Removal of Cd(II) from Micro-Polluted Water by Magnetic Core-Shell Fe3O4@Prussian Blue
Abstract
1. Introduction
2. Results and Discussion
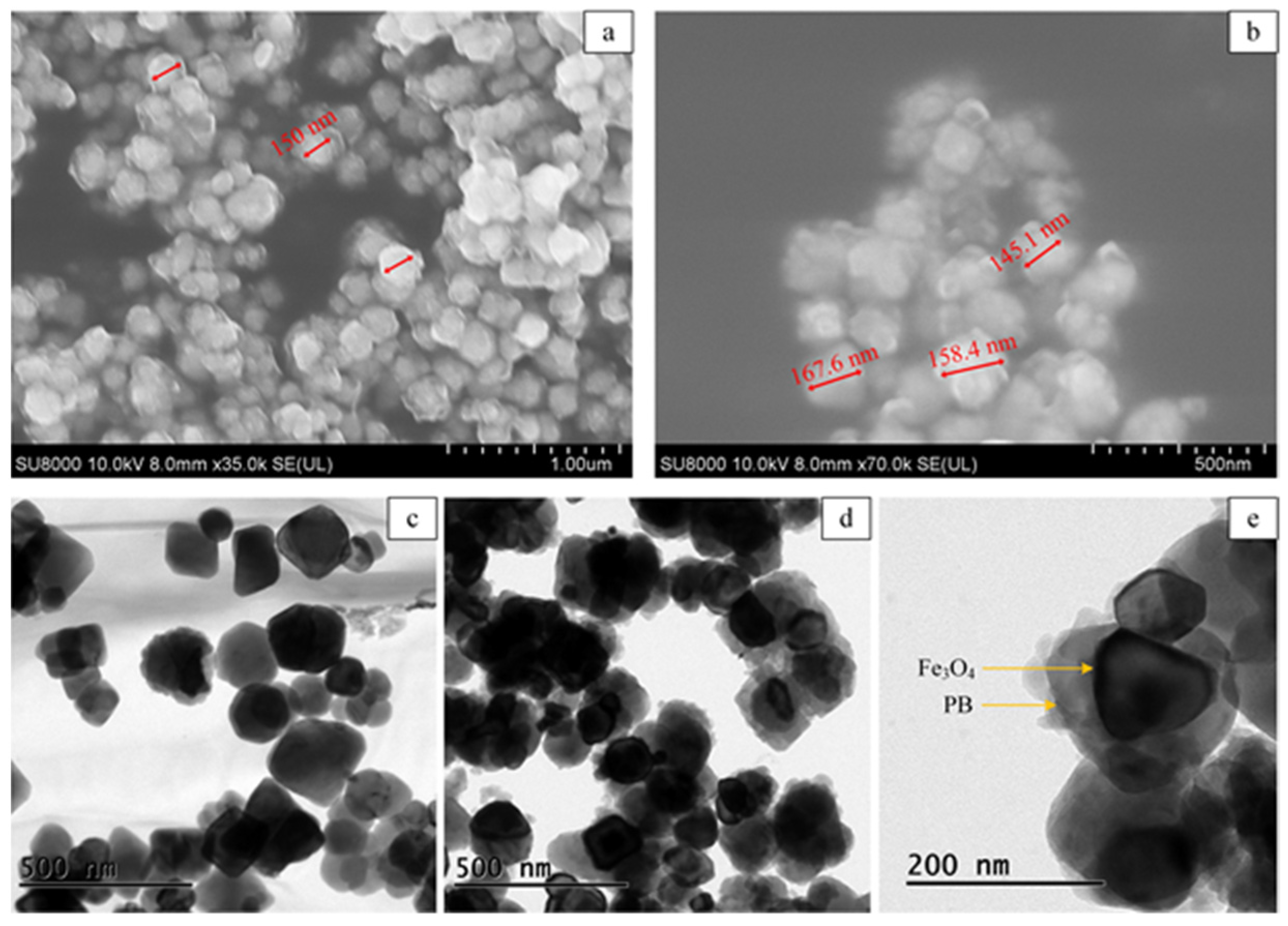
2.1. Characterization of Fe3O4@PB
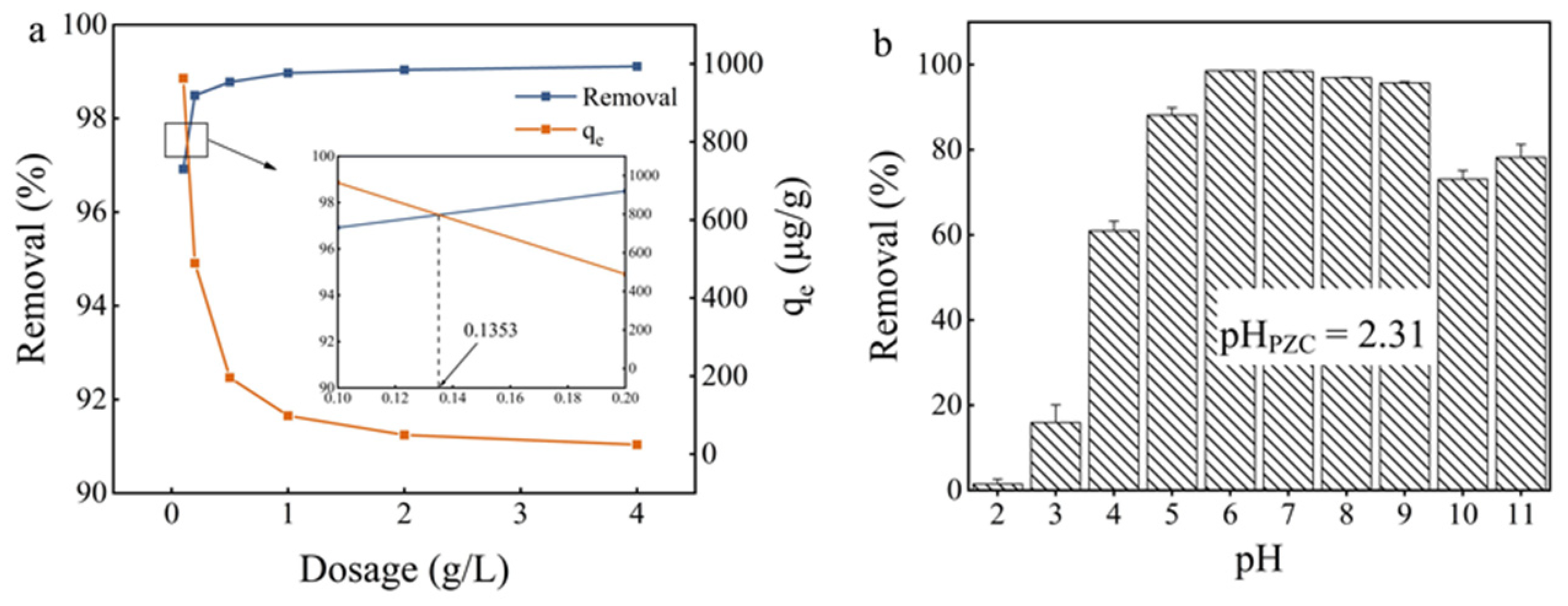
2.2. Effect of Fe3O4@PB Dosage
2.3. Effect of Initial pH
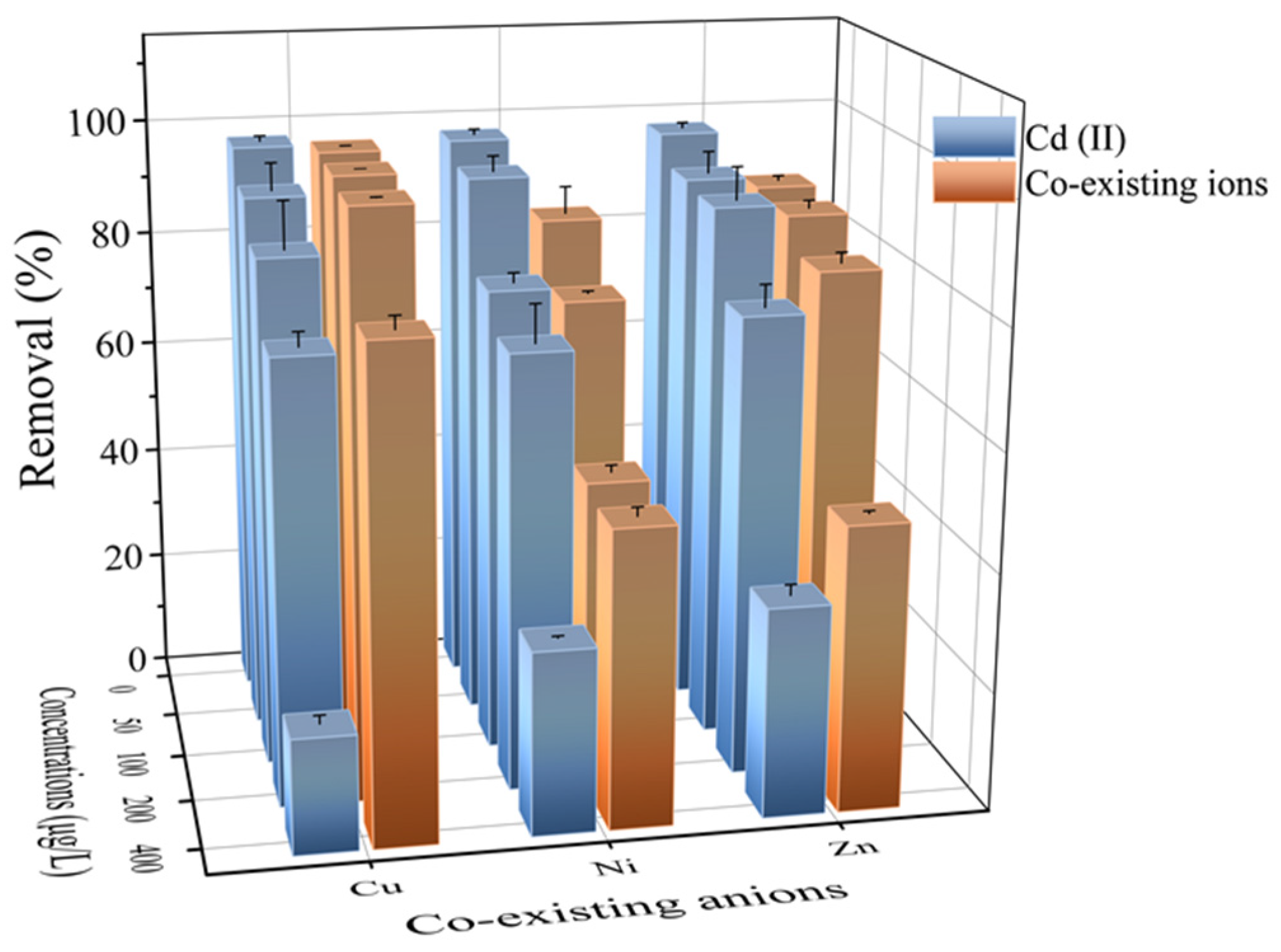
2.4. Effect of Coexisting Ions
2.5. Effect of Contact Time and Adsorption Kinetic
2.6. Effect of Initial Concentration and Adsorption Isotherm
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of Fe3O4@PB Magnetic Adsorbent
3.3. Characterization and Analytical Methods
3.4. Batch Studies and Evaluation of Adsorption Ability
3.5. Analytical Methods
3.5.1. Calculation of Removal Efficiency and Capacity
3.5.2. Adsorption Kinetics Study
3.5.3. Adsorption Isotherms Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Giller, K.E.; Witter, E.; Mcgrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 9, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.S.; Kumar, D.N.V.; Swetha, S.; Ngueagni, P.T.; Karishma, S.; Jeevanantham, S.; Yaashikaa, P.R. Ultrasonic assisted agro waste biomass for rapid removal of Cd (II) ions from aquatic environment: Mechanism and modelling analysis. Chemosphere 2021, 271, 129484. [Google Scholar] [CrossRef]
- Fu, H.; He, H.; Zhu, R.; Ling, L.; Zhang, W.; Chen, Q. Phosphate modified magnetite@ferrihydrite as an magnetic adsorbent for Cd (II) removal from water, soil, and sediment. Sci. Total Environ. 2021, 764, 142846. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Z.Y.; Yang, X.B.; Yang, L.; He, M.; Zhang, H.Y.; Wei, X.; Qin, J.; Li, X.Y.; Lu, G.D.; et al. Relation between cadmium body burden and cognitive function in older men: A cross-sectional study in China. Chemosphere 2020, 250, 126535. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.W.; Zhang, Y.; Quan, X. Health risk assessment of heavy metals and pesticides: A case study in the main drinking water source in Dalian, China. Chemosphere 2020, 242, 125113. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Sharma, H.P. Heavy metal contamination of drinking water in Kamrup district, Assam, India. Environ. Monit. Assess. 2011, 179, 479–486. [Google Scholar] [CrossRef]
- Ahmad, M.K.; Islam, S.; Rahman, S.; Haque, M.R.; Islam, M.M. Heavy metals in water, sediment and some fishes of Buriganga river, Bangladesh. Int. J. Environ. Res. 2010, 4, 321–332. [Google Scholar]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.; Ricchetti, L.; Pittman, C.U.; Anderson, R.; Mohan, D.; Mlsna, T. Lead and cadmium remediation using magnetized and non-magnetized biochar from Douglas fir. Chem. Eng. J. 2018, 331, 480–491. [Google Scholar] [CrossRef]
- European Commission. Directive 98/8/EC concerning the placing of biocidal products on the market. Off. J. Eur. Communities 1998, 23, 3–65. [Google Scholar]
- Kokkinos, E.; Chousein, C.; Simeonidis, K.; Coles, S.; Zouboulis, A.; Mitrakas, M. Improvement of manganese feroxyhyte’s surface charge with exchangeable Ca ions to maximize Cd and Pb uptake from water. Materails 2020, 13, 1762. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.H.; Amin, N.K.; El-Ashtoukhy, E.-S.Z. Removal of heavy metals from aqueous solutions by liquid cation exchanger in a jet loop contactor. Hydrometallurgy 2013, 137, 126–132. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, T.; Tang, J.J.; Zheng, Z.J.; Wang, H.M.; Shao, Q.; Chen, G.L.; Li, Z.X.; Chen, Y.Q.; Zhu, J.W.; et al. Characteristics and mechanisms of cadmium adsorption from aqueous solution using lotus seedpod-derived biochar at two pyrolytic temperatures. Environ. Sci. Pollut. Res. 2018, 25, 11854–11866. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Klapiszewski, Ł.; Moszyński, D.; Bartczak, P.; Szatkowski, T.; Majchrzak, I.; Siwińska-Stefańska, K.; Bazhenov, V.V.; Jesionowski, T. Modification of chitin with kraft lignin and development of new biosorbents for removal of cadmium (II) and nickel (II) ions. Mar. Drugs 2014, 12, 2245–2268. [Google Scholar] [CrossRef]
- Almasian, A.; Giahi, M.; Fard, G.C.; Dehdast, S.A.; Maleknia, L. Removal of heavy metal ions by modified PAN/PANI-nylon core-shell nanofibers membrane: Filtration performance, antifouling and regeneration behavior. Chem. Eng. J. 2018, 351, 1166–1178. [Google Scholar] [CrossRef]
- Sunil, K.; Karunakaran, G.; Yadav, S.; Padaki, M.; Zadorozhnyy, V.; Pai, R.K. Al-Ti2O6 a mixed metal oxide based composite membrane: A unique membrane for removal of heavy metals. Chem. Eng. J. 2018, 348, 678–684. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Z.; Hills, C.; Xue, G.; Tyrer, M. Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide. Water Res. 2009, 43, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.K.; Dahms, H.U.; Won, E.J.; Lee, J.S.; Shin, K.H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, F.M.; Roberts, E.P.; Hill, A.; Campen, A.K.; Brown, N.W. Continuous water treatment by adsorption and electrochemical regeneration. Water Res. 2011, 45, 3065–3074. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Almesfer, M.K.; Danish, M.; Ali, I.H.; Shoukry, H.; Patel, R.; Gardy, J.; Nizami, A.S.; Rehan, M. Potential of Saudi natural clay as an effective adsorbent in heavy metals removal from wastewater. Desalin. Water Treat. 2019, 158, 140–151. [Google Scholar] [CrossRef]
- Chang, J.J.; Zhang, J.; Tang, B.Q.; Wang, Q.; Liu, N.N.; Xue, Q. New insight into the removal of Cd (II) from aqueous solution by diatomite. Environ. Sci. Pollut. Res. 2020, 27, 9882–9890. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, Z.M.; Chen, M.L.; Wang, J.H. Cadmium preconcentration with bean-coat as a green adsorbent with detection by electrothermal atomic absorption spectrometry. J. Anal. Atom. Spectrom. 2011, 26, 1408–1413. [Google Scholar] [CrossRef]
- Liang, J.; Liu, M.; Zhang, Y. Technology, Cd (II) removal on surface-modified activated carbon: Equilibrium, kinetics and mechanism. Water Sci. Technol. 2016, 74, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pei, G.; Qiao, X.; Zhu, Y.; Li, H. Remediation of cadmium contaminated water and soil using vinegar residue biochar. Environ. Sci. Pollut. Res. 2018, 25, 15754–15764. [Google Scholar] [CrossRef]
- Sepehrian, H.; Ahmadi, S.J.; Waqif-Husain, S.; Faghihian, H.; Alighanbari, H. Adsorption studies of heavy metal ions on mesoporous aluminosilicate, novel cation exchanger. J. Hazard. Mater. 2010, 176, 252–256. [Google Scholar] [CrossRef]
- Alyasi, H.; Mackey, H.R.; McKay, G. Removal of cadmium from waters by adsorption using nanochitosan. Energy Environ. 2020, 31, 517–534. [Google Scholar] [CrossRef]
- Zhou, Q.X.; Zhao, X.N.; Xiao, J.P. Preconcentration of nickel and cadmium by TiO2 nanotubes as solid-phase extraction adsorbents coupled with flame atomic absorption spectrometry. Talanta 2009, 77, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ma, Q.; Tan, C.; Lim, T.T.; Huang, L.; Zhang, H. Carbon-based sorbents with three-dimensional architectures for water remediation. Small 2015, 11, 3319–3336. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; He, M.; Chen, B.; Hu, B. Magnetic Zr-MOFs nanocomposites for rapid removal of heavy metal ions and dyes from water. Chemosphere 2018, 199, 435–444. [Google Scholar] [CrossRef]
- Türk, T.; Alp, İ. Arsenic removal from aqueous solutions with Fe-hydrotalcite supported magnetite nanoparticle. J. Ind. Eng. Chem. 2014, 20, 732–738. [Google Scholar] [CrossRef]
- Shen, Y.F.; Tang, J.; Nie, Z.N.; Wang, J.D.; Ren, Y.; Zuo, L. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep. Purif. Technol. 2009, 68, 312–319. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Jiang, Q.; Zhao, L. Water-soluble Fe3O4 nanoparticles with high solubility for removal of heavy-metal ions from waste water. Dalton Trans. 2012, 41, 4544–4551. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.C.; Haldorai, Y.; Lee, G.W.; Hwang, S.K.; Han, Y.K.; Roh, C.; Huh, Y.S. Porous three-dimensional graphene foam/Prussian blue composite for efficient removal of radioactive (137) Cs. Sci. Rep. 2015, 5, 17510. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Tanaka, S. Magnetic separation of cesium ion using Prussian blue modified magnetite. Chem. Lett. 2012, 41, 32–34. [Google Scholar] [CrossRef]
- Thammawong, P.; Opaprakasit, P.; Tangboriboonrat, P.; Sreearunothai, P. Prussian blue-coated magnetic nanoparticles for removal of cesium from contaminated environment. J. Nanopart. Res. 2013, 15, 1689. [Google Scholar] [CrossRef]
- Uoginte, G.; Lujaniene, K.; Mazeika, K. Study of Cu (II), Co (II), Ni (II) and Pb (II) removal from aqueous solutions using magnetic Prussian blue nano-sorbent. J. Hazard. Mater. 2019, 369, 226–235. [Google Scholar] [CrossRef]
- Pham, T.D.; Le, T.M.; Pham, T.M.; Dang, V.H.; Vu, K.L.; Tran, T.K.; Hoang, T.H. Synthesis and characterization of novel hybridized CeO2@SiO2 nanoparticles based on rice husk and their application in antibiotic removal. Langmuir 2021, 37, 2963–2973. [Google Scholar] [CrossRef]
- Pham, T.D.; Pham, T.T.; Phan, M.N.; Ngo, T.M.V.; Dang, V.D.; Vu, C.M. Adsorption characteristics of anionic surfactant onto laterite soil with differently charged surfaces and application for cationic dye removal. J. Mol. Liq. 2020, 301, 112456. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Dao, T.H.; Truong, T.T.; Nguyen, T.M.T.; Pham, T.D. Adsorption characteristic of ciprofloxacin antibiotic onto synthesized alpha alumina nanoparticles with surface modification by polyanion. J. Mol. Liq. 2020, 309, 113150. [Google Scholar] [CrossRef]
- Pham, T.D.; Tran, T.T.; Le, V.A.; Pham, T.T.; Dao, T.H.; Le, T.S. Adsorption characteristics of molecular oxytetracycline onto alumina particles: The role of surface modification with an anionic surfactant. J. Mol. Liq. 2019, 287, 110900. [Google Scholar] [CrossRef]
- Wu, Z.L.; Wang, Y.P.; Xiong, Z.K.; Ao, Z.M.; Pu, S.Y.; Yao, G.; Lai, B. Core-shell magnetic Fe3O4@Zn/Co-ZIFs to activate peroxymonosulfate for highly efficient degradation of carbamazepine. Appl. Catal. B Environ. 2020, 277, 119136. [Google Scholar] [CrossRef]
- Chen, W.H.; Huang, J.R.; Lin, C.H.; Huang, C.P. Catalytic degradation of chlorpheniramine over GO-Fe3O4 in the presence of H2O2 in water: The synergistic effect of adsorption. Sci. Total Environ. 2020, 736, 139468. [Google Scholar] [CrossRef]
- Long, X.X.; Chen, R.Z.; Yang, S.J.; Wang, J.X.; Huang, T.J.; Lei, Q.; Tan, J.H. Preparation, characterization and application in cobalt ion adsorption using nanoparticle films of hybrid copper–nickel hexacyanoferrate. RSC Adv. 2019, 9, 7485–7494. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yuan, S.Y.; Liu, J.M.; Zhang, Y.; Du, H.; Wu, C.D.; Zhao, P.; Chen, H.Y.; Pei, Y.P. Synergistic effect and mechanism of mass transfer and catalytic oxidation of octane degradation in yolk-shell Fe3O4@C/Fenton system. Chem. Eng. J. 2020, 379, 122262. [Google Scholar] [CrossRef]
- Zhao, H.J.; Han, W.L.; Tang, Z.C. Tailored design of high-stability CoMn1.5Ox@TiO2 double-wall nanocages derived from Prussian blue analogue for catalytic combustion of o-dichlorobenzene. Appl. Catal. B Environ. 2020, 276, 119133. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Xiao, R.Y.; Wang, S.F.; Zhu, H.X.; Song, H.N.; Chen, G.N.; Lin, H.F.; Zhang, J.; Xiong, J.H. Oxygen vacancy enhancing Fenton-like catalytic oxidation of norfloxacin over Prussian blue modified CeO2: Performance and mechanism. J. Hazard. Mater. 2020, 398, 122863. [Google Scholar] [CrossRef]
- Wu, H.; Wang, T.; Jin, Y. Effects of -OH Groups on Fe3O4 particles on the heterogeneous coating in a hydrous alumina coating process. Ind. Eng. Chem. Res 2007, 46, 761–766. [Google Scholar] [CrossRef]
- Joseph, Y.; Ranke, W.; Weiss, W. Water on FeO (111) and Fe3O4 (111): Adsorption behavior on different surface terminations. J. Phys. Chem. B 2000, 104, 3224–3236. [Google Scholar] [CrossRef]
- Xu, W.H.; Wang, J.; Wang, L.; Sheng, G.P.; Liu, J.H.; Yu, H.Q.; Huang, H.J. Enhanced arsenic removal from water by hierarchically porous CeO2-ZrO2 nanospheres: Role of surface- and structure-dependent properties. J. Hazard. Mater. 2013, 260, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Lee, D.S. Magnetic Prussian blue nanocomposites for effective cesium removal from aqueous solution. Ind. Eng. Chem. Res. 2016, 55, 3852–3860. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Zaman, W.U.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb (II) and Cd (II) from water by adsorption on peels of banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef]
- Rao, M.M.; Rao, G.P.C.; Seshaiah, K.; Choudary, N.V.; Wang, M.C. Activated carbon from Ceiba pentandra hulls, an agricultural waste, as an adsorbent in the removal of lead and zinc from aqueous solutions. Waste Manag. 2008, 28, 849–858. [Google Scholar] [CrossRef]
- Chu, L.; Liu, C.B.; Zhou, G.Y.; Xu, R.; Tang, Y.H.; Zeng, Z.B.; Luo, S.L. A double network gel as low cost and easy recycle adsorbent: Highly efficient removal of Cd (II) and Pb (II) pollutants from wastewater. J. Hazard. Mater. 2015, 300, 153–160. [Google Scholar] [CrossRef]
- Li, R.H.; Liang, W.; Huang, H.; Jiang, S.C.; Guo, D.; Li, M.L.; Zhang, Z.Q.; Ali, A.; Wang, J.J. Removal of cadmium (II) cations from an aqueous solution with aminothiourea chitosan strengthened magnetic biochar. J. Appl. Polym. Sci. 2018, 135, 46239. [Google Scholar] [CrossRef]
- Gong, J.L.; Chen, L.; Zeng, G.M.; Long, F.; Deng, J.H.; Niu, Q.Y.; He, X. Shellac-coated iron oxide nanoparticles for removal of cadmium (II) ions from aqueous solution. J. Environ. Sci. 2012, 24, 1165–1173. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef]
- Huang, J.H.; Yuan, F.; Zeng, G.M.; Li, X.; Gu, Y.L.; Shi, L.X.; Liu, W.C.; Shi, Y.H. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar] [CrossRef]
- Yang, Z.H.; Chen, X.H.; Li, S.Y.; Ma, W.H.; Li, Y.; He, Z.D.; Hu, H.R.; Wang, T. Effective removal of Cd (II) from aqueous solution based on multifunctional nanoporous silicon derived from solar kerf loss waste. J. Hazard. Mater. 2020, 385, 121522. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.H.; Lei, Z.F.; Yang, Y.N.; Feng, C.P.; Zhang, Z.Y. Selective removal of cesium from aqueous solutions with nickel (II) hexacyanoferrate (III) functionalized agricultural residue-walnut shell. J. Hazard. Mater. 2014, 270, 187–195. [Google Scholar] [CrossRef]
- Gupta, P.L.; Jung, H.; Tiwari, D.; Kong, S.H.; Lee, S.M. Insight into the mechanism of Cd (II) and Pb (II) removal by sustainable magnetic biosorbent precursor to Chlorella vulgaris. J. Taiwan Inst. Chem. Eng. 2017, 71, 206–213. [Google Scholar] [CrossRef]
- Guan, Q.Q.; Gao, K.X.; Ning, P.; Miao, R.R.; He, L. Value-added utilization of paper sludge: Preparing activated carbon for efficient adsorption of Cr (VI) and further hydrogenation of furfural. Sci. Total Environ. 2020, 741, 140265. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Gharibi, F.; Gharibi, F.; McKay, G.; Gupta, V.K.; Puttaiah, S.H.; Marzban, N. Heavy metal adsorption using PAMAM/CNT nanocomposite from aqueous solution in batch and continuous fixed bed systems. Chem. Eng. J. 2018, 346, 258–270. [Google Scholar] [CrossRef]
- Yadav, N.; Maddheshiaya, D.N.; Rawat, S.; Singh, J. Adsorption and equilibrium studies of phenol and para-nitrophenol by magnetic activated carbon synthesised from cauliflower waste. Environ. Eng. Res. 2019, 25, 742–752. [Google Scholar] [CrossRef]
- Papegowda, P.K.; Syed, A.A. Isotherm, kinetic and thermodynamic studies on the removal of methylene blue dye from aqueous solution using saw palmetto spent. Int. J. Environ. Res. 2017, 11, 91–98. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu (II) and Cd (II) from aqueous solutions by ferromanganese binary oxide-biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef] [PubMed]




| Kinetic Models | Constants | Isotherm Models | Parameters | ||
|---|---|---|---|---|---|
| Pseudo-first-order | qe (μg/g) | 895.22 | Langmuir | qm (μg/g) | 9243.81 |
| k1 | 0.4517 | KL | 0.0091 | ||
| R2 | 0.9971 | R2 | 0.9474 | ||
| Pseudo-second-order | qe (μg/g) | 884.90 | Freundlich | n | 2.2847 |
| k2 | −1.53 × 1045 | KF | 562.1269 | ||
| R2 | 0.9872 | R2 | 0.9939 | ||
| Intraparticle diffusion | Kp | 9.3972 | Temkin | aT | 1.2159 |
| C | 665.4461 | bT | 2.3800 | ||
| R2 | 0.2030 | R2 | 0.9069 | ||
| Elovich | α | 1.08 × 1026 | Dubinin–Radushkevich | qm (μg/g) | 5978.5921 |
| β | 0.0702 | KDR | 4.77 × 10−5 | ||
| R2 | 0.9970 | R2 | 0.8627 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Chen, H.; Huang, T.; Zhang, Y.; Lu, Y.; Tan, J.; Chen, R. Removal of Cd(II) from Micro-Polluted Water by Magnetic Core-Shell Fe3O4@Prussian Blue. Molecules 2021, 26, 2497. https://doi.org/10.3390/molecules26092497
Long X, Chen H, Huang T, Zhang Y, Lu Y, Tan J, Chen R. Removal of Cd(II) from Micro-Polluted Water by Magnetic Core-Shell Fe3O4@Prussian Blue. Molecules. 2021; 26(9):2497. https://doi.org/10.3390/molecules26092497
Chicago/Turabian StyleLong, Xinxin, Huanyu Chen, Tijun Huang, Yajing Zhang, Yifeng Lu, Jihua Tan, and Rongzhi Chen. 2021. "Removal of Cd(II) from Micro-Polluted Water by Magnetic Core-Shell Fe3O4@Prussian Blue" Molecules 26, no. 9: 2497. https://doi.org/10.3390/molecules26092497
APA StyleLong, X., Chen, H., Huang, T., Zhang, Y., Lu, Y., Tan, J., & Chen, R. (2021). Removal of Cd(II) from Micro-Polluted Water by Magnetic Core-Shell Fe3O4@Prussian Blue. Molecules, 26(9), 2497. https://doi.org/10.3390/molecules26092497






