Triphenylantimony(V) Catecholates of the Type (3-RS-4,6-DBCat)SbPh3-Catechol Thioether Derivatives: Structure, Electrochemical Properties, and Antiradical Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. X-ray Structures
2.3. Cyclic Voltammetry
2.4. Antiradical Activity Test with DPPH
3. Materials and Methods
3.1. General Remarks
3.2. X-ray Diffraction Studies
3.3. Cyclic Voltammetry
3.4. DPPH Test
3.5. Synthesis
3.5.1. (3-(n-Butylthio)-4,6-di-tert-butyl-catecholato)triphenylantimony(V)
3.5.2. (3-(n-Hexylthio)-4,6-di-tert-butyl-catecholato)triphenylantimony(V)
3.5.3. (3-(n-Octylthio)-4,6-di-tert-butyl-catecholato)triphenylantimony(V)
3.5.4. (3-(Cyclopentylthio)-4,6-di-tert-butyl-catecholato)triphenylantimony(V)
3.5.5. (3-(Cyclohexylthio)-4,6-di-tert-butyl-catecholato)triphenylantimony(V)
3.5.6. (3-(Benzylthio)-4,6-di-tert-butyl-catecholato)triphenylantimony(V)
3.5.7. (3-(Phenylthio)-4,6-di-tert-butyl-catecholato)triphenylantimony(V)
3.5.8. (3-(Naphtyl-2-thio)-4,6-di-tert-butyl-catecholato)triphenylantimony(V)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wellington, K.W. Understanding cancer and the anticancer activities of naphthoquinones—A review. RSC Adv. 2015, 5, 20309–20338. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Pingaew, R.; Worachartcheewan, A.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, S.V. Synthesis, anticancer activity and QSAR study of 1,4-naphthoquinone derivatives. Eur. J. Med. Chem. 2014, 84, 247–263. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Li, M.-G.; Li, W.; Zhao, J.-Y.; Ding, Z.-G.; Cui, X.-L.; Wen, M.-L.; Griseusin, D. A New Pyranonaphthoquinone Derivative from a Alkaphilic Nocardiopsis sp. J. Antibiot. 2007, 60, 757–761. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, N.; Shukla, A.K.; Das, M.; Dubey, V.K. Evaluation of plumbagin and its derivative as potential modulators of redox thiol metabolism of Leishmania parasite. Parasitol. Res. 2012, 110, 341–348. [Google Scholar] [CrossRef]
- Sharma, A.; Santos, O.I.; Gaur, P.; Ferreira, V.F.; Garcia, C.R.; da Rocha, D.R. Addition of thiols to o-quinone methide: New 2-hydroxy-3-phenylsulfanylmethyl[1,4]naphthoquinones and their activity against the human malaria parasite Plasmodium falciparum (3D7). Eur. J. Med. Chem. 2013, 59, 48–53. [Google Scholar] [CrossRef]
- Jardim, G.A.M.; Reis, W.J.; Ribeiro, M.F.; Ottoni, F.M.; Alves, R.J.; Silva, T.L.; Goulart, M.O.F.; Braga, A.L.; Menna-Barreto, R.F.S.; Salomao, K.; et al. On the investigation of hybrid quinones: Synthesis, electrochemical studies and evaluation of trypanocidal activity. RSC Adv. 2015, 5, 78047–78060. [Google Scholar] [CrossRef]
- De Castro, S.L.; Emery, F.S.; da Silva Jún, E.N. Synthesis of quinoidal molecules: Strategies towards bioactive compounds with an emphasis on lapachones. Eur. J. Med. Chem. 2013, 69, 678–700. [Google Scholar] [CrossRef]
- Sacau, E.P.; Estévez-Braun, A.; Ravelo, A.G.; Ferro, E.A.; Tokuda, H.; Mukainaka, T.; Nishino, H. Inhibitory effects of lapachol derivatives on epstein-barr virus activation. Bioorg. Med. Chem. 2003, 11, 483–488. [Google Scholar] [CrossRef]
- Prati, F.; Bartolini, M.; Simoni, E.; De Simone, A.; Pinto, A.; Andrisano, V.; Bolognesi, M.L. Quinones bearing non-steroidal anti-inflammatory fragments as multitarget ligands for Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2013, 23, 6254–6258. [Google Scholar] [CrossRef]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press: Boca Raton, FL, USA, 2005; 981p. [Google Scholar]
- Maroz, A.; Anderson, R.F.; Smith, R.A.J.; Murphy, M.P. Reactivity of ubiquinone and ubiquinol with superoxide and the hydroperoxyl radical: Implications for in vivo antioxidant activity. Free Radic. Biol. Med. 2009, 46, 105–109. [Google Scholar] [CrossRef]
- Shadyro, O.I.; Sosnovskaya, A.A.; Edimicheva, I.P.; Ostrovskaya, N.I.; Kazem, K.M.; Hryntsevich, I.B.; Alekseev, A.V. Effects of quinones on free-radical processes of oxidation and fragmentation of hydroxyl-containing organic compounds. Bioorg. Med. Chem. Lett. 2007, 17, 6383–6386. [Google Scholar] [CrossRef]
- Bentinger, M.; Brismar, K.; Dallner, G. The antioxidant role of coenzyme Q. Mitochondrion 2007, 7S, S41–S50. [Google Scholar] [CrossRef]
- Espinosa-Garcia, J.; Gutierrez-Merino, C. The Trapping of the OH Radical by Coenzyme Q. A Theoretical and Experimental Study. J. Phys. Chem. A 2003, 107, 9712–9723. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Yang, T.; Yang, J. Synthesis and antioxidant activities of Coenzyme Q analogues. Eur. J. Med. Chem. 2014, 86, 710–713. [Google Scholar] [CrossRef]
- James, A.M.; Smith, R.A.J.; Murphy, M.P. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch. Biochem. Biophys. 2004, 423, 47–56. [Google Scholar] [CrossRef]
- Nohl, H.; Gille, L.; Kozlov, A.V. Antioxidant-derived prooxidant formation from ubiquinol. Free Radic. Biol. Med. 1998, 25, 666–675. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T. Formation and Biological Targets of Quinones: Cytotoxic versus Cytoprotective Effects. Chem. Res. Toxicol. 2017, 30, 13–37. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Kuzmin, V.V.; Arsenyev, M.V.; Smolyaninova, S.A.; Poddel’sky, A.I.; Berberova, N.T. Electrochemical transformations and anti/prooxidant activity of sterically hindered o-benzoquinones. Russ. Chem. Bull. 2017, 66, 1217–1229. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Cherkasov, V.K.; Kocherova, T.N.; Druzhkov, N.O.; Kurskii, Y.A.; Bubnov, M.P.; Fukin, G.K.; Abakumova, L.G. Functionalization of sterically hindered o-benzoquinones: Amino-substituted 3,6-di(tert-butyl)-o-benzoquinones. Russ. Chem. Bull. 2007, 56, 1849–1856. [Google Scholar] [CrossRef]
- Kuropatov, V.; Klementieva, S.; Fukin, G.; Mitin, A.; Ketkov, S.; Budnikova, Y.; Cherkasov, V.; Abakumov, G. Novel method for the synthesis of functionalized tetrathiafulvalenes, an acceptor–donor–acceptor molecule comprising of two o-quinone moieties linked by a TTF bridge. Tetrahedron 2010, 66, 7605–7611. [Google Scholar] [CrossRef]
- Klementieva, S.V.; Kuropatov, V.A.; Fukin, G.K.; Romanenko, G.V.; Bogomyakov, A.S.; Cherkasov, V.K.; Abakumov, G.A. Mono- and Binuclear Dimethylthallium(III) Complexes with o-Benzoquinone-TTF-o-Benzoquinone Ligand; Synthesis, Spectroscopy and X-ray Study. Z. Anorg. Allgem. Chem. 2011, 637, 232–241. [Google Scholar] [CrossRef]
- Cherkasov, V.K.; Abakumov, G.A.; Fukin, G.K.; Klementyeva, S.V.; Kuropatov, V.A. Sterically Hindered o-Quinone Annulated with Dithiete: A Molecule Comprising Diolate and Dithiolate Coordination Sites. Chem. Eur. J. 2012, 18, 13821–13827. [Google Scholar] [CrossRef]
- Nair, V.; Menon, R.S.; Bijub, A.T.; Abhilash, K.G. 1,2-Benzoquinones in Diels–Alder reactions, dipolar cycloadditions, nucleophilic additions, multicomponent reactions and more. Chem. Soc. Rev. 2012, 41, 1050–1059. [Google Scholar] [CrossRef]
- Astaf’eva, T.V.; Arsenyev, M.V.; Rumyantcev, R.V.; Fukin, G.K.; Cherkasov, V.K.; Poddel’sky, A.I. Imine-Based Catechols and o-Benzoquinones: Synthesis, Structure, and Features of Redox Behavior. ACS Omega 2020, 5, 22179–22191. [Google Scholar] [CrossRef]
- Tesema, Y.T.; Pham, D.M.; Franz, K.J. Synthesis and Characterization of Copper(II) Complexes of Cysteinyldopa and Benzothiazine Model Ligands Related to Pheomelanin. Inorg. Chem. 2006, 45, 6102–6104. [Google Scholar] [CrossRef]
- Tesema, Y.T.; Pham, D.M.; Franz, K.J. Counterions influence reactivity of metal ions with cysteinyldopa model compounds. Inorg. Chem. 2008, 47, 1087–1095. [Google Scholar] [CrossRef]
- Smolyaninov, I.; Pitikova, O.; Korchagina, E.; Poddel’sky, A.; Luzhnova, S.; Berberova, N. Electrochemical behavior and anti/prooxidant activity of thioethers with redox-active catechol moiety. Monatsh. Chem. 2018, 149, 1813–1826. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Pitikova, O.V.; Poddel’sky, A.I.; Berberova, N.T. Electrochemical transformations and antiradical activity of asymmetrical RS-substituted pyrocatechols. Russ. Chem. Bull. 2018, 67, 1857–1867. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Pitikova, O.V.; Korchagina, E.O.; Poddel’sky, A.I.; Fukin, G.K.; Luzhnova, S.A.; Tichkomirov, A.M.; Ponomareva, E.N.; Berberova, N.T. Bifunctional catechol thiothers with physiologically active fragments: Electrochemistry, antioxidant and cryoprotective activities. Bioorg. Chem. 2019, 89, 103003. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Poddel’sky, A.I.; Smolyaninova, S.A.; Arsenyev, M.V.; Fukin, G.K.; Berberova, N.T. Polyfunctional Sterically Hindered Catechols with Additional Phenolic Group and Their Triphenylantimony(V) Catecholates: Synthesis, Structure, and Redox Properties. Molecules 2020, 25, 1770. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Pitikova, O.V.; Rychagova, E.S.; Korchagina, E.O.; Poddel’sky, A.I.; Smolyaninova, S.A.; Berberova, N.T. Synthesis and antioxidant activity of sterically hindered bis-pyrocatechol thioethers. Russ. Chem. Bull. 2016, 65, 2861–2867. [Google Scholar] [CrossRef]
- Guardingo, M.; Bellido, E.; Miralles-Llumà, R.; Faraudo, J.; Sedó, J.; Tatay, S.; Verdaguer, A.; Busqué, F.; Ruiz-Molina, D. Bioinspired Catechol-Terminated Self-Assembled Monolayers with Enhanced Adhesion Properties. Small 2014, 10, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Poneti, G.; Poggini, L.; Mannini, M.; Cortigiani, B.; Sorace, L.; Otero, E.; Sainctavit, P.; Magnani, A.; Sessolia, R.; Dei, A. Thermal and optical control of electronic states in a single layer of switchable paramagnetic molecules. Chem. Sci. 2015, 6, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Loginova, N.V.; Koval’chuk, T.V.; Zheldakova, R.A.; Chernyavskaya, A.A.; Osipovich, N.P.; Glushonok, G.K.; Polozov, G.I.; Povalishev, V.N.; Sorokin, V.L.; Shadyro, O.I. Synthesis, characterization and antifungal activity of copper(II) complexes of sterically hindered o-diphenol derivatives. Polyhedron 2006, 25, 3603–3610. [Google Scholar] [CrossRef]
- Loginova, N.V.; Koval’chuk, T.V.; Faletrov, Y.V.; Halauko, Y.S.; Osipovich, N.P.; Polozov, G.I.; Zheldakova, R.A.; Gres, A.T.; Halauko, A.S.; Azarko, I.I.; et al. Redox-active metal(II) complexes of sterically hindered phenolic ligands: Antibacterial activity and reduction of cytochrome c. Part II. Metal(II) complexes of o-diphenol derivatives of thioglycolic acid. Polyhedron 2011, 30, 2581–2591. [Google Scholar] [CrossRef]
- Loginova, N.V.; Koval’chuk, T.V.; Polozov, G.I.; Osipovich, N.P.; Rytik, P.G.; Kucherov, I.I.; Chernyavskaya, A.A.; Sorokin, V.L.; Shadyro, O.I. Synthesis, characterization, antifungal and anti-HIV activities of metal(II) complexes of 4,6-di-tert-butyl-3-[(2-hydroxyethyl)thio]benzene-1,2-diol. Eur. J. Med. Chem. 2008, 43, 1536–1542. [Google Scholar] [CrossRef]
- Pierpont, C.G. Unique properties of transition metal quinone complexes of the MQ3 series. Coord. Chem. Rev. 2001, 219-221, 415–433. [Google Scholar] [CrossRef]
- Pierpont, C.G. Studies on charge distribution and valence tautomerism in transition metal complexes of catecholate and semiquinonate ligands. Coord. Chem. Rev. 2001, 216-217, 99–125. [Google Scholar] [CrossRef]
- Zanello, P.; Corsini, M. Homoleptic, mononuclear transition metal complexes of 1,2-dioxolenes: Updating their electrochemical-to-structural (X-ray) properties. Coord. Chem. Rev. 2006, 250, 2000–2022. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Cherkasov, V.K.; Abakumov, G.A. Transition metal complexes with bulky 4,6-di-tert-butyl-N-aryl(alkyl)-o-iminobenzoquinonato ligands: Structure, EPR and magnetism. Coord. Chem. Rev. 2009, 253, 291–324. [Google Scholar] [CrossRef]
- Van der Vlugt, J.I. Radical-Type Reactivity and Catalysis by Single-Electron Transfer to or from Redox-Active Ligands. Chem. Eur. J. 2019, 25, 2651–2662. [Google Scholar] [CrossRef]
- Nikolaevskaya, E.N.; Druzhkov, N.O.; Syroeshkin, M.A.; Egorov, M.P. Chemistry of diazadiene type ligands with extra coordination groups. Prospects of reactivity. Coord. Chem. Rev. 2020, 417, 213353. [Google Scholar] [CrossRef]
- Christianson, A.M.; Gabbaï, F.P. Antimony-and Bismuth-Based Materials and Applications. In Main Group Strategies Towards Functional Hybrid Materials; Baumgartner, T., Jäkle, F., Eds.; John Wiley& Sons: Chichester, UK, 2018; pp. 405–432. [Google Scholar] [CrossRef]
- Sharutin, V.V.; Poddel’sky, A.I.; Sharutina, O.K. Aryl Compounds of Pentavalent Antimony: Syntheses, Reactions, and Structures. Russ. J. Coord. Chem. 2020, 46, 663–728. [Google Scholar] [CrossRef]
- Wade, C.R.; Bond, F.P.; Gabbai, F. Two-Electron Redox Chemistry and Reversible Umpolung of a Gold–Antimony Bond. Angew. Chem. Int. Ed. 2011, 50, 7369–7372. [Google Scholar] [CrossRef]
- Furan, S.; Hupf, E.; Boidol, J.; Brünig, J.; Lork, E.; Mebs, S.; Beckmann, J. Transition metal complexes of antimony centered ligands based upon acenaphthyl scaffolds. Coordination non-innocent or not? Dalton Trans. 2019, 48, 4504–4513. [Google Scholar] [CrossRef]
- Jones, J.S.; Gabbai, F.P. Coordination- and Redox-Noninnocent Behavior of Ambiphilic Ligands Containing Antimony. Acc. Chem. Res. 2016, 49, 857–867. [Google Scholar] [CrossRef]
- Wade, C.R.; Ke, I.S.; Gabbai, F.P. Sensing of Aqueous Fluoride Anions by Cationic Stibine–Palladium Complexes. Angew. Chem. Int. Ed. 2012, 51, 478–481. [Google Scholar] [CrossRef]
- Ke, I.S.; Jones, J.S.; Gabbai, F.P. Anion-controlled switching of an X ligand into a Z ligand: Coordination non-innocence of a stiboranyl ligand. Angew. Chem. Int. Ed. 2014, 53, 2633–2637. [Google Scholar] [CrossRef]
- Hirai, M.; Gabbaï, F.P. Lewis acidic stiborafluorenes for the fluorescence turn-on sensing of fluoride in drinking water at ppm concentrations. Chem. Sci. 2014, 5, 1886–1893. [Google Scholar] [CrossRef]
- Christianson, A.M.; Rivard, E.; Gabbaï, F.P. 1λ5-Stibaindoles as Lewis Acidic, π-Conjugated, Fluoride Anion Responsive Platforms. Organometallics 2017, 36, 2670–2676. [Google Scholar] [CrossRef]
- Hirai, M.; Gabbai, F.P. Squeezing Fluoride out of Water with a Neutral Bidentate Antimony(V) Lewis Acid. Angew. Chem. Int. Ed. 2015, 54, 1205–1209. [Google Scholar] [CrossRef]
- Protasenko, N.A.; Poddel’skii, A.I.; Smolyaninov, I.V.; Berberova, N.T.; Fukin, G.K.; Cherkasov, V.K.; Abakumov, G.A. Complex of triphenylantimony(V) catecholate with 5-(2,6-dimethylphenyl)-3-(4-pyridyl)-1-phenylformazan. Russ. Chem. Bull. 2014, 63, 930–937. [Google Scholar] [CrossRef]
- Okhlopkova, L.S.; Poddel’sky, A.I.; Smolyaninov, I.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Triphenylantimony(V) Catecholato Complexes with 4-(2,6-Dimethylphenyliminomethyl)pyridine. Structure, Redox Properties: The Influence of Pyridine Ligand. J. Organomet. Chem. 2019, 897, 32–41. [Google Scholar] [CrossRef]
- Poddel’skii, A.I.; Ilyakina, E.V.; Smolyaninov, I.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Complexes of triphenylantimony(V) catecholates with ammonium salts. Spectroscopic and electrochemical investigations. Russ. Chem. Bull. 2014, 63, 923–929. [Google Scholar] [CrossRef]
- Okhlopkova, L.S.; Smolyaninov, I.V.; Baranov, E.V.; Poddel’skii, A.I. Mononuclear Antimony(V) Catecholate Complexes with Additional Pyridine Ligands. Russ. J. Coord. Chem. 2020, 46, 466–476. [Google Scholar] [CrossRef]
- Sen, S.; Ke, I.-S.; Gabbai, F.P. T-Shaped Gold Stiborane Complexes as Carbophilic Catalysts: Influence of the Peripheral Substituents. Organometallics 2017, 36, 4224–4230. [Google Scholar] [CrossRef]
- Yang, M.; Tofan, D.; Chen, C.-H.; Jack, K.M.; Gabbai, F.P. Digging the Sigma-Hole of Organoantimony Lewis Acids by Oxidation. Angew. Chem. Int. Ed. 2018, 57, 13868–13872. [Google Scholar] [CrossRef]
- Tofan, D.; Gabbai, F.P. Fluorinated antimony(V) derivatives: Strong Lewis acidic properties and application to the complexation of formaldehyde in aqueous solutions. Chem. Sci. 2016, 7, 6768–6778. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Poddel’sky, A.I.; Grunova, E.V.; Cherkasov, V.K.; Fukin, G.K.; Kurskii, Y.A.; Abakumova, L.G. Reversible Binding of Dioxygen by a Non-transition-Metal Complex. Angew. Chem. Int. Ed. 2005, 44, 2767–2771. [Google Scholar] [CrossRef]
- Cherkasov, V.K.; Abakumov, G.A.; Grunova, E.V.; Poddel’sky, A.I.; Fukin, G.K.; Baranov, E.V.; Kurskii, Y.A.; Abakumova, L.G. Triphenylantimony(V) Catecholates and o-Amidophenolates: Reversible Binding of Molecular Oxygen. Chem. Eur. J. 2006, 12, 3916–3927. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Cherkasov, V.K.; Grunova, E.V.; Poddel’sky, A.I.; Abakumova, L.G.; Kurskii, Y.A.; Fukin, G.K.; Baranov, E.V. Cyclic Endoperoxides Based on Triphenylantimony(V) Catecholates: The Reversible Binding of Dioxygen. Dokl. Chem. 2005, 405, 222–225. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Kurskii, Y.A.; Piskunov, A.V.; Somov, N.V.; Cherkasov, V.K.; Abakumov, G.A. The triphenylantimony(V) o-amidophenolates with unsymmetrical N-aryl group for a reversible dioxygen binding. Appl. Organomet. Chem. 2011, 25, 180–189. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Kurskii, Y.A.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. New morpholine- and piperazine-functionalised triphenylantimony(V) catecholates: The spectroscopic and electrochemical studies. J. Organomet. Chem. 2010, 695, 1215–1224. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Kurskii, Y.A.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. New dioxygen-inert triphenylantimony(V) catecholate complexes based on o-quinones with electron-withdrawing groups. Russ. Chem. Bull. 2009, 58, 532–537. [Google Scholar] [CrossRef]
- Fukin, G.K.; Baranov, E.V.; Jelsch, C.; Guillot, B.; Poddelskii, A.I.; Cherkasov, V.K.; Abakumov, G.A. Experimental and Theoretical Investigation of Topological and Energetic Characteristics of Sb Complexes Reversibly Binding Molecular Oxygen. J. Phys. Chem. A 2011, 115, 8271–8281. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Astaf’eva, T.V.; Smolyaninov, I.V.; Arsenyev, M.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Triphenylantimony(V) 6-alkoxymethyl-3,5-di-tert-butylcatecholates. Structure and redox-properties. J. Organomet. Chem. 2018, 873, 57–65. [Google Scholar] [CrossRef]
- Fukin, G.K.; Baranov, E.V.; Poddel’sky, A.I.; Cherkasov, V.K.; Abakumov, G.A. Reversible Binding of Molecular Oxygen to Catecholate and Amidophenolate Complexes of SbV: Electronic and Steric Factors. ChemPhysChem 2012, 13, 3773–3776. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Vavilina, N.N.; Somov, N.V.; Cherkasov, V.K.; Abakumov, G.A. Triethylantimony(V) complexes with bidentate O,N-, O,O- and tridentate O,N,O’-coordinating o-iminoquinonato/o-quinonato ligands: Synthesis, Structure and some Properties. J. Organomet. Chem. 2009, 694, 3462–3469. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Vavilina, N.N.; Kurskii, Y.A.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Triaryl- and trialkylantimony(V) Bis(catecholates) based on 1,1′-spirobis[3,3-dimethylindanequinone-5,6]: Spectroscopic and electrochemical studies. Russ. J. Coord. Chem. 2012, 38, 284–294. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V. 3,6-Di-tert-butylcatecholates of triaryl antimony(V): NMR study and redox-transformations. Russ. J. Gen. Chem. 2010, 80, 538–540. [Google Scholar] [CrossRef]
- Smolyaninova, S.A.; Poddel’sky, A.I.; Smolyaninov, I.V.; Berberova, N.T. Trialkylantimony(V) o-Amidophenolates: Electrochemical Transformations and Antiradical Activity. Russ. J. Coord. Chem. 2014, 40, 273–279. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. 3,6-Di-tert-butylcatecholates of trialkyl/triarylantimony(V). J. Organomet. Chem. 2018, 867, 238–245. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Antonova, N.A.; Poddel’sky, A.I.; Smolyaninova, S.A.; Osipova, V.P.; Berberova, N.T. Radical scavenging activity of sterically hindered catecholate and o-amidophenolate complexes of LSbVPh3 type. J. Organomet. Chem. 2011, 696, 2611–2620. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Antonova, N.A.; Poddel’sky, A.I.; Osipova, V.P.; Kolyada, M.N.; Berberova, N.T. The Influence of Ph3Sb(V)L Complexes with Redox Active Ligands on in Vivo Lipid Peroxidation. Dokl. Chem. 2012, 443, 72–76. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Antonova, N.A.; Poddel’sky, A.I.; Osipova, V.P.; Berberova, N.T.; Pimenov, Y.T. The influence of some triphenylantimony(V) catecholates and o-amidophenolates on the lipid peroxidation in vitro. Appl. Organomet. Chem. 2012, 26, 277–283. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Poddel’skii, A.I.; Antonova, N.A.; Smolyaninova, S.A.; Berberova, N.T. Antiradical activity of morpholine- and piperazine-functionalized triphenylantimony(V) catecholates. Russ. J. Coord. Chem. 2013, 39, 165–174. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Antonova, N.A.; Poddel’sky, A.I.; Smolyaninova, S.A.; Osipova, V.P.; Luzhnova, S.A.; Berberova, N.T.; Pimenov, Y.T. The influence of triphenylantimony(V) catecholate and its spiroendoperoxide on lipid peroxidation. Appl. Organomet. Chem. 2014, 28, 274–279. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Poddel’skii, A.I.; Smolyaninova, S.A.; Movchan, N.O. Redox transformations and antiradical activity of triarylantimony(V) 3,6-di-tert-butyl-4,5-dimethoxycatecholates. Russ. J. Gen. Chem. 2014, 84, 1761–1766. [Google Scholar] [CrossRef]
- Arsenyev, M.V.; Shurygina, M.P.; Poddel’sky, A.I.; Druzhkov, N.O.; Chesnokov, S.A.; Fukin, G.K.; Cherkasov, V.K.; Abakumov, G.A. New poly-o-quinone-methacrylate and its dioxygen-active antimony-containing polymer. J. Polym. Res. 2013, 20, 98. [Google Scholar] [CrossRef]
- Lenshina, N.A.; Shurygina, M.P.; Arsenyev, M.V.; Poddel’sky, A.I.; Zaitsev, S.D.; Chesnokov, S.A.; Abakumov, G.A. Optically controlled distribution of o-quinonemethacrylate metal complexes in polymer material. J. Coord. Chem. 2015, 68, 4159–4169. [Google Scholar] [CrossRef]
- Chesnokov, S.A.; Lenshina, N.A.; Arsenyev, M.V.; Kovylin, R.S.; Baten’kin, M.A.; Poddel’sky, A.I.; Abakumov, G.A. Preparation of new dioxygen-active triphenylantimony(V) catecholate-containing porous polymer. Appl. Organomet. Chem. 2017, 31, e3553. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Hall, M.; Sowerby, D.B. Synthesis and crystal structure of bis(triphenylantimony catecholate) hydrate. A new square-pyramidal antimony(V) compound. J. Am. Chem. Soc. 1980, 102, 628–632. [Google Scholar] [CrossRef]
- Tian, Z.; Tuck, D.G. Oxidation of elemental antimony by substituted ortho-benzoquinones. J. Chem. Soc. Dalton Trans. 1993, 1381–1385. [Google Scholar] [CrossRef]
- Fukin, G.K.; Zakharov, L.N.; Domrachev, G.A.; Fedorov, A.Y.; Zaburdyaeva, S.N.; Dodonov, V.A. Synthesis and structures of the six-coordinate donor-acceptor complexes R3(C6H4O2)Sb…L (R=Ph, L=OSMe2 or ONC5H5; R=Me, L=ONC5H5 or NC5H5) and R3(C2H4O2)Sb…L (R=Ph, L=ONC5H5; R=Cl or C6F5, L=OPPh3). Russ. Chem. Bull. 1999, 48, 1722–1732. [Google Scholar] [CrossRef]
- Cherkasov, V.K.; Grunova, E.V.; Poddel’sky, A.I.; Fukin, G.K.; Kurskii, Y.A.; Abakumova, L.G.; Abakumov, G.A. Oxidative addition reaction of o-quinones to triphenylantimony. Novel triphenylantimony catecholate complexes. J. Organomet. Chem. 2005, 690, 1273–1281. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Somov, N.V.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Antimony(V) catecholato complexes based on 5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthalenequinone-2,3. Crystal structure of [Ph4Sb]+[Ph2Sb(Cat)2]−. J. Organomet. Chem. 2010, 695, 530–536. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Berberova, N.T.; Fukin, G.K.; Cherkasov, V.K.; Abakumov, G.A. Triaryl/trialkylantimony(V) catecholates with electron-acceptor groups. J. Organomet. Chem. 2015, 789-790, 8–13. [Google Scholar] [CrossRef]
- Holmes, R.R.; Day, R.O.; Chandrasekhar, V.; Holmes, J.M. Pentacoordinated molecules. 68. Distortion coordinate for nonrigid five-coordinated antimony. Synthesis and structure of oxygen- and sulfur-containing cyclic organostiboranes. Inorg. Chem. 1987, 26, 163–168. [Google Scholar] [CrossRef]
- Gibbons, M.N.; Begley, M.J.; Blake, A.J.; Sowerby, D.B. New square-pyramidal organoantimony(V) compounds; crystal structures of (biphenyl-2,2′-diyl)phenylantimony(V) dibromide, dichloride and diisothiocyanate, Sb(2,2′-C12H8)PhX2 (X = Br, Cl or NCS), and of octahedral SbPh(o-O2C6Cl4)Cl2·OEt2. J. Chem. Soc. Dalton Trans. 1997, 2419–2425. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Piskunov, A.V.; Druzhkov, N.O.; Fukin, G.K.; Cherkasov, V.K.; Abakumov, G.A. New bis-o-benzoquinoid ligands with ethylene bridge and their metal complexes. Synthesis, Spectroscopy and X-ray study. Z. Anorg. Allg. Chem. 2009, 635, 2563–2571. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Baranov, E.V.; Fukin, G.K.; Cherkasov, V.K.; Abakumov, G.A. The nitro-substituted catecholates of triphenylantimony(V): Tetragonal-pyramidal vs trigonal-bipyramidal coordination. J. Organomet. Chem. 2013, 733, 44–48. [Google Scholar] [CrossRef]
- Arsen’ev, M.V.; Okhlopkova, L.S.; Poddel’skii, A.I.; Fukin, G.K. Binuclear Triphenylantimony(V) Catecholate Based on Redox-Active Bis-o-Benzoquinone, a Bis-Catechol-Aldimine Derivative. Russ. J. Coord. Chem. 2018, 44, 162–168. [Google Scholar] [CrossRef]
- Bukhvalova, S.Y.; Zhiganshina, E.R.; Astaf’eva, T.V.; Arsenyev, M.V.; Baranov, E.V.; Chesnokov, S.A.; Poddel’sky, A.I. New Sterically Hindered Bis-o-Benzoquinones with Electron-Donor Bridging Groups and Related Binuclear Triphenylantimony(V) Catecholate Complexes. Russ. J. Coord. Chem. 2020, 46, 817–827. [Google Scholar] [CrossRef]
- Brown, S.N. Metrical Oxidation States of 2-Amidophenoxide and Catecholate Ligands: Structural Signatures of Metal–Ligand π Bonding in Potentially Noninnocent Ligands. Inorg. Chem. 2012, 51, 1251–1260. [Google Scholar] [CrossRef]
- Pierpont, C.G.; Buchanan, R.M. Transition metal complexes of o-benzoquinone, o-semiquinone, and catecholate ligands. Coord. Chem. Rev. 1981, 38, 45–83. [Google Scholar] [CrossRef]
- Piskunov, A.V.; Maleeva, A.V.; Fukin, G.K.; Baranov, E.V.; Kuznetsova, O.V. Quinone complexes of aluminum: Synthesis and structures. Russ. J. Coord. Chem. 2010, 36, 161–169. [Google Scholar] [CrossRef]
- Klementyeva, S.V.; Smolentsev, A.I.; Abramov, P.A.; Konchenko, S.N. Yttrium 3,5-di-tert-butyl-catecholates supported by 2,6-diisopropylphenyl substituted β-diketiminate. Inorg. Chem. Commun. 2017, 86, 154–158. [Google Scholar] [CrossRef]
- Baryshnikova, S.V.; Bellan, E.V.; Poddel’sky, A.I.; Arsenyev, M.V.; Smolyaninov, I.V.; Fukin, G.K.; Piskunov, A.V.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Tin(IV) and antimony(V) complexes bearing catecholate ligand connected to ferrocene. Synthesis, molecular structure and electrochemical properties. Eur. J. Inorg. Chem. 2016, 5230–5241. [Google Scholar] [CrossRef]
- Kieser, J.M.; Jones, L.O.; Lin, N.J.; Zeller, M.; Schatz, G.C.; Bart, S.C. Synthesis and Characterization of Tellurium Catecholates and Their N-Oxide Adducts. Inorg. Chem. 2021, 60, 3460–3470. [Google Scholar] [CrossRef]
- Poddel’skii, A.I.; Okhlopkova, L.S.; Meshcheryakova, I.N.; Druzhkov, N.O.; Smolyaninov, I.V.; Fukin, G.K. Triphenylantimony(V) Catecholates Based on o-Quinones, Derivatives of Benzo[b][1,4]-Dioxines and Benzo[b][1,4]-Dioxepines. Russ. J. Coord. Chem. 2019, 45, 133–141. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Arsenyev, M.V.; Astaf’eva, T.V.; Chesnokov, S.A.; Fukin, G.K.; Abakumov, G.A. New sterically-hindered 6th-substituted 3,5-di-tert-butylcatechols/o-quinones with additional functional groups and their triphenylantimony(V) catecholates. J. Organomet. Chem. 2017, 835, 17–24. [Google Scholar] [CrossRef]
- Poddel’sky, A.I.; Smolyaninov, I.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Triarylantimony(V) catecholates-derivatives of 4,5-difluoro-3,6- di-tert-butyl-o-benzoquinone. J. Organomet. Chem. 2016, 824, 1–6. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.F.; Perrin, D.R. Purification of Laboratory Chemicals; Pergamon: Oxford, UK, 1980. [Google Scholar]
- Bruker; SAINT. Data Reduction and Correction Program v. 8.38A; Bruker AXS: Madison, WI, USA, 2016. [Google Scholar]
- Data Collection, Reduction and Correction Program, CrysAlisPro 1.171.38.46-Software Package; Rigaku Oxford Diffraction, 2015.
- Sheldrick, G.M. SADABS v.2016/2, Bruker/Siemens Area Detector Absorption Correction Program; Bruker AXS: Madison, WI, USA, 2016. [Google Scholar]
- SCALE3 ABSPACK: Empirical Absorption Correction, CrysAlisPro 1.171.38.46-Software Package; Rigaku Oxford Diffraction, 2015.
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL, v. 6.14, Structure Determination Software Suite; Bruker AXS: Madison, WI, USA, 2003. [Google Scholar]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanism of antioxidant activity using the DPPH free radical method. Food. Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Villano, D.; Fernandez-Pachon, M.S.; Moya, M.L.; Troncoso, A.M.; Garcya-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
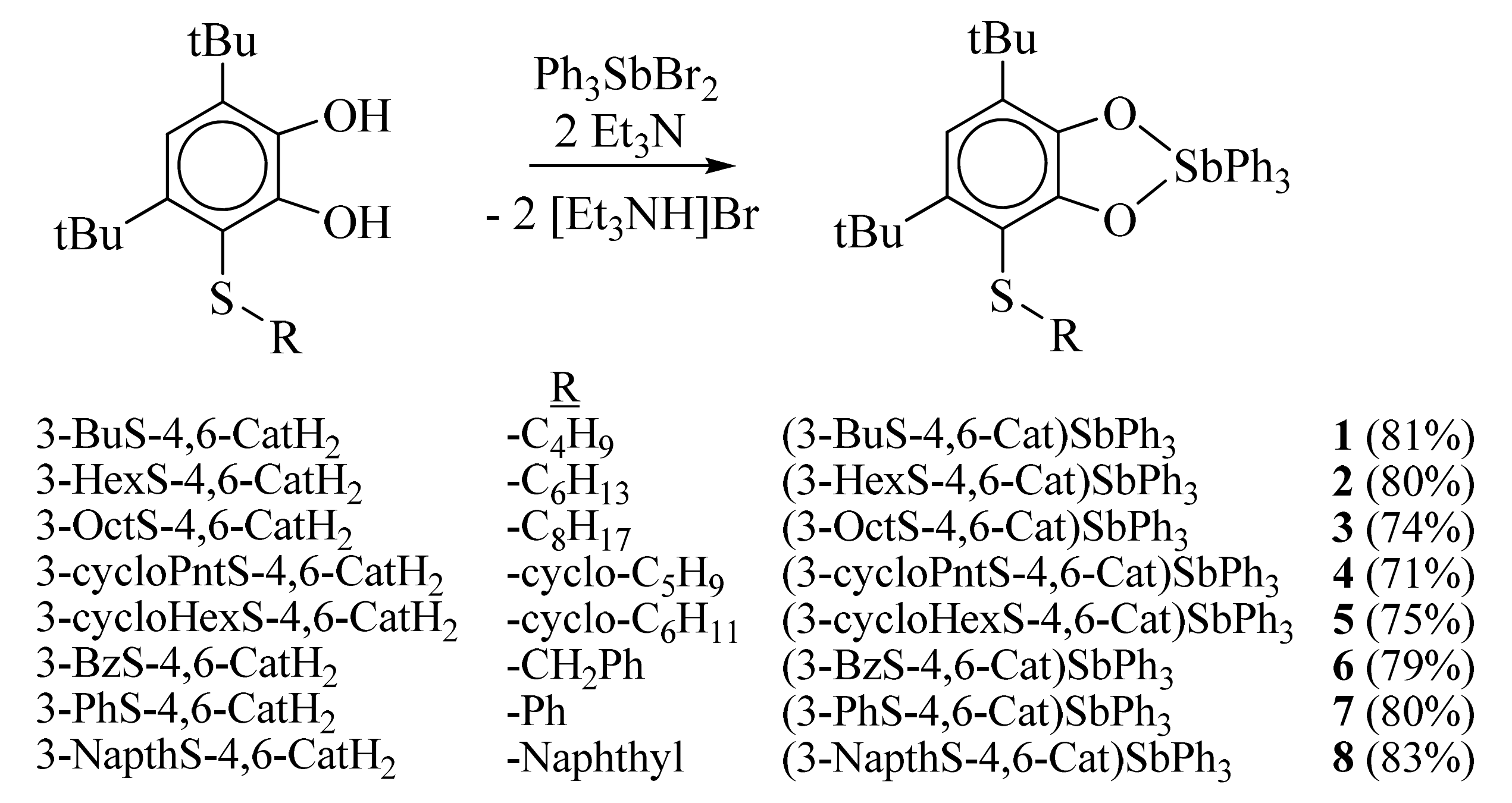
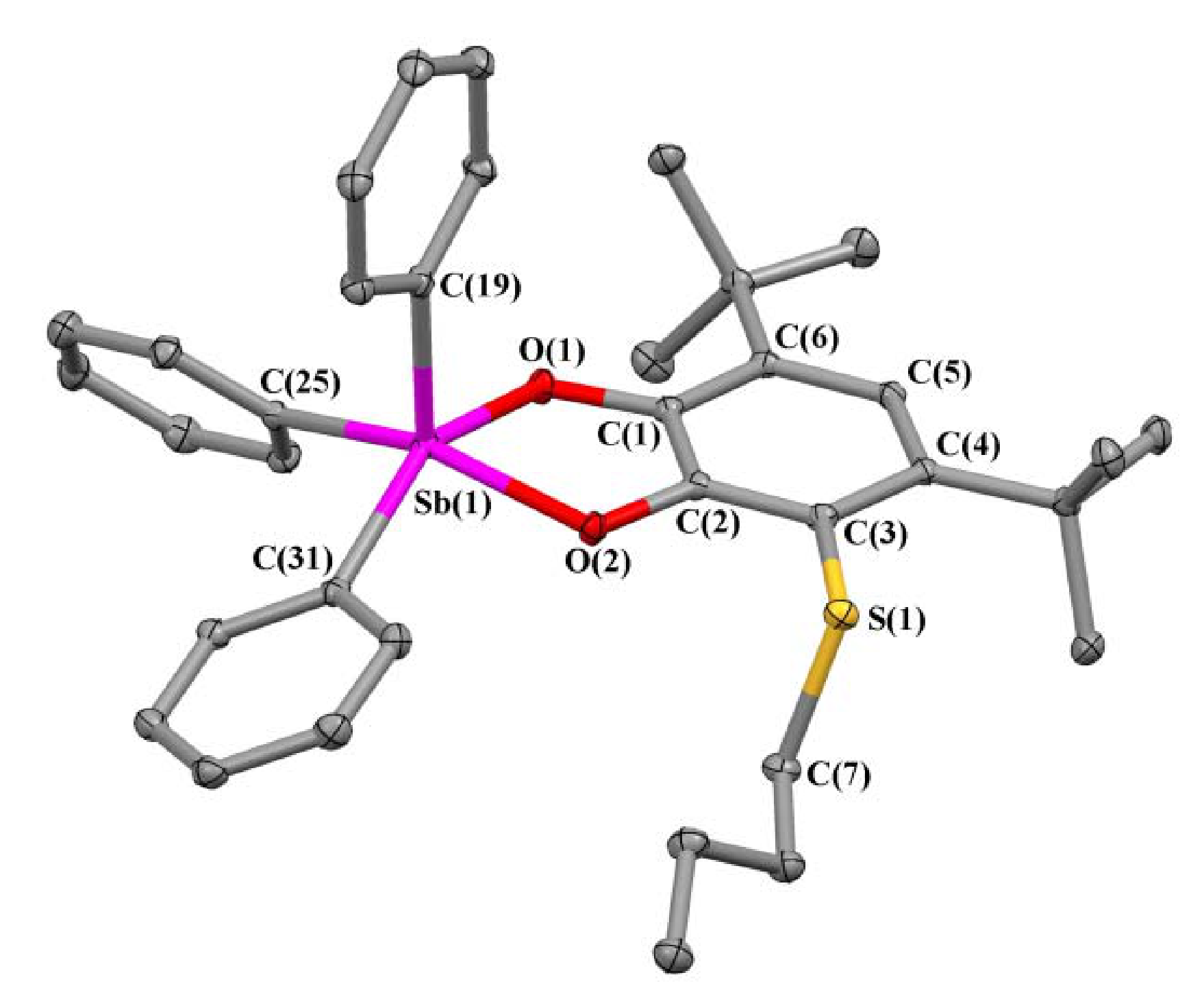
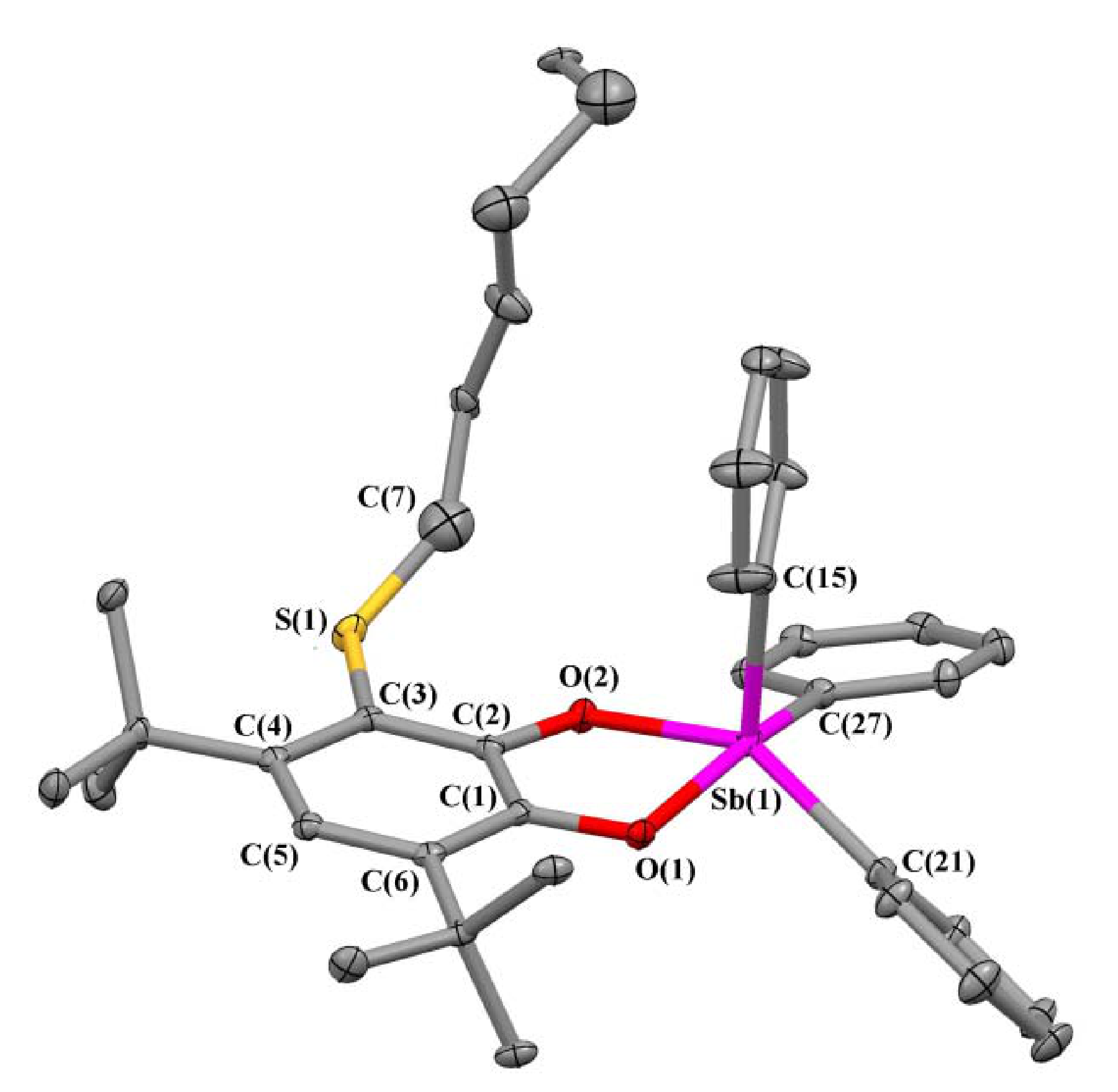
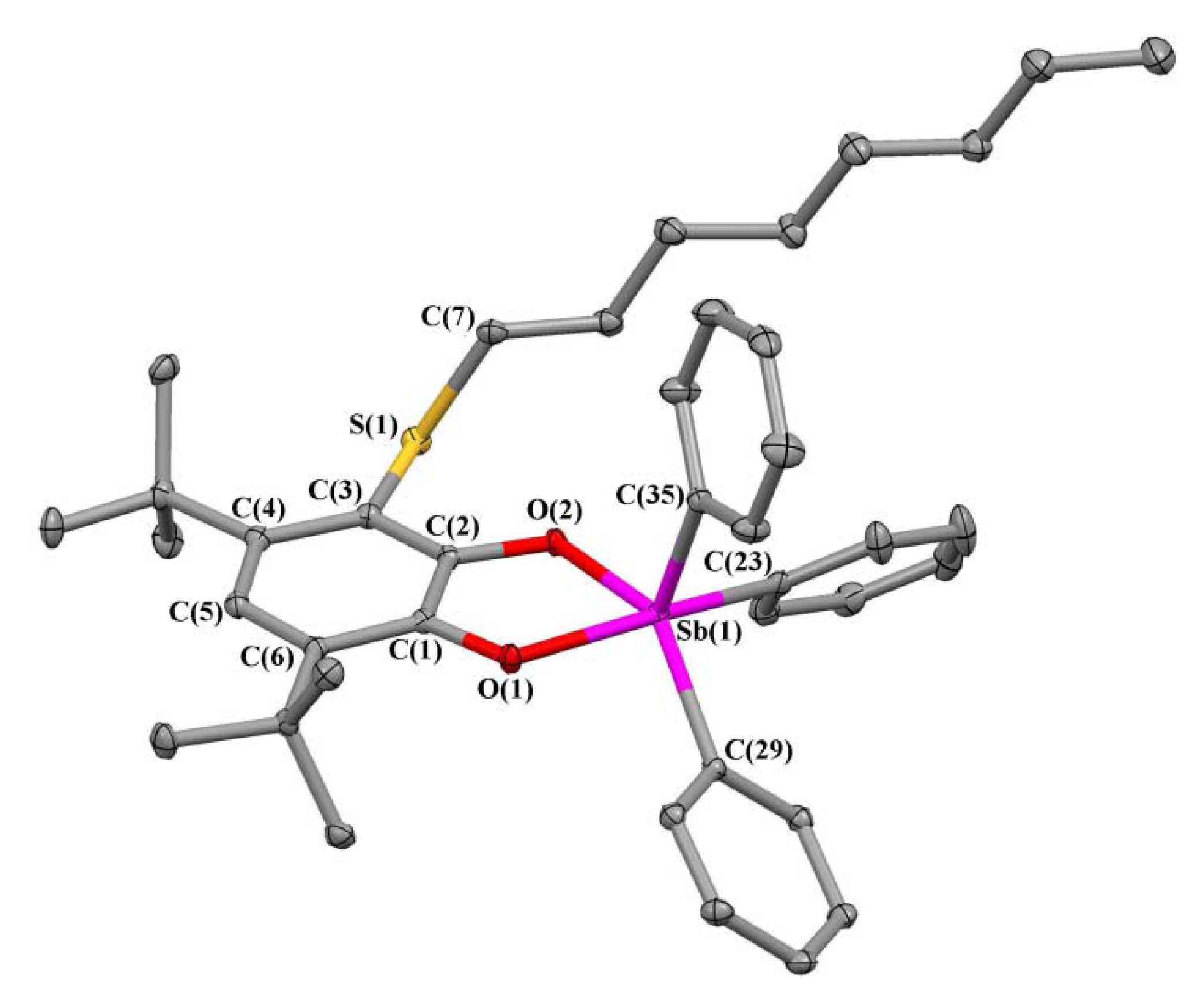
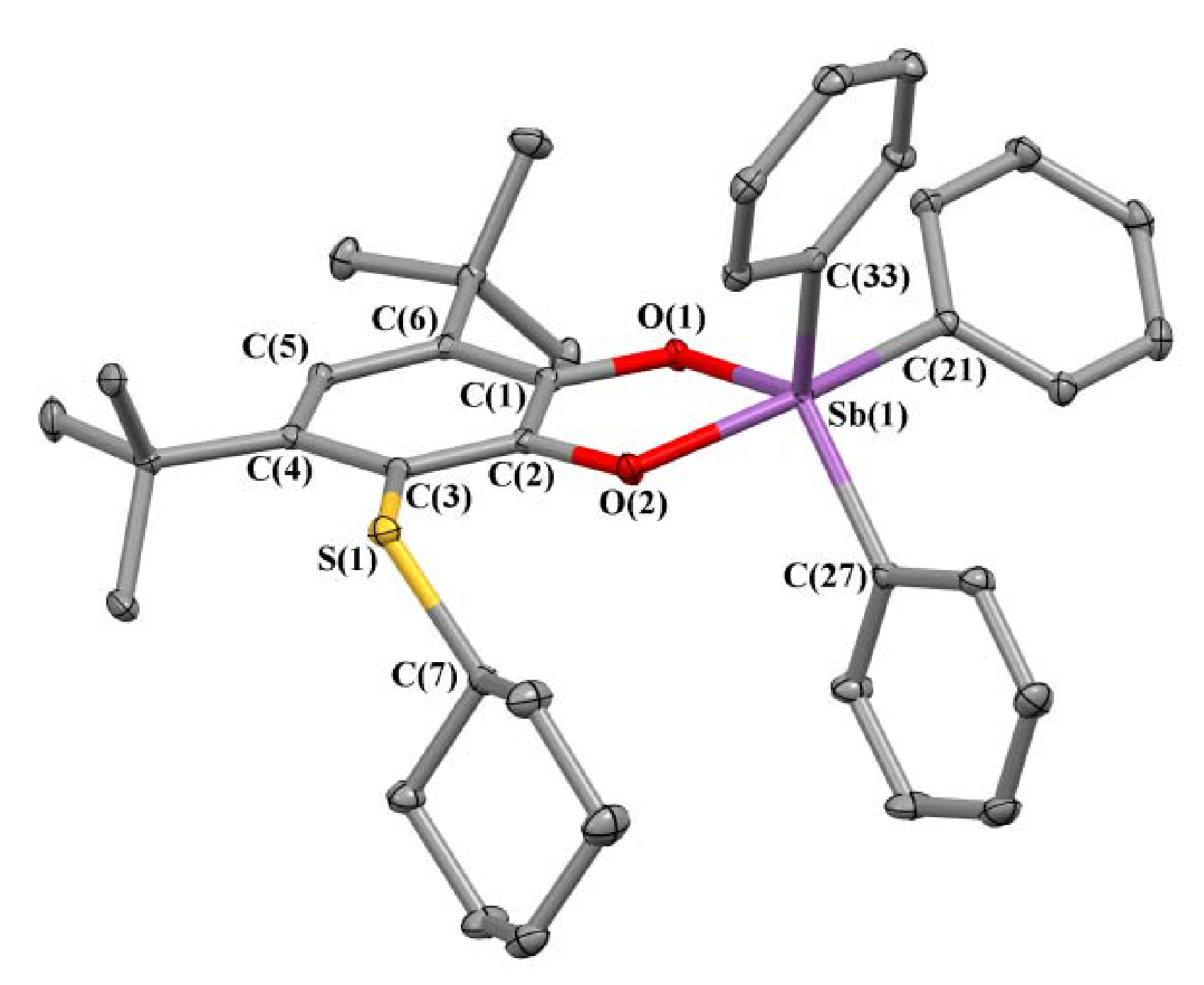
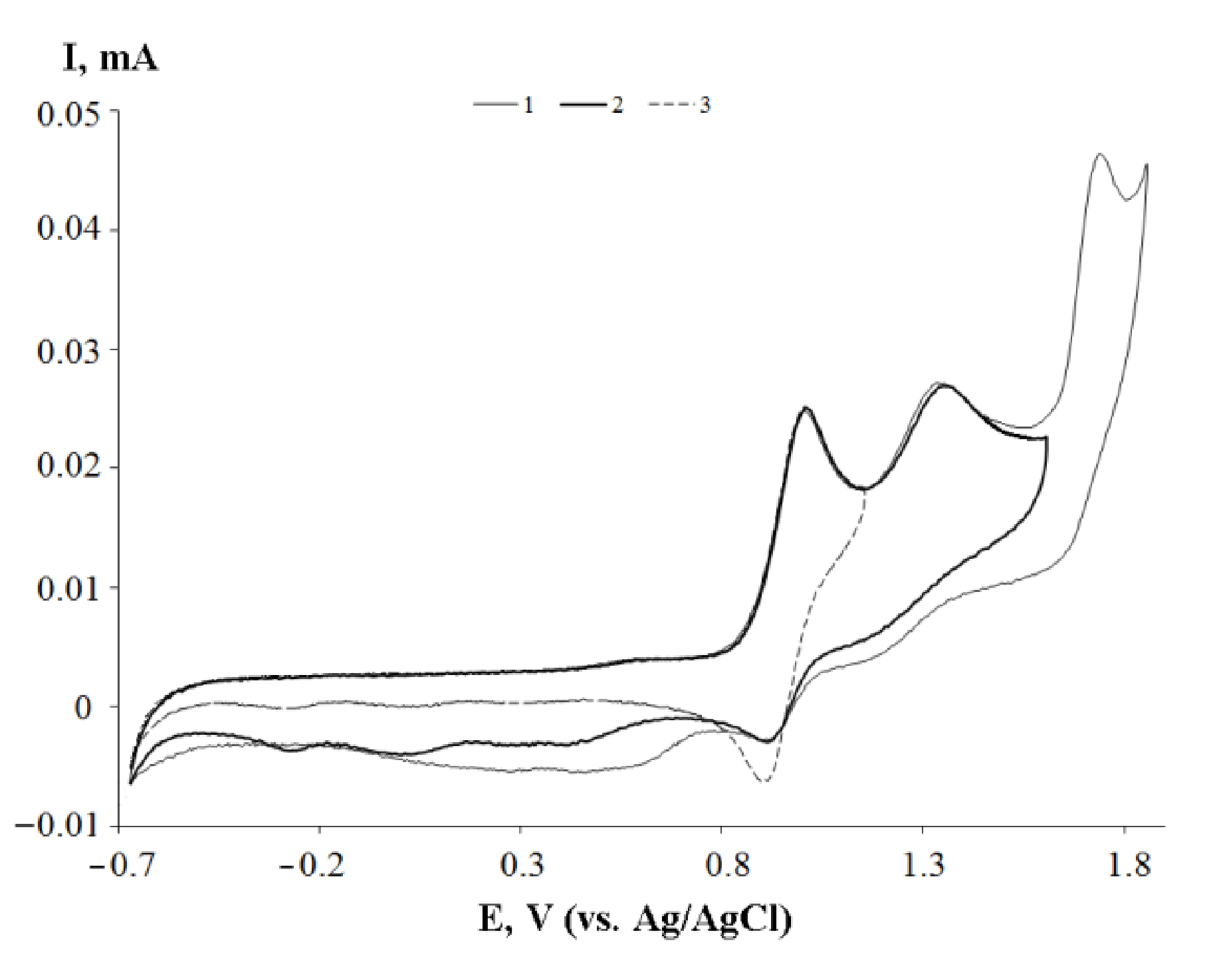
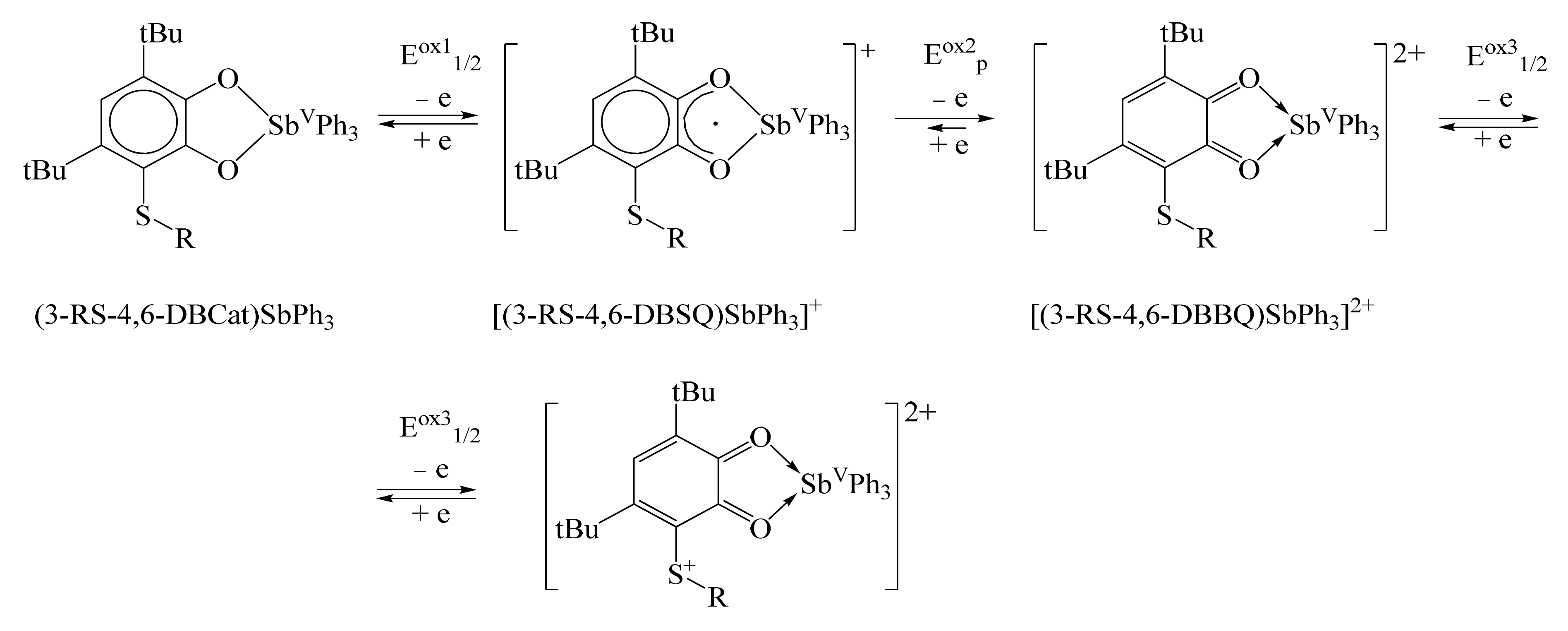
| Bond | 1 | 2 | 3 | 5 |
|---|---|---|---|---|
| Distance, Å | ||||
| Sb(1)-O(1) | 2.0272(12) | 2.0487(15) | 2.0519(14) | 2.0124(10) |
| Sb(1)-O(2) | 2.0374(12) | 2.0196(15) | 2.0175(13) | 2.0536(10) |
| Sb(1)-C(x) | Sb(1)-C(19) 2.1005(18) | Sb(1)-C(15) 2.110(2) | Sb(1)-C(23) 2.140(2) | Sb(1)-C(21) 2.1438(15) |
| Sb(1)-C(y) | Sb(1)-C(31) 2.1391(19) | Sb(1)-C(21) 2.138(2) | Sb(1)-C(29) 2.1251(19) | Sb(1)-C(27) 2.1221(15) |
| Sb(1)-C(z) | Sb(1)-C(25) 2.1407(18) | Sb(1)-C(27) 2.141(2) | Sb(1)-C(35) 2.100(2) | Sb(1)-C(33) 2.1143(15) |
| O(1)-C(1) | 1.362(2) | 1.358(3) | 1.355(2) | 1.3740(17) |
| O(2)-C(2) | 1.362(2) | 1.354(2) | 1.358(2) | 1.3500(17) |
| C(1)-C(2) | 1.406(2) | 1.402(3) | 1.403(3) | 1.401(2) |
| C(1)-C(6) | 1.395(2) | 1.392(3) | 1.400(3) | 1.391(2) |
| C(2)-C(3) | 1.394(2) | 1.398(3) | 1.397(3) | 1.402(2) |
| C(3)-C(4) | 1.417(2) | 1.416(3) | 1.418(3) | 1.418(2) |
| C(4)-C(5) | 1.401(2) | 1.403(3) | 1.399(3) | 1.400(2) |
| C(5)-C(6) | 1.399(2) | 1.404(3) | 1.400(3) | 1.404(2) |
| S(1)-C(3) | 1.7903(17) | 1.776(2) | 1.785(2) | 1.7801(15) |
| S(1)-C(7) | 1.8227(19) | 1.826(12) | 1.829(2) | 1.8343(16) |
| Angle, ° | ||||
| O(1)-Sb(1)-O(2) 79.01(5) | O(1)-Sb(1)-O(2) 78.91(6) | O(1)-Sb(1)-O(2) 78.90(5) | O(1)-Sb(1)-O(2) 78.87(4) | |
| O(1)-Sb(1)-C(31) 151.25(6) | O(2)-Sb(1)-C(21) 145.63(7) | O(2)-Sb(1)-C(29) 142.51(7) | O(2)-Sb(1)-C(27) 128.62(5) | |
| O(2)-Sb(1)-C(25) 150.38(6) | O(1)-Sb(1)-C(27) 156.27(8) | O(1)-Sb(1)-C(23) 158.06(7) | O(1)-Sb(1)-C(21) 162.40(5) | |
| № | Complex | Еox11/2, V | Iс/Iа | Еox2p, V | Еox31/2, V |
|---|---|---|---|---|---|
| 1 | (3-BuS-4,6-Cat)SbPh3 | 0.94 | 0.74 | 1.40 | 1.69 |
| 2 | (3-HexS-4,6-Cat)SbPh3 | 0.96 | 0.82 | 1.43 | 1.71 |
| 3 | (3-OctS-4,6-Cat)SbPh3 | 0.94 | 0.75 | 1.44 | 1.70 |
| 4 | (3-cycloPntS-4,6-Cat)SbPh3 | 0.94 | 0.85 | 1.45 | 1.69 |
| 5 | (3-cycloHexS-4,6-Cat)SbPh3 | 0.94 | 0.73 | 1.44 | 1.69 |
| 6 | (3-BzS-4,6-Cat)SbPh3 | 0.95 | 0.80 | 1.43 | 1.72 |
| 7 | (3-PhS-4,6-Cat)SbPh3 | 0.96 | 0.83 | 1.38 | 1.70 |
| 8 | (3-NapthS-4,6-Cat)SbPh3 | 0.96 | 0.92 | 1.32 | 1.58 |
| № | Complex | ЕС50, μmol/L | TEC50, Min | AE·103 |
|---|---|---|---|---|
| 1 | (3-BuS-4,6-Cat)SbPh3 | 29.5 ± 1.2 | 170 | 0.20 |
| 2 | (3-HexS-4,6-Cat)SbPh3 | 32.4 ± 0.9 | 180 | 0.17 |
| 3 | (3-C8H17S-4,6-Cat)SbPh3 | 32.0 ± 1.0 | 180 | 0.17 |
| 4 | (3-C5H9S-4,6-Cat)SbPh3 | 29.2 ± 1.1 | 130 | 0.26 |
| 5 | (3-C6H11S-4,6-Cat)SbPh3 | 26.3 ± 1.0 | 130 | 0.29 |
| 6 | (3-BzS-4,6-Cat)SbPh3 | 31.7 ± 1.4 | 170 | 0.19 |
| 7 | (3-PhS-4,6-Cat)SbPh3 | 31.0 ± 0.8 | 180 | 0.18 |
| 8 | (3-NapthS-4,6-Cat)SbPh3 | 30.5 ± 0.9 | 170 | 0.19 |
| (3,6-Cat)SbPh3 [75] | 17.3 ± 0.5 | 60 | 0.96 | |
| (4-MeO-3,6-Cat)SbPh3 [75] | 6.8 ± 0.8 | 22 | 6.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolyaninov, I.V.; Fukin, G.K.; Berberova, N.T.; Poddel’sky, A.I. Triphenylantimony(V) Catecholates of the Type (3-RS-4,6-DBCat)SbPh3-Catechol Thioether Derivatives: Structure, Electrochemical Properties, and Antiradical Activity. Molecules 2021, 26, 2171. https://doi.org/10.3390/molecules26082171
Smolyaninov IV, Fukin GK, Berberova NT, Poddel’sky AI. Triphenylantimony(V) Catecholates of the Type (3-RS-4,6-DBCat)SbPh3-Catechol Thioether Derivatives: Structure, Electrochemical Properties, and Antiradical Activity. Molecules. 2021; 26(8):2171. https://doi.org/10.3390/molecules26082171
Chicago/Turabian StyleSmolyaninov, Ivan V., Georgy K. Fukin, Nadezhda T. Berberova, and Andrey I. Poddel’sky. 2021. "Triphenylantimony(V) Catecholates of the Type (3-RS-4,6-DBCat)SbPh3-Catechol Thioether Derivatives: Structure, Electrochemical Properties, and Antiradical Activity" Molecules 26, no. 8: 2171. https://doi.org/10.3390/molecules26082171
APA StyleSmolyaninov, I. V., Fukin, G. K., Berberova, N. T., & Poddel’sky, A. I. (2021). Triphenylantimony(V) Catecholates of the Type (3-RS-4,6-DBCat)SbPh3-Catechol Thioether Derivatives: Structure, Electrochemical Properties, and Antiradical Activity. Molecules, 26(8), 2171. https://doi.org/10.3390/molecules26082171








