Quinazoline Derivatives Designed as Efflux Pump Inhibitors: Molecular Modeling and Spectroscopic Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Pharmacokinetic Profile
2.2. Pharmacodynamic Profile
2.2.1. Lipophilicity Evaluation
2.2.2. Molecular Docking
2.3. Spectral Characterization
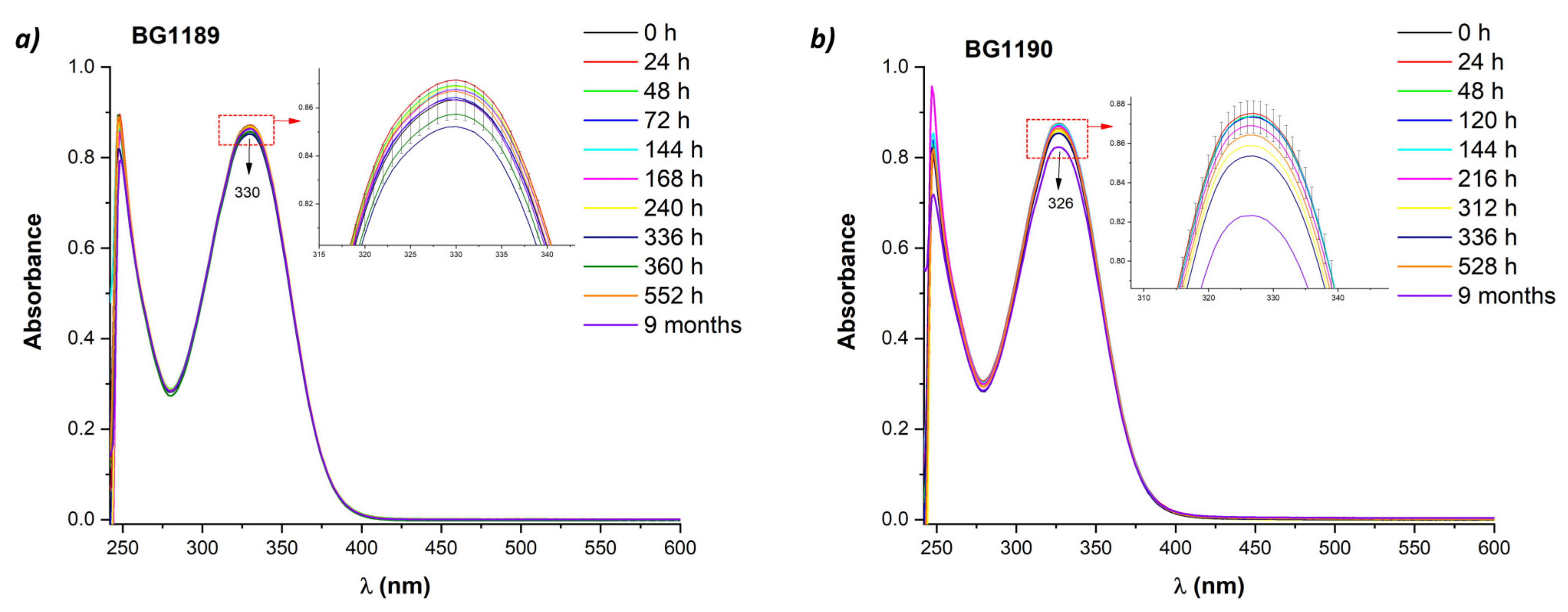
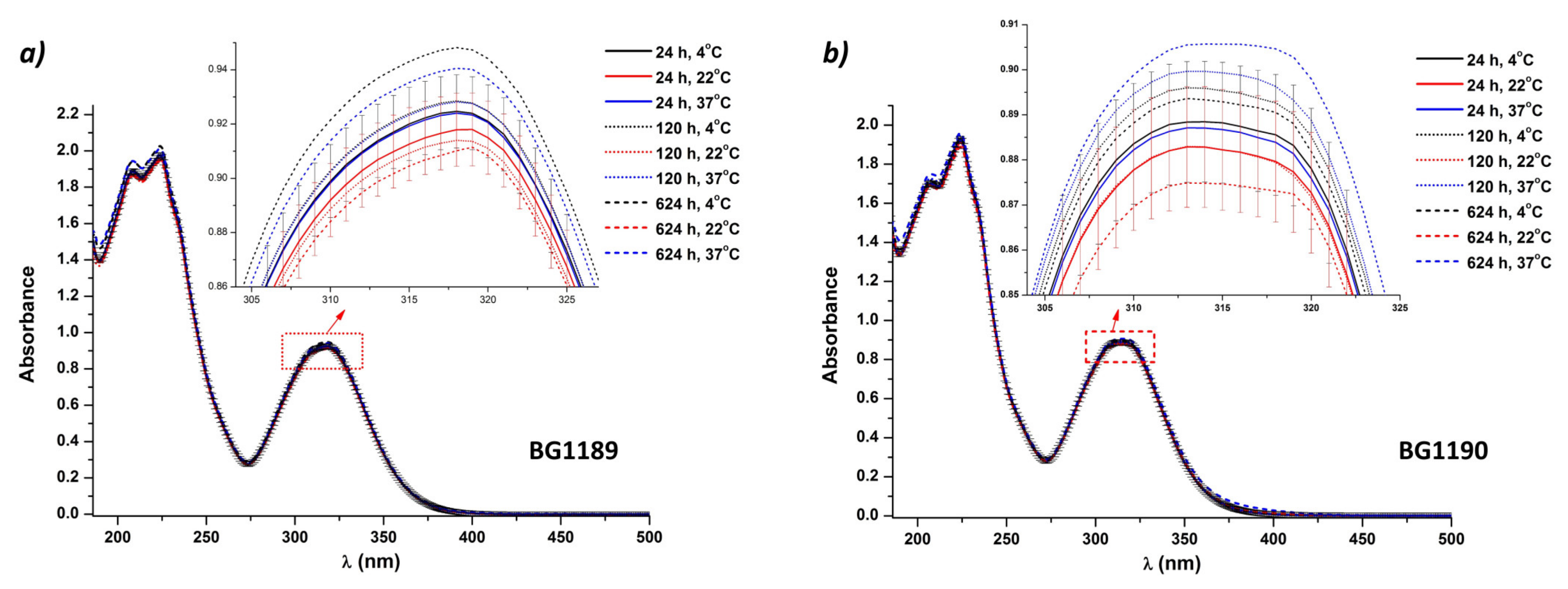
2.3.1. Stability Study Using UV-Vis Absorption Spectroscopy
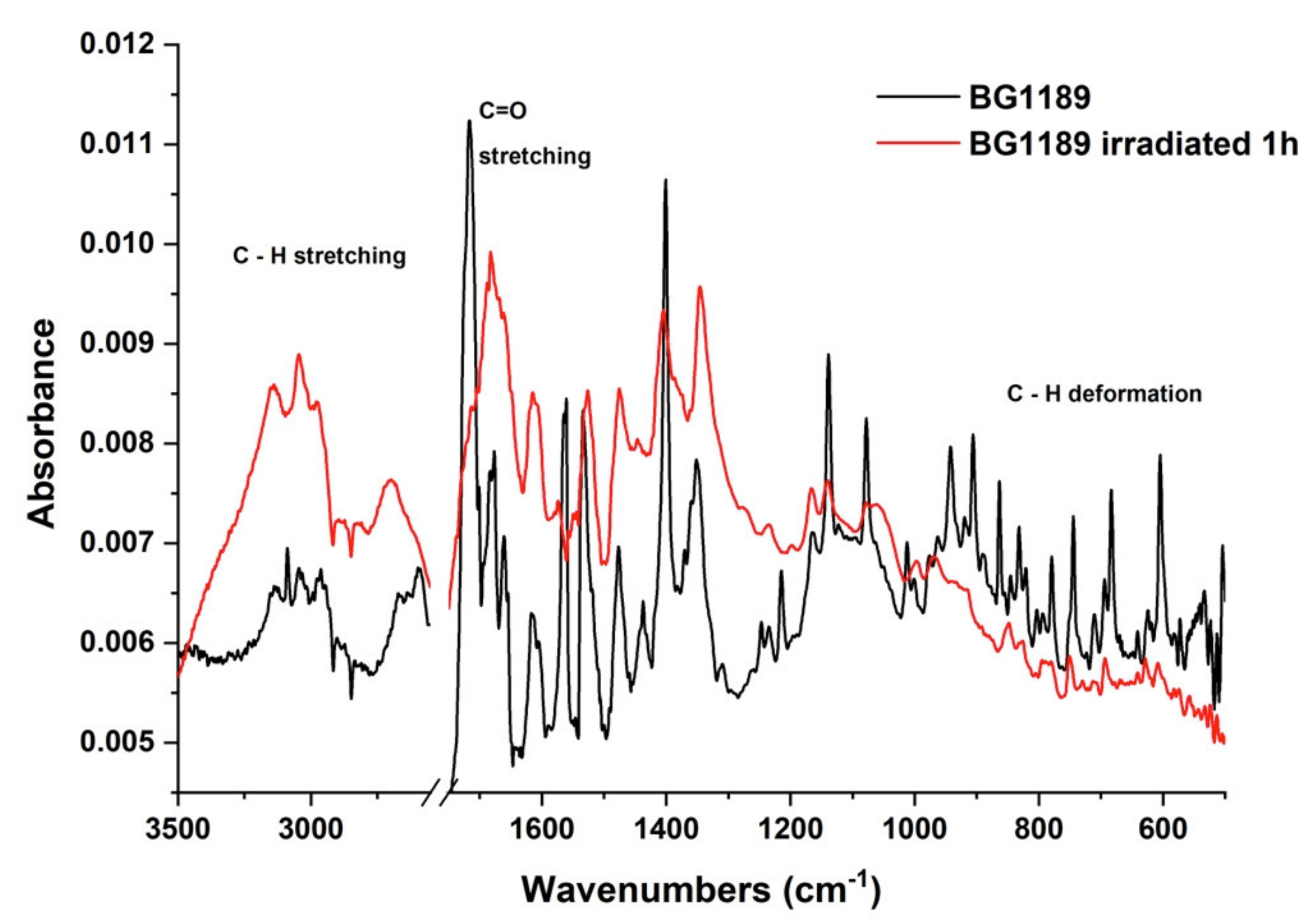
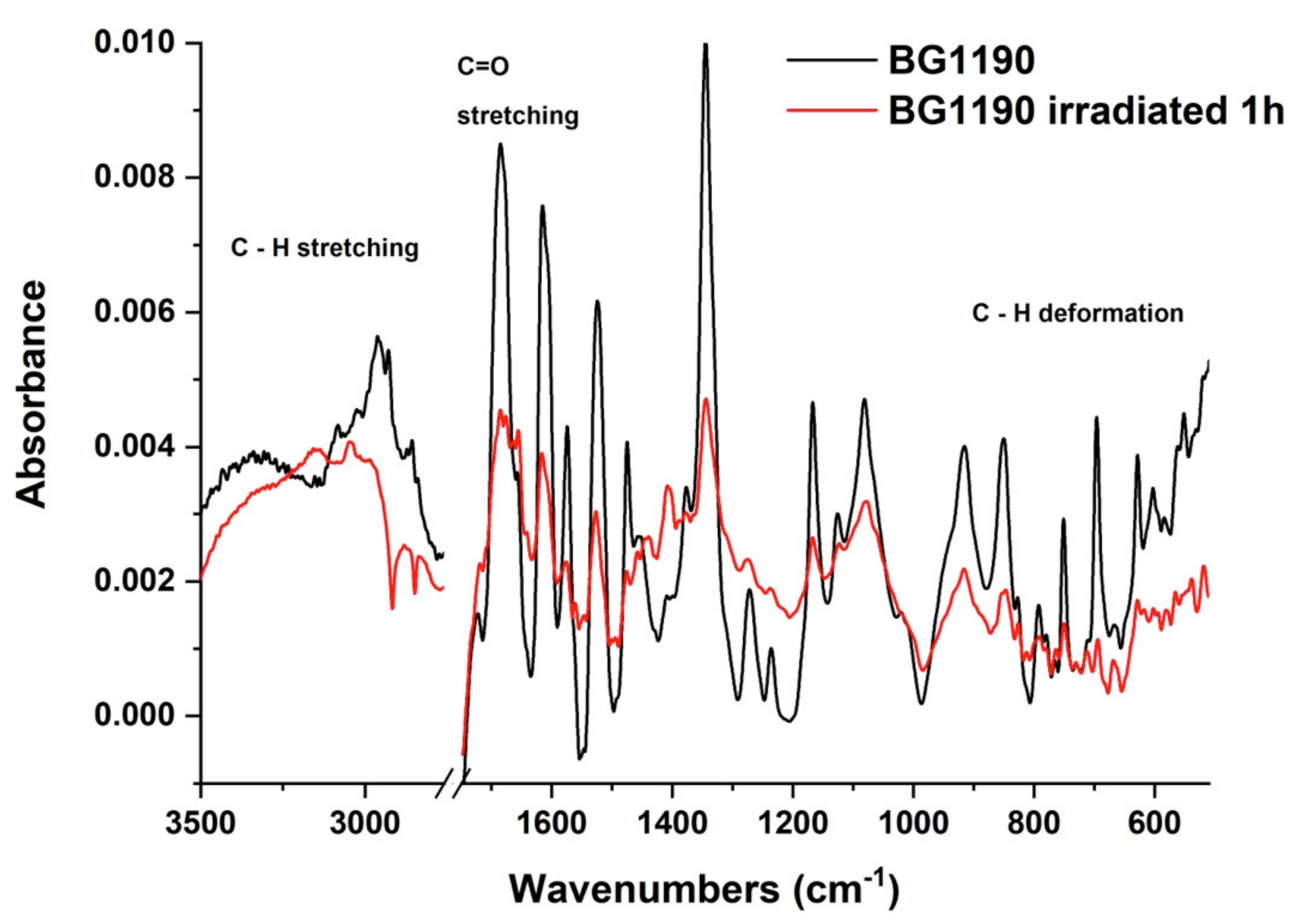
2.3.2. FTIR Spectroscopy
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Alenazy, R.; Gu, X.; Polyak, S.W.; Zhang, P.; Sykes, M.J.; Zhang, N.; Venter, H.; Ma, S. Design and Structural Optimization of Novel 2H-Benzo[h]Chromene Derivatives That Target AcrB and Reverse Bacterial Multidrug Resistance. Eur. J. Med. Chem. 2020, 113049. [Google Scholar] [CrossRef]
- Wang, Y.; Mowla, R.; Ji, S.; Guo, L.; De Barros Lopes, M.A.; Jin, C.; Song, D.; Ma, S.; Venter, H. Design, Synthesis and Biological Activity Evaluation of Novel 4-Subtituted 2-Naphthamide Derivatives as AcrB Inhibitors. Eur. J. Med. Chem. 2018, 143, 699–709. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.F. Multiple Molecular Mechanisms for Multidrug Resistance Transporters. Nature 2007, 446, 749–757. [Google Scholar] [CrossRef]
- Masi, M.; Réfregiers, M.; Pos, K.M.; Pagès, J.-M. Mechanisms of Envelope Permeability and Antibiotic Influx and Efflux in Gram-Negative Bacteria. Nat. Microbiol. 2017, 2, 17001. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug Efflux Pumps: Structure, Function and Regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Orelle, C.; Mathieu, K.; Jault, J.-M. Multidrug ABC Transporters in Bacteria. Res. Microbiol. 2019, 170, 381–391. [Google Scholar] [CrossRef]
- Nikaido, H.; Pagès, J.-M. Broad-Specificity Efflux Pumps and Their Role in Multidrug Resistance of Gram-Negative Bacteria. FEMS Microbiol. Rev. 2012, 36, 340–363. [Google Scholar] [CrossRef] [PubMed]
- Chitsaz, M.; Brown, M.H. The Role Played by Drug Efflux Pumps in Bacterial Multidrug Resistance. Essays Biochem. 2017, 61, 127–139. [Google Scholar] [CrossRef]
- Pagès, J.-M.; Masi, M.; Barbe, J. Inhibitors of Efflux Pumps in Gram-Negative Bacteria. Trends Mol. Med. 2005, 11, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Deng, Z.; Yan, A. Bacterial Multidrug Efflux Pumps: Mechanisms, Physiology and Pharmacological Exploitations. Biochem. Biophys. Res. Commun. 2014, 453, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Pagès, J.-M.; Amaral, L. Mechanisms of Drug Efflux and Strategies to Combat Them: Challenging the Efflux Pump of Gram-Negative Bacteria. Biochim. Biophys. Acta 2009, 1794, 826–833. [Google Scholar] [CrossRef]
- Spengler, G.; Kincses, A.; Gajdács, M.; Amaral, L. New Roads Leading to Old Destinations: Efflux Pumps as Targets to Reverse Multidrug Resistance in Bacteria. Molecules 2017, 22, 468. [Google Scholar] [CrossRef]
- Renau, T.E.; Léger, R.; Flamme, E.M.; Sangalang, J.; She, M.W.; Yen, R.; Gannon, C.L.; Griffith, D.; Chamberland, S.; Lomovskaya, O.; et al. Inhibitors of Efflux Pumps in Pseudomonas a Eruginosa Potentiate the Activity of the Fluoroquinolone Antibacterial Levofloxacin. J. Med. Chem. 1999, 42, 4928–4931. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Warren, M.S.; Lee, A.; Galazzo, J.; Fronko, R.; Lee, M.; Blais, J.; Cho, D.; Chamberland, S.; Renau, T.; et al. Identification and Characterization of Inhibitors of Multidrug Resistance Efflux Pumps in Pseudomonas Aeruginosa: Novel Agents for Combination Therapy. Antimicrob. Agents Chemother. 2001, 45, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Mahamoud, A.; Baitiche, M.; Adam, E.; Viveiros, M.; Smarandache, A.; Militaru, A.; Pascu, M.L.; Amaral, L.; Pagès, J.-M. Quinazoline Derivatives Are Efficient Chemosensitizers of Antibiotic Activity in Enterobacter Aerogenes, Klebsiella Pneumoniae and Pseudomonas Aeruginosa Resistant Strains. Int. J. Antimicrob. Agents 2010, 36, 164–168. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and Drug-like Compounds: The Rule-of-Five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Ma, Y.; Liu, B.; Chen, P.; Hu, Y.; Zhang, R. Synthesis, Antimicrobial Activity, Structure-Activity Relationship, and Molecular Docking Studies of Indole Diketopiperazine Alkaloids. Front. Chem. 2019, 7, 837. [Google Scholar] [CrossRef]
- Martins, A.; Spengler, G.; Martins, M.; Rodrigues, L.; Viveiros, M.; Davin-Regli, A.; Chevalier, J.; Couto, I.; Pagès, J.M.; Amaral, L. Physiological Characterisation of the Efflux Pump System of Antibiotic-Susceptible and Multidrug-Resistant Enterobacter Aerogenes. Int. J. Antimicrob. Agents 2010, 36, 313–318. [Google Scholar] [CrossRef]
- Murakami, S.; Nakashima, R.; Yamashita, E.; Yamaguchi, A. Crystal Structure of Bacterial Multidrug Efflux Transporter AcrB. Nature 2002, 419, 587–593. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, G.; Hryc, C.F.; Blaza, J.N.; Serysheva, I.I.; Schmid, M.F.; Chiu, W.; Luisi, B.F.; Du, D. An Allosteric Transport Mechanism for the AcrAB-TolC Multidrug Efflux Pump. eLife 2017, 6, e24905. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH Q1A (R2) Stability Testing of New Drug Substances and Drug Products—NOTE FOR GUIDANCE ON STABILITY TESTING: STABILITY TESTING OF NEW DRUG SUBSTANCES AND PRODUCTS (CPMP/ICH/2736/99). 2003. Available online: https://www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products (accessed on 19 April 2021).
- Pascu, M.-L.; Nastasa, V.; Smarandache, A.; Militaru, A.; Martins, A.; Viveiros, M.; Boni, M.; Andrei, I.R.; Pascu, A.; Staicu, A.; et al. Direct Modification of Bioactive Phenothiazines by Exposure to Laser Radiation. Recent Pat. Antiinfect Drug Discov. 2011, 6, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Pascu, M.L.; Danko, B.; Martins, A.; Jedlinszki, N.; Alexandru, T.; Nastasa, V.; Boni, M.; Militaru, A.; Andrei, I.R.; Staicu, A.; et al. Exposure of Chlorpromazine to 266 Nm Laser Beam Generates New Species with Antibacterial Properties: Contributions to Development of a New Process for Drug Discovery. PLoS ONE 2013, 8, e55767. [Google Scholar] [CrossRef][Green Version]
- Alexandru, T.; Staicu, A.; Pascu, A.; Radu, E.; Stoicu, A.; Nastasa, V.; Dinache, A.; Boni, M.; Amaral, L.; Pascu, M.L. Characterization of Mixtures of Compounds Produced in Chlorpromazine Aqueous Solutions by Ultraviolet Laser Irradiation: Their Applications in Antimicrobial Assays. J. Biomed. Opt. 2014, 20, 051002. [Google Scholar] [CrossRef] [PubMed]
- Andrei, I.R.; Tozar, T.; Dinache, A.; Boni, M.; Nastasa, V.; Pascu, M.L. Chlorpromazine Transformation by Exposure to Ultraviolet Laser Beams in Droplet and Bulk. Eur. J. Pharm. Sci. 2016, 81, 27–35. [Google Scholar] [CrossRef]
- Tozar, T.; Santos Costa, S.; Udrea, A.-M.; Nastasa, V.; Couto, I.; Viveiros, M.; Pascu, M.L.; Romanitan, M.O. Anti-Staphylococcal Activity and Mode of Action of Thioridazine Photoproducts. Sci. Rep. 2020, 10, 18043. [Google Scholar] [CrossRef] [PubMed]
- Nistorescu, S.; Gradisteanu Pircalabioru, G.; Udrea, A.-M.; Simon, A.; Pascu, M.L.; Chifiriuc, M.-C. Laser-Irradiated Chlorpromazine as a Potent Anti-Biofilm Agent for Coating of Biomedical Devices. Coatings 2020, 10, 1230. [Google Scholar] [CrossRef]
- Dinache, A.; Boni, M.; Alexandru, T.; Radu, E.; Stoicu, A.; Andrei, I.R.; Staicu, A.; Liggieri, L.; Nastasa, V.; Pascu, M.L.; et al. Surface Properties of Vancomycin after Interaction with Laser Beams. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 328–335. [Google Scholar] [CrossRef]
- Smarandache, A.; Pascu, A.; Andrei, I.R.; Handzlik, J.; Kiec-Kononowicz, K.; Staicu, A.; Pascu, M.-L. STUDY OF THE OPTICAL PROPERTIES OF 2-THIOHYDANTOIN DERIVATIVES. Rom. Rep. Phys. 2016, 68, 673–683. [Google Scholar]
- Smarandache, A.; Nastasa, V.; Boni, M.; Staicu, A.; Handzlik, J.; Kiec-Kononowicz, K.; Amaral, L.; Pascu, M.-L. Laser Beam Resonant Interaction of New Hydantoin Derivatives Droplets for Possible Biomedical Applications. Colloids Surf. A Physicochem. Eng. Asp. 2016, 505, 37–46. [Google Scholar] [CrossRef]
- Hunyadi, A.; Danko, B.; Boni, M.; Militaru, A.; Alexandru, T.; Nastasa, V.; Andrei, I.R.; Pascu, M.L.; Amaral, L. Rapid, Laser-Induced Conversion of 20-Hydroxyecdysone and Its Diacetonide -- Experimental Set-up of a System for Photochemical Transformation of Bioactive Substances. Anticancer Res. 2012, 32, 1291–1297. [Google Scholar]
- Udrea, A.-M.; Avram, S.; Nistorescu, S.; Pascu, M.-L.; Romanitan, M.O. Laser Irradiated Phenothiazines: New Potential Treatment for COVID-19 Explored by Molecular Docking. J. Photochem. Photobiol. B Biol. 2020, 211, 111997. [Google Scholar] [CrossRef] [PubMed]
- Dassault Systèmes BIOVIA, [Discovery Studio], [19.1.0.18287]; Dassault Systèmes: San Diego, CA, USA, 2019.
- Militaru, A.; Smarandache, A.; Mahamoud, A.; Alibert, S.; Pages, J.-M.; Pascu, M.-L. Time Stability Studies of Quinazoline Derivative Designed to Fight Drug Resistance Acquired by Bacteria. Lett. Drug Design Discov. 2011, 8, 124–129. [Google Scholar] [CrossRef]
- Aaron, J.J.; Tine, A.; Gaye, M.D.; Parkanyi, C.; Boniface, C.; Bieze, T.W.N. Effects of Solvent on the Electronic Absorption and Fluorescence Spectra of Quinazolines, and Determination of Their Ground and Excited Singlet-State Dipole Moments. Spectrochim. Acta Part A Mol. Spectrosc. 1991, 47, 419–430. [Google Scholar] [CrossRef]
- Eshimbetov, A.G.; Kristallovich, E.L.; Abdullaev, N.D.; Tulyaganov, T.S.; Shakhidoyatov, Kh. M. AM1/CI, CNDO/S and ZINDO/S Computations of Absorption Bands and Their Intensities in the UV Spectra of Some 4(3H)-Quinazolinones. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 299–307. [Google Scholar] [CrossRef] [PubMed]
- De Silva, N.; Willow, S.Y.; Gordon, M.S. Solvent Induced Shifts in the UV Spectrum of Amides. J. Phys. Chem. A 2013, 117, 11847–11855. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parson, W.W. Modern Optical Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; repr. as paperback; Wiley: Chichester, UK, 2010. [Google Scholar]
- Jafari, E.; Khajouei, M.R.; Hassanzadeh, F.; Hakimelahi, G.H.; Khodarahmi, G.A. Quinazolinone and Quinazoline Derivatives: Recent Structures with Potent Antimicrobial and Cytotoxic Activities. Res. Pharm Sci 2016, 11, 1–14. [Google Scholar]
- Baitiche, M.; Mahamoud, A.; Benachour, D.; Merbah, M.; Barbe, J. SYNTHESIS OF NEW QUINAZOLINE DERIVATIVES. Heterocycl. Commun. 2004, 10, 4–5. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Dinache, A.; Boni, M.; Pascu, M.L. Phenothiazine Derivatives Interaction with Laser Radiation. Rom. Rep. Phys. 2013, 65, 1078–1091. [Google Scholar]
- Dinache, A.; Smarandache, A.; Simon, A.; Nastasa, V.; Tozar, T.; Pascu, A.; Enescu, M.; Khatyr, A.; Sima, F.; Pascu, M.-L.; et al. Photosensitized Cleavage of Some Olefins as Potential Linkers to Be Used in Drug Delivery. Appl. Surf. Sci. 2017, 417, 136–142. [Google Scholar] [CrossRef]




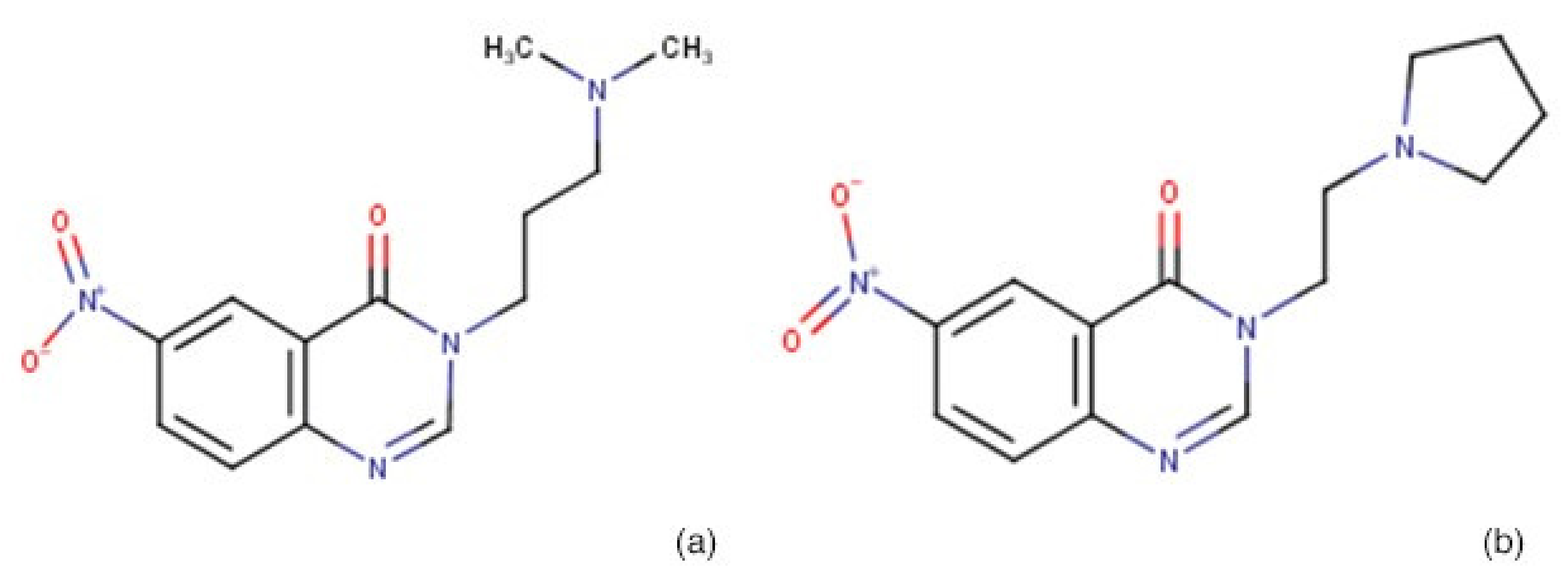
| Molecule | BG1190 | BG1189 | ||||
|---|---|---|---|---|---|---|
| Validate | Molecular Descriptors | Value | Validate | Molecular Descriptors | Value | |
| Lipinski | Yes | MW ≤ 500 | 289.31 | Yes | MW ≤ 500 | 277.30 |
| MlogPo/w ≤ 4.15 | 1.04 | MlogPo/w ≤ 4.15 | 0.37 | |||
| Acc ≤ 10 | 5 | Acc ≤ 10 | 5 | |||
| Don ≤ 5 | 1 | Don ≤ 5 | 1 | |||
| Ghose | Yes | 160 ≤ MW ≤480 | 289.31 | Yes | 160 ≤ MW ≤ 480 | 277.30 |
| −0.4 ≤ WlogPo/w ≤ 5,6 | 0.91 | -0.4 ≤ WlogPo/w≤ 5,6 | 1.15 | |||
| 40 ≤ MR ≤ 130 | 81.07 | 40 ≤ MR ≤ 130 | 74.46 | |||
| 20 ≤ atoms ≤ 70 | 38 | 20 ≤ atoms ≤ 70 | 37 | |||
| Veber | Yes | Rotatable bonds ≤ 10 | 4 | Yes | Rotatable bonds ≤ 10 | 5 |
| TPSA ≤ 140 Å2 | 78.44 Å2 | TPSA ≤ 140 Å2 | 78.44 Å2 | |||
| Model Name | Predicted Value BG1190 | Predicted Value BG1189 | Predicted Value Norfloxacin | Unit |
|---|---|---|---|---|
| Water Solubility | −1.992 | −1.549 | −3.176 | log mol/L |
| Caco2 Permeability | 0.76 | 0.597 | 1.242 | log Papp in 10−6 cm/s |
| Intestinal Absorption (Human) | 87.391 | 78.106 | 86.904 | % Absorbed |
| VDss (Human) | 0.909 | 0.84 | 0.064 | log L/kg |
| Fraction Unbound (Human) | 0.713 | 0.612 | 0.462 | Fu |
| Total Clearance | 0.811 | 0.594 | 0.366 | log ml/min/kg |
| Renal OCT2 Substrate | Yes | No | No | Yes/No |
| hERG I Inhibitor | No | No | No | Yes/No |
| hERG II Inhibitor | Yes | Yes | No | Yes/No |
| Oral Rat Acute Toxicity (LD50) | 2.55 | 2.541 | 2.217 | mol/kg |
| Hepatotoxicity | Yes | Yes | Yes | Yes/No |
| Multidrug Efflux Pump Subunit AcrB (PDB Code: 5NC5) | |
|---|---|
| AA Residues from Allosteric Situs of AcrB Subunit. | Met573; Phe617; Phe628; Phe666; Leu668. |
| Lowest EFEB BG1190 | −8.21 kcal/mol |
| AA from the Run with Lowest EFEB of BG1990 | Gln34; Pro36; Thr37; Ala39; Ser134; Ser135; Phe136; Tyr327; Thr329; Leu668; Ile671; Val672; Glu673. |
| Lowest EFEB BG1189 | −7.40 kcal/mol |
| AA from the Run with Lowest EFEB of BG1989 | Gln34; Tyr325; Pro326; Tyr327; Asp328; Thr329; Pro331; Phe332; Gly570; Val571; Gln569; Glu607; Ser608; Ser630; Leu631; Trp634; Leu668. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udrea, A.-M.; Dinache, A.; Pagès, J.-M.; Pirvulescu, R.A. Quinazoline Derivatives Designed as Efflux Pump Inhibitors: Molecular Modeling and Spectroscopic Studies. Molecules 2021, 26, 2374. https://doi.org/10.3390/molecules26082374
Udrea A-M, Dinache A, Pagès J-M, Pirvulescu RA. Quinazoline Derivatives Designed as Efflux Pump Inhibitors: Molecular Modeling and Spectroscopic Studies. Molecules. 2021; 26(8):2374. https://doi.org/10.3390/molecules26082374
Chicago/Turabian StyleUdrea, Ana-Maria, Andra Dinache, Jean-Marie Pagès, and Ruxandra Angela Pirvulescu. 2021. "Quinazoline Derivatives Designed as Efflux Pump Inhibitors: Molecular Modeling and Spectroscopic Studies" Molecules 26, no. 8: 2374. https://doi.org/10.3390/molecules26082374
APA StyleUdrea, A.-M., Dinache, A., Pagès, J.-M., & Pirvulescu, R. A. (2021). Quinazoline Derivatives Designed as Efflux Pump Inhibitors: Molecular Modeling and Spectroscopic Studies. Molecules, 26(8), 2374. https://doi.org/10.3390/molecules26082374







