Design, Synthesis, and Antimicrobial Activity of Certain New Indole-1,2,4 Triazole Conjugates
Abstract
1. Introduction
2. Results and Discussions
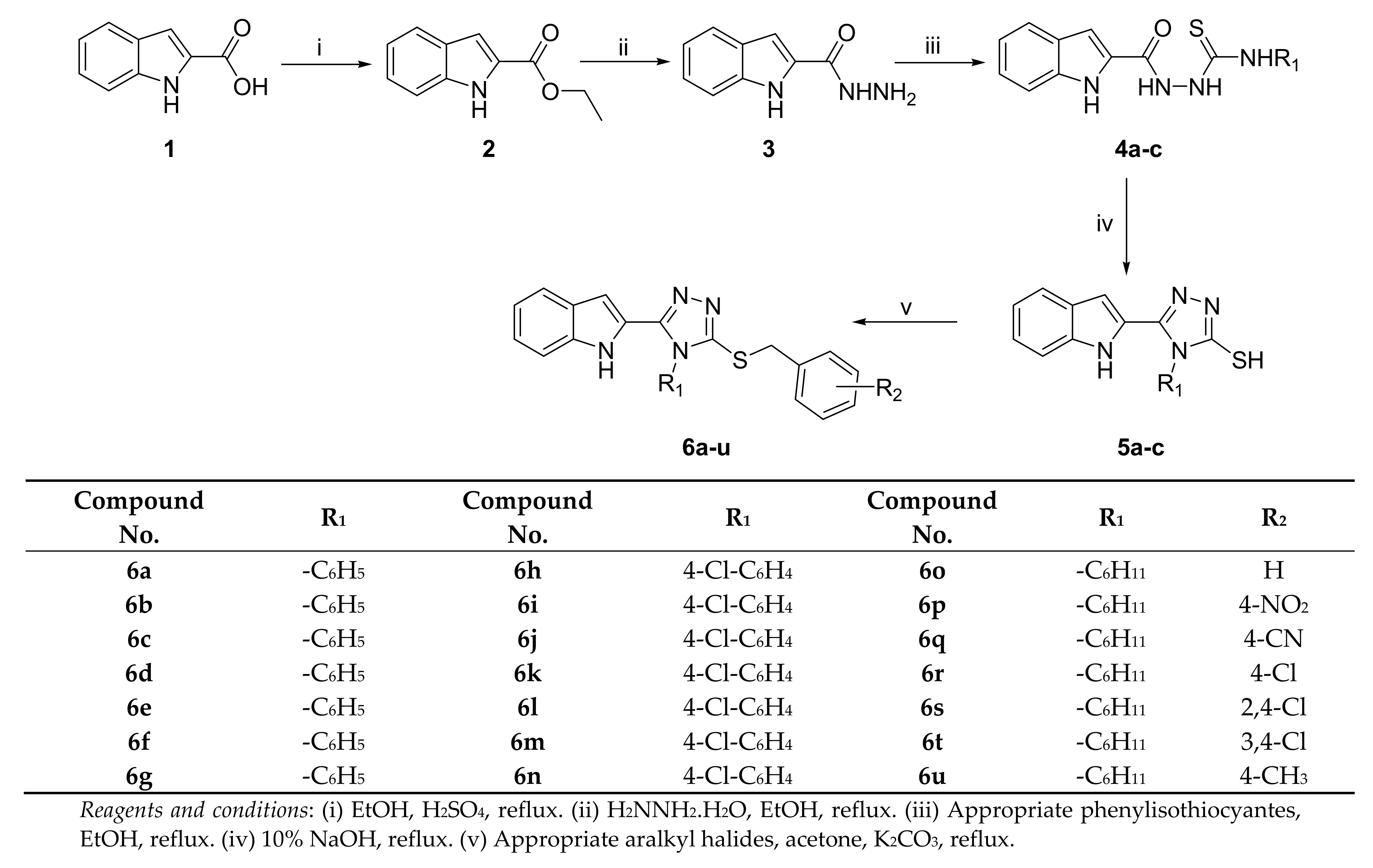
2.1. Chemistry
2.2. Antimicrobial Evaluation
3. Experimental
3.1. General
3.2. Chemistry
3.2.1. Synthesis of Ethyl 1H-Indole-2-Carboxylate (2)
3.2.2. Synthesis of 1H-Indole-2-Carbohydrazide (3)
3.2.3. General Procedure for the Synthesis of the Indole-1H-2-yl-4-Substituted-Thiosemicarbazides (4a-c)
3.2.4. General Procedure for the Synthesis of 5-(1H-Indol-2-yl)-4-Substituted-1,2,4-Triazole-3-Thiols (5a-c)
3.2.5. General Procedure for the Synthesis of the Target Compounds 6a-u
3.3. Antimicrobial Evaluation
3.3.1. Materials
3.3.2. Organisms
3.3.3. Agar Well Diffusion Technique
3.3.4. MIC Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brown, G.D.; Denning, D.W.; Levitz, S.M. Tackling human fungal infections. Science 2012, 336, 647. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.R.; Hota, B.; Ahmad, L.; Scott, D.; Foster, S.D.; Abbasi, F.; Schabowski, S.; Kampe, L.M.; Gr-Ciavarella, G.; Supino, M.; et al. Hospital and societal costs of antimicrobial-resistant infections in a chicago teaching hospital: Implications for antibiotic stewardship. Clin. Infect. Dis. 2009, 49, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Gan, L.L.; Zhou, C.H. Synthesis, antibacterial and antifungal activities of some carbazole derivatives. Bioorganic Med. Chem. Lett. 2010, 20, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52. [Google Scholar] [CrossRef] [PubMed]
- Kaku, N.; Morinaga, Y.; Takeda, K.; Kosai, K.; Uno, N.; Hasegawa, H.; Miyazaki, T.; Izumikawa, K.; Mukae, H.; Yanagihara, K. Antimicrobial and immunomodulatory effects of tedizolid against methicillin-resistant Staphylococcus aureus in a murine model of hematogenous pulmonary infection. Int. J. Med. Microbiol. 2016, 306, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Inman, M.; Moody, C.J. Indole synthesis-something old, something new. Chem. Sci. 2013, 4, 29–41. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, P.; Pathaka, D. Biological importance of the indole nucleus in recent years: A comprehensive review. J. Heterocycl. Chem. 2010, 47, 491–502. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Chen, Q.; Yang, G.F. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef]
- Frost, J.M.; Dart, M.J.; Tietje, K.R.; Garrison, T.R.; Grayson, G.K.; Daza, A.V.; El-Kouhen, O.F.; Yao, B.B.; Hsieh, G.C.; Pai, M.; et al. Indol-3-ylcycloalkyl ketones: Effects of N1 substituted indole side chain variations on CB2 cannabinoid receptor activity. J. Med. Chem. 2010, 53, 295–315. [Google Scholar] [CrossRef]
- Rathod, A.S.; Godipurge, S.S.; Biradar, J.S. Microwave Assisted, Solvent-Free, “Green” Synthesis of Novel Indole Analogs as Potent Antitubercular and Antimicrobial Agents and Their Molecular Docking Studies. Russ. J. Gen. Chem. 2018, 88, 1238–1246. [Google Scholar] [CrossRef]
- Niemyjska, M.; MacIejewska, D.; Wolska, I.; Truszkowski, P. Synthesis, structural investigations, and anti-cancer activity of new methyl indole-3-carboxylate derivatives. J. Mol. Struct. 2012, 1026, 30–35. [Google Scholar] [CrossRef]
- Varvaresou, A.; Tsantili-Kakoulidou, A.; Siatra-Papastaikoudi, T.; Tiligada, E. Synthesis and biological evaluation of indole containing derivatives of thiosemicarbazide and their cyclic 1,2,4-triazole and 1,3,4-thiadiazole analogs. Arzneimittel-Forschung/Drug Res. 2000, 50, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pintilie, O.; Profire, L.; Sunel, V.; Popa, M.; Pui, A. Synthesis and Antimicrobial Activity of Some New 1,3,4-Thiadiazole and 1,2,4-Triazole Compounds Having a D,L-Methionine Moiety. Molecules 2007, 12, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.J.; Lai, L.H.; Jin, G.Y.; Zhang, Z.X. Synthesis, fungicidal activity, and 3D-QSAR of pyridazinone-substituted 1,3,4-oxadiazoles and 1,3,4-thiadiazoles. J. Agric. Food Chem. 2002, 50, 3757–3760. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Z.; Han, Y. Synthesis and fungicidal activity against Rhizoctonia solani of 2-alkyl (alkylthio)-5-pyrazolyl-1,3,4-oxadiazoles (thiadiazoles). J. Agric. Food Chem. 2000, 48, 5312–5315. [Google Scholar] [CrossRef] [PubMed]
- Banu, K.M.; Dinakar, A.; Ananthanarayanan, C. Synthesis, characterization, antimicrobial studies and pharmacological screening of some substituted 1,2,3-triazoles. Indian J. Pharm. Sci. 1999, 61, 202–205. [Google Scholar]
- Keri, R.S.; Patil, S.A.; Budagumpi, S.; Nagaraja, B.M. Triazole: A Promising Antitubercular Agent. Chem. Biol. Drug Des. 2015, 86, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.; Dogan, H.N.; Rollas, S. Synthesis and preliminary anticancer activity of new 1,4-dihydro-3-(3-hydroxy-2-naphthyl)-4-substituted-5H-1,2,4-triazoline-5-thiones. Il Farm. 2002, 57, 559–564. [Google Scholar] [CrossRef]
- Gujjar, R.; Marwaha, A.; El Mazouni, F.; White, J.; White, K.L.; Creason, S.; Shackleford, D.M.; Baldwin, J.; Charman, W.N.; Buckner, F.S.; et al. Identification of a metabolically stable triazolopyrimidine-based dihydroorotate dehydrogenase inhibitor with antimalarial activity in mice. J. Med. Chem. 2009, 52, 1864–1872. [Google Scholar] [CrossRef]
- Johns, B.A.; Weatherhead, J.G.; Allen, S.H.; Thompson, J.B.; Garvey, E.P.; Foster, S.A.; Jeffrey, J.L.; Miller, W.H. The use of oxadiazole and triazole substituted naphthyridines as HIV-1 integrase inhibitors. Part 1: Establishing the pharmacophore. Bioorganic Med. Chem. Lett. 2009, 19, 1802–1806. [Google Scholar] [CrossRef] [PubMed]
- Schenone, S.; Bruno, O.; Ranise, A.; Bondavalli, F.; Filippelli, W.; Falcone, G.; Giordano, L.; Vitelli, M.R. 3-Arylsulphonyl-5-arylamino-1,3,4-thiadiazol-2(3H)ones as anti-inflammatory and analgesic agents. Bioorg. Med. Chem. 2001, 9, 2149–2153. [Google Scholar] [CrossRef]
- Ghodhani, D.R.; Jogel, A.A.; Sanghani, A.M.; Jignasu, P.M. Synthesis and biological screening of 1,2,4-triazole derivatives. Indian J. Chem. 2015, 54B, 556–564. [Google Scholar]
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A.M. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Shirinzadeh, H.; Süzen, S.; Altanlar, N.; Westwell, A.D. Antimicrobial Activities of New Indole Derivatives Containing 1,2,4-Triazole, 1,3,4-Thiadiazole and Carbothioamide. Turkish J. Pharm. Sci. 2018, 15, 291–297. [Google Scholar] [CrossRef]
- Mane, Y.D.; Sarnikar, Y.P.; Surwase, S.M.; Biradar, D.O.; Gorepatil, P.B.; Shinde, V.S.; Khade, B.C. Design, synthesis, and antimicrobial activity of novel 5-substituted indole-2-carboxamide derivatives. Res. Chem. Intermed. 2017, 43, 1253–1275. [Google Scholar] [CrossRef]
- Schulze, B.; Schubert, U.S. Beyond click chemistry-supramolecular interactions of 1,2,3-triazoles. Chem. Soc. Rev. 2014, 43, 2522–2571. [Google Scholar] [CrossRef]
- Küçükgüzel, G.; Çikla-Süzgün, P. Recent advances bioactive 1,2,4-triazole-3-thiones. Eur. J. Med. Chem. 2015, 97, 830–870. [Google Scholar] [CrossRef]
- Dilmaghani, K.A.; Nasuhi Pur, F.; Nezhad, M.H. Synthesis and antibacterial evaluation of new thione substituted 1,2,4-triazole schiff bases as novel antimicrobial agents. Iran. J. Pharm. Res. 2015, 14, 693–699. [Google Scholar] [CrossRef]
- Metwally, M.A.; Bondock, S.; El-Azap, H.; Kandeel, E.E.M. Thiosemicarbazides: Synthesis and reactions. J. Sulfur Chem. 2011, 32, 489–519. [Google Scholar] [CrossRef]
- Palaska, E.; Şahin, G.; Kelicen, P.; Durlu, N.T.; Altinok, G. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. Farmaco 2002, 57, 101–107. [Google Scholar] [CrossRef]
- Biagi, G.L.; Guerra, M.C.; Barbaro, A.M.; Gamba, M.F. Influence of lipophilic character on the antibacterial activity of cephalosporins and penicillins. J. Med. Chem. 1970, 13, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Asami, T.; Mizutani, M.; Shimada, Y.; Goda, H.; Kitahata, N.; Sekimata, K.; Han, S.Y.; Fujioka, S.; Takatsuto, S.; Sakata, K.; et al. Triadimefon, a fungicidal triazole-type P450 inhibitor, induces brassinosteroid deficiency-like phenotypes in plants and binds to DWF4 protein in the brassinosteroid biosynthesis pathway. Biochem. J. 2003, 369, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, A.E.W.A.O. On the synthesis and reactions of indole-2-carboxylic acid hydrazide. Monatshefte fur Chemie 2001, 132, 753–763. [Google Scholar] [CrossRef]
- Coowar, D.; Bouissac, J.; Hanbali, M.; Paschaki, M.; Mohier, E.; Luu, B. Effects of indole fatty alcohols on the differentiation of neural stem cell derived neurospheres. J. Med. Chem. 2004, 47, 6270–6282. [Google Scholar] [CrossRef] [PubMed]
- Al-Wabli, R.; Zakaria, A.; Attia, M. Synthesis, Spectroscopic Characterization and Antimicrobial Potential of Certain New Isatin-Indole Molecular Hybrids. Molecules 2017, 22, 1958. [Google Scholar] [CrossRef]
- Boraei, A.T.A.; El Ashry, E.S.H.; Duerkop, A. Regioselectivity of the alkylation of S-substituted 1,2,4-triazoles with dihaloalkanes. Chem. Cent. J. 2016, 10, 22. [Google Scholar] [CrossRef][Green Version]
- Vanden Berghe, D.A. Screening methods for antibacterial and antiviral agents from higher plants. Methods Plant. Biochem. 1991, 47–69. [Google Scholar]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
| E. coli | Pseudomonas aeruginosa | Salmonella typhimurium | Staphylococcus aureus | Bacillus subtilis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound No. | DIZ ± SD * | MIC (µg/mL) | DIZ ± SD * | MIC (µg/mL) | DIZ ± SD * | MIC (µg/mL) | DIZ ± SD * | MIC (µg/mL) | DIZ ± SD * | MIC (µg/mL) |
| 6a | 11 ± 1.06 | 1000 | 0 | - | 10 ± 0.00 | 1000 | 0 | - | 10 ± 0.70 | 1000 |
| 6b | 11 ± 0.70 | 250 | 13 ± 7.50 | 250 | 10 ± 0.70 | 250 | 0 | - | 10 ± 2.12 | 250 |
| 6c | 11 ± 2.12 | 250 | 14 ± 8.08 | 250 | 11 ± 2.88 | 250 | 0 | - | 9 ± 0.00 | 250 |
| 6d | 10 ± 1.41 | 250 | 15 ± 8.66 | 250 | 11 ± 3.46 | 250 | 0 | - | 9 ± 0.00 | 250 |
| 6e | 12 ± 1.41 | 250 | 0 | - | 11 ± 1.73 | 250 | 0 | - | 9 ± 0.00 | 500 |
| 6f | 11 ± 0.70 | 500 | 0 | - | 10 ± 0.00 | 500 | 0 | - | 10 ± 0.70 | 250 |
| 6g | 11 ± 0.70 | 250 | 10 ±5.50 | 250 | 10 ± 1.15 | 250 | 0 | - | 10 ± 0.70 | 250 |
| 6h | 12 ± 1.41 | 250 | 10 ± 1.00 | 250 | 10 ± 0.57 | 500 | 9 ± 0.57 | 1000 | 0 | - |
| 6i | 12 ± 2.12 | 250 | 11 ± 1.52 | 250 | 10 ± 0.70 | 250 | 9 ± 1.52 | 250 | 0 | - |
| 6j | 14 ± 3.53 | 1000 | 12 ± 1.32 | 500 | 11 ± 0.35 | 500 | 11 ± 1.41 | 500 | 0 | - |
| 6k | 14 ± 3.53 | 250 | 11 ± 0.57 | 250 | 10 ± 0.57 | 250 | 10 ± 1.41 | 250 | 0 | - |
| 6l | 14 ± 3.53 | 250 | 10 ± 1.15 | 250 | 9 ± 0.00 | 250 | 11 ± 1.00 | 500 | 0 | - |
| 6m | 12 ± 2.82 | 250 | 10 ± 0.57 | 250 | 10 ± 1.73 | 250 | 10 ± 0.57 | 250 | 0 | - |
| 6n | 13 ± 2.12 | 500 | 12 ± 1.15 | 250 | 11 ± 1.00 | 250 | 11 ± 1.00 | 250 | 0 | - |
| 6o | 11 ± 0.70 | 1000 | 13 ± 2.51 | 1000 | 11 ± 1.00 | 1000 | 0 | - | 0 | - |
| 6p | 11 ± 0.00 | 500 | 12 ± 2.00 | 500 | 11 ± 2.00 | 500 | 0 | - | 0 | - |
| 6q | 12 ± 1.06 | 500 | 12 ± 1.52 | 250 | 11 ± 1.15 | 250 | 0 | - | 0 | - |
| 6r | 12 ± 0.70 | 250 | 13 ± 2.64 | 500 | 12 ± 1.52 | 250 | 0 | - | 0 | - |
| 6s | 10 ± 1.41 | 250 | 14 ± 3.60 | 250 | 11 ± 1.52 | 500 | 0 | - | 0 | - |
| 6t | 11 ± 0.00 | 500 | 13 ± 3.01 | 500 | 11 ± 1.73 | 500 | 0 | - | 0 | - |
| 6u | 11 ± 1.41 | 1000 | 13 ± 2.00 | 1000 | 12 ± 1.15 | 1000 | 0 | - | 12 ± 1.00 | 250 |
| Ampicillin | 08 ± 0.57 | 500 | 24 ± 0.40 | 1000 | 14 ± 0.60 | 500 | 0 | 500 | 08 ± 0.28 | 500 |
| Candida albicans | Candida tropicalis | Candida glabrata | ||||
|---|---|---|---|---|---|---|
| Compound No. | DIZ ± SD * | MIC (µg/mL) | DIZ ± SD * | MIC (µg/mL) | DIZ ± SD * | MIC (µg/mL) |
| 6a | 26 ± 0.28 | 1000 | 13 ± 0.57 | 2 | 10 ± 0.57 | 1000 |
| 6b | 26 ± 0.11 | 250 | 14 ± 1.52 | 2 | 10 ± 0.00 | 250 |
| 6c | 26 ± 0.28 | 250 | 14 ± 2.08 | 2 | 10 ± 0.57 | 250 |
| 6d | 27 ± 0.00 | 250 | 14 ± 3.46 | 4 | 10 ± 1.00 | 125 |
| 6e | 26 ± 0.28 | 250 | 14 ± 3.21 | 4 | 11 ± 1.15 | 250 |
| 6f | 26 ± 0.28 | 2 | 14 ± 2.00 | 4 | 10 ± 0.00 | 250 |
| 6g | 26 ± 0.11 | 250 | 14 ± 1.15 | 4 | 10 ± 0.57 | 1000 |
| 6h | 0 | - | 12 ± 0.57 | 2 | 0 | |
| 6i | 0 | - | 11 ± 0.57 | 4 | 0 | - |
| 6j | 0 | - | 11 ± 0.57 | 4 | 0 | - |
| 6k | 0 | - | 10 ± 0.00 | 2 | 0 | - |
| 6l | 0 | - | 10 ± 0.00 | 2 | 0 | - |
| 6m | 0 | - | 10 ± 0.57 | 2 | 0 | - |
| 6n | 0 | - | 11 ± 0.57 | 2 | 0 | - |
| 6o | 11 ± 0.70 | 1000 | 11 ± 0.57 | 16 | 0 | - |
| 6p | 10 ± 0.00 | 32 | 11 ± 0.57 | 4 | 0 | - |
| 6q | 10 ± 0.70 | 32 | 11 ± 0.57 | 12.5 | 0 | - |
| 6r | 10 ± 0.70 | 32 | 14 ± 0.57 | 2 | 0 | - |
| 6s | 10 ± 0.00 | 250 | 12 ± 1.52 | 2 | 10 ± 0.57 | 62.5 |
| 6t | 11 ± 0.70 | 250 | 11 ± 0.57 | 2 | 10 ± 0.57 | 32 |
| 6u | 10 ± 0.00 | 1000 | 17 ± 0.28 | 2 | 13 ± 1.00 | 16 |
| Fluconazole | 07 ± 0.28 | 250 | 15 ± 0.11 | 1000 | 15 ± 0.57 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wabli, R.I.; Alsulami, M.A.; Bukhari, S.I.; Moubayed, N.M.S.; Al-Mutairi, M.S.; Attia, M.I. Design, Synthesis, and Antimicrobial Activity of Certain New Indole-1,2,4 Triazole Conjugates. Molecules 2021, 26, 2292. https://doi.org/10.3390/molecules26082292
Al-Wabli RI, Alsulami MA, Bukhari SI, Moubayed NMS, Al-Mutairi MS, Attia MI. Design, Synthesis, and Antimicrobial Activity of Certain New Indole-1,2,4 Triazole Conjugates. Molecules. 2021; 26(8):2292. https://doi.org/10.3390/molecules26082292
Chicago/Turabian StyleAl-Wabli, Reem I., Mona A. Alsulami, Sarah I. Bukhari, Nadine M. S. Moubayed, Maha S. Al-Mutairi, and Mohamed I. Attia. 2021. "Design, Synthesis, and Antimicrobial Activity of Certain New Indole-1,2,4 Triazole Conjugates" Molecules 26, no. 8: 2292. https://doi.org/10.3390/molecules26082292
APA StyleAl-Wabli, R. I., Alsulami, M. A., Bukhari, S. I., Moubayed, N. M. S., Al-Mutairi, M. S., & Attia, M. I. (2021). Design, Synthesis, and Antimicrobial Activity of Certain New Indole-1,2,4 Triazole Conjugates. Molecules, 26(8), 2292. https://doi.org/10.3390/molecules26082292






