Biological Properties and Applications of Betalains
Abstract
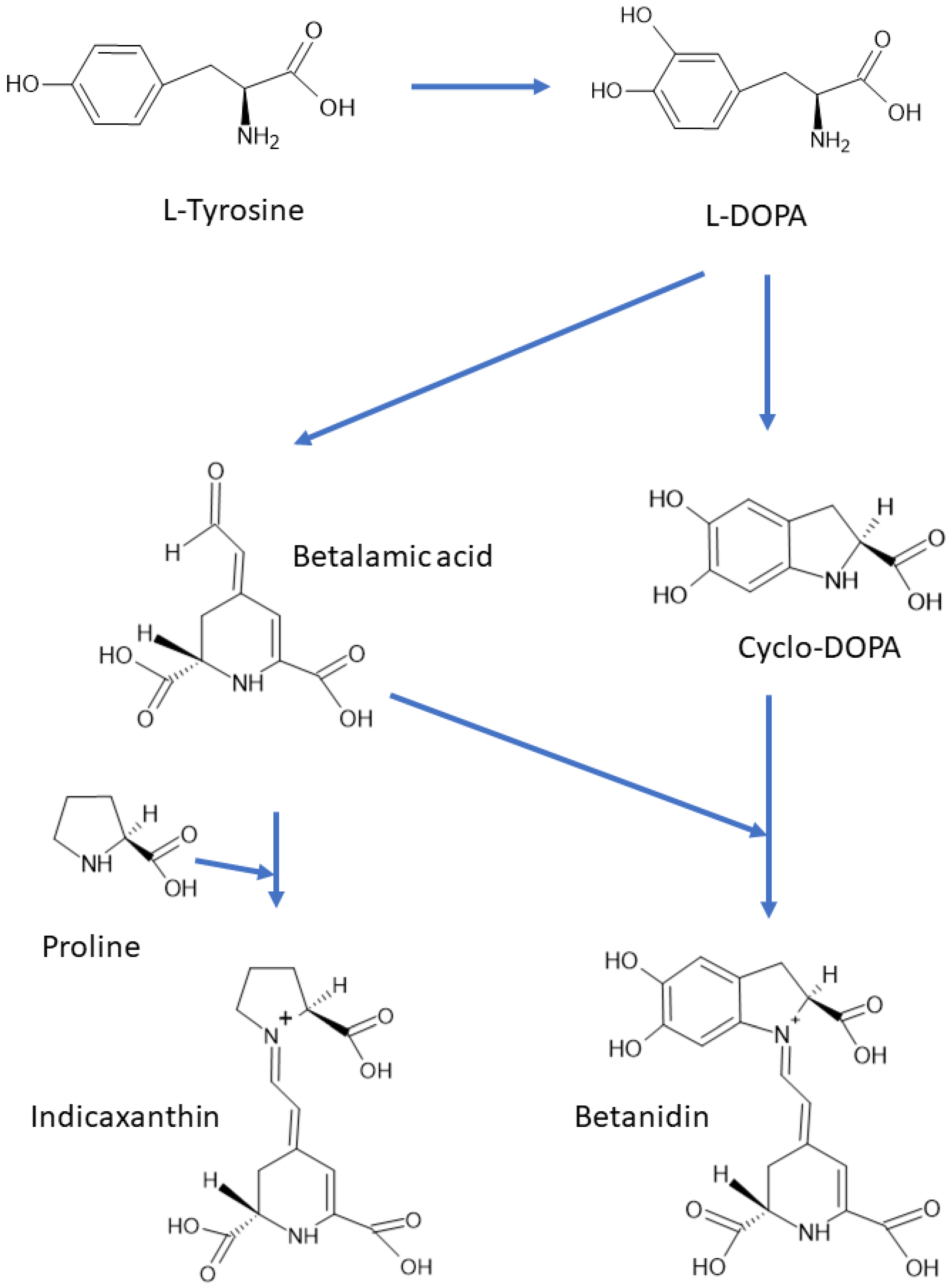
1. Occurrence of Betalains
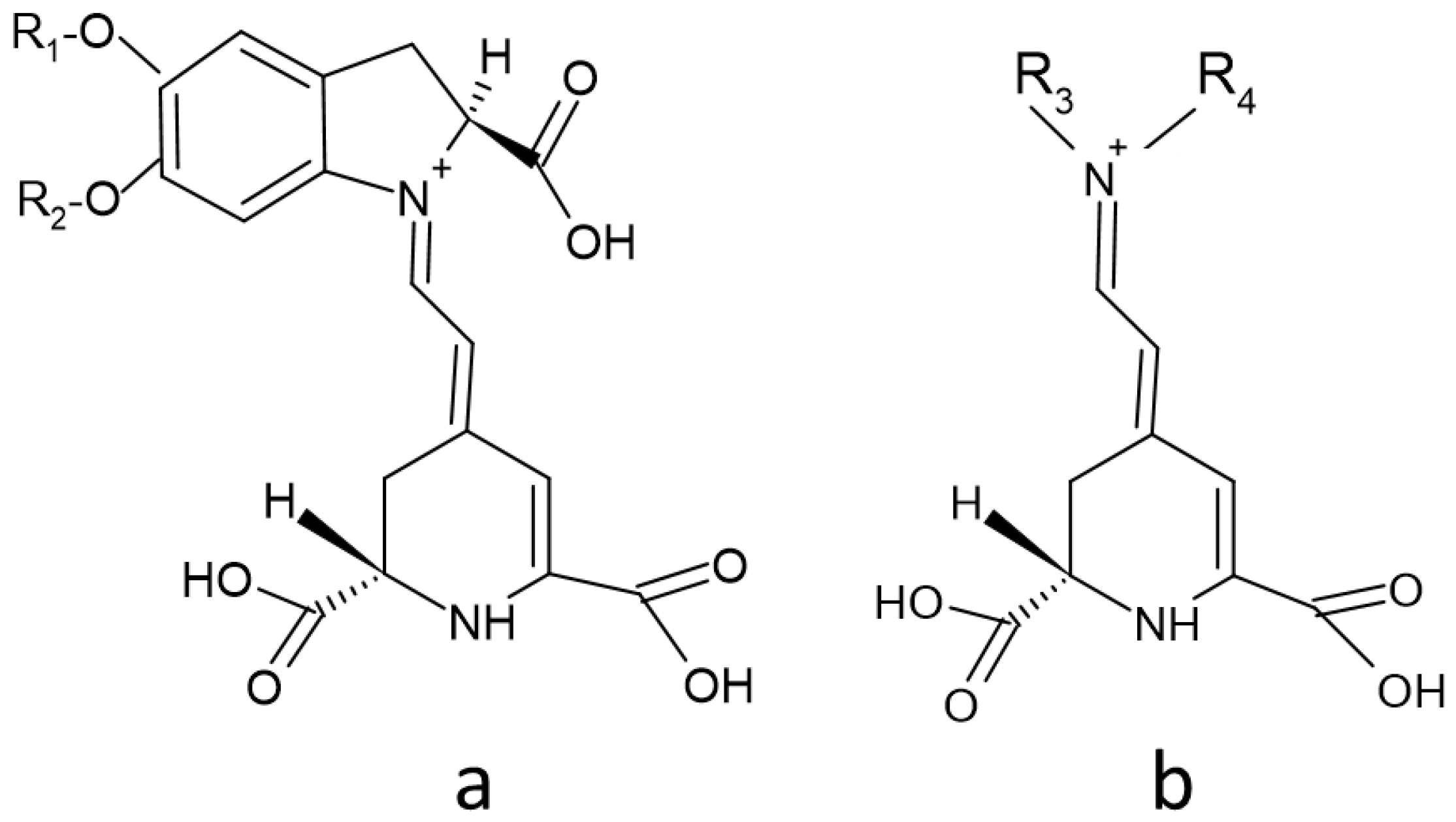
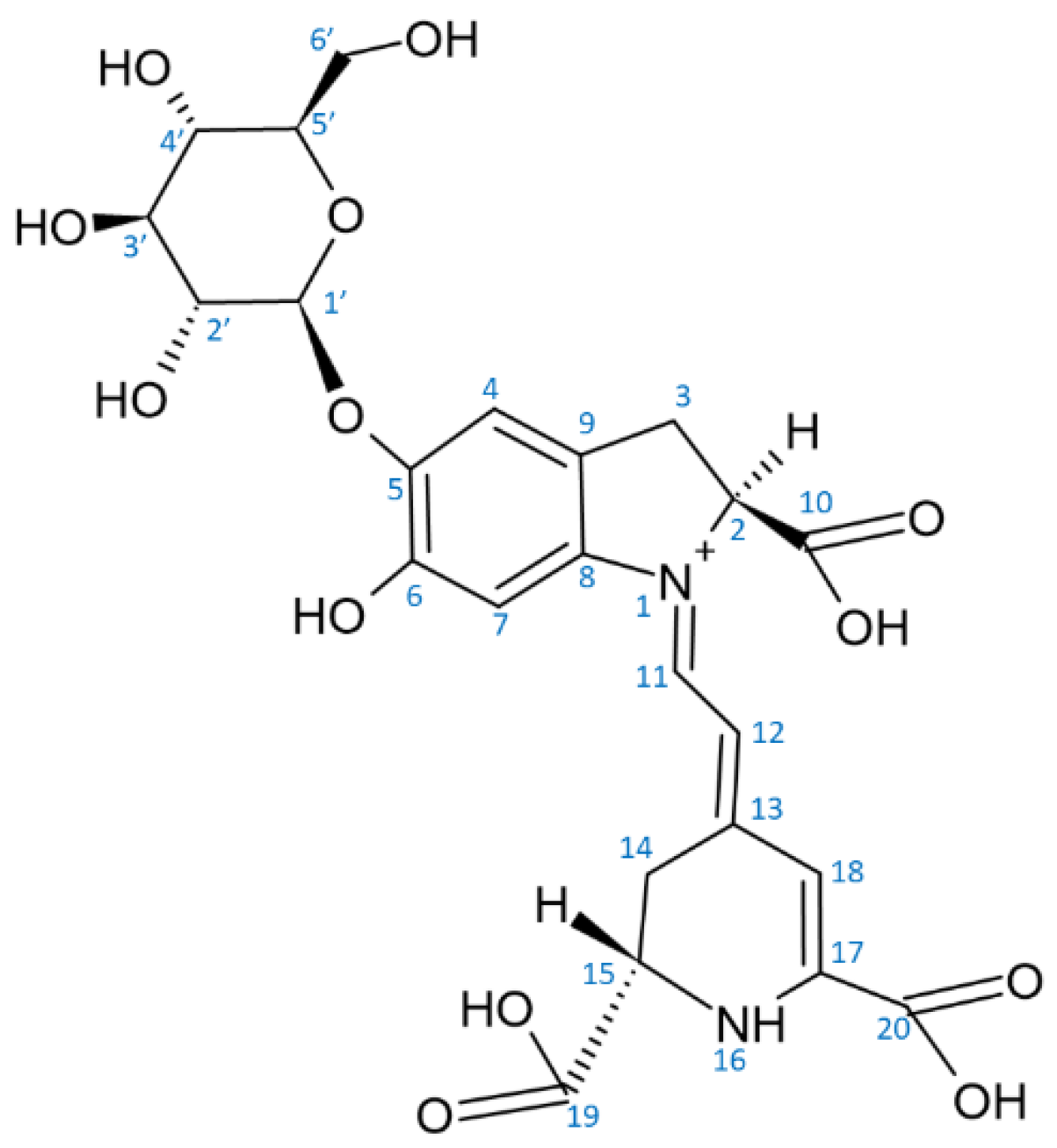
2. Structure of Betalains
3. Spectroscopic and Fluorescent Properties of Betalains
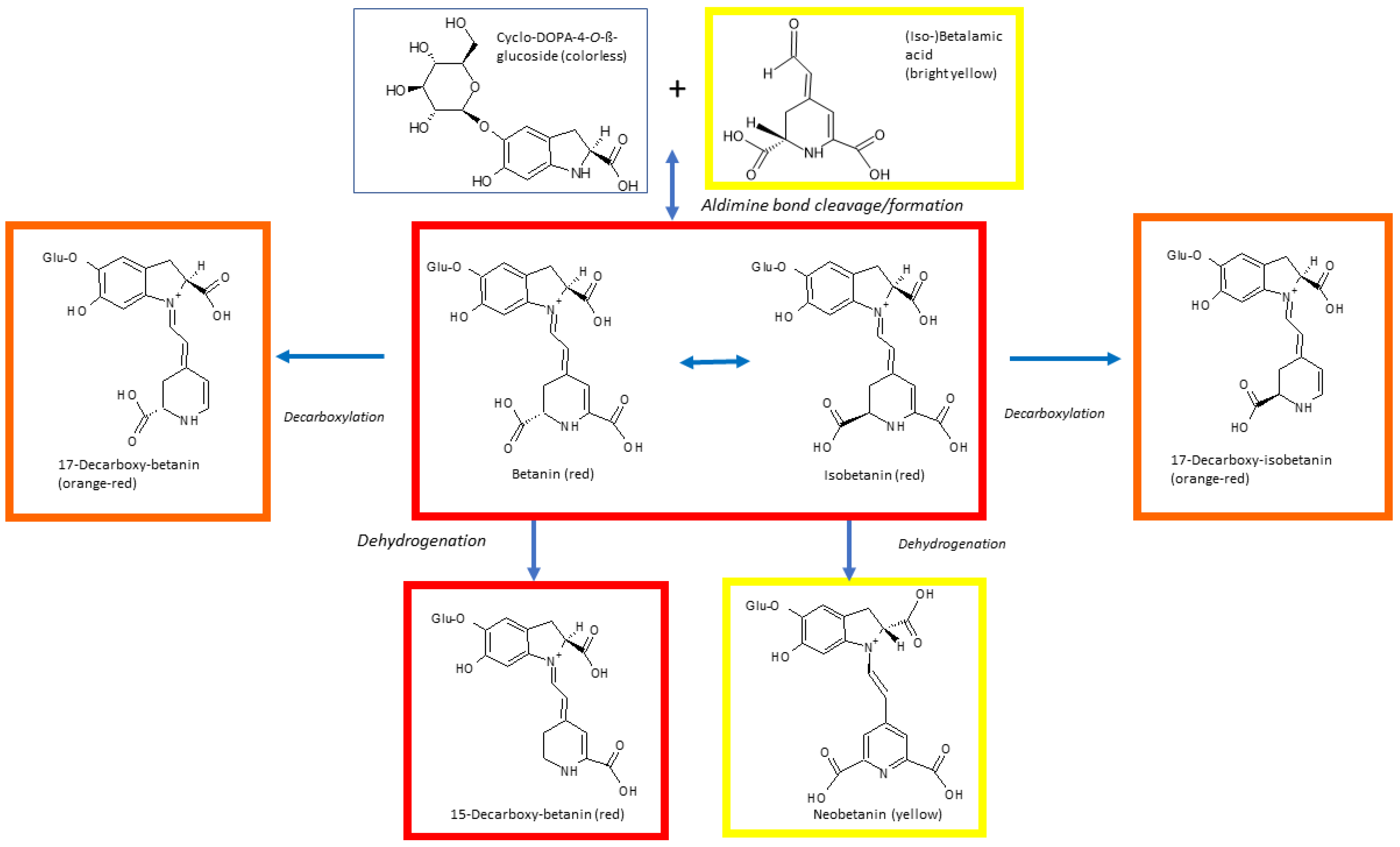
4. Betalain Stability
5. Antioxidant Activity
6. Bioavailability
7. Health Benefits
8. Betalains as Food Colorants
9. Food Safety
10. Other Applications
11. Environmental Role and Fate of Betalains
12. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Biological Activities of Plant Pigments Betalains. Crit. Rev. Food Sci. Nutr. 2016, 56, 937–945. [Google Scholar] [CrossRef]
- Wink, M. Compartmentation of secondary metabolites and xenobiotics in plant vacuoles. Adv. Bot. Res. 1997, 25, 141–169. [Google Scholar]
- Piattelli, M.; Minale, L.; Nicolaus, R.A. Betaxanthins from Mirabilis jalapa L. Phytochemistry 1965, 4, 817–823. [Google Scholar] [CrossRef]
- Impellizzeri, G.; Piattelli, M.; Sciuto, S. A new betaxanthin from Glottiphyllum longum. Phytochemistry 1973, 12, 2293–2294. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta 2005, 222, 586–593. [Google Scholar] [CrossRef]
- Osorio-Esquivel, O.; Alicia-Ortiz-Moreno Alvarez, V.B.; Dorantes-Alvarez, L.; Giusti, M.M. Phenolics, betacyanins and antioxidant activity in Opuntia joconostle fruits. Food Res. Int. 2011, 44, 2160–2168. [Google Scholar] [CrossRef]
- Felker, P.; Stintzing, F.C.; Müssig, E.; Leitenberger, M.; Carle, R.; Vogt, T.; Bunch, R. Colour inheritance in cactus pear (Opuntia ficus-indica) fruits. Ann. Appl. Biol. 2008, 152, 307–318. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Identification of betalains from yellow beet (Beta vulgaris L.) and cactus pear [Opuntia ficus-indica (L.) Mill.] by high-performance liquid chromatography–electrospray ionization mass spectroscopy. J. Agric. Food Chem. 2002, 50, 2302–2307. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Betacyanins in fruits from red-purple pitaya, Hylocereus polyrhizus (Weber) Britton & Rose. Food Chem. 2002, 77, 101–106. [Google Scholar]
- Masson, L.; Salvatierra, M.A.; Robert, P.; Encina, C.; Camilo, C. Chemical and nutritional composition of copao fruit (Eulychnia acida Phil.) under three environmental conditions in the Coquimbo region. Chil. J. Agric. Res. 2011, 71, 521–529. [Google Scholar] [CrossRef]
- Wybraniec, S.; Mizrahi, Y. Fruit flesh betacyanin pigments in Hylocereus cacti. J. Agric. Food Chem. 2002, 50, 6086–6089. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Nowak-Wydra, B.; Mitka, K.; Kowalski, P.; Mizrahi, Y. Minor betalains in fruits of Hylocereus species. Phytochemistry 2007, 68, 251–259. [Google Scholar] [CrossRef]
- Kugler, F.; Stintzing, F.C.; Carle, R. Identification of betalains from petioles of differently colored Swiss chard (Beta vulgaris L. ssp. cicla [L.] Alef. Cv. Bright Lights) by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2004, 52, 2975–2981. [Google Scholar] [CrossRef]
- Schliemann, W.; Cai, Y.; Degenkold, T.; Schmidt, J.; Corke, H. Betalains of Celosia argentea. Phytochemistry 2001, 58, 159–165. [Google Scholar] [CrossRef]
- Kugler, F.; Stintzing, F.C.; Carle, R. Characterization of betalain patterns of differently colored inflorescences from Gomphrena globose L. and Bougainvillea sp. by HPLC-DAD-ESI-MS. Anal. Bioanal. Chem. 2007, 387, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Svenson, J.; Smallfield, B.M.; Joyce, N.I.; Sanson, C.E.; Perry, N.B. Betalains in red and yellow varieties of the Andean tuber crop ulluco (Ullucus tuberosus). J. Agric. Food Chem. 2008, 56, 7730–7737. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Joseph, K.M.D.; Muralidhara, R.H.P.; Giridhar, P.; Ravishankar, G.A. Acute, subacute and subchronic safety assessment of betalains rich Rivina humilis L. berry juice in rats. Food Chem. Toxicol. 2011, 49, 3154–3157. [Google Scholar] [CrossRef] [PubMed]
- Amin, I.; Norazaidah, Y.; Hainida, K.I.E. Antioxidant activity and phenolic content of raw and blanched Amaranthus species. Food Chem. 2006, 94, 47–52. [Google Scholar] [CrossRef]
- Sang-Uk, C.; Buk-Gu, H.; Yong-Seo, P.; Dong-Kwan, K.; Shela, G. Total phenolics level, antioxidant activities and cytotoxicity of young sprouts of some traditional Korean salad plants. Plant Food Hum. Nutr. 2009, 64, 25–31. [Google Scholar]
- Cai, Y.; Sun, M.; Corke, H. Colourant properties and stability of Amaranthus betacyanin pigments. J. Agric. Food Chem. 1998, 46, 4491–4495. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Chzouoo, W.S. Betalains: Natural plant pigments with potential application in functional foods. LWT J. Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Adachi, T.; Nakatsukasa, M. High-performance liquid chromatographic separation of betalains and their distribution in Portulaca grandiflora and related species. Z. Pflanzenphysiol. 1983, 109, 155–162. [Google Scholar] [CrossRef]
- Trezzini, G.F.; Zrÿd, J.-P. Two betalains from Portulaca grandiflora. Phytochemistry 1991, 30, 1897–1899. [Google Scholar] [CrossRef]
- Swarna, J.; Lokeswari, T.S.; Smita, M.; Ravindhran, R. Characterisation and determination of in vitro antioxidant potential of betalains from Talinum triangulare (Jacq.) Willd. Food Chem. 2013, 141, 4382–4390. [Google Scholar] [CrossRef] [PubMed]
- Von Ardenne, R.; Döpp, H.; Musso, H.; Steiglich, W. Über das Vorkommen von Muscaflavin bei Hygrocyben (Agaricales) und seine Dihydroazepin-Struktur. Z. Naturforsch. 1974, 29, 637–639. [Google Scholar] [CrossRef]
- Musso, H. Pigments of fly agaric, Amanita muscaria. Tetrahedron 1979, 35, 2843–2853. [Google Scholar] [CrossRef]
- Stintzing, F.; Schliemann, W. Pigments of fly agaric (Amanita muscaria). Z. Naturforsch. 2007, 62, 779–785. [Google Scholar] [CrossRef]
- Babos, M.; Halasz, K.; Zagyva, T.; Zold-Balogh, A.; Szego, D.; Bratek, Z. Preliminary notes on dual relevance of ITS sequences and pigments in Hygrocybe taxonomy. Persoonia 2011, 26, 99–107. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Gill, M. Pigments of fungi (Macromycetes). Nat. Prod. Rep. 1994, 11, 67–90. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Llano, L.E.; Guerrero-Rubio, M.A.; Lozada-Ramírez, J.D.; García-Carmona, F.; Gandía-Herrero, F. First Betalain-Producing Bacteria Break the Exclusive Presence of the Pigments in the Plant Kingdom. mBio 2019, 10, e00345-19. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, J.; Xie, S.Y.; Zhang, T.Y.; Soladoye, O.P.; Aluko, R.E. Red Beetroot Betalains: Perspectives on Extraction, Processing, and Potential Health Benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins, and betalains—Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Von Elbe, J.H. Stability of betalaines as food colors. Food Technol. 1975, 5, 42–44. [Google Scholar]
- Ninfali, P.; Angelino, D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Pszczola, D.E. Natural colours: Pigments of imagination. Food Technol. 1998, 52, 70–76. [Google Scholar]
- Gaertner, V.; Goldman, I.L. Pigment distribution and total dissolved solids of selected cycles of table beet from a recurrent selection program for increased pigment. J. Am. Soc. Hortic. Sci. 2005, 130, 424–433. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Xu, H.; Wang, Q.; Yang, B. Impact of high pressure carbon dioxide combined with thermal treatment on degradation of red beet (Beta vulgaris L.) pigments. J. Agric. Food Chem. 2008, 56, 6480–6487. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Sawicki, T.; Martinez-Villaluenga, C.; Frias, J.; Wiczkowski, W.; Peñas, E.; Bączek, N.; Zieliński, H. The effect of processing and in vitro digestion on the betalain profile and ACE inhibition activity of red beetroot products. J. Funct. Foods 2019, 55, 229–237. [Google Scholar] [CrossRef]
- Kujala, T.S.; Vienola, M.S.; Klika, K.D.; Loponen, J.M.; Pihlaja, K. Betalain and phenolic compositions of our beetroot (Beta vulgaris) cultivars. Eur. Food Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Slatnar, A.; Stampar, F.; Vebaric, R.; Jakopic, J. HPLC-MSn identification of betalains profile of different beetroot (Beta vulgaris L. ssp. vulgaris) parts and cultivars. J. Food Sci. 2015, 80, 1952–1958. [Google Scholar]
- Sawicki, T.; Wiczkowski, W. The effects of boiling and fermentation on betalain profiles and antioxidant capacities of red beetroot products. Food Chem. 2018, 259, 292–303. [Google Scholar] [CrossRef]
- Wyler, H. Neobetanin: A new natural plant constituent? Phytochemistry 1986, 25, 2238. [Google Scholar] [CrossRef]
- Strack, D.; Engel, U.; Wray, V. Neobetanin: A new natural plant constituent. Phytochemistry 1987, 26, 2399–2400. [Google Scholar] [CrossRef]
- Vieira Teixeira da Silva, D.; Dos Santos Baião, D.; de Oliveira Silva, F.; Alves, G.; Perrone, D.; Mere Del Aguila, E.; Flosi Paschoalin, V.M. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef]
- Slavov, A.; Karagyozov, V.; Denev, P.; Kratchanova, M.; Kratchanov, C. Antioxidant activity of red beet juices obtained after microwave and thermal pretreatments. Czech J. Food Sci. 2013, 31, 139–147. [Google Scholar] [CrossRef]
- Acree, T.E.; Lee, C.Y.; Butts, R.M.; Barnard, J. Geosmin, the earth component of table beet odour. J. Agric. Food Chem. 1976, 24, 430–431. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Abderrabba, M. Chemical and Antioxidant Properties of Betalain. J. Agric. Food Chem. 2017, 65, 675–689, Correction in 2017, 65, 1466. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liu, X.; Li, S.; Zhang, X.; Yu, S.; Zhao, G.R. Metabolic Engineering of Escherichia coli for de Novo Production of Betaxanthins. J. Agric. Food Chem. 2020, 68, 8370–8380. [Google Scholar] [CrossRef]
- Grewal, P.S.; Modavi, C.; Russ, Z.N.; Harris, N.C.; Dueber, J.E. Bioproduction of a betalain color palette in Saccharomyces cerevisiae. Metab. Eng. 2018, 45, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Polturak, G.; Grossman, N.; Vela-Corcia, D.; Dong, Y.; Nudel, A.; Pliner, M.; Levy, M.; Rogachev, I.; Aharoni, A. Engineered gray mold resistance, antioxidant capacity, and pigmentation in betalain-producing crops and ornamentals. Proc. Natl. Acad. Sci. USA 2017, 114, 9062–9067. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.S.; Fu, X.Y.; Yang, Z.Q.; Wang, B.; Gao, J.J.; Wang, M.Q.; Xu, J.; Han, H.J.; Li, Z.J.; Yao, Q.H.; et al. Metabolic engineering of rice endosperm for betanin biosynthesis. New Phytol. 2020, 225, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Leathers, R.R.; Davin, C.; Zryd, J.P. Betalain producing cell cultures of Beta vulgaris L. var. bikores monogerm (red beet). In Vitro Plant. 1992, 28, 39–45. [Google Scholar] [CrossRef]
- Akita, T.; Hina, Y.; Nishi, T. Production of betacyanins by a cell suspension culture of table beet (Beta vulgaris L.). Biosci. Biotechnol. Biochem. 2000, 64, 1807–1812. [Google Scholar] [CrossRef][Green Version]
- Dörnenburg, H.; Knorr, D. Challenges and opportunities of metabolite production from plant cell and tissue culture. Food Technol. 1997, 51, 47–54. [Google Scholar]
- Gasztonyi, M.N.; Daood, H.; Hajos, M.T.; Biacs, P. Comparison of red beet (Beta vulgaris var. conditiva) varieties on the basis of their pigment components. J. Sci. Food Agric. 2001, 81, 932–933. [Google Scholar] [CrossRef]
- Fischer, N.; Dreiding, A.S. Biosynthesis of betalaines. On the cleavage of the aromatic ring during the enzymatic transformation of dopa into betalamic acid. Helv. Chim. Acta 1972, 55, 649–658. [Google Scholar] [CrossRef]
- Hatlestad, G.J.; Sunnadeniya, R.M.; Akhavan, N.A.; Gonzalez, A.; Goldman, I.L.; McGrath, J.M.; Lloyd, A.M. The beet R locus encodes a new cytochrome P450 required for red betalain production. Nat. Genet. 2012, 44, 816–820. [Google Scholar] [CrossRef]
- Christinet, L.; Burdet, F.X.; Zaiko, M.; Hinz, U.; Zrÿd, J.P. Characterization and functional identification of a novel plant 4, 5-extradiol dioxygenase involved in betalain pigment biosynthesis in Portulaca grandiflora. Plant Physiol. 2004, 134, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, H.W.; Matschiner, B.; Schutte, H.R. Beitrage zur Physiologie und Biosynthese des Betanins. Z. Pflanzenphysiol. 1969, 61, 269–278. [Google Scholar]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Betaxanthins as substrates for tyrosinase. An approach to the role of tyrosinase in the biosynthetic pathway of betalains. Plant Physiol. 2005, 138, 421–432. [Google Scholar] [CrossRef]
- Jackman, R.L.; Smith, J.L. Anthocyanins and betalains. In Natural Food Colourants; Hendry, G.F., Houghton, J.D., Eds.; Blackie Academic & Professional: London, UK, 1996; pp. 244–309. [Google Scholar]
- Stintzing, F.C.; Kammerer, D.; Schieber, A.; Adama, H.; Nacoulma, O.G.; Carle, R. Betacyanins and phenolic compounds from Amaranthus spinosus L. and Boerhavia erecta L. Z. Naturforsch. C J. Biosci. 2004, 59, 1–8. [Google Scholar] [CrossRef]
- Kobayashi, N.; Schmidt, J.; Wray, V.; Schliemann, W. Formation and occurrence of dopamine derived betacyanins. Phytochemistry 2001, 56, 429–436. [Google Scholar] [CrossRef]
- Alard, D.; Wray, V.; Grotjahn, L.; Reznik, H.; Strack, D. Neobetanin: Isolation and identification from Beta vulgaris. Phytochemistry 1985, 24, 2383–2385. [Google Scholar] [CrossRef]
- Guerrero-Rubio, M.A.; Escribano, J.; García-Carmona, F.; Gandía-Herrero, F. Light Emission in Betalains: From Fluorescent Flowers to Biotechnological Applications. Trends Plant Sci. 2020, 25, 159–175. [Google Scholar] [CrossRef]
- Wybraniec, S.; Platzner, I.; Geresh, S.; Gottlieb, H.E.; Haimberg, M.; Mogilnitzki, M.; Mizrahi, Y. Betacyanins from vine cactus Hylocereus polyrhizus. Phytochemistry 2001, 58, 1209–1212. [Google Scholar] [CrossRef]
- Schliemann, W.; Strack, D. Intramolecular stabilization of acylated betacyanins. Phytochemistry 1998, 49, 585–588. [Google Scholar] [CrossRef]
- Castellar, M.R.; Obón, J.M.; Alacid, M.; Fernández-López, J.A. Color properties and stability of betacyanins from Opuntia fruits. J. Agric. Food Chem. 2003, 51, 2772–2776. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.S.; von Elbe, J.H. Effect of pH on the degradation and regeneration of betanine. J. Food Sci. 1987, 52, 1689–1693. [Google Scholar] [CrossRef]
- Von Elbe, J.H.; Maing, I.; Amundson, C.H. Colour stability of betanin. J. Food Sci. 1974, 39, 334–337. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F.; Escribano, J. A novel method using high-performance liquid chromatography with fluorescence detection for the determination of betaxanthins. J. Chromatogr. A 2005, 1078, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, M.; Minale, L.; Prota, G. Pigments of Centrospermae—III: Betaxanthins from Beta vulgaris L. Phytochemistry 1965, 4, 121–125. [Google Scholar] [CrossRef]
- Rodrigues, A.C.B.; Mariz, I.F.A.; Maçoas, E.M.S.; Tonelli, R.R.; Martinho, J.M.G.; Quina, F.H.; Bastos, E.L. Bioinspired water-soluble two-photon fluorophores. Dyes Pigment. 2018, 150, 105–111. [Google Scholar] [CrossRef]
- Moßhammer, M.R.; Stintzing, F.C.; Carle, R. Colour studies on fruit juice blends from Opuntia and Hylocereus cacti and betalain-containing model solutions derived therefrom. Food Res Int. 2005, 38, 975–981. [Google Scholar] [CrossRef]
- Niziński, S.; Popenda, Ł.; Rode, M.F.; Kumorkiewicz, A.; Fojud, Z.; Paluch-Lubawa, E.; Wybraniec, S.; Burdziński, G. Structural studies on the stereoisomerism of a natural dye miraxanthin I. New J. Chem. 2019, 43, 18165–18174. [Google Scholar] [CrossRef]
- Wendel, M.; Nizinski, S.; Tuwalska, D.; Starzak, K.; Szot, D.; Prukala, D.; Sikorski, M.; Wybraniec, S.; Burdzinski, G. Time-resolved spectroscopy of the singlet excited state of betanin in aqueous and alcoholic solutions. Phys. Chem. Chem. Phys. 2015, 17, 18152–18158. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Purification and antiradical properties of the structural unit of betalains. J. Nat. Prod. 2012, 75, 1030–1036. [Google Scholar] [CrossRef]
- Lemos, M.A.; Sárniková, K.; Bot, F.; Anese, M.; Hungerford, G. Use of Time-Resolved Fluorescence to Monitor Bioactive Compounds in Plant Based Foodstuffs. Biosensors 2015, 5, 367–397. [Google Scholar] [CrossRef]
- Rabasović, M.S.; Šević, D.; Terzić, M.; Marinković, B.P. Comparison of beetroot extracts originating from several sites using time-resolved laser-induced fluorescence spectroscopy. Phys. Scr. 2012, 2012, T149. [Google Scholar]
- Das, A.; De, D.; Ghosh, A.; Goswami, M.M. An innovative cell imaging by beet root extracted pigment. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 230, 118037. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Rubio, M.A.; Hernández-García, S.; Escribano, J.; Jiménez-Atiénzar, M.; Cabanes, J.; García-Carmona, F.; Gandía-Herrero, F. Betalain health-promoting effects after ingestion in Caenorhabditis elegans are mediated by DAF-16/FOXO and SKN-1/Nrf2 transcription factors. Food Chem. 2020, 330, 127228. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.N.; Javellana, J.; Davies, K.M.; Lewis, D.H.; Jameson, P.E.; Deroles, S.C.; Calcott, K.E.; Gould, K.S.; Schwinn, K.E. Betalain production is possible in anthocyanin-producing plant species given the presence of DOPA-dioxygenase and L-DOPA. BMC Plant Biol. 2012, 12, 34. [Google Scholar] [CrossRef]
- Stücheli, P.; Sieber, S.; Fuchs, D.W.; Scheller, L.; Strittmatter, T.; Saxena, P.; Gademann, K.; Fussenegger, M. Genetically encoded betaxanthin-based small-molecular fluorescent reporter for mammalian cells. Nucleic Acids Res. 2020, 48, e67. [Google Scholar] [CrossRef]
- Gonçalves, L.C.; Tonelli, R.R.; Bagnaresi, P.; Mortara, R.A.; Ferreira, A.G.; Bastos, E.L. A nature-inspired betalainic probe for live-cell imaging of Plasmodium-infected erythrocytes. PLoS ONE 2013, 8, e53874. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Jiménez-Atiénzar, M.; Cabanes, J.; Escribano, J.; García-Carmona, F. Fluorescence detection of tyrosinase activity on dopamine-betaxanthin purified from Portulaca oleracea (common purslane) flowers. J. Agric. Food Chem. 2009, 57, 2523–2528. [Google Scholar] [CrossRef]
- DeLoache, W.C.; Russ, Z.N.; Narcross, L.; Gonzales, A.M.; Martin, V.J.; Dueber, J.E. An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose. Nat. Chem. Biol. 2015, 11, 465–471. [Google Scholar] [CrossRef]
- Hawkins, K.M.; Smolke, C.D. Production of benzylisoquinoline alkaloids in Saccharomyces cerevisiae. Nat. Chem. Biol. 2008, 4, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Liu, Q.; Li, Y.; Yang, J.; Song, X.; Liu, X.; Xu, H.; Qiao, M. A high-throughput method for screening of L-tyrosine high-yield strains by Saccharomyces cerevisiae. J. Gen. Appl. Microbiol. 2018, 64, 198–201. [Google Scholar] [CrossRef]
- Cabanes, J.; Gandía-Herrero, F.; Escribano, J.; García-Carmona, F.; Jiménez-Atiénzar, M. Fluorescent bioinspired protein labeling with betalamic acid. Derivatization and characterization of novel protein-betaxanthins. Dyes Pigment. 2016, 133, 458–466. [Google Scholar] [CrossRef]
- Chen, P.-H.; Lin, C.; Guoa, K.-H.; Yeh, Y.-C. Development of a pigment-based whole-cell biosensor for the analysis of environmental copper. RSC Adv. 2017, 7, 29302–29305. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation—Structural and chromatic aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Saguy, I.; Kopelman, I.J.; Mizrahi, S. Thermal kinetic degradation of betanin and betalamic acid. J. Agric. Food Chem. 1978, 26, 360–362. [Google Scholar] [CrossRef]
- Havlíková, L.; Míková, K.; Kyzlink, V. Heat stability of betacyanins. Z. Lebensmitt. Untersuch. Forsch. 1983, 177, 247–250. [Google Scholar] [CrossRef]
- García Barrera, F.A.; Reynoso, C.R.; González de Mejía, E. Estabilidad de las betalaínas extraídas del garambullo (Myrtillocactus geometrizans). Food Sci. Technol. Int. 1998, 4, 115–120. [Google Scholar] [CrossRef]
- Huang, A.S.; von Elbe, J.H. Kinetics of the degradation and regeneration of betanine. J. Food Sci. 1985, 50, 1115–1120. [Google Scholar] [CrossRef]
- Drdák, M.; Vallová, M. Kinetics of the thermal degradation of betanine. Nahrung 1990, 34, 307–310. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Thermal degradation of betacyanins in juices from purple pitaya (Hylocereus polyrhizus [Weber] Britton and Rose) monitored by high-performance liquid chromatography-tandem mass spectrometric analyses. Eur. Food Res. Technol. 2004, 219, 377–385. [Google Scholar] [CrossRef]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Jiratanan, T.; Liu, R.H. Antioxidant activity of processed table beets (Beta vulgaris var. conditiva) and green beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2004, 52, 2659–2670. [Google Scholar] [CrossRef]
- Gokhale, S.V.; Lele, S.S. Betalain content and antioxidant activity of Beta vulgaris: Effect of hot air convective drying and storage. J. Food Proc. Pres. 2014, 38, 585–590. [Google Scholar] [CrossRef]
- Pitalua, E.; Jimenez, M.; Vernon-Carter, E.J.; Beristain, C.I. Antioxidative activity of microcapsules with beet root juice using gum Arabic as wall material. Food Bioprod. Process. 2010, 88, 253–258. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Impact of thermal treatment on colour and pigment pattern of red beet (Beta vulgaris L.) preparations. J. Food Sci. 2004, 69, C491–C498. [Google Scholar] [CrossRef]
- Altamirano, R.C.; Drdák, M.; Simon, P.; Rajniakova, A.; Karovicová, J.; Preclík, L. Thermal degradation of betanine in various water alcohol model systems. Food Chem. 1993, 46, 73–75. [Google Scholar] [CrossRef]
- Wybraniec, S. Formation of decarboxylated betacyanins in heated purified betacyanin fractions from red beet root (Beta vulgaris L.) monitored by LC-MS ⁄ MS. J. Agric. Food Chem. 2005, 53, 3483–3487. [Google Scholar] [CrossRef]
- Wybraniec, S.; Mizrahi, Y. Generation of decarboxylated betacyanins in thermally treated purified fruit extract from purple pitaya (Hylocereus polyrhizus) monitored by LC-MS/MS. J. Agric. Food Chem. 2005, 53, 6704–6712. [Google Scholar] [CrossRef]
- Wybraniec, S.; Nowak-Wydra, B.; Mizrahi, Y. 1H and 13C NMR spectroscopic structural elucidation of new decarboxylated betacyanins. Tetrahedron Lett. 2006, 47, 1725–1728. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Identification of heat-induced degradation products from purified betanin, phyllocactin and hylocerenin by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2603–2616. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.J.; von Elbe, J.H. Identification of betanin degradation products. Eur. Food Res. Technol. 1983, 176, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Stability and color changes of thermally treated betanin, hyllocactin, and hylocerenin solutions. J. Agric. Food Chem. 2006, 54, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Heuer, S.; Richter, S.; Metzger, J.W.; Wray, V.; Nimtz, M.; Strack, D. Betacyanins from bracts of Bougainvillea glabra. Phytochemistry 1994, 37, 761–767. [Google Scholar] [CrossRef]
- Reynoso, R.; Garcia, F.A.; Morales, D.; Gonzalez de Mejia, E. Stability of betalain pigments from a Cactacea fruit. J. Agric. Food Chem. 1997, 45, 2884–2889. [Google Scholar] [CrossRef]
- Sapers, G.M.; Hornstein, J.S. Varietal differences in colorant properties and stability of red beet pigments. J. Food Sci. 1979, 44, 1245–1248. [Google Scholar] [CrossRef]
- Singer, J.W.; von Elbe, J.H. Degradation rates of vulgaxanthine I. J. Food Sci. 1980, 45, 489–491. [Google Scholar] [CrossRef]
- Cai, Y.; Corke, H. Amaranthus betacyanin pigments applied in model food systems. J. Food Sci. 1999, 64, 869–873. [Google Scholar] [CrossRef]
- Vaillant, F.; Perez, A.; Davila, I.; Dornier, M.; Reynes, M. Colourant and antioxidant properties of red-purple pitahaya (Hylocereus sp.). Fruits 2005, 60, 1–10. [Google Scholar] [CrossRef]
- Von Elbe, J.H.; Attoe, E.L. Oxygen involvement in betanine degradation—Measurement of active oxygen species and oxidation-reduction potentials. Food Chem. 1985, 16, 49–67. [Google Scholar] [CrossRef]
- Schliemann, W.; Kobayashi, N.; Strack, D. The decisive step in betaxanthin biosynthesis is a spontaneous reaction. Plant Physiol. 1999, 119, 1217–1232. [Google Scholar] [CrossRef]
- Wyler, H.; Dreiding, A.S. Deuterierung von Betanidin und Indicaxanthin. (E/Z)-stereoisomerie in Betalainen. Helv. Chim. Acta 1984, 67, 1793–1800. [Google Scholar] [CrossRef]
- Mabry, T.J.; Wyler, H.; Parikh, I.; Dreiding, A.S. The conversion of betanidin and betanin to neobetanidin derivatives. Tetrahedron 1967, 23, 3111–3127. [Google Scholar] [CrossRef]
- Huang, A.S.; von Elbe, J.H. Stability comparison of two betacyanine pigments—Amaranthine and betanine. J. Food Sci. 1986, 51, 670–674. [Google Scholar] [CrossRef]
- Wyler, H.; Dreiding, A.S. Constitution of the beet pigment betanin. IV. Degradation products of betanidin. Helv. Chim. Acta 1962, 45, 638–640. [Google Scholar] [CrossRef]
- Piattelli, M.; Minale, L. Pigments of Centrospermae-II. Distribution of betacyanins. Phytochemistry 1964, 3, 547–557. [Google Scholar] [CrossRef]
- Mabry, T.J.; Wyler, H.; Sassu, G.; Mercier, M.; Parikh, I.; Dreiding, A.S. Die Struktur des Neobetanins. 5. Über die Konstitution des Randenfarbstoffes Betanin. Helv. Chim. Acta 1962, 45, 640–647. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Schliemann, W.; Corke, H. Chemical stability and colorant properties of betaxanthin pigments from Celosia argentea. J. Agric. Food Chem. 2001, 49, 4429–4435. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, K.; Kuusi, T. stability properties of golden beet and red beet pigments: Influence of pH, temperature, and some stabilizers. Z. Lebensm. Untersuch. Forsch. 1978, 166, 19–22. [Google Scholar] [CrossRef]
- Saguy, I.; Goldman, M.; Bord, A.; Cohen, E. Effect of oxygen retained on beet powder on the stability of betanine and vulgaxanthine-I. J. Food Sci. 1984, 49, 99–101. [Google Scholar] [CrossRef]
- Saguy, I. Thermostability of red beet pigments (betanine and vulgaxanthin-I): Influence of pH and temperature. J. Food Sci. 1979, 44, 1554–1555. [Google Scholar] [CrossRef]
- Piattelli, M.; Minale, L.; Nicolaus, R.A. Pigmenti gialli delle centrosperme (betaxantine) sull’isolamento e sulla costituzione della opunzia xantina. Rend. Acad. Sci. Fis. Mat. 1963, 30, 23–28. [Google Scholar]
- Attoe, E.L.; von Elbe, J.H. Oxygen involvement in betanin degradation. Z. Lebensmitteluntersuch. Forsch. A 1984, 179, 232–236. [Google Scholar] [CrossRef]
- Sobkowska, E.; Czapski, J.; Kaczmarek, R. Red table beet pigment as food colorant. Int. Food Ingred. 1991, 3, 24–28. [Google Scholar]
- Pasch, J.H.; von Elbe, J.H. Betanine stability in buffered solutions containing organic acids, metal cations, antioxidants, or sequestrants. J. Food Sci. 1979, 44, 72–75. [Google Scholar] [CrossRef]
- Czapski, J. Heat stability of betacyanins in red beet juice and in betanin solutions. Eur. Food Res. Technol. 1990, 191, 275–278. [Google Scholar] [CrossRef]
- Han, D.; Kim, S.J.; Kim, D.M. Repeated regeneration of degraded red beet juice pigments in the presence of antioxidants. J. Food Sci. 1998, 63, 69–72. [Google Scholar] [CrossRef]
- Herbach, K.M.; Rohe, M.; Stintzing, F.C.; Carle, R. Structural and chromatic stability of purple pitaya (Hylocereus polyrhizus [Weber] Britton & Rose) betacyanins as affected by the juice matrix and selected additives. Food Res. Int. 2006, 39, 667–677. [Google Scholar]
- Attoe, E.L.; von Elbe, J.H. Oxygen involvement in betanine degradation: Effect of antioxidants. J. Food Sci. 1985, 50, 106–110. [Google Scholar] [CrossRef]
- Attoe, E.L.; von Elbe, J.H. Degradation kinetics of betanine in solutions as influenced by oxygen. J. Agric. Food Chem. 1982, 30, 708–712. [Google Scholar] [CrossRef]
- Drunkler, D.A.; Fett, R.; Bordignon-Luiz, M.T. Avaliação da estabilidade de betalaínas em extrato de beterraba (Beta vulgaris L.) com α-, β- e γ-ciclodextrinas 1. Bol. Centr. Pesq. Proces. Aliment. 2006, 24, 259–276. [Google Scholar]
- Altamirano, R.C.; Drdák, M.; Simon, P.; Smelík, A.; Simko, P. Stability of red beet pigment concentrate in maize starch. J. Sci. Food Agric. 1992, 58, 595–596. [Google Scholar] [CrossRef]
- Máriássyová, M.; Šilhár, S. Conversion of betalains in the presence of antioxidants. Czech J. Food Sci. 2000, 18, 220–221. [Google Scholar]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Bilyk, A.; Howard, M. Reversibility of thermal degradation of betacyanins under the influence of isoascorbic acid. J. Agric. Food Chem. 1982, 30, 906–908. [Google Scholar] [CrossRef]
- Shenoy, V.R. Anthocyanins—Prospective food colours. Curr. Sci. 1993, 64, 575–579. [Google Scholar]
- Bartosz, G.; Grzesik-Pietrasiewicz, M.; Sadowska-Bartosz, I. Fluorescent products of anthocyanidin and anthocyanin oxidation. J. Agric. Food Chem. 2020, 68, 12019–12027. [Google Scholar] [CrossRef] [PubMed]
- Attoe, E.L.; von Elbe, J.H. Photochemical degradation of betanine and selected anthocyanins. J. Food Sci. 1981, 46, 1934–1937. [Google Scholar] [CrossRef]
- Boyles, C.; Sobeck, S.J.S. Photostability of organic red food dyes. Food Chem. 2020, 315, 126249. [Google Scholar] [CrossRef] [PubMed]
- Merin, U.; Gagel, S.; Popel, G.; Bernstein, S.; Rosenthal, I. Thermal degradation kinetics of prickly-pear fruit red pigment. J. Food Sci. 1987, 52, 485–486. [Google Scholar] [CrossRef]
- Kearsley, M.W.; Katsaboxakis, K.Z. Stability and use of natural colours in foods red beet powder, copper chlorophyII powder and cochineal. Int. J. Food Sci. Technol. 1980, 15, 501–514. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Recent development in microencapsulation of food ingredients. Drying Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Cai, Y.; Corke, H. Production and properties of spray-dried Amaranthus betacyanin pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Castellar, M.R.; Obón, J.M.; Fernández-López, J.A. The isolation and properties of a concentrated red-purple betacyanin food colourant from Opuntia stricta fruits. J. Sci. Food Agric. 2006, 86, 122–128. [Google Scholar] [CrossRef]
- Lee, C.Y.; Smith, N.L. Blanching effect on polyphenol oxidase activity in table beets. J. Food Sci. 1979, 44, 82–86. [Google Scholar] [CrossRef]
- Martínez-Parra, J.; Muñoz, R. Characterization of betacyanin oxidation catalyzed by a peroxidase from Beta vulgaris L. roots. J. Agric. Food Chem. 2001, 49, 4064–4068. [Google Scholar] [CrossRef]
- Escribano, J.; Gandía-Herrero, F.; Caballero, N.; Pedreno, M.A. Subcellular localization and isoenzyme pattern of peroxidase and polyphenol oxidase in beet root (Beta vulgaris L.). J. Agric. Food Chem. 2002, 50, 6123–6129. [Google Scholar] [CrossRef]
- Shih, C.C.; Wiley, R.C. Betacyanine and betaxanthine decolorizing enzymes in the beet (Beta vulgaris L.) root. J. Food Sci. 1981, 47, 164–172. [Google Scholar] [CrossRef]
- Wasserman, B.P.; Eiberger, L.L.; Guilfoy, M.P. Effect of hydrogen peroxide and phenolic compounds on horseradish peroxidase-catalysed decolorization of betalain pigments. J. Food Sci. 1984, 49, 536–538. [Google Scholar] [CrossRef]
- Minale, L.; Piattelli, M.; de Stefano, S.; Nicolaus, R.A. Pigments of Centrospermae VI. Acylated betacyanins. Phytochemistry 1966, 5, 1037–1052. [Google Scholar] [CrossRef]
- Minale, L.; Piattelli, M.; de Stefano, S. Pigments of Centrospermae VII. Betacyanins from Gomphrena globosa L. Phytochemistry 1967, 6, 703–708. [Google Scholar] [CrossRef]
- Czyżowska, A.; Klewicka, E.; Libudzisz, Z. The influence of lactic acid fermentation process of red beet juice on the stability of biologically active colorants. Eur. Food Res. Technol. 2006, 223, 110–116. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Nithyanandam, R.; Sarbatly, R. A critical review on the spray drying of fruit extract: Effect of additives on physicochemical properties. Crit. Rev. Food Sci. Nutr. 2014, 54, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef]
- Lejeune, B.; Pouget, M.-P.; Pourrat, A. Le rouge de betterave. Essais de stabilisation et utilisation dans la formulation des gels. Probl. Technol. 1983, 31, 638–643. [Google Scholar]
- Díaz, F.; Santos, E.; Kerstupp, S.; Villagómez, R.; Scheinvar, L. Colorant extraction from red prickly pear (Opuntia lasiacantha) for food application. EJEAF Che. Electron. J. Environ. Agric. Food Chem. 2006, 5, 1330–1337. [Google Scholar]
- Gandía-Herrero, F.; Cabanes, J.; Escribano, J.; García-Carmona, F.; Jiménez-Atiénzar, M. Encapsulation of the Most Potent Antioxidant Betalains in Edible Matrixes as Powders of Different Colors. J. Agric. Food Chem. 2013, 61, 4294–4302. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Jiménez-Atiénzar, M.; Cabanes, J.; García-Carmona, F.; Escribano, J. Stabilization of the bioactive pigment of Opuntia fruits through maltodextrin encapsulation. J. Agric. Food Chem. 2010, 58, 10646–10652. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear Opuntia ficus-indica. Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Granit, R. Betalains—A new class of dietary cationized antioxidants. J. Agric. Food Chem. 2001, 49, 5178–5185. [Google Scholar] [CrossRef]
- Gliszczyńska-Swigło, A.; Szymusiak, H.; Malinowska, P. Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical-scavenging activity. Food Addit. Contam. 2006, 23, 1079–1087. [Google Scholar] [CrossRef]
- Escribano, J.; Pedreño, M.A.; García-Carmona, F.; Muñoz, R. Characterization of the antiradical activity of betalains from Beta vulgaris L. roots. Phytochem. Anal. 1998, 9, 124–127. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. HPLC characterization of betalains from plants in the Amaranthaceae. J. Chromatogr. Sci. 2005, 43, 454–460. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem. Toxicol. 2014, 73, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Frank, T.; Stintzing, F.C.; Carle, R.; Bitsch, I.; Quas, D.; Strab, G.; Bitsch, R.; Netzel, M. Urinary pharmacokinetics of betalains following consumption of red beet juice in healthy humans. Pharmacol. Res. 2005, 52, 290–297. [Google Scholar] [CrossRef]
- Taira, J.; Tsuchida, E.; Katoh, M.C.; Uehara, M.; Ogi, T. Antioxidant capacity of betacyanins as radical scavengers for peroxyl radical and nitric oxide. Food Chem. 2015, 166, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; Tesoriere, L.; Livrea, M.A. Betanin inhibits the myeloperoxidase/nitrite-induced oxidation of human low-density lipoproteins. Free Radic. Res. 2007, 41, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Sakihama, Y.; Maeda, M.; Hashimoto, M.; Tahara, S.; Hashidoko, Y. Beetroot betalain inhibits peroxynitrite-mediated tyrosine nitration and DNA strand cleavage. Free Radic. Res. 2012, 46, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Kumorkiewicz-Jamro, A.; Starzak, K.; Sutor, K.; Nemzer, B.; Pietrzkowski, Z.; Popenda, Ł.; Wybraniec, S. Structural Study on Hypochlorous Acid-Mediated Chlorination of Betanin and Its Decarboxylated Derivatives from an Anti-Inflammatory Beta vulgaris L. Extract. Molecules 2020, 25, 378. [Google Scholar] [CrossRef]
- Allegra, M.; Furtmüller, P.G.; Jantschko, W.; Zederbauer, M.; Tesoriere, L.; Livrea, M.A.; Obinger, C. Mechanism of interaction of betanin and indicaxanthin with human myeloperoxidase and hypochlorous acid. Biochem. Biophys. Res. Commun. 2005, 332, 837–844. [Google Scholar] [CrossRef]
- Wendel, M.; Nizinski, S.; Gierszewski, M.; Prukala, D.; Sikorski, M.; Starzak, K.; Wybraniec, S.; Burdzinski, G. Chemical quenching of singlet oxygen by betanin. Photochem. Photobiol. Sci. 2016, 15, 872–878. [Google Scholar] [CrossRef]
- Tesoriere, L.; Butera, D.; D’Arpa, D.; Di Gaudio, F.; Allegra, M.; Gentile, C.; Livrea, M.A. Increased resistance to oxidation of betalain-enriched human low density lipoproteins. Free Radic. Res. 2003, 37, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.W.; Han, X.X.; Du, G.M.; Wei, C.Y.; Zhu, X.; Ren, W.; Zeng, L.J.; Zhang, Y.T. Antioxidant activities and immunomodulatory effects in mice of betalain in vivo. Food Sci. Chin. 2019, 40, 196–201. [Google Scholar]
- Tesoriere, L.; Allegra, M.; Butera, D.; Livrea, M.A. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: Potential health effects of betalains in humans. Am. J. Clin. Nutr. 2004, 80, 941–945. [Google Scholar] [CrossRef]
- Tesoriere, L.; Butera, D.; Allegra, M.; Fazzari, M.; Livrea, M.A. Distribution of betalain pigments in red blood cells after consumption of cactus pear fruits and increased resistance of the cells to ex vivo induced oxidative hemolysis in humans. J. Agric. Food Chem. 2005, 53, 1266–1270. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Paluszczak, J.A.; Szaefer, H.; Baer-Dubowska, W. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br. J. Nutr. 2013, 110, 2138–2149. [Google Scholar] [CrossRef]
- Zielińska-Przyjemska, M.; Olejnik, A.; Kostrzewa, A.; Łuczak, M.; Jagodziński, P.P.; Baer-Dubowska, W. The beetroot component betanin modulates ROS production, DNA damage and apoptosis in human polymorphonuclear neutrophils. Phytother. Res. 2012, 26, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Czapski, J.; Mikołajczyk, K.; Kaczmarek, M. Relationship between antioxidant capacity of red beet juice and contents of its betalain pigments. Pol. J. Food Nutr. Sci. 2009, 59, 119–122. [Google Scholar]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Khan, M.I. Plant Betalains: Safety, Antioxidant Activity, Clinical Efficacy, and Bioavailability. Compr. Rev. Food Sci. Food Safety 2016, 15, 316–330. [Google Scholar] [CrossRef]
- Allegra, M.; Ianaro, A.; Tersigni, M.; Panza, E.; Tesoriere, L.; Livrea, M.A. Indicaxanthin from cactus pear fruit exerts anti-inflammatory effects in carrageenin-induced rat pleurisy. J. Nutr. 2014, 144, 185–192. [Google Scholar] [CrossRef]
- Sawicki, T.; Topolska, J.; Bączek, N.; Szawara-Nowak, D.; Juśkiewicz, J.; Wiczkowski, W. Characterization of the profile and concentration of betacyanin in the gastric content, blood and urine of rats after an intragastric administration of fermented red beet juice. Food Chem. 2020, 313, 126169. [Google Scholar] [CrossRef] [PubMed]
- Krantz, C.; Monier, M.; Wahlstrom, B. Absorption, excretion, metabolism and cardiovascular effects of beetroot extract in the rat. Food Cosmet. Toxicol. 1980, 18, 363–366. [Google Scholar] [CrossRef]
- Clifford, T.; Constantinou, C.M.; Keane, K.M.; West, D.J.; Howatson, G.; Stevenson, E.J. The plasma bioavailability of nitrate and betanin from Beta vulgaris rubra in humans. Eur. J. Nutr. 2017, 56, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Reynoso, R.C.; Giner, T.V.; de Mejia, E.G. Safety of a filtrate of fermented garambullo fruit: Biotransformation and toxicity studies. Food Chem. Toxicol. 1999, 37, 825–830. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Angileri, F.; Gentile, C.; Livrea, M.A. In vitro digestion of betalainic foods. Stability and bioaccessibility of betaxanthins and betacyanins and antioxidative potential of food digesta. J. Agric. Food Chem. 2008, 56, 10487–10492. [Google Scholar] [CrossRef]
- Cserni, G.; Kocsis, L. The case of the purple colon. Virchows Arch. 2008, 452, 703. [Google Scholar] [CrossRef]
- Roemmelt, A.T.; Franckenberg, S.; Steuer, A.E.; Kraemer, T. Purple discoloration of the colon found during autopsy: Identification of betanin, its aglycone and metabolites by liquid chromatography–high-resolution mass spectrometry. Forens. Sci. Intl. 2014, 240, e1–e6. [Google Scholar] [CrossRef]
- Tesoriere, L.; Gentile, C.; Angileri, F.; Attanzio, A.; Tutone, M.; Allegra, M.; Livrea, M.A. Trans-epithelial transport of the betalain pigments indicaxanthin and betanin across Caco-2 cell monolayers and influence of food matrix. Eur. J. Nutr. 2013, 52, 1077–1087. [Google Scholar] [CrossRef]
- Hayek, S.; Ibrahim, S.A. Antimicrobial activity of xoconostle pears (Opuntia matudae) against Escherichia coli O157:H7 in laboratory medium. Int. J. Microbiol. 2012, 2012, 368472. [Google Scholar] [CrossRef]
- Velićanski, A.S.; Cvetković, D.D.; Markov, S.L.; Vulić, J.J.; Djilas, S.M. Antibacterial activity of Beta vulgaris L. pomace extract. Acta Period. Technol. 2011, 42, 263–269. [Google Scholar] [CrossRef]
- Čanadanović-Brunet, J.M.; Savatović, S.S.; Ćetković, G.S.; Vulić, J.J.; Djilas, S.M.; Markov, S.L.; Cvetković, D.D. Antioxidant and antimicrobial activities of beet root pomace extracts. Czech J. Food Sci. 2011, 29, 575–585. [Google Scholar] [CrossRef]
- Tenore, G.C.; Novellino, E.; Basile, A. Nutraceutical potential and antioxidant benefits of red pitaya (Hylocereus polyrhizus) extracts. J. Funct. Foods 2012, 4, 129–136. [Google Scholar] [CrossRef]
- Kumar, S.; Brooks, M.S.L. Use of red beet (Beta vulgaris L.) for antimicrobial applications-a critical review. Food Bioprocess Technol. 2018, 11, 17–42. [Google Scholar] [CrossRef]
- Chang, Y.J.; Pong, L.Y.; Hassan, S.S.; Choo, W.S. Antiviral activity of betacyanins from red pitahaya (Hylocereus polyrhizus) and red spinach (Amaranthus dubius) against dengue virus type 2 (GenBank accession no. MH488959). Access Microbiol. 2019, 2, acmi000073. [Google Scholar] [CrossRef]
- Hilou, A.; Nacoulma, O.G.; Guiguemde, T.R. In vivo antimalarial activities of extracts from Amaranthus spinosus L. and Boerhaavia erecta L. in mice. J. Ethnopharmacol. 2006, 103, 236–240. [Google Scholar] [CrossRef]
- Gentile, C.; Tesoriere, L.; Allegra, M.; Livrea, M.A.; D’Alessio, P. Antioxidant betalains from cactus pear (Opuntia ficus-indica) inhibit endothelial ICAM-1 expression. Ann. N. Y. Acad. Sci. 2004, 1028, 481–486. [Google Scholar] [CrossRef]
- Khan, M.I.; Sri Harsha, P.S.C.; Giridhar, P.; Ravishankar, G.A. Pigment identification, nutritional composition, bioactivity, and in vitro cancer cell cytotoxicity of Rivina humilis L. berries, potential source of betalains. LWT J. Food Sci. Technol. 2012, 47, 315–323. [Google Scholar] [CrossRef]
- Nowacki, L.; Vigneron, P.; Rotellini, L.; Cazzola, H.; Merlier, F.; Prost, E.; Ralanairina, R.; Gadonna, J.-P.; Rossi, C.; Vayssade, M. Betanin-enriched red beetroot (Beta vulgaris L.) extract induces apoptosis and autophagic cell death in MCF-7 cells. Phytother. Res. 2015, 9, 1964–1973. [Google Scholar] [CrossRef]
- Naselli, F.; Tesoriere, L.; Caradonna, F.; Bellavia, D.; Attanzio, A.; Gentile, C.; Livrea, M.A. Anti-proliferative and pro-apoptotic activity of whole extract and isolated indicaxanthin from Opuntia ficus-indica associated with re-activation of the onco-suppressor p16INK4a gene in human colorectal carcinoma (Caco-2) cells. Biochem. Biophys. Res. Commun. 2014, 450, 652–658. [Google Scholar] [CrossRef]
- Lee, E.J.; An, D.; Nguyen, C.T.T.; Patil, B.S.; Kim, J.; Yoo, K.S. Betalain and betaine composition of greenhouse- or field-produced beetroot (Beta vulgaris L.) and inhibition of HepG2 cell proliferation. J. Agric. Food Chem. 2014, 62, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, D.; Arunasree, M.K.; Roy, K.R.; Chandramohan Reddy, T.; Reddy, G.V.; Reddanna, P. Betanin, a betacyanin pigment purified from fruits of Opuntia ficus-indica induces apoptosis in human chronic myeloid leukemia cell line-K562. Phytomedicine 2007, 14, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, G.J.; Tokuda, H.; Konoshima, T.; Nishino, H. Chemoprevention of lung and skin cancer by Beta vulgaris (beet) root extract. Cancer Lett. 1996, 100, 211–214. [Google Scholar] [CrossRef]
- Zou, D.M.; Brewer, M.; Garcia, F.; Feugang, J.M.; Wang, J.; Zang, R.; Liu, H.; Zou, C. Cactus pear: A natural product in cancer chemoprevention. Nutr. J. 2005, 4, 25. [Google Scholar] [CrossRef]
- Wu, L.-C.; Hsu, H.-W.; Chen, Y.-C.; Chiu, C.-C.; Lin, Y.-I.; Ho, J.-A.A. Antioxidant and antiproliferative activities of red pitaya. Food Chem. 2006, 95, 319–327. [Google Scholar] [CrossRef]
- Reddy, M.K.; Alexander-Lindo, R.L.; Nair, M.G. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J. Agric. Food Chem. 2005, 53, 9268–9273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Pan, J.; Wang, Y.; Lubet, R.; You, M. Beetroot red (betanin) inhibits vinyl carbamate- and benzo(a)pyrene-induced lung tumorigenesis through apoptosis. Mol. Carcinog. 2013, 52, 686–691. [Google Scholar] [CrossRef]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M.A. Phytochemical indicaxanthin suppresses 7-ketocholesterol-induced THP-1 cell apoptosis by preventing cytosolic Ca2+ increase and oxidative stress. Br. J. Nutr. 2013, 110, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, G.J.; Azuine, M.A.; Sridhar, R.; Okuda, Y.; Tsuruta, A.; Ichiishi, E.; Mukainake, T.; Takasaki, M.; Konoshima, T.; Nishino, H.; et al. Chemoprevention of DMBA-induced UV-B promoted, NOR-1-induced TPA promoted skin carcinogenesis, and DEN-induced phenobarbital promoted liver tumors in mice by extract of beetroot. Pharmacol. Res. 2003, 47, 141–148. [Google Scholar] [CrossRef]
- Lechner, J.F.; Wang, L.S.; Rocha, C.M.; Larue, B.; Henry, C.; McIntyre, C.M.; Riedl, K.M.; Schwartz, S.J.; Stoner, G.D. Drinking water with red beetroot food color antagonizes esophageal carcinogenesis in N-nitrosomethylbenzylamine-treated rats. J. Med. Food. 2010, 13, 733–739. [Google Scholar] [CrossRef]
- Henarejos-Escudero, P.; Hernández-García, S.; Guerrero-Rubio, M.A.; García-Carmona, F.; Gandía-Herrero, F. Antitumoral Drug Potential of Tryptophan-Betaxanthin and Related Plant Betalains in the Caenorhabditis elegans Tumoral Model. Antioxidants 2020, 9, 646. [Google Scholar] [CrossRef] [PubMed]
- Wróblewska, M.; Juskiewicz, J.; Wiczkowski, W. Physiological properties of beetroot crisps applied in standard and dyslipidaemic diets of rats. Lipids Health Dis. 2011, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Sani, H.A.; Baharom, A.; Ahmad, M.A.; Ismail, I.I. Effectiveness of Hylocereus polyrhizus extract in decreasing serum lipids and liver MDA-TBAR level in hypercholesterolemic rats. Sains Malays. 2009, 38, 271–279. [Google Scholar]
- Clemente, A.C.; Desai, P.V. Evaluation of the haematological, hypoglycemic, hypolipidemic and antioxidant properties of Amaranthus tricolor leaf extract in rat. Trop. J. Pharm. Res. 2011, 10, 595–602. [Google Scholar]
- Pietrzkowski, Z.; Thresher, W.C. Solid Betalains Composition and Methods. US Patent US 2010/0076050A1, 2010. [Google Scholar]
- Budinsky, A.; Wolfram, R.; Oguogho, A.; Efthimiou, Y.; Stamatopoulos, Y.; Sinzinger, H. Regular ingestion of Opuntia robusta lowers oxidation injury. Prostagland. Leukotr. Essent. Fatty Acids 2001, 65, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Przyjemska, M.; Olejnik, A.; Dobrowolska-Zachwieja, A.; Grajek, W. In vitro effects of beetroot juice and chips on oxidative metabolism and apoptosis in neutrophils from obese individuals. Phytother. Res. 2009, 23, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Holy, B.; Nnamdi Isaac, N.; Ojoye Ngoye, B. Post-prandial effect of beetroot (Beta vulgaris) juice on glucose and lipids levels of apparently healthy subjects. Eur. J. Pharm. Med. Res. 2017, 4, 60–62. [Google Scholar]
- De Castro, A.P.R.B.; da Cunha, D.T.; Antunes, A.E.C.; Corona, L.P.; Bezerra, R.M.N. Effect of Freeze-Dried Red Beet (Beta vulgaris L.) Leaf Supplementation on Biochemical and Anthropometrical Parameters in Overweight and Obese Individuals: A Pilot Study. Plant Foods Hum Nutr. 2019, 74, 232–234. [Google Scholar] [CrossRef]
- Rahimi, P.; Mesbah-Namin, S.A.; Ostadrahimi, A.; Separham, A.; Asghari Jafarabadi, M. Betalain- and betacyanins-rich supplements′ impacts on the PBMC SIRT 1 and LOX1 genes expression and Sirtuin-1 protein levels in coronary artery disease patients: A pilot crossover clinical trial. J. Funct. Foods 2019, 60, 103401. [Google Scholar] [CrossRef]
- Yahaghi, L.; Yaghmaei, P.; Hayati-Roodbari, N.; Irani, S.; Ebrahim-Habibi, A. Betanin effect on PPAR-α and SREBP-1c expression in NMRI mice model of steatohepatitis with fibrosis. Physiol. Int. 2020, 107, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Ignatowicz, E.; Murias, M.; Ewertowska, M.; Mikołajczyk, K.; Jodynis-Liebert, J. Protective effect of red beetroot against carbon tetrachloride- and N-nitrosodiethylamine-induced oxidative stress in rats. J. Agric. Food Chem. 2009, 57, 2570–2575. [Google Scholar] [CrossRef] [PubMed]
- Szaefer, H.; Krajka-Kuźniak, V.; Ignatowicz, E.; Adamska, T.; Baer-Dubowska, W. Evaluation of the effect of beetroot juice on DMBA-induced damage in liver and mammary gland of female Sprague-Dawley rats. Phytother. Res. 2014, 28, 55–61. [Google Scholar] [CrossRef]
- Galati, E.M.; Mondello, M.R.; Lauriano, E.R.; Taviano, M.F.; Galluzzo, M.; Miceli, N. Opuntia ficus indica (L.) Mill. fruit juice protects liver from carbon tetrachloride induced injury. Phytother. Res. 2005, 19, 796–800. [Google Scholar] [CrossRef]
- Zeashan, H.; Amresh, G.; Singh, S.; Rao, C.V. Hepatoprotective activity of Amaranthus spinosus in experimental animals. Food Chem. Toxicol. 2008, 46, 3417–3421. [Google Scholar] [CrossRef] [PubMed]
- Wettasinghe, M.; Bolling, B.; Plhak, L.; Xiao, H.; Parkin, K. Phase II enzyme-inducing and antioxidant activities of beetroot (Beta vulgaris L.) extracts from phenotypes of different pigmentation. J. Agric. Food Chem. 2002, 50, 6704–6709. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Wettasinghe, M.; Bolling, B.W.; Ji, L.-L.; Parkin, K.L. Betalains, phase II enzyme-inducing components from red beetroot (I L.) extracts. Nutr. Cancer 2005, 53, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, Z.; Yang, S.; Wang, J.; Yang, X.; Tan, D. Betanin attenuates paraquat-induced liver toxicity through a mitochondrial pathway. Food Chem. Toxicol. 2014, 70, 100–106. [Google Scholar] [CrossRef]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological effects of red beetroot and betalains: A review. Phytother. Res. 2020, 34, 1847. [Google Scholar] [CrossRef]
- Hadipour, E.; Fereidoni, M.; Tayarani-Najaran, Z. Betanin Attenuates Oxidative Stress Induced by 6-OHDA in PC12 Cells via SAPK/JNK and PI3 K Pathways. Neurochem. Res. 2020, 45, 395–403. [Google Scholar] [CrossRef]
- Nade, V.S.; Kawale, L.A.; Zambre, S.S.; Kapure, A.B. Neuroprotective potential of Beta vulgaris L. in Parkinson’s disease. Indian J. Pharmacol. 2015, 47, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Madadi, E.; Mazloum-Ravasan, S.; Yu, J.S.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Therapeutic Application of Betalains: A Review. Plants 2020, 9, 1219. [Google Scholar] [CrossRef]
- Wang, C.Q.; Yang, G.Q. Betacyanins from Portulaca oleracea L. ameliorate cognition deficits and attenuate oxidative damage induced by D-galactose in the brains of senescent mice. Phytomedicine 2010, 17, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Sacan, O.; Yanardag, R. Antioxidant and antiacetylcholinesterase activities of chard (Beta vulgaris L. var. cicla). Food Chem. Toxicol. 2010, 48, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Thong-Asa, W.; Prasartsri, S.; Klomkleaw, N.; Thongwan, N. The neuroprotective effect of betanin in trimethyltin-induced neurodegeneration in mice. Metab. Brain Dis. 2020, 35, 1395–1405. [Google Scholar] [CrossRef]
- Motawi, T.K.; Ahmed, S.A.; El-Boghdady, N.A.; Metwally, N.S.; Nasr, N.N. Protective effects of betanin against paracetamol and diclofenac induced neurotoxicity and endocrine disruption in rats. Biomarkers 2019, 24, 645–651. [Google Scholar] [CrossRef]
- Jajja, A.; Sutyarjoko, A.; Lara, J.; Rennie, K.; Brandt, K.; Qadir, O.; Siervo, M. Beetroot supplementation lowers daily systolic blood pressure in older, overweight subjects. Nutr. Res. 2014, 34, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Hathan, B.S. Chemical composition, functional properties and processing of Beetroot-a review. Int. J. Sci. Eng. Res. 2014, 5, 679–684. [Google Scholar]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef]
- Frati Munari, A.C.; Gordillo, B.E.; Altamirano, P.; Ariza, C.R. Hypoglycemic effect of Opuntia streptacantha Lemaire in NIDDM. Diabetes Care 1988, 11, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Wootton-Beard, P.C.; Brandt, K.; Fell, D.; Warner, S.; Ryan, L. Effects of a beetroot juice with high neobetanin content on the early-phase insulin response in healthy volunteers. J. Nutr. Sci. 2014, 3, e9. [Google Scholar] [CrossRef] [PubMed]
- Olumese, F.E.; Oboh, H.A. Effects of daily intake of beetroot juice on blood glucose and hormones in young healthy subjects. Nigerian Quart. J. Hosp. Med. 2016, 26, 455–462. [Google Scholar]
- Lugo-Radillo, A.; Delgado-Enciso, I.; Peña-Beltrán, E. Betanidin significantly reduces blood glucose levels in BALB/c mice fed with an atherogenic diet. Nat. Prod. Bioprospect. 2012, 2, 154–155. [Google Scholar] [CrossRef][Green Version]
- Han, J.; Tan, C.; Wang, Y.; Yang, S.; Tan, D. Betanin reduces the accumulation and cross-links of collagen in high-fructose-fed rat heart through inhibiting non-enzymatic glycation. Chem. Biol. Interact. 2015, 227, 37–44. [Google Scholar] [CrossRef]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef] [PubMed]
- Sutariya, B.; Saraf, M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J. Ethnopharmacol. 2017, 198, 432–443. [Google Scholar] [CrossRef]
- Abd El-Ghffar, E.A.; Hegazi, N.M.; Saad, H.H.; Soliman, M.M.; El-Raey, M.A.; Shehata, S.M.; Barakat, A.; Yasri, A.; Sobeh, M. HPLC-ESI- MS/MS analysis of beet (Beta vulgaris) leaves and its beneficial properties in type 1 diabetic rats. Biomed. Pharmacother. 2019, 120, 109541. [Google Scholar] [CrossRef]
- Bolkent, S.; Yanardağ, R.; Tabakoğlu-Oğuz, A.; Ozsoy-Saçan, O. Effects of chard (Beta vulgaris L. var. cicla) extract on pancreatic B cells in streptozotocin-diabetic rats: A morphological and biochemical study. J. Ethnopharmacol. 2000, 73, 251–259. [Google Scholar] [CrossRef]
- Winkler, C.; Wirleitner, B.; Schroecksnadel, K.; Schennach, H.; Fuchs, D. In vitro effects of beet root juice on stimulated and unstimulated peripheral blood mononuclear cells. Am. J. Biochem. Biotech. 2005, 1, 180–185. [Google Scholar] [CrossRef]
- Vidal, P.J.; López-Nicolás, J.M.; Gandía-Herrero, F.; García-Carmona, F. Inactivation of lipoxygenase and cyclooxygenase by natural betalains and semi-synthetic analogues. Food Chem. 2014, 154, 246–254. [Google Scholar] [CrossRef]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M.A. Indicaxanthin inhibits NADPH oxidase (NOX)-1 activation and NF-κB-dependent release of inflammatory mediators and prevents the increase of epithelial permeability in IL-1β-exposed Caco-2 cells. Br. J. Nutr. 2014, 111, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Allegra, M.; D’Acquisto, F.; Tesoriere, L.; Attanzio, A.; Livrea, M.A. Pro-oxidant activity of indicaxanthin from Opuntia ficus-indica modulates arachidonate metabolism and prostaglandin synthesis through lipid peroxide production in LPS-stimulated (RAW) 264.7 macrophages. Redox Biol. 2014, 2, 892–900. [Google Scholar] [CrossRef][Green Version]
- Ahmadi, H.; Nayeri, Z.; Minuchehr, Z.; Sabouni, F.; Mohammadi, M. Betanin purification from red beeetroots and evaluation of its anti-oxidant and anti-inflammatory activity on LPS-activated microglial cells. PLoS ONE 2020, 15, e0233088. [Google Scholar] [CrossRef]
- Han, J.; Ma, D.; Zhang, M.; Yang, X.; Tan, D. Natural antioxidant betanin protects rats from paraquat-induced acute lung injury interstitial pneumonia. Biomed. Res. Int. 2015, 2015, 608174. [Google Scholar] [CrossRef]
- Raish, M.; Ahmad, A.; Ansari, M.A.; Alkharfy, K.M.; Ahad, A.; Khan, A.; Ali, N.; Ganaie, M.A.; Hamidaddin, M.A.A. Beetroot juice alleviates isoproterenol-induced myocardial damage by reducing oxidative stress, inflammation, and apoptosis in rats. 3 Biotech. 2019, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Longhi-Balbinot, D.T.; Zarpelon, A.C.; Staurengo-Ferrari, L.; Baracat, M.M.; Georgetti, S.R.; Sassonia, R.C.; Verri, W.A., Jr.; Casagrande, R. Anti-inflammatory activity of betalain-rich dye of Beta vulgaris: Effect on edema, leukocyte recruitment, superoxide anion and cytokine production. Arch. Pharm. Res. 2015, 38, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.V.T.D.; Baião, D.D.S.; Ferreira, V.F.; Paschoalin, V.M.F. Betanin as a multipath oxidative stress and inflammation modulator: A beetroot pigment with protective effects on cardiovascular disease pathogenesis. Crit. Rev. Food Sci. Nutr. 2020, 30, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pietrzkowski, Z.; Argumedo, R.; Shu, C.; Nemzer, B.; Wybraniec, S.; Reyes-Izquierdo, T. Betalain-rich redbeet concentrate improves reduced knee discomfort and joint function: A double blind, placebo-controlled pilot clinical study. Nutr. Diet. Suppl. 2014, 6, 9–13. [Google Scholar]
- Pietrzkowski, Z.; Nemzer, B.; Spórna, A.; Stalica, P.; Tresher, W.; Keller, R.; Jimenez, R.; Michalowski, T.; Wybraniec, S. Influence of betalain-rich extracts on reduction of discomfort associated with osteoarthritis. New Med. 2010, 1, 12–17. [Google Scholar]
- Pietrzkowski, Z. Betalains Composition and Uses Thereof. WO Patent 2010/014839 A1, 2010. [Google Scholar]
- Baldassano, S.; Rotondo, A.; Serio, R.; Livrea, M.A.; Tesoriere, L.; Mulè, F. Inhibitory effects of indicaxanthin on mouse ileal contractility: Analysis of the mechanism of action. Eur. J. Pharmacol. 2011, 658, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Baldassano, S.; Tesoriere, L.; Rotondo, A.; Serio, R.; Livrea, M.A.; Mulè, F. Inhibition of the mechanical activity of mouse ileum by cactus pear (Opuntia ficus indica L. Mill.) fruit extract and its pigment indicaxanthin. J. Agric. Food Chem. 2010, 58, 7565–7571. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Mondello, M.R.; Giuffrida, D.; Dugo, G.; Miceli, N.; Pergolizzi, S.; Taviano, M.F. Chemical characterization and biological effects of Sicilian Opuntia ficus-indica (L.) fruit juice: Antioxidant and antiulcerogenic activity. J. Agric. Food Chem. 2003, 51, 4903–4908. [Google Scholar] [CrossRef] [PubMed]
- Alimi, H.; Hfaiedh, N.; Bouoni, Z.; Sakly, M.; Ben Rhouma, K. Evaluation of antioxidant and antiulcerogenic activities of Opuntia ficus indica f. inermis flowers extract in rats. Environ. Toxicol. Pharmacol. 2011, 32, 406–416. [Google Scholar] [CrossRef]
- Bisson, J.F.; Daubié, S.; Hidalgo, S.; Guillemet, D.; Linares, E. Diuretic and antioxidant effects of Cacti-NeaR®, a dehydrated water extract from prickly pear fruit, in rats. Phytother. Res. 2010, 24, 587–594. [Google Scholar] [CrossRef]
- Martinez, R.M.; Hohmann, M.S.; Longhi-Balbinot, D.T.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Vicentini, F.T.M.C.; Sassonia, R.C.; Verri, W.A.J.; Casagrande, R. Analgesic activity and mechanism of action of a Beta vulgaris dye enriched in betalains in inflammatory models in mice. Inflammopharmacology 2020, 28, 1663–1675. [Google Scholar] [CrossRef]
- Montenegro, C.F.; Kwong, D.A.; Minow, Z.A.; Davis, B.A.; Lozada, C.F.; Casazza, G.A. Betalain-rich concentrate supplementation improves exercise performance and recovery in competitive triathletes. Appl. Physiol. Nutr. Metab. 2017, 42, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Y.; Zhang, Z. Radioprotective activity of betalains from red beets in mice exposed to gamma irradiation. Eur. J. Pharmacol. 2009, 615, 223–227. [Google Scholar] [CrossRef]
- Baron, M.; Davies, S.; Alexander, L.; Snellgrove, D.; Sloman, K.A. The effect of dietary pigments on the coloration and behaviour of flame-red dwarf gourami, Colisa lalia. Anim. Behav. 2008, 75, 1041–1051. [Google Scholar] [CrossRef]
- Watson, W.C.; Luke, R.G.; Inall, J.A. Beeturia: Its incidence and a clue to its mechanism. Brit. Med. J. 1963, 5363, 971–973. [Google Scholar] [CrossRef]
- Sotos, J.G. Beeturia and iron absorption. Lancet 1999, 354, 1032. [Google Scholar] [CrossRef]
- Mitchell, S.C. Food idiosyncrasies: Beetroot and asparagus. Drug Metab. Dispos. 2001, 29, 539–543. [Google Scholar]
- Watts, A.R.; Lennard, M.S.; Mason, S.L.; Tucker, G.T.; Woods, H.F. Beeturia and the biological fate of beetroot pigments. Pharmacogenetics 1993, 3, 302–311. [Google Scholar] [CrossRef]
- Eastwood, M.A.; Nyhlin, H. Beeturia and colonic oxalic acid. QJM Intl. J. Med. 1995, 88, 711–717. [Google Scholar]
- Dias, S.; Castanheira, E.M.S.; Fortes, A.G.; Pereira, D.M.; Gonçalves, M.S.T. Natural Pigments of Anthocyanin and Betalain for Coloring Soy-Based Yogurt Alternative. Foods 2020, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Comparative effect of red yeast rice (Monascus purpureus), red beet root (Beta vulgaris) and betanin (E-162) on colour and consumer acceptability of fresh pork sausages packaged in a modified atmosphere. J. Sci. Food Agric. 2006, 86, 500–508. [Google Scholar] [CrossRef]
- Prudencio, I.D.; Prudencio, E.S.; Gris, E.F.; Tomazi, T.; Bordignon-Luiz, M.T. Petit suisse manufactured with cheese whey retentate and application of betalains and anthocyanins. LWT J. Food Sci. Technol. 2008, 41, 905–910. [Google Scholar] [CrossRef]
- Junqueira-Goncalves, M.P.; Cardoso, L.P.; Pinto, M.S.; Pereira, R.M.; Soares, N.F.; Miltz, J. Irradiated beetroot extract as a colorant for cream cheese. Radiat. Phys. Chem. 2011, 80, 114–118. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Simón-Carrillo, A.; Escribano, J.; García-Carmona, F. Determination of beet root betanin in dairy products by high-performance liquid chromatography (HPLC). J. Chem. Educ. 2012, 89, 660–664. [Google Scholar] [CrossRef]
- Hendry, G.A.F.; Houghton, J.D. (Eds.) Natural Food Colours, 2nd ed.; Blackie: London, UK, 1996. [Google Scholar]
- Cerezal, M.P.; Pino, A.J.; Salabarria, Y. Red beet (Beta vulgaris L.) colourant stability in the form of a concentrated liquor. Technol. Aliment. 1994, 29, 7–16. [Google Scholar]
- Cerezal, M.P.; Nuñez, L.D. Characterización del colourante de remolacha roja (Beta vulgaris L.) em polvo. Alimentaria 1996, 1, 91–94. [Google Scholar]
- Manchali, S.; Murthy, K.N.C.; Nagaraju, S.; Neelwarne, B. Stability of betalain pigments of red beet. In Red Beet Biotechnology; Neelwarne, B., Ed.; Springer: Boston, MA, USA, 2013; pp. 55–74. [Google Scholar]
- Gómez, M.; Lorenzo, J.M. Effect of packaging conditions on shelf-life of foal fresh meat. Meat Sci. 2012, 91, 513–552. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Ito, N.; Fukushima, S.; Tsuda, H.; Shirai, T.; Tatematsu, M.; Imaida, K. Modification of chemical carcinogenesis by antioxidants. Princess Takamatsu Symp. 1983, 14, 381–389. [Google Scholar]
- Williams, G.M.; Wang, C.X.; Iatropoulos, M.J. Toxicity studies of butylated hydroxanisole and butylated hydroxytoluene. II. Chronic feeding studies. Food Chem. Toxicol. 1990, 28, 799–806. [Google Scholar] [CrossRef]
- Muíño, I.; Fuente, J.; Pérez, C.; Apeleo, E.; Pérez-Santaescolástica, C.; Cañeque, V.; Lauzurica, S.; Bermejo-Poza, R.; Díaz, A.M.T. Use of red wine polyphenols as a natural preservative in health-promoting omega-3 fatty acids-enriched lamb patties. Molecules 2018, 23, 3080. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Edwards, C.G.; Fellman, J.K.; Mattinson, D.S.; Navazio, J. Biosynthetic origin of geosmin in red beets (Beta vulgaris L.). J. Agric. Food Chem. 2003, 51, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, Y.; Nerd, A.; Nobel, P.S. Cacti as crops. Horticult. Rev. 1997, 18, 291–320. [Google Scholar]
- Stintzing, F.C.; Carle, R. Cactus fruits—More than colour. Fruit Process. 2006, 16, 166–171. [Google Scholar]
- Roriz, C.L.; Heleno, S.A.; Carocho, M.; Rokrantzdrigues, P.; Pinela, J.; Dias, M.I.; Fernandes, I.P.; Barreiro, M.F.; Morales, P.; Barros, L.; et al. Betacyanins from Gomphrena globosa L. flowers: Incorporation in cookies as natural colouring agents. Food Chem. 2020, 329, 127178. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur. Food Res. Technol. 2003, 216, 303–311. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; García-Carmona, F.; Escribano, J. Floral fluorescence effect. Nature 2005, 437, 334. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.; Čanadanović-Brunet, J.; Ćetković, G.; Tumbas, V.; Djilas, S.; Četojević-Simin, D.; Čanadanović, V. Antioxidant and cell growth activities of beet root pomace extracts. J. Funct. Food 2012, 4, 670–678. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Čanadanović, V.M.; Djilas, S.M.; Šaponjac, V.T.T. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J. Funct. Food 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Pourrat, A.; Lejeune, B.; Grand, A.; Bastide, P.; Bastide, J. Propriétés du jus et des colorants de la betterave rouge. Méd. Nutr. 1987, 23, 166–172. [Google Scholar]
- Schwartz, S.J.; von Elbe, J.H.; Pariza, M.W.; Goldsworthy, T.; Pitot, H.C. Inability of red beet betalain pigments to initiate or promote hepatocarcinogenesis. Food Chem. Toxicol. 1983, 21, 531–535. [Google Scholar] [CrossRef]
- Haveland-Smith, R.B. Evaluation of the genotoxicity of some natural food colours using bacterial assays. Mutat. Res. 1981, 91, 285–290. [Google Scholar] [CrossRef]
- Von Elbe, J.H.; Schwartz, S.J. Absence of mutagenic activity and a short-term toxicity study of beet pigments as food colorants. Arch. Toxicol. 1981, 49, 93–98. [Google Scholar] [CrossRef]
- Zampini, I.C.; Ordoñez, R.; Giannini, N.P.; Blendinger, P.G.; Isla, M.I. Nutraceutical properties and toxicity studies of fruits from four Cactaceae species grown in Argentine Northwestern. Food Res. Intl. 2011, 44, 2345–2351. [Google Scholar] [CrossRef]
- Hor, S.Y.; Ahmad, M.; Farsi, E.; Yam, M.F.; Hashim, M.A.; Lim, C.P.; Sadikun, A.; Asmawi, M.Z. Safety assessment of methanol extract of red dragon fruit (Hylocereus polyrhizus): Acute and subchronic toxicity studies. Regul. Toxicol. Pharmacol. 2012, 63, 106–114. [Google Scholar] [CrossRef]
- Forni, E.; Trifilo, A.; Polesello, A. Researches on the utilisation of the pigment from Phytolacca decandra L. as a food colorant: Part 1—preparation of an extract free from toxic substances. Food Chem. 1983, 10, 35–46. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Betalains—Emerging prospects for food scientists. Trends Food Sci. Technol. 2007, 18, 514–525. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Zhang, X.; Liu, J. Development of active and intelligent packaging by incorporating betalains from red pitaya (Hylocereus polyrhizus) peel. Food Hydrocoll. 2020, 100, 105410. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Cejudo-Bastante, C.; Cran, M.J.; Heredia, F.J.; Bigger, S.W. Optical, structural, mechanical and thermal characterization of antioxidant ethylene vinyl alcohol copolymer films containing betalain-rich beetroot. Food Pack. Shelf Life 2020, 24, 100502. [Google Scholar] [CrossRef]
- Sivakumar, V.; Anna, J.L.; Vijayeeswarri, J.; Swaminathan, G. Ultrasound assisted enhancement in natural dye extraction from beetroot for industrial applications and natural dyeing of leather. Ultrason. Sonochem. 2009, 116, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lanier, S.M.; Downing, J.A.; Avent, J.L.; Lumc, J.; McHalea, J.L. Betalain pigments for dye-sensitized solar cells. J. Photochem. Photobiol. Chem. 2008, 195, 72–80. [Google Scholar] [CrossRef]
- Güzel, E.; Arslan, B.S.; Durmaz, V.; Cesur, M.; Tutar, Ö.F.; Sarı, T.; İşleyen, M.; Nebioğlu, M.; Şişman, I. Photovoltaic performance and photostability of anthocyanins, isoquinoline alkaloids and betalains as natural sensitizers for DSSCs. Solar Energy 2018, 173, 34–41. [Google Scholar] [CrossRef]
- García-Salinas, M.J.; Ariza, M.J. Optimizing a Simple Natural Dye Production Method for Dye-Sensitized Solar Cells: Examples for Betalain (Bougainvillea and Beetroot Extracts) and Anthocyanin Dyes. Appl. Sci. 2019, 9, 2515. [Google Scholar] [CrossRef]
- Calogero, G.; Di Marco, G.; Caramori, S.; Cazzanti, S.; Argazzic, R.; Bignozzi, C.A. Natural dye senstizers for photoelectrochemical cells. Energy Environ. Sci. 2009, 2, 1162–1172. [Google Scholar] [CrossRef]
- Calogero, G.; Di Marco, G.; Cazzanti, S.; Caramori, S.; Argazzi, R.; Di Carlo, A.; Bignozzi, C.A. Efficient dye-sensitized solar cells using red turnip and purple wild Sicilian prickly pear fruits. Int. J. Mol. Sci. 2010, 11, 254–267. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, L.; Gao, Y.; Ma, T. Dye-sensitized solar cells using 20 natural dyes as sensitizers. J. Photochem. Photobiol. Chem. 2011, 219, 188–194. [Google Scholar] [CrossRef]
- Calogero, G.; Yumb, J.-H.; Sinopoli, A.; Di Marco, G.; Gr€atzel, M.; Nazeeruddin, M.K. Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Sol. Energy 2012, 86, 1563–1575. [Google Scholar] [CrossRef]
- Sandquist, C.; McHale, J.L. Improved efficiency of betanin-based dye-sensitized solar cells. J. Photochem. Photobiol. Chem. 2011, 221, 90–97. [Google Scholar] [CrossRef]
- Wang, C.Q.; Chen, M.; Wang, B.S. Betacyanin accumulationin the leaves of C3 halophyte Suaeda salsa L. is induced by watering roots with H2O2. Plant Sci. 2007, 172, 1–7. [Google Scholar] [CrossRef]
- Ibdah, M.; Krins, A.; Seidlitz, H.K.; Heller, W.; Strack, D.; Vogt, T. Spectral dependence of flavonol and betacyanin accumulation in Mesembryanthemum crystallinum under enhanced ultraviolet radiation. Plant Cell Environ. 2002, 25, 1145–1154. [Google Scholar] [CrossRef]
- Nakashima, T.; Arakim, T.; Ueno, O. Photoprotective function of betacyanin in leaves of Amaranthus cruentus L. under water stress. Photosynthetica 2011, 49, 497–506. [Google Scholar] [CrossRef]
- Hayakawa, K.; Agarie, S. Physiological Roles of Betacyanin in a Halophyte, Suaeda japonica Makino. Plant Prod. Sci. 2010, 13, 351–359. [Google Scholar] [CrossRef]
- Jain, G.; Gould, K.S. Functional significance of betalain biosynthesis in leaves of Disphyma australe under salinity stress. Environ. Exp. Bot. 2015, 109, 131–140. [Google Scholar] [CrossRef]
- Marchesini, V.A.; Yin, C.; Colmer, T.D.; Veneklaas, E.J. Drought tolerances of three stem-succulent halophyte species of an inland semiarid salt lake system. Funct. Plant Biol. 2014, 41, 1230–1238. [Google Scholar] [CrossRef]
- Li, G.; Meng, X.; Zhu, M.; Li, Z. Research Progress of Betalain in Response to Adverse Stresses and Evolutionary Relationship Compared with Anthocyanin. Molecules 2019, 24, 3078. [Google Scholar] [CrossRef]
- Sepúlveda-Jiménez, G.; Rueda-Benítez, P.; Porta, H.; Rocha-Sosa, M. Betacyanin synthesis in red beet (Beta vulgaris) leaves induced by wounding and bacterial infiltration is preceded by an oxidative burst. Physiol. Mol. Plant Pathol. 2004, 64, 125–133. [Google Scholar] [CrossRef]
- Kragh, K.M.; Nielsen, J.E.; Nielsen, K.K.; Dreboldt, S.; Mikkelsen, J.D. Characterization and localization of new antifungal cysteine-rich proteins from Beta vulgaris. Mol. Plant Microbe Inter. 1995, 8, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Gould, K.S. Are betalain pigments the functional homologues of anthocyanins in plants? Environ. Exp. Bot. 2015, 119, 48–53. [Google Scholar] [CrossRef]
- El-Wakeel, M.A.; Dawood, M.G.; El-Rokiek, K.G.; El-Awadi, M.E.-S.; El-Din, S.A.S. Use of Beta vulgaris allelopathic properties to control some weeds associated with Lupinus albus plant comparing with two recommended herbicides. AgricEngInt CIGR J. 2019, 21, 216–222. [Google Scholar]
| Factors Enhancing Stability | Factors Decreasing Stabilty |
|---|---|
| Presence of matrix | Absence of matrix |
| High pigment concentration | Low pigment concentration |
| Low water activity | High water activity |
| High extent of glycosylation | Low extent of glycosylation |
| High extent of acyltion | Low extent of acylation |
| 3 < pH < 7 | pH < 3 or pH > 7 |
| Low temperature | High temperature |
| Darkness | UV and light |
| Absence of oxygen | Oxygen |
| Antioxidants | H2O2, other oxidants |
| Metal chelators | Metal cations |
| Degrading enzymes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. https://doi.org/10.3390/molecules26092520
Sadowska-Bartosz I, Bartosz G. Biological Properties and Applications of Betalains. Molecules. 2021; 26(9):2520. https://doi.org/10.3390/molecules26092520
Chicago/Turabian StyleSadowska-Bartosz, Izabela, and Grzegorz Bartosz. 2021. "Biological Properties and Applications of Betalains" Molecules 26, no. 9: 2520. https://doi.org/10.3390/molecules26092520
APA StyleSadowska-Bartosz, I., & Bartosz, G. (2021). Biological Properties and Applications of Betalains. Molecules, 26(9), 2520. https://doi.org/10.3390/molecules26092520