The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends
Abstract
1. Introduction
2. Results
2.1. Standard Quality Parameters
2.2. Antioxidant Properties
2.3. Protein Content and Enzyme Activities
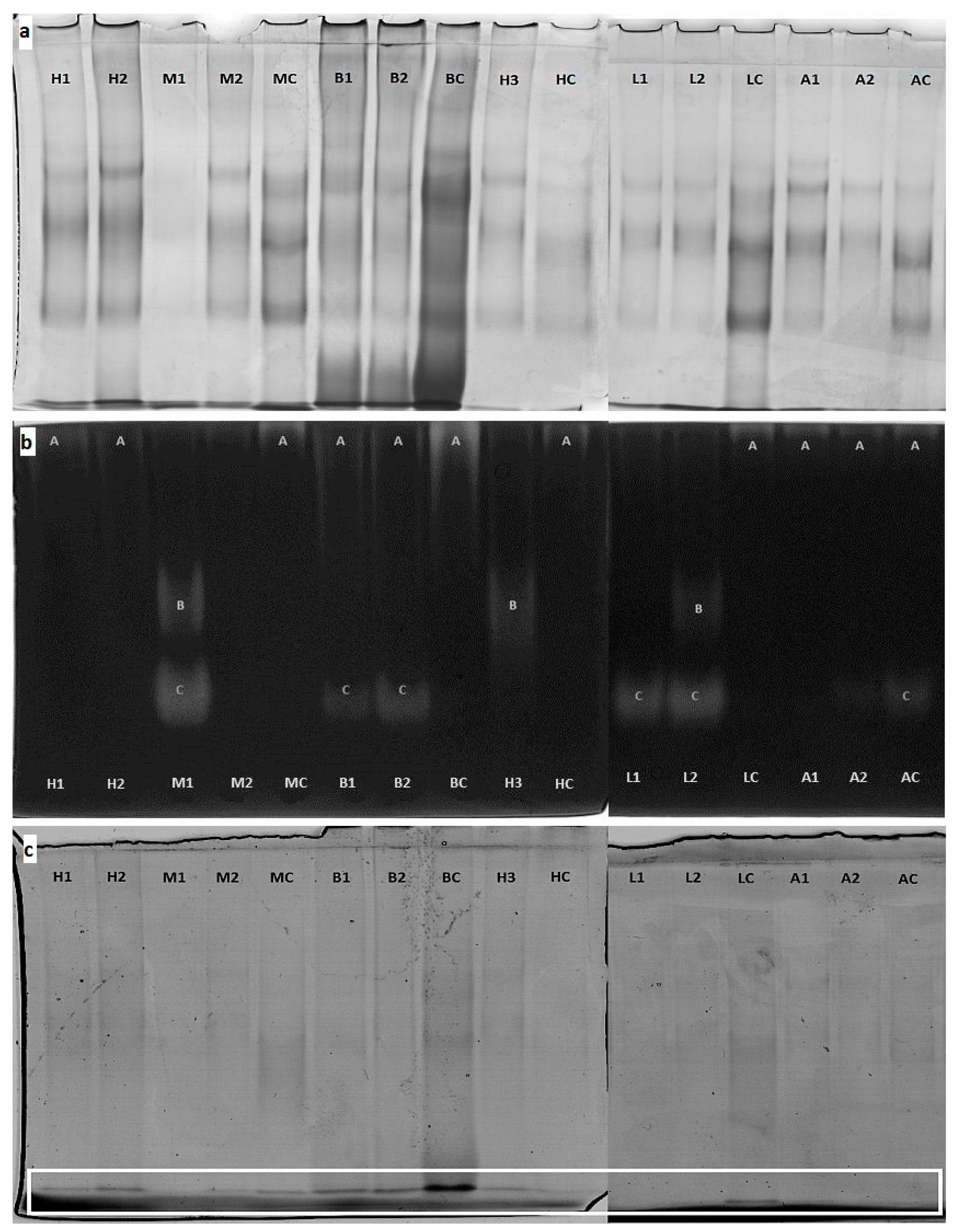
2.4. Protein Profile by PAGE
2.5. Native Enzymes Detection by PAGE
2.6. Multivariate Statistical Analysis of Obtained Results
3. Materials and Methods
3.1. Honey
3.2. Refractometric Determination of the Water Content in Honey
3.3. Active and Free Acidity
3.4. Conductivity
3.5. HMF Determination
3.6. Total Protein Content
3.7. Diastase Number Determination
3.8. Acid Phosphatase Activity Assay
3.9. Total Phenolic Content Determination
3.10. Antioxidant Assays
3.10.1. DPPH Test
3.10.2. FRAP Assay
3.11. Electrophoretic Analyses
3.11.1. Native Protein Electrophoresis
3.11.2. Amylase Electrophoretic Detection
3.11.3. Acid Phosphatase Electrophoretic Detection
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zábrodská, B.; Vorlová, L. Adulteration of honey and available methods for detection—A review. Acta Vet. Brno. 2014, 83, S85–S102. [Google Scholar] [CrossRef]
- Directive 2014/63/EU of the European Parliament and of the Council amending Council Directive2001/110/EC relating to honey. Off. J. Eur. Communities 2014, 57, L164/1.
- Dżugan, M.; Ruszel, A.; Tomczyk, M. Quality of imported honeys obtainable on the market in the podkarpacie region. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2018, 25, 127–139. [Google Scholar] [CrossRef]
- Asha’Ari, Z.A.; Ahmad, M.Z.; Wan Din, W.S.J.; Che Hussin, C.M.; Leman, I. Ingestion of honey improves the symptoms of allergic rhinitis: Evidence from a randomized placebo-controlled trial in the East Coast of Peninsular Malaysia. Ann. Saudi Med. 2013, 33, 469–475. [Google Scholar] [CrossRef]
- Newman, T.J. Local, Unpasteurized Honey as a Treatment for Allergic Rhinitis: A Systematic Review Local, Unpasteurized Honey as a Treatment for Allergic Rhinitis. Master’s Thesis, Pacific University Oregon, Forest Grove, OR, USA, 2014. [Google Scholar]
- Majewska, E.; Drużyńska, B.; Wołosiak, R. Determination of the botanical origin of honeybee honeys based on the analysis of their selected physicochemical parameters coupled with chemometric assays. Food Sci. Biotechnol. 2019, 28, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, A.; Muzolf-Panek, M.; Tomaszewska-Gras, J.; Konieczny, P. Predicting the botanical origin of honeys with chemometric analysis according to their antioxidant and physicochemical properties. Pol. J. Food Nutr. Sci. 2019, 69, 191–201. [Google Scholar] [CrossRef]
- Mai, Z.; Lai, B.; Sun, M.; Shao, J.; Guo, L. Food adulteration and traceability tests using stable carbon isotope technologies. Trop. J. Pharm. Res. 2019, 18, 1771–1784. [Google Scholar] [CrossRef]
- Wu, L.; Du, B.; Vander Heyden, Y.; Chen, L.; Zhao, L.; Wang, M.; Xue, X. Recent advancements in detecting sugar-based adulterants in honey—A challenge. Trac. Trends Anal. Chem. 2017, 86, 25–38. [Google Scholar] [CrossRef]
- Guler, A.; Kocaokutgen, H.; Garipoglu, A.V.; Onder, H.; Ekinci, D.; Biyik, S. Detection of adulterated honey produced by honeybee (Apis mellifera L.) colonies fed with different levels of commercial industrial sugar (C3 and C4 plants) syrups by the carbon isotope ratio analysis. Food Chem. 2014, 155, 155–160. [Google Scholar] [CrossRef]
- Baroni, M.V.; Chiabrando, G.A.; Costa, C.; Wunderlin, D.A. Assessment of the floral origin of honey by SDS-page immunoblot techniques. J. Agric. Food Chem. 2002, 50, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. Physical properties of honey: Honey composition. In The Honey Book; University of Belgrade: Belgrade, Serbia, 2011. [Google Scholar]
- Machado De-Melo, A.A.; Almeida-Muradian, L.B.D.; Sancho, M.T.; Pascual-Maté, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2018, 57, 5–37. [Google Scholar] [CrossRef]
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislation of honey criteria and standards. J. Apic. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Bocian, A.; Buczkowicz, J.; Jaromin, M.; Hus, K.K.; Legáth, J. An Effective Method of Isolating Honey Proteins. Molecules 2019, 24, 2399. [Google Scholar] [CrossRef]
- Wesołowska, M.; Dżugan, M. Activity and thermal stability of diastase present in honey from Podkarpacie region. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2017, 24, 103–112. [Google Scholar] [CrossRef]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Czech J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- Eshete, Y.; Eshete, T. A Review on the Effect of Processing Temperature and Time duration on Commercial Honey Quality. Madr. J. Food Technol. 2019, 4, 158–162. [Google Scholar] [CrossRef]
- Sanz, M.L.; Del Castillo, M.D.; Corzo, N.; Olano, A. 2-Furoylmethyl Amino Acids and Hydroxymethylfurfural As Indicators of Honey Quality. J. Agric. Food Chem. 2003, 51, 4278–4283. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar]
- Dżugan, M.; Grabek-Lejko, D.; Swacha, S.; Tomczyk, M.; Bednarska, S.; Kapusta, I. Physicochemical quality parameters, antibacterial properties and cellular antioxidant activity of Polish buckwheat honey. Food Biosci. 2020, 34, 100538. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitability for Romanian honey authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Cabrera, M.; Perez, M.; Gallez, L.; Andrada, A.; Balbarrey, G. Colour, antioxidant capacity, phenolic and flavonoid content of honey from the Humid Chaco Region, Argentina. Phyton 2017, 86, 124–130. [Google Scholar] [CrossRef]
- Cimpoiu, C.; Hosu, A.; Miclaus, V.; Puscas, A. Determination of the floral origin of some Romanian honeys on the basis of physical and biochemical properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 100, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Flanjak, I.; Strelec, I.; Kenjerić, D.; Primorac, L. Croatian produced unifloral honeys characterised according to the protein and proline content and enzyme activities. J. Apic. Sci. 2016, 60, 39–48. [Google Scholar] [CrossRef]
- White, J.W. Quality evaluation of honey: Role of HMF and diastase assays. Am. Bee J. 1992, 132, 737–743. [Google Scholar] [CrossRef]
- Bonta, V.; Dezmirean, D.S.; Marghitas, L.A.; Urcan, A.C.; Bobis, O. Sugar Spectrum, Hydroxymethylfurfural and Diastase Activity in Honey: A Validated Approach as Indicator of Possible Adulteration. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Anim. Sci. Biotechnol. 2020, 77, 44. [Google Scholar] [CrossRef]
- Alonso-Torre, S.R.; Cavia, M.M.; Fernández-Muiño, M.A.; Moreno, G.; Huidobro, J.F.; Sancho, M.T. Evolution of acid phosphatase activity of honeys from different climates. Food Chem. 2006, 97, 750–755. [Google Scholar] [CrossRef]
- Dżugan, M.; Wesołowska, M. Jakość Miodów Produkowanych na Podkarpaciu (The Quality of Honeys Produced in Podkarpackie Region, in Polish); Uniwersytet Rzeszowski: Rzeszów, Poland, 2016; pp. 52–54. [Google Scholar]
- Saxena, S.; Gautam, S. A Preliminary Cost Effective Qualitative Assay for Diastase Analysis in Honey. Insights Enzym. Res. 2018, 01, 1–4. [Google Scholar] [CrossRef]
- Voldřich, M.; Rajchl, A.; Čižkova, H.; Cuhra, P. Detection of Foreign Enzyme Addition into the Adulterated Honey. Czech J. Food Sci. 2009, 27, S280–S282. [Google Scholar] [CrossRef]
- Jimenez, D.R.; Gilliam, M. Ultrastructure of the ventriculus of the honey bee, Apis mellifera (L.): Cytochemical localization of acid phosphatase, alkaline phosphatase, and nonspecific esterase. Cell Tissue Res. 1990, 261, 431–443. [Google Scholar] [CrossRef]
- Borutinskaite, V.; Treigyte, G.; Čeksteryte, V.; Kurtinaitiene, B.; Navakauskiene, R. Proteomic identification and enzymatic activity of buckwheat (Fagopyrum esculentum) honey based on different assays. J. Food Nutr. Res. 2018, 57, 57–69. [Google Scholar]
- Nisbet, C.; Guler, A.; Ciftci, G.; Yarim, G.F. The Investigation of Protein Prophile of Different Botanic Origin Honey and Density Saccharose-Adulterated Honey by SDS-PAGE Method. Kafkas Univ. Vet.Fak. Derg. 2009, 15, 443–446. [Google Scholar]
- Wang, J.; Kliks, M.M.; Qu, W.; Jun, S.; Shi, G.; Li, Q.X. Rapid determination of the geographical origin of honey based on protein fingerprinting and barcoding using MALDI TOF MS. J. Agric. Food Chem. 2009, 57, 10081–10088. [Google Scholar] [CrossRef]
- Di Girolamo, F.; D’Amato, A.; Righetti, P.G. Assessment of the floral origin of honey via proteomic tools. J. Proteom. 2012, 75, 3688–3693. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.S.; Valentim-Neto, P.A.; Seraglio, S.K.T.; da Luz, C.F.P.; Arisi, A.C.M.; Costa, A.C.O. Proteome comparison for discrimination between honeydew and floral honeys from botanical species Mimosa scabrella Bentham by principal component analysis. J. Sci. Food Agric. 2017, 97, 4515–4519. [Google Scholar] [CrossRef] [PubMed]
- Regulation 2009/17/94 of the Minister of Agriculture and Rural Development of Poland regarding methods analyzes related to honey assessment. J. Laws Repub. Poland 2009, 17, 94.
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein—Dye binding. Anal. Biochem. 1979, 10, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Neuhoff, V.; Arold, N.; Taube, D.; Ehrhardt, W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 1988, 9, 255–262. [Google Scholar] [CrossRef]
- Rafiei, B.; Ghadamyari, M.; Imani, S.; Hosseininaveh, V.; Ahadiyat, A. Purification and characterization of α-amylase in Moroccan locust, Dociostaurus maroccanus Thunberg (Orthoptera: Acrididae) and its inhibition by inhibitors from Phaseolus vulgaris L. Toxin Rev. 2016, 35, 90–97. [Google Scholar] [CrossRef][Green Version]
- Kalinowski, A.; Bocian, A.; Kosmala, A.; Winiarczyk, K. Two-dimensional patterns of soluble proteins including three hydrolytic enzymes of mature pollen of tristylous Lythrum salicaria. Sex. Plant Reprod. 2007, 20, 51–62. [Google Scholar] [CrossRef]

| Honey Sample | Moisture Content [%] | pH | Free Acidity [mval/kg] | Electrical Conductivity [mS/cm] | HMF Content [mg/kg] | |
|---|---|---|---|---|---|---|
| Nectar Honey | A1 | 17.20 ± 0.00 | 3.99 ± 0.02 * | 16.00 ± 1.40 * | 0.40 ± 0.00 * | 5.82 ± 0.00 * |
| A2 | 18.75 ± 0.05 * | 4.23 ± 0.01 * | 10.50 ± 0.70 * | 0.31 ± 0.02 | 35.20 ± 0.00 * | |
| AC | 17.17 ± 0.30 | 3.77 ± 0.03 | 20.85 ± 6.50 | 0.31 ± 0.18 | 22.66 ± 0.00 | |
| B1 | 19.80 ± 0.00 | 4.01 ± 0.02 * | 21.50 ± 0.70 * | 0.54 ± 0.00 * | 6.40 ± 0.60 * | |
| B2 | 19.70 ± 0.00 | 3.83 ± 0.00 | 23.50 ± 0.70 * | 0.54 ± 0.01 * | 34.18 ± 0.00 * | |
| BC | 20.50 ± 0.00 | 4.04 ± 0.00 * | 26.50 ± 0.00 | 0.43 ± 0.01 | 20.02 ± 0.00 | |
| L1 | 20.50 ± 0.10 | 4.32 ± 0.00 | 14.50 ± 0.70 * | 0.25 ± 0.02 * | 19.33 ± 0.00 * | |
| L2 | 19.05 ± 0.15 * | 4.42 ± 0.02 | 14.50 ± 0.70 * | 0.23 ± 0.02 * | 23.23 ± 0.00 * | |
| LC | 20.30 ± 0.10 | 4.13 ± 0.00 | 25.50 ± 0.00 | 0.64 ± 0.00 | 13.20 ± 0.00 | |
| M1 | 18.85 ± 0.05 * | 4.30 ± 0.02 | 10.50 ± 2.10 * | 0.79 ± 0.02 * | 73.92 ± 0.00 * | |
| M2 | 18.35 ± 0.05 * | 3.75 ± 0.02 * | 16.50 ± 0.70 * | 0.75 ± 0.01 * | 47.55 ± 0.48 * | |
| MC | 20.20 ± 0.00 | 4.11 ± 0.00 | 29.50 ± 0.00 | 0.67 ± 0.00 | 12.50 ± 0.00 | |
| Applicable limits [2] | 20% | <50 | <0.8 | <40 | ||
| Honeydew Honey | H1 | 17.55 ± 0.05 * | 4.54 ± 0.02 * | 29.50 ± 0.70 * | 1.70 ± 0.01 * | 11.78 ± 0.12 * |
| H2 | 17.55 ± 0.05 * | 4.44 ± 0.01 * | 38.00 ± 1.40 * | 1.95 ± 0.06 * | 35.38 ± 0.36 * | |
| H3 | 18.75 ± 0.15 * | 4.68 ± 0.00 * | 29.50 ± 2.10 * | 1.99 ± 0.07 * | 15.04 ± 0.14 | |
| HC | 19.90 ± 0.00 | 3.64 ± 0.00 | 30.50 ± 0.00 | 1.16± 0.00 | 19.80± 0.00 | |
| Applicable limits [2] | 20% | <50 | >0.8 | <40 | ||
| Honey Sample | Protein Content [mg/100 g] | Enzymatic Activity | Antioxidant Activity | |||
|---|---|---|---|---|---|---|
| Diastase Activity (as Diastase Number DN) | Acid Phosphatase Activity (mmol/g/min) | Total Phenolic Content (mg GAE/kg) | FRAP (μmol TE/kg) | DPPH (% Radical Inhibition) | ||
| A1 | 27.27 ± 2.57 * | 8.3 ± 2.3 * | 5.0 ± 1.9 | 58.56 ± 0.56 * | 203.85 ± 2.04 * | 10.82 ± 0.22 * |
| A2 | 19.09 ± 2.57 * | 2.7 ± 0.6 * | 7.5 ± 2.6 | 98.20 ± 2.95 * | 317.31 ± 6.35 * | 11.51 ± 0.12 * |
| AC | 37.73 ± 0.64 | 5.63 ± 1.8 | 7.7 ± 0.5 | 140.81 ± 11.41 | 531.96 ± 18.50 | 19.64 ± 6.13 |
| B1 | 224.55 ± 6.43 * | 13.9 ± 3.8 * | 20.7 ± 0.4 * | 516.22 ± 5.02 * | 1113.46 ± 33.40 * | 33.99 ± 1.02 * |
| B2 | 175.46 ± 14.14 * | 10.3 ± 2.2 * | 19.5 ± 1.7 * | 653.15 ± 6.53 * | 623.08 ± 11.98 * | 39.55 ± 4.00 * |
| BC | 475.456 ± 3.86 | 66.43 ± 5.7 | 31.8 ± 2.6 | 2075.70 ± 19.90 | 4973.10 ± 46.73 | 59.69 ± 2.51 |
| L1 | 23.18 ± 0.64 * | 4.3 ± 1.1 * | 6.7 ± 0.9 * | 125.23 ± 1.50 * | 615.38 ± 12.31 * | 17.76 ± 1.60 * |
| L2 | 23.634 ± 3.856 * | 8.0 ± 1.2 * | 5.2 ± 1.2 * | 102.70 ± 1.13 * | 607.69 ± 18.23 * | 17.92 ± 0.36 * |
| LC | 89.55 ± 1.93 | 28.94 ± 3.8 | 17.8 ± 2.6 | 436.90 ± 4.25 | 1555.80 ± 14.82 | 23.30 ± 0.76 |
| M1 | 10.91 ± 0.00 * | 2.7 ± 0.7 * | 9.8 ± 1.7 * | 161.26 ± 2.01 * | 425.00 ± 8.50 * | 13.73 ± 0.14 * |
| M2 | 53.18 ± 3.21 * | 3.1 ± 0.9 * | 10.3 ± 1.4 | 117.20 ± 1.18 * | 334.62 ± 6.69 * | 13.41 ± 0.11 * |
| MC | 133.18 ± 4.50 | 29.51 ± 4.0 | 11.5 ± 0.2 | 496.80 ± 5.00 | 1470.20 ± 14.93 | 19.52 ± 0.26 |
| H1 | 89.09 ± 6.43 * | 16.3 ± 2.2 * | 16.7 ± 0.5 | 310.81 ± 2.89 * | 1390.38 ± 41.71 * | 79.37 ± 4.76 |
| H2 | 105.456 ± 21.86 * | 12.5 ± 1.9 * | 17.3 ± 2.8 * | 597.30 ± 11.95 | 1934.62 ± 77.38 * | 72.32 ± 0,71 |
| H3 | 70.46 ± 8.36 | 14.6 ± 1.0 | 16.3 ± 1.9 | 568.47 ± 6.25 * | 1488.46 ± 29.77 * | 12.73 ± 0.10 * |
| HC | 70.91 ± 2.57 | 17.45± 2.9 | 14.0 ± 1.9 | 646.80 ± 5.87 | 1526.00 ± 16.97 | 69.58 ± 0.35 |
| Original Group | Predicted Classification (Number of Samples) | Correct Classification (%) | |
|---|---|---|---|
| Imported | Local | ||
| imported | 11 | 0 | 100 |
| local | 1 | 4 | 80 |
| Total | 12 | 4 | 93.75 |
| Honey Samples | Original Group | Predicted Classification | |
| A1 | imported | imported | |
| A2 | imported | imported | |
| AC * | local | imported | |
| B1 | imported | imported | |
| B2 | imported | imported | |
| BC | local | local | |
| L1 | imported | imported | |
| L2 | imported | imported | |
| LC | local | local | |
| M1 | imported | imported | |
| M2 | imported | imported | |
| MC | local | local | |
| H1 | imported | imported | |
| H2 | imported | imported | |
| H3 | imported | imported | |
| HC | local | local | |
| Symbol | Variety | Type | Origin | Color | Crystallization State |
|---|---|---|---|---|---|
| A1 | acacia | blend, imported | from outside the EU | white | liquid |
| A2 | acacia | blend, imported | EU and from outside the EU | white | liquid |
| AC | acacia | raw, local | Podkarpackie, Poland | white | liquid |
| B1 | buckwheat | blend, imported | EU and from outside the EU | dark amber | partially crystallized |
| B2 | buckwheat | blend, imported | EU and from outside the EU | dark amber | crystallized |
| BC | buckwheat | raw, local | Podkarpackie, Poland | dark amber | partially crystallized |
| L1 | linden | blend, imported | EU and from outside the EU | white | half crystallized |
| L2 | linden | blend, imported | EU and from outside the EU | extra light amber | half crystallized |
| LC | linden | raw, local | Podkarpackie, Poland | extra light amber | liquid |
| M1 | multifloral | blend, imported | EU and from outside the EU | amber | crystallized |
| M2 | multifloral | blend, imported | EU and from outside the EU | light amber | crystallized |
| MC | multifloral | raw, local | Podkarpackie, Poland | light amber | crystallized |
| H1 | honeydew | blend, imported | EU and from outside the EU | dark amber | crystallized |
| H2 | honeydew | blend imported | EU | dark amber | liquid |
| H3 | honeydew | blend, imported | EU | dark amber | liquid |
| HC | honeydew | raw, local | Podkarpackie, Poland | dark amber | crystallized |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miłek, M.; Bocian, A.; Kleczyńska, E.; Sowa, P.; Dżugan, M. The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends. Molecules 2021, 26, 2423. https://doi.org/10.3390/molecules26092423
Miłek M, Bocian A, Kleczyńska E, Sowa P, Dżugan M. The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends. Molecules. 2021; 26(9):2423. https://doi.org/10.3390/molecules26092423
Chicago/Turabian StyleMiłek, Michał, Aleksandra Bocian, Ewelina Kleczyńska, Patrycja Sowa, and Małgorzata Dżugan. 2021. "The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends" Molecules 26, no. 9: 2423. https://doi.org/10.3390/molecules26092423
APA StyleMiłek, M., Bocian, A., Kleczyńska, E., Sowa, P., & Dżugan, M. (2021). The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends. Molecules, 26(9), 2423. https://doi.org/10.3390/molecules26092423