1,3,4-Oxadiazole N-Mannich Bases: Synthesis, Antimicrobial, and Anti-Proliferative Activities
Abstract
1. Introduction
2. Results and Discussion
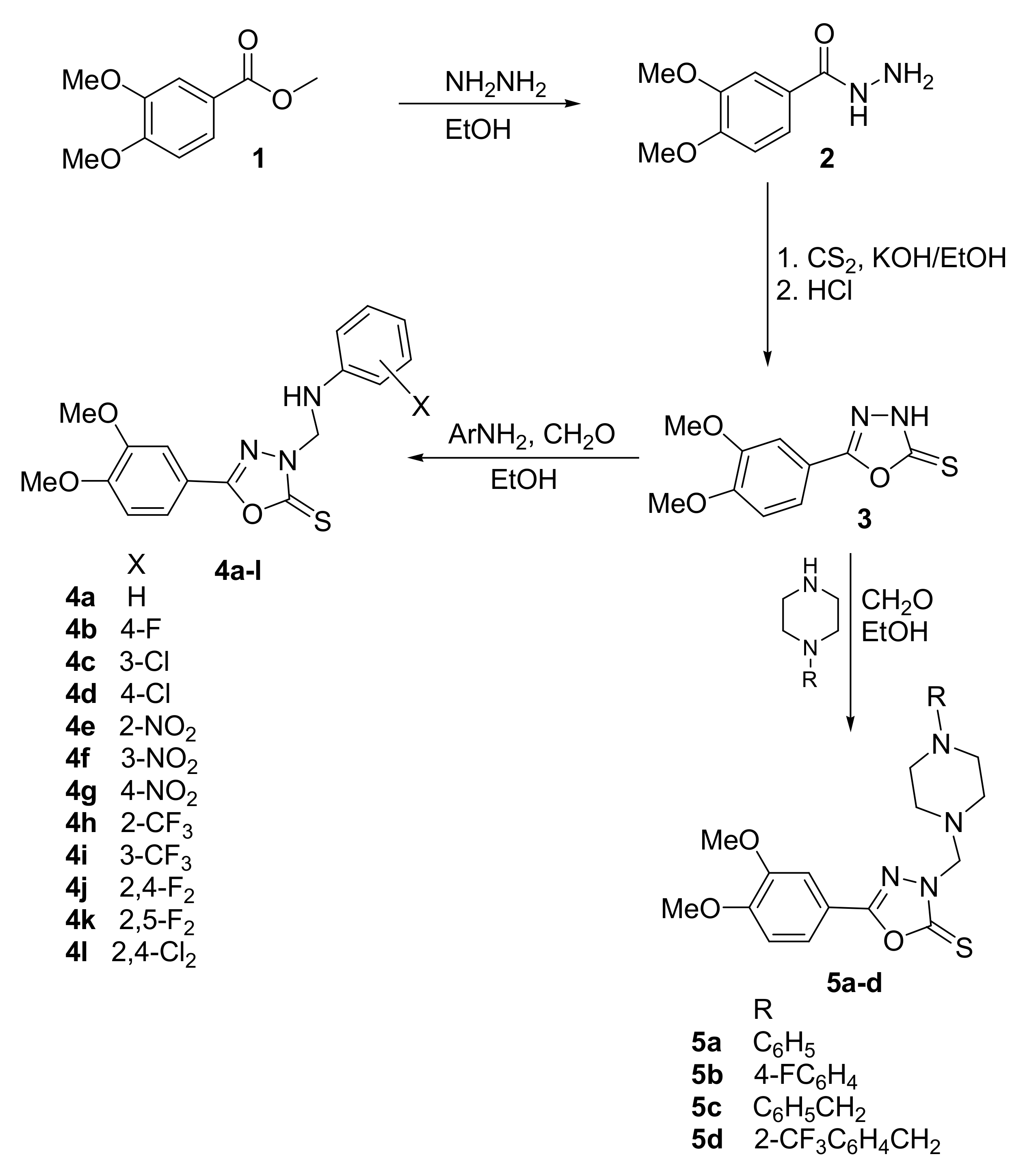
2.1. Chemical Synthesis
2.2. In Vitro Antibacterial and Antifungal Activities
2.3. In Vitro Anti-proliferative Activity
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 3-(Arylaminomethyl)-5-(3,4-Dimethoxyphenyl)-1,3,4-Oxadiazole-2(3H)-Thiones 4a–l and 3-[(4-Substituted Piperazin-1-yl)Methyl]-5-(3,4-Dimethoxyphenyl}-1,3,4-Oxadiazole- 2(3H)-Thiones 5a–d
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Verma, G.; Khan, M.F.; Akhtar, W.; Alam, M.M.; Akhter, M.; Shaquiquzzaman, M. A Review Exploring Therapeutic Worth of 1,3,4-Oxadiazole Tailored Compounds. Mini-Reviews Med. Chem. 2019, 19, 477–509. [Google Scholar] [CrossRef]
- De Oliveira, C.S.; Lira, B.F.; Barbosa-Filho, J.M.; Lorenzo, J.G.F.; De Athayde-Filho, P.F. Synthetic Approaches and Pharmacological Activity of 1,3,4-Oxadiazoles: A Review of the Literature from 2000–2012. Molecules 2012, 17, 10192–10231. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Pati, A.; Patra, M.; Behera, R.K.; Behera, A.K. Acid Hydrazides, Potent Reagents for Synthesis of Oxygen-, Nitrogen-, and/or Sulfur-Containing Heterocyclic Rings. Chem. Rev. 2014, 114, 2942–2977. [Google Scholar] [CrossRef] [PubMed]
- Ogata, M.; Atobe, H.; Kushida, H.; Yamamoto, K. In Vitro Sensitivity of Mycoplasmas Isolated from Various Animals and Sewage to Antibiotics and Nitrofurans. J. Antibiot. 1971, 24, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Summa, V.; Petrocchi, A.; Bonelli, F.; Crescenzi, B.; Donghi, M.; Ferrara, M.; Fiore, F.; Gardelli, C.; Paz, O.G.; Hazuda, D.J.; et al. Discovery of Raltegravir, a Potent, Selective Orally Bioavailable HIV-Integrase Inhibitor for the Treatment of HIV-AIDS Infection. J. Med. Chem. 2008, 51, 5843–5855. [Google Scholar] [CrossRef]
- Shepard, D.R.; Dreicer, R. Zibotentan for the treatment of castrate-resistant prostate cancer. Expert Opin. Investig. Drugs 2010, 19, 899–908. [Google Scholar] [CrossRef] [PubMed]
- McCoull, W.; Addie, M.S.; Birch, A.M.; Birtles, S.; Buckett, L.K.; Butlin, R.J.; Bowker, S.S.; Boyd, S.; Chapman, S.; Davies, R.D.; et al. Identification, optimisation and in vivo evaluation of oxadiazole DGAT-1 inhibitors for the treatment of obesity and diabetes. Bioorganic Med. Chem. Lett. 2012, 22, 3873–3878. [Google Scholar] [CrossRef]
- Vardan, S.; Smulyan, H.; Mookherjee, S.; Eich, R. Effects of tiodazosin, a new antihypertensive, hemodynamics and clinical variables. Clin. Pharmacol. Ther. 1983, 34, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Schlecker, R.; Thieme, P.C. The synthesis of antihypertensive 3-(1,3,4-oxadiazol-2-yl)phenoxypropanolahines. Tetrahedron 1988, 44, 3289–3294. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, Q.; Kim, W.; Tharmalingam, N.; Fuchs, B.B.; Mylonakis, E. Antimicrobial activity of 1,3,4-oxadiazole derivatives against planktonic cells and biofilm of Staphylococcus aureus. Futur. Med. Chem. 2018, 10, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Bhatt, N.; Somani, H.; Trivedi, A. Synthesis, antimicrobial and cytotoxic activities of some novel thiazole clubbed 1,3,4-oxadiazoles. Eur. J. Med. Chem. 2013, 67, 54–59. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Wei, C.; Wu, S.; Wu, R.; Wang, S.; Hu, D.; Song, B. Novel sulfone derivatives containing a 1,3,4-oxadiazole moiety: Design and synthesis based on the 3D-QSAR model as potential antibacterial agent. Pest Manag. Sci. 2020, 76, 3188–3198. [Google Scholar] [CrossRef]
- Xu, H.; Jia, A.; Hou, E.; Liu, Z.; Yang, R.; Yang, R.; Guo, Y. Natural Product-Based Fungicides Discovery: Design, Synthesis and Antifungal Activities of Some Sarisan Analogs Containing 1,3,4-Oxadiazole Moieties. Chem. Biodivers. 2019, 17, e1900570. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Shao, W.-B.; Zhu, J.-J.; Long, Z.-Q.; Liu, L.-W.; Wang, P.-Y.; Li, Z.; Yang, S. Novel 1,3,4-Oxadiazole-2-carbohydrazides as Prospective Agricultural Antifungal Agents Potentially Targeting Succinate Dehydrogenase. J. Agric. Food Chem. 2019, 67, 13892–13903. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.Y.; Ahmad, A.; Shiekh, R.A.; Al-Ghamdi, K.J.; Sobral, A.J. Imidazole clubbed 1,3,4-oxadiazole derivatives as potential antifungal agents. Bioorganic Med. Chem. 2015, 23, 4172–4180. [Google Scholar] [CrossRef]
- Sonawane, A.D.; Rode, N.D.; Nawale, L.; Joshi, R.R.; Joshi, R.A.; Likhite, A.P.; Sarkar, D. Synthesis and biological evaluation of 1,2,4-triazole-3-thione and 1,3,4-oxadiazole-2-thione as antimycobacterial agents. Chem. Biol. Drug Des. 2017, 90, 200–209. [Google Scholar] [CrossRef]
- Desai, N.; Somani, H.; Trivedi, A.; Bhatt, K.; Nawale, L.; Khedkar, V.M.; Jha, P.C.; Sarkar, D. Synthesis, biological evaluation and molecular docking study of some novel indole and pyridine based 1,3,4-oxadiazole derivatives as potential antitubercular agents. Bioorganic Med. Chem. Lett. 2016, 26, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, F.; Eydoux, C.; Querat, G.; de Lamballerie, X.; Canard, B.; Alvarez, K.; Guillemot, J.-C.; Barral, K. Novel 2-phenyl-5-[(E)-2-(thiophen-2-yl)ethenyl]-1,3,4-oxadiazole and 3-phenyl-5-[(E)-2-(thiophen-2-yl)ethenyl]-1,2,4-oxadiazole derivatives as dengue virus inhibitors targeting NS5 polymerase. Eur. J. Med. Chem. 2016, 109, 146–156. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Q.; Tai, A.; Jiang, G.; Ouyang, G. Synthesis and antiviral activity of 2-substituted methylthio-5-(4-amino-2-methylpyrimidin-5-yl)-1,3,4-oxadiazole derivatives. Bioorganic Med. Chem. Lett. 2015, 25, 2243–2246. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Dou, D.; Aravapalli, S.; Teramoto, T.; Lushington, G.H.; Mwania, T.M.; Alliston, K.R.; Eichhorn, D.M.; Padmanabhan, R.; Groutas, W.C. Design, synthesis and characterization of novel 1,2-benzisothiazol-3(2H)-one and 1,3,4-oxadiazole hybrid derivatives: Potent inhibitors of Dengue and West Nile virus NS2B/NS3 proteases. Bioorganic Med. Chem. 2013, 21, 102–113. [Google Scholar] [CrossRef]
- Benassi, A.; Doria, F.; Pirota, V. Groundbreaking Anticancer Activity of Highly Diversified Oxadiazole Scaffolds. Int. J. Mol. Sci. 2020, 21, 8692. [Google Scholar] [CrossRef] [PubMed]
- Glomb, T.; Szymankiewicz, K.; Świątek, P. Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole. Molecules 2018, 23, 3361. [Google Scholar] [CrossRef]
- Akhtar, J.; Siddiqui, A.A.; Khan, A.A.; Ali, Z.; Dewangan, R.P.; Pasha, S.; Yar, M.S. Design, synthesis, docking and QSAR study of substituted benzimidazole linked oxadiazole as cytotoxic agents, EGFR and erbB2 receptor inhibitors. Eur. J. Med. Chem. 2017, 126, 853–869. [Google Scholar] [CrossRef]
- Ruel, R.; Thibeault, C.; L’Heureux, A.; Martel, A.; Cai, Z.-W.; Wei, D.; Qian, L.; Barrish, J.C.; Mathur, A.; D’Arienzo, C.; et al. Discovery and preclinical studies of 5-isopropyl-6-(5-methyl-1,3,4-oxadiazol-2-yl)-N-(2-methyl-1H-pyrrolo[2,3-b]pyridin-5-yl)pyrrolo[2,1-f][1,2,4]triazin-4-amine (BMS-645737), an in vivo active potent VEGFR-2 inhibitor. Bioorganic Med. Chem. Lett. 2008, 18, 2985–2989. [Google Scholar] [CrossRef]
- Altıntop, M.D.; Sever, B.; Çiftçi, G.A.; Turan-Zitouni, G.; Kaplancıklı, Z.A.; Özdemir, A. Design, synthesis, in vitro and in silico evaluation of a new series of oxadiazole-based anticancer agents as potential Akt and FAK inhibitors. Eur. J. Med. Chem. 2018, 155, 905–924. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ren, S.-Z.; Lu, X.-Y.; Li, J.-J.; Shen, F.-Q.; Xu, C.; Zhu, H.-L. Discovery of a series of 1,3,4-oxadiazole-2(3 H )-thione derivatives containing piperazine skeleton as potential FAK inhibitors. Bioorganic Med. Chem. 2017, 25, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Valente, S.; Trisciuoglio, D.; De Luca, T.; Nebbioso, A.; Labella, D.; Lenoci, A.; Bigogno, C.; Dondio, G.; Miceli, M.; Brosch, G.; et al. 1,3,4-Oxadiazole-Containing Histone Deacetylase Inhibitors: Anticancer Activities in Cancer Cells. J. Med. Chem. 2014, 57, 6259–6265. [Google Scholar] [CrossRef]
- Pidugu, V.R.; Yarla, N.S.; Bishayee, A.; Kalle, A.M.; Satya, A.K. Novel histone deacetylase 8-selective inhibitor 1,3,4-oxadiazole-alanine hybrid induces apoptosis in breast cancer cells. Apoptosis 2017, 22, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, M.-H.; Qian, S.-S.; Guo, F.-J.; Dang, X.-F.; Wang, X.-M.; Xue, Y.-R.; Zhu, H.-L. Synthesis and antitumor activity of 1,3,4-oxadiazole possessing 1,4-benzodioxan moiety as a novel class of potent methionine aminopeptidase type II inhibitors. Bioorganic Med. Chem. Lett. 2013, 23, 2876–2879. [Google Scholar] [CrossRef]
- Mohan, C.D.; Anilkumar, N.C.; Rangappa, S.; Shanmugam, M.K.; Mishra, S.; Chinnathambi, A.; Alharbi, S.A.; Bhattacharjee, A.; Sethi, G.; Kumar, A.P.; et al. Novel 1,3,4-Oxadiazole Induces Anticancer Activity by Targeting NF-κB in Hepatocellular Carcinoma Cells. Front. Oncol. 2018, 8, 42. [Google Scholar] [CrossRef]
- Yadav, N.; Kumar, P.; Chhikara, A.; Chopra, M. Development of 1,3,4-oxadiazole thione based novel anticancer agents: Design, synthesis and in-vitro studies. Biomed. Pharmacother. 2017, 95, 721–730. [Google Scholar] [CrossRef]
- Ullah, H.; Rahim, F.; Taha, M.; Uddin, I.; Wadood, A.; Shah, S.A.A.; Farooq, R.K.; Nawaz, M.; Wahab, Z.; Khan, K.M. Synthesis, molecular docking study and in vitro thymidine phosphorylase inhibitory potential of oxadiazole derivatives. Bioorganic Chem. 2018, 78, 58–67. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, H.; Yang, Z.-M.; Zhu, H.-L. Synthesis, molecular modeling and biological evaluation of 2-aminomethyl-5-(quinolin-2-yl)-1,3,4-oxadiazole-2(3H)-thione quinolone derivatives as novel anticancer agent. Eur. J. Med. Chem. 2013, 60, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.-R.; Li, D.-D.; Pi, Y.-Z.; Li, J.-R.; Sun, J.; Fang, F.; Zhong, W.-Q.; Gong, H.-B.; Zhu, H.-L. Novel 1,3,4-oxadiazole thioether derivatives targeting thymidylate synthase as dual anticancer/antimicrobial agents. Bioorganic Med. Chem. 2013, 21, 2286–2297. [Google Scholar] [CrossRef]
- Haibara, H.; Yamazaki, R.; Nishiyama, Y.; Ono, M.; Kobayashi, T.; Hokkyo-Itagaki, A.; Nishisaka, F.; Nishiyama, H.; Kurita, A.; Matsuzaki, T.; et al. YPC-21661 and YPC-22026, novel small molecules, inhibit ZNF143 activityin vitroandin vivo. Cancer Sci. 2017, 108, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, M.; Metwally, K.A.; Gamal-Eldeen, A.M.; Aly, O.M. 1,3,4-oxadiazole-2-thione Derivatives; Novel Approach for Anticancer and Tubulin Polymerization Inhibitory Activities. Anti-Cancer Agents Med. Chem. 2015, 16, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Ozyazici, T.; Gurdal, E.E.; Orak, D.; Sipahi, H.; Ercetin, T.; Gulcan, H.O.; Koksal, M. Synthesis, anti-inflammatory activity, and molecular docking studies of some novel Mannich bases of the 1,3,4-oxadiazole-2(3 H )-thione scaffold. Arch. der Pharm. 2020, 353, e2000061. [Google Scholar] [CrossRef] [PubMed]
- Koksal, M.; Ozkan-Dagliyan, I.; Ozyazici, T.; Kadioglu, B.; Sipahi, H.; Bozkurt, A.; Bilge, S.S. Some Novel Mannich Bases of 5-(3,4-Dichlorophenyl)-1,3,4-oxadiazole-2(3H )-one and Their Anti-Inflammatory Activity. Arch. der Pharm. 2017, 350, 1700153. [Google Scholar] [CrossRef] [PubMed]
- Dewangan, D.; Nakhate, K.; Tripathi, D.; Kashyap, P.; Dhongde, H. Synthesis, Characterization and Screening for Analgesic and Anti-inflammatory activities of 2, 5-disubstituted 1, 3, 4-oxadiazole derivatives. Anti-Inflammatory Anti-Allergy Agents Med. Chem. 2015, 14, 138–145. [Google Scholar] [CrossRef]
- Ma, L.; Xiao, Y.; Li, C.; Xie, Z.-L.; Li, D.-D.; Wang, Y.-T.; Ma, H.-T.; Zhu, H.-L.; Wang, M.-H.; Ye, Y.-H. Synthesis and antioxidant activity of novel Mannich base of 1,3,4-oxadiazole derivatives possessing 1,4-benzodioxan. Bioorganic Med. Chem. 2013, 21, 6763–6770. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Imran, S.; Wadood, A.; Rahim, F.; Saad, S.M.; Khan, K.M.; Nasir, A. Synthesis, molecular docking and α-glucosidase inhibition of 5-aryl-2-(6′-nitrobenzofuran-2′-yl)-1,3,4-oxadiazoles. Bioorganic Chem. 2016, 66, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.-R.; He, H.-B.; Yang, L.-L.; Gao, L.-X.; Chang, L.; Tang, J.; Li, J.-Y.; Li, J.; Yang, F. Synthesis and structure-activity relationship of non-phosphorus-based fructose-1,6-bisphosphatase inhibitors: 2,5-Diphenyl-1,3,4-oxadiazoles. Eur. J. Med. Chem. 2014, 83, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, E.; Alcaro, S.; Cirilli, R.; Vigo, S.; Cardia, M.C.; Sanna, M.L.; Meleddu, R.; Yanez, M.; Costa, G.; Casu, L.; et al. 3-Acetyl-2,5-diaryl-2,3-dihydro-1,3,4-oxadiazoles: A New Scaffold for the Selective Inhibition of Monoamine Oxidase B. J. Med. Chem. 2011, 54, 6394–6398. [Google Scholar] [CrossRef]
- Distinto, S.; Meleddu, R.; Yanez, M.; Cirilli, R.; Bianco, G.; Sanna, M.L.; Arridu, A.; Cossu, P.; Cottiglia, F.; Faggi, C.; et al. Drug design, synthesis, in vitro and in silico evaluation of selective monoaminoxidase B inhibitors based on 3-acetyl-2-dichlorophenyl-5-aryl-2,3-dihydro-1,3,4-oxadiazole chemical scaffold. Eur. J. Med. Chem. 2016, 108, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, L.; Hua, Y.; Wang, C.; Batsanov, A.S.; Bryce, M.R. A carbazole-oxadiazole diad molecule for single-emitting-component white organic light-emitting devices (WOLEDs). Tetrahedron 2014, 70, 2015–2019. [Google Scholar] [CrossRef][Green Version]
- Kamtekar, K.T.; Monkman, A.P.; Bryce, M.R. Recent Advances in White Organic Light-Emitting Materials and Devices (WOLEDs). Adv. Mater. 2010, 22, 572–582. [Google Scholar] [CrossRef]
- Anticancer Research. Anticancer. Res. 2018, 40, 5035–5041. [CrossRef]
- Karaaslan, C.; Duydu, Y.; Ustundag, A.; Yalcin, C.O.; Kaskatepe, B.; Goker, H. Synthesis & Anticancer Evaluation of New Substituted 2-(3,4- Dimethoxyphenyl)benzazoles. Med. Chem. 2019, 15, 287–297. [Google Scholar] [CrossRef]
- Chitti, S.; Singireddi, S.; Reddy, P.S.K.; Trivedi, P.; Bobde, Y.; Kumar, C.; Rangan, K.; Ghosh, B.; Sekhar, K.V.G.C. Design, synthesis and biological evaluation of 2-(3,4-dimethoxyphenyl)-6 (1,2,3,6-tetrahydropyridin-4-yl)imidazo[1,2-a]pyridine analogues as antiproliferative agents. Bioorganic Med. Chem. Lett. 2019, 29, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.S.; Tiong, K.H.; Muruhadas, A.; Randhawa, N.; Choo, H.L.; Bradshaw, T.D.; Stevens, M.F.; Leong, C.-O. CYP2S1 and CYP2W1 Mediate 2-(3,4-Dimethoxyphenyl)-5-Fluorobenzothiazole (GW-610, NSC 721648) Sensitivity in Breast and Colorectal Cancer Cells. Mol. Cancer Ther. 2011, 10, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Aly, O.M.; Beshr, E.A.; Maklad, R.M.; Mustafa, M.; Gamal-Eldeen, A. Synthesis, Cytotoxicity, Docking Study, and Tubulin Polymerization Inhibitory Activity of Novel 1-(3,4-Dimethoxyphenyl)-5-(3,4,5-trimethoxyphenyl)-1 H -1,2,4-triazole-3-carboxanilides. Arch. der Pharm. 2014, 347, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Alsaid, M.S.; Nissan, Y.M.; Ashour, A.E.; Al-Mishari, A.A.; Kumar, A.; Ahmed, S.F. Novel Sulfonamide Derivatives Carrying a Biologically Active 3,4-Dimethoxyphenyl Moiety as VEGFR-2 Inhibitors. Chem. Pharm. Bull. 2016, 64, 1747–1754. [Google Scholar] [CrossRef]
- Bansal, S.; Sinha, D.; Singh, M.; Cheng, B.; Tse-Dinh, Y.-C.; Tandon, V. 3,4-Dimethoxyphenyl bis-benzimidazole, a novel DNA topoisomerase inhibitor that preferentially targets Escherichia coli topoisomerase I. J. Antimicrob. Chemother. 2012, 67, 2882–2891. [Google Scholar] [CrossRef]
- Felicetti, T.; Cannalire, R.; Burali, M.S.; Massari, S.; Manfroni, G.; Barreca, M.L.; Tabarrini, O.; Schindler, B.D.; Sabatini, S.; Kaatz, G.W.; et al. Searching for Novel Inhibitors of theS. aureusNorA Efflux Pump: Synthesis and Biological Evaluation of the 3-Phenyl-1,4-benzothiazine Analogues. ChemMedChem 2017, 12, 1293–1302. [Google Scholar] [CrossRef]
- Shcherbakov, K.V.; Artemyeva, M.A.; Burgart, Y.V.; Saloutin, V.I.; Volobueva, A.S.; Misiurina, M.A.; Esaulkova, Y.L.; Sinegubova, E.O.; Zarubaev, V.V. 7-Imidazolyl-substituted 4′-methoxy and 3′,4′-dimethoxy-containing polyfluoroflavones as promising antiviral agents. J. Fluor. Chem. 2020, 240, 109657. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, A.A.; Al-Deeb, O.A.; Al-Omar, M.; Lehmann, J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorganic Med. Chem. 2004, 12, 5107–5113. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdullah, E.S.; Sebastian, S.; Al-Wabli, R.I.; El-Emam, A.A.; Panicker, C.; Van Alsenoy, C. Vibrational spectroscopic studies (FT-IR, FT-Raman) and quantum chemical calculations on 5-(Adamantan-1-yl)-3-[(4-fluoroanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, a potential chemotherapeutic agent. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2014, 133, 605–618. [Google Scholar] [CrossRef]
- Al-Omary, F.A.; Karakaya, M.; Sert, Y.; Haress, N.G.; El-Emam, A.A.; Çırak, Ç. Structural and spectroscopic analysis of 3-[(4-phenylpiperazin-1-yl)methyl]-5-(thiophen-2-yl)-2,3-dihydro-1,3,4-oxadiazole-2-thione with experimental (FT-IR, Laser-Raman) techniques and ab initio calculations. J. Mol. Struct. 2014, 1076, 664–672. [Google Scholar] [CrossRef]
- Al-Omary, F.A.; Mary, Y.S.; Panicker, C.Y.; El-Emam, A.A.; Al-Swaidan, I.A.; Al-Saadi, A.A.; Van Alsenoy, C. Spectroscopic investigations, NBO, HOMO-LUMO, NLO analysis and molecular docking of 5-(adamantan-1-yl)-3-anilinomethyl-2,3-dihydro-1,3,4-oxadiazole-2-thione, a potential bioactive agent. J. Mol. Struct. 2015, 1096, 1–14. [Google Scholar] [CrossRef]
- Qadeer, G.; Rama, N.H.; Malik, M.A.; Raftery, J. 3,4-Dimethoxybenzohydrazide. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, 3026. [Google Scholar] [CrossRef]
- Abdullah, M.A.; Abuo-Rahma, G.E.-D.A.; Abdelhafez, E.-S.M.; Hassan, H.A.; El-Baky, R.M.A. Design, synthesis, molecular docking, anti- Proteus mirabilis and urease inhibition of new fluoroquinolone carboxylic acid derivatives. Bioorganic Chem. 2017, 70, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Goswami, B.N.; Kataky, J.C.S.; Baruah, J.N.; Nath, S.C. Synthesis of 3,5-disubstituted 1,3,4-oxadiazole-2-thiones as potential fungicidal agents. J. Heterocycl. Chem. 1984, 21, 205–208. [Google Scholar] [CrossRef]
- Woods, G.L.; Washington, J.A. Antibacterial susceptibility tests: Dilution and disk diffusion methods. In Manual of Clinical Microbiology; Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; American Society of Microbiology: Washington, DC, USA, 1995. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Approved standard document M-7A.; NCCS: Villanova, PA, USA, 1985.
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Berridge, M.; Tan, A. Characterization of the Cellular Reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular Localization, Substrate Dependence, and Involvement of Mitochondrial Electron Transport in MTT Reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2012, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
| Compound No. | X/R | Crystallization Solvents | M.P. (°C) | Yield (%) | Mol. Formula (Mol. Wt.) |
|---|---|---|---|---|---|
| 4a | H | EtOH/H2O | 147–149 | 82 | C17H17N3O3S (343.40) |
| 4b | 4-F | EtOH/H2O | 135–137 | 84 | C17H16FN3O3S (361.39) |
| 4c | 3-Cl | EtOH | 143–145 | 77 | C17H16ClN3O3S (377.85) |
| 4d | 4-Cl | EtOH | 166–168 | 79 | C17H16ClN3O3S (377.85) |
| 4e | 2-NO2 | EtOH/CHCl3 | 211–213 | 88 | C17H16N4O5S (388.40) |
| 4f | 3-NO2 | EtOH/CHCl3 | 181–183 | 85 | C17H16N4O5S (388.40) |
| 4g | 4-NO2 | EtOH/CHCl3 | 222–224 | 92 | C17H16N4O5S (388.40) |
| 4h | 2-CF3 | EtOH/H2O | 220–222 | 90 | C18H16F3N3O3S (411.40) |
| 4i | 3-CF3 | EtOH/H2O | 206–208 | 86 | C18H16F3N3O3S (411.40) |
| 4j | 2,4-F2 | EtOH | 169–171 | 92 | C17H15F2N3O3S (379.38) |
| 4k | 2,5-F2 | EtOH | 212–214 | 90 | C17H15F2N3O3S (379.38) |
| 4l | 2,4-Cl2 | EtOH | 227–229 | 94 | C17H15Cl2N3O3S (412.29) |
| 5a | C6H5 | EtOH | 151–153 | 85 | C21H24N4O3S (412.51) |
| 5b | 4-FC6H4 | EtOH | 118–120 | 78 | C21H23FN4O3S (430.50) |
| 5c | C6H5CH2 | EtOH/H2O | 121–123 | 75 | C22H26N4O3S (426.53) |
| 5d | 2-CF3C6H4CH2 | EtOH | 141–143 | 89 | C23H25F3N4O3S (494.53) |
| Comp. No. | C log Pc | Diameter of Growth Inhibition Zone (mm) a | |||||
|---|---|---|---|---|---|---|---|
| SA | BS | ML | EC | PA | CA | ||
| 4a | 3.701 | 14 | 13 | 15 | - | - | 10 |
| 4b | 4.146 | 15 | 16 | 17 | - | - | 12 |
| 4c | 4.716 | 14 | 17 | 13 | - | - | - |
| 4d | 4.716 | 15 | 13 | 14 | - | - | - |
| 4e | 4.242 | 12 | 11 | 10 | - | - | 13 |
| 4f | 4.092 | 11 | 12 | - | - | - | 12 |
| 4g | 4.092 | 13 | 12 | 11 | - | - | 16 |
| 4h | 5.113 | 15 | 12 | - | - | - | - |
| 4i | 5.113 | 16 | 12 | - | - | - | - |
| 4j | 4.395 | 19 (4) b | 12 | 16 | 12 | 13 | 11 |
| 4k | 4.395 | 17 | 14 | 15 | 14 | - | 12 |
| 4l | 5.535 | 19 (4) b | 21 (2) b | 18 (16) b | 15 | 12 | 11 |
| 5a | 3.789 | 23 (1) b | 26 (1) b | 21 (1) b | 14 | 13 | - |
| 5b | 4.103 | 20 (8) b | 23 (1) b | 20 (1) b | 16 | 17 | - |
| 5c | 4.712 | 26 (1) b | 30 (0.5) b | 22 (1) b | 19 (4) b | 18 (4) b | - |
| 5d | 5.595 | 28 (1) b | 29 (1) b | 26 (1) b | 22 (1) b | 20 (2) b | - |
| Gentamicin sulfate | 27 (1) b | 26 (2) b | 20 (2) b | 22 (0.5) b | 21 (0.5) b | NT | |
| Ampicillin trihydrate | 22 (2) b | 23 (1) b | 20 (2) b | 16 (8) b | 16 (8) b | NT | |
| Clotrimazole | NT | NT | NT | NT | NT | 21 (4) b | |
| Comp. No. | IC50 (µM) a | ||||
|---|---|---|---|---|---|
| PC3 | HCT-116 | HePG-2 | HeLa | MCF7 | |
| 4a | 63.94 ± 3.8 | 53.17 ± 3.3 | 47.23 ± 3.1 | 28.31 ± 2.0 | 55.34 ± 2.8 |
| 4b | 71.80 ± 4.0 | 77.52 ± 4.1 | 56.34 ± 3.3 | 49.47 ± 3.0 | 68.26 ± 3.4 |
| 4c | 84.52 ± 4.8 | 39.44 ± 2.6 | 78.86 ± 4.1 | 44.69 ± 2.8 | 33.86 ± 2.3 |
| 4d | 78.35 ± 4.5 | 48.30 ± 3.0 | 73.80 ± 3.9 | 54.02 ± 3.2 | 40.52 ± 2.5 |
| 4e | >100 | 92.11 ± 4.9 | >100 | 75.61 ± 3.9 | 88.33 ± 3.9 |
| 4f | 74.67 ± 4.4 | 69.38 ± 3.8 | 58.41 ± 3.5 | 57.26 ± 2.5 | 65.35 ± 3.2 |
| 4g | >100 | 92.11 ± 4.9 | >100 | 75.61 ± 3.9 | 88.33 ± 3.9 |
| 4h | 59.48 ± 3.5 | 35.01 ± 2.7 | 42.74 ± 2.9 | 31.72 ± 2.2 | 29.10 ± 2.1 |
| 4i | 59.48 ± 3.5 | 35.01 ± 2.7 | 42.74 ± 2.9 | 31.72 ± 2.2 | 29.10 ± 2.1 |
| 4j | 95.61 ± 5.1 | 64.07 ± 3.5 | 86.45 ± 4.5 | 61.98 ± 3.5 | 59.87 ± 2.7 |
| 4k | 75.22 ± 4.1 | 61.07 ± 3.4 | 77.40 ± 3.6 | 49.55 ± 3.2 | 29.56 ± 3.6 |
| 4l | 34.60 ± 2.3 | 19.95 ± 1.8 | 17.42 ± 1.4 | 10.96 ± 1.1 | 12.97 ± 1.0 |
| 5a | 52.53 ± 3.3 | 27.49 ± 2.3 | 36.08 ± 2.5 | 24.09 ± 1.8 | 17.80 ± 1.3 |
| 5b | >100 | 89.26 ± 4.6 | 91.78 ± 4.9 | 67.53 ± 3.7 | 79.16 ± 3.6 |
| 5c | 23.92 ± 1.9 | 14.69 ± 1.2 | 11.93 ± 1.0 | 9.50 ± 0.8 | 6.49 ± 0.4 |
| 5d | 38.02 ± 2.5 | 32.81 ± 2.6 | 22.91 ± 1.6 | 18.37 ± 1.4 | 24.33 ± 1.9 |
| Doxorubicin | 8.87 ± 0.6 | 5.23 ± 0.3 | 4.50 ± 0.2 | 5.57 ± 0.4 | 4.17 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wahaibi, L.H.; Mohamed, A.A.B.; Tawfik, S.S.; Hassan, H.M.; El-Emam, A.A. 1,3,4-Oxadiazole N-Mannich Bases: Synthesis, Antimicrobial, and Anti-Proliferative Activities. Molecules 2021, 26, 2110. https://doi.org/10.3390/molecules26082110
Al-Wahaibi LH, Mohamed AAB, Tawfik SS, Hassan HM, El-Emam AA. 1,3,4-Oxadiazole N-Mannich Bases: Synthesis, Antimicrobial, and Anti-Proliferative Activities. Molecules. 2021; 26(8):2110. https://doi.org/10.3390/molecules26082110
Chicago/Turabian StyleAl-Wahaibi, Lamya H., Ahmed A. B. Mohamed, Samar S. Tawfik, Hanan M. Hassan, and Ali A. El-Emam. 2021. "1,3,4-Oxadiazole N-Mannich Bases: Synthesis, Antimicrobial, and Anti-Proliferative Activities" Molecules 26, no. 8: 2110. https://doi.org/10.3390/molecules26082110
APA StyleAl-Wahaibi, L. H., Mohamed, A. A. B., Tawfik, S. S., Hassan, H. M., & El-Emam, A. A. (2021). 1,3,4-Oxadiazole N-Mannich Bases: Synthesis, Antimicrobial, and Anti-Proliferative Activities. Molecules, 26(8), 2110. https://doi.org/10.3390/molecules26082110






