Biocide Use in the Antimicrobial Era: A Review
Abstract
1. Introduction
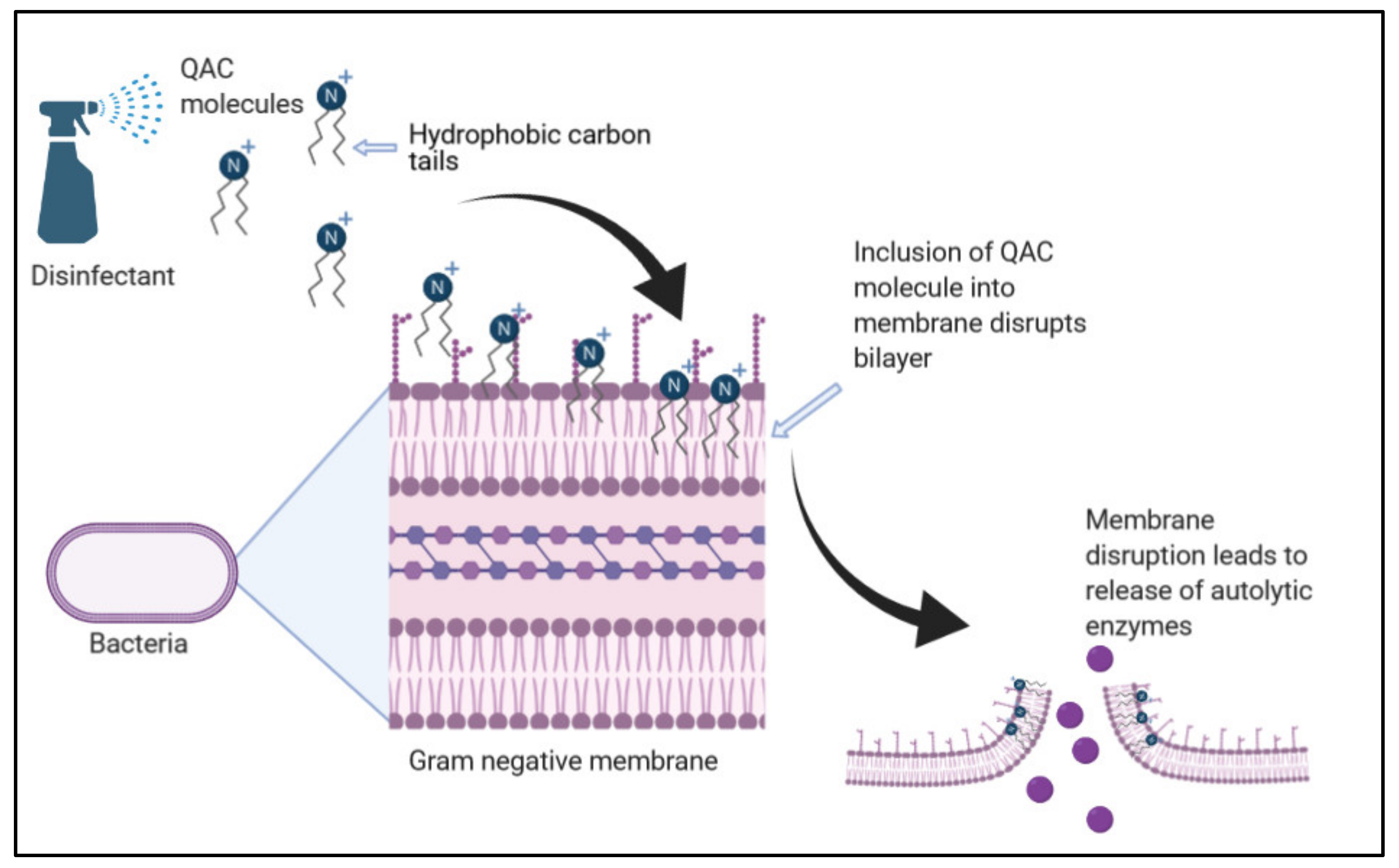
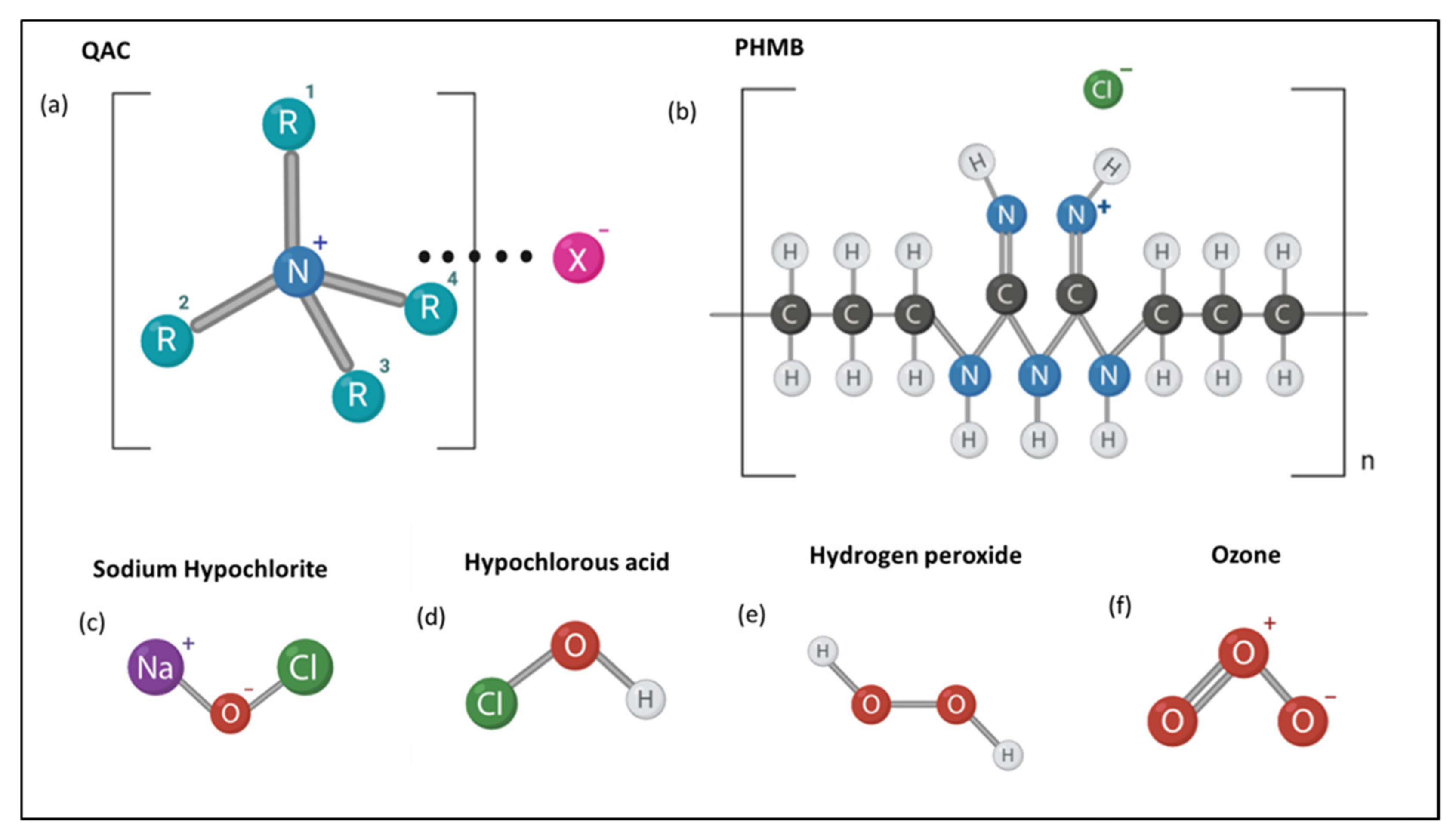
2. Quaternary Ammonium Compounds (QACs)
3. Biguanides
4. Chlorine-Releasing Agents
5. Hydrogen Peroxide
6. Ozone
7. Emerging Biocide Resistance and Impacts on AMR
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Maillard, J.-Y. Antimicrobial biocides in the healthcare environment: Efficacy, usage, policies, and perceived problems. Ther. Clin. Risk Manag. 2005, 1, 307–320. [Google Scholar] [PubMed]
- Fraise, A.P.; Lambert, P.A.; Maillard, J.Y. (Eds.) Russell, Hugo & Ayliffe’s Principles and Practice of Disinfection, Preservation and Sterilization; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Cookson, B. Clinical significance of emergence of bacterial antimicrobial resistance in the hospital environment. J. Appl. Microbiol. 2005, 99, 989–996. [Google Scholar] [CrossRef]
- Fraise, A.P. Biocide abuse and antimicrobial resistance—A cause for concern? J. Antimicrob. Chemother. 2002, 49, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Wesgate, R.; Grasha, P.; Maillard, J.-Y. Use of a predictive protocol to measure the antimicrobial resistance risks associated with biocidal product usage. Am. J. Infect. Control 2016, 44, 458–464. [Google Scholar] [CrossRef]
- Gilbert, P.; McBain, A.J.; Bloomfield, S.F. Biocide abuse and antimicrobial resistance: Being clear about the issues. J. Antimicrob. Chemother. 2002, 50, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Heathman, L.S.; Pierce, G.O.; Kabler, P. Resistance of Various Strains of E. Typhi and Coli aerogenes to Chlorine and Chloramine. Public Health Rep. (1896–1970) 1936, 51, 1367. [Google Scholar] [CrossRef]
- Poole, K. Mechanisms of bacterial biocide and antibiotic resistance. J. Appl. Microbiol. 2002, 92, 55S–64S. [Google Scholar] [CrossRef] [PubMed]
- Bock, L.J. Bacterial biocide resistance: A new scourge of the infectious disease world? Arch. Dis. Child. 2019, 104, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niu, L.-N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.-H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary Ammonium Biocides: Efficacy in Application. Appl. Environ. Microbiol. 2014, 81, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Kwaśniewska, D.; Chen, Y.-L.; Wieczorek, D. Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, S.; Jennings, M.C.; Granata, D.; Klein, M.; Wuest, W.M.; Minbiole, K.P.C.; Carnevale, V. Analysis of the Destabilization of Bacterial Membranes by Quaternary Ammonium Compounds: A Combined Experimental and Computational Study. ChemBioChem 2019, 21, 1510–1516. [Google Scholar] [CrossRef]
- Knauf, G.A.; Cunningham, A.L.; Kazi, M.I.; Riddington, I.M.; Crofts, A.A.; Cattoir, V.; Trent, M.S.; Davies, B.W. Exploring the Antimicrobial Action of Quaternary Amines against Acinetobacter baumannii. mBio 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Lineback, C.B.; Nkemngong, C.A.; Wu, S.T.; Li, X.; Teska, P.J.; Oliver, H.F. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob. Resist. Infect. Control 2018, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Obłąk, E.; Piecuch, A.; Rewak-Soroczyńska, J.; Paluch, E. Activity of gemini quaternary ammonium salts against microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 625–632. [Google Scholar] [CrossRef]
- Williams, G.J.; Denyer, S.P.; Hosein, I.K.; Hill, D.W.; Maillard, J.-Y. Limitations of the Efficacy of Surface Disinfection in the Healthcare Setting. Infect. Control Hosp. Epidemiol. 2009, 30, 570–573. [Google Scholar] [CrossRef]
- Dawson, L.F.; Valiente, E.; Donahue, E.H.; Birchenough, G.; Wren, B.W. Hypervirulent Clostridium difficile PCR-Ribotypes Exhibit Resistance to Widely Used Disinfectants. PLoS ONE 2011, 6, e25754. [Google Scholar] [CrossRef]
- Wesgate, R.; Robertson, A.; Barrell, M.; Teska, P.; Maillard, J.-Y. Impact of test protocols and material binding on the efficacy of antimicrobial wipes. J. Hosp. Infect. 2019, 103, e25–e32. [Google Scholar] [CrossRef]
- Kampf, G. Acquired resistance to chlorhexidine—Is it time to establish an ‘antiseptic stewardship’ initiative? J. Hosp. Infect. 2016, 94, 213–227. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef]
- Demarco, C.E.; Cushing, L.A.; Frempong-Manso, E.; Seo, S.M.; Jaravaza, T.A.A.; Kaatz, G.W. Efflux-Related Resistance to Norfloxacin, Dyes, and Biocides in Bloodstream Isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 3235–3239. [Google Scholar] [CrossRef]
- Kulik, E.M.; Waltimo, T.; Weiger, R.; Schweizer, I.; Lenkeit, K.; Filipuzzi-Jenny, E.; Walter, C. Development of resistance of mutans streptococci and Porphyromonas gingivalis to chlorhexidine digluconate and amine fluoride/stannous fluoride-containing mouthrinses, in vitro. Clin. Oral Investig. 2014, 19, 1547–1553. [Google Scholar] [CrossRef]
- Allen, M.J.; White, G.F.; Morby, A.P. The response of Escherichia coli to exposure to the biocide polyhexamethylene biguanide. Microbiology 2006, 152, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Chindera, K.; Mahato, M.; Sharma, A.K.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.F.; Kumar, S.; McFarlane, A.; Stach, J.; Bentin, T.; et al. The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci. Rep. 2016, 6, 23121. [Google Scholar] [CrossRef] [PubMed]
- Machuca, J.; Lopez-Rojas, R.; Fernandez-Cuenca, F.; Pascual, Á. Comparative activity of a polyhexanide–betaine solution against biofilms produced by multidrug-resistant bacteria belonging to high-risk clones. J. Hosp. Infect. 2019, 103, e92–e96. [Google Scholar] [CrossRef]
- Ng, I.-S.; Ooi, C.W.; Liu, B.-L.; Peng, C.-T.; Chiu, C.-Y.; Chang, Y.-K. Antibacterial efficacy of chitosan- and poly(hexamethylene biguanide)-immobilized nanofiber membrane. Int. J. Biol. Macromol. 2020, 154, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Fjeld, H.; Lingaas, E. Polyheksanid—Sikkerhet og effekt som antiseptikum. Tidsskr. Den Nor. legeforening 2016, 136, 707–711. [Google Scholar] [CrossRef]
- Hübner, N.-O.; Kramer, A. Review on the Efficacy, Safety and Clinical Applications of Polihexanide, a Modern Wound Antiseptic. Ski. Pharmacol. Physiol. 2010, 23, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, A.; Serralta, V.; Davis, S.; Orr, R.; Eaglstein, W.; Mertz, P.M. The effect of an antimicrobial gauze dressing impregnated with 0.2-percent polyhexamethylene biguanide as a barrier to prevent Pseudomonas aeruginosa wound invasion. Wounds 2002, 14, 169–176. Available online: https://www.woundsresearch.com/article/550 (accessed on 1 November 2020).
- Renzoni, A.; Von Dach, E.; Landelle, C.; Diene, S.M.; Manzano, C.; Gonzales, R.; Abdelhady, W.; Randall, C.P.; Bonetti, E.J.; Baud, D.; et al. Impact of Exposure of Methicillin-Resistant Staphylococcus aureus to Polyhexanide In Vitro and In Vivo. Antimicrob. Agents Chemother. 2017, 61, e00272-17. [Google Scholar] [CrossRef]
- Fukuzaki, S. Mechanisms of Actions of Sodium Hypochlorite in Cleaning and Disinfection Processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Estrela, C.; Estrela, C.R.; Barbin, E.L.; Spanó, J.C.E.; Marchesan, M.A.; Pecora, J.D. Mechanism of action of sodium hypochlorite. Braz. Dent. J. 2002, 13, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, S.; Uso, E. The antibacterial properties of sodium hypochlorite and sodium dichloroisocyanurate as hospital disinfectants. J. Hosp. Infect. 1985, 6, 20–30. [Google Scholar] [CrossRef]
- Gallandat, K.; Kolus, R.C.; Julian, T.R.; Lantagne, D.S. A systematic review of chlorine-based surface disinfection efficacy to inform recommendations for low-resource outbreak settings. Am. J. Infect. Control 2021, 49, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Observations on research with spores of Bacillales and Clostridiales species. J. Appl. Microbiol. 2019, 126, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, S.F.; Arthur, M. Interaction of Bacillus subtilis spores with sodium hypochlorite, sodium dichloroisocyanurate and chloramine-T. J. Appl. Bacteriol. 1992, 72, 166–172. [Google Scholar] [CrossRef]
- Department of Health and Health Protection Agency. Clostridiodies Difficile: How to Deal with the Problem. 2008. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/340851/Clostridium_difficile_infection_how_to_deal_with_the_problem.pdf (accessed on 1 November 2020).
- Joshi, L.; Welsch, A.; Hawkins, J.; Baillie, L. The effect of hospital biocide sodium dichloroisocyanurate on the viability and properties ofClostridium difficilespores. Lett. Appl. Microbiol. 2017, 65, 199–205. [Google Scholar] [CrossRef][Green Version]
- Dyer, C.; Hutt, L.P.; Burky, R.; Joshi, L.T. Biocide Resistance and Transmission of Clostridium difficile Spores Spiked onto Clinical Surfaces from an American Health Care Facility. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Da Cruz Nizer, W.S.D.C.; Inkovskiy, V.; Overhage, J. Surviving Reactive Chlorine Stress: Responses of Gram-Negative Bacteria to Hypochlorous Acid. Microorganisms 2020, 8, 1220. [Google Scholar] [CrossRef]
- Ranieri, M.R.; Whitchurch, C.B.; Burrows, L.L. Mechanisms of biofilm stimulation by subinhibitory concentrations of antimicrobials. Curr. Opin. Microbiol. 2018, 45, 164–169. [Google Scholar] [CrossRef]
- Mahdizadeh, S.; Sawford, K.; Van Andel, M.; Browning, G.F. Efficacy of citric acid and sodium hypochlorite as disinfectants against Mycoplasma bovis. Vet. Microbiol. 2020, 243, 108630. [Google Scholar] [CrossRef]
- Severing, A.-L.; Rembe, J.-D.; Koester, V.; Stuermer, E.K. Safety and efficacy profiles of different commercial sodium hypochlorite/hypochlorous acid solutions (NaClO/HClO): Antimicrobial efficacy, cytotoxic impact and physicochemical parametersin vitro. J. Antimicrob. Chemother. 2019, 74, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Similarities and differences in the responses of microorganisms to biocides. J. Antimicrob. Chemother. 2003, 52, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, M.; Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Mode of action of hydrogen peroxide and other oxidizing agents: Differences between liquid and gas forms. J. Antimicrob. Chemother. 2010, 65, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Assadian, O.; Zatorska, B.; Presterl, E.; Schahawi, M.D.-E. A novel micellar formulation based on natural plant extracts enhances the efficacy of hydrogen peroxide against biofilms of Staphylococcus spp. and Pseudomonas aeruginosa. Biofouling 2020, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; Tavares, R.R.; Borges, A.; Mergulhão, F.; Simões, M. Current and emergent strategies for disinfection of hospital environments. J. Antimicrob. Chemother. 2013, 68, 2718–2732. [Google Scholar] [CrossRef] [PubMed]
- Kenters, N.; Huijskens, E.; De Wit, S.C.J.; Sanders, I.G.J.M.; Van Rosmalen, J.; Kuijper, E.J.; Voss, A. Effectiveness of various cleaning and disinfectant products on Clostridium difficile spores of PCR ribotypes 010, 014 and 027. Antimicrob. Resist. Infect. Control 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Ríos-Castillo, A.G.; González-Rivas, F.; Rodríguez-Jerez, J.J. Bactericidal Efficacy of Hydrogen Peroxide-Based Disinfectants Against Gram-Positive and Gram-Negative Bacteria on Stainless Steel Surfaces. J. Food Sci. 2017, 82, 2351–2356. [Google Scholar] [CrossRef]
- Le Toquin, E.; Faure, S.; Orange, N.; Gas, F. New Biocide Foam Containing Hydrogen Peroxide for the Decontamination of Vertical Surface Contaminated with Bacillus thuringiensis Spores. Front. Microbiol. 2018, 9, 2295. [Google Scholar] [CrossRef]
- Skowron, K.; Wałecka-Zacharska, E.; Grudlewska, K.; Białucha, A.; Wiktorczyk, N.; Bartkowska, A.; Kowalska, M.; Kruszewski, S.; Gospodarek-Komkowska, E. Biocidal Effectiveness of Selected Disinfectants Solutions Based on Water and Ozonated Water against Listeria monocytogenes Strains. Microorganisms 2019, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Fontes, B.; Heimbecker, A.M.C.; Brito, G.D.S.; Costa, S.F.; Van Der Heijden, I.M.; Levin, A.S.; Rasslan, S. Effect of low-dose gaseous ozone on pathogenic bacteria. BMC Infect. Dis. 2012, 12, 358. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Janex, M.-L.; Savoye, P.; Cockx, A.; Lazarova, V. Wastewater disinfection by ozone: Main parameters for process design. Water Res. 2002, 36, 1043–1055. [Google Scholar] [CrossRef]
- Noszticzius, Z.; Wittmann, M.; Kály-Kullai, K.; Beregvári, Z.; Kiss, I.; Rosivall, L.; Szegedi, J. Chlorine Dioxide Is a Size-Selective Antimicrobial Agent. PLoS ONE 2013, 8, e79157. [Google Scholar] [CrossRef]
- Schwaiger, K.; Harms, K.S.; Bischoff, M.; Preikschat, P.; Mölle, G.; Bauer-Unkauf, I.; Lindorfer, S.; Thalhammer, S.; Bauer, J.; Hölzel, C.S. Insusceptibility to disinfectants in bacteria from animals, food and humans-is there a link to antimicrobial resistance? Front. Microbiol. 2014, 5, 88. [Google Scholar] [CrossRef] [PubMed]
| Biocide | Mode of Action | Advantages | Disadvantages |
|---|---|---|---|
| Quaternary Ammonium Compounds | Cationic action destabilizes cell membrane resulting in cell lysis [11,12,13,14]. | Does not produce free radicals; therefore, they are not carcinogenic or genotoxic [11,12]. Generally inexpensive to use [1]. | Less effective against biofilms [16]. Efficacy can be strain specific [19]. Efficacy may vary with temperature [17,20]. |
| Polyhexamethylene Biguanides | Adherence to lipids within cell membranes leading to non-specific cell membrane disruption, allowing cellular entry of PHMB [25,26]. | Broad antimicrobial specificity [24]. Low toxicity [25,26,27]. Water soluble, thermostable and pH stable [26]. Presents activity against certain biofilms including that of antimicrobial resistant strains [27]. | Efficacy is temperature sensitive [28]. Efficacy may be altered by presence of organic matter [29,31]. |
| NaOCl | Oxidative damage to cell membrane, as well as intracellular proteins and amino acids. Membrane damage leads to entry of NaOCl to damage organelles [33,35]. | Suitable for household use due to appropriate shelf life and stability at average household temperatures [34,35]. Safe for human hygiene [35]. | Efficacy may be altered by presence of organic matter [38]. Efficacy may be altered depending on contaminated surface material [41,47,48]. |
| ClO2 (chlorine dioxide gas) | Oxidative damage to cell membrane, as well as intracellular proteins and amino acids. Membrane damage leads to entry of ClO2 to damage organelles [33]. | Safe for human hygiene. Not cytotoxic. Can be active against biofilms. Oxidative mechanism is greatly specific thus less product is required. [58] | Gas generation is expensive [58] |
| Hypochlorous acid (HClO) | Oxidative damage to cell membrane, as well as intracellular proteins and amino acids. Membrane damage leads to entry of HClO to damage organelles [33,46]. | Generally inexpensive and non-toxic [33]. Safe for human hygiene [46]. Can be effective against enveloped viruses [58]. | Reduced oxidative specificity means more product is required [58]. |
| Peroxides (H2O2) | Hydroxyl radicals cause oxidative damage to cell membrane components as well as intracellular molecules [48,49]. | Only degrades into water and hydrogen—environmentally friendly [48]. Broad antimicrobial specificity [55]. Can be applied in aqueous or vaporized form [54]. Vaporized form enables disinfection of ‘hard to reach’ places [53,54]. | Typically unstable therefore difficult to store [54]. Presents strain specificity [49]. Efficacy varies with application method [48]. |
| Ozone (gas) | Induces cell lysis via membrane oxidation [56]. | Broad antimicrobial specificity [55]. Easy to produce with a 20-min half -life [56]. Enables easier disinfection of ‘hard to reach’ places [56]. | Toxic at high concentrations [55]. Efficacy may vary in the presence of organic matter depending on whether the ozone is in gaseous or aqueous form [55,57]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, I.A.; Joshi, L.T. Biocide Use in the Antimicrobial Era: A Review. Molecules 2021, 26, 2276. https://doi.org/10.3390/molecules26082276
Jones IA, Joshi LT. Biocide Use in the Antimicrobial Era: A Review. Molecules. 2021; 26(8):2276. https://doi.org/10.3390/molecules26082276
Chicago/Turabian StyleJones, Imogen Anne, and Lovleen Tina Joshi. 2021. "Biocide Use in the Antimicrobial Era: A Review" Molecules 26, no. 8: 2276. https://doi.org/10.3390/molecules26082276
APA StyleJones, I. A., & Joshi, L. T. (2021). Biocide Use in the Antimicrobial Era: A Review. Molecules, 26(8), 2276. https://doi.org/10.3390/molecules26082276






