRef]
- Bicer, N.; Yildiz, E.; Yegani, A.A.; Aksu, F. Synthesis of curcumin complexes with iron(III) and manganese(II), and effects of curcumin-iron(III) on Alzheimer’s disease. New J. Chem. 2018, 42, 8098–8104. [Google Scholar] [CrossRef]
- Refat, M.S. Synthesis and characterization of ligational behavior of curcumin drug towards some transition metal ions: Chelation effect on their thermal stability and biological activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 105, 326–337. [Google Scholar] [CrossRef]
- Bagchi, A.; Mukherjee, P.; Bhowmick, S.; Raha, A. Synthesis, characterization and antibacterial activity of a novel curcumin metal complex. Int. J. Drug Dev. Res. 2015, 7, 11–14. [Google Scholar]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.F.V.; De Giovani, W.F. Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep. 2004, 9, 97–104. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.F.V.; De Giovani, W.F. Synthesis, spectral and electrochemical properties of Al(III) and Zn(II) complexes with flavonoids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1985–1990. [Google Scholar] [CrossRef] [PubMed]
- Macáková, K.; Mladěnka, P.; Filipský, T.; Říha, M.; Jahodář, L.; Trejtnar, F.; Bovicelli, P.; Proietti Silvestri, I.; Hrdina, R.; Saso, L. Iron reduction potentiates hydroxyl radical formation only in flavonols. Food Chem. 2012, 135, 2584–2592. [Google Scholar] [CrossRef]
- Mira, L.; Fernandez, M.T.; Santos, M.; Rocha, R.; Florêncio, M.H.; Jennings, K.R. Interactions of Flavonoids with Iron and Copper Ions: A Mechanism for their Antioxidant Activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef]
- Lopes, G.K.B.; Schulman, H.M.; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim. Biophys. Acta Gen. Subj. 1999, 1472, 142–152. [Google Scholar] [CrossRef]
- Pȩkal, A.; Biesaga, M.; Pyrzynska, K. Interaction of quercetin with copper ions: Complexation, oxidation and reactivity towards radicals. BioMetals 2011, 24, 41–49. [Google Scholar] [CrossRef]
- MacCarrone, G.; Caruso, R.; Contino, A.; Giuffrida, A.; Messina, M.; Cucinotta, V. The contribution of electrospray mass spectrometry to the study of metal complexes: The case of copper(II)-dipeptide systems. Eur. J. Inorg. Chem. 2009, 2009, 2612–2620. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Bernabé-Pineda, M.; Ramírez-Silva, M.T.; Romero-Romo, M.; González-Vergara, E.; Rojas-Hernández, A. Determination of acidity constants of curcumin in aqueous solution and apparent rate constant of its decomposition. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 1091–1097. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, photochemistry and photobiology of curcumin: Studies from organic solutions, bio-mimetics and living cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Shakeri, A.; Panahi, Y.; Johnston, T.P.; Sahebkar, A. Biological properties of metal complexes of curcumin. BioFactors 2019, 45, 304–317. [Google Scholar] [CrossRef]
- Saithongdee, A.; Praphairaksit, N.; Imyim, A. Electrospun curcumin-loaded zein membrane for iron(III) ions sensing. Sensors Actuators B Chem. 2014, 202, 935–940. [Google Scholar] [CrossRef]



| Al(III) | ||||
|---|---|---|---|---|
| Ligand | (pqr) | log β | Temperature | Reference |
| CA | (111) | 13.40 ± 0.03 | 37 °C | [13] |
| (121) | 22.26 ± 0.06 | |||
| (131) | 30.87 ± 0.09 | |||
| (142) | 42.53 ± 0.09 | |||
| FA | (121) | 21.3 ± 0.3 | 37 °C | [13] |
| (131) | 30.31 ± 0.02 | |||
| (142) | 42.24 ± 0.02 | |||
| p-CA | (131) | 30.21 ± 0.09 | 37 °C | [13] |
| HCCA | (121) | 25.9 ± 0.2 | 37 °C | [14] |
| (131) | 36.5 ± 0.3 | |||
| Que | (121) | 29.25 ± 0.06 | 37 °C | [34] |
| (111) | 16.1 ± 0.1 | 37 °C | [26] | |
| (101) | 23.0 ± 0.5 | 25 °C | [27] | |
| Cur | (111) | 16.4 ± 0.1 | 37 °C | [45] |
| Fe(III) | ||||
| HCCA | (122) | 28.35 ± 0.06 | 37 °C | [14] |
| Que | (121) | 37.24 ± 0.06 | 37 °C | [26] |
| (112) | 43.9 ± 0.1 | |||
| (122) | 53.1 ± 0.1 | |||
| (101) | 5.5 | 25 °C | [46] | |
| (102) | 9.56 | |||
| Lut | (101) | 8.4 | 25 °C | [47] |
| Cur | (131) | 41.4 ± 0.3 | 37 °C | [45] |
| Al(III) | ||||
|---|---|---|---|---|
| Ligand (L) | Complex | ΔGf | Level of Theory | Reference |
| CA | [Al(H2O)3(OH)(LA)]+ | −119.4 | M052X/6-31+G(d) − SMD, water | [13] |
| FA | [Al(H2O)(OH)3(LA)]− | −158.4 | M052X/6-31+G(d) − SMD, water | [13] |
| p-CA | [Al(H2O)(OH)3(LA)]− | −159.5 | M052X/6-31+G(d) − SMD, water | [13] |
| HCCA | [Al(H2O)2(OH)2(LB)] | −145.3 | M052X/6-31+G(d) − SMD, water | [14] |
| [Al(H2O)2(OH)2(LA)] | −139.5 | |||
| [Al(H2O)(OH)3(LB)]− | −157.7 | |||
| [Al(OH)3(η1-LA)]− | −159.0 | |||
| Que | [Al(H2O)2(OH)2(LB)] | −123.7 | M052X/6-31+G(d) − SMD, ethanol | [31] |
| [Al(H2O)2(OH)2(LC)] | −119.0 | |||
| [Al(H2O)3(OH)(LB)]+ | −71.2 | M052X/6-31+G(d) − SMD, water | [34] | |
| [Al(H2O)3(OH)(LC)]+ | −68.6 | |||
| [Al(H2O)4(LA)]+ | −55.9 | |||
| Lut | [Al(H2O)4(LA)]2+ | −1.5 | B3LYP/6-31G ** − CPCM, water | [51] a |
| [Al(H2O)4(LB)]2+ | 2.5 | |||
| [Al(H2O)2(LB)2]+ | −6.8 | |||
| [Al(H2O)2(LA)2]+ | −1.1 | |||
| Cur | [Al(H2O)3(OH)(LA)]+ | −135.1 | M052X/6-31+G(d) − SMD, water | [45] |
| [Al(H2O)3(OH)(LB)]+ | −124.9 | |||
| Fe(III) | ||||
| CA | [Fe(H2O)4(LA)]2+ | 5.8 | M052X/6-31+G(d) − SMD, water | This work |
| [Fe(H2O)4(LA)2]+ | 16.0 | |||
| FA | [Fe(H2O)4(LA)]2+ | –49.7 | M05/6-311++G(d,p) − SMD, water | [50] |
| [Fe(H2O)4(LA)2]+ | –85.4 | |||
| HCCA | [Fe(OH)2(η1-L)2]− | −27.1 | M052X/6-31+G(d) − SMD, water | [14] |
| [Fe(OH)2(η2-L)2]− | −19.2 | |||
| Que | [Fe(H2O)2(OH)2(LA)]− | −99.4 | M052X/6-31+G(d) − SMD, water | [34] |
| [Fe(H2O)(OH)(LA)2]− | −113.7 | |||
| [Fe(OH)2(LA)2]− | −125.8 | |||
| Cur | [Fe(H2O)(OH)3(LA)]− | −57.1 | M052X/6-31+G(d) − SMD, water | [45] |
| [Fe(H2O)(OH)3(LB)]− | −55.5 | |||
| Al(III) | ||||
|---|---|---|---|---|
| Ligand | M:L | Solvent | Characterization Methods | Reference |
| CA | 1:1 1:2 | water | UV–Vis, 1H-NMR | [13] |
| FA | 1:1 1:2 | water | UV–Vis, 1H-NMR | [13] |
| p-CA | 1:1 | water | UV–Vis, 1H-NMR | [13] |
| HCCA | 1:1 | water | UV–Vis, 1H, 13C-NMR | [14] |
| Que | 1:2 | methanol | FTIR, 1H, 13C MAS NMR | [57] |
| 1:1 | water | UV–Vis, 1H, 13C-NMR | [34] | |
| Lut | 1:1 2:1 | methanol:water (9:1) | UV–Vis, FT-IR, RAMAN | [58] |
| 1:2 | ethanol | UV–Vis, FTIR, fluorescence, ESI-MS | [59] | |
| Cur | 1:1 2:1 3:1 | methanol | 1H, 13C, 27Al-NMR, MALDI-TOF | [60] |
| 1:1 1:2 | water | UV-Vis, ESI MS/MS, LD-MS, MS/MS | [45] | |
| Fe(III) | ||||
| HCCA | 1:2 | water | UV–Vis, 1H, 13C-NMR | [14] |
| Que | 1:2 | methanol | UV–Vis, FTIR, ESI MS, 1H-NMR | [29] |
| 1:2 | water | UV–Vis, 1H, 13C-NMR | [34] | |
| Lut | 1:1 | ethanol | UV–Vis, FT-IR, ESI MS | [56] |
| 1:2 1:2 a | water | ESI MS, ESI-TOF MS | [37] | |
| Cur | 1:2 | water | UV–Vis, ESI MS/MS, LD-MS, MS/MS | [45] |
| 1:2 | methanol | FTIR | [61] | |
| 1:2 | water:methanol (1:1) | UV–Vis, FTIR, RAMAN, ESR, 1H-NMR, X-ray | [62] | |
| 1:2 | methanol | UV–Vis, FTIR | [63] | |
| Experimental Methods | Methodology | Principle | Analysis Results | Reliability and Quality of the Results on the Complex Formation |
|---|---|---|---|---|
| Potentiometry |
| The complexation equilibria are studied by measuring, with a glass electrode, the competition of the ligand for H+ and metal cations. | The experimental data, obtained at different metal and ligand total concentrations, and processed by numerical procedures, were rationalized according to a general equilibrium, to obtain the complexes existing in solution at a given pH. | Speciation studies allow to predict the most probable stoichiometric coefficients of the complexes and the corresponding stability constants. |
| UV-Vis |
| Absorption of ultraviolet and visible photons by a molecule causes a change from its fundamental electronic state to an excited electronic state. | In a typical UV–visible spectrum the wavelength is reported in the abscissa (190 < λ < 780 nm) and the absorbance (or the transmittance) in the ordinate. The recorded spectrum will be characterized by a series of bands of variable intensity. | Complexation leads to specific band shifts in the spectrum of the ligand that, in several cases, can be directly related to the involvement of a specific complexation site of the ligand. In addition, metal-to-ligand charge transfer transitions and d-d transition bands may occur |
| 1H, 13C, 27Al-NMR |
| Absorption of a radio frequency radiation is measured after immersing a molecule in a strong static magnetic field, which causes nuclear spin transitions. | An NMR spectrum shows the frequency absorbed and then emitted by the atoms of the nucleus under examination, which depends on the chemical environment around it (chemical shift). | Upon complexation huge shielding or deshielding effects may be induced on the magnetic nuclei of the ligand, generally placed close to the binding site. However, significant chemical shift displacement can occur far from the binding site when resonance structures are involved in the ligand. Quantitative information on the structural properties of the complexes may also be obtained from the spectra of magnetic metals such as 27Al. |
| FTIR |
| Absorption of an infrared photon by a molecule, causes its transition from its fundamental vibrational state to an excited vibrational state. | In a typical IR spectrum, the percentage of transmittance is plotted against the wave number (4000 cm−1 < λ < 400 cm−1). Each peak in the spectrum can be assigned to a specific functional group. | Formation of a complex usually leads to the appearance of specific peaks related to the metal-heteroatom bond (e.g., Al-O). Moreover, metal binding affects the bond vibrational energies of the functional groups of the ligand involved in the complexation, leading to related peak shifts. |
| Mass spectrometry |
| This technique allows separating a mixture of ions according to their mass/charge ratio. Molecules are ionized and fragmented into lighter ions according to typical patterns depending on their chemical structure. | The diagram showing the abundance of each ion as a function of the mass/charge ratio (m/z) is the so-called mass spectrum, typical of each compound as it is directly related to its chemical structure and to the ionization conditions it has been subjected. | Upon complexation, specific m/z fragments containing the metal can be detected, indicative of the specific binding site involved. The stoichiometry of the complex (M:L ratio) can be easily determined. |
| Fluorescence |
| An incident photon excites the fluorophore from the ground state to a higher energy state (electronically and vibrationally) with the same spin. In a few nanoseconds, the excited electron returns to the ground electronic state passing through one or more excited states at intermediate energy. All decays except one are usually non-radiative, while the last one emits light with a longer wavelength than the incident radiation, that is the fluorescence. | In a typical fluorescence spectrum, the fluorescence intensity is plotted vs. the wavelength of emission. | Complex formation between a metal and a fluorophore may lead to a significant fluorescence enhancement due to restricted intramolecular rotations of the ligand, as well as a huge shift of the emission maximum. |
| RAMAN |
| This technique is based on the diffusion (scattering) of a monochromatic electromagnetic radiation by the analyzed sample. The diffused radiation contains the components with different energy (Rayleigh, Stokes and anti-Stokes) associated to molecular vibrations of different functional groups. | In a Raman spectrum the intensity of the signals proportional to the number of Stokes photons, are plotted against the Raman shift (in cm−1), corresponding to the energy difference associated to transitions between fundamental vibrational levels. | Upon complexation specific band shifts (i.e., inplane skeletal vibrations as well as stretching vibrations) occurs in the RAMAN spectra indicating which part of the ligand takes part in the metal binding. |
| ESR |
| It detects the induced transition by a microwave radiation between the energy levels of electron spins under a static magnetic field. It allows the study of organic and inorganic free radicals, odd electrons molecules, molecules in the triplet state, transition metal complexes, etc. | The EPR spectrum is the first derivative of the absorption spectrum obtained in the microwave range | ESR measurements of the magnetic moment, is indicative of low or high spin configuration around the metal, thus providing information on the coordination geometry |
| X-ray Diffraction |
| It allows the structural characterization of crystalline materials. It is based on the constructive interference of an x-ray monochromatic beam which is scattered at specific angles from each set of lattice planes in a sample. | The x-ray pattern is the fingerprint of the periodic atomic arrangement in the material | X-ray diffraction provides insight on the coordination geometry around the metal ion as well as on the degree of crystallinity of the complexes. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malacaria, L.; Corrente, G.A.; Beneduci, A.; Furia, E.; Marino, T.; Mazzone, G. A Review on Coordination Properties of Al(III) and Fe(III) toward Natural Antioxidant Molecules: Experimental and Theoretical Insights. Molecules 2021, 26, 2603. https://doi.org/10.3390/molecules26092603
Malacaria L, Corrente GA, Beneduci A, Furia E, Marino T, Mazzone G. A Review on Coordination Properties of Al(III) and Fe(III) toward Natural Antioxidant Molecules: Experimental and Theoretical Insights. Molecules. 2021; 26(9):2603. https://doi.org/10.3390/molecules26092603
Chicago/Turabian StyleMalacaria, Luana, Giuseppina Anna Corrente, Amerigo Beneduci, Emilia Furia, Tiziana Marino, and Gloria Mazzone. 2021. "A Review on Coordination Properties of Al(III) and Fe(III) toward Natural Antioxidant Molecules: Experimental and Theoretical Insights" Molecules 26, no. 9: 2603. https://doi.org/10.3390/molecules26092603
APA StyleMalacaria, L., Corrente, G. A., Beneduci, A., Furia, E., Marino, T., & Mazzone, G. (2021). A Review on Coordination Properties of Al(III) and Fe(III) toward Natural Antioxidant Molecules: Experimental and Theoretical Insights. Molecules, 26(9), 2603. https://doi.org/10.3390/molecules26092603










