Prospects for the Personalized Multimodal Therapy Approach to Pain Management via Action on NO and NOS
Abstract
1. Introduction
2. Materials
3. Results
3.1. Back Pain
3.1.1. The Role of NO and NOSs in the Development of Back Pain
3.1.2. Association of SNVs of NOSs Family Genes with Back Pain
3.1.3. Prospects for the Use of NOS Inhibitors to Modulate the Effect of Drugs Used to Treat Back Pain
3.2. Neuropathic Pain
3.2.1. Role of NO and NOSs in the Development of Neuropathic Pain
3.2.2. Association of SNVs of NOSs Family Genes with Neuropathic Pain
3.2.3. Prospects for the Use of NOS Inhibitors to Modulate the Effect of Drugs Used to Treat Neuropathic Pain
3.3. Post-Traumatic Pain
3.3.1. The Role of NO and NOSs in the Development of Pain after Muscle, Tendon, and Joint Sports Injuries
3.3.2. Association of SNVs of NOSs Family Genes with Pain after Muscle, Tendon, and Joint Sports Injuries
3.3.3. Prospects for the Use of NOS Inhibitors to Modulate the Effect of Drugs Used to Treat Posttraumatic Pain
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Motina, A.N.; Astashchenko, Y.A.; Masaleva, I.O.; Tretyakova, E.E. The social hygienic characteristic of patients with osteochondrosis of spine. Probl. Soc. Hyg. Public Health Hist. Med. 2020, 28, 396–399. [Google Scholar] [CrossRef]
- Dagenais, S.; Caro, J.; Haldeman, S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008, 8, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Balyazin, V. Pain—multidisciplinary problem of medicine. Chief Physician South Russ. 2018, 2, 4–6. (In Russian) [Google Scholar]
- Dutmer, A.L.; Preuper, H.R.S.; Soer, R.; Brouwer, S.; Bültmann, U.; Dijkstra, P.U.; Coppes, M.H.; Stegeman, P.; Buskens, E.; Van Asselt, A.D.; et al. Personal and Societal Impact of Low Back Pain. Spine 2019, 44, E1443–E1451. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.D.; Stefanovic-Racic, M.; McIntyre, L.A.; Georgescu, H.I.; Evans, C.H. Toward a Biochemical Understanding of Human Intervertebral Disc Degeneration and Herniation. Spine 1997, 22, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- González-Castro, T.B.; Genis-Mendoza, A.D.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; López-Narvaez, M.L.; Pérez-Hernández, N.; Rodríguez-Pérez, J.M.; Martínez-Magaña, J.J. Association between polymorphisms of NOS1, NOS2 and NOS3 genes and suicide behavior: A systematic review and meta-analysis. Metab. Brain Dis. 2019, 34, 967–977. [Google Scholar] [CrossRef]
- Castania, V.; Issy, A.C.; Silveira, J.W.; Ferreira, F.R.; Titze-De-Almeida, S.S.; Resende, F.F.B.; Ferreira, N.R.; Titze-De-Almeida, R.; Defino, H.L.A.; Del Bel, E. The Presence of the Neuronal Nitric Oxide Synthase Isoform in the Intervertebral Disk. Neurotox. Res. 2016, 31, 148–161. [Google Scholar] [CrossRef]
- Brisby, H.; Ashley, H.; Diwan, A.D. In Vivo Measurement of Facet Joint Nitric Oxide in Patients with Chronic Low Back Pain. Spine 2007, 32, 1488–1492. [Google Scholar] [CrossRef]
- Miclescu, A.; Gordh, T. Nitric oxide and pain: ‘Something old, something new’. Acta Anaesthesiol. Scand. 2009, 53, 1107–1120. [Google Scholar] [CrossRef]
- Gühring, H.; Tegeder, I.; Lotsch, J.; Pahl, A.; Werner, U.; Reeh, P.; Rehse, K.; Brune, K.; Geisslinger, G. Role of nitric oxide in zymosan induced paw inflammation and thermal hyperalgesia. Inflamm. Res. 2001, 50, 83–88. [Google Scholar] [CrossRef]
- De Alba, J.; Clayton, N.M.; Collins, S.D.; Colthup, P.; Chessell, I.; Knowles, R.G. GW274150, a novel and highly selective inhibitor of the inducible isoform of nitric oxide synthase (iNOS), shows analgesic effects in rat models of inflammatory and neuropathic pain. Pain 2006, 120, 170–181. [Google Scholar] [CrossRef]
- Jensen, L.; Andersen, L.L.; Schrøder, H.D.; Frandsen, U.; Sjøgaard, G. Neuronal Nitric Oxide Synthase Is Dislocated in Type I Fibers of Myalgic Muscle but Can Recover with Physical Exercise Training. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, K.; Saura, R.; Doita, M.; Mizuno, K. Intervertebral disc cell apoptosis by nitric oxide: Biological understanding of intervertebral disc degeneration. Kobe J. Med Sci. 2000, 46, 283–295. [Google Scholar]
- Yang, X.; Jin, L.; Yao, L.; Shen, F.H.; Shimer, A.L.; Li, X. Antioxidative nanofullerol prevents intervertebral disk degeneration. Int. J. Nanomed. 2014, 9, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Nerlich, A.G.; Bachmeier, B.E.; Schleicher, E.D.; Rohrbach, H.; Paesold, G.; Boos, N. Immunomorphological Analysis of RAGE Receptor Expression and NF- B Activation in Tissue Samples from Normal and Degenerated Intervertebral Discs of Various Ages. Ann. N. Y. Acad. Sci. 2007, 1096, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, N.; Baba, H.; Miyoshi, N.; Maezawa, Y.; Uchida, K.; Kokubo, Y.; Fukuda, M. Herniation of Cervical Intervertebral Disc. Spine 2001, 26, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-Z.; Ishihara, H.; Osada, R.; Kimura, T.; Tsuji, H. Nitric Oxide Mediates the Change of Proteoglycan Synthesis in the Human Lumbar Intervertebral Disc in Response to Hydrostatic Pressure. Spine 2001, 26, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Rannou, F.; Richette, P.; Benallaoua, M.; Francois, M.; Genries, V.; Korwin-Zmijowska, C.; Revel, M.; Corvol, M.; Poiraudeau, S. Cyclic tensile stretch modulates proteoglycan production by intervertebral disc annulus fibrosus cells through production of nitrite oxide. J. Cell. Biochem. 2003, 90, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Studer, R.K.; Gilbertson, L.G.; Georgescu, H.; Sowa, G.; Vo, N.; Kang, J.D. p38 MAPK inhibition modulates rabbit nucleus pulposus cell response to IL-1. J. Orthop. Res. 2008, 26, 991–998. [Google Scholar] [CrossRef]
- Aparicio, J.P.; Bances, I.F.; Fernández, E.L.-A.; Montes, A.H.; Vázquez, J.P.; García, S.R.; Fernández, P.L.; Valle-Garay, E.; Asensi, V.; Aparicio, A.P.; et al. The IL-1β (+3953 T/C) gene polymorphism associates to symptomatic lumbar disc herniation. Eur. Spine J. 2011, 20, 383–389. [Google Scholar] [CrossRef]
- Han, I.; Ropper, A.; Teng, Y.; Shin, D.A.; Jeon, Y.; Park, H.; Shin, D.; Park, Y.; Kim, K.; Kim, N.K. Association between VEGF and eNOS gene polymorphisms and lumbar disc degeneration in a young Korean population. Genet. Mol. Res. 2013, 12, 2294–2305. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, S.; Barbato, D.L.; Tatulli, G.; Aquilano, K.; Ciriolo, M.R. The role of nNOS and PGC-1 in skeletal muscle cells. J. Cell Sci. 2014, 127, 4813–4820. [Google Scholar] [CrossRef] [PubMed]
- Vandivier, R.W.; Eidsath, A.; Banks, S.M.; Preas, H.L.; Leighton, S.B.; Godin, P.J.; Suffredini, A.F.; Danner, R.L. Down-regulation of nitric oxide production by ibuprofen in human volunteers. J. Pharmacol. Exp. Ther. 1999, 289, 1398–1403. [Google Scholar]
- Gühring, H.; Hamza, M.; Sergejeva, M.; Ates, M.; E Kotalla, C.; Ledent, C.; Brune, K. A role for endocannabinoids in indomethacin-induced spinal antinociception. Eur. J. Pharmacol. 2002, 454, 153–163. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Lee, J.H.; Seok, J.H.; Hong, J.H.; Lee, Y.S.; Lim, J.H.; Kim, Y.M.; Hur, G.M. Acetaminophen Inhibits iNOS Gene Expression in RAW 264.7 Macrophages: Differential Regulation of NF-κB by Acetaminophen and Salicylates. Biochem. Biophys. Res. Commun. 2000, 272, 758–764. [Google Scholar] [CrossRef]
- Granados-Soto, V.; Flores-Murrieta, F.J.; Castañeda-Hernández, G.; López-Muñoz, F.J. Evidence for the involvement of nitric oxide in the antinociceptive effect of ketorolac. Eur. J. Pharmacol. 1995, 277, 281–284. [Google Scholar] [CrossRef]
- Ventura-Martinez, R.; Déciga-Campos, M.; Díaz-Reval, M.I.; González-Trujano, M.E.; López-Muñoz, F.J. Peripheral involvement of the nitric oxide–cGMP pathway in the indomethacin-induced antinociception in rat. Eur. J. Pharmacol. 2004, 503, 43–48. [Google Scholar] [CrossRef]
- Hamza, M.; Wang, X.-M.; Wu, T.; Brahim, J.S.; Rowan, J.S.; A Dionne, R. Nitric Oxide is Negatively Correlated to Pain during Acute Inflammation. Mol. Pain 2010, 6, 55. [Google Scholar] [CrossRef]
- Aley, K.O.; McCarter, G.; Levine, J.D. Nitric Oxide Signaling in Pain and Nociceptor Sensitization in the Rat. J. Neurosci. 1998, 18, 7008–7014. [Google Scholar] [CrossRef]
- Holthusen, H.; Arndt, J.O. Nitric oxide evokes pain in humans on intracutaneous injection. Neurosci. Lett. 1994, 165, 71–74. [Google Scholar] [CrossRef]
- Berrazueta, J.R.; Losada, A.; Poveda, J.; Ochoteco, A.; Riestra, A.; Salas, E.; Amado, J.A. Successful treatment of shoulder pain syndrome due to supraspinatus tendinitis with transdermal nitroglycerin. A double blind study. Pain 1996, 66, 63–67. [Google Scholar] [CrossRef]
- Fujioka, Y.; Stahlberg, A.; Ochi, M.; Olmarker, K. Expression of Inflammation/Pain-Related Genes in the Dorsal Root Ganglion following Disc Puncture in Rats. J. Orthop. Surg. 2016, 24, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Nerlich, A.G.; Schleicher, E.D.; Boos, N. 1997 Volvo Award Winner in Basic Science Studies. Spine 1997, 22, 2781–2795. [Google Scholar] [CrossRef] [PubMed]
- Ziskoven, C.; Jäger, M.; Kircher, J.; Patzer, T.; Bloch, W.; Brixius, K.; Krauspe, R. Physiology and pathophysiology of nitrosative and oxidative stress in osteoarthritic joint destruction. Can. J. Physiol. Pharmacol. 2011, 89, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Freemont, T.J.; Le Maitre, C.; Watkins, A.; Hoyland, J.A. Degeneration of intervertebral discs: Current understanding of cellular and molecular events, and implications for novel therapies. Expert Rev. Mol. Med. 2001, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Slade, S.C.; Keating, J.L. Unloaded Movement Facilitation Exercise Compared to No Exercise or Alternative Therapy on Outcomes for People with Nonspecific Chronic Low Back Pain: A Systematic Review. J. Manip. Physiol. Ther. 2007, 30, 301–311. [Google Scholar] [CrossRef]
- Kim, K.-W.; Ha, K.-Y.; Lee, J.-S.; Rhyu, K.-W.; An, H.S.; Woo, Y.-K. The Apoptotic Effects of Oxidative Stress and Antiapoptotic Effects of Caspase Inhibitors on Rat Notochordal Cells. Spine 2007, 32, 2443–2448. [Google Scholar] [CrossRef]
- Gruber, H.E.; Hoelscher, G.L.; Ingram, J.A.; Bethea, S.; Hanley, E.N. IGF-1 rescues human intervertebral annulus cells fromin vitrostress-induced premature senescence. Growth Factors 2008, 26, 220–225. [Google Scholar] [CrossRef]
- Levin, O.S. Diabetic polyneuropathy: Current approaches to diagnosis and pathogenetic therapy. Clinician 2014, 7, 54–63. [Google Scholar]
- Merkulov, Y.A.; Zavalishin, I.A.; Merkulova, D.M. The role of axonopathy in the mechanisms of development of demyelination processes in the central and peripheral nervous system. Neurosci. Behav. 2009, 39, 31–34. [Google Scholar] [CrossRef]
- Popelyansky, Y.Y. Diseases of the Peripheral Nervous System: A Guide for Doctors; MEDpress-Inform: Moscow, Russia, 2009; p. 352. [Google Scholar]
- Beregovsky, V.B.; Khramilin, V.N.; Demidova, I.Y.; Strokov, I.A.; Guryeva, I.V. Distal diabetic neuropathy: Review of evidence-based recommendations. Ann. Clin. Exp. Neurol. 2015, 9, 60–68. [Google Scholar]
- Baig, F.; Knopp, M.; A Rajabally, Y. Diagnosis, epidemiology and treatment of inflammatory neuropathies. Br. J. Hosp. Med. 2012, 73, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Chance, P.; Fischbeck, K. Molecular genetics of Charcot-Marie-Tooth disease and related neuropathies. Hum. Mol. Genet. 1994, 3, 1503–1507. [Google Scholar] [CrossRef]
- Marques, W., Jr.; Funayama, C.A.; Secchin, J.B.; Lourenço, C.M.; Gouvêa, S.P.; Marques, V.D.; Barreira, A.A. Coexistence of two chronic neuropathies in a young child: Charcot–Marie–Tooth disease type 1A and chronic inflammatory demyelinating polyneuropathy. Muscle Nerve 2010, 42, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Mathey, E.K.; Park, S.B.; Hughes, R.A.; Pollard, J.D.; Armati, P.J.; Barnett, M.H.; Lin, C.S. Chronic inflammatory demyelinating polyradiculoneuropathy: From pathology to phenotype. J. Neurol. Neurosurg. Psychiatry 2015, 86, 973–985. [Google Scholar] [CrossRef]
- Vinik, A.I.; Strotmeyer, E.S.; Nakave, A.A.; Patel, C.V. Diabetic Neuropathy in Older Adults. Clin. Geriatr. Med. 2008, 24, 407–435. [Google Scholar] [CrossRef]
- Chin, R.L.; Latov, N.; Sander, H.W.; Hays, A.P.; Croul, S.E.; Magda, P.; Brannagan, T.H. Sensory CIDP presenting as cryptogenic sensory polyneuropathy. J. Peripher. Nerv. Syst. 2004, 9, 132–137. [Google Scholar] [CrossRef]
- Shnayder, N.A.; Petrova, M.M.; Morozova, G.A. Diagnostics of Diabetic Neuropathy. NMF MBN: Moscow, Russia, 2014; p. 286. [Google Scholar]
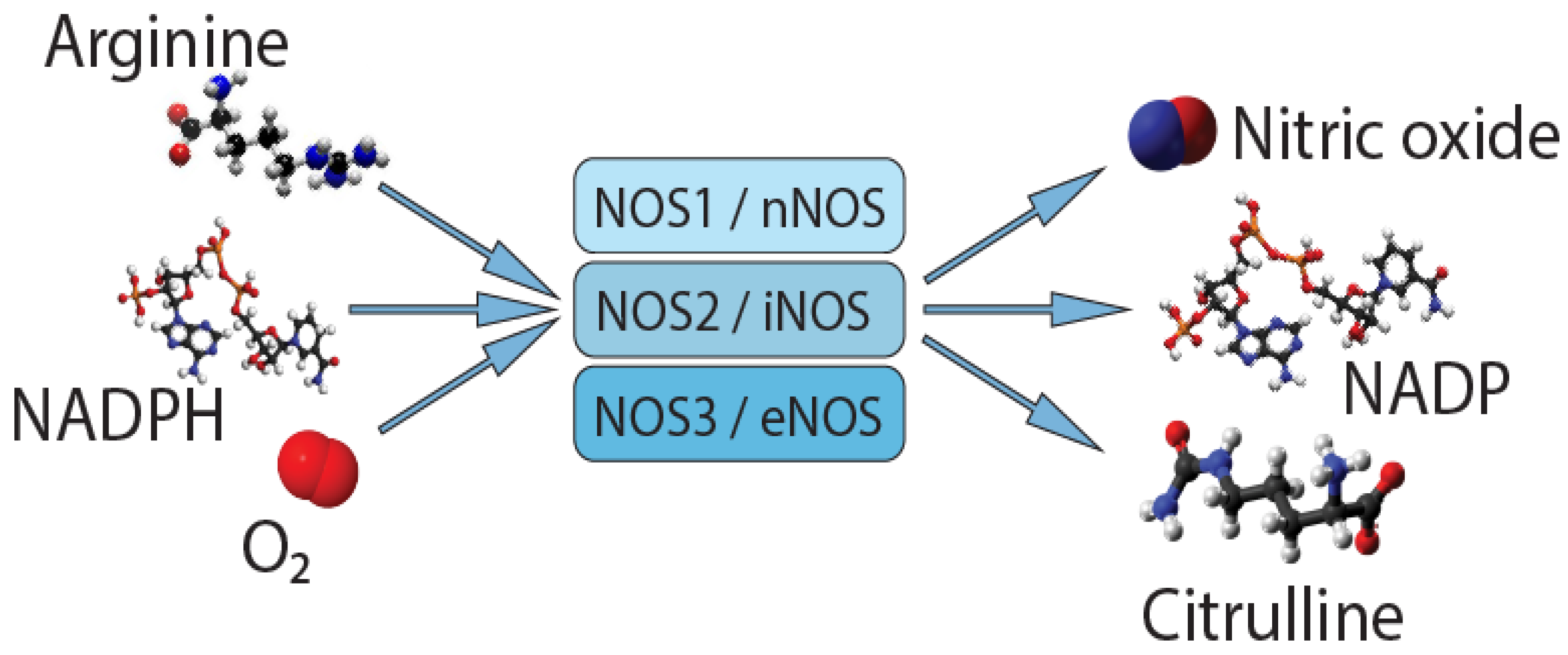
- Nathan, C.; Xie, Q.W. Nitric oxide synthases: Roles, tolls, and controls. Cell 1994, 78, 915–918. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Dolan, S.; Kelly, J.G.; Huan, M.; Nolan, A.M. Transient Up-regulation of Spinal Cyclooxygenase-2 and Neuronal Nitric Oxide Synthase following Surgical Inflammation. J. Am. Soc. Anesthesiol. 2003, 98, 170–180. [Google Scholar] [CrossRef]
- Inoue, T.; Mashimo, T.; Shibata, M.; Shibuta, S.; Yoshiya, I. Rapid development of nitric oxide-induced hyperalgesia de-pends on an alternate to the cGMP-mediated pathway in the rat neuropathic pain model. Brain Res. 1998, 792, 263–270. [Google Scholar] [CrossRef]
- Roche, A.K.; Cook, M.; Wilcox, G.L.; Kajander, K.C. A nitric oxide synthesis inhibitor (L-NAME) reduces licking behavior and FOSlabeling in the spinal cord of rats during formalin-induced inflammation. Pain 1996, 66, 331–341. [Google Scholar] [CrossRef]
- Fan, W.; Huang, F.; Li, C.; Qu, H.; Gao, Z.; Leng, S.; Li, D.; He, H. Involvement of NOS/NO in the development of chronic dental inflammatory pain in rats. Brain Res. Rev. 2009, 59, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, F.; Wu, Z.; Zhu, X.; Li, D.; He, H. The role of nitric oxide in orofacial pain. Nitric Oxide 2012, 26, 32–37. [Google Scholar] [CrossRef]
- Chakravortty, D.; Hensel, M. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect. 2003, 5, 621–627. [Google Scholar] [CrossRef]
- Kolesnikov, Y.A.; Pick, C.G.; Pasternak, G.W. NG-Nitro-L-arginine prevents morphine tolerance. Eur. J. Pharmacol. 1992, 221, 399–400. [Google Scholar] [CrossRef]
- Kolesnikov, Y.A.; Pick, C.G.; Ciszewska, G.; Pasternak, G.W. Blockade of tolerance to morphine but not to kappa opioids by a nitric oxide synthase inhibitor. Proc. Natl. Acad. Sci. USA 1993, 90, 5162–5166. [Google Scholar] [CrossRef]
- Cotter, M.A.; Ekberg, K.; Wahren, J.; Cameron, N.E. Effects of proinsulin C-peptide in experimental diabetic neuropathy: Vascular actions and modulation by nitric oxide synthase inhibition. Diabetes 2003, 52, 1812–1817. [Google Scholar] [CrossRef]
- Maxfield, E.K.; Cameron, N.E.; Cotter, M.A. Effects of diabetes on reactivity of sciatic vasa nervorum in rats. J. Diabetes Complicat. 1997, 11, 47–55. [Google Scholar] [CrossRef]
- Lam, H.; Hanley, D.; Trapp, B.; Saito, S.; Raja, S.; Dawson, T.; Yamaguchi, H. Induction of spinal cord neuronal nitric oxide synthase (NOS) after formalin injection in the rat hind paw. Neurosci. Lett. 1996, 210, 201–204. [Google Scholar] [CrossRef]
- Čížková, D.; Lukáčová, N.; Maršala, M.; Maršala, J. Neuropathic pain is associated with alterations of nitric oxide synthase immunoreactivity and catalytic activity in dorsal root ganglia and spinal dorsal horn. Brain Res. Bull. 2002, 58, 161–171. [Google Scholar] [CrossRef]
- Kolesnikov, Y.A.; Chereshnev, I.; Criesta, M.; Pan, Y.-X.; Pasternak, G.W. Opposing actions of neuronal nitric oxide synthase isoforms in formalin-induced pain in mice. Brain Res. 2009, 1289, 14–21. [Google Scholar] [CrossRef] [PubMed]
- O’Rielly, D.D.; Loomis, C.W. Increased Expression of Cyclooxygenase and Nitric Oxide Isoforms, and Exaggerated Sensitivity to Prostaglandin E2, in the Rat Lumbar Spinal Cord 3 Days after L5–L6 Spinal Nerve Ligation. J. Am. Soc. Anesthesiol. 2006, 104, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Lui, P.-W.; Lee, C.-H. Preemptive effects of intrathecal cyclooxygenase inhibitor or nitric oxide synthase inhibitor on thermal hypersensitivity following peripheral nerve injury. Life Sci. 2004, 75, 2527–2538. [Google Scholar] [CrossRef]
- Infante, C.; Díaz, M.; Hernández, A.; Constandil, L.; Pelissier, T. Expression of nitric oxide synthase isoforms in the dorsal horn of monoarthritic rats: Effects of competitive and uncompetitive N-methyl-D-aspartate antagonists. Arthritis Res. Ther. 2007, 9, R53. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-Y.; Chen, Z.-J.; Xiao, H.; Lin, Y.-H.; Hu, Y.-Y.; Chang, L.; Wu, H.-Y.; Wang, P.; Lu, W.; Zhu, D.-Y.; et al. nNOS-expressing neurons in the vmPFC transform pPVT-derived chronic pain signals into anxiety behaviors. Nat. Commun. 2020, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Höke, A.; Zochodne, D.W. Local expression of inducible nitric oxide synthase in an animal model of neuropathic pain. Neurosci. Lett. 1999, 260, 207–209. [Google Scholar] [CrossRef]
- Meller, S.; Dykstra, C.; Grzybycki, D.; Murphy, S.; Gebhart, G. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology 1994, 33, 1471–1478. [Google Scholar] [CrossRef]
- Costacou, T.; Chang, Y.; Ferrell, R.E.; Orchard, T.J. Identifying Genetic Susceptibilities to Diabetes-related Complications among Individuals at Low Risk of Complications: An Application of Tree-Structured Survival Analysis. Am. J. Epidemiol. 2006, 164, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Zotova, E.V.; Voron’ko, O.E.; Bursa, T.R.; Galeev, I.V.; Strokov, I.A.; Nosikov, V.V. Polymorphic markers of the NO synthase genes and genetic predisposition to diabetic polyneuropathy in patients with type 1 diabetes mellitus. Mol. Biol. (Mosk.) 2005, 39, 224–229. (In Russian) [Google Scholar] [CrossRef]
- Staunton, C.; Djouhri, L.; Barrett-Jolley, R.; Thippeswami, T. 1400W alleviates pain and increases the expression levels of anti-nociceptive and neuromodulatory mediators. Physiol. Soc. 2014, 31, 126. [Google Scholar]
- Pettipher, E.R.; Hibbs, T.A.; Smith, M.A.; Griffiths, R.J. Analgesic activity of 2-amino-4-methylpyridine, a novel NO synthase inhibitor. Inflamm. Res. 1997, 46, 135–136. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Blasko, E.; Glaser, C.B.; Devlin, J.J.; Xia, W.; Feldman, R.I.; Polokoff, M.A.; Phillips, G.B.; Whitlow, M.; Auld, D.S.; McMillan, K.; et al. Mechanistic Studies with Potent and Selective Inducible Nitric-oxide Synthase Dimerization Inhibitors. J. Biol. Chem. 2002, 277, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Davey, D.D.; Adler, M.; Arnaiz, D.; Eagen, K.; Erickson, S.; Guilford, W.; Kenrick, M.; Morrissey, M.M.; Ohlmeyer, M.; Pan, G.; et al. Design, Synthesis, and Activity of 2-Imidazol-1-ylpyrimidine Derived Inducible Nitric Oxide Synthase Dimerization Inhibitors. J. Med. Chem. 2007, 50, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Bonnefous, C.; Payne, J.E.; Roppe, J.; Zhuang, H.; Chen, X.; Symons, K.T.; Nguyen, P.M.; Sablad, M.; Rozenkrants, N.; Zhang, Y.; et al. Discovery of Inducible Nitric Oxide Synthase (iNOS) Inhibitor Development Candidate KD7332, Part 1: Identification of a Novel, Potent, and Selective Series of Quinolinone iNOS Dimerization Inhibitors that are Orally Active in Rodent Pain Models. J. Med. Chem. 2009, 52, 3047–3062. [Google Scholar] [CrossRef]
- Mogil, J.S. Animal models of pain: Progress and challenges. Nat. Rev. Neurosci. 2009, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Rocha, P.; Ferreira, A.; Da Silva, J.; Alves, A.; Martins, D.; Britto, L.; Chacur, M. Effects of selective inhibition of nNOS and iNOS on neuropathic pain in rats. Mol. Cell. Neurosci. 2020, 105, 103497. [Google Scholar] [CrossRef]
- Romero, T.R.; Resende, L.C.; Duarte, I.D. The neuronal NO synthase participation in the peripheral antinociception mechanism induced by several analgesic drugs. Nitric Oxide 2011, 25, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Silvagno, F.; Xia, H.; Bredt, D.S. Neuronal Nitric-oxide Synthase-μ, an Alternatively Spliced Isoform Expressed in Differentiated Skeletal Muscle. J. Biol. Chem. 1996, 271, 11204–11208. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Park, J.W.; Swanson, K.M.; Dey, S.; Noguchi, C.T.; Schechter, A.N. Skeletal muscle as an endogenous nitrate reservoir. Nitric Oxide 2015, 47, 10–16. [Google Scholar] [CrossRef]
- Stamler, J.S.; Meissner, G. Physiology of Nitric Oxide in Skeletal Muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef]
- Chao, D.S.; Gorospe, J.R.; E Brenman, J.; A Rafael, J.; Peters, M.F.; Froehner, S.C.; Hoffman, E.P.; Chamberlain, J.S.; Bredt, D.S. Selective loss of sarcolemmal nitric oxide synthase in Becker muscular dystrophy. J. Exp. Med. 1996, 184, 609–618. [Google Scholar] [CrossRef]
- Ihara, H.; Kuwamura, M.; Atsuta, M.; Nihonmatsu, I.; Okada, T.; Mukamoto, M.; Kozaki, S. Expression of neuronal nitric oxide synthase variant, nNOS-μ, in rat brain. Nitric Oxide 2006, 15, 13–19. [Google Scholar] [CrossRef]
- Thomas, G.D.; Shaul, P.W.; Yuhanna, I.S.; Froehner, S.C.; Adams, M.E. Vasomodulation by Skeletal Muscle–Derived Nitric Oxide Requires α-Syntrophin–Mediated Sarcolemmal Localization of Neuronal Nitric Oxide Synthase. Circ. Res. 2003, 92, 554–560. [Google Scholar] [CrossRef]
- Szomor, Z.L.; Appleyard, R.C.; Murrell, G.A.C. Overexpression of nitric oxide synthases in tendon overuse. J. Orthop. Res. 2005, 24, 80–86. [Google Scholar] [CrossRef]
- Barouch, L.A.; Harrison, R.W.; Skaf, M.W.; Rosas, G.O.; Cappola, T.P.; Kobeissi, Z.A.; Hobai, I.A.; Lemmon, C.A.; Burnett, A.L.; O’Rourke, B.; et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nat. Cell Biol. 2002, 416, 337–339. [Google Scholar] [CrossRef]
- Bokhari, A.R.; Murrell, G.A. The role of nitric oxide in tendon healing. J. Shoulder Elb. Surg. 2012, 21, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, M.-X.; Wei, A.; Zhu, W.; Murrell, G.A.C. The cell specific temporal expression of nitric oxide synthase isoforms during Achilles tendon healing. Inflamm. Res. 2001, 50, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Abramson, S.B. Osteoarthritis and nitric oxide. Osteoarthr. Cartil. 2008, 16, S15–S20. [Google Scholar] [CrossRef]
- Mazzetti, I.; Grigolo, B.; Pulsatelli, L.; Dolzani, P.; Silvestri, T.; Roseti, L.; Meliconi, R.; Facchini, A. Differential roles of nitric oxide and oxygen radicals in chondrocytes affected by osteoarthritis and rheumatoid arthritis. Clin. Sci. (Lond.) 2001, 101, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Taskiran, D.; Stefanovicracic, M.; Georgescu, H.; Evans, C. Nitric-Oxide Mediates Suppression of Cartilage Proteoglycan Synthesis by Interleukin-1. Biochem. Biophys. Res. Commun. 1994, 200, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Häuselmann, H.J.; Stefanovic-Racic, M.; A Michel, B.; Evans, C.H. Differences in nitric oxide production by superficial and deep human articular chondrocytes: Implications for proteoglycan turnover in inflammatory joint diseases. J. Immunol. 1998, 160, 1444–1448. [Google Scholar] [PubMed]
- Saunders, C.J.; Xenophontos, S.L.; Cariolou, M.A.; Anastassiades, L.C.; Noakes, T.D.; Collins, M. The bradykinin β2 receptor (BDKRB2) and endothelial nitric oxide synthase 3 (NOS3) genes and endurance performance during Ironman Triathlons. Hum. Mol. Genet. 2006, 15, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Brookes, C.; Ribbans, W.J.; El Khoury, L.Y.; Raleigh, S.M.; Stuart, M.R. Variability within the human iNOS gene and Achilles tendon injuries: Evidence for a heterozygous advantage effect. J. Sci. Med. Sport 2020, 23, 342–346. [Google Scholar] [CrossRef]
- Balke, J.E.; Zhang, L.; Percival, J.M. Neuronal nitric oxide synthase (nNOS) splice variant function: Insights into nitric oxide signaling from skeletal muscle. Nitric Oxide 2019, 82, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Motohashi, N.; Uezumi, A.; Fukada, S.-I.; Yoshimura, T.; Itoyama, Y.; Aoki, M.; Miyagoe-Suzuki, Y.; Takeda, S. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J. Clin. Investig. 2007, 117, 2468–2476. [Google Scholar] [CrossRef]
- Lau, K.S.; Grange, R.W.; Chang, W.-J.; Kamm, K.E.; Sarelius, I.; Stull, J.T. Skeletal muscle contractions stimulate cGMP formation and attenuate vascular smooth muscle myosin phosphorylation via nitric oxide. FEBS Lett. 1998, 431, 71–74. [Google Scholar] [CrossRef]
- Murrell, G.A. Oxygen free radicals and tendon healing. J. Shoulder Elb. Surg. 2007, 16, S208–S214. [Google Scholar] [CrossRef]
- Paoloni, J.A.; Appleyard, R.C.; Nelson, J.; Murrell, G.A.C. Topical Nitric Oxide Application in the Treatment of Chronic Extensor Tendinosis at the Elbow. Am. J. Sports Med. 2003, 31, 915–920. [Google Scholar] [CrossRef]
- Xia, W.; Szomor, Z.; Wang, Y.; Murrell, G.A. Nitric oxide enhances collagen synthesis in cultured human tendon cells. J. Orthop. Res. 2006, 24, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Yaster, M.; Raja, S.N.; Tao, Y.-X. Genetic Knockout and Pharmacologic Inhibition of Neuronal Nitric Oxide Synthase Attenuate Nerve Injury-Induced Mechanical Hypersensitivity in Mice. Mol. Pain 2007, 3, 29. [Google Scholar] [CrossRef]
- Levy, D.; Kubes, P.; Zochodne, D.W. Delayed peripheral nerve degeneration, regeneration, and pain in mice lacking in-ducible nitric oxide synthase. J. Neuropathol. Exp. Neurol. 2001, 60, 411–421. [Google Scholar] [CrossRef]
- Klepper, L. Nitric oxide (NO) and nitrogen dioxide (NO2) emissions from herbicide-treated soybean plants. Atmospheric Environ. (1967) 1979, 13, 537–542. [Google Scholar] [CrossRef]
- Gordh, T. The role of nitric oxide in neuropathic pain and neurodegeneration. Acta Anaesthesiol. Scand. Suppl. 1998, 113, 29–30. [Google Scholar] [PubMed]
- Barinaga, M. Is nitric oxide the “retrograde messenger”? Science 1991, 254, 1296–1297. [Google Scholar] [CrossRef]
- MacMicking, J.; Xie, Q.-W.; Nathan, C. Nitric Oxide and Macrophage Function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef]
- Chen, J.; Zacharek, A.; Zhang, C.; Jiang, H.; Li, Y.; Roberts, C.; Lu, M.; Kapke, A.; Chopp, M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor ex-pression and neurogenesis after stroke in mice. J. Neurosci. 2005, 25, 2366–2375. [Google Scholar] [CrossRef]
- Hughes, M.N. Chemistry of Nitric Oxide and Related Species. Methods Enzymol. 2008, 436, 3–19. [Google Scholar] [CrossRef]
- Salvemini, D.; Doyle, T.; Cuzzocrea, S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem. Soc. Trans. 2006, 34, 965–970. [Google Scholar] [CrossRef]
- Ridger, V.C.; Greenacre, S.A.B.; Handy, R.L.C.; Halliwell, B.; Moore, P.K.; Whiteman, M.; Brain, S.D. Effect of peroxynitrite on plasma extravasation, microvascular blood flow and nociception in the rat. Br. J. Pharmacol. 1997, 122, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.X.; Xin, W.J.; Hu, N.W.; Zhang, T.; Xu, J.T.; Liu, X.G. Clonidine depresses LTP of C-fiber evoked potentials in spinal dorsal horn via NO-cGMP pathway. Brain Res. 2006, 1118, 58–65. [Google Scholar] [CrossRef]
- Ortiz, M.I.; Medina-Tato, D.A.; Sarmiento-Heredia, D.; Palma-Martínez, J.; Granados-Soto, V. Possible activation of the NO–cyclic GMP–protein kinase G–K+ channels pathway by gabapentin on the formalin test. Pharmacol. Biochem. Behav. 2006, 83, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, J. Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci. 2008, 27, 2783–2802. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, O.A.; Doreulee, N.; Chepkova, A.N.; Kazmierczak, T.; Haas, H.L. Long-term depression of cortico-striatal synaptic transmission by DHPG depends on endocannabinoid release and nitric oxide synthesis. Eur. J. Neurosci. 2007, 26, 1889–1894. [Google Scholar] [CrossRef]
- Paul-Clark, M.J.; Gilroy, D.W.; Willis, D.; Willoughby, D.A.; Tomlinson, A. Nitric Oxide Synthase Inhibitors Have Opposite Effects on Acute Inflammation Depending on Their Route of Administration. J. Immunol. 2001, 166, 1169–1177. [Google Scholar] [CrossRef]
- Iadecola, C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997, 20, 132–139. [Google Scholar] [CrossRef]
- Kawabata, A.; Manabe, S.; Manabe, Y.; Takagi, H. Effect of topical administration of l-arginine on formalin-induced nociception in the mouse: A dual role of peripherally formed NO in pain modulation. Br. J. Pharmacol. 1994, 112, 547–550. [Google Scholar] [CrossRef]
- Koch, A.; Zacharowski, K.; Boehm, O.; Stevens, M.; Lipfert, P.; Von Giesen, H.J.; Wolf, A.; Freynhagen, R. Nitric oxide and pro-inflammatory cytokines correlate with pain intensity in chronic pain patients. Inflamm. Res. 2007, 56, 32–37. [Google Scholar] [CrossRef]
- Handy, R.L.C.; Moore, P.K. Effects of selective inhibitors of neuronal nitric oxide synthase on carrageenan-induced me-chanical and thermal hyperalgesia. Neuropharmacology 1998, 37, 37–43. [Google Scholar] [CrossRef]
- Mollace, V.; Muscoli, C.; Masini, E.; Cuzzocrea, S.; Salvemini, D. Modulation of Prostaglandin Biosynthesis by Nitric Oxide and Nitric Oxide Donors. Pharmacol. Rev. 2005, 57, 217–252. [Google Scholar] [CrossRef] [PubMed]
- Dudhgaonkar, S.P.; Tandan, S.K.; Kumar, D.; Naik, A.K.; Raviprakash, V. Ameliorative effect of combined administration of inducible nitric oxide synthase inhibitor with cyclooxygenase-2 inhibitors in neuropathic pain in rats. Eur. J. Pain 2007, 11, 528–534. [Google Scholar] [CrossRef] [PubMed]

| Drug | Molecule |
|---|---|
| Ketorolac |  |
| L-NG-Nitroarginine methyl ester (L-NAME) | 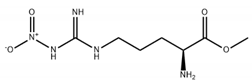 |
| Nanofullerol | 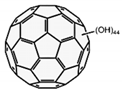 |
| Carboxymethyl lysine (CML) | 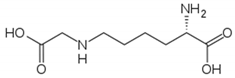 |
| N (ω)-propyl-L-arginine (NPLA) | 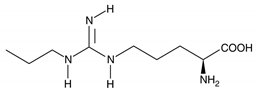 |
| 4-Methylaminopyridine | 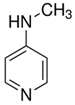 |
| Glycerol trinitrate (GTN) | 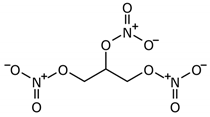 |
| Nitro flurbiprofen(HCT-1026) |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shnayder, N.A.; Petrova, M.M.; Popova, T.E.; Davidova, T.K.; Bobrova, O.P.; Trefilova, V.V.; Goncharova, P.S.; Balberova, O.V.; Petrov, K.V.; Gavrilyuk, O.A.; et al. Prospects for the Personalized Multimodal Therapy Approach to Pain Management via Action on NO and NOS. Molecules 2021, 26, 2431. https://doi.org/10.3390/molecules26092431
Shnayder NA, Petrova MM, Popova TE, Davidova TK, Bobrova OP, Trefilova VV, Goncharova PS, Balberova OV, Petrov KV, Gavrilyuk OA, et al. Prospects for the Personalized Multimodal Therapy Approach to Pain Management via Action on NO and NOS. Molecules. 2021; 26(9):2431. https://doi.org/10.3390/molecules26092431
Chicago/Turabian StyleShnayder, Natalia A., Marina M. Petrova, Tatiana E. Popova, Tatiana K. Davidova, Olga P. Bobrova, Vera V. Trefilova, Polina S. Goncharova, Olga V. Balberova, Kirill V. Petrov, Oksana A. Gavrilyuk, and et al. 2021. "Prospects for the Personalized Multimodal Therapy Approach to Pain Management via Action on NO and NOS" Molecules 26, no. 9: 2431. https://doi.org/10.3390/molecules26092431
APA StyleShnayder, N. A., Petrova, M. M., Popova, T. E., Davidova, T. K., Bobrova, O. P., Trefilova, V. V., Goncharova, P. S., Balberova, O. V., Petrov, K. V., Gavrilyuk, O. A., Soloveva, I. A., Medvedev, G. V., & Nasyrova, R. F. (2021). Prospects for the Personalized Multimodal Therapy Approach to Pain Management via Action on NO and NOS. Molecules, 26(9), 2431. https://doi.org/10.3390/molecules26092431








