Grindstone Chemistry: Design, One-Pot Synthesis, and Promising Anticancer Activity of Spiro[acridine-9,2′-indoline]-1,3,8-trione Derivatives against the MCF-7 Cancer Cell Line
Abstract
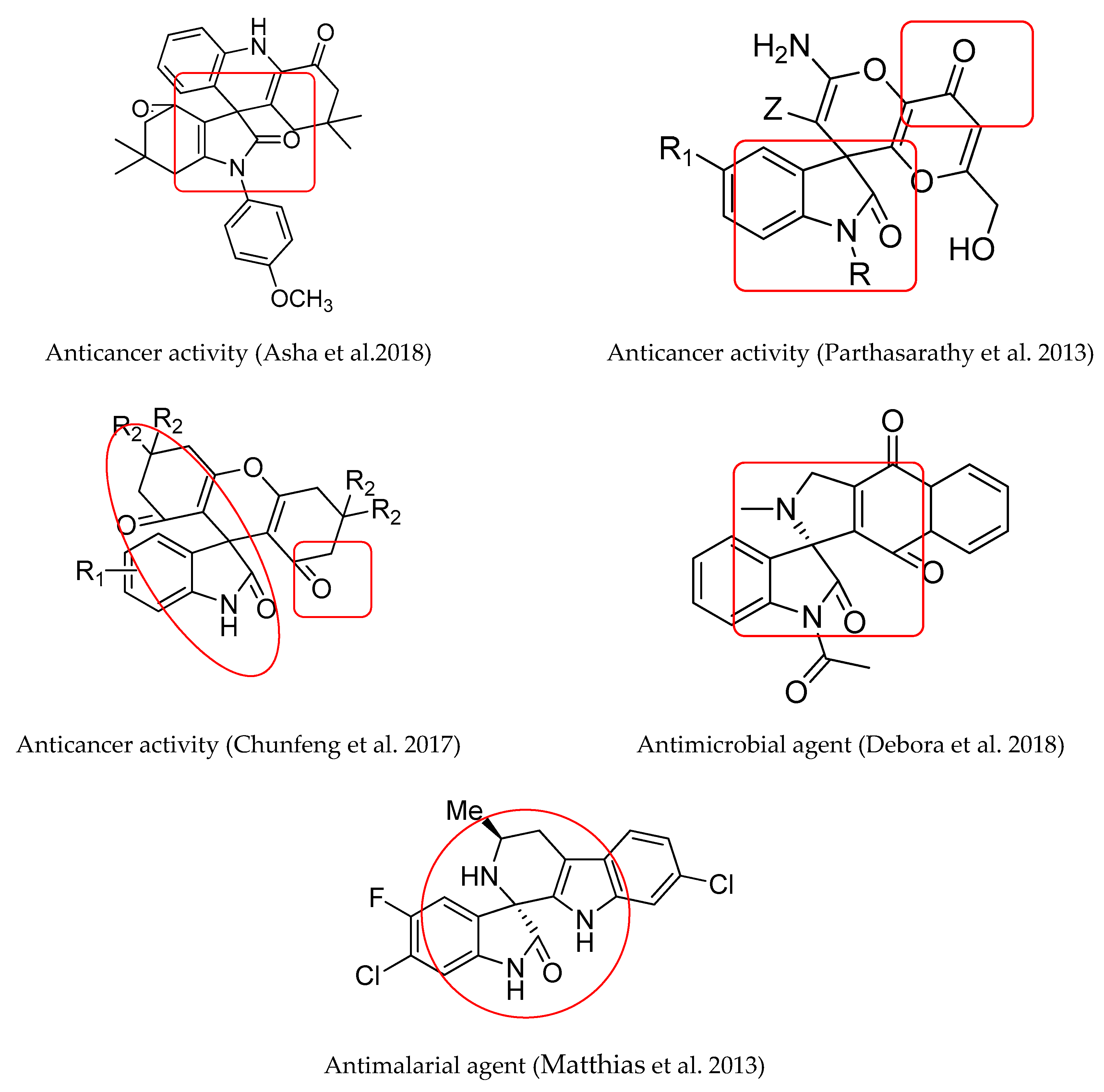
1. Introduction
2. Results and Discussion
2.1. Chemistry
Anticancer Activity
3. Experimental
3.1. Synthesis of Compounds 1a–1j
3.2. Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shanmugam, P.; Viswambharan, B.; Madhavan, S. Synthesis of Novel Functionalized 3-Spiropyrrolizidine and 3-Spiropyrrolidine Oxindoles from Baylis−Hillman Adducts of Isatin and Heteroaldehydes with Azomethine Ylides via [3 + 2]-Cycloaddition. Org. Lett. 2007, 9, 4095–4098. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, M.M.; Abd El-Wahas, A.H.; Eid, F.A.; El-Agrody, A.M. Synthesis of halogen derivatives of benzo[h]chromene and benzo[a]anthracene with promising antimicrobial activities. Farmaco 2002, 57, 715–722. [Google Scholar] [CrossRef]
- Kang, T.H.; Matsumoto, K.; Murakami, Y.; Takayama, H.; Kitajima, M.; Aimi, N.; Watanabe, H. Pteropodine and isopteropodine positively modulate the function of rat muscarinic M1 and 5-HT2 receptors expressed in Xenopus oocyte. Eur. J. Pharmacol. 2002, 444, 39–45. [Google Scholar] [CrossRef]
- Da Silva, J.F.M.; Garden, S.J.; Pinto, A.C. The chemistry of isatins: A review from 1975 to 1999. J. Braz. Chem. Soc. 2001, 12, 273–324. [Google Scholar] [CrossRef]
- Kornet, M.J.; Thio, A.P. Oxindole-3-spiropyrrolidines and -piperidines. Synthesis and local anesthetic activity. J. Med. Chem. 1976, 19, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Okita, T.; Isobe, M. Synthesis of the pentacyclic intermediate for dynemicin a and unusual formation of spiro-oxindole ring. Tetrahedron 1994, 50, 11143–11152. [Google Scholar] [CrossRef]
- Rosenmond, P.; Hosseini-Merescht, M.; Bub, C. A Simple Entry into the Series of Tetracyclic Hetero- and Secoyohimbanes, Strychnos and Oxindole Alkaloids. Ann. Chem. 1994, 2, 151–154. [Google Scholar] [CrossRef]
- Parteek, P.; Mousmee, S. Medicinal chemistry of acridine and its analogues. MedChemComm 2018, 9, 1589–1618. [Google Scholar]
- Kirk, S.R.; Luedtke, N.W.; Toy, Y.J. Neomycin−Acridine Conjugate: A Potent Inhibitor of Rev-RRE Binding. Am. Chem. Soc. 2000, 122, 980–981. [Google Scholar] [CrossRef]
- Albert, A. The Acridine; Edward Arnold: London, UK, 1966. [Google Scholar]
- Inman, W.D.; O’Neill-Johnson, M.; Crews, P. Novel marine sponge alkaloids. 1. Plakinidine A and B, anthelmintic active alkaloids from a Plakortis sponge. J. Am. Chem. Soc. 1990, 112, 1–4. [Google Scholar] [CrossRef]
- Martins, E.T.; Baruah, H.; Kramarczyk, J.; Saaluta, G.; Day, C.S.; Kucera, G.L.; Bierbach, U. Design, Synthesis, and Biological Activity of a Novel Non-Cisplatin-Type Platinum−Acridine Pharmacophore. J. Med. Chem. 2001, 44, 4492–4496. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.K.; Pednekar, S.; Ganguly, S.N.; Chakraborty, G.; Manhas, M.S. A simplified green chemistry approach to the Biginelli reaction using ‘Grindstone Chemistry’. Tetrahedron Lett. 2004, 45, 8351–8353. [Google Scholar] [CrossRef]
- Varughese, D.J.; Manhas, M.S.; Bose, A.K. Microwave enhanced greener synthesis of indazoles via nitrenes. Tetrahedron Lett. 2006, 47, 6795–6797. [Google Scholar] [CrossRef]
- Toda, F.; Tanaka, K.; Sekikawa, A. Host–guest complex formation by a solid–solid reaction. J. Chem. Soc. Chem. Commun. 1987, 279–280. [Google Scholar] [CrossRef]
- Bazgir, A.; Khanaposhtani, M.M.; Soorki, A.A. One-pot synthesis and antibacterial activities of pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine-dione derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 5800–5803. [Google Scholar] [CrossRef]
- Bazgir, A.; Seyyedhamzeh, M.; Yasaei, Z.; Mirzaei, P. A novel three-component method for the synthesis of triazolo[1,2-a]indazole-triones. Tetrahedron Lett. 2007, 48, 8790–8794. [Google Scholar] [CrossRef]
- Sayyafi, M.; Seyyedhamzeh, M.; Khavasi, H.R.; Bazgir, A. One-pot, three-component route to 2H-indazolo[2,1-b]phthalazine-triones. Tetrahedron 2008, 64, 2375–2378. [Google Scholar] [CrossRef]
- Dabiri, M.; Arvin-Nezhad, H.; Khavasi, H.R.; Bazgir, A. A facile three-components, One pot synthesis of pyrimido[4,5-d]pyrimidine-2,5-dione derivatives under microwave-assisted conditions. J. Heterocycl. Chem. 2007, 44, 1009–1011. [Google Scholar] [CrossRef]
- Dabiri, M.; Azimi, S.C.; Arvin-Nezhad, H.; Bazgir, A. An efficient three-component, one-pot synthesis of new pyrimido[4,5-d]pyrimidine-2,4-diones. Heterocycles 2008, 75, 87–93. [Google Scholar] [CrossRef]
- Dabiri, M.; Delbari, A.S.; Bazgir, A. A novel three-component, one-pot synthesis of 1,2-dihydro-1-aryl-naphtho[1, 2-e][1,3]oxazine-3-one derivatives under microwave-assisted and thermal solvent-free conditions. Synlett 2007, 5, 821–823. [Google Scholar]
- Dabiri, M.; Arvin-Nezhad, H.; Khavasi, H.R.; Bazgir, A. A novel and efficient synthesis of pyrimido[4,5-d]pyrimidine-2,4,7-trione and pyrido[2,3-d:6,5-d] dipyrimidine-2,4,6,8-tetrone derivatives. Tetrahedron 2007, 63, 1770–1774. [Google Scholar] [CrossRef]
- Dabiri, M.; Delbari, A.S.; Bazgir, A. A Simple and Environmenttally Benign Method for the Synthesis of Naphthoxazin-3-one Derivatives. Heterocycles 2007, 71, 543–548. [Google Scholar]
- Ghahremanzadeh, R.; Imani Shakibaei, G.; Bazgir, A. An Efficient One-Pot Synthesis of 1H-Pyrazolo[1,2-b]phthalazine-5,10-dione Derivatives. Synlett 2008, 1129–1132. [Google Scholar] [CrossRef]
- Bazgir, A.; NorooziTisseh, Z.; Mirzaei, P. An efficient synthesis of spiro[dibenzo[b,i] xanthenes-13,3′-indoline]-pentaones and 5H-dibenzo[b,i]xanthene-tetraones. Tetrahedron Lett. 2008, 49, 5165–5168. [Google Scholar] [CrossRef]
- Jadidi, K.; Ghahremanzadeh, R.; Bazgir, A. Spirooxindoles: Reaction of 2,6-diamino pyrimidin-4(3H)-one and isatins. Tetrahedron 2009, 65, 2005–2009. [Google Scholar] [CrossRef]
- Dabiri, M.; Azimi, S.C.; Khavasi, H.R.; Bazgir, A. A novel reaction of 6-amino-uracils and isatins. Tetrahedron 2008, 64, 7307–7311. [Google Scholar] [CrossRef]
- Jadidi, K.; Ghahremanzadeh, R.; Bazgir, A. Efficient Synthesis of Spiro[chromeno[2,3-d]pyrimi dine-5,3′-indoline]-tetraones by a One-Pot and Three-Component Reaction. J. Comb. Chem. 2009, 11, 341–344. [Google Scholar] [CrossRef]
- Ghahremanzadeh, R.; Sayyafi, M.; Ahadi, S.; Bazgir, A. Novel One-Pot, Three-Component Synthesis of Spiro[Indoline-pyrazolo[4′,3′:5,6]pyrido[2,3-d]pyrimidine]trione Library. J. Comb. Chem. 2009, 11, 393–396. [Google Scholar] [CrossRef]
- Chate, A.V.; Kamdi, S.P.; Bhagat, A.N.; Jadhav, C.K.; Nipte, A.; Sarkate, A.P.; Tiwari, S.V.; Gill, C.H. Design, Synthesis and SAR Study of Novel Spiro [Pyrimido[5,4-b]Quinoline-10,50 -Pyrrolo[2,3-d]Pyrimidine] Derivatives as Promising Anticancer Agents. J. Heterocycl. Chem. 2018, 55, 1–6. [Google Scholar] [CrossRef]
- Parthasarathy, K.; Praveen, C.; Balachandran, C.; Senthil Kumar, P.; Ignacimuthu, S.; Perumal, P.T. Cu(OTf)2 catalyzed three component reaction: Efficient synthesis of spiro [indo line-3,4′- pyrano[3,2-b]pyran derivatives and their anticancer potency towards A549 human Lung cancer cell lines. Bioorg. Med. Chem. Lett. 2013, 23, 2708–2713. [Google Scholar] [CrossRef]
- Chunfeng, C.; Chunlei, L.; Jianfeng, L.; Jianqing, J.; Lijun, W.; Chunlei, W.; Runpu, S. An Efficient Synthesis of Spiro[indoline-3,9′-xanthene]trione Derivatives Catalyzed by Magnesium Per chlorate. Molecules 2017, 22, 1–7. [Google Scholar]
- Futuro, D.O.; Ferreira, P.G.; Nicoletti, C.D.; Borba-Santos, L.P.; Silva, F.C.; Rozental, S.; Ferreira, V.F. The Antifungal Activity of Naphthoquinones: An Integrative Review. An. Acad. Bras. Ciências 2018, 90, 1187–1214. [Google Scholar] [CrossRef]
- Rottmann, M.; McNamara, C.; Yeung, B.K.; Lee, M.C.; Zou, B.; Russell, B.; Seitz, P.; Plouffe, D.M.; Dharia, N.V.; Tan, J.; et al. Spiroindolones, a new and potent chemotype for the treatment of malaria. NIH Public Access. 2010, 3, 1175–1180. [Google Scholar]
- Ghahremanzadeh, R.; Ahadi, S.; Shakibaei, G.I.; Bazgir, A. Grindstone chemistry: One-pot synthesis of spiro[diindenopyridine-indoline]triones and spiro[acenaphthylene-diindenopyridine]triones. Tetrahedron Lett. 2010, 51, 499–502. [Google Scholar] [CrossRef]
- Surendra Kumar, R.; Moydeen, M.; Al-Deyab, S.S.; Aseer, M.; Idhayadhulla, A. Synthesis of new morpholine-connected pyrazolidine derivatives and their antimicrobial, antioxidant, and cytotoxic activities. Bioorg. Med. Chem. Lett. 2017, 27, 66–71. [Google Scholar] [CrossRef] [PubMed]

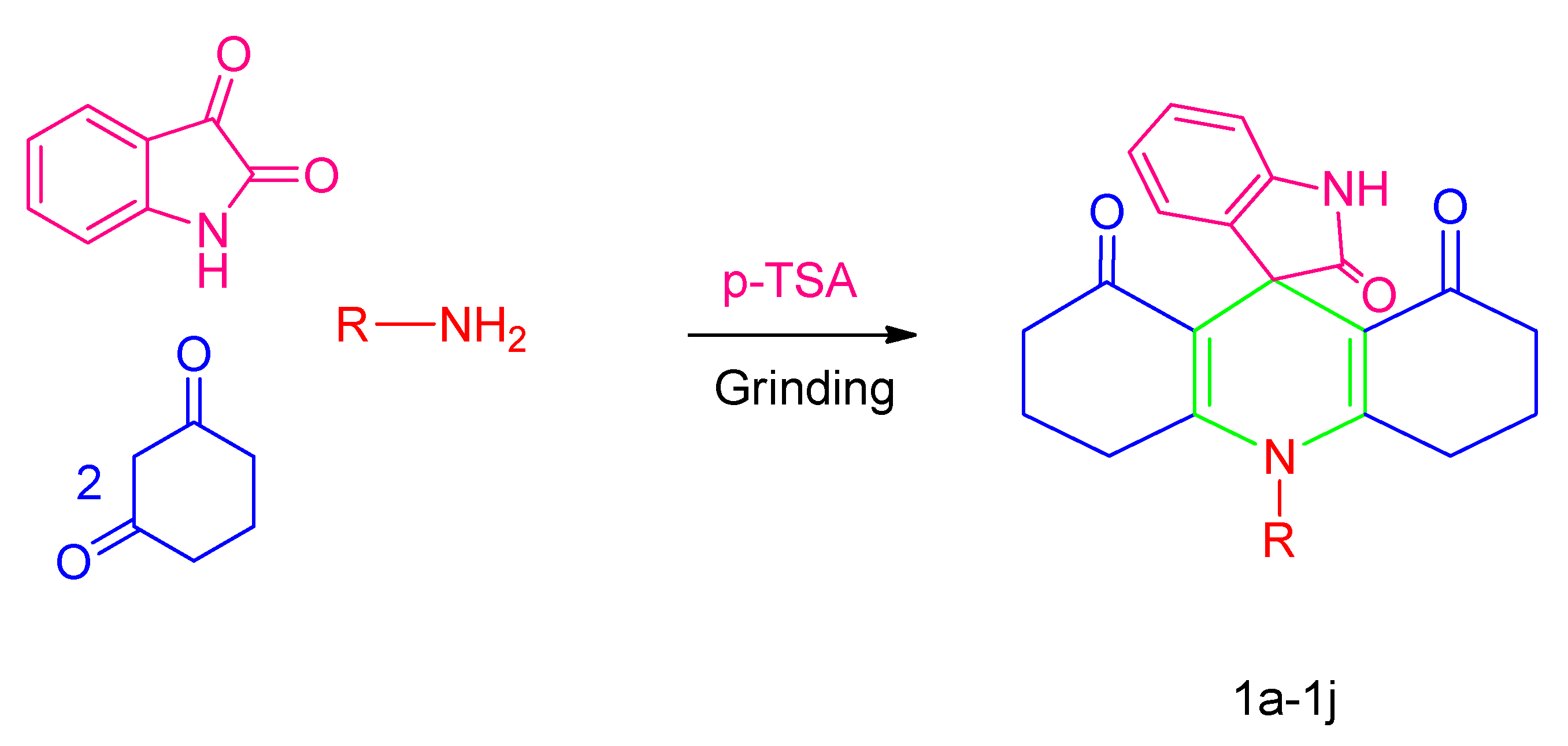
| Compounds | R | Time (Min) | Yield (%) |
|---|---|---|---|
| 1a | Ph | 10 | 92 |
| 1b | -4-NO2-C6H4 | 10 | 91 |
| 1c | -4-OCH3-C6H4 | 10 | 88 |
| 1d | -2-OCH3-C6H4 | 10 | 87 |
| 1e | -3-CH3-C6H4 | 10 | 95 |
| 1f | -2-NO2-C6H4 | 10 | 82 |
| 1g | -4-CH3-C6H4 | 10 | 94 |
| 1h | -2-Cl-C6H4 | 10 | 87 |
| 1i | -2,4-NO2-C6H4 | 10 | 78 |
| 1j | -4-Br-C6H4 | 10 | 91 |
| Compounds | MCF-7 Cell Line | ||
|---|---|---|---|
| GI50 (µM) | TGI (µM) | LC50 (µM) | |
| 1a | 02.7 ± 0.11 | 06.5 ± 1.06 | 08.1 ± 0.02 |
| 1b | 0.02 ± 0.29 | 0.41 ± 2.32 | 0.87 ± 0.24 |
| 1c | 0.01 ± 0.34 | 0.02± 1.19 | 0.71 ± 1.21 |
| 1d | 0.09 ± 0.61 | 0.55 ± 0.38 | 1.40 ± 1.15 |
| 1e | 0.19 ± 0.93 | 0.20 ± 1.32 | 0.70 ± 1.17 |
| 1f | 0.52 ± 0.09 | 10.1 ± 2.22 | 22.1 ± 0.12 |
| 1g | 0.35 ± 0.83 | 0.66 ± 1.12 | 14.2 ± 0.82 |
| 1h | 0.26 ± 0.14 | 04.4 ± 1.09 | 09.7 ± 0.28 |
| 1i | 0.03 ± 0.49 | 0.69 ± 0.61 | 2.12 ± 0.21 |
| 1j | 0.04 ± 0.34 | 0.34 ± 0.55 | 1.01 ± 0.18 |
| Doxorubicin | 0.02 ± 0. 70 | 0.21 ± 0. 19 | 0.74 ± 0.35 |
Sample Availability: Samples of the compounds are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobinath, P.; Packialakshmi, P.; Daoud, A.; Alarifi, S.; Idhayadhulla, A.; Radhakrishnan, S. Grindstone Chemistry: Design, One-Pot Synthesis, and Promising Anticancer Activity of Spiro[acridine-9,2′-indoline]-1,3,8-trione Derivatives against the MCF-7 Cancer Cell Line. Molecules 2020, 25, 5862. https://doi.org/10.3390/molecules25245862
Gobinath P, Packialakshmi P, Daoud A, Alarifi S, Idhayadhulla A, Radhakrishnan S. Grindstone Chemistry: Design, One-Pot Synthesis, and Promising Anticancer Activity of Spiro[acridine-9,2′-indoline]-1,3,8-trione Derivatives against the MCF-7 Cancer Cell Line. Molecules. 2020; 25(24):5862. https://doi.org/10.3390/molecules25245862
Chicago/Turabian StyleGobinath, Perumal, Ponnusamy Packialakshmi, Ali Daoud, Saud Alarifi, Akbar Idhayadhulla, and Surendrakumar Radhakrishnan. 2020. "Grindstone Chemistry: Design, One-Pot Synthesis, and Promising Anticancer Activity of Spiro[acridine-9,2′-indoline]-1,3,8-trione Derivatives against the MCF-7 Cancer Cell Line" Molecules 25, no. 24: 5862. https://doi.org/10.3390/molecules25245862
APA StyleGobinath, P., Packialakshmi, P., Daoud, A., Alarifi, S., Idhayadhulla, A., & Radhakrishnan, S. (2020). Grindstone Chemistry: Design, One-Pot Synthesis, and Promising Anticancer Activity of Spiro[acridine-9,2′-indoline]-1,3,8-trione Derivatives against the MCF-7 Cancer Cell Line. Molecules, 25(24), 5862. https://doi.org/10.3390/molecules25245862







