PyPLIF HIPPOS-Assisted Prediction of Molecular Determinants of Ligand Binding to Receptors
Abstract
1. Introduction
2. Results
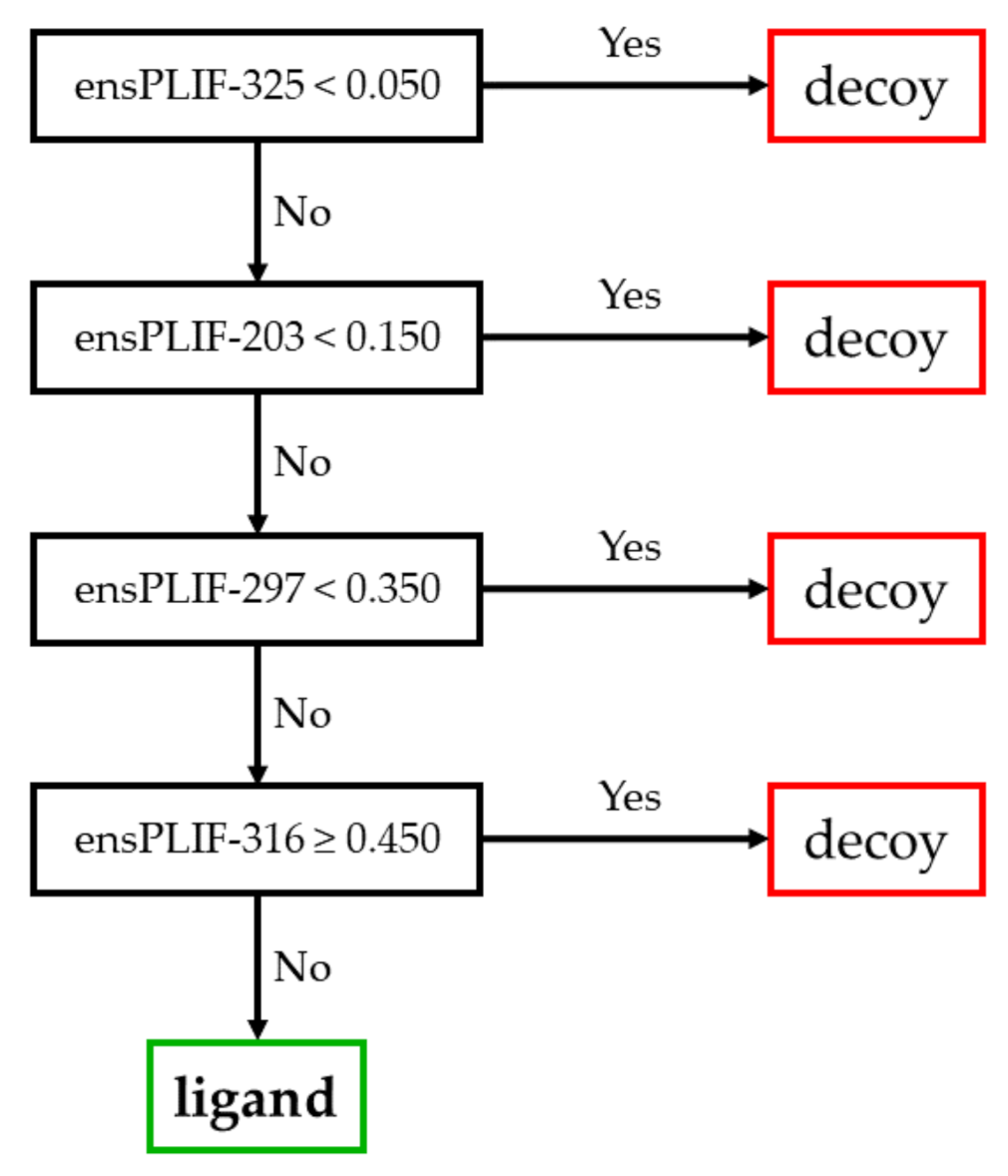
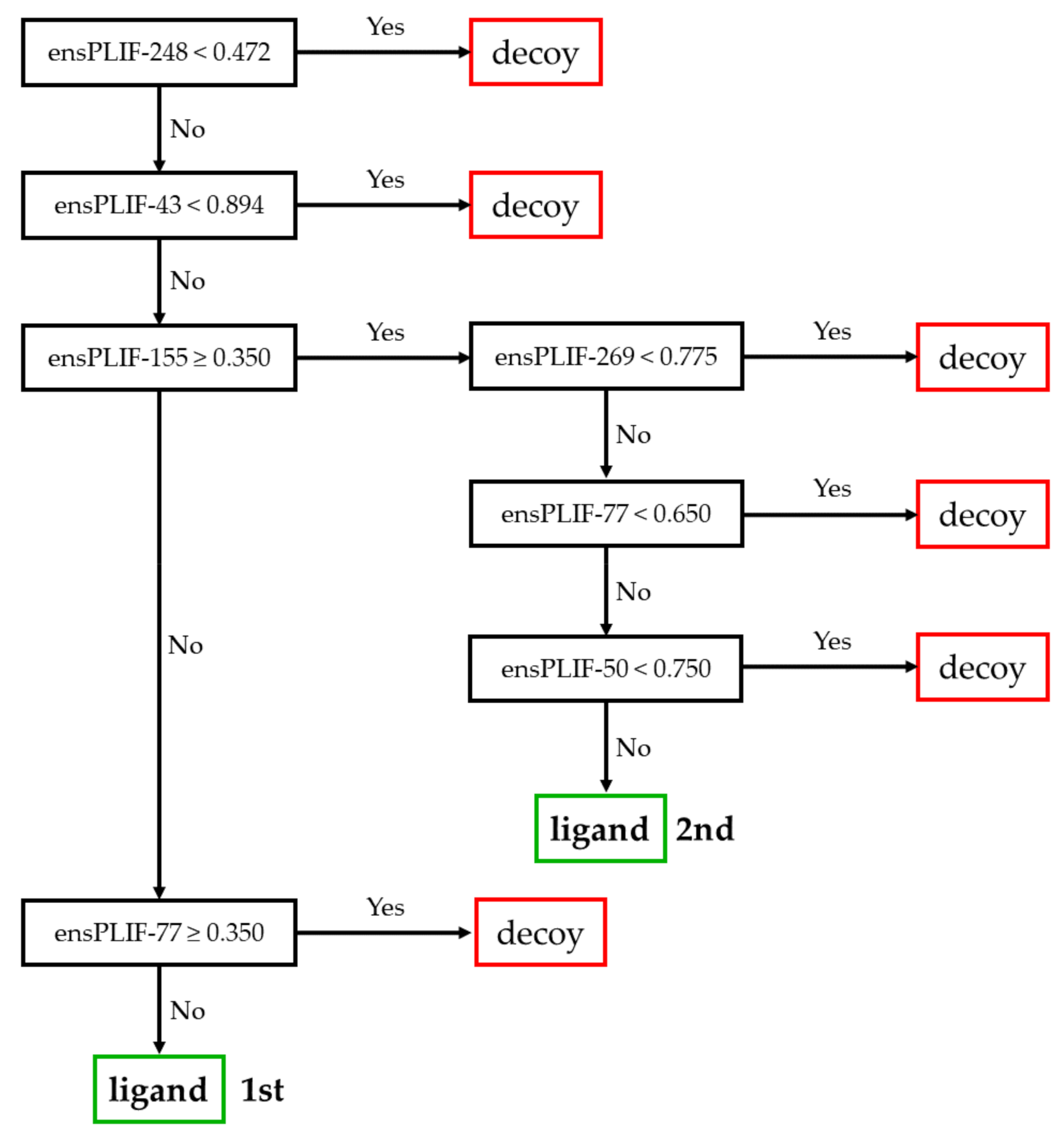
2.1. The Best Decision Tree Related to AA2AR
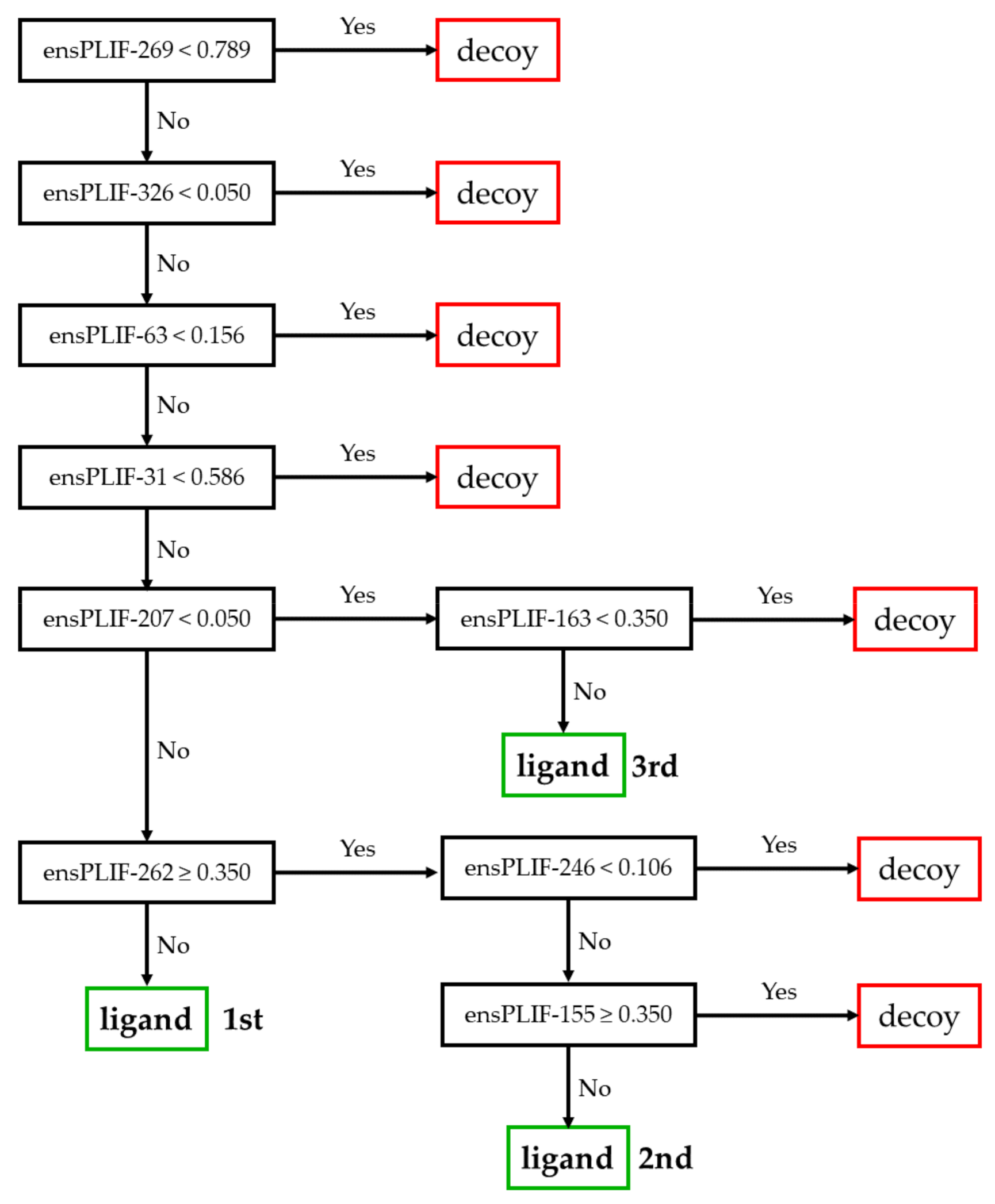
2.2. The Best Decision Tree Related to ADRB2
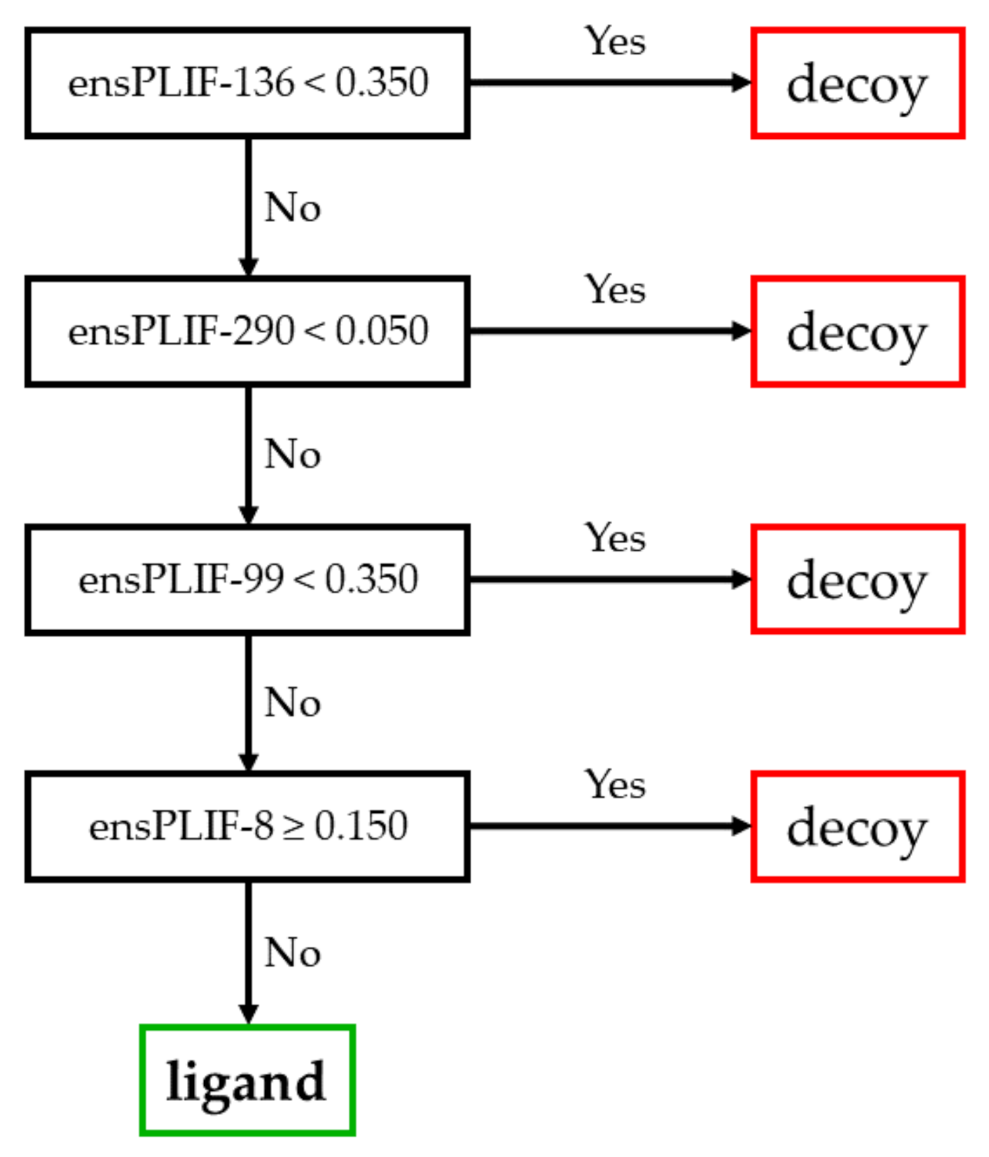
2.3. The Best Decision Tree Related to CXCR4
2.4. The Best Decision Tree Related to DRD3
3. Discussion
3.1. The Identified Molecular Determinants of the Ligand Binding to AA2AR
3.2. The Identified Molecular Determinants of the Ligand Binding to ADRB2
3.3. The Identified Molecular Determinants of the Ligand Binding to CXCR4
3.4. The Identified Molecular Determinants of the Ligand Binding to DRD3
4. Materials and Methods
4.1. Materials
4.2. Methods
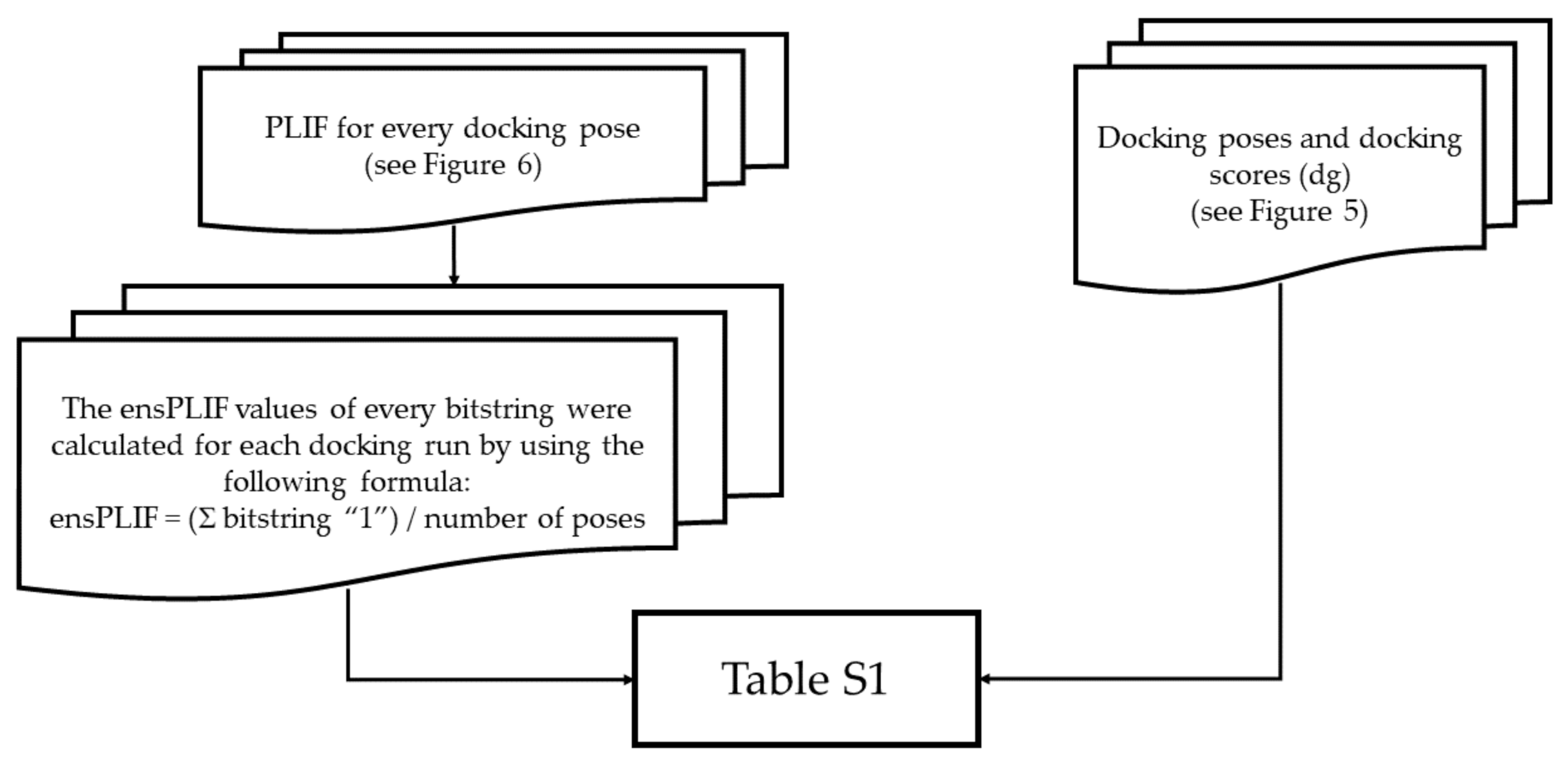
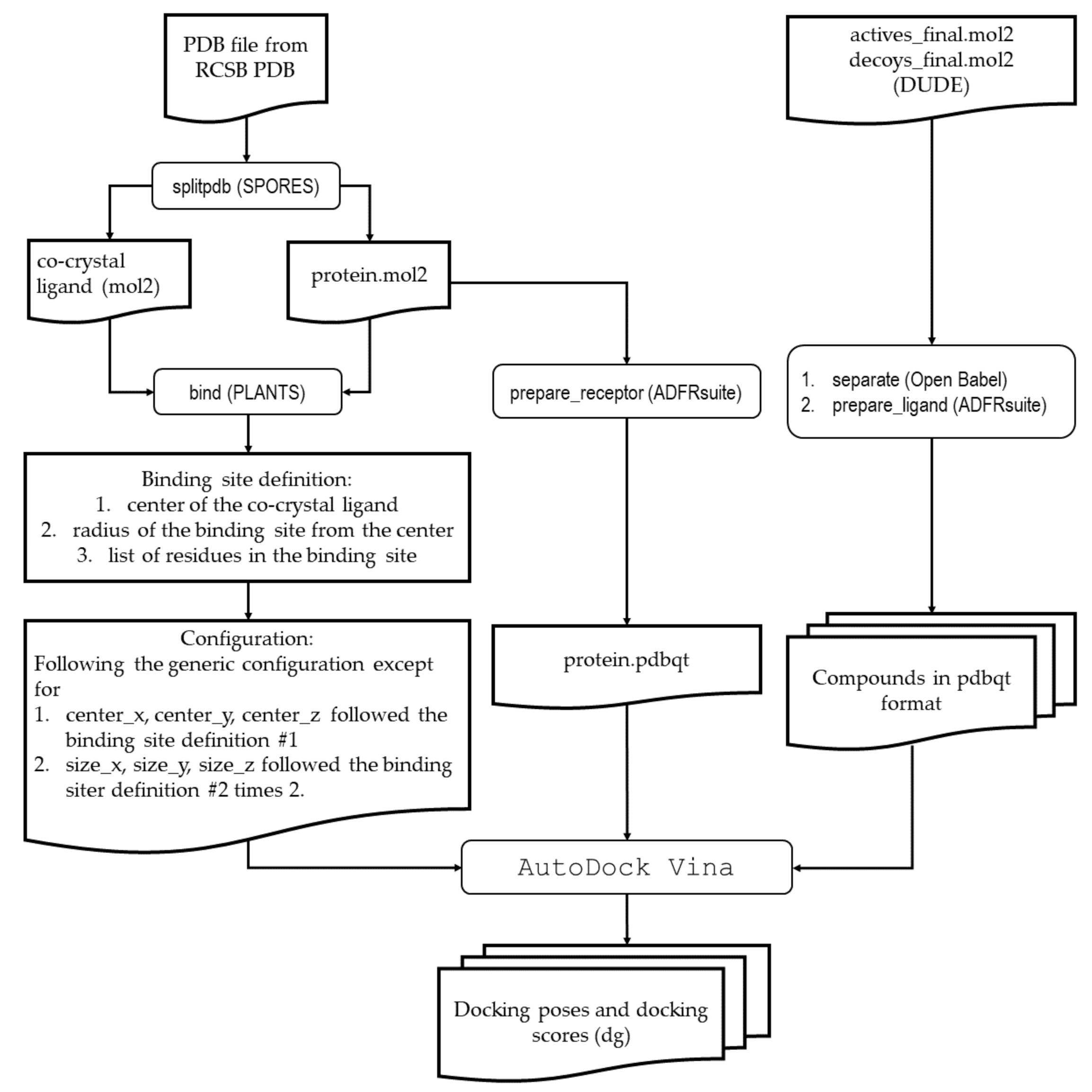
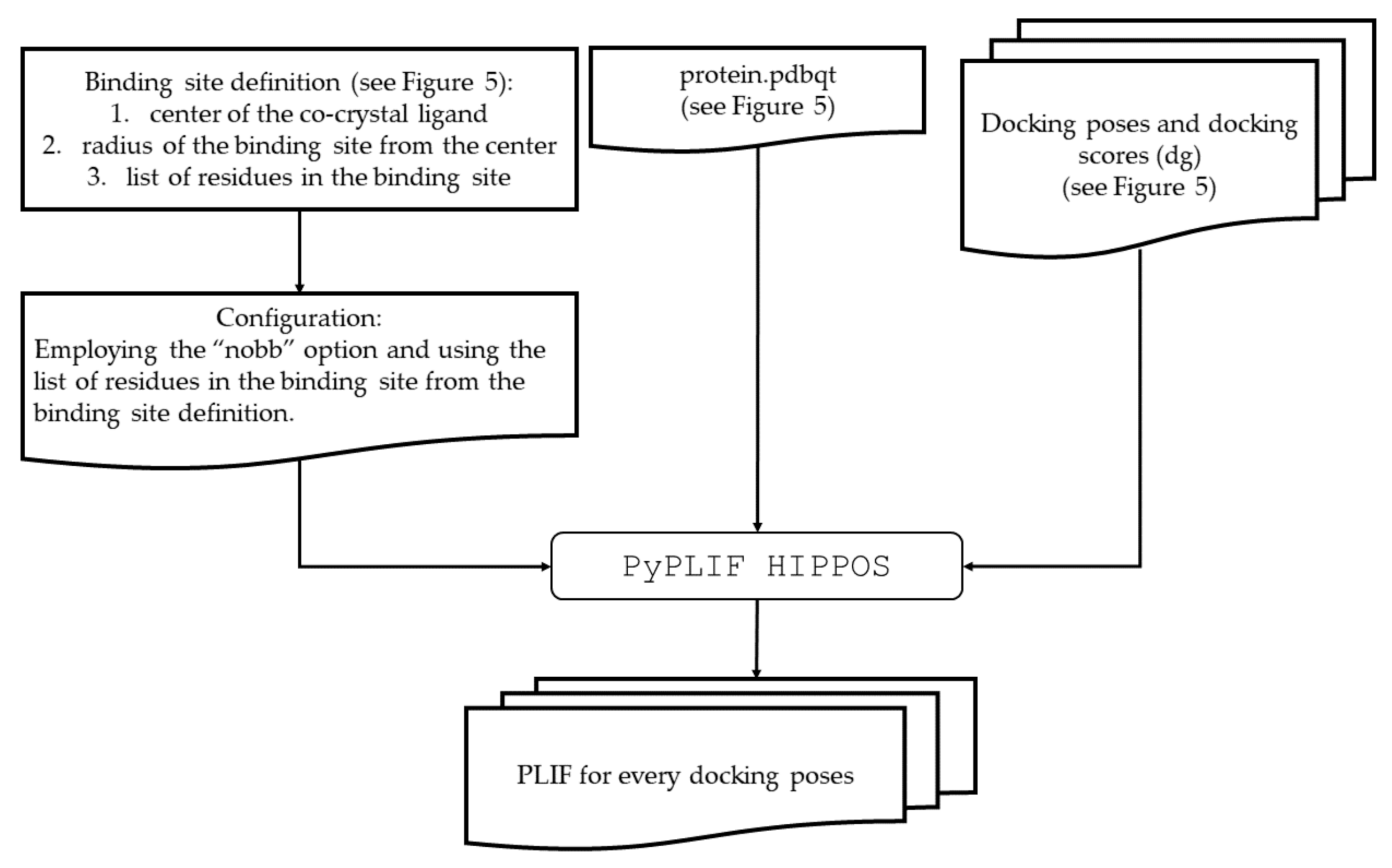
4.2.1. Generic Procedure
4.2.2. Identification of Molecular Determinant of Ligand Binding to AA2AR
4.2.3. Identification of Molecular Determinant of Ligand Binding to ADRB2
4.2.4. Identification of Molecular Determinant of Ligand Binding to CXCR4
4.2.5. Identification of Molecular Determinant of Ligand Binding to DRD3
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rognan, D. Fragment-based approaches and computer-aided drug discovery. Top. Curr. Chem. 2012, 317, 201–222. [Google Scholar] [PubMed]
- de Graaf, C.; Kooistra, A.J.; Vischer, H.F.; Katritch, V.; Kuijer, M.; Shiroishi, M.; Iwata, S.; Shimamura, T.; Stevens, R.C.; de Esch, I.J.P.; et al. Crystal structure-based virtual screening for fragment-like ligands of the human histamine H1 receptor. J. Med. Chem. 2011, 54, 8195–8206. [Google Scholar] [CrossRef] [PubMed]
- Sirci, F.; Istyastono, E.P.; Vischer, H.F.; Kooistra, A.J.; Nijmeijer, S.; Kuijer, M.; Wijtmans, M.; Mannhold, R.; Leurs, R.; de Esch, I.J.P.; et al. Virtual fragment screening: Discovery of histamine H3 receptor ligands using ligand-based and protein-based molecular fingerprints. J. Chem. Inf. Model. 2012, 52, 3308–3324. [Google Scholar] [CrossRef] [PubMed]
- Istyastono, E.P.; Kooistra, A.J.; Vischer, H.; Kuijer, M.; Roumen, L.; Nijmeijer, S.; Smits, R.; de Esch, I.; Leurs, R.; de Graaf, C. Structure-based virtual screening for fragment-like ligands of the G protein-coupled histamine H4 receptor. Med. Chem. Commun. 2015, 6, 1003–1017. [Google Scholar] [CrossRef]
- Isberg, V.; Mordalski, S.; Munk, C.; Rataj, K.; Harpsøe, K.; Hauser, A.S.; Vroling, B.; Bojarski, A.J.; Vriend, G.; Gloriam, D.E. GPCRdb: An information system for G protein-coupled receptors. Nucleic Acids Res. 2016, 44, D356–D364. [Google Scholar] [CrossRef]
- Bakker, R.A.; Dees, G.; Carrillo, J.J.; Booth, R.G.; López-Gimenez, J.F.; Milligan, G.; Strange, P.G.; Leurs, R. Domain swapping in the human histamine H1 receptor. J. Pharmacol. Exp. Ther. 2004, 311, 131–138. [Google Scholar] [CrossRef]
- Kooistra, A.J.; Kuhne, S.; De Esch, I.J.P.; Leurs, R.; De Graaf, C. A structural chemogenomics analysis of aminergic GPCRs: Lessons for histamine receptor ligand design. Br. J. Pharmacol. 2013, 170, 101–126. [Google Scholar] [CrossRef]
- Kooistra, A.J.; Mordalski, S.; Pándy-Szekeres, G.; Esguerra, M.; Mamyrbekov, A.; Munk, C.; Keserű, G.M.; Gloriam, D.E. GPCRdb in 2021: Integrating GPCR sequence, structure and function. Nucleic Acids Res. 2021, 49, D335–D343. [Google Scholar] [CrossRef]
- Munk, C.; Harpsøe, K.; Hauser, A.S.; Isberg, V.; Gloriam, D.E. Integrating structural and mutagenesis data to elucidate GPCR ligand binding. Curr. Opin. Pharmacol. 2016, 30, 51–58. [Google Scholar] [CrossRef]
- Shin, N.; Coates, E.; Murgolo, N.J.; Morse, K.L.; Bayne, M.; Strader, C.D.; Monsma, F.J. Molecular modeling and site-specific mutagenesis of the histamine-binding site of the histamine H4 receptor. Mol. Pharmacol. 2002, 62, 38–47. [Google Scholar] [CrossRef]
- Vroling, B.; Sanders, M.; Baakman, C.; Borrmann, A.; Verhoeven, S.; Klomp, J.; Oliveira, L.; de Vlieg, J.; Vriend, G. GPCRDB: Information system for G protein-coupled receptors. Nucleic Acids Res. 2011, 39, D309–D319. [Google Scholar] [CrossRef]
- Istyastono, E.P.; Nijmeijer, S.; Lim, H.D.; van de Stolpe, A.; Roumen, L.; Kooistra, A.J.; Vischer, H.F.; de Esch, I.J.P.; Leurs, R.; de Graaf, C. Molecular determinants of ligand binding modes in the histamine H4 receptor: Linking ligand-based three-dimensional quantitative structure−activity relationship (3D-QSAR) models to in silico guided receptor mutagenesis studies. J. Med. Chem. 2011, 54, 8136–8147. [Google Scholar] [CrossRef]
- Istyastono, E.P.; Yuniarti, N.; Hariono, M.; Yuliani, S.H.; Riswanto, F.D.O. Binary quantitative structure-activity relationship analysis in retrospective structure based virtual screening campaigns targeting estrogen receptor alpha. Asian J. Pharm. Clin. Res. 2017, 10, 206–211. [Google Scholar] [CrossRef]
- Korb, O.; Stützle, T.; Exner, T.E. Empirical scoring functions for advanced protein-ligand docking with PLANTS. J. Chem. Inf. Model. 2009, 49, 84–96. [Google Scholar] [CrossRef]
- Radifar, M.; Yuniarti, N.; Istyastono, E.P. PyPLIF-assisted redocking indomethacin-(R)-alpha-ethyl-ethanolamide into cyclooxygenase-1. Indones. J. Chem. 2013, 13, 283–286. [Google Scholar] [CrossRef]
- Radifar, M.; Yuniarti, N.; Istyastono, E.P. PyPLIF: Python-based protein-ligand interaction fingerprinting. Bioinformation 2013, 9. [Google Scholar] [CrossRef]
- Therneau, T.; Atkinson, B.; Ripley, B. rpart: Recursive Partitioning and Regression Trees; R Package Version 4.1-9. 2015. Available online: http://CRAN.R-project.org/package=rpart (accessed on 28 September 2019).
- Istyastono, E.P.; Radifar, M.; Yuniarti, N.; Prasasty, V.D.; Mungkasi, S. PyPLIF HIPPOS: A molecular interaction fingerprinting tool for docking results of AutoDock Vina and PLANTS. J. Chem. Inf. Model. 2020, 60, 3697–3702. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Mysinger, M.M.; Carchia, M.; Irwin, J.J.; Shoichet, B.K. Directory of useful decoys, enhanced (DUD-E): Better ligands and decoys for better benchmarking. J. Med. Chem. 2012, 55, 6582–6594. [Google Scholar] [CrossRef]
- Cannon, E.O.; Amini, A.; Bender, A.; Sternberg, M.J.E.; Muggleton, S.H.; Glen, R.C.; Mitchell, J.B.O. Support vector inductive logic programming outperforms the naive Bayes classifier and inductive logic programming for the classification of bioactive chemical compounds. J. Comput. Aided Mol. Des. 2007, 21, 269–280. [Google Scholar] [CrossRef]
- Istyastono, E.P. Employing recursive partition and regression tree method to increase the quality of structure-based virtual screening in the estrogen receptor alpha ligands identification. Asian J. Pharm. Clin. Res. 2015, 8, 21–24. [Google Scholar]
- Koshland, D.E. The key–lock theory and the induced fit theory. Angew. Chem. Int. Ed. Engl. 1994, 33, 2375–2378. [Google Scholar] [CrossRef]
- Riswanto, F.D.O.; Hariono, M.; Yuliani, S.H.; Istyastono, E.P. Computer-aided design of chalcone derivatives as lead compounds targeting acetylcholinesterase. Indones. J. Pharm. 2017, 28, 100–111. [Google Scholar] [CrossRef]
- Bafna, D.; Ban, F.; Rennie, P.S.; Singh, K.; Cherkasov, A. Computer-aided ligand discovery for estrogen receptor alpha. Int. J. Mol. Sci. 2020, 21, 4193. [Google Scholar] [CrossRef]
- Istyastono, E.P.; Riswanto, F.D.O.; Yuliani, S.H. Computer-aided drug repurposing: A cyclooxygenase-2 inhibitor celecoxib as a ligand for estrogen receptor alpha. Indones. J. Chem. 2015, 15, 274–280. [Google Scholar] [CrossRef]
- Riswanto, F.D.O.; Rawa, M.S.A.; Murugaiyah, V.; Salin, N.H.; Istyastono, E.P.; Hariono, M.; Wahab, H.A. Anti-cholinesterase activity of chalcone derivatives: Synthesis, in vitro assay and molecular docking study. Med. Chem. 2021, 17, 442–452. [Google Scholar] [CrossRef]
- Prasasty, V.D.; Istyastono, E.P. Structure-based design and molecular dynamics simulations of pentapeptide AEYTR as a potential acetylcholinesterase inhibitor. Indones. J. Chem. 2020, 20, 953–959. [Google Scholar] [CrossRef]
- Istyastono, E.P.; Prasasty, V.D. Computer-aided discovery of pentapeptide AEYTR as a potent acetylcholinesterase inhibitor. Indones. J. Chem. 2021, 21, 243–350. [Google Scholar] [CrossRef]
- Korb, O.; Stützle, T.; Exner, T.E. An ant colony optimization approach to flexible protein–ligand docking. Proc. IEEE Swarm Intell. Symp. 2007, 1, 115–134. [Google Scholar] [CrossRef]
- Gabel, J.; Desaphy, J.; Rognan, D. Beware of machine learning-based scoring functions-on the danger of developing black boxes. J. Chem. Inf. Model. 2014, 54, 2807–2815. [Google Scholar] [CrossRef]
- Smits, R.A.; Adami, M.; Istyastono, E.P.; Zuiderveld, O.P.; van Dam, C.M.E.; de Kanter, F.J.J.; Jongejan, A.; Coruzzi, G.; Leurs, R.; de Esch, I.J.P. Synthesis and QSAR of quinazoline sulfonamides as highly potent human histamine H4 receptor inverse agonists. J. Med. Chem. 2010, 53, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.P.; Brown, G.A.; Christopher, J.A. Structure-based and fragment-based GPCR drug discovery. ChemMedChem 2014, 9, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Hariono, M.; Yuliani, S.H.; Istyastono, E.P.; Riswanto, F.D.O.; Adhipandito, C.F. Matrix metalloproteinase 9 (MMP9) in wound healing of diabetic foot ulcer: Molecular target and structure-based drug design. Wound Med. 2018, 22, 1–13. [Google Scholar] [CrossRef]
- Jones, J.I.; Nguyen, T.T.; Peng, Z.; Chang, M. Targeting MMP-9 in diabetic foot ulcers. Pharmaceuticals 2019, 12, 79. [Google Scholar] [CrossRef]
- Li, N.; Wang, L.J.; Jiang, B.; Li, X.; Guo, C.; Guo, S.; Shi, D. Recent progress of the development of dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Eur. J. Med. Chem. 2018, 151, 145–157. [Google Scholar] [CrossRef]
- Istyastono, E.P. Docking studies of curcumin as a potential lead compound to develop novel dipeptydyl peptidase-4 inhibitors. Indones. J. Chem. 2010, 9, 132–136. [Google Scholar] [CrossRef]
- ten Brink, T.; Exner, T.E. Influence of protonation, tautomeric, and stereoisomeric states on protein-ligand docking results. J. Chem. Inf. Model. 2009, 49, 1535–1546. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33–47. [Google Scholar] [CrossRef]
- Ravindranath, P.A.; Forli, S.; Goodsell, D.S.; Olson, A.J.; Sanner, M.F. AutoDockFR: Advances in protein-ligand docking with explicitly specified binding site flexibility. PLoS Comput. Biol. 2015, 11, 1–28. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2019; Available online: http://www.r-project.org (accessed on 28 September 2019).
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef]
| Receptor | SBVS Prediction Quality | Molecular Determinant | |||
|---|---|---|---|---|---|
| EF 1 | F-Measure 2 | Residue | Interaction Type 3 | Retrospective Verification 4 | |
| AA2AR | 272.286 | 0.184 | Glu169 | ionic (protein as anion) | verified |
| Trp246 | aromatic edge-to-face | verified | |||
| Leu249 | hydrophobic | verified | |||
| His250 | aromatic edge-to-face | verified | |||
| ADRB2 | 465.151 | 0.307 | Trp109 | aromatic edge-to-face | n.a. 5 |
| Asp113 | ionic (protein as anion) | verified 6 | |||
| Asp192 | hydrophobic | n.a. 5 | |||
| Phe193 | aromatic face-to-face | n.a. 5 | |||
| Ser204 | h-bond (protein as donor) | verified | |||
| Trp286 | hydrophobic | n.a. 5 | |||
| Phe289 | aromatic edge-to-face | n.a. 5 | |||
| Phe290 | aromatic edge-to-face | n.a. 5 | |||
| Asn312 | h-bond (protein as donor) | verified | |||
| CXCR4 | n.d. 7 | 0.333 | Glu32 | hydrophobic | verified |
| Asp97 | hydrophobic | verified | |||
| Trp102 | aromatic edge-to-face | n.a. 5 | |||
| Tyr255 | aromatic edge-to-face | verified | |||
| DRD3 | 455.652 | 0.169 | Phe106 | hydrophobic | n.a. 5 |
| Val107 | hydrophobic | n.a. 5 | |||
| Asp110 | ionic (protein as anion) | verified 6 | |||
| Ile183 | hydrophobic | verified | |||
| Phe346 | aromatic edge-to-face | n.a. 5 | |||
| His349 | aromatic edge-to-face | verified | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Istyastono, E.P.; Yuniarti, N.; Prasasty, V.D.; Mungkasi, S. PyPLIF HIPPOS-Assisted Prediction of Molecular Determinants of Ligand Binding to Receptors. Molecules 2021, 26, 2452. https://doi.org/10.3390/molecules26092452
Istyastono EP, Yuniarti N, Prasasty VD, Mungkasi S. PyPLIF HIPPOS-Assisted Prediction of Molecular Determinants of Ligand Binding to Receptors. Molecules. 2021; 26(9):2452. https://doi.org/10.3390/molecules26092452
Chicago/Turabian StyleIstyastono, Enade P., Nunung Yuniarti, Vivitri D. Prasasty, and Sudi Mungkasi. 2021. "PyPLIF HIPPOS-Assisted Prediction of Molecular Determinants of Ligand Binding to Receptors" Molecules 26, no. 9: 2452. https://doi.org/10.3390/molecules26092452
APA StyleIstyastono, E. P., Yuniarti, N., Prasasty, V. D., & Mungkasi, S. (2021). PyPLIF HIPPOS-Assisted Prediction of Molecular Determinants of Ligand Binding to Receptors. Molecules, 26(9), 2452. https://doi.org/10.3390/molecules26092452