Tuning the Acceptor Unit of Push–Pull Porphyrazines for Dye-Sensitized Solar Cells
Abstract
1. Introduction
2. Results
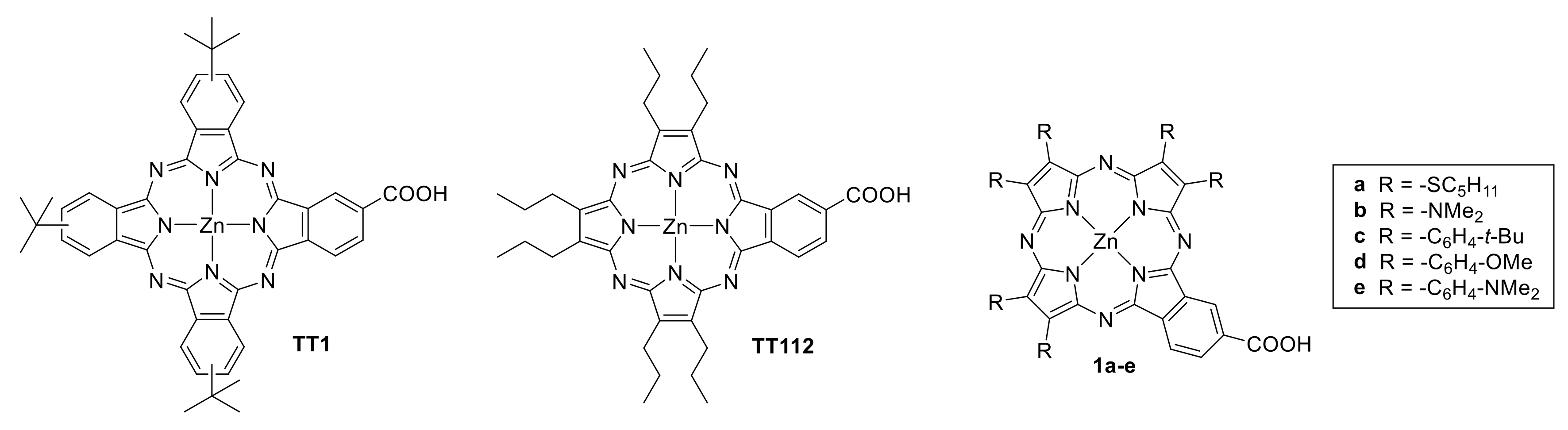
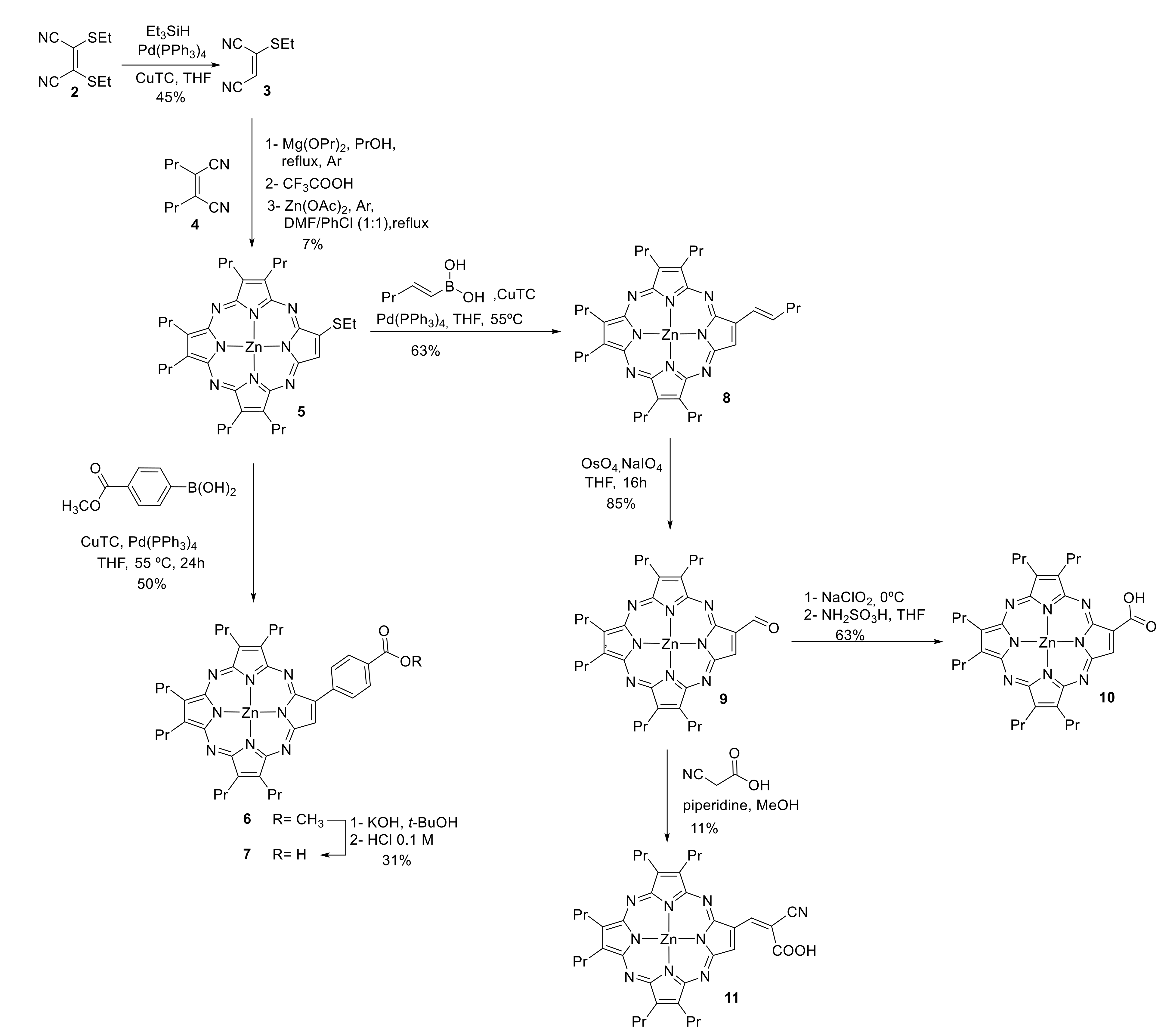
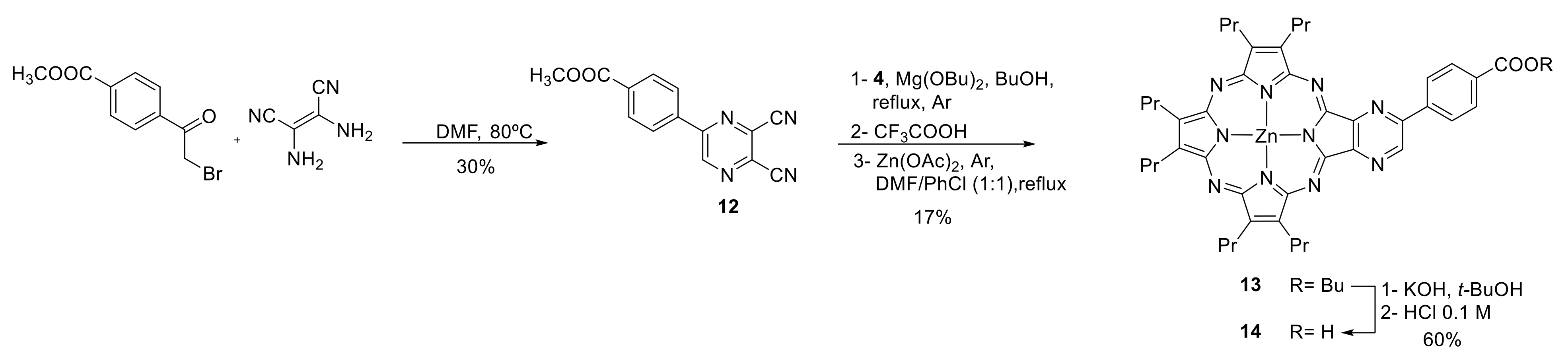
2.1. Synthesis
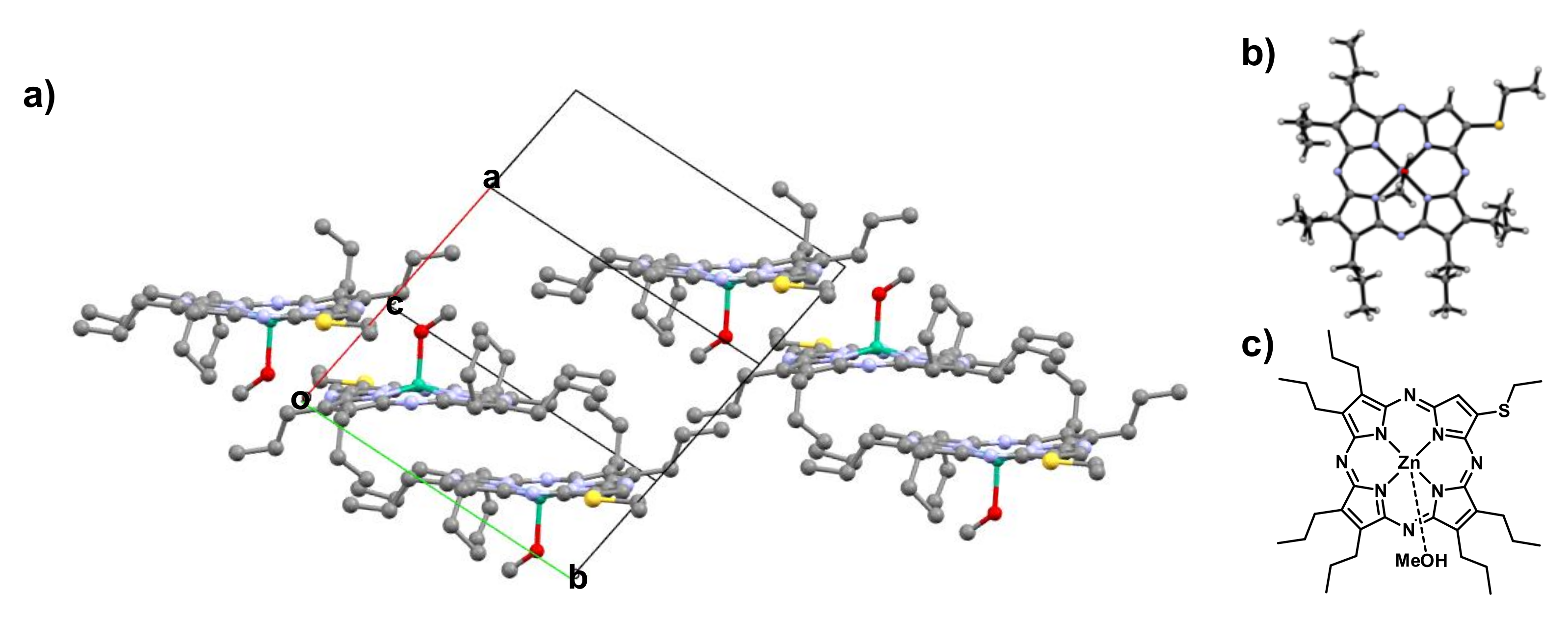
2.2. Optical Properties
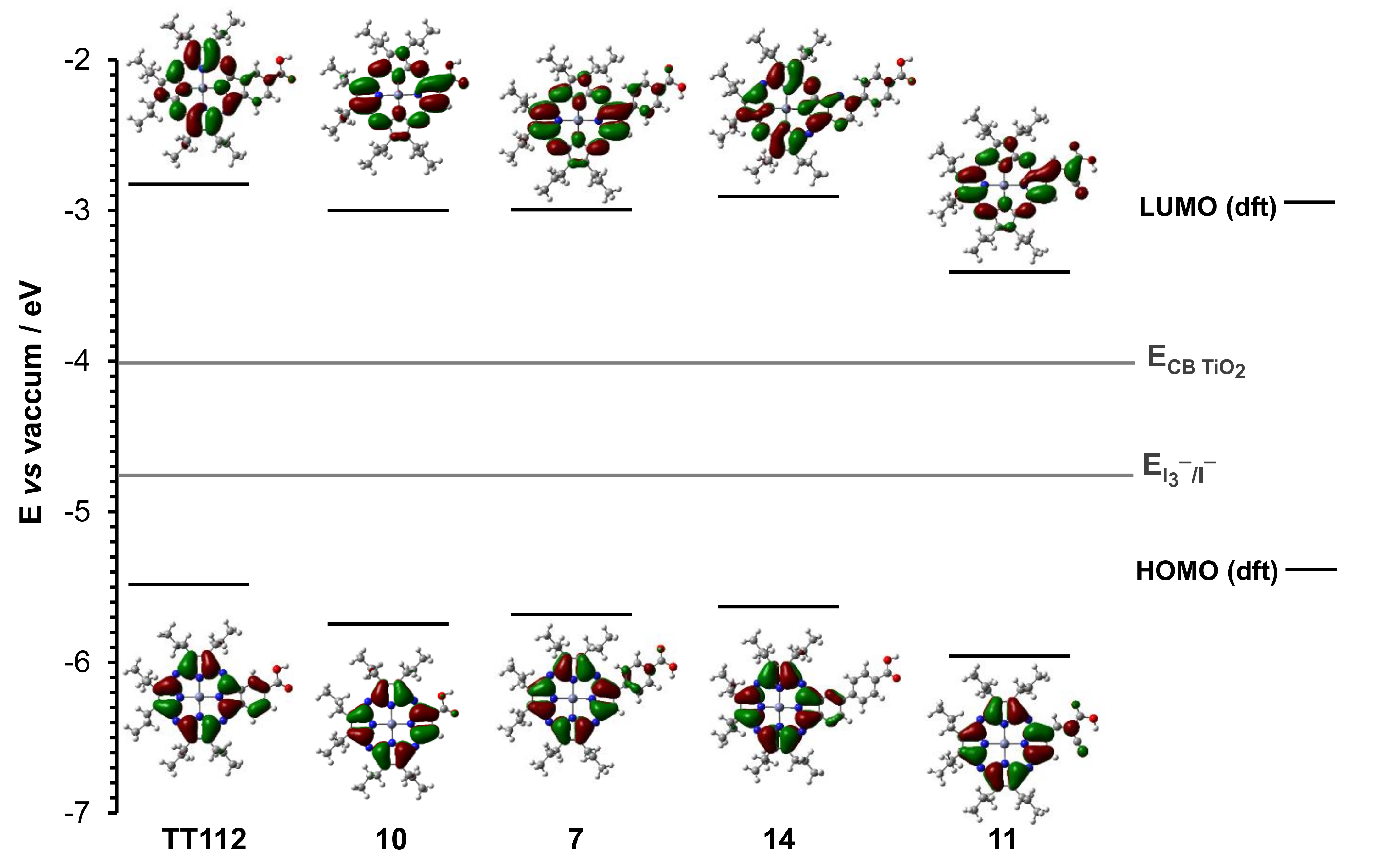
2.3. Theoretical Calculations
3. Materials and Methods
3.1. Instrumentation and Materials
3.2. Synthesis
3.2.1. Thioethylmaleonitrile (3)
3.2.2. [7,8,12,13,17,18-Hexapropyl-2-thioethylporphyrazinato]Zn(II) (5)
3.2.3. [7,8,12,13,17,18-Hexapropyl-2-(4-methoxycarbonylphenyl)porphyrazinato]Zn(II) (6)
3.2.4. [7,8,12,13,17,18-Hexapropyl-2-(4-carboxyphenyl)porphyrazinato]Zn(II) (7)
3.2.5. [7,8,12,13,17,18-Hexapropyl-2-pent-1-enylporphyrazinato]Zn(II) (8)
3.2.6. [7,8,12,13,17,18-Hexapropyl-2-formylporphyrazinato]Zn(II) (9)
3.2.7. [7,8,12,13,17,18-Hexapropyl-2-carboxyporphyrazinato]Zn(II) (10)
3.2.8. [7,8,12,13,17,18-Hexapropyl-2-(2-carboxy-2-cyanovinyl)porphyrazinato]Zn(II) (11)
3.2.9. Methyl 4-(5,6-dicyanopyrazine-2-yl)benzoate (12)
3.2.10. [7,8,12,13,17,18-Hexapropyl-(4-butoxycarbonylphenyl[2,3b]-pyrazine)porphyrazinato] Zn(II) (13)
3.2.11. [7,8,12,13,17,18-Hexapropyl-(4-carboxyphenyl[2,3b]-pyrazine)porphyrazinato]Zn(II) (14)
3.3. Device Preparation
3.4. Photovoltaic Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, N. Meso-Azaporphyrins and Their Analogues. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 2, pp. 301–360. [Google Scholar]
- Rodriguez-Morgade, M.S.; Stuzhin, P.A. The chemistry of porphyrazines: An overview. J. Porphyrins Phthalocyanines 2004, 8, 1129–1165. [Google Scholar] [CrossRef]
- Fuchter, M.J.; Zhong, C.; Zong, H.; Hoffman, B.M.; Barrett, A.G.M. Porphyrazines: Designer macrocycles by peripheral substituent change. Aust. J. Chem. 2008, 61, 235–255. [Google Scholar] [CrossRef]
- Pietrangeli, D.; Rosa, A.; Ristori, S.; Salvati, A.; Altieri, S.; Ricciardi, G. Carboranyl-porphyrazines and derivatives for boron neutron capture therapy: From synthesis to in vitro tests. Coord. Chem. Rev. 2013, 257, 2213–2231. [Google Scholar] [CrossRef]
- Rodriguez-Morgade, M.S.; Torres, T. Phthalocyanines and related compounds (update 2017). Sci. Synth. Knowl. Updates 2017, 5, 72–81. [Google Scholar]
- Kadish, K.M.; Smith, K.M.; Guilard, R. (Eds.) The Porphyrin Handbook; Academic Press: San Diego, CA, USA, 2003; Volume 15–20. [Google Scholar]
- Fukuda, T.; Kobayashi, N. UV-Visible absorption spectroscopic properties of phthalocyanines and related macrocycles. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific Publishing Co.: Singapore, 2010; Volume 9. [Google Scholar]
- Rio, Y.; Rodríguez-Morgade, M.S.; Torres, T. Modulating the electronic properties of porphyrinoids: A voyage from the violet to the infrared regions of the electromagnetic spectrum. Org. Biomol. Chem. 2008, 6, 1877–1894. [Google Scholar] [CrossRef]
- Garrido Montalban, A.; Lange, S.J.; Beall, L.S.; Mani, N.S.; Williams, D.J.; White, A.J.P.; Barrett, A.G.M.; Hoffman, B.M. Seco-Porphyrazines: Synthetic, structural, and spectroscopic investigations. J. Org. Chem. 1997, 62, 9284–9289. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, W.; Wang, K.; Jiang, J. Unprecedented Phthalocyanines Bearing Eight Di-butylamino Peripheral Substituents: Synthesis, Spectroscopy, and Structure. Inorg. Chem. 2015, 54, 9962–9967. [Google Scholar] [CrossRef]
- Garrido Montalban, A.; Jarrell, W.; Riguet, E.; McCubbin, Q.J.; Anderson, M.E.; White, A.J.P.; Williams, D.J.; Barrett, A.G.M.; Hoffman, B.M. Bis(dimethylamino)porphyrazines: Synthetic, structural, and spectroscopic investigations. J. Org. Chem. 2000, 65, 2472–2478. [Google Scholar] [CrossRef]
- Angeloni, S.; Ercolani, C. New classes of porphyrazine macrocycles with annulated heterocyclic rings. J. Porphyrins Phthalocyanines 2000, 4, 474–483. [Google Scholar] [CrossRef]
- Donzello, M.P.; Ercolani, C.; Stuzhin, P.A. Novel families of phthalocyanine-like macrocycles-Porphyrazines with annulated strongly electron-withdrawing 1,2,5-thia/selenodiazole rings. Coord. Chem. Rev. 2006, 250, 1530–1561. [Google Scholar] [CrossRef]
- Leng, F.; Wang, X.; Jin, L.; Yin, B. The synthesis and properties of unsymmetrical porphyrazines annulated with a tetrathiafulvalene bearing two tetraethylene glycol units. Dyes Pigments 2010, 87, 89–94. [Google Scholar] [CrossRef]
- Stuzhin, P.A.; Ercolani, C. Porphyrazines with annulated heterocycles. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2003; Volume 15, pp. 263–364. [Google Scholar]
- Donzello, M.P.; Ercolani, C.; Novakova, V.; Zimcik, P.; Stuzhin, P.A. Tetrapyrazinoporphyrazines and their metal derivatives. Part I: Synthesis and basic structural information. Coord. Chem. Rev. 2016, 309, 107–179. [Google Scholar] [CrossRef]
- Novakova, V.; Donzello, M.P.; Ercolani, C.; Zimcik, P.; Stuzhin, P.A. Tetrapyrazinoporphyrazines and their metal derivatives. Part II: Electronic structure, electrochemical, spectral, photophysical and other application related properties. Coord. Chem. Rev. 2018, 361, 1–73. [Google Scholar] [CrossRef]
- Gal’pern, M.G.; Luk’yanets, E.A. Phthalocyanines and related compounds. VII. Tetra-2,3-benzo[g]quinoxalinoporphyrazines. Zh. Obshch. Khim. 1971, 41, 2549–2552. [Google Scholar]
- Kobayashi, N.; Higashi, Y.; Osa, T. Planar phthalocyanine-pyrazinoporphyrazine heterodinucleates. J. Chem. Soc. Chem. Commun. 1994, 15, 1785–1786. [Google Scholar] [CrossRef]
- Garrido Montalban, A.; Meunier, H.G.; Ostler, R.B.; Barrett, A.G.M.; Hoffman, B.M.; Rumbles, G. Photoperoxidation of a diamino zinc porphyrazine to the seco-zinc porphyrazine: Suicide or murder? J. Phys. Chem. A 1999, 103, 4352–4358. [Google Scholar] [CrossRef]
- Trabanco, A.A.; Montalban, A.G.; Rumbles, G.; Barrett, A.G.M.; Hoffman, B.M. A seco-porphyrazine: Superb sensitizer for singlet oxygen generation and endoperoxide synthesis. Synlett 2000, 5, 1010–1013. [Google Scholar]
- Tasso, T.T.; Schlothauer, J.C.; Junqueira, H.C.; Matias, T.A.; Araki, K.; Liandra-Salvador, E.; Antonio, F.C.T.; Homem-de-Mello, P.; Baptista, M.S. Photobleaching efficiency parallels the enhancement of membrane damage for porphyrazine photosensitizers. J. Am. Chem. Soc. 2019, 141, 15547–15556. [Google Scholar] [CrossRef]
- Ariza, J.F.; Urbani, M.; Grätzel, M.; Rodríguez-Morgade, M.S.; Nazeeruddin, M.K.; Torres, T. An unsymmetrical, push–pull porphyrazine for dye-sensitized solar cells. ChemPhotoChem 2017, 1, 164–166. [Google Scholar] [CrossRef]
- Reddy, Y.; Giribabu, L.; Lyness, C.; Snaith, H.; Vijaykumar, C.; Chandrasekharam, M.; Lakshmikantam, M.; Yum, J.H.; Kalyanasundaram, K.; Grätzel, M.; et al. Efficient sensitization of nanocrystalline TiO2 films by a near-IR-absorbing unsymmetrical zinc phthalocyanine. Angew. Chem. Int. Ed. 2007, 119, 373–376. [Google Scholar] [CrossRef]
- Cid, J.-J.; Yum, J.-H.; Jang, S.-R.; Nazeeruddin, M.K.; Martinez-Ferrero, E.; Palomares, E.; Ko, J.; Grätzel, M.; Torres, T. Molecular cosensitization for efficient panchromatic dye-sensitized solar cells. Angew. Chem. Int. Ed. 2007, 46, 8358–8362. [Google Scholar] [CrossRef] [PubMed]
- Higashino, T.; Rodríguez-Morgade, M.S.; Osuka, A.; Torres, T. Peripheral arylation of Subporphyrazines. Chem. Eur. J. 2013, 19, 10353–10359. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ariza, F.; Urbani, M.; Rodríguez-Morgade, M.S.; Torres, T. Panchromatic photosensitizers based on push–pull, unsymmetrically substituted Porphyrazines. Chem. Eur. J. 2018, 24, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.-P.; Papadopoulos, I.; Lavarda, G.; Gotfredsen, H.; Rami, P.R.; Tykwinski, R.R.; Rodríguez-Morgade, M.S.; Guldi, D.M.; Torres, T. Light-harvesting porphyrazines to enable intramolecular singlet fission. Nanoscale 2019, 11, 22286–22292. [Google Scholar] [CrossRef]
- Yella, A.; Mai, C.L.; Zakeeruddin, S.M.; Chang, S.N.; Hsieh, C.H.; Yeh, C.Y.; Grätzel, M. Molecular engineering of push-pull porphyrin dyes for highly efficient dye-sensitized solar cells: The role of benzene spacers. Angew. Chem. Int. Ed. 2014, 53, 2973–2977. [Google Scholar] [CrossRef]
- Lu, J.; Liu, S.; Wang, M. Push-pull zinc porphyrins as light-harvesters for efficient dye-sensitized solar cells. Front. Chem. 2018, 6, 541. [Google Scholar] [CrossRef]
- Nkhahle, R.; Nyokong, T. Creating the ideal push-pull system for electrocatalysis: A comparative study on symmetrical and asymmetrical cardanol-based cobalt Phthalocyanines. Electroanalysis 2021, 33, 11–22. [Google Scholar] [CrossRef]
- Fuse, S.; Takizawa, M.; Matsumura, K.; Sato, S.; Okazaki, S.; Nakamura, H. Thiophene-based organic D–π–A dyes as potent sensitizers for photodynamic therapy. Eur. J. Org. Chem. 2017, 2017, 5170–5177. [Google Scholar] [CrossRef]
- Veliz Montes, C.; Memczak, H.; Gyssels, E.; Torres, T.; Madder, A.; Schneider, R.J. Photoinduced cross-linking of short furan-modified DNA on surfaces. Langmuir 2017, 33, 1197–1201. [Google Scholar] [CrossRef]
- Kimura, M.; Suzuki, H.; Tohata, Y.; Ikeuchi, T.; Yamamoto, S.; Kobayashi, N. Carbazole-fused zinc(II)–Phthalocyanine sensitizers. Asian J. Org. Chem. 2017, 6, 544–550. [Google Scholar] [CrossRef]
- Guo, Y.; Song, S.; Zheng, Y.; Li, R.; Peng, T. Synthesis and characterization of an A2BC type phthalocyanine and its visible-light-responsive photocatalytic H2 production performance on graphitic carbon nitride. Dalton Trans. 2016, 45, 14071–14079. [Google Scholar] [CrossRef]
- Llamas, E.M.; Tome, J.P.C.; Rodrigues, J.M.M.; Torres, T.; Madder, A. Porphyrin-based photosensitizers and their DNA conjugates for singlet oxygen induced nucleic acid interstrand crosslinking. Org. Biomol. Chem. 2017, 15, 5402–5409. [Google Scholar] [CrossRef]
- Belviso, S.; Santoro, E.; Penconi, M.; Righetto, S.; Tessore, F. Thioethyl porphyrazines: Attractive chromophores for second-order nonlinear optics and DSSCs. J. Phys. Chem. C 2019, 123, 13074–13082. [Google Scholar] [CrossRef]
- Liebeskind, L.S.; Srogl, J. Thiol ester—boronic acid coupling. A mechanistically unprecedented and general ketone synthesis. J. Am. Chem. Soc. 2000, 122, 11260–11261. [Google Scholar] [CrossRef]
- Arroyo, I.J.; Hu, R.; Merino, G.; Tang, B.Z.; Peña-Cabrera, E. The smallest and one of the brightest. efficient preparation and optical description of the parent borondipyrromethene system. J. Org. Chem. 2009, 74, 5719–5722. [Google Scholar] [CrossRef]
- Dai, H.-F.; Chen, W.-X.; Zhao, L.; Xiong, F.; Sheng, H.; Chen, F.-E. Synthetic studies on (+)-biotin, part 11: Application of cinchona alkaloid-mediated asymmetric alcoholysis of meso-cyclic anhydride in the total synthesis of (+)-Biotin. Adv. Synth. Catal. 2008, 350, 1635–1641. [Google Scholar] [CrossRef]
- Mori, Y.; Seki, M. Synthesis of multi-functionalized ketones through the Fukuyama coupling reaction catalyzed by Pearlman’s catalyst: Preparation of ethyl 6-oxotridecanoate. Org. Synth. 2007, 84, 285. [Google Scholar]
- Mori, Y.; Seki, M. A Practical synthesis of multifunctional ketones through the Fukuyama coupling reaction. Adv. Synth. Catal. 2007, 349, 2027–2038. [Google Scholar] [CrossRef]
- Fukuyama, T.; Toyukama, H. Palladium-mediated synthesis of aldehydes and ketones from thiol esters. Aldrichim. Acta 2004, 37, 87–96. [Google Scholar] [CrossRef]
- Gouloumis, A.; Liu, S.-G.; Sastre, A.; Vázquez, P.; Echegoyen, L.; Torres, T. Synthesis and electrochemical properties of phthalocyanine–fullerene hybrids. Chem. Eur. J. 2000, 6, 3600–3607. [Google Scholar]
- Luo, J.H.; Li, Q.S.; Yang, L.N.; Sun, Z.Z.; Li, Z.S. Theoretical design of porphyrazine derivatives as promising sensitizers for dye-sensitized solar cells. RSC Adv. 2014, 4, 20200–20207. [Google Scholar] [CrossRef]
- Urbani, M.; Ragoussi, M.E.; Nazeeruddin, M.K.; Torres, T. Phthalocyanines for dye-sensitized solar cells. Coord. Chem. Rev. 2019, 381, 1–64. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, Inc., Wallingford, CT, USA. 2016. Available online: https://gaussian.com/citation/ (accessed on 1 April 2021).
- Theisen, R.F.; Huang, L.; Fleetham, T.; Adams, J.B.; Li, J. Ground and excited states of zinc phthalocyanine, zinc tetrabenzoporphyrin, and azaporphyrin analogs using DFT and TDDFT with Franck-Condon analysis. J. Chem. Phys. 2015, 142, 094310. [Google Scholar] [CrossRef]
- Fischer, S.A.; Cramer, C.J.; Govind, N. Excited-State Absorption from Real-Time Time-Dependent Density Functional Theory: Optical Limiting in Zinc Phthalocyanine. J. Phys. Chem. Lett. 2016, 7, 1387–1391. [Google Scholar] [CrossRef]
- Covezzi, A.; Orbelli Biroli, A.; Tessore, F.; Forni, A.; Marinotto, D.; Biagini, P.; Di Carlo, G.; Pizzottia, M. 4D–π–1A type β-substituted ZnII-porphyrins: Ideal green sensitizers for building-integrated photovoltaics. Chem. Commun. 2016, 52, 12642–12645. [Google Scholar] [CrossRef]
- Guo, L.; Ellis, D.E.; Hoffman, B.M.; Ishikawa, Y. Ligand Substitution Effect on Electronic Structure and Optical Properties of Nickel, Porphyrazines. Inorg. Chem. 1996, 35, 5304–5312. [Google Scholar] [CrossRef]
- Doppelt, P.; Huille, S. Mesogenic octakis(octylthio)-tetraazametalloporphyrins. New J. Chem. 1990, 14, 607–609. [Google Scholar]
- Lange, S.J.; Nie, H.; Stern, C.L.; Barrett, A.G.M.; Hoffman, B.M. Peripheral palladium(II) and platinum(II) complexes of bis(dimethylamino)porphyrazine. Inorg. Chem. 1998, 37, 6435–6443. [Google Scholar] [CrossRef]
- Medina, P. Fotovoltaica Molecular: Una de las Respuestas a la Problemática Energética y Ambiental, in Biogás: Nanotecnología, Beneficios y Producción; González, E.E., Forero, E., Eds.; ACCEFYN, Editorial Gente Nueva: Bogotá, Colombia, 2021; pp. 284–300. [Google Scholar]
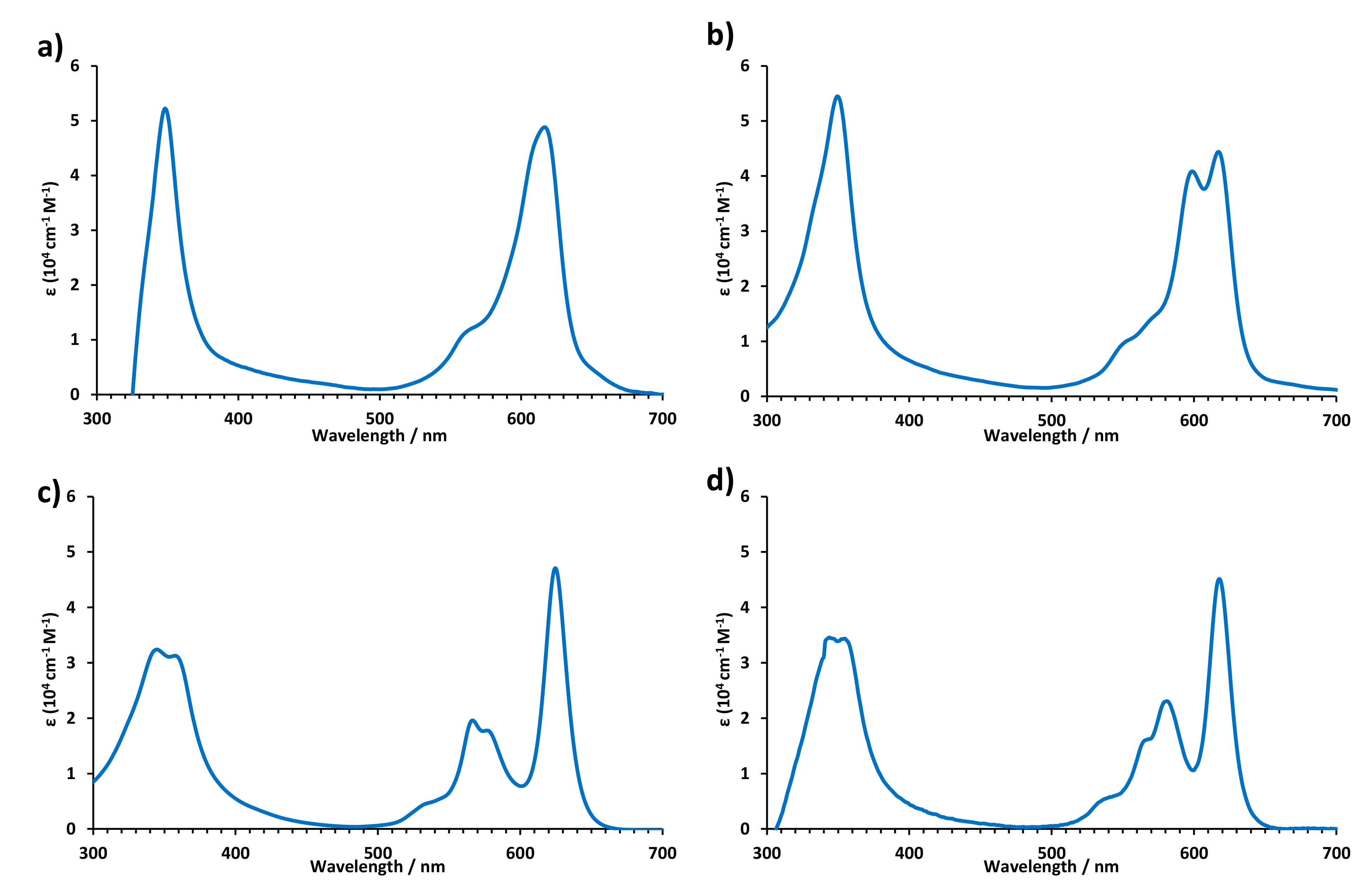
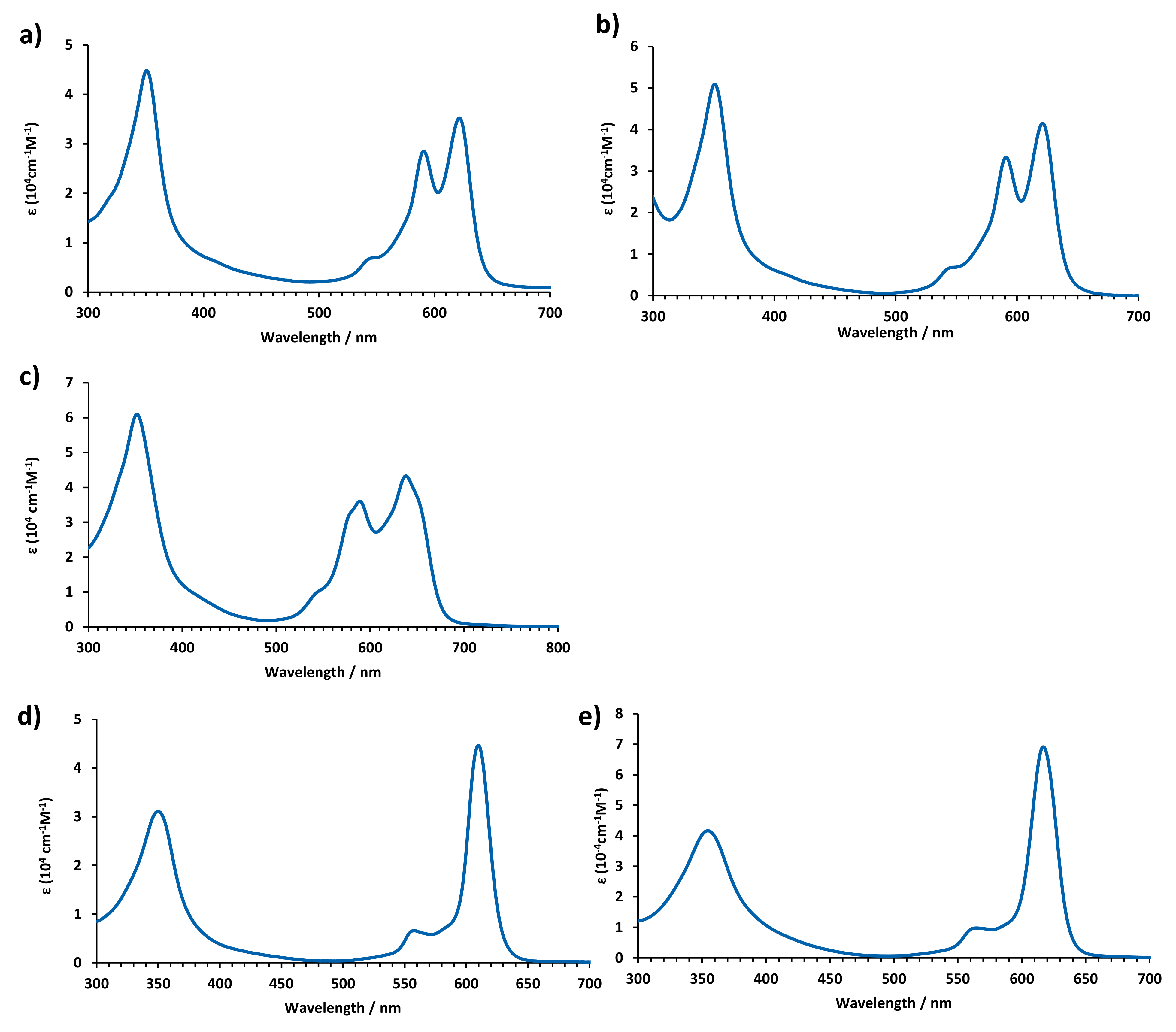
| Pz | Absorption λ (nm) (ε [104 M−1cm−1]) |
|---|---|
| TT112 [23] | 615 (5.07), 560 (4.25), 346 (4.78) |
| 5 | 619 (4.69), 567 (sh), 350 (4.72). |
| 6 | 622 (4.5), 591 (4.4), 544 (3.8), 415, (sh), 351 (4.6) |
| 7 | 622 (4.6), 591 (4.5), 544 (3.8), 414 (sh), 351 (4.7) |
| 8 | 617 (4.64), 599 (4.60), 570 (sh), 555 (sh), 350 (4.73) |
| 9 | 625 (4.67), 577 (4.25), 567 (sh), 539 (4.29), 358 (4.50), 345 (4.51) |
| 10 | 618 (4.65), 581 (4.36), 570 (sh), 544 (3.83), 355 (4.54), 344 (4.54) |
| 11 | 654 (sh), 638 (4.6), 589 (4.5), 578 (sh), 541 (sh), 352 (4.8) |
| 13 | 610 (4.6), 582 (sh), 558 (3.8), 350 (4.5). |
| 14 | 616 (4.8), 564 (4.0) 354 (4.6) |
| Pz | HOMO (eV vs. Vac.) | LUMO (eV vs. Vac) |
|---|---|---|
| TT112 | −5.48 | −2.81 |
| 10 | −5.74 | −3.02 |
| 7 | −5.69 | −3.00 |
| 14 | −5.62 | −2.91 |
| 11 | −5.96 | −3.41 |
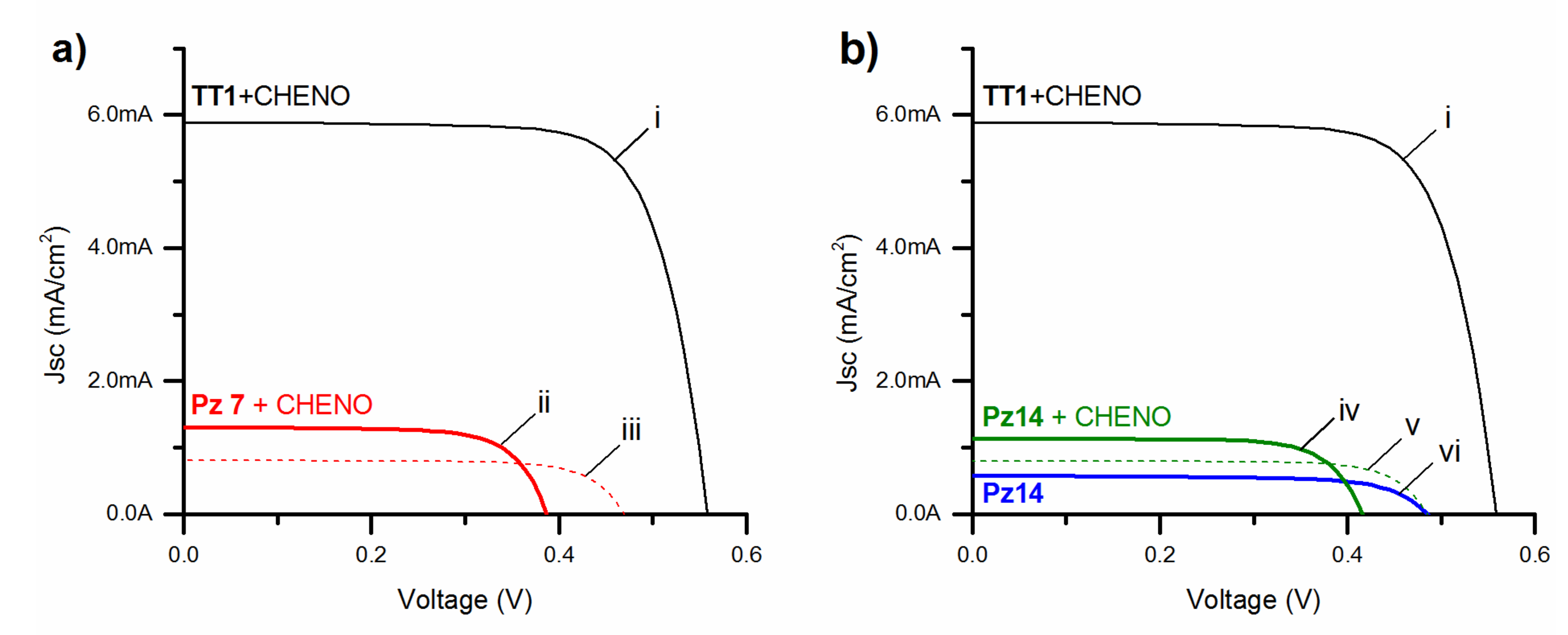
| Entry | Dye a (0.1 mM) | [CHENO] b (mM) | Elect. c (LiI Conc.) | JSC (mA∙cm−2) | VOC (mV) | F.F. (%) | Pin (mW∙cm−2) | η (%) |
|---|---|---|---|---|---|---|---|---|
| (i) | TT1 | 10 | A (0.1 M) | 5.89 | 558 | 74.6 | 88.9 | 2.76 |
| (ii) | 7 | 10 | B (0.5 M) | 1.31 | 387 | 71.3 | 89.1 | 0.41 |
| (iii) | 7 | 10 | A (0.1 M) | 0.806 | 470 | 74.3 | 88.7 | 0.32 |
| (iv) | 14 | 10 | B (0.5 M) | 1.13 | 416 | 73.6 | 89.1 | 0.39 |
| (v) | 14 | 10 | A (0.1 M) | 0.797 | 485 | 75.2 | 88.9 | 0.33 |
| (vi) | 14 | 0 | A (0.1 M) | 0.579 | 485 | 69.8 | 88.9 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, D.-P.; Fernández-Ariza, J.; Urbani, M.; Sauvage, F.; Torres, T.; Rodríguez-Morgade, M.S. Tuning the Acceptor Unit of Push–Pull Porphyrazines for Dye-Sensitized Solar Cells. Molecules 2021, 26, 2129. https://doi.org/10.3390/molecules26082129
Medina D-P, Fernández-Ariza J, Urbani M, Sauvage F, Torres T, Rodríguez-Morgade MS. Tuning the Acceptor Unit of Push–Pull Porphyrazines for Dye-Sensitized Solar Cells. Molecules. 2021; 26(8):2129. https://doi.org/10.3390/molecules26082129
Chicago/Turabian StyleMedina, Diana-Paola, Javier Fernández-Ariza, Maxence Urbani, Frédéric Sauvage, Tomás Torres, and M. Salomé Rodríguez-Morgade. 2021. "Tuning the Acceptor Unit of Push–Pull Porphyrazines for Dye-Sensitized Solar Cells" Molecules 26, no. 8: 2129. https://doi.org/10.3390/molecules26082129
APA StyleMedina, D.-P., Fernández-Ariza, J., Urbani, M., Sauvage, F., Torres, T., & Rodríguez-Morgade, M. S. (2021). Tuning the Acceptor Unit of Push–Pull Porphyrazines for Dye-Sensitized Solar Cells. Molecules, 26(8), 2129. https://doi.org/10.3390/molecules26082129








