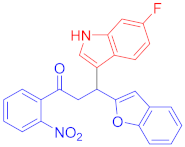
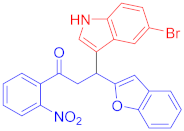
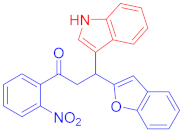
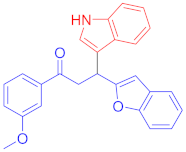
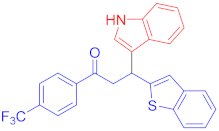
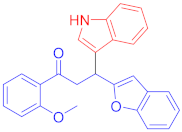
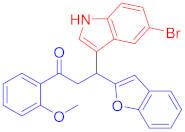
Abstract
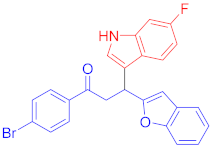
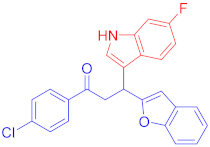
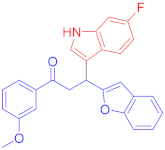
The Friedel–Crafts reaction between substituted indoles as nucleophiles with chalcones-based benzofuran and benzothiophene scaffolds was carried out by employing a highly efficient bimetallic iron–palladium catalyst system. This catalytic approach produced the desired bis-heteroaryl products with low catalyst loading, a simple procedure, and with acceptable yield. All synthesized indole scaffolds 3a–3s were initially evaluated for their cytotoxic effect against human fibroblast BJ cell lines and appeared to be non-cytotoxic. All non-cytotoxic compounds 3a–3s were then evaluated for their anticancer activities against cervical cancer HeLa, prostate cancer PC3, and breast cancer MCF-7 cell lines, in comparison to standard drug doxorubicin, with IC50 values 1.9 ± 0.4 µM, 0.9 ± 0.14 µM and 0.79 ± 0.05 µM, respectively, and appeared to be moderate to weak anticancer agents. Fluoro-substituted chalcone moiety-containing compounds, 3b appeared to be the most active member of the series against cervical HeLa (IC50 = 8.2 ± 0.2 µM) and breast MCF-7 cancer cell line (IC50 = 12.3 ± 0.04 µM), whereas 6-fluroindol-4-bromophenyl chalcone-containing compound 3e (IC50 = 7.8 ± 0.4 µM) appeared to be more active against PC3 prostate cancer cell line.
1. Introduction
Nitrogen heterocycles (natural and synthetic) are key components of several biochemical, and therefore possess interesting biological properties. Indoles are one such class of organic heterocyclic compounds that occur frequently as a privileged structural motif in many synthetic and natural products with versatile pharmacological activities [1]. In many cases they have widespread uses as cholesterol lowering agents, antiviral, antibacterial, antifungal, and as anticancer compounds [2]. Some of them regulate cellular signaling and numerous neuropsychological processes. The indole structures have a rich history in chemistry beginning with the study of dyes in the mid-19th century. Despite the reported toxicity of some indole-containing compounds, several of them possess clinically beneficial properties. The natural products vinblastine and vincristine, isolated from C. roseus [3], are active against leukemia, lymphoma, breast, lung and other cancers (Figure 1) [4]. Analogues of these compounds are in use for the treatment of a variety of cancers [5]. Numerous other indole-containing compounds have been shown to possess anti-cancer activity, as well as a myriad of other biological properties [6,7,8]. Because of the prevalence of the indole moiety in biologically active compounds, it is considered a “privileged structure” and that makes it a logical motif to improve biological activity of synthetic compounds [9]. The indole moiety is also found in many natural products and comprises a major subset for the alkaloid class of natural products. For example, ajmalicine is an anti-hypertensive alkaloid [10], asperazine has an unusual profile of cytotoxicity [11,12], and dragmacidin exhibits anti-tumor activity against P-388 cell lines with IC50 value of 15 μg/mL, and IC50 of 1–10 μg/mL against MDAMB (human mammary), HCT-8 (human colon) and A-549 (human lung) cancer cell lines [13,14,15].
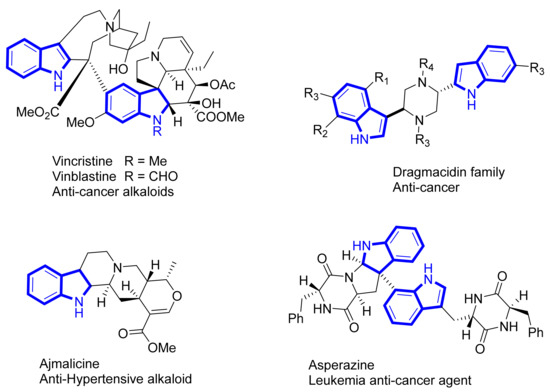
Figure 1.
Bioactive compounds incorporating indole skeleton.
Cancer is one of the leading causes of death globally, with almost 10 million deaths worldwide in 2020 [16]. By 2030, it will become the leading cause of death [17]. 70% of cancer deaths occur in middle or low-resource countries, where as 80% of cases are diagnosed too late for effective treatment. Cancer related deaths are increasing globally [17].
The heterogeneity of tumors, the lack of selectivity of drugs, and the development of drug resistance are some of the major obstacles impeding the effective cure of this deadly disease group. The fact of cell-death makes the situation even worse, because anti-cancer drugs follow first-order kinetics; that is, a fixed percentage, rather than a fixed number of cells are killed by a given treatment. Moreover, individual tumors may contain subpopulations of neoplastic cells that differ in terms of crucial features, any one of which could cause recurrence of the disease [17,18].
Although significant progress has been made in cancer research; still urgent need for a new drug with the advantage of less side effect, safe, overcome drug resistance and high efficacy are challenge to the researcher [19].
In this manuscript, we describe the synthesis of the new hits based on indole/benzofuran/benzothiophene analogues, along with the cytotoxities of the newly synthesized compounds against selected cancer cell lines.
2. Results and Discussion
2.1. Chemistry
Michaël-type Friedel–Crafts addition of nucleophiles (especially indoles) with electron-deficient alkenes are fundamentally important chemical transformations [20,21,22,23,24]. These methods have extensive uses in the scientific work on a number of potential nucleophilic species. Stabilized carbanions, organometallic reagents, and heteroatom-based nucleophiles have all been applied to a range of Michael acceptors in previous years [20,21]. The desired chalcones 1a–1l in this study were synthesized by following our previous reported method by Barakat et al [23] (Figure 2). Next, the achiral Friedel–Crafts reaction was explored with the chalcones-based benzofuran or thiophene analogues and the substituted indole by employing Fe-Pd bimetallic catalyst system [23] in MeOH at 60 °C to afford the Friedel–Crafts adducts. To check the productivity and the generality of this approach, the reaction of substituted indoles (indole 2a; 5-bromoindole 2b; 6-fluroroindole 2c) and chalcones based benzofuran or thiophene analogues was carried out under the reaction conditions (10 mol% of FeCl3, 10 mol% of PdCl2 and 15 mol% ethyl acetoacetate (EAA), 1.0 equiv. chalcone and 1.1 equiv. substituted indole in CH3OH at 60 °C; Scheme 1). A range of heteroaryl enone substituents further demonstrated a broad substrate scope. Both electron-deficient and electron-rich heterocycles afforded the Friedel–Crafts products in high yield.
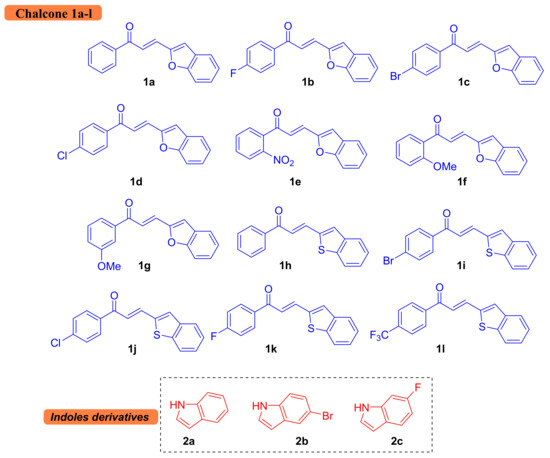
Figure 2.
List of synthesized chalcones and substituted indoles utilized in this study.
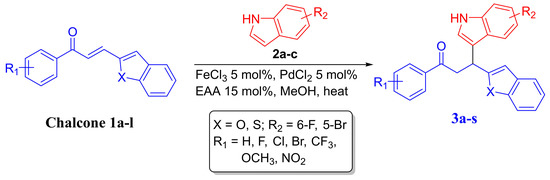
Scheme 1.
Synthetic routes to 3a–3s. The reaction carried out with optimum reaction conditions as reported by Barakat et al. [23].
The structures of requisite Friedel–Crafts adducts were determined by using spectroscopic data, including 1H-, 13C-NMR, Mass, and FT-IR spectroscopy.
2.2. Anti-Cancer Activity
All synthesized indoles 3a–3s were first evaluated for their cytotoxic effect against human fibroblast BJ cell line, and appeared to be either non-toxic (3a, 3i–3k, 3r and 3s) or slightly toxic than (30%) 3b–3i, and 3f–3q at a concentration of 30 µM. Based on the largely non-toxic nature of newly synthesized compounds against human fibroblast cell line compounds 3a–3s were further evaluated for their anticancer activities against HeLa, PC3, and MCF-7 cancer cell lines in comparison to standard drug doxorubicin, with IC50 values 1.9 ± 0.4 µM, 0.9 ± 0.14 µM and 0.79 ± 0.05 µM, respectively, and appeared to be moderate to weak anticancer agents. Results of cytotoxic analysis and anti-cancer activities are summarized in Table 1.

Table 1.
The IC50 results of the synthesized against 4 cancer cell lines.
Among series of substituted indoles and chalcone-based benzofurans compounds, 3a–3m and 3r–3s, 6-fluoroindol-4-bromophenyl chalcone-containing compound 3e (IC50 = 7.8 ± 0.4 µM) appeared to be the most active anti-cancer agent against prostate PC3 cancer cell line in comparison to non-substituted indoles and chalcone-based benzofuran (3a, IC50 = 11.4 ± 0.2 µM) and drug doxorubicin (IC50 = 1.9 ± 0.4 µM). However, decrease in activity was observed in compounds 3b (IC50 = 22.2 ± 0.4 µM), 3d (IC50 = 26.3 ± 0.8 µM), and 3f (IC50 = 27.0 ± 0.4 µM) having non-fluorinated indole moieties with fluoro-, bromo-, and chloro-substituted chalcone moieties, respectively. Similar pattern of decrease of activity was observed in compounds 3g (IC50 = 20.0 ± 1.3 µM), having chloro substituted phenyl rings of chalcones instead of bromo-substituted chalcone moiety of containing compound 3e (IC50 = 7.8 ± 0.4 µM). A complete loss of anticancer activity against PC3 cells was observed when fluoro-substituted chalcone moieties were replaced by o-nitro phenyl ring of containing 3i. Anti-cancer potential was restored in indole and chalcone-based benzofuran derivative 3j (IC50 = 16.0 ± 0.1 µM) with bromo-substituted indole ring instead of fluorine substituent 3i. Among series of compounds having methoxy-substituted chalcone moiety 3m (IC50 = 19.3 ± 0.1 µM) appeared as weak anti-cancer agents whereas compounds 3r and 3s appeared to be inactive. The pattern of activity of compound 3f (IC50 = 16.3 ± 1.6 µM) and 3r (in active) clearly demonstrated the positional effect of methoxy substituent of the chalcone moiety towards anti-cancer activity. All members of the series of the indole and chalcone-based thiophene derivatives 3n–3q, appeared as weak anti-cancer agents against prostate cancer PC3 cell line in comparison to doxorubicin (IC50 = 1.9 ± 0.4 µM) as standard drug.
All synthesized substituted indole and chalcone-based benzofurans 3a–3m and 3r–3s were further evaluated for their anticancer activities against cervical cancer HeLa cell lines and appeared to be moderate to weak anticancer agents against standard doxorubicin (IC50 = 0.9 ± 0.14 µM). Non-substituted indole and chalcone-based benzofurans 3a appeared as weak anti-cancer agent with IC50 = 22.5 ± 0.9 µM. However, substitution of fluorine on phenyl ring of chalcone moiety contributed towards drastic increase in anti-cancer potential as observed in 3b (IC50 = 8.2 ± 0.2 µM), followed by gradual decrease in anti-cancer potential by various substituents on chalcone and indole moieties as observed in 6-bromoindol-4-flurophenyl chalcone-containing 3c (IC50 = 10.8 ± 0.1 µM), 4-bromophenyl chalcone-containing 3d (IC50 = 12.6 ± 0.4 µM), 6-fluroindol-4-bromophenyl chalcone-containing 3e (IC50 = 13.6 ± 0.4 µM), 4-chlorophenyl chalcone-containing 3f (IC50 = 11.9 ± 0.2 µM), 6-fluroindol-4-chlorophenyl chalcone-containing 3g (IC50 = 14.7 ± 1.1 µM), 4-aminophenyl chalcone-containing 3h (IC50 = 29.7 ± 0.4 µM), 6-fluroindol-2-nitrophenyl chalcone-containing 3i (IC50 = 15.2 ± 0.6 µM), 5-bromoroindol-2-nitrophenyl chalcone-containing 3j (IC50 = 17.3 ± 0.7 µM), 4-trifluoromethylphenyl chalcone-containing 3k (IC50 = 14.9 ± 0.6 µM), 3-methoxyphenyl chalcone-containing 3l (IC50 = 11.1 ± 0.6 µM), 6-fluoroindol-3-methoxyphenyl chalcone-containing 3m (IC50 = 15.2 ± 0.2 µM), and 5-bromoindol-2-methoxyphenyl chalcone-containing 3s (IC50 = 18.4 ± 0.2 µM). Similar activity pattern was observed in non-substituted indole and chalcone-based thiophene derivative 3n (IC50 = 12.4 ± 0.2 µM), 4-chlorophenyl chalcone-containing compound 3o (IC50 = 13.8 ± 0.3 µM), 4-fluorophenyl chalcone-containing 3p (IC50 = 12.7 ± 0.1 µM), and 4-trifluoromethylphenyl chalcone-containing 3q (IC50 = 18.8 ± 0.2 µM against cervical cancer cell line HeLa in comparison to doxorubicin (IC50 = 1.9 ± 0.4 µM) as standard drug.
Many of the tested compounds appeared to be inactive against MCF-7 breast cancer cell lines, except 3b, 3c, 3f, 3m and 3s. Non-substituted indole and chalcone-based benzofurans 3a, appeared to be inactive against MCF-7 breast cancer cell lines; however, substitution of fluorine on phenyl ring of chalcone moiety contributed towards drastic increase in anti-cancer potential, as observed in 3b (IC50 = 12.37 ± 0.04 µM), followed by gradual decrease in anti-cancer potential due to various substituents on chalcone and indole moieties, as observed in 4-chlorophenyl chalcone-containing 3f (IC50 = 19.28 ± 0.78 µM), 6-fluroindol-3-methoxyphenyl chalcone-containing 3m (IC50 = 22.9 ± 0.43 µM), 6-bromoindol-4-flurophenyl chalcone-containing 3c (IC50 = 25.87 ± 0.8 µM), and 5-bromoindol-2-methoxyphenyl chalcone-containing compound 3s (IC50 = 28.64 ± 0.3 µM) against standard doxorubicin (IC50 = 0.79 ± 0.4 µM). All synthesized indole and chalcone-based thiophene derivatives 3n–3q appeared to be inactive against breast cancer cell line MCF-7.
The comparison of substituted indole and chalcone-based benzofurans (3a, 3b, 3g, and 3k) with substituted indole and chalcone-based thiophenes (3r–3s) disclosed that non-substituted indole and chalcone-based benzofuran 3a (IC50 = 11.4 ± 0.2 µM) and fluoro-, and chloro-substituted chalcone moieties, containing compounds 3b (IC50 = 22.2 ± 0.5 µM) and 3g (IC50 = 22.0 ± 1.3 µM), respectively, appeared to be more active against PC3 prostate cancer cell lines, in comparison to non-substituted indole and chalcone-based thiophene 3r (IC50 = 20.4 ± 0.4 µM) and fluoro-, and chloro-substituted chalcone moieties, containing compounds 3p (IC50 = 27.6 ± 0.5 µM) and 3s (IC50 = 29.7 ± 0.5 µM), respectively, that appeared to have more anti-cancer potential against cervical cancer HeLa cell line 3r (IC50 = 12.4 ± 0.2 µM), 3o (IC50 = 13.8 ± 0.3 µM) and 3p (IC50 = 12.7 ± 0.1 µM)]. Among fluoro-, and chloro-substituted chalcone-containing compounds 3b and 3s, respectively. Whereas trifluoro-substituted chalcone-containing benzofuran 3k (IC50 = 14.9 ± 0.6 µM) showed more activity against cervical cancer HeLa cell line, in comparison to thiophene analogue (3q, IC50 = 18.8 ± 0.2 µM). Among benzofurans (3a, 3b, 3g, and 3k) and thiophene 3r–3s structural analogues, 4-fluorophenyl chalcone-containing 3b (IC50 = 12.37 ± 0.04 µM) appeared to be the only active member against MCF-7 breast cancer cell line. All results are summarized in Table 1.
3. Materials and Methods
3.1. General Procedure (GP1)
Chalcones 1a–1l (0.5 mmol), indoles (0.55 mmol), and dry methanol (20 mL) were charged into a 100 mL round bottom flask, equipped with condenser under inert atmosphere. FeCl3 (5 mol%), PdCl2 (5 mol%), and 15 mol% of ethyl-acetoacetate (EAA) were added to the reaction mixture. Then the reaction was heated at 60–80 °C for 2–3 h. The reaction was monitored by TLC until the complete consumption of starting materials. Then the reaction mixture was allowed to cool and 20 mL of distilled water was added. The products were extracted in CH2Cl2 (3 × 20 mL) and the organics part were dried over anhydrous Mg2SO4. The organic phases were concentrated under reduced pressure and subjected to column chromatography using 100–200 mesh silica gel and 20–30% Ethyl acetate in n-hexane to afford moderate to good yields (60–90%).
3.2. Synthesis of 3-(Benzofuran-2-yl)-3-(1H-indol-3-yl)-1-phenylpropan-1-one (3a)
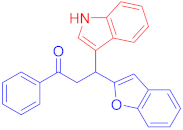
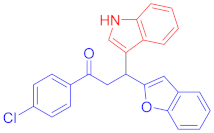
Following the general procedure (GP1) chalcone 1a (124 mg, 0.5 mmol) and indole 2a (64 mg, 0.55 mmol) produces Friedel–Crafts product-3a (yield 159 mg, 87%); m.p. 114–115 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.97 (d, J = 2.4 Hz, 1H, NH), 8.08–8.02 (m, 2H, Ar-H), 7.66–7.60 (m, 2H, Ar-H), 7.55–7.48 (m, 3H, Ar-H), 7.44–7.40 (m, 1H, Ar-H), 7.38–7.32 (m, 2H, Ar-H), 7.16 (pd, J = 7.3, 1.5 Hz, 2H, Ar-H), 7.06 (ddd, J = 8.1, 7.0, 1.2 Hz, 1H, Ar-H), 6.96 (ddd, J = 7.9, 6.9, 1.0 Hz, 1H, Ar-H), 6.70 (d, J = 1.0 Hz, 1H, Ar-H), 5.12 (t, J = 7.2 Hz, 1H, CH), 4.07 (dd, J = 17.5, 7.6 Hz, 1H, CH2(a)), 3.93 (dd, J = 17.5, 7.0 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, DMSO-d6) δ 197.7 (CO), 161.0, 153.9, 136.6, 136.3, 133.3, 128.7, 128.4, 128.1, 126.1, 123.3, 122.9, 122.6, 121.1, 120.5, 118.7, 118.6, 114.3, 111.6, 110.7, 101.8, 41.9 (CH), 31.8 (CH2); IR (KBr, cm−1) νmax = 3404, 3056, 29.04,2848, 1678, 1589, 1450, 1415, 1334, 1253; [Anal. Calcd. for C25H19NO2: C, 82.17; H, 5.24; N, 3.83; Found: C, 82.29; H, 5.12; N, 3.65]; LC/MS (ESI, m/z): 366.15 [M + H]+, exact mass 365.14 for C25H19NO2.
3.3. Synthesis of 3-(Benzofuran-2-yl)-1-(4-fluorophenyl)-3-(1H-indol-3-yl)propan-1-one (3b)
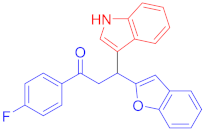
Following the general procedure (GP1) chalcone 1b (133 mg, 0.5 mmol) indole 2a (64 mg, 0.55 mmol) produces Friedel–Crafts product-3b (yield 174 mg, 91%); m.p. 157–158 oC; 1H-NMR (500 MHz, DMSO-d6) δ 10.98 (d, J = 2.4 Hz, 1H, NH), 8.16−8.10 (m, 2H, Ar-H), 7.64 (d, J = 7.9 Hz, 1H, Ar-H), 7.50 (dd, J = 6.8, 2.2 Hz, 1H, Ar-H), 7.44−7.40 (m, 1H, Ar-H), 7.38–7.32 (m, 4H, Ar-H), 7.16 (td, J = 7.0, 1.6 Hz, 2H, Ar-H), 7.06 (ddd, J = 8.1, 6.9, 1.2 Hz, 1H, Ar-H), 6.98–6.93 (m, 1H, Ar-H), 6.70 (d, J = 1.0 Hz, 1H, Ar-H), 5.11 (t, J = 7.2 Hz, 1H, CH), 4.07 (dd, J = 17.5, 7.5 Hz, 1H, CH2(a)), 3.92 (dd, J = 17.5, 6.9 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, DMSO-d6) δ 196.9 (CO), 166.6 & 164.6 (C1-F, JC-F = 200.3 Hz), 161.5, 154.4, 136.9, 133.9, 133.9, 131.7 & 131.6 (C4-F, JC-F = 7.4 Hz), 128.9, 126.6, 123.9, 123.5, 123.10, 121.6, 121.1, 119.2 &119.1 (C3-F, JC-F = 11.1 Hz), 116.3 & 116.2 (C2-F, JC-F = 17.4 Hz), 114.8, 112.1, 111.3, 102.4, 42.4 (CH), 32.3(CH2); IR (KBr, cm−1) νmax = 3331, 3055, 2904, 1677, 1591, 1502, 1452, 1363, 1340, 1234, 1225; [Anal. Calcd. for C25H18FNO2: C, 78.31; H, 4.73; N, 3.65; Found: C, 78.19; H, 4.65; N, 3.52]; LC/MS (ESI, m/z): 384.14 [M + H]+, exact mass 383.13 for C25H18FNO2.
3.4. Synthesis of 3-(Benzofuran-2-yl)-3-(5-bromo-1H-indol-3-yl)-1-(4-fluorophenyl)propan-1-one (3c)
The general procedure (GP1) chalcone 1b (133 mg, 0.5 mmol) 5-bromoindole 2b (108 mg, 0.55 mmol) produces Friedel–Crafts product-3c (yield 191 mg, 83%); m.p. 167–168 °C; 1H-NMR (400 MHz, DMSO-d6) δ 11.21 (d, J = 2.4 Hz, 1H, NH), 8.13 (dd, J = 8.7, 5.8 Hz, 2H, Ar-H), 7.82 (d, J = 2.0 Hz, 1H, Ar-H), 7.54–7.50 (m, 1H, Ar-H), 7.47–7.41 (m, 2H, Ar-H), 7.34 (t, J = 8.9 Hz, 3H, Ar-H), 7.22–7.12 (m, 3H, Ar-H), 6.73 (s, 1H, Ar-H), 5.10 (t, J = 7.1 Hz, 1H, CH), 4.06 (dd, J = 17.7, 7.3 Hz, 1H, CH2(a)), 3.93 (dd, J = 17.7, 7.2 Hz, 1H, CH2(b)); 13C-NMR (101 MHz, DMSO-d6) δ 196.2 (CO), 166.4 & 163.9 (C1-F, JC-F = 250.7 Hz), 160.6, 153.9, 134.9, 133.3, 131.2 & 131.1 (C4-F, JC-F = 9.6 Hz), 128.4, 127.9, 124.8, 123.6, 123.4, 122.6, 120.9, 120.6, 115.8 & 115.6 (C2-F, JC-F = 22.2 Hz), 114.2, 113.6, 111.3, 110.7, 101.9, 41.9 (CH), 31.4 (CH2); IR (KBr, cm−1) νmax = 3396, 3066, 2921, 1675, 1591, 1504, 1452, 1413, 1226; [Anal. Calcd. for C25H17BrFNO2: C, 64.95; H, 3.71; N, 3.03; Found: C, 65.17; H, 3.62; N, 2.93]; LC/MS (ESI, m/z): 462.05 [M + H] +, exact mass 461.04 for C25H17BrFNO2.
3.5. Synthesis of 3-(Benzofuran-2-yl)-1-(4-bromophenyl)-3-(1H-indol-3-yl)propan-1-one (3d)
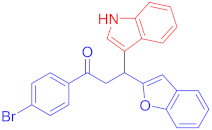
Following the general procedure (GP1) chalcone 1c (164 mg, 0.5 mmol) indole 2a (64 mg, 0.55 mmol) produces Friedel–Crafts product-3d (yield 189 mg, 85%); m.p. 108–109 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.99–10.93 (m, 1H, NH), 8.00–7.94 (m, 2H, Ar-H), 7.73 (d, J = 8.5 Hz, 2H, Ar-H), 7.62 (d, J = 8.0 Hz, 1H, Ar-H), 7.51–7.47 (m, 1H, Ar-H), 7.41 (d, J = 7.6 Hz, 1H, Ar-H), 7.37–7.31 (m, 2H, Ar-H), 7.16 (td, J = 7.1, 1.7 Hz, 2H, Ar-H), 7.05 (t, J = 7.6 Hz, 1H, Ar-H), 6.95 (t, J = 7.5 Hz, 1H, Ar-H), 6.70 (s, 1H, Ar-H), 5.08 (t, J = 7.3 Hz, 1H, CH), 4.06 (dd, J = 17.5, 7.5 Hz, 1H, CH2(a)), 3.91 (dd, J = 17.5, 6.8 Hz, 1H CH2(b)); 13C-NMR (126 MHz, DMSO-d6) δ 197.0 (CO), 160.9, 153.9, 136.3, 135.5, 131.8, 130.2, 128.4, 127.4, 126.1, 123.4, 123.0, 122.6, 121.1, 120.5, 118.7, 118.6, 114.2, 111.6, 110.7, 101.9, 41.9 (CH), 31.7 (CH2); IR (KBr, cm−1) νmax = 3323, 3057, 2920, 2852, 1678, 1580, 1452, 1394, 1363, 1338, 1252, 1197; [Anal. Calcd. for C25H18BrNO2: C, 67.58; H, 4.08; N, 3.15; Found: C, 67.45; H, 4.19; N, 3.01]; LC/MS (ESI, m/z): 444.06 [M + H] +, exact mass 443.05 for C25H18BrNO2.
3.6. Synthesis of 3-(Benzofuran-2-yl)-1-(4-bromophenyl)-3-(6-fluoro-1H-indol-3-yl)propan-1-one (3e)
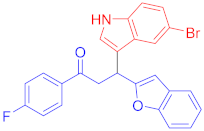
Following the general procedure (GP1) chalcone 1c (164 mg, 0.5 mmol) 6-fluoroindole 2c (74 mg, 0.55 mmol) produces Friedel–Crafts product-3e (yield 166 mg, 72%); m.p. 123–124 °C; 1H-NMR (400 MHz, DMSO-d6) δ 11.05 (d, J = 2.5 Hz, 1H, NH), 7.97 (d, J = 8.1 Hz, 2H, Ar-H), 7.72 (d, J = 8.1 Hz, 2H, Ar-H), 7.62 (dd, J = 8.8, 5.3 Hz, 1H, Ar-H), 7.50 (d, J = 7.1 Hz, 1H, Ar-H), 7.41 (d, J = 7.5 Hz, 1H, Ar-H), 7.37 (d, J = 2.6 Hz, 1H, Ar-H), 7.22–7.08 (m, 3H, Ar-H), 6.82 (td, J = 9.4, 2.5 Hz, 1H, Ar-H), 6.71 (s, 1H, Ar-H), 5.08 (t, J = 7.2 Hz, 1H, CH), 4.06 (dd, J = 17.6, 7.4 Hz, 1H, CH2(a)), 3.90 (dd, J = 17.6, 6.8 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, DMSO-d6) δ 196.9 (CO), 160.7, 159.8 & 157.9 (C1-F, JC-F = 186.3 Hz), 153.9, 136.2 & 136.1 (C3-F, JC-F = 10.3 Hz), 135.5, 131.8, 130.2, 128.4, 127.5, 123.6 & 123.6 (C4-F, JC-F = 2.6 Hz), 123.4, 122.9, 122.6, 120.6, 119.7 & 119.7 (C5-F, JC-F = 8.2 Hz), 114.5, 110.7, 107.2 & 106.9 (C2-F, JC-F = 19.5 Hz), 101.9, 97.6 & 97.4 (C6-F, JC-F = 20.2 Hz), 41.9 (CH), 31.6 (CH2); IR (KBr, cm−1) νmax = 3359, 2918, 2850, 1678, 1624, 1581, 1454, 1394, 1336, 1250; [Anal. Calcd. for C25H17BrFNO2: C, 64.95; H, 3.71; N, 3.03; Found: C, 64.84; H, 3.66; N, 3.21]; LC/MS (ESI, m/z): 462.05 [M + H], exact mass 461.04 for C25H17BrFNO2.
3.7. Synthesis of 3-(Benzofuran-2-yl)-1-(4-chlorophenyl)-3-(1H-indol-3-yl)propan-1-one (3f)
Following the general procedure (GP1) chalcone 1d (141 mg, 0.5 mmol) indole 2a (64 mg, 0.55 mmol) produces Friedel–Crafts product-3f (yield 158 mg, 79%); m.p. 126–127 °C; 1H-NMR (400 MHz, DMSO-d6) δ 10.98 (d, J = 2.4 Hz, 1H, NH), 8.05 (d, J = 8.2 Hz, 2H, Ar-H), 7.63 (d, J = 8.0 Hz, 1H, Ar-H), 7.58 (d, J = 8.6 Hz, 2H, Ar-H), 7.52–7.47 (m, 1H, Ar-H), 7.44–7.40 (m, 1H, Ar-H), 7.38–7.33 (m, 2H, Ar-H), 7.16 (t, J = 5.6 Hz, 2H, Ar-H), 7.06 (t, J = 7.7 Hz, 1H, Ar-H), 6.96 (t, J = 7.4 Hz, 1H, Ar-H), 6.70 (s, 1H, Ar-H), 5.10 (t, J = 7.2 Hz, 1H, CH), 4.07 (dd, J = 17.6, 7.4 Hz, 1H, CH2(a)), 3.93 (dd, J = 17.6, 7.1 Hz, 1H, CH2(b)); 13C-NMR (101 MHz, DMSO-d6) δ 197.4 (CO), 161.5, 154.5, 138.8, 136.9, 135.8, 130.6, 129.4, 128.9, 126.7, 123.9, 123.6, 123.2, 121.7, 121.1, 119.3, 119.1, 114.8, 112.1, 111.3, 102.5, 42.6 (CH), 32.3 (CH2); IR (KBr, cm−1) νmax = 3323, 2935, 2918, 2854, 1680, 1585, 1456, 1396, 1362, 1340, 1250; [Anal. Calcd. for C25H18ClNO2: C, 75.09; H, 4.54; N, 3.50; Found: C, 75.23; H, 4.46; N, 3.39]; LC/MS (ESI, m/z): 400.14 [M + H] +, exact mass 399.10 for C25H18ClNO2.
3.8. Synthesis of 3-(Benzofuran-2-yl)-1-(4-chlorophenyl)-3-(6-fluoro-1H-indol-3-yl)propan-1-one (3g)
Following the general procedure (GP1) chalcone 1d (141 mg, 0.5 mmol) 6-fluoroindole 2c (74 mg, 0.55 mmol) produces Friedel–Crafts product-3g (yield 146 mg, 70%); m.p. 112–113 °C; 1H-NMR (400 MHz, DMSO-d6) δ 11.08 (d, J = 2.4 Hz, 1H, NH), 8.05 (d, J = 8.6 Hz, 2H, Ar-H), 7.64 (dd, J = 8.8, 5.6 Hz, 1H, Ar-H), 7.57 (d, J = 8.3 Hz, 2H, Ar-H), 7.50 (d, J = 7.4 Hz, 1H, Ar-H), 7.44–7.36 (m, 2H, Ar-H), 7.21–7.10 (m, 3H, Ar-H), 6.84 (td, J = 9.2, 2.6 Hz, 1H, Ar-H), 6.71 (s, 1H, Ar-H), 5.11 (t, J = 7.2 Hz, 1H, CH), 4.07 (dd, J = 17.6, 7.7 Hz, 1H, CH2(a)), 3.92 (dd, J = 17.6, 7.1 Hz, 1H, CH2(b)); 13C-NMR (101 MHz, DMSO-d6) δ 196.7 (CO), 160.7, 160.0 & 157.7 (C1-F, JC-F = 233.3 Hz), 153.9, 138.3, 136.3 & 136.1 (C3-F, JC-F = 12.6 Hz), 135.2, 130.0, 128.8, 128.4, 123.6 & 123.4 (C4-F, JC-F = 2.4 Hz), 122.9, 122.6, 120.6, 119.8 & 119.7 (C5-F, JC-F = 10.1 Hz), 114.5, 110.7, 107.2 & 106.9 (C2-F, JC-F = 24.3 Hz), 101.9, 97.6 & 97.4 (C6-F, JC-F = 25.2 Hz), 41.9 (CH), 31.7 (CH2); IR (KBr, cm−1) νmax = 3304, 3028, 2910, 2850, 1665, 1554, 1450, 1394, 1363, 1338, 1252; [Anal. Calcd. for C25H17ClFNO2: C, 71.86; H, 4.10; N, 3.35; Found: C, 71.69; H, 4.25; N, 3.19]; LC/MS (ESI, m/z): 418.10 [M + H] +, exact mass 417.09 for C25H17ClFNO2.
3.9. Synthesis of 3-(Benzo[b]thiophen-2-yl)-1-(4-bromophenyl)-3-(1H-indol-3-yl)propan-1-one (3h)
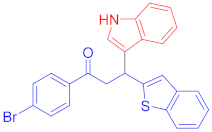
Following the general procedure (GP1) chalcone 1i (172 mg, 0.5 mmol), indole 2a (108 mg, 0.55 mmol) produces Friedel–Crafts product-3h (yield 156 mg, 68%); m.p. 121–122 °C; 1H-NMR (400 MHz, DMSO-d6) δ 10.97 (s, 1H, NH), 7.98 (d, J = 8.2 Hz, 2H, Ar-H), 7.72 (dt, J = 18.7, 9.0 Hz, 4H, Ar-H), 7.54 (d, J = 8.0 Hz, 1H, Ar-H), 7.41 (d, J = 2.5 Hz, 1H, Ar-H), 7.35–7.19 (m, 4H, Ar-H), 7.05 (t, J = 7.6 Hz, 1H, Ar-H), 6.92 (t, J = 7.4 Hz, 1H, Ar-H), 5.23 (t, J = 7.3 Hz, 1H, CH), 4.01 (dd, J = 10.8, 7.1 Hz, 2H, CH2); 13C-NMR (126 MHz, DMSO-d6) δ 197.0 (CO), 150.76, 139.5, 138.5, 136.4, 135.6, 131.8, 130.2, 127.5, 126.1, 124.1, 123.6, 123.0, 122.5, 122.2, 121.2, 120.2, 118.7, 118.5, 116.8, 111.5, 44.5 (CH), 33.4 (CH2); IR (KBr, cm−1) νmax = 3335, 3038, 2907, 2852, 1678, 1580, 1455, 1440, 1360, 1348, 1255; [Anal. Calcd. for C25H18BrNOS: C, 65.22; H, 3.94; N, 3.04; Found: C, 65.04; H, 3.86; N, 2.91]; LC/MS (ESI, m/z): 460.04 [M + H] +, exact mass 459.03 for C25H18BrNOS.
3.10. Synthesis of 3-(Benzofuran-2-yl)-3-(6-fluoro-1H-indol-3-yl)-1-(2-nitrophenyl)propan-1-one (3i)
Following the general procedure (GP1) chalcone 1e (147 mg, 0.5 mmol) and 6-fluoroindole 2c (74 mg, 0.55 mmol) produces Friedel–Crafts product-3i (yield 146 mg, 68%); m.p. 139–140 °C; 1H-NMR (500 MHz, DMSO-d6) δ 11.10 (d, J = 2.5 Hz, 1H, NH), 8.06 (dd, J = 8.0, 1.2 Hz, 1H, Ar-H), 7.78 (td, J = 7.5, 1.2 Hz, 1H, Ar-H), 7.71 (ddd, J = 12.9, 7.5, 1.6 Hz, 2H, Ar-H), 7.61 (dd, J = 8.8, 5.4 Hz, 1H, Ar-H), 7.54–7.50 (m, 1H, Ar-H), 7.44 (dt, J = 7.2, 1.3 Hz, 1H, Ar-H), 7.40 (d, J = 2.5 Hz, 1H, Ar-H), 7.22–7.12 (m, 3H, Ar-H), 6.83 (ddd, J = 9.7, 8.7, 2.4 Hz, 1H, Ar-H), 6.78–6.74 (m, 1H, Ar-H), 5.05 (t, J = 7.1 Hz, 1H, CH), 4.01 (dd, J = 17.9, 7.6 Hz, 1H, CH2(a)), 3.82 (dd, J = 18.0, 6.6 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, DMSO-d6) δ 199.5 (CO), 160.1, 159.8 &157.9 (C1-F, JC-F = 186.4 Hz), 154.0, 146.2, 136.3 & 136.2 (C3-F, JC-F = 10.0 Hz), 135.4, 134.1, 131.8, 128.4 & 128.4 (C4-F, JC-F = 3.8 Hz), 124.3, 123.9, 123.8, 123.5, 122.9, 122.7, 120.7, 119.7 & 119.6(C5-F, JC-F = 8.0 Hz), 114.1, 110.8, 107.3 & 107.1 (C2-F, JC-F = 19.5 Hz), 102.3, 97.7 & 97.5 (C6-F, JC-F = 20.0 Hz), 45.2 (CH), 31.6 (CH2); IR (KBr, cm−1) νmax = 3305, 3062,2915, 2858, 1699, 1621, 1527, 1452, 1338, 1255, 1144, 798, 748, 696; [Anal. Calcd. for C25H17FN2O4: C, 70.09; H, 4.00; N, 6.54; Found: C, 69.89; H, 4.11; N, 6.45]; LC/MS (ESI, m/z): 429.13 [M + H] +, exact mass 428.12 for C25H17FN2O4.
3.11. Synthesis of 3-(Benzofuran-2-yl)-3-(5-bromo-1H-indol-3-yl)-1-(2-nitrophenyl)propan-1-one (3j)
Following the general procedure (GP1) chalcone 1e (147 mg, 0.5 mmol) 5-bromoindole 2b (108 mg, 0.55 mmol) produces Friedel–Crafts product-3j (yield 154 mg, 63%); m.p. 173–174 °C; 1H-NMR (400 MHz, DMSO-d6) δ 11.25 (d, J = 2.6 Hz, 1H, NH), 8.06 (d, J = 7.9 Hz, 1H, Ar-H), 7.84–7.76 (m, 2H, Ar-H), 7.73 (d, J = 7.6 Hz, 2H, Ar-H), 7.54 (d, J = 6.8 Hz, 1H, Ar-H), 7.50–7.40 (m, 2H, Ar-H), 7.33 (d, J = 8.7 Hz, 1H, Ar-H), 7.17 (q, J = 7.3, 6.7 Hz, 3H, Ar-H), 6.77 (s, 1H, Ar-H), 5.06 (t, J = 6.9 Hz, 1H, CH), 4.02 (dd, J = 18.0, 7.5 Hz, 1H, CH2(a)), 3.83 (dd, J = 17.8, 6.7 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, DMSO-d6) δ 199.4 (CO), 159.9, 153.9, 146.1, 135.3, 134.9, 133.9, 131.7, 128.4, 128.4, 127.8, 124.9, 124.2, 123.7, 123.5, 122.6, 120.8, 120.6, 113.7, 113.6, 111.3, 110.7, 102.2, 45.1 (CH), 31.2 (CH2); IR (KBr, cm−1) νmax = 3324, 3062, 2918, 2850, 1695, 1570, 1519, 1452, 1334, 1245, 1099, 879, 854, 791, 746, 694, 604; [Anal. Calcd. for C25H17BrN2O4: C, 61.36; H, 3.50; N, 5.72; Found: C, 61.21; H, 3.63; N, 5.62]; LC/MS (ESI, m/z): 489.05 [M + H], exact mass 488.04 for C25H17BrN2O4.
3.12. Synthesis of 3-(Benzofuran-2-yl)-3-(1H-indol-3-yl)-1-(2-nitrophenyl)propan-1-one (3k)
Following the general procedure (GP1) chalcone 1e (147 mg, 0.5 mmol) indole 2a (64 mg, 0.55 mmol) produces Friedel–Crafts product-3k (yield 135 mg, 66%); m.p. 154–155 °C; 1H-NMR (500 MHz, DMSO-d6) δ 11.01 (d, J = 2.5 Hz, 1H, NH), 8.06 (dd, J = 8.0, 1.2 Hz, 1H, Ar-H), 7.78 (td, J = 7.5, 1.2 Hz, 1H, Ar-H), 7.74–7.68 (m, 2H, Ar-H), 7.60 (d, J = 7.9 Hz, 1H, Ar-H), 7.54–7.50 (m, 1H, Ar-H), 7.45–7.41 (m, 1H, Ar-H), 7.39–7.33 (m, 2H, Ar-H), 7.17 (pd, J = 7.2, 1.4 Hz, 2H, Ar-H), 7.08–7.03 (m, 1H, Ar-H), 6.95 (t, J = 7.4 Hz, 1H, Ar-H), 6.75 (s, 1H, Ar-H), 5.05 (t, J = 7.1 Hz, 1H, CH), 4.01 (dd, J = 17.9, 7.8 Hz, 1H, CH2(a)), 3.82 (dd, J = 17.9, 6.4 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, DMSO-d6) δ 199.6 (CO), 160.3, 153.9, 146.2, 136.4, 135.4, 134.1, 131.8, 128.5, 128.4, 126.0, 124.3, 123.5, 123.2, 122.7, 121.2, 120.7, 118.7, 118.6, 113.8, 111.7, 110.8, 102.2, 45.3 (CH), 31.6 (CH2); IR (KBr, cm−1) νmax = 3310, 3055, 2901, 2850, 1684, 1625, 1530, 145, 1331, 1253; [Anal. Calcd. for C25H18N2O4: C, 73.16; H, 4.42; N, 6.83; Found: C, 73.10; H, 4.38; N, 6.79]; LC/MS (ESI, m/z): 411.13 [M + H] +, exact mass 410.13 for C25H18N2O4.
3.13. Synthesis of 3-(Benzofuran-2-yl)-3-(1H-indol-3-yl)-1-(3-methoxyphenyl)propan-1-one (3l)
Following the general procedure (GP1) chalcone 1g (139 mg, 0.5 mmol) indole 2a (64 mg, 0.55 mmol) produces Friedel–Crafts product-3l (yield 162 mg, 82%); m.p. 162–163 °C; 1H-NMR (500 MHz, DMSO-d6) δ 10.97 (d, J = 2.5 Hz, 1H, NH), 7.68–7.61 (m, 2H, Ar-H), 7.52–7.47 (m, 2H, Ar-H), 7.46–7.40 (m, 2H, Ar-H), 7.37–7.33 (m, 2H, Ar-H), 7.21–7.14 (m, 3H, Ar-H), 7.05 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H, Ar-H), 6.95 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H, Ar-H), 6.70 (d, J = 1.0 Hz, 1H, Ar-H), 5.10 (t, J = 7.2 Hz, 1H, CH), 4.04 (dd, J = 17.5, 7.5 Hz, 1H, CH2(a)), 3.93 (dd, J = 17.5, 7.0 Hz, 1H, CH2(b)), 3.79 (s, 3H, OCH3); 13C-NMR (126 MHz, DMSO-d6) δ 197.5 (CO), 161.0, 159.4, 153.9, 138.0, 136.3, 129.9, 128.4, 126.1, 123.3, 123.0, 122.5, 121.1, 120.6, 120.5, 119.4, 118.7, 118.5, 114.2, 112.4, 111.6, 110.7, 101.8, 55.3 (OCH3), 42.0 (CH), 31.8 (CH2); IR (KBr, cm−1) νmax = 3392, 3055, 2960, 2916, 2852, 1674, 1591, 1452,1423, 1333, 1265, 1190, 1161, 1095, 1008, 813, 790, 744, 609, 544; [Anal. Calcd. for C26H21NO3: C, 78.97; H, 5.35; N, 3.54; Found: C, 79.13; H, 5.25; N, 3.44]; LC/MS (ESI, m/z): 396.16 [M + H], exact mass 395.15 for C26H21NO3.
3.14. Synthesis of 3-(Benzofuran-2-yl)-3-(6-fluoro-1H-indol-3-yl)-1-(3-methoxyphenyl)propan-1-one (3m)
Following the general procedure (GP1) chalcone 1g (139 mg, 0.5 mmol) 6- fluoroindole 2c (74 mg, 0.55 mmol) produces Friedel–Crafts product-3m (yield 153 mg, 74%); m.p. 154–155 °C; 1H-NMR (400 MHz, DMSO-d6) δ 11.04 (d, J = 2.4 Hz, 1H, NH), 7.67–7.58 (m, 2H, Ar-H), 7.53–7.46 (m, 2H, Ar-H), 7.46–7.40 (m, 2H, Ar-H), 7.36 (d, J = 2.5 Hz, 1H, Ar-H), 7.22–7.09 (m, 4H, Ar-H), 6.82 (td, J = 9.4, 2.5 Hz, 1H, Ar-H), 6.71 (s, 1H, Ar-H), 5.08 (t, J = 7.1 Hz, 1H, CH), 4.03 (dd, J = 17.5, 7.5 Hz, 1H, CH2(a)), 3.95–3.87 (m, 1H, CH2(b)), 3.79 (s, 3H, CH3); 13C-NMR (126 MHz, DMSO-d6) δ 197.5 (CO), 160.8, 159.4, 159.78 &157.9(C1-F, JC-F = 186.0 Hz), 153.9, 137.9, 136.2 & 136.1 (C3-F, JC-F = 10.1 Hz), 129.9, 128.4, 123.7, 123.4, 123.0, 122.6, 120.6 & 120.6 (C4-F, JC-F = 7.4 Hz), 119.7 & 119.6 (C5-F, JC-F = 8.1 Hz), 119.5, 114.5, 112.5, 110.7, 107.2 & 106.9 (C2-F, JC-F = 19.4 Hz), 101.9, 97.6 & 97.4 (C6-F, JC-F = 20.3 Hz), 55.3 (OCH3), 42.0 (CH), 31.7 (CH2); IR (KBr, cm−1) νmax = 3409, 3053, 2922, 2836, 1666, 1513, 1483, 1454, 1430, 1286, 1244, 1163, 1101, 1016, 806, 740, 586; [Anal. Calcd. for C26H20FNO3: C, 75.53; H, 4.88; N, 3.39; Found: C, 75.41; H, 4.99; N, 3.27]; LC/MS (ESI, m/z): 414.15 [M + H] +, exact mass 413.14 for C26H20FNO3.
3.15. Synthesis of 3-(Benzo[b]thiophen-2-yl)-3-(1H-indol-3-yl)-1-phenylpropan-1-one (3n)
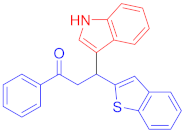
Following the general procedure (GP1) chalcone 1h (132 mg, 0.5 mmol) indole 2a (64 mg, 0.55 mmol) produces Friedel–Crafts product-3n (yield 164 mg, 86%); m.p. 159–160 °C; 1H-NMR (400 MHz, DMSO-d6) δ 10.90 (s, 1H, NH), 7.97 (d, J = 7.7 Hz, 2H, Ar-H), 7.68 (d, J = 8.1 Hz, 1H, Ar-H), 7.60 (d, J = 8.0 Hz, 1H, Ar-H), 7.54 (t, J = 7.4 Hz, 1H, Ar-H), 7.50–7.40 (m, 3H, Ar-H), 7.35 (s, 1H, Ar-H), 7.30–7.22 (m, 2H, Ar-H), 7.19 (t, J = 7.5 Hz, 1H, Ar-H), 7.13 (t, J = 7.6 Hz, 1H, Ar-H), 6.98 (t, J = 7.7 Hz, 1H, Ar-H), 6.86 (t, J = 7.5 Hz, 1H, Ar-H), 5.19 (t, J = 7.2 Hz, 1H, CH), 4.02–3.87 (m, 2H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ 197.8 (CO), 150.9, 139.6, 138.6, 136.7, 136.475, 133.4, 128.7, 128.1, 126.2, 124.1, 123.7, 122.9, 122.4, 122.1, 121.1, 120.2, 118.8, 118.6, 116.9, 111.5, 44.6 (CH), 33.4 (CH2); IR (KBr, cm−1) νmax = 3427, 3394, 3053, 2885, 1668, 1597, 1450, 1334, 1265, 1194; [Anal. Calcd. for C25H19NOS: C, 78.71; H, 5.02; N, 3.67; Found: C, 78.62; H, 5.17; N, 3.61]; LC/MS (ESI, m/z): 382.13 [M + H] +, exact mass 381.12 for C25H19NOS.
3.16. Synthesis of 3-(Benzo[b]thiophen-2-yl)-1-(4-chlorophenyl)-3-(1H-indol-3-yl)propan-1-one (3o)
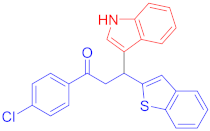
Following the general procedure (GP1) chalcone 1j (149 mg, 0.5 mmol) indole 2a (64 mg, 0.55 mmol) produces Friedel–Crafts product-3o (yield 160 mg, 77%); m.p. 150–151 °C; 1H-NMR (400 MHz, DMSO-d6) δ 10.99 (d, J = 2.5 Hz, 1H, NH), 8.09–8.03 (m, 2H, Ar-H), 7.76 (d, J = 8.0 Hz, 1H, Ar-H), 7.69 (d, J = 7.9 Hz, 1H, Ar-H), 7.57 (dd, J = 11.7, 8.4 Hz, 3H, Ar-H), 7.43 (d, J = 2.7 Hz, 1H, Ar-H), 7.35 (d, J = 8.2 Hz, 1H, Ar-H), 7.32 (s, 1H, Ar-H), 7.26 (d, J = 7.9 Hz, 1H, Ar-H), 7.22 (d, J = 7.4 Hz, 1H, Ar-H), 7.06 (t, J = 7.6 Hz, 1H, Ar-H), 6.94 (t, J = 7.5 Hz, 1H, Ar-H), 5.25 (t, J = 7.1 Hz, 1H, CH), 4.11–3.95 (m, 2H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ 196.8 (CO), 150.8, 139.6, 138.5, 138.2, 136.4, 135.3, 130.1, 128.8, 126.1, 124.1, 123.5, 123.1, 122.6, 122.2, 121.1, 120.3, 118.7, 118.6, 116.8, 111.6, 44.6 (CH), 33.4 (CH2).; IR (KBr, cm−1) νmax = 3383, 3055, 2954, 2921, 2852, 1676, 1583, 1483, 1454, 1402, 1356, 1328, 1261, 1194; [Anal. Calcd. for C25H18ClNOS: C, 72.19; H, 4.36; N, 3.37; Found: C, 72.05; H, 4.24; N, 3.33]; LC/MS (ESI, m/z): 416.10 [M + H] +, exact mass 415.08 for C25H18ClNOS.
3.17. Synthesis of 3-(Benzo[b]thiophen-2-yl)-1-(4-fluorophenyl)-3-(1H-indol-3-yl)propan-1-one (3p)
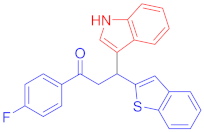
Following the general procedure (GP1) chalcone 1k (141 mg, 0.5 mmol) indole 2a (64 mg, 0.55 mmol) produces Friedel–Crafts product-3p (yield 164 mg, 82%); m.p. 142–143 °C; 1H-NMR (400 MHz, DMSO-d6) δ 10.98 (s, 1H, NH), 8.14 (dd, J = 8.5, 5.6 Hz, 2H, Ar-H), 7.76 (d, J = 8.0 Hz, 1H, Ar-H), 7.68 (d, J = 7.9 Hz, 1H, Ar-H), 7.55 (d, J = 8.0 Hz, 1H, Ar-H), 7.43 (s, 1H, Ar-H), 7.40–7.30 (m, 4H, Ar-H), 7.30–7.24 (m, 1H, Ar-H), 7.21 (t, J = 7.6 Hz, 1H, Ar-H), 7.06 (t, J = 7.6 Hz, 1H, Ar-H), 6.93 (t, J = 7.5 Hz, 1H, Ar-H), 5.25 (t, J = 7.2 Hz, 1H, CH), 4.02 (dd, J = 11.1, 7.2 Hz, 2H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ 196.4 (CO), 166.3 & 163.8 (C1-F, JC-F = 250.2 Hz), 150.8, 139.6, 138.5, 136.4, 133.4 & 133.4 (C4-F, JC-F = 2.9 Hz), 131.2, 126.1, 124.1, 123.6, 123.0, 122.5, 122.5, 122.2, 121.1, 120.2 & 120.2 (C3-F, JC-F = 6.3 Hz), 118.5, 116.9, 115.8 & 115.6 (C2-F, JC-F = 22.0 Hz), 111.5, 44.5 (CH), 33.5(CH2); IR (KBr, cm−1) νmax = 3400, 3059, 2904, 1670, 1591, 1502, 1454, 1411, 1356, 1335, 1226, 1153; [Anal. Calcd. for C25H18FNOS: C, 75.16; H, 4.54; N, 3.51; Found: C, 75.26; H, 4.43; N, 3.47]; LC/MS (ESI, m/z): 400.13 [M + H] +, exact mass 399.11 for C25H18FNOS.
3.18. Synthesis of 3-(Benzo[b]thiophen-2-yl)-3-(1H-indol-3-yl)-1-(4-(trifluoromethyl)phenyl)propan-1-one (3q)
Following the general procedure (GP1) chalcone 1l (162 mg, 0.5 mmol) indole 2a (64 mg, 0.55 mmol) produces Friedel–Crafts product-3q (yield 171 mg, 76%); m.p. 165–166 °C; 1H-NMR (400 MHz, DMSO-d6) δ 10.98 (d, J = 2.5 Hz, 1H, NH), 8.23 (d, J = 8.1 Hz, 2H, Ar-H), 7.89 (d, J = 8.3 Hz, 2H, Ar-H), 7.77 (d, J = 8.0 Hz, 1H, Ar-H), 7.69 (d, J = 7.9 Hz, 1H, Ar-H), 7.56 (d, J = 8.0 Hz, 1H, Ar-H), 7.43 (d, J = 2.6 Hz, 1H, Ar-H), 7.35 (d, J = 8.6 Hz, 2H, Ar-H), 7.30–7.19 (m, 2H, Ar-H), 7.06 (t, J = 7.6 Hz, 1H, Ar-H), 6.94 (t, J = 7.4 Hz, 1H, Ar-H), 5.26 (t, J = 7.1 Hz, 1H, CH), 4.10 (t, J = 7.4 Hz, 2H, CH2); 13C-NMR (101 MHz, DMSO-d6) δ 197.3 (CO), 150.6, 139.7, 139.5, 138.5, 136.4, 132.7, 132.4, 128.9, 126.1, 125.70, 124.1, 123.6, 123.0, 122.5, 122.1, 121.2, 120.2, 118.6, 116.7, 111.5, 44.9 (CH), 33.4 (CH2); IR (KBr, cm−1) νmax = 3458, 3415, 3050, 2960, 2902, 1689, 1454, 1409, 1319, 1261, 1166; [Anal. Calcd. for C26H18F3NOS: C, 69.47; H, 4.04; N, 3.12; Found: C, 69.59; H, 3.97; N, 3.07]; LC/MS (ESI, m/z): 450.11 [M + H] +, exact mass 449.11 for C26H18F3NOS.
3.19. Synthesis of 3-(Benzofuran-2-yl)-3-(1H-indol-3-yl)-1-(2-methoxyphenyl)propan-1-one (3r):
Following the general procedure (GP1) chalcone 1f (139 mg, 0.5 mmol) indole 2a (64 mg, 0.55 mmol) produces Friedel–Crafts product-3r (yield 158 mg, 80%); m.p. 67–68 °C; 1H-NMR (400 MHz, DMSO-d6) δ 10.96 (d, J = 2.5 Hz, 1H, NH), 7.56–7.48 (m, 3H, Ar-H), 7.47–7.40 (m, 2H, Ar-H), 7.35 (d, J = 8.1 Hz, 1H, Ar-H), 7.26 (d, J = 2.6 Hz, 1H, Ar-H), 7.16 (dd, J = 8.1, 4.9 Hz, 3H, Ar-H), 7.06 (t, J = 7.4 Hz, 1H, Ar-H), 6.96 (dt, J = 14.9, 7.2 Hz, 2H, Ar-H), 6.62 (s, 1H, Ar-H), 5.06 (t, J = 7.2 Hz, 1H, CH), 3.94 (dd, J = 17.1, 7.6 Hz, 1H, CH2(a)), 3.86 (s, 3H, OCH3), 3.78 (dd, J = 17.0, 7.1 Hz, 1H, CH2(b)); 13C-NMR (101 MHz, DMSO-d6) δ 199.5 (CO), 160.9, 158.0, 153.9, 136.3, 133.6, 129.6, 129.4, 128.4, 127.7, 126.0, 122.9, 122.7, 121.1, 120.6, 120.4, 118.6, 118.4, 114.2, 112.5, 111.7, 110.6, 101.9, 55.8 (OCH3), 47.1 (CH), 31.9 (CH2); IR (KBr, cm−1) νmax = 3408, 3051, 2918, 2837, 1666, 1599, 1483, 1454, 1430, 1340, 1286, 1244, 1161; [Anal. Calcd. for C26H21NO3: C, 78.97; H, 5.35; N, 3.54; Found: C, 78.81; H, 5.19; N, 3.39]; LC/MS (ESI, m/z): 396.16 [M + H] +, exact mass 395.15 for C26H21NO3.
3.20. Synthesis of 3-(Benzofuran-2-yl)-3-(5-bromo-1H-indol-3-yl)-1-(2-methoxyphenyl)propan-1-one (3s)
Following the general procedure (GP1) chalcone 1f (139 mg, 0.5 mmol) 5-bromoindole 2b (108 mg, 0.55 mmol) produces Friedel–Crafts product-3s (yield 180 mg, 76%); m.p. 60–61 °C; 1H-NMR (400 MHz, DMSO-d6) δ 11.20 (d, J = 2.3 Hz, 1H, NH), 7.72 (s, 1H, Ar-H), 7.51 (t, J = 7.5 Hz, 2H, Ar-H), 7.47–7.41 (m, 2H, Ar-H), 7.37–7.31 (m, 2H, Ar-H), 7.17 (dd, J = 11.8, 7.6 Hz, 4H, Ar-H), 6.98 (t, J = 7.4 Hz, 1H, Ar-H), 6.65 (s, 1H, Ar-H), 5.05 (t, J = 7.3 Hz, 1H, CH), 3.91 (dd, J = 17.3, 7.4 Hz, 1H, CH2(a)), 3.87 (s, 3H, OCH3), 3.81 (dd, J = 17.1, 7.3 Hz, 1H, CH2(b)); 13C-NMR (126 MHz, DMSO-d6) δ 199.4, 160.6, 158.1, 153.9, 135.0, 133.7, 129.6, 128.3, 127.9, 127.6, 124.7, 123.6, 123.5, 122.6, 120.8, 120.6, 120.5, 114.0, 113.6, 112.4, 111.2, 110.7, 101.9, 55.8, 47.0, 31.8; IR (KBr, cm−1) νmax = 3320, 3045, 2915, 2857, 1668, 1575, 1452, 1395, 1365, 1338, 1252; [Anal. Calcd. for C26H20BrNO3: C, 65.83; H, 4.25; N, 2.95; Found: C, 66.02; H, 4.11; N, 2.86]; LC/MS (ESI, m/z): 474.07 [M + H] +, exact mass 473.06 for C26H20BrNO3
3.21. The Biological Activity Assays Protocols
Cytotoxicity against BJ Human fibroblast cells, and anti-cancer activity against PC3, HeLa and MCF-7 cancer cell lines were evaluated by following the procedure as described in the literature [25,26,27,28,29] (Supplementary Materials).
4. Conclusions
We have successfully synthesized multi-scaffold-based indoles, benzofurans and benzothiophenes. The anti-cancer activity showed promising results which make them candidates for further research. Fluorine atoms apparently play a crucial role in the bioactivity of this class of compounds with 3b appearing to be the most active member of the series against cervical cancer HeLa (IC50 = 8.2 ± 0.2 µM) and breast cancer MCF-7 cell lines (IC50 = 12.3 ± 0.04 µM), whereas hit 3e (IC50 = 7.8 ± 0.4 µM) appeared more active against PC3 prostate cancer cell line. The mechanism of action and in vivo study are required to further validate results of in vitro status.
Supplementary Materials
The following are available online, 1H-NMR and 13C-NMR for compounds 3a–3s along with the biological activity assays protocols are provided in the Supplementary Materials.
Author Contributions
Conceptualization, A.B. and M.I.C.; methodology, M.S.I.; M.A.; A.M.A.-M.; A.S.A.; S.A.; validation, M.S.I.; M.A.; A.M.A.-M.; A.S.A.; S.A.; formal analysis, M.S.I.; M.A.; A.M.M.A.-M.; A.S.A.; S.A.; investigation, M.S.I.; M.A.; A.M.A.-M.; A.S.A.; S.A.; resources, A.B.; data curation, M.S.I.; M.A.; A.M.A.-M.; A.S.A.; writing—original draft preparation, A.B.; and S.Y.; writing—review and editing, M.I.C.; visualization, A.B. and M.I.C.; project administration, M.A.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (14-BIO128-02).
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Acknowledgments
This Project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (14-BIO128-02).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 3a–3s are available from the authors.
References
- Lounasmaa, M.; Tolvanen, A. Simple indole alkaloids and those with a nonrearranged monoterpenoid unit (July 1997 to December 1998). Nat. Prod. Rep. 2000, 17, 175–191. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorganic Chem. 2019, 89, 103021. [Google Scholar] [CrossRef]
- Noble, R.L.; Beer, C.T.; Cutts, J.H. Role of chance observations in chemotherapy: Vinca rosea. Ann. New York Acad. Sci. 1958, 76, 882–894. [Google Scholar] [CrossRef]
- Gueritte, F.; Fahy, J. The Vinca Alkaloids. In Anticancer agents from natural products; Cragg, G.M., Kingston, D.G.I., Newman, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2005; p. 123. [Google Scholar]
- Duflos, A.; Kruczynski, A.; Barret, J.-M. Novel aspects of natural and modified vinca alkaloids. Curr. Med. Chem. Agents 2012, 2, 55–70. [Google Scholar] [CrossRef]
- Sharma, V.; Kumar, P.; Pathak, D. Biological importance of the indole nucleus in recent years: A comprehensive review. J. Heterocycl. Chem. 2010, 47, 491–502. [Google Scholar] [CrossRef]
- Brancale, A.; Silvestri, R. Indole, a core nucleus for potent inhibitors of tubulin polymerization. Med. Res. Rev. 2007, 27, 209–238. [Google Scholar] [CrossRef]
- Gul, W.; Hamann, M.T. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci. 2005, 78, 442–453. [Google Scholar] [CrossRef]
- Rodrigues de, S.A.F.; Barreiro, E.J.; Fraga, C.A.M. From Nature to Drug Discovery: The Indole Scaffold as a ‘Privileged Structure’. Mini Rev Med Chem. 2009, 9, 782–793. [Google Scholar] [CrossRef]
- Noble, R.L. The discovery of the Vinca alkaloids—chemotherapeutic agents against cancer. Biochem. Cell Biol. 1990, 68, 1344–1351. [Google Scholar] [CrossRef]
- Varoglu, M.; Corbett, T.H.; Valeriote, F.A.; Crews, P. Asperazine, a selective cytotoxic alkaloid from a sponge-derived culture of Aspergillus niger. J. Org. Chem. 1997, 62, 7078–7079. [Google Scholar] [CrossRef]
- Valeriote, F.; Corbett, T.; Lorusso, P.; Moore, R.E.; Scheuer, P.; Patterson, G.; Paul, V.; Grindey, G.; Bonjouklian, R.; Pearce, H.; et al. Discovery of anticancer agents from natural products. Int. J. Pharmacogn. 1995, 33, 59–66. [Google Scholar] [CrossRef]
- Valeriote, F.; Corbett, T.; Edelstein, M.; Baker, L. New in vitro screening model for the discovery of antileukemic anticancer agents. Cancer Investig. 1996, 14, 124–141. [Google Scholar] [CrossRef]
- Govek, S.P.; Overman, L.E. Total synthesis of (+)-asperazine. Tetrahedron 2007, 63, 8499–8513. [Google Scholar] [CrossRef]
- Kohmoto, S.; Kashman, Y.; McConnell, O.J.; Rinehart, J.; Wright, A.; Koehn, F. Dragmacidin, a new cytotoxic bis (indole) alkaloid from a deep water marine sponge, Dragmacidon sp. J. Org. Chem. 1988, 53, 3116–3118. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 68, 394–424. [Google Scholar] [CrossRef]
- WHO (February 2006). Cancer. World Health Organization. Retrieved on 2007-06-25. Available online: https://www.cancer.org/research/cancer-facts-statistics/global (accessed on 1 June 2020).
- Garcia, M.; Jemal, A.; Ward, E.M. Global cancer facts and figures 2007; American Cancer Society: Atlanta, GA, USA, 2007; Available online: https://www.cancer.org/research/cancer-facts-statistics/global (accessed on 1 June 2020).
- World Health Organization. Preventing Chronic Diseases: A Vital Investment; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Barakat, A.; Islam, M.S.; Al-Majid, M.A.; Al-Othman, Z.A. Highly enantioselective Friedel–Crafts alkylation of indoles with α, β-unsaturated ketones with simple Cu (II)–oxazoline–imidazoline catalysts. Tetrahedron 2013, 69, 5185–5192. [Google Scholar] [CrossRef]
- Islam, M.S.; Al-Majid, A.M.; Al-Othman, Z.A.; Barakat, A. Highly enantioselective Friedel-Crafts alkylation of indole with electron deficient trans-β-nitroalkenes under simple Zn(II)-oxazoline-imidazoline catalysts. Tetrahedron Asymmetry 2014, 25, 245–251. [Google Scholar] [CrossRef]
- Barakat, A.; Al-Majid, A.M.A.; Al-Qahatany, F.M.; Islam, M.S.; Al-Agamy, M.H. Synthesis, characterization and antimicrobial activity of novel pharmacophores incorporating imidazoline-oxazoline scaffold. Bull. Korean Chem. Soc. 2014, 35, 562–568. [Google Scholar] [CrossRef]
- Badria, F.A.; Atef, S.; Al-Majid, A.M.; Islam, M.S.; Ali, M.; Elshaier, Y.A.M.M.; Ghabbour, H.A.; Barakat, A. Synthesis and inhibitory effect of some indole-pyrimidine-based hybrid heterocycles on α-glucosidase and α-amylase as potential hypoglycemic agents. ChemistryOpen 2019, 8, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Badria, F.A.; Soliman, S.M.; Atef, S.; Islam, M.S.; Al-Majid, A.M.; Dege, N.; Ghabbour, H.A.; Ali, M.; El-Senduny, F.F.; Barakat, A. Anticancer indole-based chalcones: A structural and theoretical analysis. Molecules 2019, 24, 3728. [Google Scholar] [CrossRef]
- Foye, W.O.; Lemke, T.L.; Williams, D.A. Principles of Medicinal Chemistry, 4th ed.; Williams and Wilkins: Philadelphia, PA, USA, 2002; p. 822. [Google Scholar]
- Mannerström, M.; Toimela, T.; Sarkanen, J.-R.; Heinonen, T. Human BJ Fibroblasts is an Alternative to Mouse BALB/c 3T3 Cells inIn VitroNeutral Red Uptake Assay. Basic Clin. Pharmacol. Toxicol. 2017, 121, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Price, P.; McMillan, T.J. Use of the tetrazolium assay in measuring the response of human tumor cells to ionizing radiation. Cancer Res. 1990, 50, 1392–1396. Available online: https://cancerres.aacrjournals.org/content/canres/50/5/1392.full.pdf (accessed on 1 June 2020).
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).