Abstract
The development of Multi-Target Directed Ligand is of clear interest for the treatment of multifactorial pathology such as Alzheimer’s disease (AD). In this context, acetylcholinesterase (AChE) inhibitors have been modulated in order to generate novel pleiotropic compounds targeting a second protein of therapeutic interest in AD. Among them, donecopride was the first example of a dual acetylcholinesterase inhibitor and 5-HT4 receptor agonist. In order to explore the structural diversity around this preclinical candidate we have explored the preparation of novel constrained analogs through late-stage rigidification strategy. A series of phenylpyrazoles was prepared in a late-stage functionalization process and all compounds were evaluated in vitro towards AChE and 5-HTRs. A docking study was performed in order to better explain the observed SAR towards AChE, 5-HT4R and 5-HT6R and this study led to the description of novel ligand targeting both AChE and 5-HT6R.
1. Introduction
In the field of neurodegenerative diseases, the prototype of multifactorial pathologies, the development of Multi-Target Directed Ligands (MTDLs) offered great promise and therapeutic opportunities [1]. Indeed, these compounds are rationally designed to display multiple activities by modulating several biological targets of interest in the treatment of a specific disease. Chosen to demonstrate a therapeutic synergy, the association of these biological targets needs to be rigorously determined [2]. In the field of Alzheimer’s disease (AD), multiple in vitro or in vivo MTDL candidates have been generated over the last years and could lead to preclinical or clinical opportunities [3]. Because of its validated interest in the treatment of AD symptoms, acetylcholinesterase (AChE) inhibitors, such as donepezil, have been chosen as a starting point to develop novel analogs with a second biological target such as receptors [4]. As an example, we recently described the preclinical candidate donecopride, the first MTDL able to demonstrate AChE inhibition and activating serotoninergic receptors 5-HT4 (Figure 1) [5,6,7].
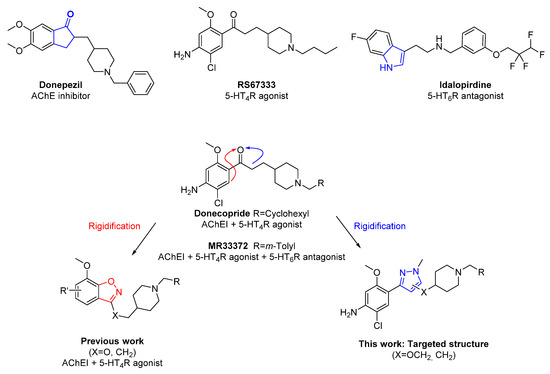
Figure 1.
Structures of reference ligands and MTDL (Donecopride, MR33372, benzisoxazole) and targeted structure.
This secondary target was chosen because of the ability of 5-HT4R agonists to favor the “non-amyloidogenic” cleavage of the amyloid precursor protein (APP) by α-secretase, inducing then a decrease in amyloid pathology. [8] This positive effect has already been demonstrated with the use of RS67333 a reference 5-HT4R agonist, in primary neurons, [9] and led to the in vivo improvement of memory in several animal models of AD [10]. Indeed, in the context of AD, the activation of 5-HT4Rs with agonist has led to the increase in neurotransmitter release as well as a clear impact of amyloid load in transgenic mice models after chronic administration. Several 5-HT4R agonists have been studied in clinical trials in recent years to demonstrate both symptomatic and disease-modifying effect against AD [11]. Beside this receptor subtypes, other serotonin receptors have been studied in the field of neurodegenerative diseases [12], such as the 5-HT6R [13,14]. Specifically, 5-HT6R antagonists increase extracellular levels of acetylcholine, glutamate, and norepinephrine in forebrain regions, whereas activation of 5-HT6R inhibits corticostriatal glutamatergic transmission. In the end, these various elements (notably, distribution in limbic areas, modulation of different systems of neurotransmission) are in favor of the implication of 5-HT6R in the modulation of the cognitive processes as well as mood regulation and of numerous behaviors (eating behavior, addictive behavior, etc.). Indeed, they appear as a valuable target to treat cognitive impairments since their blockade confers to 5-HT6R antagonists’, such as idalopirdine (Figure 1), procognitive effects. [15] It is important to note that during the clinical trials the potential synergistic effect to act simultaneously on 5-HT6R and AChE has been evaluated since idalopirdine has been tested along with donepezil [16].
In this context, we recently described initial modulations of donecopride by introducing a series of substituent on the piperidine ring, which yielded the description of a first 5-HT4R agonist and 5-HT6R antagonist, [17] and MR33372, the first compound able to modulate simultaneously AChE, 5-HT4R and 5-HT6R [18]. In parallel with these first works, we also decided to assess the impact of conformational restriction on the aromatic ring, inspired by both bicyclic donepezil and idalopirdine (Figure 1). Indeed, such restriction are classical in drug discovery [19], and we recently described the preparation and biological evaluation of benzisoxazole analog possessing an oxygen or a methylene linker [20]. We would like to describe in this article, a novel rigidification strategy with the construction of a novel heterocycle between the ketone and its alpha-position. The preparation of novel phenylpyrazole derivatives possessing either a methylene or an oxygen linker (Figure 1), as well as their biological evaluation against AChE and both 5-HT4R and 5-HT6R will be described and compared with other members of this series.
2. Results
2.1. Chemistry
The access to targeted compounds was achieved starting from Boc-protected benzophenone 1 that we previously described (Scheme 1) [5]. The latter was treated by DMF–DMA [21] to generate an intermediate dimethyl enamines, which was not isolated, but reacted in boiling ethanol with methylhydrazine to generate the expected pyrazole 2 with 38% yield [22,23]. In order to obtain the final derivatives, the Boc protecting group was removed with TFA, and the resulted piperidine was alkylated with the appropriate bromide to generate the final cyclohexyl and m-tolyl substituted analog 3a–b. These particular substituents were chosen for a better comparison with either donecopride and MR33372. In order to verify the impact of the aniline both on the reactivity and the biological activities, the corresponding acetamides 4–5 were obtained in acetic anhydride with good yields. Following the same procedure, the cyclohexyl substituted analogs 6 and 7 were prepared after deprotection and alkylation (Scheme 1).
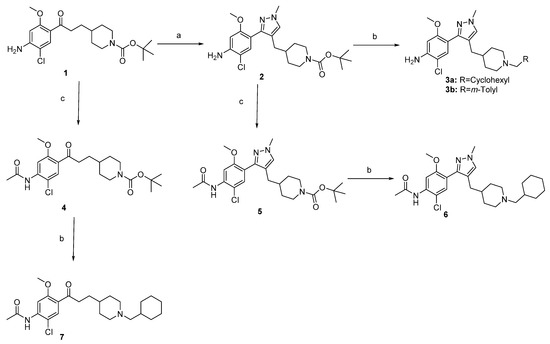
Scheme 1.
Synthetic pathways for access to compounds 2–7. Reagents and conditions: (a) i. DMF.DMA, Rflx, ii. CH3NHNH2, EtOH, Rflx, mw, 38%; (b) i. TFA, DCM, TA, ii. RCH2Br, K2CO3, DMF, or acetone, 110 °C, 31–46%; (c) Ac2O, rt, 24 h, 78–83%.
As already described in the benzisoxazole [20] and donecopride series [6], the influence of the linker could be of great importance on the ability of the ligand to bind to AChE. We then investigated the possibility to modulate both the linker and its position on the pyrazole ring. Indeed, the β-ketoester 8 is a crucial intermediate in the preparation of the benzophenone 1 and was then already available [6]. Afterwards, 8 was treated with methylhydrazine in refluxing ethanol to obtain the pyrazolone 9a with 57% yield (Scheme 2). Unfortunately, the O-alkylation procedure tested was ineffective to generate the expected piperidine 10a, due to the reactivity of the aniline moiety. The pyrazolone 9a was then treated with acetic anhydride to obtain the ester 11 with 94% yield, before being hydrolyzed with LiOH to generate the acetamide 9b. The latter was then alkylated with N-Boc 4-iodomethylpiperidine in DMF to generate this time the expected ether 10b with good conversion. Finally, the protecting group was removed with trifluoroacetic acid, and the m-tolyl analog 12b was obtained after an N-alkylation reaction with the corresponding bromide. Unfortunately, all attempts aiming at deprotecting the N-acetamide group of 12b failed. In order to verify this time the impact of the substitution of the aromatic ring, commercial pyrazolone 9c–d were engaged in the same procedure. The 3-4-dimethoxyphenyl group of 9d was chosen by analogy with donepezil, a reference AChE inhibitor (AChEI). The expected m-tolyl analogs 12c–d were obtained in a two-step synthesis.
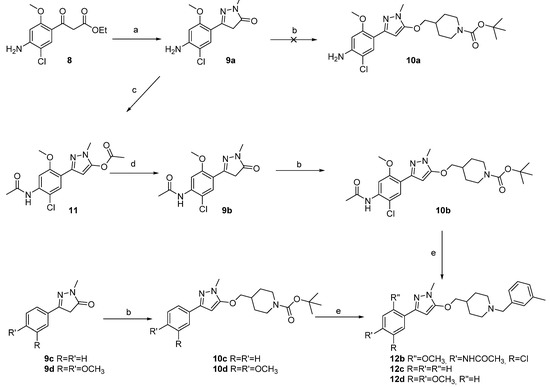
Scheme 2.
Synthesis pathways for access to compounds 9–12. Reagents and conditions: (a) CH3NHNH2, EtOH, 160 °C, mw, 57%; (b) N-Boc 4-iodomethylpiperidine, K2CO3, DMF, 60 °C, 21–27%; (c) Ac2O, 94%, (d) LiOH, EtOH/H2O, Rfx, 76%, (e) i. TFA, DCM, TA, ii. m-TolylCH2Br, K2CO3, acetone, 110 °C, 32–67%.
2.2. In Vitro Results
The inhibitory activity of novel analogs towards hAChE was evaluated according to the Ellman test [24]. Their affinity for human 5-HT4R and 5-HT6R was assessed using a radioligand displacement assay (Table 1). In these tests, donepezil (DPZ) was used as a reference AChEI, RS67333 as a 5-HT4R ligand, and idalopirdine as a 5-HT6R ligand, respectively.

Table 1.
hAChE inhibitory activity and affinity for 5-HT4R and 5-HT6R.
As already introduced, we demonstrated that pharmacomodulation of the preclinical candidate donecopride could greatly affect the ability of its analog to interact with ChE and 5-HT receptors. Indeed, in our initial study [6], we showed that its ester or amide analogs lose their ability to inhibit AChE. The same tendency has also been observed in the benzisoxazole family with a clear impact of the modulation of the benzenic position on both AChE and 5-HT4R binding. [20] In this context, we decided to explore the modulation of the α-position of the ketone and the possibility to generate conformationally constrained derivatives to increase the elucidation of SAR around donecopride. The preparation of first series of phenylpyrazoles 3a–b and 6, was then achieved from the advance intermediate 1. In this synthesis, the protection of the basic nitrogen with a Boc group is mandatory to obtain the pyrazole cycle, since all attempts to modulate the final donecopride analog in a late stage process failed. For this reason, we also explored in parallel the impact of the protection of the aniline moiety with an acetamide group, with no clear benefit in terms of synthesis of compound 6. The preparation of the Boc protected 2 was, however, of interest since it allowed us to introduce in a convergent way both the cyclohexyl and the m-tolyl moieties. Indeed if the cyclohexyl present in donecopride yielded low nanomolar affinities for both AChE and 5-HT4R, [6] its replacement by a m-tolyl generated triple activity this time towards 5-HT6R [18]. If MR33372 possesses affinities in the hundred nM range for the three targets, this compound demonstrated an in vivo anti-amnesic effect in a model of scopolamine induced deficit.
Compared with donecopride, the introduction of the acetamide group on 7 had little impact on AChE inhibition (IC50 = 16 vs. 34 nM respectively), but greatly affected the affinity for 5-HT4R (Table 1). The introduction of the methylpyrazole ring on 3a led to a complete loss of activities for all targets. Interestingly, however, the introduction of the m-tolyl moiety is again of interest for 3b. Compared to MR33372 this time, 3b is a slightly better AChEI (IC50 = 161 vs. 111 nM respectively) and possesses a decreased affinity for 5-HT6R. Contrary, the conformational restriction greatly affects the ability of the compound to bind to 5-HT4R. Due to its synthetic accessibility from intermediate 8, a second series of ether substituted analog 12b–d was generated bearing the m-tolyl substituent previously identified. Unfortunately, this modulation led to a complete loss of activities for the three targets.
2.3. In Silico Results
In order to better understand the impact of these SAR on AChE binding, a docking study was performed with 3b in comparison with donecopride (Figure 2). The docking study was carried out with the aim to predict the AChE inhibition of synthesized compounds compared to donecopride, the initial scaffold of the carried out modulations. The docking study into the hAChE active site (PDB code: 4EY7) [25] was performed using Gold 5.7.2 software and the ligand 3D models were built from donecopride X-ray structure solved previously. Firstly, the correctness of employed docking procedure was checked by redocking the co-crystallized ligand, donepezil. Gold program regained the crystallographic donepezil position with a RMSD smaller to 1 Å and with a score fit value of 110.02 [6]. Next, the docking procedure was applied to donecopride and to the newly synthesized compounds (3b, 7, and 12b). The docking generated positions closed to donepezil one for donecopride (score fit ~105.14, Figure 2) and for compound 7 (score fit ~ 117.63, Figure 2) and 12b (score fit~86.79, Figure 2). Indeed, donecopride and compound 7 under the docking results reproduces well the donepezil key binding interactions: (i) the charged nitrogen of the piperidine ring is oriented in a position suitable for an Hbond with the water molecule in the proximity of Tyr337 and Tyr341, (ii) the carbonyl group of both ligands forms an Hbond with NH of the Phe295 backbone, (iii) the benzene ring is positioned in a parallel way to the Trp286 indole ring to favor the π-stacking interaction and (iv) the cyclohexane ring occupied the donepezil benzyl ring place in neighboring of Trp86. Even if compound 12b was positioned systematically with the substituted phenyl ring towards the binding cavity’s bottom, its position was higher comparing to previous three compounds. Consequently, 12b lost, more to the loss of Hbond due to carbonyl group absence in its scaffold, also the interaction through the piperidine’s protonated nitrogen as well as π-stacking with Trp86 (Figure 2).
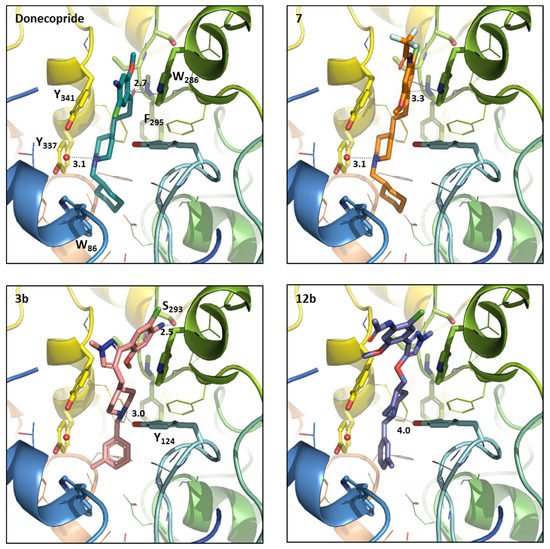
Figure 2.
Donecopride (green) position compared to the compound 7 (orange), 3b (pink), and 12b (purple) positioned in hAChE binding sites using the docking studies. The compound and the selected side chains of the binding site residues are in stick and the protein in ribbon representation. This figure was made with PYMOL (DeLano Scientific, South San Francisco, CA, USA, 2002, San Carlo, Redwood City, CA, USA) and the protein is presented in the same orientation in all four images.
However, the first docking does not produce conclusive results for compound 3b. Compound 3b was systematically placed with aniline moiety at the bottom of the binding site cavity. Therefore, a second docking with a greater number of docking cycles was tested for compound 3b. This docking generated even some more logical poses of compound 3b with aniline moiety placed at the binding cavity entrance. The generated solution with good orientation of aniline moiety corresponded to the score fit 91.78 is present in Figure 2.
The compound 3b docked in the hAChE active site higher compared to the donecopride in the same way as compound 12b. Like 12b, 3b compound lost the interaction through the piperidine’s protonated nitrogen, π-stacking with Trp86, and the Hbond interaction due to carbonyl group absence in its scaffold (Figure 2). However, on the other hand with compound 12b, 3b establishes new interactions through hydrogen bonds with the hydroxy group of Tyr124 and Ser293.
Based on this study we could state that the introduction of the methylpyrazole ring led to the loss of one of the crucial hydrogen bonds with the backbone NH of Phe295. In this series, the classical cation-π interaction with Tyr337 is not enough to maintain the affinity of the cyclohexyl 3a. The replacement of the cyclohexyl by the aromatic m-tolyl led to clear impact on AChE inhibition surely by increasing the stacking of the aromatic ring of 3b with Phe86 (Figure 2). Both interactions as well as hydrophobic interactions in the aromatic gorge could then justify the interesting properties of the novel phenyl pyrazole 3b.
In order to better understand their worst profile on serotonergic receptors, docking studies were performed in a homology model of the 5-HT4 and 5-HT6 receptors. The docking results on 5-HT4R for compounds 7, 3b, and 12b were compared to donecopride one [6]. From donecopride docking, the selected pose was that where donecopride is placed horizontally in the cavity interacting on one side through an H-bond with Asp100 on the transmembrane helix 3 (TM3, consistent with the constraint used during docking) and on other side through another H-bond with Ser197 on TM5 (in yellow in Figure 3). The donecopride’s group assuring this second H-bond is NH2 substituent on the benzene ring. Therefore, the addition of the substituent on NH2 group as well as the lengthening of the connector between the benzene cycle and the piperidine increase the compounds’ length and the compounds can no longer be placed horizontally as donecopride. They take a curve position (Figure 3) and lost the interaction with TM5 in binding site. For compound 7, the position of its carbonyl group allowed it to maintain some electrostatic interactions with Ser197 and Asn279, which is impossible for compound 12b and 3b through the replacement of this carbonyl group by the methylpyrazole ring. Hence, the hypothesis of their loss of affinity.
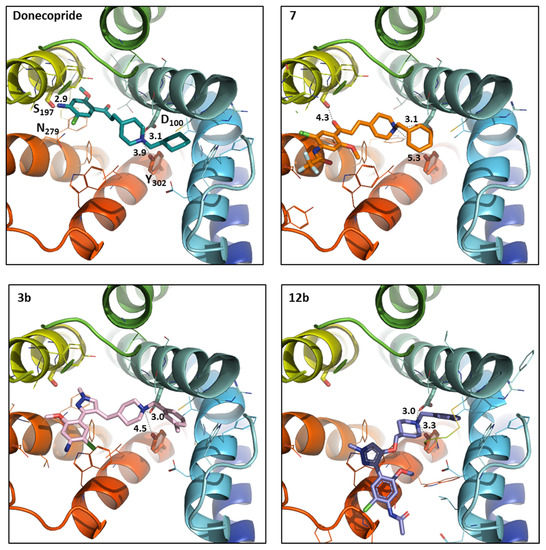
Figure 3.
Donecopride (green) position compared to the compound 7 (orange), 3b (pink), and 12b (purple) positioned in h5-HT4R binding sites using the docking studies. The compound and the selected side chains of the binding site residues are in stick and the protein in ribbon representation. This figure was made with PYMOL (DeLano Scientific, 2002, San Carlo, CA, USA) and the protein is presented in the same orientation in all four images.
The comparison of the binding site of 5-HT6R and 5-HT4R showed that the various amino acids change and so modify the binding site topology. For example, in the 5-HT6R, two tryptophan residues are in proximity of TM3 helix, Trp102 on TM3, and Trp92 in the ECL1 loop (Figure 4), and these two residues with a bulky lateral chains obstruct the space between the helices TM2 and TM3, which leads to the ligands no longer accessing this pocket, unlike the 5-HT4R. The binding cavity in 5-HT6R is smaller than that of 5-HT4R and therefore the ligands must take curved conformations to be able to access Asp106. A supplementary interaction through π stacking with Trp102 was only observed for ligand 3b (Figure 4), which could explain the micromolar binding affinity of this ligand to 5-HT6R.
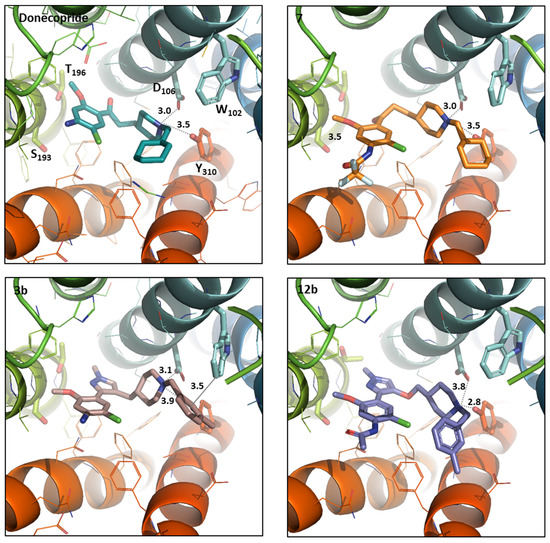
Figure 4.
Donecopride (green) position compared to the compound 7 (orange), 3b (pink) and 12b (purple) positioned in h-5HT6R binding sites using the docking studies. The compound and the selected side chains of the binding site residues are in stick and the protein in ribbon representation. This figure was made with PYMOL (DeLano Scientific, 2002, San Carlo, CA, USA) and the protein is presented in the same orientation in all four images.
3. Experimental
3.1. Chemistry
3.1.1. General Methods
All chemical reagents and solvents were purchased from commercial sources and used without further purification. Melting points were determined on a STUART SMP50 melting point apparatus (Cole-Parmer, Vernon Hills, IL, USA). 1H, 13C, and 19F NMR spectra were recorded on a BRUKER AVANCE III 400 MHz (Bruker, Billerica, MA, USA) apparatus with chemical shifts expressed in parts per million downfield from TMS as an internal standard and coupling in Hertz. IR spectra were recorded on a Perkin-Elmer BX FT-IR apparatus (Perkin Elmer, Wellesley, MA, USA) using KBr pellets. High-resolution mass spectra (HRMS) were obtained by electrospray on a BrukermaXis (Bruker, Billerica, MA, USA). The purities of all tested compounds were analyzed by LC−MS, with the purity all being higher than 95%. Analyses were performed using a Waters Alliance 2695 (Waters corporation, Milford, MA, USA) as separating module (column XBridge C18 2.5 μM/4.6 × 50 mM) using the following gradients: A (95%)/B (5%) to A (5%)/B (95%) in 4.00 min. This ratio was hold during 1.50 min before return to initial conditions in 0.50 min. Initial conditions were then maintained for 2.00 min (A = H2O, B = CH3CN; each containing HCOOH: 0.1%). MS were obtained on a SQ detector by positive ESI.
3.1.2. Synthesis of Compounds (2–7, 9–12)
- tert-butyl 4-((3-(4-amino-5-chloro-2-methoxyphenyl)-1-methyl-1H-pyrazol-4-yl)methyl)piperidine-1-carboxylate2. An amount of 375 mg of compound 1 (0.947 mmol) were refluxed in 5 mL of DMF.DMA overnight. After evaporation under reduced pressure, the residue was dissolved in 10 mL of EtOH and 26 mL of methylhydrazine (3.788 mmol, 4 eq) were added. The mixture was refluxed for 4 h. After concentration, the new residue was dissolved in 20 mL of EtOH and 645 mg of ZnCl2 (4.735 mmol, 5 eq) were added. The new reactional mixture was refluxed for 2 h and then concentrated. The residue was dissolved in DCM and washed 3 times with water. The organic phase was dried on MgSO4, concentrated and purified on neutral Al2O3 to afford 155 mg of product 2 as a brown solid. Yield = 38%; m.p. = 67–69 °C; 1H-NMR (CDCl3, 400 MHz): 7.17 (s, 1H), 7.15 (s, 1H), 6.34 (s, 1H), 4.10 (br s, 2H), 3.99 (br s, 2H), 3.87 (s, 3H), 3.71 (s, 3H), 2.57 (t, J = 13.0 Hz, 2H), 2.29 (d, J = 7.0 Hz, 2H), 1.54 (d, J = 13.1 Hz, 2H), 1.45−1.38 (m, 10H), 0.97 (qd, J = 12.2 Hz, J’ = 4.2 Hz, 2H); 13C-NMR (CDCl3, 100 MHz): 156.8, 154.9, 147.4, 143.5, 131.7, 129.3, 118.7, 114.4, 110.7, 98.7, 79.2, 55.6, 44.0 (2C), 38.9, 37.0, 32.0 (2C), 31.1, 28.5 (3C); MS [M + H]+ = 434.86; IR (KBr, cm−1): 3468, 3351, 2931, 1686, 1624, 1461, 1444, 1426, 1365, 1247, 1211, 1173, 1211.
General Procedure for Acetylation
The aniline was stirred in anhydric acetic (6 mL·mmol−1) at room temperature overnight. The mixture was concentrated under reduced pressure. The residue i was then dissolved in EtOAc and washed twice with aq. NaHCO3 saturated and once with water. The organic phase was dried, filtrated, and evaporated in vacuo. The crude product is purified on silica gel.
- tert-butyl 4-(3-(4-acetamido-5-chloro-2-methoxyphenyl)-3-oxopropyl)piperidine-1-carboxylate4. From 150 mg of compound 1 (0.379 mmol), 129 mg of compound 4 were obtained as a white solid (gradient of elution: DCM to DCM/EtOAc 9/1). Yield = 78%; m.p. = 123–125 °C; 1H-NMR (CDCl3, 400 MHz): 8.26 (s, 1H), 7.80 (br s, 1H), 7.77 (s, 1H), 4.07 (br s, 2H), 3.90 (s, 3H), 2.94 (m, 2H), 2.65 (m, 2H), 2.26 (s, 3H), 1.66–1.56 (m, 4H), 1.43 (s, 9H), 1.42 (m, 1H), 1.08 (m, 2H); 13C-NMR (CDCl3, 100 MHz): 199.6, 168.7, 158.5, 154.9, 138.8, 130.6, 123.4, 113.7, 104.0, 79.2, 56.0, 44.0 (2C), 40.9, 35.7, 32.1 (2C), 30.9, 28.5 (3C), 25.2; MS [M + H]+ = 438.84; IR (KBr, cm−1): 3323, 2975, 2930, 2853, 1690, 1674, 1599, 1578, 1513, 1451, 1402, 1242, 1163, 1017. HRMS [M + H + Na]+calcd for C22H31ClN2NaO5, 461.1819, exp 461.1813.
- tert-butyl 4-((3-(4-acetamido-5-chloro-2-methoxyphenyl)-1-methyl-1H-pyrazol-4-yl)methyl)piperidine-1-carboxylate5. From 38 mg of compound 2 (0.088 mmol), 35 mg of compound 5 were obtained as a white solid (gradient of elution: DCM to DCM/EtOAc 6/4). Yield = 83%; m.p. = 147–149 °C; 1H-NMR (CDCl3, 400 MHz): 8.20 (s, 1H), 7.71 (s, 1H), 7.32 (s, 1H), 7.17 (s, 1H), 3.98 (br s, 2H), 3.88 (s, 3H), 3.81 (s, 3H), 2.56 (t, J = 12.8 Hz, 2H), 2.31 (d, J = 7.0 Hz, 2H), 2.26 (s, 3H), 1.53 (d, J = 13.2 Hz, 2H), 1.45−1.38 (m, 10H), 0.96 (qd, J = 12.2 Hz, J’ = 4.3 Hz, 2H); 13C-NMR (CDCl3, 100 MHz): 168.4, 155.7, 154.8, 154.4, 144.7, 134.4, 127.4, 119.3, 113.8, 104.4, 86.0, 79.5, 75.7, 55.9, 43.5 (2C), 36.1, 33.7, 28.6 (2C), 28.5 (3C), 25.1; MS [M + H]+ = 476.82; IR (KBr, cm−1): 3420, 2930, 1688, 1584, 1527, 1450, 1388, 1244, 1161.
- 5-(4-amino-5-chloro-2-methoxyphenyl)-2-methyl-2,4-dihydro-3H-pyrazol-3-one9a. A total of 300 mg of compound 8 (1.107 mmol), 54 µL of methylhydrazine (0.996 mmol, 0.9 eq), and 3 mL of EtOH were stirred under microwaves irradiation at 160 °C for 45 min. The solvent was eliminated under reduced pressure and the residue was purified on silica gel (gradient of elution: DCM to EtOAc) to afford 143 mg of compound 9a as a white solid. Yield = 57%; m.p. = 203–205°C; 13C-NMR (CDCl3, 400 MHz): 7.81 (s, 1H), 6.27 (s, 1H), 4.31 (br s, 2H), 3.78 (s, 3H), 3.68 (s, 2H), 3.36 (s, 3H); 13C-NMR (CDCl3, 100 MHz): 172.5, 157.5, 152.4, 145.6, 128.0, 111.7, 111.4, 98.0, 55.6, 42.1, 31.3; MS [M + H]+ = 253.92; IR (KBr, cm−1): 3448, 3331, 2958, 2229, 1677, 1631, 1603, 1572, 1458, 1348, 1214, 1049; HRMS [M + H]+calcd for C11H13ClN3O2, 254.0696, exp 254.0693.
- 3-(4-acetamido-5-chloro-2-methoxyphenyl)-1-methyl-1H-pyrazol-5-yl acetate11. A total of 200 mg of compound 9a (0.80 mmol) were stirred in 2 mL of anhydric acetic at room temperature overnight. Water was added to obtain a white suspension. This latter was filtrated and washed with water. A total of 250 mg of product 11 were then obtained as a white solid. Yield = 94%. m.p. = 191–193°C; 1H-NMR (CDCl3, 400 MHz): 8.21 (s, 1H), 7.92 (s, 1H), 7.68 (s, 1H), 6.61 (s, 1H), 3.89 (s, 3H), 3.73 (s, 3H), 2.35 (s, 3H), 2.25 (s, 3H); 13C-NMR (CDCl3, 100 MHz): 168.4, 166.1, 155.8, 145.1, 144.8, 134.6, 127.3, 118.8, 113.6, 104.3, 95.5, 55.9, 34.7, 25.1, 20.7; MS [M + H]+ = 337.92; IR (KBr, cm−1): 3422, 2945, 1787, 1772, 1699, 1585, 1511, 1483, 1450, 1352, 1243, 1191, 1011. HRMS [M + H]+ calcd for C15H17ClN3O4, 338.0908, exp 338.0914.
- N-(2-chloro-5-methoxy-4-(1-methyl-5-oxo-4,5-dihydro-1H-pyrazol-3-yl)phenyl)acetamide9b. A total of 250 mg of compound 11 (0.742 mmol) and 21 mg of LiOH (0.890 mmol, 1.2 eq) were dissolved in 14 mL of EtOH/water (5/2). The reactional mixture was stirred at room temperature for 1 h 45 min. After evaporation in vacuo, water was added, adjusted at pH 4–5 and extracted 3 times with CHCl3. The organic phases were combined, washed once with water, dried on MgSO4, and concentrated to obtain 176 mg of monoprotected compound 9b and used without further purification. Yield = 76%; m.p. = 219 -221 °C; 1H-NMR (CDCl3, 400 MHz): 8.26 (s, 1H), 7.94 (s, 1H), 7.73 (br s, 1H), 3.88 (s, 3H), 3.74 (s, 2H), 3.39 (s, 3H), 2.27 (s, 3H); 13C-NMR (CDCl3, 100 MHz) 172.6, 168.6, 156.8, 151.6, 136.9, 126.9, 116.2, 114.0, 104.1, 56.0, 42.0, 31.3, 25.2; MS [M + H]+ = 295.85; IR (KBr, cm−1): 3316, 2340, 1681, 1606, 1586, 1547, 1506, 1450, 1392, 1209, 1015. HRMS [M + H]+ calcd for C13H15ClN3O3, 296.0802, exp 296.0803.
General Procedure for the O-Alkylation
The pyrazolone was dissolved in DMF (3 mL·mmol−1) and K2CO3 (2 eq) and N-Boc-4-iodomethylpiperidine (1.2 eq) were added. The mixture was stirred for 3 h at 60 °C. After cooling, the brown solution was diluted in water and extracted 3 times with EtOAc. The organic phases were combined, washed 5 times with water, dried on MgSO4, and concentrated under reduced pressure. The crude product was then purified on silica gel (eluant DCM to DCM/EtOAc 8/2).
- Tert-butyl 4-(((3-(4-acetamido-5-chloro-2-methoxyphenyl)-1-methyl-1H-pyrazol-5-yl)oxy)methyl)piperidine-1-carboxylate10b. From 167 mg of compound 9b (0.379 mmol), 60 mg of compound 10b were obtained as a white solid. Yield = 22%; m.p. = 182–184 °C; 1H-NMR (CDCl3, 400 MHz): 8.19 (s, 1H), 7.89 (s, 1H), 7.69 (br s, 1H), 6.00 (s, 1H), 4.15 (br s, 2H), 3.92 (d, J = 6.3 Hz, 2H), 3.89 (s, 3H), 3.66 (s, 3H), 2.73 (t, J = 13.0 Hz, 2H), 2.23 (s, 3H), 1.98 (m, 1H), 1.78 (d, J = 12.6 Hz, 2H), 1.45 (s, 9H), 1.27 (qd, J = 12.1 Hz, J’ = 4.2 Hz, 2H); 13C-NMR (CDCl3, 100 MHz): 168.4, 155.7, 154.8, 154.4, 144.7, 134.4, 127.4, 119.3, 113.8, 104.4, 86.0, 79.5, 75.7, 55.9, 43.5 (2C), 36.1, 33.7, 28.6 (2C), 28.5 (3C), 25.1; [M + H]+ = 492.76; IR (KBr, cm−1): 3420, 2936, 2853, 1690, 1585, 1557, 1528, 1450, 1436, 1365, 1247, 1173, 1147, 1018.
- Tert-butyl 4-(((1-methyl-3-phenyl-1H-pyrazol-5yl)oxy)methyl)piperidine-1-carboxylate10c. From 218 mg of compound 9c (0.647 mmol), 129 mg of compound 10c were obtained as a yellow solid. Yield = 27%; m.p. = 133–135 °C; 1H-NMR (CDCl3, 400 MHz): 7.73 (m, 2H), 7.37 (m, 2H), 7.27 (m, 1H), 5.80 (s, 1H), 4.16 (br s, 2H), 3.93 (d, J = 6.4 Hz, 2H), 3.68 (s, 3H), 2.75 (t, J = 12.8 Hz, 2H), 2.00 (m, 1H), 1.80 (d, J = 13.0 Hz, 2H), 1.47 (s, 9H), 1.29 (qd, J = 11.8 Hz, J’ = 4.6 Hz, 2H); 13C-NMR (CDCl3, 400 MHz): 155.1, 154.8, 149.3, 133.8, 128.5 (2C), 127.6, 125.2 (2C), 82.0, 79.6, 75.9, 43.5 (2C), 36.0, 33.8, 28.6 (2C), 28.5 (3C); MS [M + H]+ = 372.01; IR (KBr, cm−1): 3426, 2925, 2853, 1742, 1690, 1626, 1558, 1512, 1454, 1422, 1367, 1275.
- Tert-butyl 4-(((3-(3,4-dimethoxyphenyl)-1-methyl-1H-pyrazol-5-yl)oxy)methyl)piperidine-1-carboxylate10d. From 151 mg of compound 9d (0.379 mmol), 80 mg of compound 10d were obtained as a yellow oil. Yield = 21%; 1H-NMR (CDCl3, 400 MHz): 7.33 (d, J = 2.0 Hz, 1H), 7.22 (d, J = 8.2 Hz, J’ = 2.0 Hz, 1H), 6.86 (d, J = 8.3 Hz, 1H), 5.74 (s, 1H), 4.18 (br s, 2H), 3.95 (s, 3H), 3.93 (d, J = 6.4 Hz, 2H), 3.90 (s, 3H), 3.67 (s, 3H), 2.75 (t, J = 13.0 Hz, 2H), 2.00 (m, 1H), 1.79 (d, J = 10.3 Hz, 2H), 1.47 (s, 9H), 1.29 (qd, J = 11.7 Hz, J = 3.9 Hz, 2H); MS [M + H]+ = 431.91;
General Procedure for the N-Alkylation
The protected compound (0.167 mmol) was dissolved in DCM (15 mL·mmol−1) and TFA (2 mL·mmol−1) was added. The solution was stirred for 15 min. After concentration, the residue was dissolved in acetone (for the benzylation) or DMF (for the alkylation) and K2CO3 (10 eq) and 3-methylbenzyl bromide (1.2 eq) or (bromomethyl)cyclohexane (1.3 eq) were added. The mixture was stirred at room temperature (for the benzylation) or warmed at 110 °C (for the alkylation) for 3 h and then concentrated under reduced pressure. The residue was dissolved in a mixture of NaCl sat/EtOAc. The organic phase was dried and evaporated. The crude was then purified on neutral Al2O3 (gradient of elution: DCM to DCM/EtOAc 8/2).
- 2-chloro-4-(4-((1-(cyclohexylmethyl)piperidin-4-yl)methyl)-1-methyl-1H-pyrazol-3-yl)-5-methoxyaniline3a. From 59 mg of compound 2 (0.136 mmol), 18 mg of compound 3a were obtained as a pale brown gum. Yield = 31%; 1H-NMR (CDCl3, 400 MHz): 7.17 (s, 1H), 7.15 (s, 1H), 6.34 (s, 1H), 4.09 (br s, 2H), 3.88 (br s, 2H), 3.72 (s, 3H), 2.83 (m, 2H), 2.29 (d, J = 6.6 Hz, 2H), 2.10 (m, 2H), 1.78−1.10 (m, 17 H), 0.89−0.80 (m, 2H); 13C-NMR (CDCl3, 100 MHz): 156.8, 147.4, 143.4, 131.8, 129.4, 119.1, 114.6, 110.7, 98.7, 66.0, 55.6, 54.4 (2C), 38.9, 36.9, 35.1, 32.0 (2C), 31.9 (2C), 31.1, 26.7, 26.1 (2C); MS [M + H]+ = 431.02; IR (KBr, cm−1): 3438, 2921, 2849, 1622, 1525, 1461, 1445, 1402, 1211. HRMS [M + H]+ calcd for C24H36ClN4O, 431.2578, exp 431.2576.
- 2-chloro-5-methoxy-4-(1-methyl-4-((1-(3-methylbenzyl)piperidin-4-yl)methyl)-1H-pyrazol-3-yl)aniline 3b. From 41 mg of compound 2 (0.095 mmol), 19 mg of compound 3b were obtained as a pale yellow gum. Yield = 46%; 1H-NMR (CDCl3, 400 MHz): 7.18 (t, J = 7.6 Hz, 1H), 7.17 (s, 1H), 7.14 (s, 1H), 7.10 (s, 1H), 7.05 (m, 2H), 6.33 (s, 1H), 4.09 (br s, 2H), 3.86 (s, 3H), 3.71 (s, 3H), 3.40 (s, 2H), 2.81 (m, 2H), 2.33 (s, 3H), 2.28 (d, J = 6.8 Hz, 2H), 1.82 (td, J = 11.8 Hz, J’ = 2.4 Hz, 2H), 1.55 (d, J = 12.7 Hz, 2H), 1.28 (m, 1H), 1.15 (qd, J = 12.3 Hz, J’ = 3.6 Hz, 2H). 13C-NMR (CDCl3, 100 MHz): 156.8, 147.4, 143.4, 138.4, 137.7, 131.8, 130.0, 129.3, 128.0, 127.6, 126.4, 119.2, 114.6, 110.7, 98.7, 63.6, 55.6, 53.9 (2C), 38.9, 36.9, 32.2 (2C), 31.1, 21.4; MS [M + H]+ = 439.74; IR (KBr, cm−1): 3434, 2925, 1623, 1525, 1460, 1445, 1402, 1211. HRMS [M + H]+ calcd for C25H32ClN4O, 439.2265, exp 439.2268.
- N-(2-chloro-4-(4-((1-(cyclohexylmethyl)piperidin-4-yl)methyl)-1-methyl-1H-pyrazol-3-yl)-5-methoxyphenyl)acetamide 6. From 60 mg of compound 5 (0.126 mmol), 32 mg of compound 6 were obtained as a beige solid. Yield = 46%; m.p. = 148–150 °C; 1H-NMR (CDCl3, 400 MHz): 8.20 (s, 1H), 7.70 (br s, 1H), 7.31 (s, 1H), 7.16 (s, 1H), 3.87 (s, 3H), 3.80 (s, 3H), 2.77 (d, J = 11.8 Hz, 2H), 2.29 (d, J = 6.8 Hz, 2H), 2.26 (s, 3H), 2.03 (d, J = 7.0 Hz, 2H), 1.74−1.61 (m, 6H), 1.52 (d, J = 12.6 Hz, 2H), 1.43 (m, 1H), 1.28−1.08 (m, 7H), 0.87−0.77 (m, 2H); 13C-NMR (CDCl3, 100 MHz): 168.5, 156.4, 146.8, 135.1, 130.9, 129.6, 119.8, 119.4, 112.9, 103.8, 66.2, 55.8, 54.5 (2C), 38.9, 37.0, 35.3, 32.3 (2C), 32.1 (2C), 31.2, 26.8, 26.2 (2C), 25.2; MS [M + H]+ = 472.92; IR (KBr, cm−1): 3420, 2921, 2849, 1694, 1625, 1584, 1527, 1449, 1388, 1243; HRMS [M + H]+calcd for C26H34N3O3, 473.2683, exp 473.2682.
- N-(2-chloro-4-(3-(1-(cyclohexylmethyl)piperidin-4-yl)propanoyl)-5-methoxyphenyl)acetamide 7. From 81 mg of compound 4 (0.185 mmol), 34 mg of compound 7 were obtained as a beige gum. Yield = 42%; 1H-NMR (CDCl3, 400 MHz): 8.26 (s, 1H), 7.77 (s, 1H), 7.75 (br s, 1H), 3.90 (s, 3H), 2.93 (t, J = 7.6 Hz, 2H), 2.83 (d, J = 11.0 Hz, 2H), 2.25 (s, 3H), 2.05 (d, J = 7.1 Hz, 2H), 1.79–1.45 (m, 10H), 1.23–1.05 (m, 8H), 0.83 (m, 2H); 13C-NMR (CDCl3, 100 MHz): 200.1, 168.6, 158.4, 138.6, 130.6, 123.5, 113.5, 103.9, 66.2, 55.9, 53.3 (2C), 41.2, 35.7, 35.2, 32.2 (2C), 32.1 (2C), 31.0, 26.8, 26.2 (2C), 25.2; MS [M + H]+ = 434.82; IR (KBr, cm−1): 3345, 2921, 2849, 1704, 1672, 1599, 1578, 1512, 1450, 1401, 1263, 1241, 1176, 1014; HRMS [M + H]+calcd for C24H36ClN2O3, 435.2414, exp 435.2415.
- N-(2-chloro-5-methoxy-4-(1-methyl-5-((1-(3-methylbenzyl)piperidin-4-yl)methoxy)-1H-pyrazol-3-yl)phenyl)acetamide 12b. From 70 mg of compound 10b (0.126 mmol), 45 mg of compound 12b were obtained as a beige solid. Yield = 64%; m.p. = 174–176 °C; 1H-NMR (CDCl3, 400 MHz): 8.22 (s, 1H), 7.90 (s, 1H), 7.67 (br s, 1H), 7.21 (t, J = 7.4 Hz, 1H), 7.14−7.06 (m, 3H), 6.00 (s, 1H), 3.93 (d, J = 6.3 Hz, 2H), 3.91 (s, 3H), 3.67 (s, 3H), 3.48 (s, 2H), 2.94 (d, J = 11.2 Hz, 2H), 2.35 (s, 3H), 2.25 (s, 3H), 2.00 (td, J = 11.9 Hz, J’ = 2.5 Hz, 2H), 1.85−1.77 (m 3H), 1.44 (qd, J = 11.9 Hz, J’ = 3.7 Hz, 2H); 13C-NMR (CDCl3, 100 MHz): 168.4, 155.7, 154.7, 144.7, 138.2, 137.8, 134.4, 130.0, 128.1, 127.8, 127.4, 126.3, 119.4, 113.7, 104.3, 86.0, 76.2, 63.5, 56.0, 53.3 (2C), 35.8, 33.7, 28.9 (2C), 25.1, 21.4; MS [M + H]+ = 496.82; IR (KBr, cm−1): 3415, 2937, 1694, 1680, 1585, 1557, 1527, 1449, 1361, 1243; HRMS [M + H]+calcd for C27H34ClN4O3, 497.2319, exp 497.2319.
- 4-(((1-methyl-3-phenyl-1H-pyrazol-5-yl)oxy)methyl)-1-(3-methylbenzyl)piperidine 12c. From 62 mg of compound 10c (0.126 mmol), 20 mg of compound 12c were obtained as an oil. Yield = 32%; 1H-NMR (CDCl3, 400 MHz): 7.73 (m, 2H), 7.37 (m, 2H), 7.27 (m, 1H), 7.22 (t, J = 7.5 Hz, 1H), 7.15 (s, 1H), 7.13 (d, J = 7.5 Hz, 1H), 7.08 (d, J = 7.5 Hz, 1H), 5.80 (s, 1H), 3.94 (d, J = 6.4 Hz, 2H), 3.68 (s, 3H), 3.52 (s, 2H), 2.98 (d, J = 10.7 Hz, 2H), 2.35 (s, 3H), 2.04 (t, J = 1.6 HZ, 2H), 1.86 (m, 1H), 1.80 (d, J = 13.5 Hz, 2H), 1.48 (m, 2H); 13C-NMR (CDCl3, 100 MHz): 155.3, 149.3, 137.9, 137.6, 133.9, 130.1, 128.5 (2C), 128.1, 127.9, 127.5, 126.5, 125.2 (2C), 82.0, 76.2, 63.4, 53.2 (2C), 35.7, 33.8, 28.7 (2C), 21.4; MS [M + H]+ = 376.00; HRMS [M + H]+calcd for C24H30N3O, 376.2389, exp 376.2392.
- 4-(((3-(3,4-dimethoxyphenyl)-1_methyl-1H-pyrazol-5-yl)oxy)methyl)-1-(3-methylbenzyl)piperidine12d. From 70 mg of compound 10d (0.126 mmol), 35 mg of compound 12d were obtained as a pale brown oil. Yield = 67%; 1H-NMR (CDCl3, 400 MHz): 7.33 (d, J = 2.0 Hz, 1H), 7.24−7.19 (m, 2H), 7.14−7.06 (m, 3H), 6.87 (d, J = 8.4 Hz, 1H), 5.73 (s, 1H), 3.95 (s, 3H), 3.93 (d, J = 6.4 Hz, 2H), 3.90 (s, 3H), 3.67 (s, 3H), 3.49 (s, 2H), 2.95 (d, J = 11.7 Hz, 2H), 2.35 (s, 3H), 2.00 (td, J = 11.7 Hz, J’ = 2.4 Hz, 2H), 1.85 (m, 1H), 1.81 (d, J = 13.0 Hz, 2H), 1.44 (qd, J = 12.3 Hz, J = 3.7 Hz, 2H); 13C-NMR (CDCl3, 100 MHz): 155.3, 149.2, 149.0, 148.7, 138.1, 137.8, 130.0, 128.1, 127.8, 127.1, 126.4, 117.8, 111.0, 108.1, 81.7, 76.3, 63.5, 55.9, 55.9, 53.2 (2C), 35.8, 33.7, 28.8 (2C), 21.4; MS [M + H]+ = 435.94; IR (KBr, cm−1): 2935, 1609, 1589, 1558, 1525, 1466, 1435, 1258, 1234, 1029; HRMS [M + H]+calcd for C26H34N3O3, 436.2600, exp 436.2596.
3.2. Biological Evaluation
3.2.1. In Vitro Tests of AChE Biological Activity
Inhibitory capacity of compounds on AChE biological activity was evaluated through the use of the spectrometric method of Ellman [24]. Acetylthiocholine iodide and 5,5-dithiobis-(2-nitrobenzoic) acid (DTNB) were purchased from Sigma Aldrich. AChE from human erythrocytes (buffered aqueous solution, ≥500 units/mg protein (BCA), Sigma Aldrich) was diluted in 20 mM HEPES buffer pH 8, 0.1% Triton X-100 such as to have enzyme solution with 0.25 unit/mL enzyme activity. In the procedure, 100 μL of 0.3 mM DTNB dissolved in phosphate buffer pH 7.4 were added into the 96 wells plate followed by 50 μL of test compound solution and 50 μL of enzyme (0.05 U final). After 5 min of preincubation at 25 °C, the reaction was then initiated by the injection of 50 μL of 1 mM acetylthiocholine iodide solution. The hydrolysis of acetylthiocholine was monitored by the formation of yellow 5-thio-2-nitrobenzoate anion as the result of the reaction of DTNB with thiocholine, released by the enzymatic hydrolysis of acetylthiocholine, at a wavelength of 412 nm using a 96-well microplate plate reader (Synergy 2, Biotek, Colmar, France). Test compounds were dissolved in analytical grade DMSO. Donepezil was used as a reference standard. The rate of absorbance increase at 412 nm was followed every minute for 10 min. Assays were performed with a blank containing all components except acetylthiocholine, in order to account for non-enzymatic reaction. The reaction slopes were compared and the percent inhibition due to the presence of test compounds was calculated by the following expression: 100 − (vi/v0 × 100) where vi is the rate calculated in the presence of inhibitor and v0 is the enzyme activity.
First screening of AChE activity was carried out at a 10−6 M concentration of compounds under study. For the compounds with significant inhibition (≥50%), IC50 values were determined graphically by plotting the % inhibition versus the logarithm of six inhibitor concentrations in the assay solution using the GraphPad Prism 6 software.
3.2.2. Pharmacological Characterization of Drugs on Human 5-HT4R
For competition studies, 2.5 µg of proteins (5-HT4(b) membrane preparations, HTS110M, Eurofins. Eurofins’ 5-HT4(b) membrane preparations are crude membrane preparations made from their proprietary stable recombinant cell lines to ensure high-level of GPCR surface expression.) were incubated in duplicate at 25 °C for 60 min in the absence or the presence of 10−6 or 10−8 M of each drug (RS67333 was used as a reference standard) and 0.5 nM [3H]-GR113808 (NET 1152, Perkin Elmer) in 25 mM Tris buffer (pH 7.4, 25 °C). At the end of the incubation, homogenates were filtered through Whatman GF/C filters (Alpha Biotech) presoaked with 0.5% polyethylenimine using a Brandel cell harvester. Filters were subsequently washed three times with 1 mL of ice-cold 25 mM Tris buffer (pH 7.4, 4 °C). Non-specific binding was evaluated in parallel in the presence of 30 µM serotonin.
3.2.3. Pharmacological Characterization of Drugs on Human 5-HT6R
Drugs were evaluated through their possibility to compete for the binding of [3H]-LSD on membranes of HEK-293 cells transiently expressing the human 5-HT6 receptors (ref. RBHS6M, Perkin Elmer). In brief, 4 µg of proteins were incubated at 37 °C for 60 min in duplicate in the absence or the presence of 10−6 or 10−8 M of each drug (serotonin was used as a reference standard) and 2.5 nM [3H]-LSD (ref. NET638250UC, Perkin Elmer), in 25 mM Tris-HCl buffer (pH 7.4) supplemented with 0.5 mM EDTA. At the end of the incubation, the homogenates were then filtered through Whatman GF/C filters and washed five times with ice-cold 25 mM Tris-HCl buffer. Non-specific binding was evaluated in the presence of 100 µM serotonin. Radioactivity associated to proteins was then quantified and expressed as the percentage of inhibition of the drugs under study.
For some of these compounds, affinity constants were calculated from five-point inhibition curves using the GraphPad Prism 6 software and expressed as Ki ± SD.
3.3. In Silico Study
The X-ray structure of donecopride was used as a 3D model during the docking and four conformers present in the crystal cell were used. The initial model of compounds 7, 3b, and 12b were built from donecopride X-ray structure [6]. The compound’s protonation state at pH 7.4 was predicted using standard tools of the ChemAxon Package (http://www.chemaxon.com/, accessed on 23 February 2021). The majority microspecie protonated on piperidine nitrogen at this pH was used for docking studies of each compounds.
The crystallographic coordinates of human acetylcholinesterase used in this study were obtained from X-ray structure of the donepezil/AChE complex (PDB ID 4EY7, a structure refined to 2.35 Å with an R factor of 17.7%) [25]. The AChE amino acid protonation state was checked before the docking study using the ProPKA software and the proposed protonation for Glu202 was applied.
The docking of the donepezil, donecopride, and compounds 3b, 7, and 12b into the AChE was carried out with the GOLD program (v2020.2.0) using the default parameters [26,27]. This program applies a genetic algorithm to explore conformational spaces and ligand binding modes. To evaluate the proposed ligand positions, the ChemPLP fitness function was used. The binding site in the AChE model was defined as a 7 Å sphere from the co-crystallized donepezil ligand and a water molecule interacting with protonated piperidine ring of donepezil was conserved during the docking (residue number 931). The second docking with the higher number of docking cycles (20 cycles) was carried out for compound 3b.
For the docking studies into 5-HT4 receptor, the 3D model of the human 5-HT4R built previously by homology sequence approach [28] was used. This model was generated using the crystal structure (PBD: 2RH1) of the human β2 adrenergic receptor-T4 lysozyme fusion protein complexed with the carazolol [29] as a template. The docking of the donecopride and 7, 3b, 12b compounds into the generated model was carried out with the GOLD program (v2020.2.0) using the default parameters [26,27] and the proposed ligand positions were evaluated by the ChemPLP fitness function. The binding site in the 5-HT4R model was defined as a 10 Å sphere centered on the aspartic acid residue Asp100. As the mutagenesis studies have shown that the interaction between the positively ionizable amine of ligands and Asp100 of 5-HT4R is crucial for ligand binding, a hydrogen bond constraint between positively ionizable amine ligand and OD atom of Asp100 was used during the docking [30] Furthermore, special attention was paid during the docking procedure to the following amino acids in the binding site, which were kept flexible: Arg96, Asp100, Thr104, Tyr192, Ser197, and Trp294.
For the docking studies on the 5-HT6 receptor first a homology model was built. The sequence of the human 5-HT6R was retrieved from the UniProt Knowledgebase (UniProtKB) [31] (ID: P50406_HUMAN). Using screening methods like FUGUE [32], SP3 [33], PSIBLAST, [34,35] HHSEARCH [36], and the @tome-2 server [37], the β2 adrenergic receptor was identified as the best 3D experimental template for the homology modeling also for the 5-HT6R (Sequence identity = 32%). The high-resolution (2.4 A) crystal structure of the human β2 adrenergic receptor (β2AR)-T4 lysozyme fusion protein bound to the carazolol (PDB: 2RH1) [29] was used as the 3D template. The alignment between the two sequences was manually optimized to avoid insertions and deletions in secondary structure elements. Disulfide bond: Cys99-Cys180 between the transmembrane helix 3 (TM3) and the extracellular loop (ECL2) was conserved. This alignment (Figure S2) was used as the basis for the homology modeling with the Modeller software [38]. The resulting model was then evaluated by methods like verify3D [39] and Eval23D [40].
The docking of the donecopride and 7, 3b, 12b compounds into the generated model was carried out with the GOLD program (v2020.2.0) [26,27] using the same procedure as for 5-HT4R. The binding site in the 5-HT6R model was defined as a 10 Å sphere centered on the cysteine residue Cys110. A hydrogen bond constraint between positively ionizable amine ligand and OD atom of Asp100 was also applied during the docking and the following amino acids were kept flexible: Trp102, Asp106, Ser193, Thr196, and Trp307.
4. Conclusions
In conclusion, we prepared two novel series of conformationally constrained donecopride analogs using a late stage functionalization process. The introduction of the novel pyrazole ring instead of the ketone linked reduces the ability of novel ligands to interact both with AChE and 5-HT4R. These results were confirmed thanks to our docking study. The replacement of the cyclohexyl moiety of donecopride with a m-tolyl group led, however, to a novel phenyl pyrazole 3b as a dual active compound toward AChE and 5-HT6R. According to our docking studies, the introduction of a substituted benzyl moiety on the piperidine ring enhanced the ability of 3b to interact with AChE and 5-HT6R active site thanks to stronger hydrophobic interactions. This preliminary result is of clear interest in the journey toward the identification of valuable pleiotropic compounds for the treatment of neurodegenerative diseases. Indeed, the simultaneous modulation of 5-HT6R and AChE could lead to an interesting synergistic effect in the treatment of AD, as demonstrated with the use of idalopirdine and donepezil in the clinical trials.
Based on these promising preliminary results, a future pharmacomodulation of 3b will be envisaged in order to increase its interaction with 5-HT6R and with AChE.
Supplementary Materials
The following data are available online. Including analytical spectrum of the compounds, superimposition of 7, 12b and 3b with donecopride in AChE binding site and amino-acid sequences alignment of 5-HT6R and human β2-adrenergic receptor. Reference [41] is cited in the Supplementary Materials.
Author Contributions
Conceptualization, C.R. and P.D.; methodology, C.L., R.L., A.D., and J.S.-d.O.S.; software, J.S.-d.O.S.; validation, C.R. and P.D.; writing—original draft preparation, C.R. and P.D.; writing—review and editing, C.L., J.S.-d.O.S., C.R. and P.D.; funding acquisition, C.R. and P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the Région Normandie (RIN GreenChem) and FEDER through the CERMN’s analytical platform.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article and Supplementary Materials.
Acknowledgments
The present work was performed thanks to the generous computing resources provided by the CRIANN (Normandy, France). The authors gratefully acknowledge the European Community (FEDER) for the molecular modeling software.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Cavalli, A.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V.; Recanatini, M.; Melchiorre, C. Multi-target-Directed Ligands To Combat Neurodegenerative Diseases. J. Med. Chem. 2008, 51, 347–372. [Google Scholar] [CrossRef]
- Albertini, C.; Salerno, A.; de Sena Murteira Pinheiro, P.; Bolognesi, M.L. From combinations to multitarget-directed ligands: A continuum in Alzheimer’s disease polypharmacology. Med. Res. Rev. 2020, 1–28. [Google Scholar] [CrossRef]
- Lalut, J.; Rochais, C.; Dallemagne, P. Multiple Ligands in Neurodegenerative Diseases. In Drug Selectivity–An Evolving Concept in Drug Discovery, Book Series “Methods and Principles in Medicinal Chemistry; Wiley-VCH Publishing House: Hoboken, NJ, USA, 2017; pp. 477–508. [Google Scholar]
- Rodríguez-Soacha, D.A.; Scheiner, M.; Decker, M. Multi-target-directed-ligands acting as enzyme inhibitors and receptor ligands. Eur. J. Med. Chem. 2019, 180, 690–706. [Google Scholar] [CrossRef]
- Lecoutey, C.; Hedou, D.; Freret, T.; Giannoni, P.; Gaven, F.; Since, M.; Bouet, V.; Ballandonne, C.; Corvaisier, S.; Fréon, A.M.; et al. Design of donecopride, a dual serotonin subtype 4 receptor agonist/acetylcholinesterase inhibitor with potential interest for Alzheimer’s disease treatment. Proc. Natl. Acad. Sci. USA 2014, 111, E3825–E3830. [Google Scholar] [CrossRef]
- Rochais, C.; Lecoutey, C.; Gaven, F.; Giannoni, P.; Hamidouche, K.; Hedou, D.; Dubost, E.; Genest, D.; Yahiaoui, S.; Freret, T.; et al. Novel Multitarget-Directed Ligands (MTDLs) with Acetylcholinesterase (AChE) Inhibitory and Serotonergic Subtype 4 Receptor (5-HT4R) Agonist Activities As Potential Agents against Alzheimer’s Disease: The Design of Donecopride. J. Med. Chem. 2015, 58, 3172–3187. [Google Scholar] [CrossRef] [PubMed]
- Rochais, C.; Lecoutey, C.; Hamidouche, K.; Giannoni, P.; Gaven, F.; Cem, E.; Mignani, S.; Baranger, K.; Freret, T.; Bockaert, J.; et al. Donecopride, a Swiss army knife with potential against Alzheimer’s disease. Br. J. Pharmacol. 2020, 177, 1988–2005. [Google Scholar] [CrossRef] [PubMed]
- Cachard-Chastel, M.; Lezoualc’H, F.; Dewachter, I.; Deloménie, C.; Croes, S.; Devijver, H.; Langlois, M.; Van Leuven, F.; Sicsic, S.; Gardier, A.M. 5-HT4 receptor agonists increase sAPPα levels in the cortex and hippocampus of male C57BL/6j mice. Br. J. Pharmacol. 2007, 150, 883–892. [Google Scholar] [CrossRef]
- Maillet, M.; Robert, S.J.; Cacquevel, M.; Gastineau, M.; Vivien, D.; Bertoglio, J.; Zugaza, J.L.; Fischmeister, R.; Lezoualc’H, F. Crosstalk between Rap1 and Rac regulates secretion of sAPPα. Nat. Cell Biol. 2003, 5, 633–639. [Google Scholar] [CrossRef]
- Egiannoni, P.; Egaven, F.; Bundel, D.E.; Ebaranger, K.; Emarchetti-Gauthier, E.; Roman, F.S.; Evaljent, E.; Emarin, P.; Ebockaert, J.; Erivera, S.; et al. Early administration of RS 67333, a specific 5-HT4 receptor agonist, prevents amyloidogenesis and behavioral deficits in the 5XFAD mouse model of Alzheimer’s disease. Front. Aging Neurosci. 2013, 5, 96. [Google Scholar] [CrossRef]
- Lalut, J.; Karila, D.; Dallemagne, P.; Rochais, C. Modulating 5-HT4and 5-HT6receptors in Alzheimer’s disease treatment. Futur. Med. Chem. 2017, 9, 781–795. [Google Scholar] [CrossRef]
- Barnes, N.M.; Ahern, G.P.; Becamel, C.; Bockaert, J.; Camilleri, M.; Chaumont-Dubel, S.; Claeysen, S.; Cunningham, K.A.; Fone, K.C.; Gershon, M.; et al. International Union of Basic and Clinical Pharmacology. CX. Classification of Receptors for 5-hydroxytryptamine; Pharmacology and Function. Pharmacol. Rev. 2021, 73, 310–520. [Google Scholar] [CrossRef]
- Karila, D.; Freret, T.; Bouet, V.; Boulouard, M.; Dallemagne, P.; Rochais, C. Therapeutic Potential of 5-HT6Receptor Agonists. J. Med. Chem. 2015, 58, 7901–7912. [Google Scholar] [CrossRef] [PubMed]
- Benhamú, B.; Martín-Fontecha, M.; Vázquez-Villa, H.; Pardo, L.; López-Rodríguez, M.L. Serotonin 5-HT6Receptor Antagonists for the Treatment of Cognitive Deficiency in Alzheimer’s Disease. J. Med. Chem. 2014, 57, 7160–7181. [Google Scholar] [CrossRef]
- Arnt, J.; Bang-Andersen, B.; Grayson, B.; Bymaster, F.P.; Cohen, M.P.; Giethlen, B.; Kreilgaard, M.; McKinzie, D.L.; Neill, J.C.; Nielsen, S.M.; et al. Lu AE58054, a 5-HT6 antagonist, reverses cognitive impairment induced by subchronic phencyclidine in a novel object recognition test in rats. Int. J. Neuropsychopharmacol. 2010, 13, 1021–1033. [Google Scholar] [CrossRef]
- Ferris, C.F.; Kulkarni, P.; Yee, J.R.; Nedelman, M.; De Jong, I.E.M. The Serotonin Receptor 6 Antagonist Idalopirdine and Acetylcholinesterase Inhibitor Donepezil Have Synergistic Effects on Brain Activity—A Functional MRI Study in the Awake Rat. Front. Pharmacol. 2017, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Yahiaoui, S.; Hamidouche, K.; Ballandonne, C.; Davis, A.; Sopkova-de Oliveira Santos, J.; Freret, T.; Boulouard, M.; Rochais, C.; Dallemagne, P. Design, synthesis, and pharmacological evaluation of multitarget-directed ligands with both serotonergic subtype 4 receptor (5-HT4R) partial agonist and 5-HT6R antagonist activities, as potential treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2016, 121, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Hatat, B.; Yahiaoui, S.; Lecoutey, C.; Davis, A.; Freret, T.; Boulouard, M.; Claeysen, S.; Rochais, C.; Dallemagne, P. A Novel in vivo Anti-amnesic Agent, Specially Designed to Express Both Acetylcholinesterase (AChE) Inhibitory, Serotonergic Subtype 4 Receptor (5-HT4R) Agonist and Serotonergic Subtype 6 Receptor (5-HT6R) Inverse Agonist Activities, With a Potential Interest Against Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 148. [Google Scholar] [CrossRef]
- Fang, Z.; Song, Y.; Zhan, P.; Zhang, Q.; Liu, X. Conformational restriction: An effective tactic in ’follow-on’-based drug discovery. Futur. Med. Chem. 2014, 6, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Lalut, J.; Payan, H.; Davis, A.; Lecoutey, C.; Legay, R.; Santos, J.S.-D.O.; Claeysen, S.; Dallemagne, P.; Rochais, C. Rational design of novel benzisoxazole derivatives with acetylcholinesterase inhibitory and serotoninergic 5-HT4 receptors activities for the treatment of Alzheimer’s disease. Sci. Rep. 2020, 10, 3014. [Google Scholar] [CrossRef]
- Bredereck, H.; Simchen, G.; Rebsdat, S.; Kantlehner, W.; Horn, P.; Wahl, R.; Hoffmann, H.; Grieshaber, P. Darstellung und Eigenschaften der Amidacetale und Aminalester. Chem. Ber. 1968, 101, 41–50. [Google Scholar] [CrossRef]
- Beria, I.; Ballinari, D.; Bertrand, J.A.; Borghi, D.; Bossi, R.T.; Brasca, M.G.; Cappella, P.; Caruso, M.; Ceccarelli, W.; Ciavolella, A.; et al. Identification of 4,5-Dihydro-1H-pyrazolo[4,3-h]quinazoline Derivatives as a New Class of Orally and Selective Polo-Like Kinase 1 Inhibitors. J. Med. Chem. 2010, 53, 3532–3551. [Google Scholar] [CrossRef]
- Genest, D.; Rochais, C.; Lecoutey, C.; Sopkova Oliveira Santos, J.S.; Ballandonne, C.; Butt-Gueulle, S.; Legay, R.; Since, M.; Dallemagne, P. Design, synthesis and biological evaluation of novel indano- and thiaindano-pyrazoles with potential interest for Alzheimer’s disease. Med. Chem. Comm. 2013, 4, 925. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C. Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J. Mol. Biol. 1995, 245, 43–53. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Dubost, E.; Dumas, N.; Fossey, C.; Magnelli, R.; Butt-Gueulle, S.; Ballandonne, C.; Caignard, D.H.; Dulin, F.; Santos, J.S.D.-O.; Millet, P.; et al. Synthesis and Structure–Affinity Relationships of Selective High-Affinity 5-HT4 Receptor Antagonists: Application to the Design of New Potential Single Photon Emission Computed Tomography Tracers. J. Med. Chem. 2012, 55, 9693–9707. [Google Scholar] [CrossRef] [PubMed]
- Cherezov, V.; Rosenbaum, D.M.; Hanson, M.A.; Rasmussen, S.G.F.; Thian, F.S.; Kobilka, T.S.; Choi, H.-J.; Kuhn, P.; Weis, W.I.; Kobilka, B.K.; et al. High-Resolution Crystal Structure of an Engineered Human 2-Adrenergic G Protein-Coupled Receptor. Science 2007, 318, 1258–1265. [Google Scholar] [CrossRef]
- Rivail, L.; Giner, M.; Gastineau, M.; Berthouze, M.; Soulier, J.-L.; Fischmeister, R.; Lezoualc’H, F.; Maigret, B.; Sicsic, S.; Berque-Bestel, I. New insights into the human 5-HT4 receptor binding site: Exploration of a hydrophobic pocket. Br. J. Pharmacol. 2004, 143, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Jain, E.; Bairoch, A.; Duvaud, S.; Phan, I.; Redaschi, N.; Suzek, B.E.; Martin, M.J.; McGarvey, P.; Gasteiger, E. Infrastructure for the life sciences: Design and implementation of the UniProt website. BMC Bioinform. 2009, 10, 1–19. [Google Scholar] [CrossRef]
- Shia, J.; Blundell, T.L.; Mizuguchia, K. FUGUE: Sequence-structure homology recognition using environment-specific substitution tables and structure-dependent gap penalties11Edited by B. Honig. J. Mol. Biol. 2001, 310, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhou, Y. Fold recognition by combining sequence profiles derived from evolution and from depth-dependent structural alignment of fragments. Proteins Struct. Funct. Bioinform. 2004, 58, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Schäaffer, A.A.; Aravind, L.; Madden, T.L.; Shavirin, S.; Spouge, J.L.; Wolf, Y.I.; Koonin, E.V.; Altschul, S.F. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 2001, 29, 2994–3005. [Google Scholar] [CrossRef] [PubMed]
- Söding, J. Protein homology detection by HMM-HMM comparison. Bioinformatics 2004, 21, 951–960. [Google Scholar] [CrossRef]
- Pons, J.-L.; Labesse, G. @TOME-2: A new pipeline for comparative modeling of protein-ligand complexes. Nucleic Acids Res. 2009, 37, W485–W491. [Google Scholar] [CrossRef] [PubMed]
- Eswar, N.; Eramian, D.; Webb, B.; Shen, M.; Sali, A. Protein structure modeling with MODELLER. Methods Mol. Biol. 2008, 426, 145–159. [Google Scholar] [CrossRef]
- Eisenberg, D.; Lüthy, R.; Bowie, J.U. VERIFY3D: Assessment of protein models with three-dimensional profiles. Methods Enzymol. 1997, 277, 396–404. [Google Scholar] [CrossRef]
- Gracy, J.; Chiche, L.; Sallantin, J. Improved alignment of weakly homologous protein sequences using structural information. Protein Eng. Des. Sel. 1993, 6, 821–829. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucl. Acids Res. 2014, 42, 320–324. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).