Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Kraft Lignin towards 4,4′ MDI
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Sample Preparation
2.3. 31P Nuclear Magnetic Resonance (31P NMR)
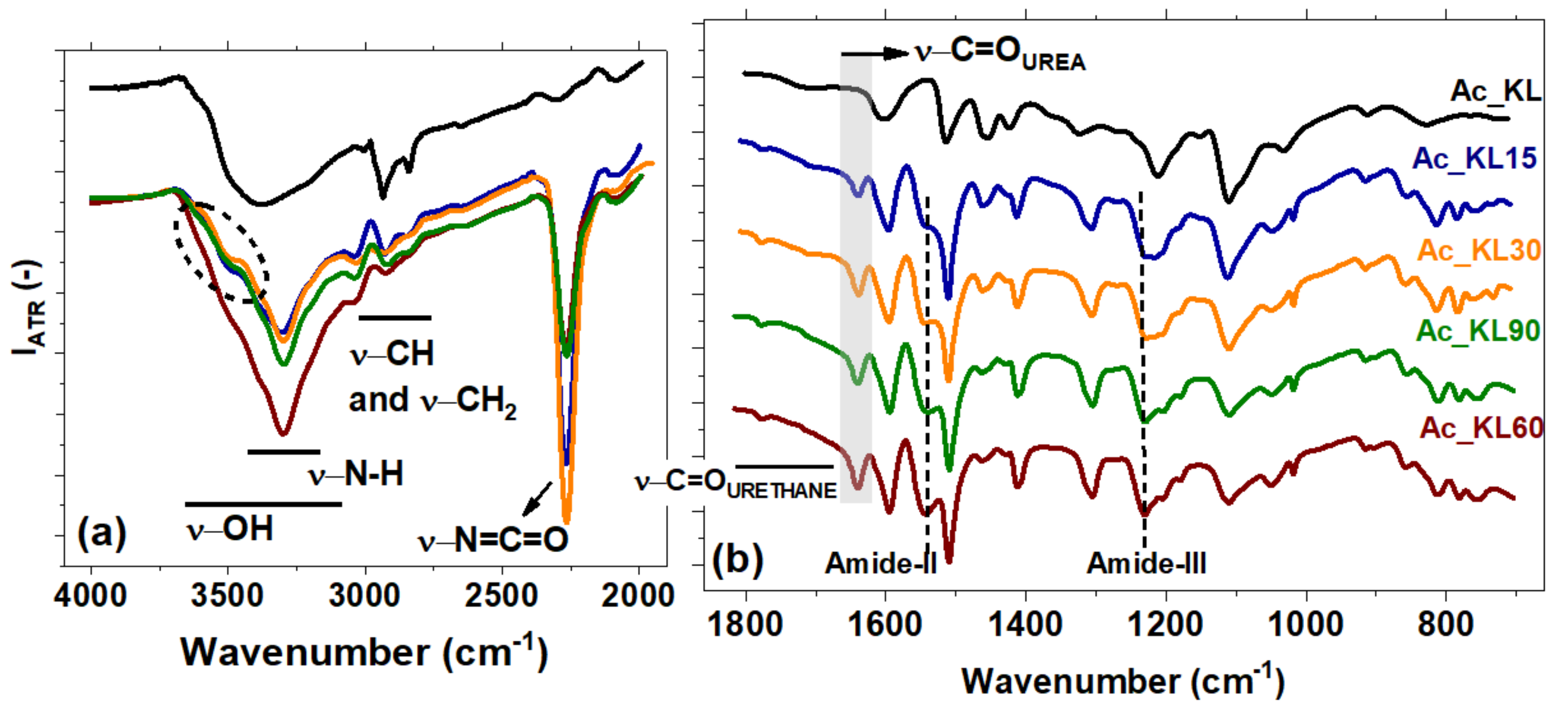
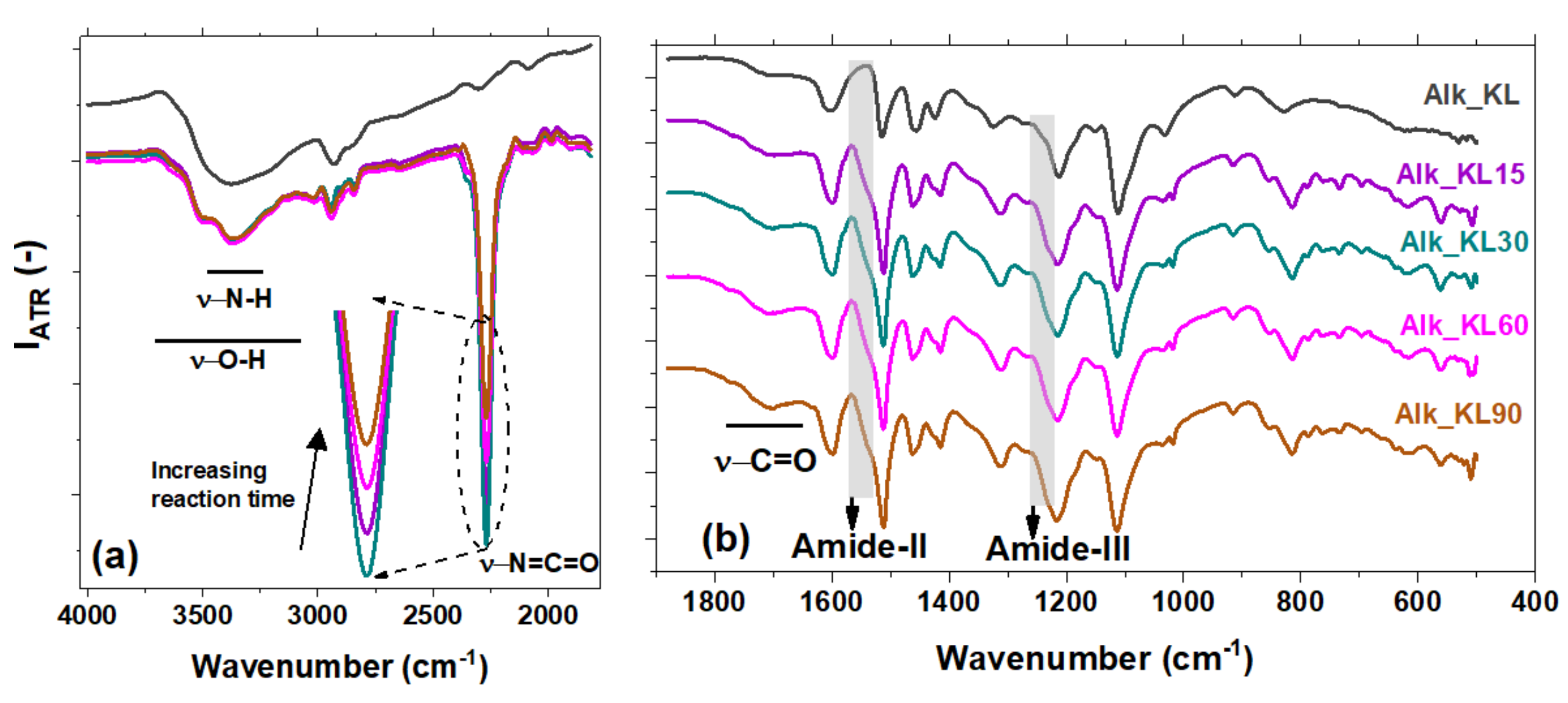
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
3. Results and Discussion
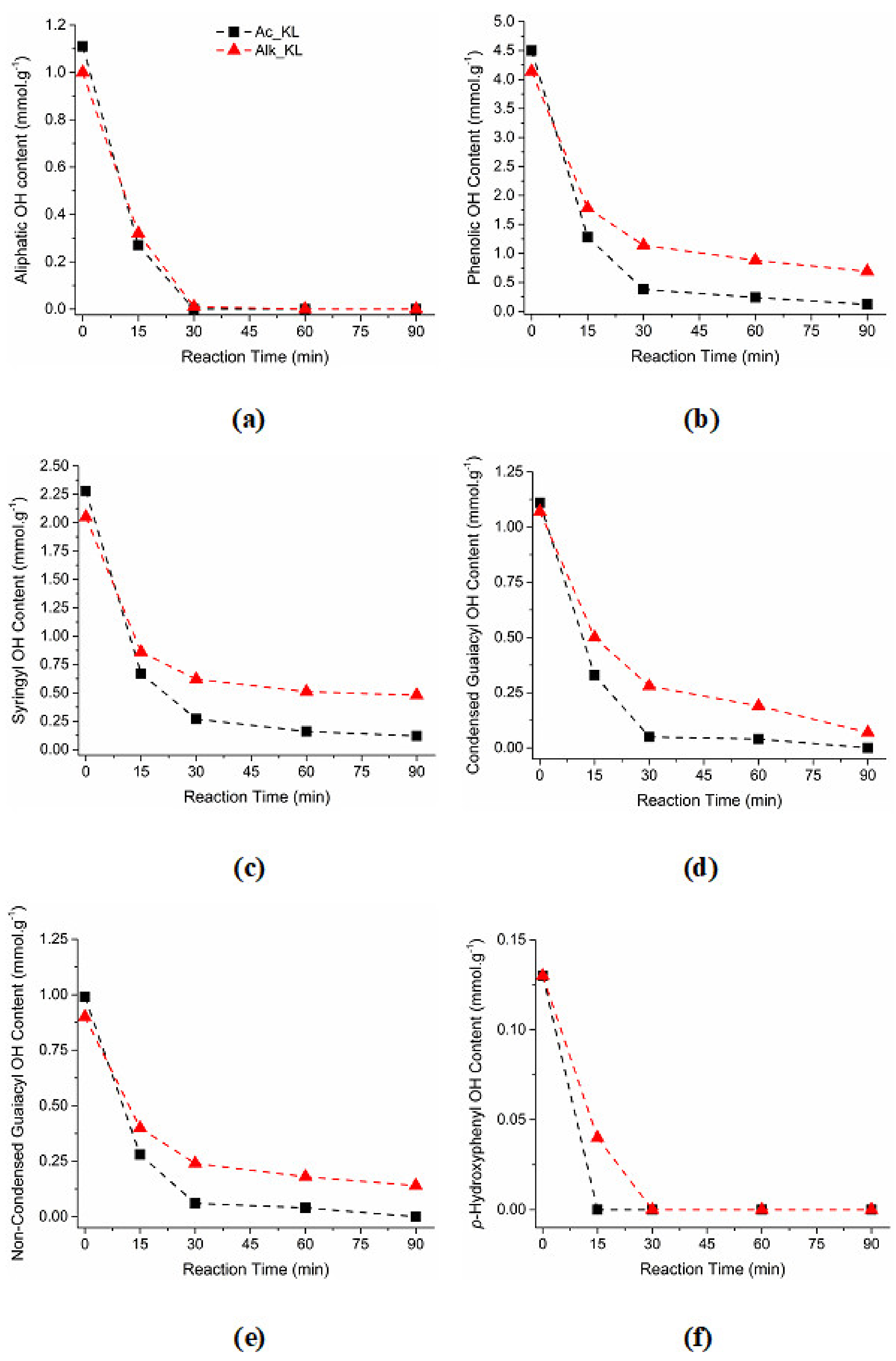
3.1. 31P NMR
3.2. FTIR-ATR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-based polyurethanes: Opportunities for bio-based foams, elastomers, coatings and adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.; Ungureanu, M.; Ignat, L.; Ungureanu, D.; Popa, V.I. Properties of lignin-polyurethane films prepared by casting method. Ind. Crops Prod. 2004, 20, 231–241. [Google Scholar] [CrossRef]
- Hirose, S.; Kobashigawa, K.; Izuta, Y.; Hatakeyama, H. Thermal degradation of polyurethanes containing lignin studied by TG-FTIR. Polym. Int. 1998, 47, 247–256. [Google Scholar] [CrossRef]
- De Silva, E.A.B.; Zabkova, M.; Araújo, J.D.; Cateto, C.A.; Barreiro, M.F.; Belgacem, M.N.; Rodrigues, A.E. An integrated process to produce vanillin and lignin-based polyurethanes from Kraft lignin. Chem. Eng. Res. Des. 2009, 87, 1276–1292. [Google Scholar] [CrossRef]
- Gouveia, J.R.; da Costa, C.L.; Tavares, L.B.; dos Santos, D.J. Synthesis of lignin-based polyurethanes: A mini-review. Mini. Rev. Org. Chem. 2018, 16, 345–352. [Google Scholar] [CrossRef]
- Jeong, H.; Park, J.; Kim, S.; Lee, J.; Ahn, N.; Roh, H. Preparation and characterization of thermoplastic polyurethanes using partially acetylated kraft lignin. Fibers Polym. 2013, 14, 1082–1093. [Google Scholar] [CrossRef]
- Gouveia, J.R.; de Sousa, R.R., Jr.; Ribeiro, A.O.; Saraiva, S.A.; dos Santos, D.J. Effect of soft segment molecular weight and NCO:OH ratio on thermomechanical properties of lignin-based thermoplastic polyurethane adhesive. Eur. Polym. J. 2020, 131, 109690. [Google Scholar] [CrossRef]
- Saito, T.; Perkins, J.H.; Jackson, D.C.; Trammel, N.E.; Hunt, M.A.; Naskar, A.K. Development of lignin-based polyurethane thermoplastics. RSC Adv. 2013, 3, 21832–21840. [Google Scholar] [CrossRef]
- Gouveia, J.R.; Antonino, L.D.; Garcia, G.E.S.; Tavares, L.B.; Santos, A.N.B.; Dos Santos, D.J. Kraft lignin-containing polyurethane adhesives: The role of hydroxypropylation on thermomechanical properties. J. Adhes. 2020. [Google Scholar] [CrossRef]
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Dos Santos, D.J. Bio-based polyurethane prepared from Kraft lignin and modified castor oil. Express Polym. Lett. 2016, 10, 927–940. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Kasbe, P.S.; Mahanwar, P.A.; Gadekar, P.T. Synthesis and characterization of lignin-polyurethane based wood adhesive. Int. J. Adhes. Adhes. 2019, 95, 102427. [Google Scholar] [CrossRef]
- Nacas, A.M.; Ito, N.M.; Sousa, R.R.D.; Spinacé, M.A.; Dos Santos, D.J. Effects of NCO:OH ratio on the mechanical properties and chemical structure of Kraft lignin–based polyurethane adhesive. J. Adhes. 2017, 93, 18–29. [Google Scholar] [CrossRef]
- Chahar, S.; Dastidar, M.G.; Choudhary, V.; Sharma, D.K. Synthesis and characterisation of polyurethanes derived from waste black liquor lignin. J. Adhes. Sci. Technol. 2004, 18, 169–179. [Google Scholar] [CrossRef]
- Griffini, G.; Passoni, V.; Suriano, R.; Levi, M.; Turri, S. Polyurethane coatings based on chemically unmodified fractionated lignin. ACS Sustain. Chem. Eng. 2015, 3, 1145–1154. [Google Scholar] [CrossRef]
- Li, Y.; Ragauskas, A.J. Kraft lignin-based rigid polyurethane foam. J. Wood Chem. Technol. 2012, 32, 210–224. [Google Scholar] [CrossRef]
- Bernardini, J.; Cinelli, P.; Anguillesi, I.; Coltelli, M.B.; Lazzeri, A. Flexible polyurethane foams green production employing lignin or oxypropylated lignin. Eur. Polym. J. 2015, 64, 147–156. [Google Scholar] [CrossRef]
- Cinelli, P.; Anguillesi, I.; Lazzeri, A. Green synthesis of flexible polyurethane foams from liquefied lignin. Eur. Polym. J. 2013, 49, 1174–1184. [Google Scholar] [CrossRef]
- Nadji, H.; Bruzzèse, C.; Belgacem, M.N.; Benaboura, A.; Gandini, A. Oxypropylation of lignins and preparation of rigid polyurethane foams from the ensuing polyols. Macromol. Mater. Eng. 2005, 290, 1009–1016. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Glasser, W.G. About making lignin great again—Some lessons from the past. Front. Chem. 2019, 7, 1–17. [Google Scholar] [CrossRef]
- Crestini, C.; Crucianelli, M.; Orlandi, M.; Saladino, R. Oxidative strategies in lignin chemistry: A new environmental friendly approach for the functionalisation of lignin and lignocellulosic fibers. Catal. Today 2010, 156, 8–22. [Google Scholar] [CrossRef]
- Gandini, A.; Belgacem, M.N.; Guo, Z.-X.; Montanari, S. Lignins as Macromonomers for Polyesters and Polyurethanes. In Chemical Modification, Properties, and Usage of Lignin; Springer: Boston, MA, USA, 2002; pp. 57–80. [Google Scholar]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Belgacem, M.N. Optimization study of lignin oxypropylation in view of the preparation of polyurethane rigid foams. Ind. Eng. Chem. Res. 2009, 48, 2583–2589. [Google Scholar] [CrossRef]
- Zhang, X.; Kim, Y.; Elsayed, I.; Taylor, M.; Eberhardt, T.L.; Hassan, E.B.; Shmulsky, R. Rigid polyurethane foams containing lignin oxyalkylated with ethylene carbonate and polyethylene glycol. Ind. Crops Prod. 2019, 141, 111797. [Google Scholar] [CrossRef]
- Chauhan, M.; Gupta, M.; Singh, B.; Singh, A.K.; Gupta, V.K. Effect of functionalized lignin on the properties of lignin–isocyanate prepolymer blends and composites. Eur. Polym. J. 2014, 52, 32–43. [Google Scholar] [CrossRef]
- Gómez-Fernández, S.; Ugarte, L.; Calvo-Correas, T.; Peña-Rodríguez, C.; Corcuera, M.A.; Eceiza, A. Properties of flexible polyurethane foams containing isocyanate functionalized kraft lignin. Ind. Crops Prod. 2017, 100, 51–64. [Google Scholar] [CrossRef]
- Zieglowski, M.; Trosien, S.; Rohrer, J.; Mehlhase, S.; Weber, S.; Bartels, K.; Siegert, G.; Trellenkamp, T.; Albe, K.; Biesalski, M. Reactivity of isocyanate-functionalized lignins: A key factor for the preparation of lignin-based polyurethanes. Front. Chem. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Takahashi, M.; Yang, S.; Yamamoto, K.; Ohara, K.; Phuc, N.H.H.; Watanabe, T.; Uchiyama, T.; Sakuda, A.; Hayashi, A.; Tatsumisago, M.; et al. Improvement of lithium ionic conductivity of Li3PS4 through suppression of crystallization using low-boiling-point solvent in liquid-phase synthesis. Solid State Ion. 2021, 361, 115568. [Google Scholar] [CrossRef]
- Granata, A.; Argyropoulos, D.S. 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, a reagent for the accurate determination of the uncondensed and condensed phenolic moieties in lignins. J. Agric. Food Chem. 1995, 43, 1538–1544. [Google Scholar] [CrossRef]
- Zawadzki, M.; Ragauskas, A. N-hydroxy compounds as new internal standards for the 31P-NMR determination of lignin hydroxy functional groups. Holzforschung 2001, 55, 283–285. [Google Scholar] [CrossRef]
- Hofmann, K.; Glasser, W.G. Engineering plastics from lignin. 21.1 synthesis and properties of epoxidized lignin- poly (propylene oxide) copolymers. J. Wood Chem. Technol. 1993, 13, 73–95. [Google Scholar] [CrossRef]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Belgacem, M.N. Kinetic study of the formation of lignin-based polyurethanes in bulk. React. Funct. Polym. 2011, 71, 863–869. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Montanari, S.; Baradie, B.; Andréolèty, J.-P.; Gandini, A. Star-Shaped and Crosslinked Polyurethanes Derived from Lignins and Oligoether Isocyanates. In The Chemistry and Processing of Wood and Plant Fibrous Material; Elsevier: Amsterdam, The Netherlands, 1996; pp. 351–358. [Google Scholar]
- Lundquist, K.; Parkås, J. Different types of phenolic units in lignins. BioResources 2011, 6, 920–926. [Google Scholar] [CrossRef]
- Elrhayam, Y.; Elharfi, A. 3D-QSAR studies of the chemical modification of hydroxyl groups of biomass (cellulose, hemicelluloses and lignin) using quantum chemical descriptors. Heliyon 2019, 5, e02173. [Google Scholar] [CrossRef] [PubMed]
- Maia, R.A.; Ventorim, G.; Batagin-Neto, A. Reactivity of lignin subunits: The influence of dehydrogenation and formation of dimeric structures. J. Mol. Model. 2019, 25, 1–11. [Google Scholar] [CrossRef]
- Martinez, C.; Rivera, J.L.; Herrera, R.; Rico, J.L.; Flores, N.; Rutiaga, J.G.; López, P. Evaluation of the chemical reactivity in lignin precursors using the Fukui function. J. Mol. Model. 2008, 14, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Qiu, X.; Fang, Z.; Yang, D. Effect of molecular weight on the reactivity and dispersibility of sulfomethylated alkali lignin modified by horseradish peroxidase. ACS Sustain. Chem. Eng. 2018, 6, 14197–14202. [Google Scholar] [CrossRef]
- Pan, X.; Saddler, J.N. Effect of replacing polyol by organosolv and kraft lignin on the property and structure of rigid polyurethane foam. Biotechnol. Biofuels 2013, 6, 12. [Google Scholar] [CrossRef]
- Jardim, J.M.; Hart, P.W.; Lucia, L.; Jameel, H. Insights into the potential of hardwood kraft lignin to be a green platform material for emergence of the biorefinery. Polymers 2020, 12, 1795. [Google Scholar] [CrossRef]
- Zimmer, B.; Nies, C.; Schmitt, C.; Paulo, C.; Possart, W. Chemistry, polymer dynamics and mechanical properties of a two-part polyurethane elastomer during and after crosslinking. Part II: Moist conditions. Polymer 2018, 149, 238–252. [Google Scholar] [CrossRef]
- Cachet, N.; Camy, S.; Benjelloun-Mlayah, B.; Condoret, J.S.; Delmas, M. Esterification of organosolv lignin under supercritical conditions. Ind. Crops Prod. 2014, 58, 287–297. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, Z.; Yin, Z.; Qian, H.; Ma, D. Amide II and amide III bands in polyurethane model soft and hard segments. Polym. Bull. 2008, 60, 97–101. [Google Scholar] [CrossRef]
- Yilgör, I.; Yilgör, E.; Wilkes, G.L. Critical parameters in designing segmented polyurethanes and their effect on morphology and properties: A comprehensive review. Polymer 2015, 58, A1–A36. [Google Scholar] [CrossRef]
- Giroto, A.S.; do Valle, S.F.; Ribeiro, T.; Ribeiro, C.; Mattoso, L.H.C. Towards urea and glycerol utilization as “building blocks” for polyurethane production: A detailed study about reactivity and structure for environmentally friendly polymer synthesis. React. Funct. Polym. 2020, 153, 104629. [Google Scholar] [CrossRef]
- Zimmer, B.; Nies, C.; Schmitt, C.; Possart, W. Chemistry, polymer dynamics and mechanical properties of a two-part polyurethane elastomer during and after crosslinking. Part I: Dry conditions. Polymer 2017, 115, 77–95. [Google Scholar] [CrossRef]
- Maillard, D.; Osso, E.; Faye, A.; Li, H.; Ton-That, M.T.; Stoeffler, K. Influence of lignin’s pH on polyurethane flexible foam formation and how to control it. J. Appl. Polym. Sci. 2020, 138, 50319. [Google Scholar] [CrossRef]
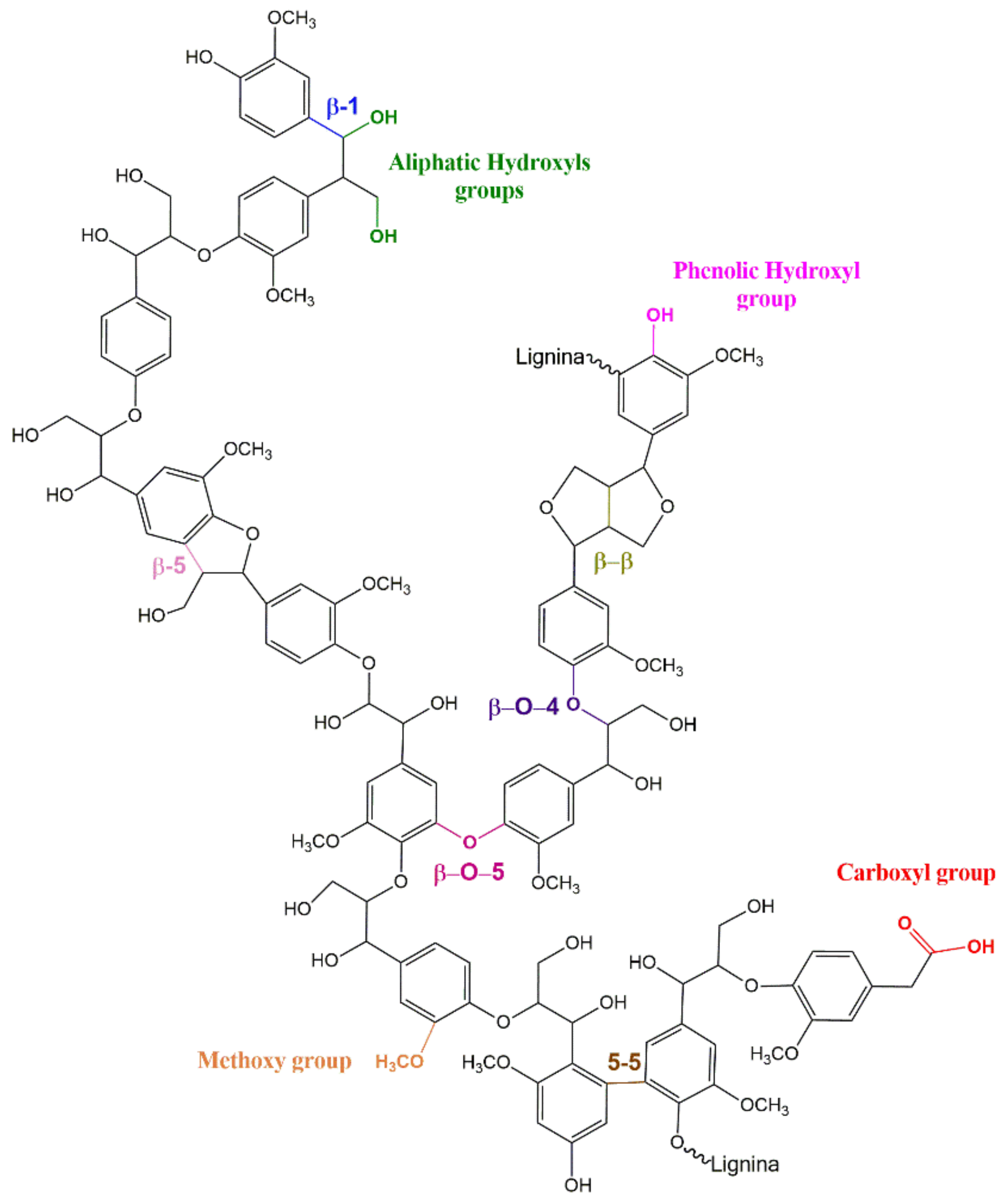
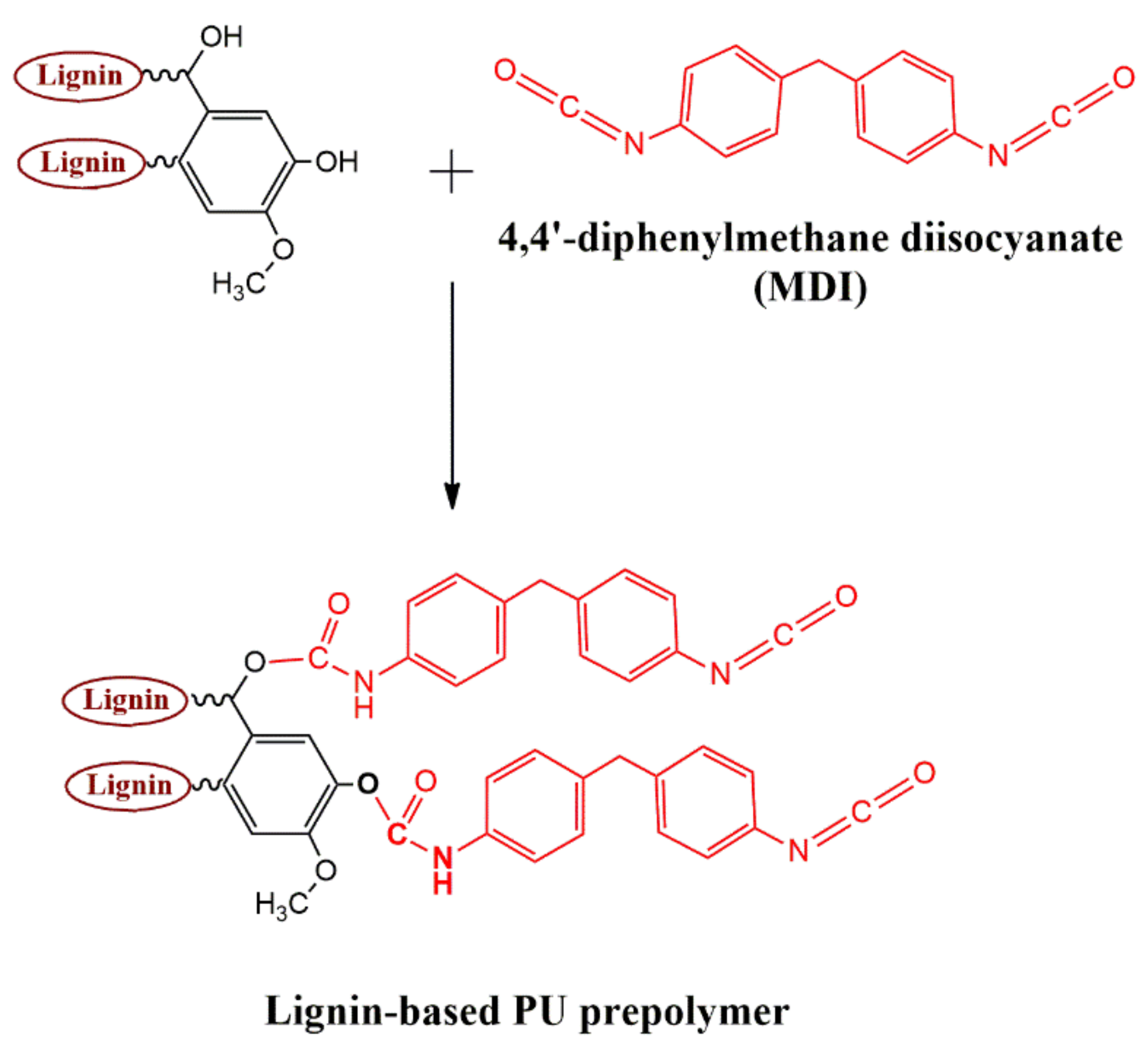
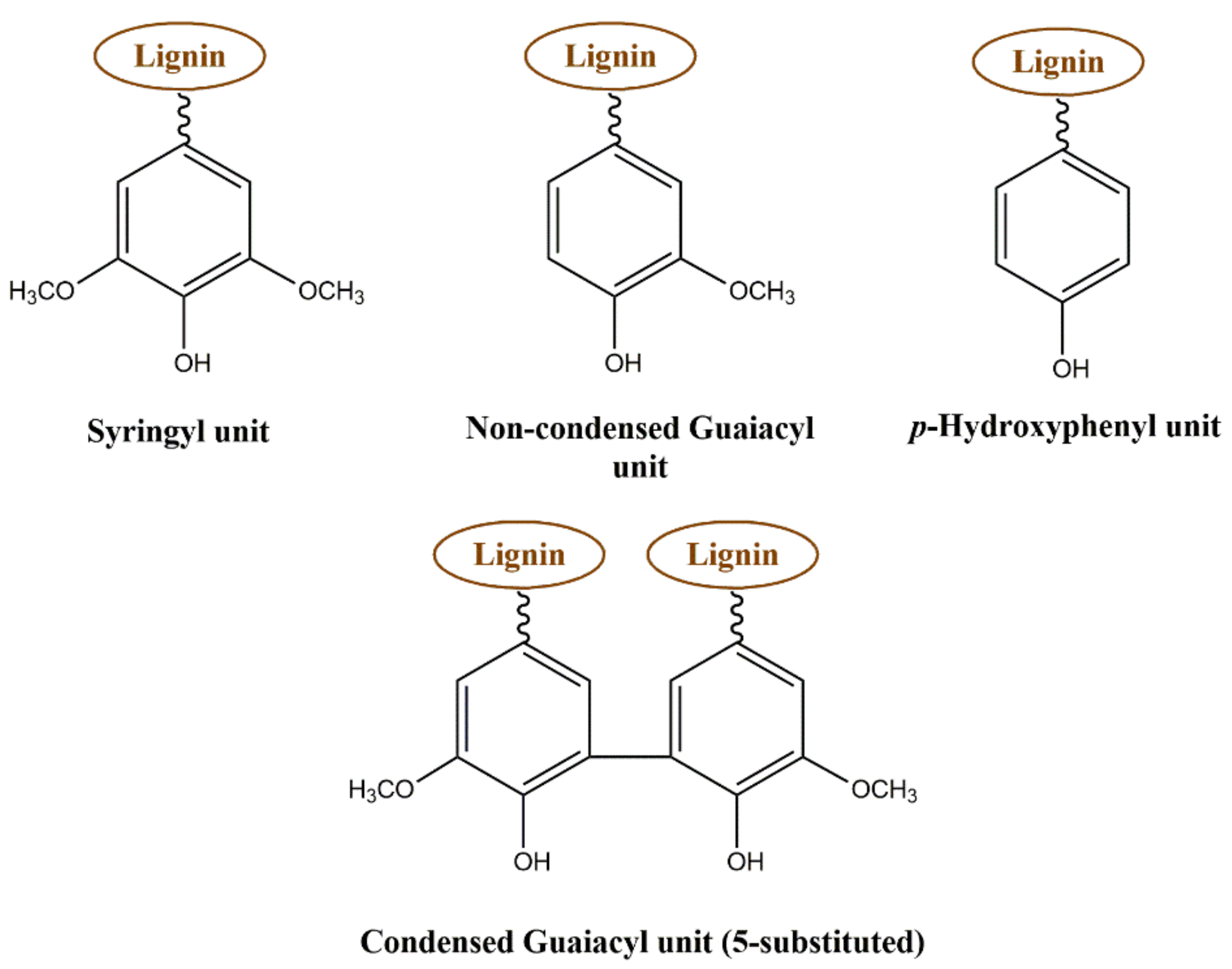

| Lignin Sample | Aliphatic OH (mmol/g) | Phenolics OH (mmol/g) | Total OH (mmol/g) | ||||
|---|---|---|---|---|---|---|---|
| Syringyl | Condensed Guaiacyl | Uncondensed Guaiacyl | p-Hydroxy Phenyl | Total Phenolics | |||
| AC_KL | 1.11 | 2.28 | 1.11 | 0.99 | 0.13 | 4.50 | 5.61 |
| Alk_KL | 1.00 | 2.05 | 1.07 | 0.9 | 0.13 | 4.14 | 5.14 |
| Band Positions (cm−1) | Assignment |
|---|---|
| 3400 | –OH stretching of aromatic and aliphatic |
| 3300 | –NH amine stretching |
| 2930 | –CH asymmetric stretching vibration of methyl/methylene groups |
| 2840 | –CH symmetric stretching of methyl/methylene groups |
| 2270 | –N=C=O isocyanate asymmetric stretching |
| 1765–1650 | –C=O urethane carbonyl stretching vibration |
| 1702 | –C=O lignin carbonyl stretching vibration |
| 1640 | –C=O urea carbonyl stretching vibration |
| 1540 | Amide II |
| 1235 | Amide III |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonino, L.D.; Gouveia, J.R.; de Sousa Júnior, R.R.; Garcia, G.E.S.; Gobbo, L.C.; Tavares, L.B.; dos Santos, D.J. Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Kraft Lignin towards 4,4′ MDI. Molecules 2021, 26, 2131. https://doi.org/10.3390/molecules26082131
Antonino LD, Gouveia JR, de Sousa Júnior RR, Garcia GES, Gobbo LC, Tavares LB, dos Santos DJ. Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Kraft Lignin towards 4,4′ MDI. Molecules. 2021; 26(8):2131. https://doi.org/10.3390/molecules26082131
Chicago/Turabian StyleAntonino, Leonardo Dalseno, Júlia Rocha Gouveia, Rogério Ramos de Sousa Júnior, Guilherme Elias Saltarelli Garcia, Luara Carneiro Gobbo, Lara Basílio Tavares, and Demetrio Jackson dos Santos. 2021. "Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Kraft Lignin towards 4,4′ MDI" Molecules 26, no. 8: 2131. https://doi.org/10.3390/molecules26082131
APA StyleAntonino, L. D., Gouveia, J. R., de Sousa Júnior, R. R., Garcia, G. E. S., Gobbo, L. C., Tavares, L. B., & dos Santos, D. J. (2021). Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Kraft Lignin towards 4,4′ MDI. Molecules, 26(8), 2131. https://doi.org/10.3390/molecules26082131






