Efficient Red Thermally Activated Delayed Fluorescence Emitters Based on a Dibenzonitrile-Substituted Dipyrido[3,2-a:2′,3′-c]phenazine Acceptor
Abstract
1. Introduction
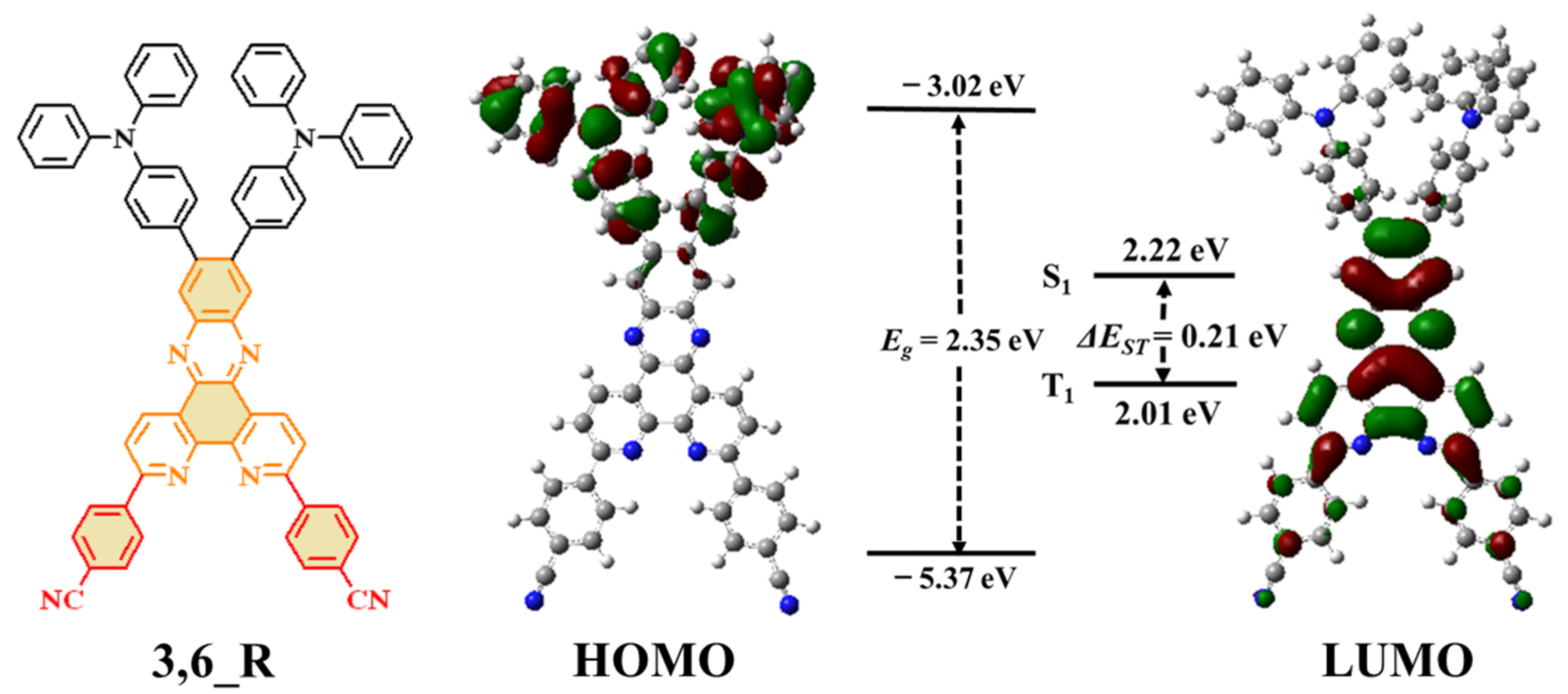
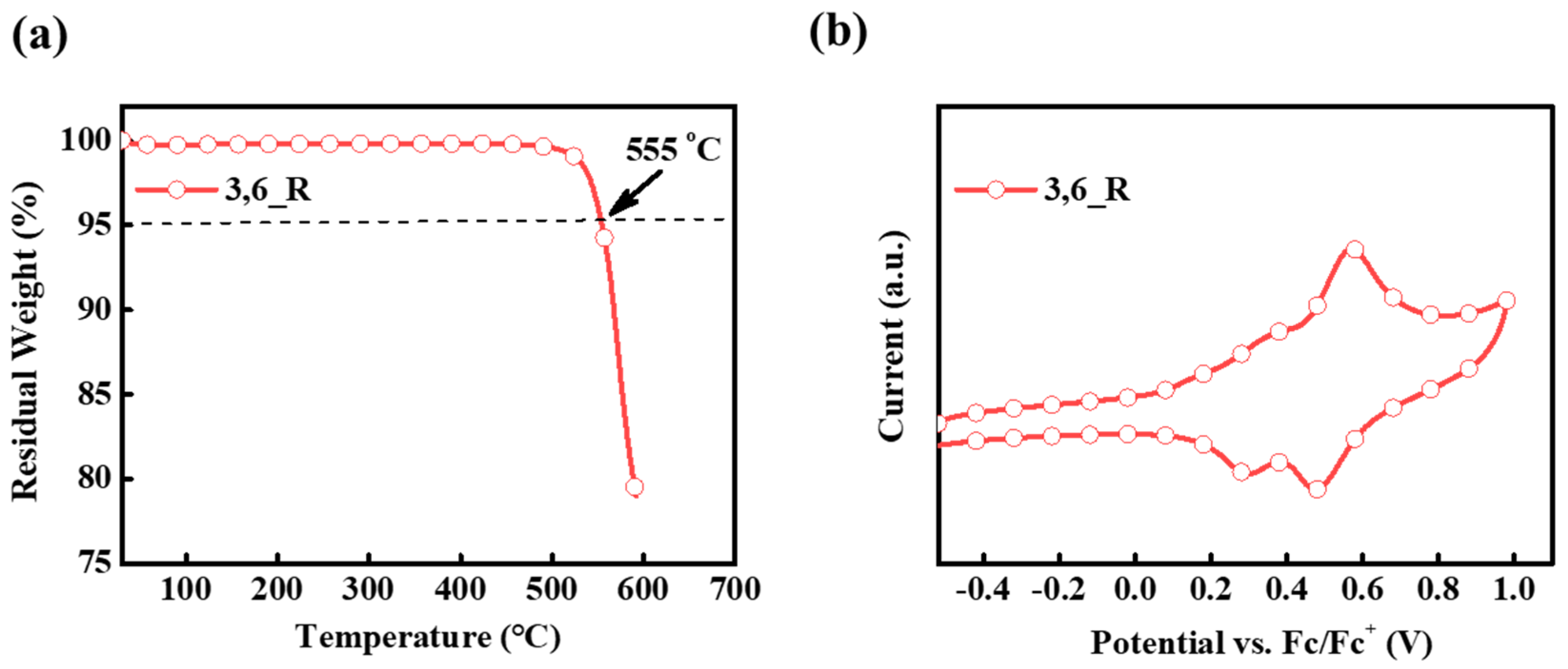
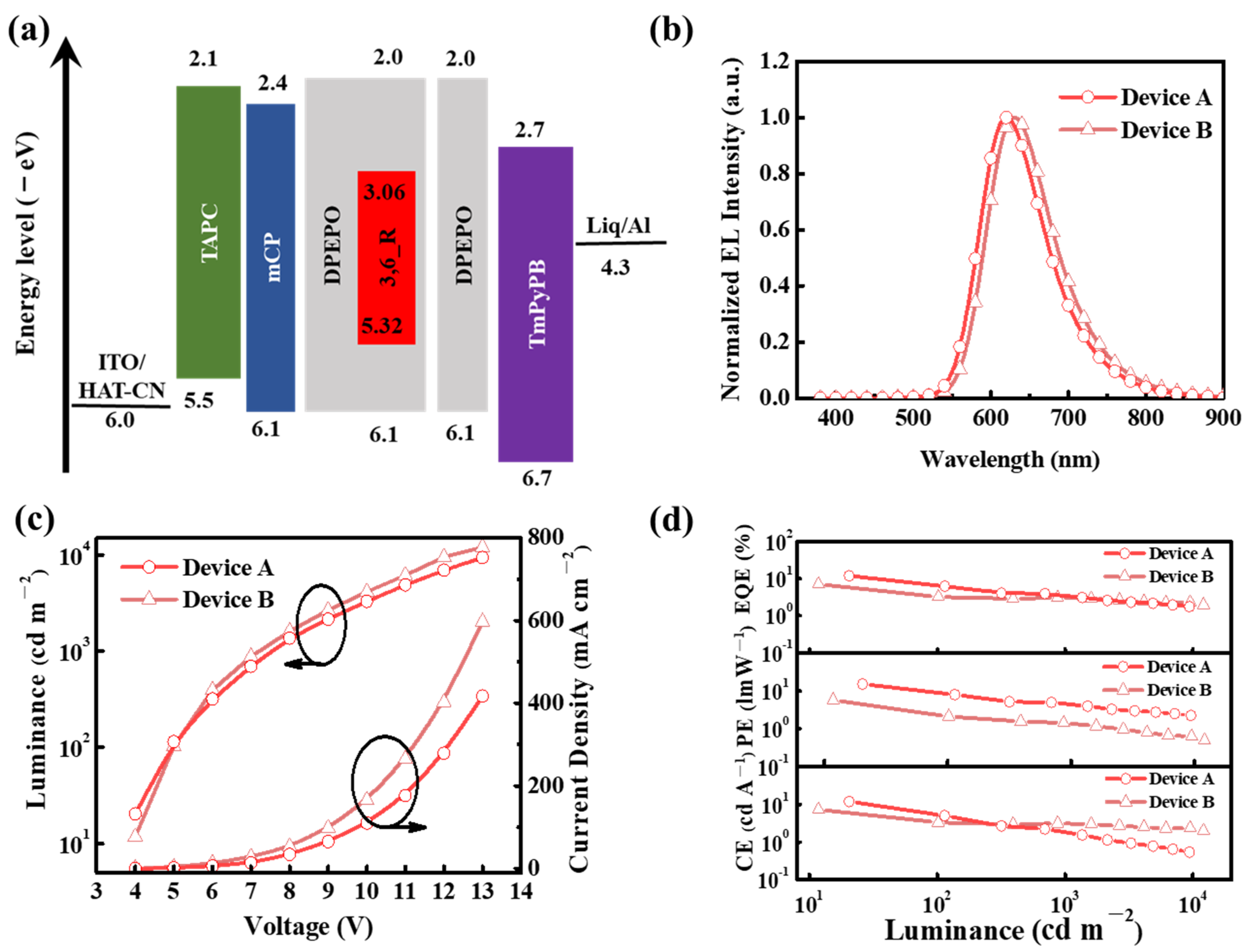
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light emitting diodes from dela-yed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W. Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics. Adv. Mater. 2014, 26, 7931–7958. [Google Scholar] [CrossRef]
- Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M.P. Recent advances in organic the-rmally activated delayed fluorescence materials. Chem. Soc. Rev. 2017, 46, 915–1016. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Ren, Z.; Yan, S.; Bryce, M.R. All-organic thermally activated delayed fluorescence materials for organ-ic light-emitting diodes. Nat. Rev. Mater. 2018, 3, 1038–1058. [Google Scholar] [CrossRef]
- Wong, M.Y.; Zysman-Colman, E. Purely organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.-S.; Gillett, A.J.; Zhang, S.-F.; Ye, H.; Liu, Y.; Chen, X.-K.; Lin, Z.-S.; Evans, E.W.; Myers, W.K.; Ronson, T.K.; et al. Fast spin-flip enables efficient and stable organic electroluminescence from charge-transfer states. Nat. Photon. 2020, 14, 636–642. [Google Scholar] [CrossRef]
- Wada, Y.; Nakagawa, H.; Matsumoto, S.; Wakisaka, Y.; Kaji, H. Organic light emitters exhibiting very fast reverse i-ntersystem crossing. Nat. Photon. 2020, 14, 643–649. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, T.; Wang, Z.; Wang, L.; Zhan, L.; Gong, S.; Zhong, C.; Lu, Z.-H.; Zhang, S.; Yang, C. De novo des-ign of excited-state intramolecular proton transfer emitters via a thermally activated delayed fluorescence channel. J. Am. Chem. Soc. 2018, 140, 8877–8886. [Google Scholar] [CrossRef]
- Yang, M.; Park, I.S.; Yasuda, T. Full-color, narrowband, and high-efficiency electroluminescence from boron and carb-azole embedded polycyclic heteroaromatics. J. Am. Chem. Soc. 2020, 142, 19468–19472. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.; Kim, S.; Lee, H.; Ko, I.; Karthik, D.; Lee, J.; Kwon, J.H. Highly efficient blue thermally activated delayed f-luorescence emitters based on symmetrical and rigid oxygen-bridged boron acceptors. Nat. Photon. 2019, 13, 540–546. [Google Scholar] [CrossRef]
- Lin, T.-A.; Chatterjee, T.; Tsai, W.-L.; Lee, W.-K.; Wu, M.-J.; Jiao, M.; Pan, K.-C.; Yi, C.-L.; Chung, C.-L.; Wong, K.-T.; et al. Sky-blue organic light emitting diode with 37% external quantum efficiency using thermally activated dela-yed fluorescence from spiroacridine-triazine hybrid. Adv. Mater. 2016, 28, 6976–6983. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, P.; Gong, S.; Huang, Y.-H.; Wang, C.-Y.; Zhong, C.; Zeng, W.; Chen, Z.; Lee, W.-K.; Yin, X.; et al. Ac-ceptor plane expansion enhances horizontal orientation of thermally activated delayed fluorescence emitter. Sci. Adv. 2020, 6, eaba7855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, D.; Wei, J.; Liu, Z.; Lu, Y.; Duan, L. Multi-resonance induced thermally activated delayed fluoro-phores for narrowband green OLEDs. Angew. Chem. Int. Ed. 2019, 58, 16912–16917. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-L.; Huang, M.-J.; Lin, C.-C.; Huang, P.-Y.; Chou, T.-Y.; Chen-Cheng, R.-W.; Lin, H.-W.; Liu, R.-S.; Cheng, C.-H. Diboron compound-based organic light-emitting diodes with high efficiency and reduced efficiency roll-off. Nat. Photon. 2018, 12, 235–240. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Ran, Q.; Wang, Q.; Liu, Y.; Hänisch, C.; Reineke, S.; Fan, J.; Liao, L.-S. High-efficiency red organic lig-ht-emitting diodes with external quantum efficiency close to 30% based on a novel thermally activated delayed fluo-rescence emitter. Adv. Mater. 2019, 31, 1902368. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Liang, Q.; Wang, R.; Hou, J.; Li, W.; Peng, Q.; Shuai, Z.; Qiao, J. Highly efficient thermally activated delay-ed fluorescence via J-aggregates with strong intermolecular charge transfer. Adv. Mater. 2019, 31, 1808242. [Google Scholar] [CrossRef]
- Zeng, W.; Zhou, T.; Ning, W.; Zhong, C.; He, J.; Gong, S.; Xie, G.; Yang, C. Realizing 22.5% external quantum effic-iency for solution-processed thermally activated delayed-fluorescence OLEDs with red emission at 622 nm via a syn-ergistic strategy of molecular engineering and host selection. Adv. Mater. 2019, 31, 1901404. [Google Scholar] [CrossRef]
- Zhang, Q.; Kuwabara, H.; Potscavage, W.J.; Huang, S.; Hatae, Y.; Shibata, T.; Adachi, C. Anthraquinone-based intra-molecular charge-transfer compounds: Computational molecular design, thermally activated delayed fluorescence, and highly efficient red electroluminescence. J. Am. Chem. Soc. 2014, 136, 18070. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, J.H.; Lee, J.Y. Recent progress of highly efficient red and near-infrared thermally activated delayed fluorescent emitters. Adv. Opt. Mater. 2018, 6, 1800255. [Google Scholar] [CrossRef]
- Chen, J.-X.; Tao, W.-W.; Chen, W.-C.; Xiao, Y.-F.; Wang, K.; Cao, C.; Yu, J.; Li, S.; Geng, F.-X.; Adachi, C.; et al. Re-d/Near-infrared thermally activated delayed fluorescence OLEDs with near 100 % internal quantum efficiency. Angew. Chem. Int. Ed. 2019, 58, 14660–14665. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Duan, R.; Liang, B.; Han, G.; Wang, S.; Ye, K.; Liu, Y.; Yi, Y.; Wang, Y. Deep-red to near-infrared thermally activated delayed fluorescence in organic solid films and electroluminescent devices. Angew. Chem. Int. Ed. 2017, 56, 11525–11529. [Google Scholar] [CrossRef]
- Gong, X.; Lu, C.-H.; Lee, W.-K.; Li, P.; Huang, Y.-H.; Chen, Z.; Zhan, L.; Wu, C.-C.; Gong, S.; Yang, C. High-efficie-ncy red thermally activated delayed fluorescence emitters based on benzothiophene-fused spiro-acridine donor. Chem. Eng. J. 2021, 405, 126663. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, B.; Ma, P.; Li, Z.; Wang, X.; Zhao, C.; Fan, X.; Tao, L.; Duan, C.; Zhang, J.; et al. A red thermally activated delayed fluorescence emitter employing dipyridophenazine with a gradient multi-induct-ive effect to impr-ove radiation efficiency. J. Mater. Chem. C 2019, 7, 7525–7530. [Google Scholar] [CrossRef]
- Chen, J.-X.; Wang, K.; Zheng, C.-J.; Zhang, M.; Shi, Y.-Z.; Tao, S.-L.; Lin, H.; Liu, W.; Tao, W.-W.; Ou, X.-M.; et al. Red organic light-emitting diode with external quantum efficiency beyond 20% based on a novel thermally activated delayed fluorescence emitter. Adv. Sci. 2018, 5, 1800436. [Google Scholar] [CrossRef]
- Jin, J.; Wang, W.; Xue, P.; Yang, Q.; Jiang, H.; Tao, Y.; Zheng, C.; Xie, G.; Huang, W.; Chen, R. Intermolecular locking design of red thermally activated delayed fluorescence molecules for high-performance solution-processed organic light-emitting diodes. J. Mater. Chem. C 2021, 9, 2291–2297. [Google Scholar] [CrossRef]
- Wang, S.; Yan, X.; Cheng, Z.; Zhang, H.; Liu, Y.; Wang, Y. Highly efficient near-infrared delayed fluorescence orga-nic light emitting diodes using a phenanthrene-based charge-transfer compound. Angew. Chem. Int. Ed. 2015, 54, 13068–13072. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nakagawa, T.; MacDonald, J.; Zhang, Q.; Nomura, H.; Miyazaki, H.; Adachi, C. Highly efficient organic light-emitting diode based on a hidden thermally activated delayed fluorescence channel in a heptazine derivative. Adv. Mater. 2013, 25, 3319–3323. [Google Scholar] [CrossRef] [PubMed]
- Furue, R.; Matsuo, K.; Ashikari, Y.; Ooka, H.; Amanokura, N.; Yasuda, T. Highly efficient red-orange delayed fluore-scence emitters based on strong π-accepting dibenzophenazine and dibenzoquinoxaline cores: Toward a rational pur-ered OLED design. Adv. Opt. Mater. 2018, 6, 1701147. [Google Scholar] [CrossRef]
- Kothavale, S.; Chung, W.J.; Lee, J.Y. Rational molecular design of highly efficient yellow-red thermally activated del-ayed fluorescent emitters: A combined effect of auxiliary fluorine and rigidified acceptor unit. ACS Appl. Mater. Interfaces 2020, 12, 18730–18738. [Google Scholar] [CrossRef]
- Balijapalli, U.; Nagata, R.; Yamada, N.; Nakanotani, H.; Tanaka, M.; D’Aléo, A.; Placide, V.; Mamada, M.; Tsuchiya, Y.; Adachi, C. Highly efficient near-infrared electrofluorescence from a thermally-activated delayed fluorescence mole-cule. Angew. Chem. Int. Ed. 2021, 60, 8477–8482. [Google Scholar] [CrossRef]
- Gong, X.; Li, P.; Huang, Y.-H.; Wang, C.-Y.; Lu, C.-H.; Lee, W.-K.; Zhong, C.; Chen, Z.; Ning, W.; Wu, C.-C.; et al. A red thermally activated delayed fluorescence emitter simultaneously having high photoluminescence quantum effic-iency and preferentially horizontal emitting dipole orientation. Adv. Funct. Mater. 2020, 30, 1908839. [Google Scholar] [CrossRef]
- Noda, H.; Nakanotani, H.; Adachi, C. Excited state engineering for efficient reverse intersystem crossing. Sci. Adv. 2018, 4, eaao6910. [Google Scholar] [CrossRef] [PubMed]
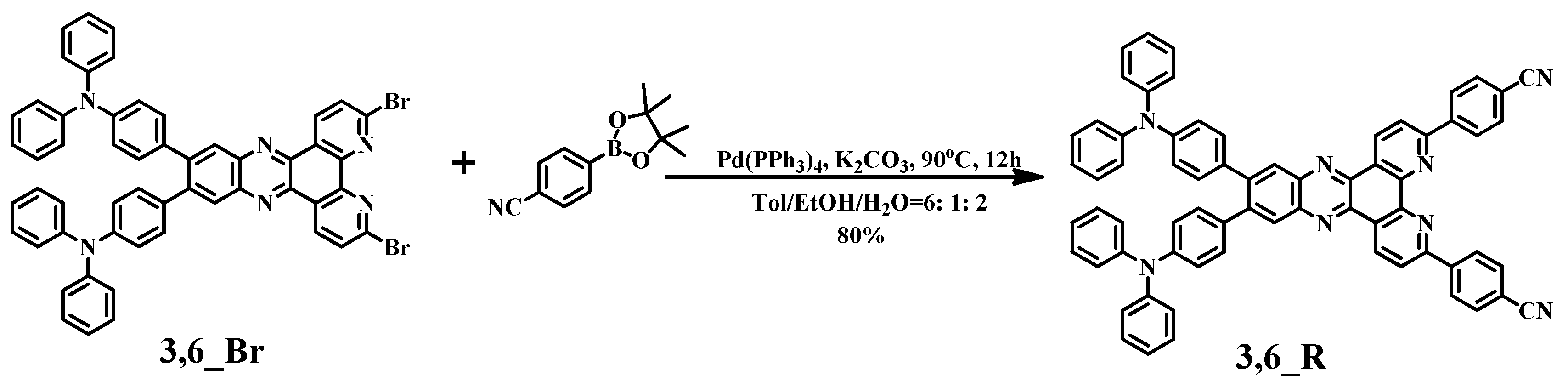

| Compound | λabs [nm] a | λem [nm] b | τp/τd [ns]/[μs] c | Φp/Φd [%] d | ΦPL [%] e | kr,S/kRISC [107 s−1] f |
|---|---|---|---|---|---|---|
| 3,6_R | 355/480 | 571/613 | 20/1.04 | 72/14 | 86 | 3.6/0.067 |
| Devices | Von [V] a | λEL [nm] b | Lmax [cd m−2] c | CE [cd A−1] d | PE [lm W−1] e | EQE [%] f | CIE (x,y) g |
|---|---|---|---|---|---|---|---|
| A | 4.0 | 619 | 9382 | 15.3/5.5/1.9 | 12.0/8.7/4.5 | 12.0/6.6/3.5 | (0.62, 0.38) |
| B | 4.0 | 629 | 12100 | 7.4/2.1/1.4 | 5.8/3.6/3.2 | 7.1/3.2/3.0 | (0.64, 0.36) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Zeng, X.; Ning, W.; Ying, A.; Luo, Y.; Gong, S. Efficient Red Thermally Activated Delayed Fluorescence Emitters Based on a Dibenzonitrile-Substituted Dipyrido[3,2-a:2′,3′-c]phenazine Acceptor. Molecules 2021, 26, 2427. https://doi.org/10.3390/molecules26092427
He L, Zeng X, Ning W, Ying A, Luo Y, Gong S. Efficient Red Thermally Activated Delayed Fluorescence Emitters Based on a Dibenzonitrile-Substituted Dipyrido[3,2-a:2′,3′-c]phenazine Acceptor. Molecules. 2021; 26(9):2427. https://doi.org/10.3390/molecules26092427
Chicago/Turabian StyleHe, Lin, Xuan Zeng, Weimin Ning, Ao Ying, Yunbai Luo, and Shaolong Gong. 2021. "Efficient Red Thermally Activated Delayed Fluorescence Emitters Based on a Dibenzonitrile-Substituted Dipyrido[3,2-a:2′,3′-c]phenazine Acceptor" Molecules 26, no. 9: 2427. https://doi.org/10.3390/molecules26092427
APA StyleHe, L., Zeng, X., Ning, W., Ying, A., Luo, Y., & Gong, S. (2021). Efficient Red Thermally Activated Delayed Fluorescence Emitters Based on a Dibenzonitrile-Substituted Dipyrido[3,2-a:2′,3′-c]phenazine Acceptor. Molecules, 26(9), 2427. https://doi.org/10.3390/molecules26092427







