Radiation-Induced Metabolic Shifts in the Hepatic Parenchyma: Findings from 18F-FDG PET Imaging and Tissue NMR Metabolomics in a Mouse Model for Hepatocellular Carcinoma
Abstract
1. Introduction
2. Results
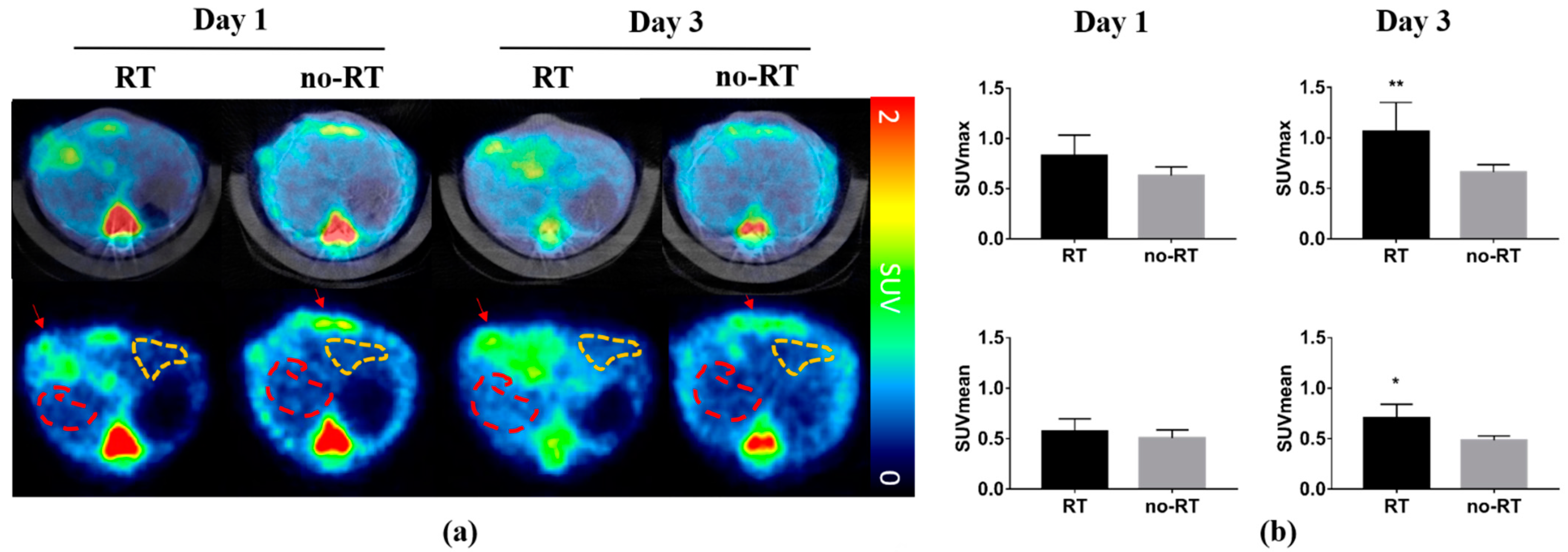
2.1. 18F-FDG Uptake in Irradiated and Not-Irradiated Liver Parenchyma
2.2. Metabolic Changes in Irradiated and Not-Irradiated Liver Parenchyma
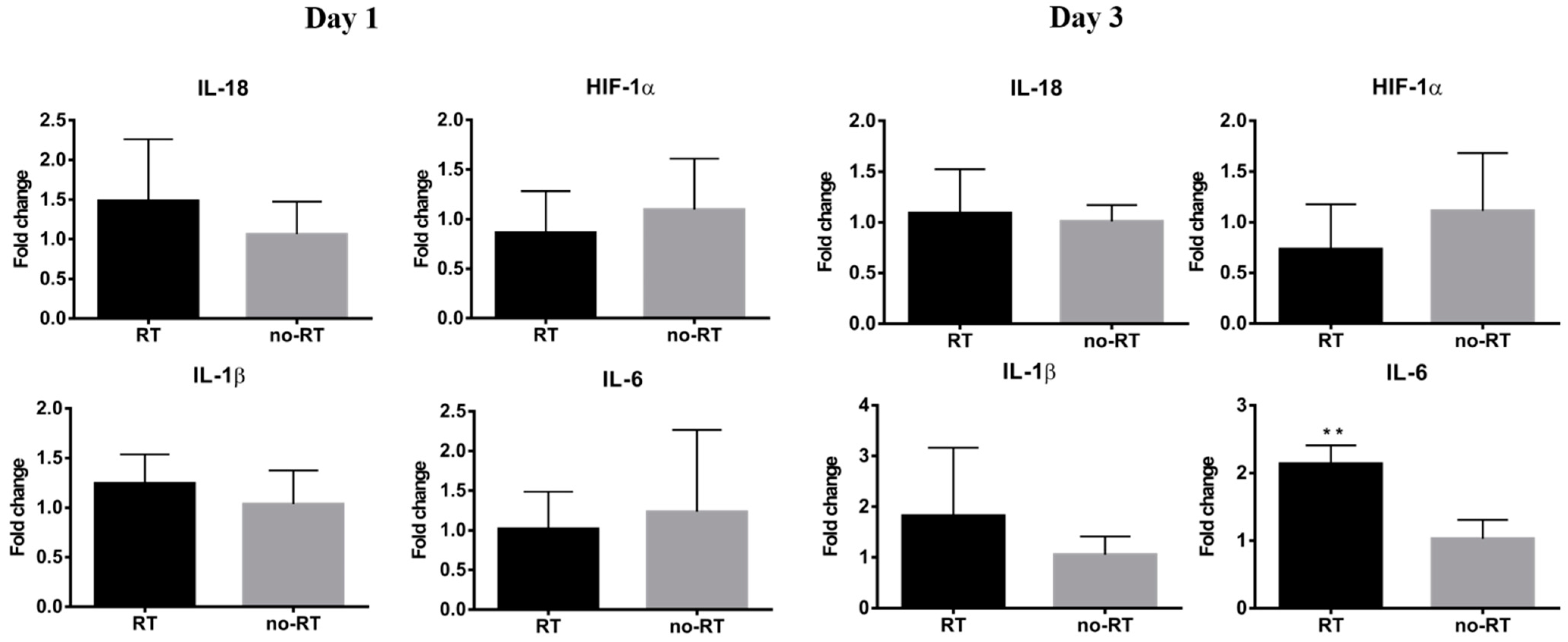
2.3. Markers of Inflammation and Gluconeogenesis in Irradiated and Not-Irradiated Liver Parenchyma
2.4. Assessment of Liver Enzymes in Irradiated Mice Bearing Orthotopic HCC
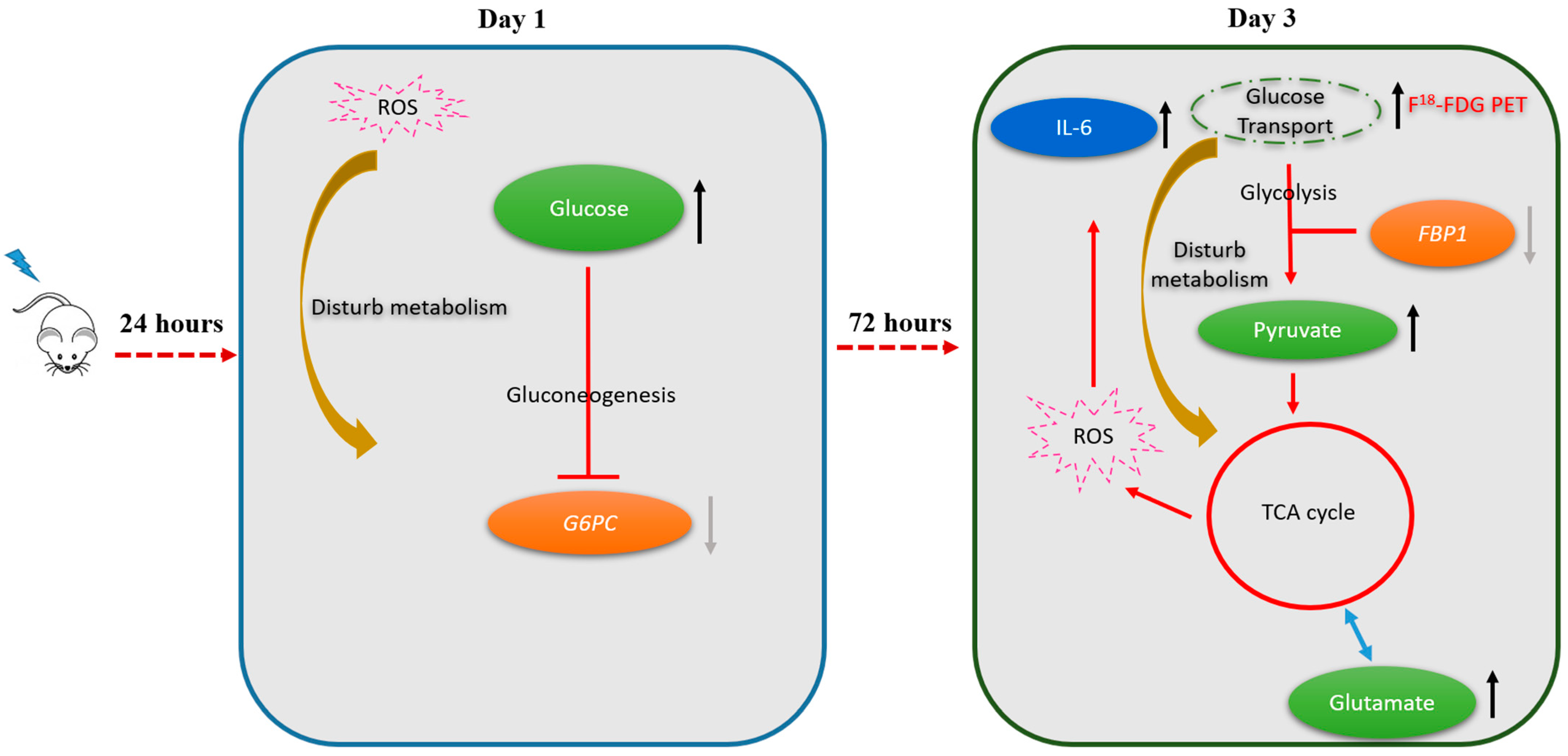
2.5. Alterations in Hepatic Metabolic Pathways in Response to Irradiation
3. Discussion
3.1. Metabolic Switch to Glycolysis Following Irradiation of the Liver Parenchyma
3.2. Inflammatory Response in Irradiated Liver Parenchyma
3.3. Bystander Effects in the Left Liver Lobe
3.4. Future Research Directions
4. Materials and Methods
4.1. Experimental Design
4.2. Animal Model and Procedures for Irradiation
4.3. 18F-FDG PET/CT Imaging Protocol and Analysis
4.4. Collection and Extraction of the Liver Tissue for NMR Metabolomics
4.5. NMR Metabolomics Data Processing
4.6. Serum Biomarkers of Liver Damage
4.7. Immunohistochemical Staining of Inflammatory Markers
4.8. RNA Extraction and Gene Expression Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S. Hepatocellular carcinoma in Taiwan. Hepatol. Res. 2007, 37, S101–S105. [Google Scholar] [CrossRef]
- Benson, R.; Madan, R.; Kilambi, R.; Chander, S. Radiation induced liver disease: A clinical update. J. Egypt. Natl. Cancer Inst. 2016, 28, 7–11. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y. Radiation-induced liver disease: Current understanding and future perspectives. Exp. Mol. Med. 2017, 49, e359. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T.S.; Robertson, J.M.; Anscher, M.S.; Jirtle, R.L.; Ensminger, W.D.; Fajardo, L.F. Hepatic toxicity resulting from cancer treatment. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1237–1248. [Google Scholar] [CrossRef]
- Cheng, W.; Xiao, L.; Ainiwaer, A.; Wang, Y.; Wu, G.; Mao, R.; Yang, Y.; Bao, Y. Molecular responses of radiation-induced liver damage in rats. Mol. Med. Rep. 2015, 11, 2592–2600. [Google Scholar] [CrossRef]
- Kim, J.H.; Jenrow, K.A.; Brown, S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014, 32, 103–115. [Google Scholar] [CrossRef]
- Castro, N.P.; Osorio, C.A.; Torres, C.; Bastos, E.P.; Mourao-Neto, M.; Soares, F.A.; Brentani, H.P.; Carraro, D.M. Evidence that molecular changes in cells occur before morphological alterations during the progression of breast ductal carcinoma. Breast Cancer Res. 2008, 10, 1–14. [Google Scholar] [CrossRef]
- Norregaard, K.; Jorgensen, J.T.; Simon, M.; Melander, F.; Kristensen, L.K.; Bendix, P.M.; Andresen, T.L.; Oddershede, L.B.; Kjaer, A. 18F-FDG PET/CT-based early treatment response evaluation of nanoparticle-assisted photothermal cancer therapy. PLoS ONE 2017, 12, e0177997. [Google Scholar] [CrossRef]
- Agrawal, A.; Rangarajan, V. Appropriateness criteria of FDG PET/CT in oncology. Indian J. Radiol. Imaging 2015, 25, 88–101. [Google Scholar] [CrossRef]
- Kinahan, P.E.; Fletcher, J.W. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin. Ultrasound CT MRI 2010, 31, 496–505. [Google Scholar] [CrossRef]
- Kiyohara, S.; Nagamachi, S.; Wakamatsu, H.; Nishii, R.; Fujita, S.; Futami, S.; Tamura, S. Usefulness of metabolic volume and total lesion glycolysis for predicting therapeutic response in cancer therapy by 18F-FDG PET/CT. Kaku Igaku Jpn. J. Nucl. Med. 2010, 47, 453–461. [Google Scholar]
- Gupta, N.C.; Graeber, G.M.; Bishop, H.A. Comparative efficacy of positron emission tomography with fluorodeoxyglucose in evaluation of small (<1 cm), intermediate (1 to 3 cm), and large (>3 cm) lymph node lesions. Chest 2000, 117, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Dona, A.C.; Kyriakides, M.; Scott, F.; Shephard, E.A.; Varshavi, D.; Veselkov, K.; Everett, J.R. A guide to the identification of metabolites in NMR-based metabonomics/metabolomics experiments. Comput. Struct. Biotechnol. J. 2016, 14, 135–153. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Yu, C.F.; Chiu, S.C.; Chiu, H.; Hsu, S.T.; Wu, C.R.; Yang, C.L.; Hong, J.H.; Yen, T.C.; Chen, F.H. Diffusion-weighted MRI and (18)F-FDG PET correlation with immunity in early radiotherapy response in BNL hepatocellular carcinoma mouse model: Timeline validation. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1733–1744. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Li, C.; Wu, J.; Kuo, J.E.; Wang, C. Assessment of early triage for acute radiation injury in rat model based on urinary amino acid target analysis. Mol. Biosyst. 2014, 10, 1441–1449. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Perez-Felpete, N.; Fernandez-Fernandez, C.; Donapetry-Garcia, C.; Pazos-Garcia, C. Liver glucose metabolism in humans. Biosci. Rep. 2016, 36, e00416. [Google Scholar] [CrossRef]
- Paulikova, E.; Ahlers, I.; Praslicka, M. Gluconeogenesis in lethally X-irradiated rats. Physiol. Bohemoslov. 1983, 32, 73–79. [Google Scholar]
- Kay, R.E.; Entenman, C. Hyperglycemia and increased liver glycogen in rats after x-irradiation. Proc. Soc. Exp. Biol. Med. 1956, 91, 143–146. [Google Scholar] [CrossRef]
- Kurland, I.J.; Broin, P.O.; Golden, A.; Su, G.; Meng, F.; Liu, L.; Mohney, R.; Kulkarni, S.; Guha, C. Integrative Metabolic Signatures for Hepatic Radiation Injury. PLoS ONE 2015, 10, e0124795. [Google Scholar] [CrossRef]
- Ferreira, S.J.; Sonnewald, U. The mode of sucrose degradation in potato tubers determines the fate of assimilate utilization. Front. Plant Sci. 2012, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, C. Gluconeogenesis in Cancer: Function and Regulation of PEPCK, FBPase, and G6Pase. Trends Cancer 2019, 5, 30–45. [Google Scholar] [CrossRef]
- Grasmann, G.; Smolle, E.; Olschewski, H.; Leithner, K. Gluconeogenesis in cancer cells—Repurposing of a starvation-induced metabolic pathway? Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Mossenta, M.; Busato, D.; Dal Bo, M.; Toffoli, G. Glucose Metabolism and Oxidative Stress in Hepatocellular Carcinoma: Role and Possible Implications in Novel Therapeutic Strategies. Cancers 2020, 12, 1668. [Google Scholar] [CrossRef]
- Wei, J.; Wang, H.; Wang, H.; Wang, B.; Meng, L.; Xin, Y.; Jiang, X. The role of NLRP3 inflammasome activation in radiation damage. Biomed. Pharmacother. 2019, 118, 109217. [Google Scholar] [CrossRef]
- Xiao, M. The Role of Proinflammatory Cytokine Interleukin-18 in Radiation Injury. Health Phys. 2016, 111, 212–217. [Google Scholar] [CrossRef]
- Cha, H.; Lee, E.J.; Seong, J. Multi-analyte analysis of cytokines that predict outcomes in patients with hepatocellular carcinoma treated with radiotherapy. World J. Gastroenterol. 2017, 23, 2077–2085. [Google Scholar] [CrossRef]
- Kesner, A.L.; Lau, V.K.; Speiser, M.; Hsueh, W.A.; Agazaryan, N.; DeMarco, J.J.; Czernin, J.; Silverman, D.H. Time-course of effects of external beam radiation on [18F]FDG uptake in healthy tissue and bone marrow. J. Appl. Clin. Med. Phys. 2008, 9, 147–156. [Google Scholar] [CrossRef]
- Ando, M.; Uehara, I.; Kogure, K.; Asano, Y.; Nakajima, W.; Abe, Y.; Kawauchi, K.; Tanaka, N. Interleukin 6 enhances glycolysis through expression of the glycolytic enzymes hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3. J. Nippon Med. Sch. 2010, 77, 97–105. [Google Scholar] [CrossRef]
- Basu, A.; Shah, P.; Nielsen, M.; Basu, R.; Rizza, R.A. Effects of type 2 diabetes on the regulation of hepatic glucose metabolism. J. Investig. Med. 2004, 52, 366–374. [Google Scholar] [CrossRef]
- Tappy, L.; D’Alessio, D. How are we going to understand (dys)regulation of glucose metabolism? Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wu, T.; Wu, J.; Zhao, L.; Li, Q.; Varghese, Z.; Moorhead, J.F.; Powis, S.H.; Chen, Y.; Ruan, X.Z. Chronic inflammation exacerbates glucose metabolism disorders in C57BL/6J mice fed with high-fat diet. J. Endocrinol. 2013, 219, 195–204. [Google Scholar] [CrossRef]
- Li, J.; He, M.; Shen, B.; Yuan, D.; Shao, C. Alpha particle-induced bystander effect is mediated by ROS via a p53-dependent SCO2 pathway in hepatoma cells. Int. J. Radiat. Biol. 2013, 89, 1028–1034. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Fu, J.; Wang, J.; Ye, S.; Liu, W.; Shao, C. Role of ROS-mediated autophagy in radiation-induced bystander effect of hepatoma cells. Int. J. Radiat. Biol. 2015, 91, 452–458. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Mychasiuk, R.; Muhammad, A.; Hossain, S.; Ilnytskyy, S.; Ghose, A.; Kirkby, C.; Ghasroddashti, E.; Kovalchuk, O.; Kolb, B. Liver irradiation causes distal bystander effects in the rat brain and affects animal behaviour. Oncotarget 2016, 7, 4385–4398. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Jeoung, N.H. Pyruvate Dehydrogenase Kinases: Therapeutic Targets for Diabetes and Cancers. Diabetes Metab. J. 2015, 39, 188–197. [Google Scholar] [CrossRef]
- Michel, R.; Francoise, I.; Laure, P.; Anouchka, M.; Guillaume, P.; Sylvain, K. Dose to organ at risk and dose prescription in liver SBRT. Rep. Pract. Oncol. Radiother. 2017, 22, 96–102. [Google Scholar] [CrossRef]
- Boll, H.; Figueiredo, G.; Fiebig, T.; Nittka, S.; Doyon, F.; Kerl, H.U.; Nolte, I.; Forster, A.; Kramer, M.; Brockmann, M.A. Comparison of Fenestra LC, ExiTron nano 6000, and ExiTron nano 12,000 for micro-CT imaging of liver and spleen in mice. Acad. Radiol. 2013, 20, 1137–1143. [Google Scholar] [CrossRef]


| Metabolite | VIP Score | Fold Change (|RT/no-RT| > 1.1) | p | Metabolite | VIP Score | Fold change (|RT/no-RT| > 1.1) | p |
|---|---|---|---|---|---|---|---|
| Right liver | Day 1 | Day 3 | |||||
| Glucose | 3.456 | 16.930 | 0.003 | Pyruvate | 1.818 | 6.996 | 0.008 |
| Sucrose | 2.244 | 13.483 | 0.015 | Glutamate | 1.043 | 3.487 | 0.024 |
| Galactarate | 1.396 | 2.523 | 0.003 | Sucrose | 1.153 | 2.812 | 0.019 |
| Galactitol | 1.931 | 4.776 | 0.001 | Malonate | 0.879 | 0.565 | 1.6 × 10−4 |
| Pyridoxine | 0.836 | 0.512 | 0.006 | ||||
| Choline | 0.955 | 0.479 | 0.009 | ||||
| Niacinamide | 1.244 | 0.345 | 1.7 × 10−7 | ||||
| Hypoxanthine | 1.763 | 0.341 | 0.028 | ||||
| Betaine | 1.449 | 0.252 | 1.4 × 10−4 | ||||
| Guanidoacetate | 2.690 | 0.121 | 0.001 | ||||
| Sarcosine | 1.992 | 0.090 | 3.8 × 10−4 | ||||
| Glycocholate | 2.190 | 0.059 | 2.0 × 10−4 | ||||
| Left liver | Day 1 | Day 3 | |||||
| Glucose | 2.561 | 17.774 | 0.008 | Fumarate | 1.108 | 1.844 | 0.001 |
| Malonate | 2.597 | 9.836 | 0.001 | Niacinamide | 0.981 | 0.577 | 0.005 |
| Succinate | 2.437 | 3.979 | 0.004 | Riboflavin | 1.062 | 0.452 | 0.029 |
| Galactitol | 1.539 | 3.506 | 0.001 | Succinate | 1.200 | 0.428 | 0.008 |
| Glycylproline | 1.350 | 3.018 | 1.7 × 10−4 | Succinylacetone | 1.326 | 0.415 | 0.043 |
| Galactarate | 1.404 | 2.866 | 0.001 | Betaine | 1.492 | 0.364 | 3.2 × 10−4 |
| Alanine | 1.185 | 2.093 | 0.018 | Sarcosine | 1.448 | 0.349 | 0.002 |
| N-Methylhydantoin | 0.919 | 1.832 | 0.049 | Guanidoacetate | 2.401 | 0.335 | 0.036 |
| Anserine | 0.613 | 1.433 | 0.046 |
| Experimental Design | Number of Animals | ||||
|---|---|---|---|---|---|
| Post-irradiaton | 1 day | 3 day | |||
| Longitudinal imaging data | FDG PET | 1RT | 5 | 5 | |
| no-RT | 5 | 5 | |||
| Time-points data collection | NMR metabolomics | 1RT | 6 | 6 | |
| no-RT | 6 | 6 | |||
| Biomarkers of liver damage | 1RT | 3 | 3 | ||
| no-RT | 3 | 3 | |||
| Cytokines qPCR | 1RT | ≥3 | ≥3 | ||
| no-RT | ≥3 | ≥3 | |||
| Gluconeogenesis qPCR | 1RT | ≥3 | ≥3 | ||
| no-RT | ≥3 | ≥3 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, Y.-H.; Tsai, C.-K.; Yu, C.-F.; Wang, W.-L.; Yang, C.-L.; Hong, J.-H.; Yen, T.-C.; Chen, F.-H.; Lin, G. Radiation-Induced Metabolic Shifts in the Hepatic Parenchyma: Findings from 18F-FDG PET Imaging and Tissue NMR Metabolomics in a Mouse Model for Hepatocellular Carcinoma. Molecules 2021, 26, 2573. https://doi.org/10.3390/molecules26092573
Chung Y-H, Tsai C-K, Yu C-F, Wang W-L, Yang C-L, Hong J-H, Yen T-C, Chen F-H, Lin G. Radiation-Induced Metabolic Shifts in the Hepatic Parenchyma: Findings from 18F-FDG PET Imaging and Tissue NMR Metabolomics in a Mouse Model for Hepatocellular Carcinoma. Molecules. 2021; 26(9):2573. https://doi.org/10.3390/molecules26092573
Chicago/Turabian StyleChung, Yi-Hsiu, Cheng-Kun Tsai, Ching-Fang Yu, Wan-Ling Wang, Chung-Lin Yang, Ji-Hong Hong, Tzu-Chen Yen, Fang-Hsin Chen, and Gigin Lin. 2021. "Radiation-Induced Metabolic Shifts in the Hepatic Parenchyma: Findings from 18F-FDG PET Imaging and Tissue NMR Metabolomics in a Mouse Model for Hepatocellular Carcinoma" Molecules 26, no. 9: 2573. https://doi.org/10.3390/molecules26092573
APA StyleChung, Y.-H., Tsai, C.-K., Yu, C.-F., Wang, W.-L., Yang, C.-L., Hong, J.-H., Yen, T.-C., Chen, F.-H., & Lin, G. (2021). Radiation-Induced Metabolic Shifts in the Hepatic Parenchyma: Findings from 18F-FDG PET Imaging and Tissue NMR Metabolomics in a Mouse Model for Hepatocellular Carcinoma. Molecules, 26(9), 2573. https://doi.org/10.3390/molecules26092573








