Nutritional Value and Preventive Role of Nigella sativa L. and Its Main Component Thymoquinone in Cancer: An Evidenced-Based Review of Preclinical and Clinical Studies
Abstract
1. Introduction
2. Literature Search
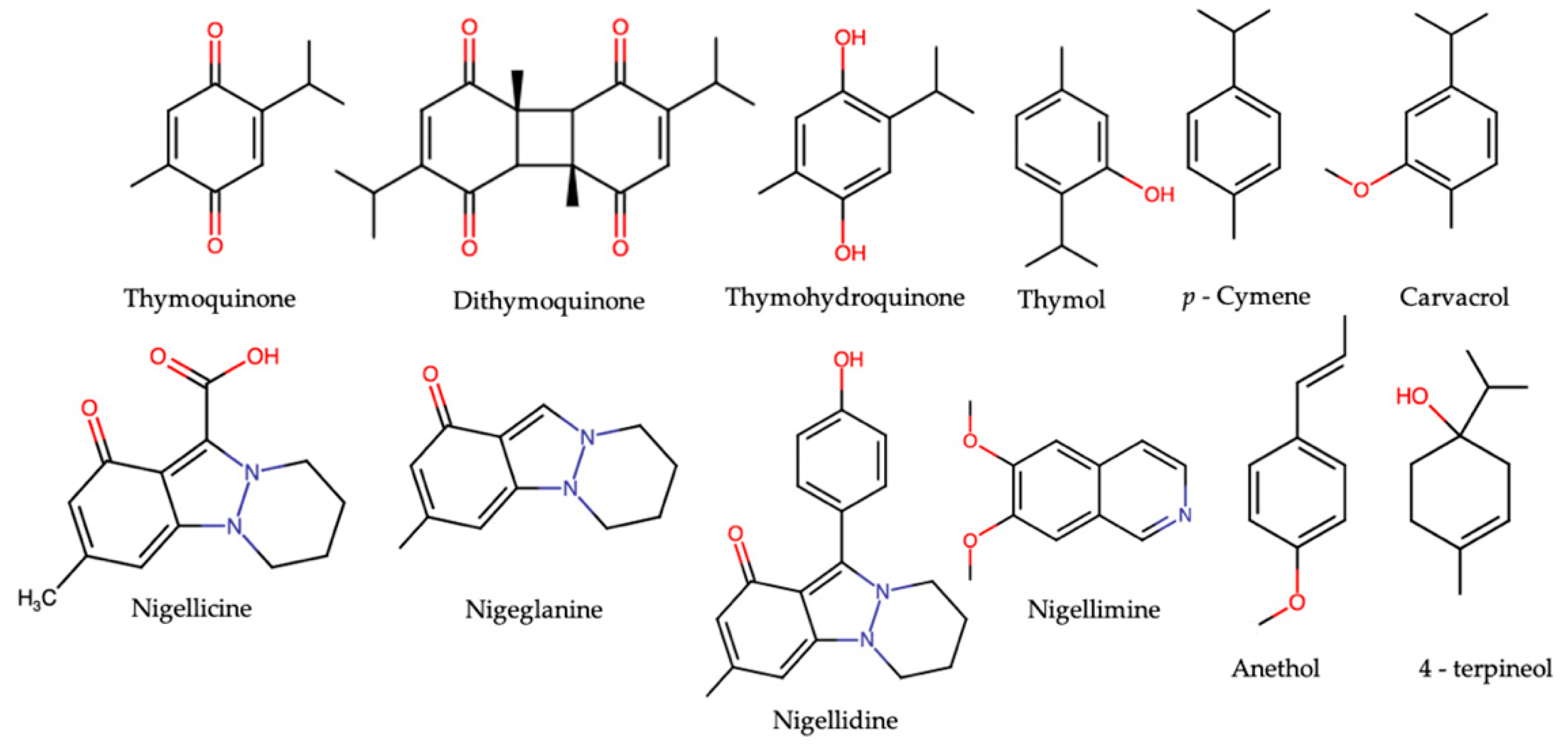
3. N. sativa and Its Major Constituents
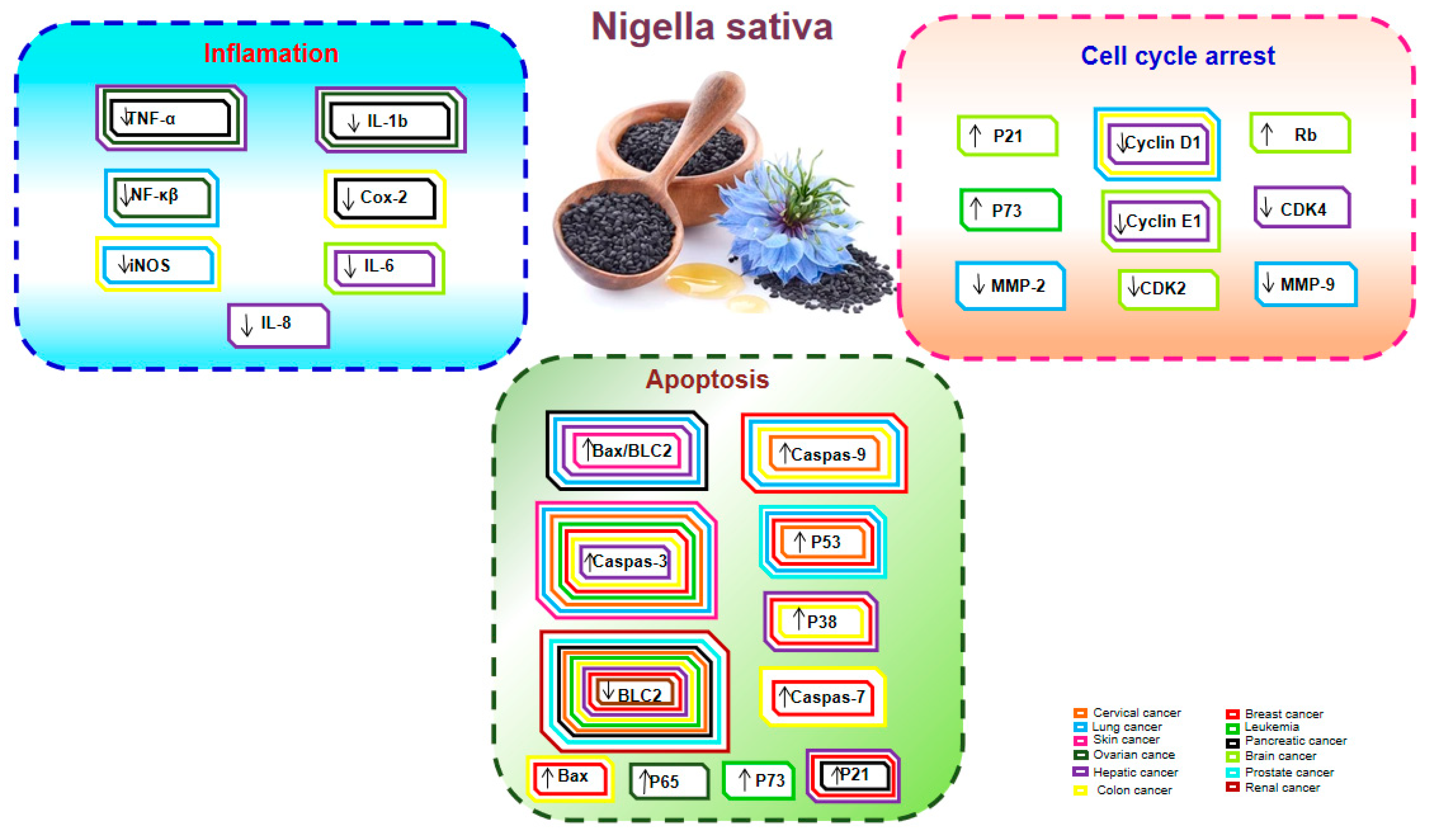
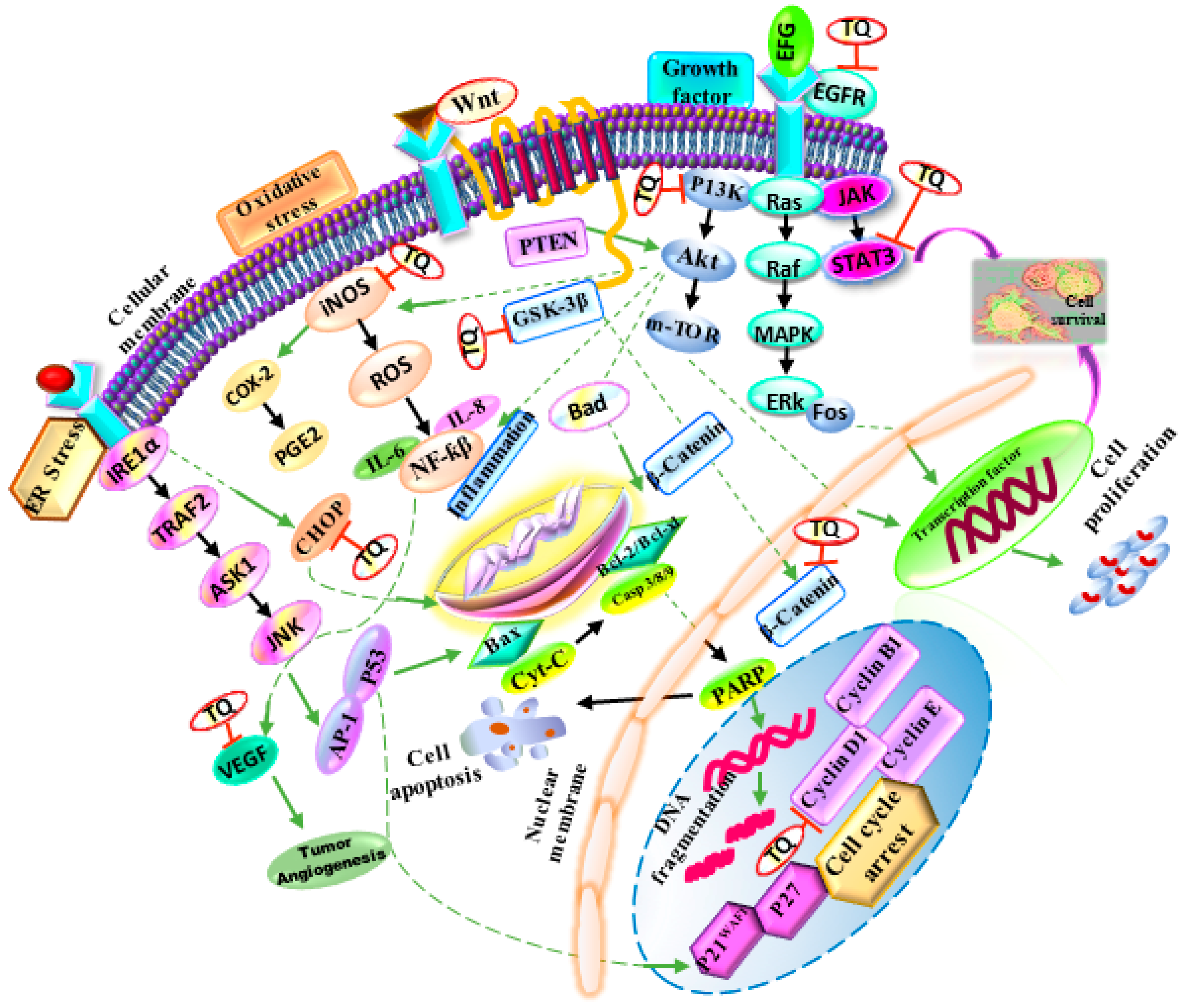
4. N. sativa as an Anticancer Agent
4.1. Breast Cancer
4.2. Colon Cancer
4.3. Hepatic Cancer
4.4. Lung Cancer
4.5. Pancreatic Cancer
4.6. Cervical Cancer
4.7. Leukemia and Blood Cancer
4.8. Kidney and Bladder Cancer
4.9. Skin Cancer
4.10. Ovarian Cancer
4.11. Prostate Cancer
4.12. N. sativa and Other Cancers
5. Combined Therapy
6. Clinical Studies
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef]
- Khan, A.; Chen, H.C.; Tania, M.; Zhang, D.Z. Anticancer activities of Nigella sativa (black cumin). Afr. J. Tradit. Complement Altern. Med. 2011, 8, 226–232. [Google Scholar] [CrossRef] [PubMed]
- You, J.S.; Jones, P.A. Cancer genetics and epigenetics: Two sides of the same coin? Cancer Cell 2012, 22, 9–20. [Google Scholar] [CrossRef]
- Das, T.; Sa, G.; Saha, B.; Das, K. Multifocal signal modulation therapy of cancer: Ancient weapon, modern targets. Mol. Cell Biochem. 2010, 336, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Fayyad, M.W.; Nasrallah, G.K. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit. Rev. Food Sci. Nutr. 2017, 57, 3911–3928. [Google Scholar] [CrossRef]
- Bose, S.; Panda, A.K.; Mukherjee, S.; Sa, G. Curcumin and tumor immune-editing: Resurrecting the immune system. Cell Div. 2015, 10, 6. [Google Scholar] [CrossRef]
- Hossain, D.M.; Bhattacharyya, S.; Das, T.; Sa, G. Curcumin: The multi-targeted therapy for cancer regression. Front. Biosci. Schol. Ed. 2012, 4, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernández, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Zhang, J.; Manna, P.P.; Daglia, M.; Atanasov, A.G.; et al. Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnol. Adv. 2018, 38, 107322. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.H.; Memar, B.; Ataee, R.; Karami, M. Protective effect of thymoquinone, the main component of Nigella sativa, against diazinon cardio-toxicity in rats. Drug Chem. Toxicol. 2018, 42, 585–591. [Google Scholar] [CrossRef]
- Tabassum, H.; Ahmad, A.; Ahmad, I.Z. Nigella sativa L. and its bioactive constituents as hepato protectant: A review. Curr. Pharm. Biotechnol. 2018, 19, 43–67. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Khan, I.A.; Shahbaz, M.; Qaisrani, T.B.; Fatmawati, S.; Abu-Izneid, T.; Imran, A.; Rahman, K.U.; Gondal, T.A. Thymoquinone: A novel strategy to combat cancer: A review. Biomed. Pharmacother. 2018, 106, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Greenish, H.G. Contribution to the chemistry of Nigella sativa. Pharmac. J. Trans. 1880, 10, 909–911. [Google Scholar]
- Al-Jassir, M.S. Chemical composition and microflora of black cumin (Nigella sativa L.) seeds growing in Saudi Arabia. Food Chem. 1992, 45, 239–242. [Google Scholar] [CrossRef]
- Ustun, G.; Kent, L.; Cekin, N.; Civelekoglu, H. Investigation of the technological properties of Nigella sativa (Black Cumin) seed oil. J. Am. Oil Chem. 1990, 67, 958–960. [Google Scholar] [CrossRef]
- Dandik, L.; Aksoy, H.A. The kinetics of hydrolysis of Nigella sativa (Black cumin) seed oil catalyzed by native lipase in ground seed. J. Am. Oil Chem. Soc. 1992, 69, 1239–1241. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.; Attia, R.S. Characterization of black cumin (Nigella sativa) seeds 1-chemical composition and lipids. Alex. Sci. Exch. 1993, 14, 467. [Google Scholar]
- Nergiz, C.; Ötleş, S. Chemical composition of Nigella sativa L. seeds. Food Chem. 1993, 48, 259–261. [Google Scholar] [CrossRef]
- Dandik, L.; Aksoy, H.A. Applications of Nigella sativa seed lipase in oleochemical reactions. Enz. Microb. Technol. 1996, 19, 277–281. [Google Scholar] [CrossRef]
- El-Dhaw, Z.Y.; Abdel-Munaem, N.M. Chemical and biological values of black cumin seeds. J. Agric. Sci. Mansoura Univ. 1996, 21, 4149–4159. [Google Scholar]
- Takruri, H.R.H.; Dameh, M.A.F. Study of nutritional value of black cumin seeds (Nigella sativa L.). J. Sci. Food Agric. 1998, 76, 404–410. [Google Scholar] [CrossRef]
- Üstün, G.; Turkay, S.; Karaali, A. Nigella sativa seeds: A potential source for oil and oleochemicals. In Proceedings of the World Conference on Oil Seed and Edible Oil Processing, Istanbul, Turkey, 6–10 October 1996; AOCS Press: Champaign, IL, USA, 1998; Volume 2, pp. 155–160. [Google Scholar]
- Jadayil, S.A.; Tukan, S.K.; Takruri, H.R. Bioavailability of iron from four different local food plants in Jordan. Plant Foods Hum. Nutr. 1999, 54, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.B. Some characteristics of nigella (Nigella sativa L.) seed cultivated in Egypt and its lipid profile. Food Chem. 2003, 83, 63–68. [Google Scholar] [CrossRef]
- Nickavar, B.; Mojab, F.; Javidnia, K.; Amoli, M.A.R. Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Z. Naturforsch. C J. Biosci. 2003, 58, 629–631. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Morsel, J.T. Analysis of glycolipids from black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and niger (Guizotia abyssinica Cass.) oilseeds. Food. Chem. 2003, 80, 197–204. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Morsel, J.T. Oxidative stability of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and niger (Guizotia abyssinica Cass.) crude seed oils upon stripping. Eur. J. Lipid Sci. Technol. 2004, 106, 35–43. [Google Scholar] [CrossRef]
- Al-Saleh, I.A.; Billedo, G.; El–Doush, I.I. Levels of selenium, DLa-tocopherol, DL-g-tocopherol, all-trans-retinol, thymoquinone and thymol in different brands of Nigella sativa seeds. J. Food Comp. Anal. 2006, 19, 167–175. [Google Scholar] [CrossRef]
- Ashraf, M.; Ali, Q.; Iqbal, Z. Effect of nitrogen application rate on the content and composition of oil, essential oil and minerals in black cumin (Nigella sativa L.) seeds. J. Sci. Food Agric. 2006, 86, 871–876. [Google Scholar] [CrossRef]
- Cheikh-Rouhou, S.; Besbes, S.; Hentati, B.; Blecker, C.; Deroanne, C.; Attia, H. Nigella sativa L.: Chemical composition and physicochemical characteristics of lipid fraction. Food Chem. 2007, 101, 673–681. [Google Scholar] [CrossRef]
- Ghosheh, O.A.; Houdi, A.A.; Crooks, P.A. High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L.). J. Pharm. Biomed. Anal. 1999, 19, 757–762. [Google Scholar] [CrossRef]
- Malik, S.; Cun-Heng, H.; Clardy, J. Isolation and structure determination of nigellicine, a novel alkaloid from the seeds of nigella sativa. Tetrahedron Lett. 1985, 26, 2759–2762. [Google Scholar]
- Butt, M.S.; Sultan, M.T. Nigella sativa: Reduces the risk of various maladies. Crit. Rev. Food Sci. Nut. 2010, 50, 654–665. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Estimates; World Health Organization: Geneva, Switzerland, 2013; Available online: http://www.who.int/healthinfo/global_burden_disease/en/ (accessed on 14 July 2020).
- Woo, C.C.; Hsu, A.; Kumar, A.P.; Sethi, G.; Tan, K.H.B. Thymoquinone inhibits tumor growth and induces apoptosis in a breast cancer xenograft mouse model: The role of p38 MAPK and ROS. PLoS ONE 2013, 8, e75356. [Google Scholar] [CrossRef]
- Rajput, S.; Kumar, B.P.; Dey, K.K.; Pal, I.; Parekh, A.; Mandal, M. Molecular targeting of Akt by thymoquinone promotes G1 arrest through translation inhibition of cyclin D1 and induces apoptosis in breast cancer cells. Life Sci. 2013, 93, 783–790.–790. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Y.; Syed, B.; Simjee, S.U.; Beg, O.; Ahmed, A. Antiproliferative and apoptotic effects of proteins from black seeds (Nigella sativa) on human breast MCF-7 cancer cell line. BMC Complement. Med. Ther. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Woo, C.C.; Loo, S.Y.; Gee, V.; Yap, C.W.; Sethi, G.; Kumar, A.P.; Tan, K.H.B. Anticancer activity of thymoquinone in breast cancer cells: Possible involvement of PPAR-γ pathway. Biochem. Pharmacol. 2011, 82, 464–475. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone inhibits bone metastasis of breast cancer cells through abrogation of the CXCR4 signaling axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Tania, M.; Wei, C.; Mei, Z.; Fu, S.; Cheng, J.; Xu, J.; Fu, J. Thymoquinone inhibits cancer metastasis by downregulating TWIST1 expression to reduce epithelial to mesenchymal transition. Oncotarget 2015, 6, 19580. [Google Scholar] [CrossRef]
- Sutton, K.M.; Greenshields, A.L.; Hoskin, D.W. Thymoquinone, a bioactive component of black caraway seeds, causes G1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr. Cancer 2014, 66, 408–418. [Google Scholar] [CrossRef]
- Arafa, E.S.A.; Zhu, Q.; Shah, Z.I.; Wani, G.; Barakat, B.M.; Racoma, I.; El-Mahdy, M.A.; Wani, A.A. Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat. Res. 2011, 706, 28–35. [Google Scholar] [CrossRef]
- El-Aziz, M.A.A.; Hassan, H.A.; Mohamed, M.H.; Meki, A.R.M.; Abdel-Ghaffar, S.K.; Hussein, M.R. The biochemical and morphological alterations following administration of melatonin, retinoic acid and Nigella sativa in mammary carcinoma: An animal model. Int. J. Exp. Pathol. 2005, 86, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Rooney, S.; Ryan, M.F. Effects of alpha-hederin and thymoquinone, constituents of Nigella sativa, on human cancer cell lines. Anticancer Res. 2005, 25, 2199–2204. [Google Scholar] [PubMed]
- Chen, M.-C.; Lee, N.-H.; Hsu, H.-H.; Ho, T.J.; Tu, C.C.; Hsieh, D.J.; Lin, Y.M.; Chen, L.M.; Kuo, W.W.; Huang, C.Y. Thymoquinone induces caspase-independent, autophagic cell death in CPT-11-resistant lovo colon cancer via mitochondrial dysfunction and activation of JNK and p38. J. Agric. Food Chem. 2015, 63, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Chen, R.J.; Yueh-Min Lin, Y.M.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Inhibition of NF-κB and metastasis in irinotecan (CPT-11)-resistant LoVo colon cancer cells by thymoquinone via JNK and p38. Environ. Toxicol. 2017, 32, 669–678. [Google Scholar] [CrossRef]
- El-Najjar, N.; Chatila, M.; Moukadem, H.; Vuorela, H.; Ocker, M.; Gandesiri, M.; Schneider-Stock, R.; Gali-Muhtasib, H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis 2010, 15, 183–195. [Google Scholar] [CrossRef]
- Hsu, H.H.; Chen, M.C.; Day, C.H.; Lin, Y.M.; Li, S.Y.; Tu, C.C.; Padma, V.V.; Shih, H.N.; Kuo, W.W.; Huang, C.Y. Thymoquinone suppresses migration of LoVo human colon cancer cells by reducing prostaglandin E2 induced COX-2 activation. World J. Gastroenterol. 2017, 23, 71171–71179. [Google Scholar] [CrossRef]
- Kundu, J.; Choi, B.Y.; Jeong, C.H.; Kundu, J.K.; Chun, K.S. Thymoquinone induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2-and Src-mediated phosphorylation of EGF receptor tyrosine kinase. Oncol. Rep. 2014, 32, 821–828. [Google Scholar] [CrossRef]
- Al-Johar, D.; Shinwari, N.; Arif, J.; Al-Sanea, N.; Jabbar, A.A.; El-Sayed, R.A.; Mashhour, A.; Billedo, G.; El-Doush, I.; Al-Saleh, I. Role of Nigella sativa and a number of its antioxidant constituents towards azoxymethane-induced genotoxic effects and colon cancer in rats. Phytother. Res. 2008, 22, 1311–1323. [Google Scholar] [CrossRef]
- Asfour, W.; Almadi, S.; Haffar, L. Thymoquinone suppresses cellular proliferation, inhibits VEGF production and obstructs tumor progression and invasion in the rat model of DMH-induced colon carcinogenesis. Pharm. Pharmacol. 2013, 4, 7–17. [Google Scholar] [CrossRef]
- Hassan, M.I.; Mabrouk, G.M.; Shehata, H.H.; Aboelhussein, M.M. Antineoplastic effects of bee honey and Nigella sativa on hepatocellular carcinoma cells. Integr. Cancer Ther. 2012, 11, 354–363. [Google Scholar] [CrossRef]
- Ashour, A.E.; Abd-Allah, A.R.; Korashy, H.M.; Attia, S.M.; Alzahrani, A.Z.; Saquib, Q.; Bakheet, S.A.; Abdel-Hamied, H.E.; Jamal, S.; Rishi, A.K. Thymoquinone suppression of the human hepatocellular carcinoma cell growth involves inhibition of IL-8 expression, elevated levels of TRAIL receptors, oxidative stress and apoptosis. Mol. Cell. Biochem. 2014, 389, 85–98. [Google Scholar] [CrossRef]
- ElKhoely, A.; Hafez, H.F.; Ashmawy, A.M.; Badary, O.; Abdelaziz, A.; Mostafa, A.; Shouman, S.A. Chemopreventive and therapeutic potentials of thymoquinone in HepG2 cells: Mechanistic perspectives. J. Nat. Med. 2015, 69, 313–323. [Google Scholar] [CrossRef]
- Palabiyik, S.S.; Karakus, E.; Halici, Z.; Cadirci, E.; Bayir, Y.; Ayaz, G.; Cinar, I. The protective effects of carvacrol and thymol against paracetamol-induced toxicity on human hepatocellular carcinoma cell lines (HepG2). Hum. Exp. Toxicol. 2016, 35, 1252–1263. [Google Scholar] [CrossRef]
- Shahin, Y.R.; Elguindy, N.M.; Abdel Bary, A.; Balbaa, M. The protective mechanism of Nigella sativa against diethylnitrosamine-induced hepatocellular carcinoma through its antioxidant effect and EGFR/ERK1/2 signaling. Environ. Toxicol. Chem. 2018, 33, 885–898. [Google Scholar] [CrossRef]
- Sayed-Ahmed, M.M.; Aleisa, A.M.; Al-Rejaie, S.S.; Al-Yahya, A.A.; Al-Shabanah, O.A.; Hafez, M.M.; Nagi, M.N. Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxid. Med. Cell. Longev. 2010, 3, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Nikaido, T. In vivo attenuation of angiogenesis in hepatocellular carcinoma by Nigella sativa. Turk. J. Med. Sci. 2018, 48, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Raghunandhakumar, S.; Paramasivam, A.; Senthilraja, S.; Naveenkumar, C.; Asokkumar, S.; Binuclara, J.; Jagan, S.; Anandakumar, P.; Devaki, T. Thymoquinone inhibits cell proliferation through regulation of G1/S phase cell cycle transition in N-nitrosodiethylamine-induced experimental rat hepatocellular carcinoma. Toxicol. Lett. 2013, 223, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Thymoquinone-induced antitumor and apoptosis in human lung adenocarcinoma cells. J. Cell Physiol. 2019, 234, 10421–10431. [Google Scholar] [CrossRef]
- Jafri, S.H.; Glass, J.; Shi, R.; Zhang, S.; Prince, M.; Kleiner-Hancock, H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J. Exp. Clin. Cancer Res. 2010, 29, 87. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kuang, X.R.; Lv, P.T.; Yan, X.X. Thymoquinone inhibits proliferation and invasion of human nonsmall-cell lung cancer cells via ERK pathway. Tumor Biol. 2015, 36, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.R.; Chatterjee, A.; Ganguli, A.; Bhattacharya, S.; Chakrabarti, G. Thymoquinone inhibits microtubule polymerization by tubulin binding and causes mitotic arrest following apoptosis in A549 cells. Biochimie 2014, 97, 78–91. [Google Scholar] [CrossRef]
- Zhu, N.; Zhao, X.; Xiang, Y.; Ye, S.; Huang, J.; Hu, W.; Lv, L.; Zeng, C. Thymoquinone attenuates monocrotaline-induced pulmonary artery hypertension via inhibiting pulmonary arterial remodeling in rats. Int. J. Cardiol. 2016, 221, 587–596. [Google Scholar] [CrossRef]
- Torres, M.P.; Ponnusamy, M.P.; Chakraborty, S.; Smith, L.M.; Das, S.; Arafat, H.A.; Batra, S.K. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: Implications for the development of novel cancer therapies. Mol. Cancer Ther. 2010, 9, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Chehl, N.; Chipitsynam, G.; Gongm, Q.; Yeom, C.J.; Arafatm, H.A. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB 2009, 11, 373–381. [Google Scholar] [CrossRef]
- Banerjee, S.; Azmi, A.S.; Padhye, S.; Singh, M.W.; Baruah, J.B.; Philip, P.A.; Sarkar, F.H.; Mohammad, R.M. Structure-activity studies on therapeutic potential of Thymoquinone analogs in pancreatic cancer. Pharm. Res. 2010, 27, 1146–1158. [Google Scholar] [CrossRef]
- Yusufi, M.; Banerjee, S.; Mohammad, M.; Khatal, S.; Swamy, K.V.; Khan, E.M.; Aboukameel, A.; Sarkar, F.H.; Padhye, S. Synthesis, characterization and anti-tumor activity of novel thymoquinone analogs against pancreatic cancer. Bioorg. Med. Chem. Lett. 2013, 23, 3101–3104. [Google Scholar] [CrossRef]
- Shafi, G.; Munshi, A.; Hasan, T.N.; Alshatwi, A.A.; Jyothy, A.; Lei, D.K. Induction of apoptosis in HeLa cells by chloroform fraction of seed extracts of Nigella sativa. Cancer Cell Int. 2009, 9, 29. [Google Scholar] [CrossRef]
- Sakalar, C.; Yuruk, M.; Kaya, T.; Aytekin, M.; Kuk, S.; Canatan, H. Pronounced transcriptional regulation of apoptotic and TNF–NF-kappa-B signaling genes during the course of thymoquinone mediated apoptosis in HeLa cells. Mol. Cell. Biochem. 2013, 383, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.; Benghuzzi, H.; Tucci, M. Effects of thymoquinone, lycopene, and selenomethione in the presence of estrogen on the viability of SiHa cells in vitro. Biomed. Sci. Instrum. 2006, 42, 37–41. [Google Scholar]
- Ng, W.K.; Yazan, L.S.; Ismail, M. Thymoquinone from Nigella sativa was more potent than cisplatin in eliminating of SiHa cells via apoptosis with down-regulation of Bcl-2 protein. Toxicol. In Vitro 2011, 25, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Ichwan, S.J.; Al-Ani, I.M.; Bilal, H.G.; Suriyah, W.H.; Taher, M.; Ikeda, M.A. Apoptotic activities of thymoquinone, an active ingredient of black seed (Nigella sativa), in cervical cancer cell lines. Chin. J. Physiol. 2014, 57, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Khan, M.; Wei, C.; Cheng, J.; Chen, H.; Yang, L.; Ijaz, I.; Fu, J. Thymoquinone inhibits the migration and invasive characteristics of cervical cancer cells SiHa and CaSki in vitro by targeting epithelial to mesenchymal transition associated transcription factors Twist1 and Zeb1. Molecules 2017, 22, 2105. [Google Scholar] [CrossRef]
- Reindl, W.; Yuan, J.; Krämer, A.; Strebhardt, K.; Berg, T. Inhibition of polo-like kinase 1 by blocking polo-box domain-dependent protein-protein interactions. Chem. Biol. 2008, 15, 459–466. [Google Scholar] [CrossRef]
- Abusnina, A.; Alhosin, M.; Keravis, T.; Muller, C.D.; Fuhrmann, G.; Bronner, C.; Lugnier, C. Down-regulation of cyclic nucleotide phosphodiesterase PDE1A is the key event of p73 and UHRF1 deregulation in thymoquinone-induced acute lymphoblastic leukemia cell apoptosis. Cell Signal. 2011, 23, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Salim, L.; Mohan, S.; Othman, R.; Abdelwahab, S.; Kamalidehghan, B.; Sheikh, B.; Ibrahim, M. Thymoquinone induces mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in vitro. Molecules 2013, 18, 11219–11240. [Google Scholar] [CrossRef]
- Alhosin, M.; Ibrahim, A.; Boukhari, A.; Sharif, T.; Gies, J.P.; Auger, C.; Schini-Kerth, V.B. Anti-neoplastic agent thymoquinone induces degradation of α and β tubulin proteins in human cancer cells without affecting their level in normal human fibroblasts. Investig. New Drugs. 2012, 30, 1813–1819. [Google Scholar] [CrossRef]
- Pang, J.; Shen, N.; Yan, F.; Zhao, N.; Dou, L.; Wu, L.C.; Seiler, C.L.; Yu, L.; Yang, K.; Bachanova, V.; et al. Thymoquinone exerts potent growth-suppressive activity on leukemia through DNA hypermethylation reversal in leukemia cells. Oncotarget 2017, 8, 34453. [Google Scholar]
- Salim, L.Z.A.; Othman, R.; Abdulla, M.A.; Al-Jashamy, K.; Ali, H.M.; Hassandarvish, P.; Dehghan, F.; Ibrahim, M.Y.; Omer, F.A.; Mohan, S. Thymoquinone inhibits murine leukemia WEHI-3 cells in vivo and in vitro. PLoS ONE 2014, 9, e115340. [Google Scholar] [CrossRef] [PubMed]
- Qadi, S.A.; Hassan, M.A.; Sheikh, R.A.; Baothman, O.A.; Zamzami, M.A.; Choudhry, H.; Al-Malki, A.L.; Albukhari, A.; Alhosin, M. Thymoquinone-Induced Reactivation of Tumor Suppressor Genes in Cancer Cells Involves Epigenetic Mechanisms. Epigenet. Insights 2019, 12, 2516865719839011. [Google Scholar] [CrossRef]
- Badr, G.; Lefevre, E.A.; Mohany, M. Thymoquinone inhibits the CXCL12-induced chemotaxis of multiple myeloma cells and increases their susceptibility to Fas-mediated apoptosis. PLoS ONE 2011, 6, e23741. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, K.; Breyer, S.; Schobert, R. Terpene conjugates of the Nigella sativa seed-oil constituent thymoquinone with enhanced efficacy in cancer cells. Chem. Biodivers. 2010, 7, 129–139. [Google Scholar] [CrossRef]
- Park, E.J.; Chauhan, A.K.; Min, K.J.; Park, D.C.; Kwon, T.K. Thymoquinone induces apoptosis through downregulation of c-FLIP and Bcl-2 in renal carcinoma Caki cells. Oncol. Rep. 2016, 36, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, G.H.; Park, E.J.; Oh, T.I.; Lee, S.; Kan, S.Y.; Kang, H.; Kim, B.M.; Kim, J.H.; Lim, J.H. Thymoquinone Selectively Kills Hypoxic Renal Cancer Cells by Suppressing HIF-1α-Mediated Glycolysis. Int. J. Mol. Sci. 2019, 20, 1092. [Google Scholar] [CrossRef]
- Chae, I.G.; Song, N.Y.; Kim, D.H.; Lee, M.Y.; Park, J.M.; Chun, K.S. Thymoquinone induces apoptosis of human renal carcinoma Caki-1 cells by inhibiting JAK2/STAT3 through pro-oxidant effect. Food Chem. Toxicol. 2020, 139, 111253. [Google Scholar] [CrossRef] [PubMed]
- Kou, B.; Kou, Q.; Ma, B.; Zhang, J.; Sun, B.; Yang, Y.; Li, J.; Zhou, J.; Liu, W. Thymoquinone inhibits metastatic phenotype and epithelial mesenchymal transition in renal cell carcinoma by regulating the LKB1/AMPK signaling pathway. Oncol. Rep. 2018, 40, 1443–1450. [Google Scholar] [CrossRef]
- Shahraki, S.; Khajavirad, A.; Shafei, M.N.; Mahmoudi, M.; Tabasi, N.S. Effect of total hydroalcholic extract of Nigella sativa and its n-hexane and ethyl acetate fractions on ACHN and GP-293 cell lines. J. Tradit. Complement. Med. 2016, 6, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Iskender, B.; Izgi, K.; Hizar, E.; Jauch, J.; Arslanhan, A.; Yuksek, E.H.; Canatan, H. Inhibition of epithelial-mesenchymal transition in bladder cancer cells via modulation of mTOR signalling. Tumour Biol. 2016, 37, 8281–8291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Du, H.; Wang, L.; Yue, Y.; Zhang, P.; Huang, Z.; Lv, W.; Ma, J.; Shao, Q.; Ma, M.; et al. Thymoquinone suppresses invasion and metastasis in bladder cancer cells by reversing EMT through the Wnt/β-catenin signaling pathway. Chem. Biol. Interact. 2020, 320, 109022. [Google Scholar] [CrossRef]
- Zhang, M.; Du, H.; Huang, Z.; Zhang, P.; Yue, Y.; Wang, W.; Liu, W.; Zeng, J.; Ma, J.; Chen, G.; et al. Thymoquinone induces apoptosis in bladder cancer cell via endoplasmic reticulum stress-dependent mitochondrial pathway. Chem. Biol. Interact. 2018, 292, 65–75. [Google Scholar] [CrossRef]
- Sang, Y.; Deng, Y. Current insights into the epigenetic mechanisms of skin cancer. Dermatol. Ther. 2019, 32, e12964. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Batool, R.; Mahmood, T.; Ali, B.; Khalil, A.T.; Kanwal, S.; Shaha, S.A.; Alam, M.M.; et al. Potential phytochemicals in the fight against skin cancer: Current landscape and future perspectives. Biomed. Pharmacother. 2019, 109, 1381–1393. [Google Scholar] [CrossRef]
- Das, S.; Dey, K.K.; Dey, G.; Pal, I.; Majumder, A.; Maiti Choudhury, S.; Mandal, M. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgenin in squamous cell carcinoma. PLoS ONE 2012, 7, e46641. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Saskowski, J.; Barham, W.; Yull, F.; Khabele, D. Thymoquinone enhances cisplatin-response through direct tumor effects in a syngeneic mouse model of ovarian cancer. J. Ovarian Res. 2015, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Ajinwo, O.R.; Ullah, I.; Mbye, H.; Richardson, A.; Horrocks, P.; Li, W.W. The synthesis and evaluation of thymoquinone analogues as anti-ovarian cancer and antimalarial agents. Bioorg. Med. Chem. Lett. 2018, 28, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Barham, W.; Saskowski, J.; Tikhomirov, O.; Chen, L.; Lee, H.J.; Yull, F.; Khabele, D. Tracking NF-κβ activity in tumor cells during ovarian cancer progression in a syngeneic mouse model. J. Ovarian Res. 2013, 6, 63. [Google Scholar] [CrossRef]
- Kou, B.; Liu, W.5; Zhao, W.; Duan, P.; Yang, Y.; Yi, Q.; Guo, F.; Li, J.; Zhou, J.; Kou, Q. Thymoquinone inhibits epithelial-mesenchymal transition in prostate cancer cells by negatively regulating the TGF-β/Smad2/3 signaling pathway. Oncol. Rep. 2017, 38, 3592–3598. [Google Scholar] [CrossRef]
- Dirican, A.; Atmaca, H.; Bozkurt, E.; Erten, C.; Karaca, B.; Uslu, R. Novel combination of docetaxel and thymoquinone induces synergistic cytotoxicity and apoptosis in DU-145 human prostate cancer cells by modulating PI3K–AKT pathway. Clin. Transl. Oncol. 2015, 17, 145–151. [Google Scholar] [CrossRef]
- Kus, G.; Ozkurt, M.; Kabadere, S.; Erkasap, N.; Goger, G.; Demirci, F. Antiproliferative and antiapoptotic effect of thymoquinone on cancer cells in vitro. Bratisl. Lek. Listy 2018, 119, 312–316. [Google Scholar] [CrossRef]
- Koka, P.S.; Mondal, D.; Schultz, M.; Abdel-Mageed, A.B.; Agrawal, K.C. Studies on molecular mechanisms of growth inhibitory effects of thymoquinone against prostate cancer cells: Role of reactive oxygen species. Exp. Biol. Med. 2010, 235, 751–760. [Google Scholar] [CrossRef]
- Zubair, H.; Khan, H.Y.; Sohail, A.; Azim, S.; Ullah, M.F.; Ahmad, A.; Sarkar, F.H.; Hadi, S.M. Redox cycling of endogenous copper by thymoquinone leads to ROS-mediated DNA breakage and consequent cell death: Putative anticancer mechanism of antioxidants. Cell Death Dis. 2013, 4, e660. [Google Scholar] [CrossRef]
- Peng, L.; Liu, A.; Shen, Y.; Xu, H.Z.; Yang, S.Z.; Ying, X.Z.; Liao, W.; Liu, H.X.; Lin, Z.Q.; Chen, Q.Y.; et al. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncol. Rep. 2013, 29, 571–578. [Google Scholar] [CrossRef]
- Salim, E.I. Cancer chemopreventive potential of volatile oil from black cumin seeds, Nigella sativa L., in a rat multi-organ carcinogenesis bioassay. Oncol. Lett. 2010, 1, 913–924. [Google Scholar] [CrossRef]
- Abdelfadil, E.; Cheng, Y.H.; Bau, D.T.; Ting, W.J.; Chen, L.M.; Hsu, H.H.; Lin, Y.M.; Chen, R.J.; Tsai, F.J.; Tsai, C.H.; et al. Thymoquinone induces apoptosis in oral cancer cells through p38β inhibition. Am. J. Chin. Med. 2013, 41, 683–696. [Google Scholar] [CrossRef]
- Chu, S.C.; Hsieh, Y.S.; Yu, C.C.; Lai, Y.Y.; Chen, P.N. Thymoquinone induces cell death in human squamous carcinoma cells via caspase activation-dependent apoptosis and LC3-II activation-dependent autophagy. PLoS ONE 2014, 9, e101579. [Google Scholar] [CrossRef]
- Rajkamal, G.; Suresh, K.; Sugunadevi, G.; Vijayaanand, M.A.; Rajalingam, K. Evaluation of chemopreventive effects of Thymoquinone on cell surface glycoconjugates and cytokeratin expression during DMBA induced hamster buccal pouch carcinogenesis. BMB Rep. 2010, 43, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Subburayan, K.; Thayyullathil, F.; Pallichankandy, S.; Rahman, A.; Galadari, S. Par-4-dependent p53 up-regulation plays a critical role in thymoquinone-induced cellular senescence in human malignant glioma cells. Cancer Lett. 2018, 426, 80–97. [Google Scholar] [CrossRef]
- Kotowski, U.; Heiduschka, G.; Kadletz, L.; Fahim, T.; Seemann, R.; Schmid, R.; Schneider, S.; Mitterbauer, A.; Thurnher, D.; Thurnher, D. Effect of thymoquinone on head and neck squamous cell carcinoma cells in vitro: Synergism with radiation. Oncol. Lett. 2017, 14, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.M.; Varghese, E.; Kubatka, P.; Triggle, C.R.; Büsselberg, D. Metformin: The answer to cancer in a flower? Current knowledge and future prospects of metformin as an anti-cancer agent in breast cancer . Biomolecules 2019, 9, 846. [Google Scholar] [CrossRef]
- Baguley, B.C. Multiple drug resistance mechanisms in cancer. Mol. Biotechnol. 2010, 46, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Zahreddine, H.; Borden, K. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef]
- Mu, G.G.; Zhang, L.L.; Li, H.Y.; Liao, Y.; Yu, H.G. Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Dig. Dis. Sci. 2015, 60, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Pandita, A.; Kumar, B.; Manvati, S.; Vaishnavi, S.; Singh, S.K.; Bamezai, R.N. Synergistic combination of gemcitabine and dietary molecule induces apoptosis in pancreatic cancer cells and down regulates PKM2 expression. PLoS ONE 2014, 9, e107154. [Google Scholar] [CrossRef] [PubMed]
- Bashmail, H.A.; Alamoudi, A.A.; Noorwali, A.; Hegazy, G.A.; AJabnoor, G.; Choudhry, H.; Al-Abd, A.M. Thymoquinone synergizes gemcitabine anti-breast cancer activity via modulating its apoptotic and autophagic activities. Sci. Rep. 2018, 8, 11674. [Google Scholar] [CrossRef]
- Şakalar, Ç.; İzgi, K.; İskender, B.; Sezen, S.; Aksu, H.; Çakır, M.; Kurt, B.; Turan, A.; Canatan, H. The combination of thymoquinone and paclitaxel shows anti-tumor activity through the interplay with apoptosis network in triple-negative breast cancer. Tumour Biol. 2016, 37, 4467–4477. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Refaat, B.A.; El-Shemi, A.G.; Kensara, O.A.; Ahmad, J.; Idris, S. Thymoquinone potentiates chemoprotective effect of Vitamin D3 against colon cancer: A pre-clinical finding. Am. J. Transl. Res. 2017, 9, 774. [Google Scholar] [PubMed]
- Kensara, O.A.; El-Shemi, A.G.; Mohamed, A.M.; Refaat, B.; Idris, S.; Ahmad, J. Thymoquinone subdues tumor growth and potentiates the chemopreventive effect of 5-fluorouracil on the early stages of colorectal carcinogenesis in rats. Drug Des. Devel. Ther. 2016, 10, 2239. [Google Scholar]
- Attoub, S.; Sperandio, O.; Raza, H.; Arafat, K.; Al-Salam, S.; Al Sultan, M.A.; Al Safi, M.; Takahashi, T.; Adem, A. Thymoquinone as an anticancer agent: Evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam. Clin. Pharmacol. 2013, 27, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Effenberger-Neidnicht, K.; Schobert, R. Combinatorial effects of thymoquinone on the anti-cancer activity of doxorubicin. Cancer Chemother. Pharmacol. 2011, 67, 867–874. [Google Scholar] [CrossRef]
- Soltani, A.; Pourgheysari, B.; Shirzad, H.; Sourani, Z. Antiproliferative and apoptosis-inducing activities of thymoquinone in lymphoblastic leukemia cell line. Indian J. Hematol. Blood Transfus. 2017, 33, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Rukoyatkina, N.; Butt, E.; Subramanian, H.; Nikolaev, V.O.; Mindukshev, I.; Walter, U.; Gambaryan, S.; Benz, P.M. Protein kinase A activation by the anti-cancer drugs ABT-737 and thymoquinone is caspase-3-dependent and correlates with platelet inhibition and apoptosis. Cell Death Dis. 2017, 8, e2898. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, Z.; Shahid, F.; Khan, A.A.; Khan, F. Oral administration of Nigella sativa oil and thymoquinone attenuates long term cisplatin treatment induced toxicity and oxidative damage in rat kidney. Biomed. Pharmacother. 2017, 96, 912–923. [Google Scholar] [CrossRef]
- Nessa, M.U.; Beale, P.; Chan, C.; Yu, J.Q.; Huq, F. Synergism from combinations of cisplatin and oxaliplatin with quercetin and thymoquinone in human ovarian tumour models. Anticancer Res. 2011, 31, 3789–3797. [Google Scholar]
- Liu, X.; Dong, J.; Cai, W.; Pan, Y.; Li, R.; Li, B. The effect of thymoquinone on apoptosis of SK-OV-3 ovarian cancer cell by regulation of Bcl-2 and Bax. Int. J. Gynecol. Cancer. 2017, 27, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Dirican, A.; Erten, C.; Atmaca, H.; Bozkurt, E.; Kucukzeybek, Y.; Varol, U.; Oktay Tarhan, M.; Karaca, B.; Uslu, R. Enhanced cytotoxicity and apoptosis by thymoquinone in combination with zoledronic acid in hormone-and drug-resistant prostate cancer cell lines. J. BUON 2014, 19, 1055–1061. [Google Scholar] [PubMed]
- Alaufi, O.M.; Noorwali, A.; Zahran, F.; Al-Abd, A.M.; Al-Attas, S. Cytotoxicity of thymoquinone alone or in combination with cisplatin (CDDP) against oral squamous cell carcinoma in vitro. Sci. Rep. 2017, 7, 1313. [Google Scholar] [CrossRef] [PubMed]
- Dogar, M.Z.; Adnan, H.; Akhtar, M.S.; Sheikh, M.A. Prelimiary assessment of efficacy of Nigella sativa seeds in acute lymphoblastic leukemia local children. Pharmacologyonline 2009, 2, 769–777. [Google Scholar]
- Clinical and Immunohisochemical Evaluation of Chemopreventive Effect of Thymoquinone on Oral Potentially Malignant Lesions. Available online: https://clinicaltrials.gov/ct2/show/NCT03208790 (accessed on 10 December 2020).
- Al-Amri, A.A.; Bamoasa, A.O. Phase I safety and clinical activity of thymoquinone in patients with advanced refractory malignant disease. Shiraz E-Med. J. 2009, 10, 107–111. [Google Scholar]
- Ansary, J.; Cianciosi, D. Natural antioxidants: Is the research going in the right direction? Med. J. Nutr. Metab. 2020, 13, 187–191. [Google Scholar] [CrossRef]
| Proximate Analysis | (%) |
| Moisture | 3.8–7.0 |
| Crude protein | 18.59–31.2 |
| Crude fat | 22.0–56.4 |
| Total ash | 4.0–4.29 |
| Crude fiber | 3.7–4.7 |
| Carbohydrates | 24.9−40.0 |
| Fat Soluble Vitamin | mg/Kg |
| DL-α-tocopherol | 0.177 |
| DL-β-tocopherol | 9.027 |
| DL-γ-tocopherol | 5.427 |
| All trans retinol | 0.277 |
| Water Soluble Vitamin | mg/Kg |
| B1 | 13–18 |
| B6 | 4–15 |
| Niacin | 33–97 |
| Folic acid | 400–870 |
| Minerals | mg/100 g |
| Iron | 9.10−15.40 |
| Copper | 1.50−3.75 |
| Sodium | 41.20−55.0 |
| Potassium | 442.3−675.0 |
| Calcium | 154.4−305.0 |
| Zinc | 3.36−6.60 |
| Phosphorus | 378.12−576.90 |
| Magnesium | 134.90−147.05 |
| Fatty Acid Profiles | Molecular Formula | % |
|---|---|---|
| Myristic acid | CH3(CH2)12COOH | 0.29–1.1 |
| Myristoleic acid | C14H26O2 | 2.42–2.65 |
| Palimitic acid | C16H32O2 | 9.9–18.4 |
| Stearic acid | C18H36O2 | 1.51–3.70 |
| Oleic acid | C18H34O2 | 18.9–25.69 |
| Linoleic acid | C18H32O2 | 47.0–67.5 |
| Linolenic acid | C18H30O2 | 0.19–2.70 |
| Arachidic acid | C20H40O2 | 0.19–0.25 |
| Eicosenoic acid | C20H38O2 | 0.32–1.0 |
| Arachidonic acid | C20H32O2 | 0.19–0.25 |
| Behenic acid | C22H44O2 | 1.80–2.60 |
| Saturated fatty acids | n.a | 16.25–26.7 |
| Monounsaturated fatty acids | n.a. | 19.22–29.11 |
| Poly unsaturated fatty acids | n.a. | 49.1−72.42 |
| N. sativa Extracts/Fraction/ Component/Analogs | Experimental Models | Intervention | Main Results | References |
|---|---|---|---|---|
| Breast cancer | ||||
| In vitro | ||||
| Thymoquinone | MCF-7 and MDA-MB-231 | 40 μM for 12 h |
| [35] |
| Thymoquinone | MDA-MB-468 and T-47D cells | 0.01–60 μM for 12, 24 and 48 h |
| [36] |
| Proteins from black seeds | MCF-7 cells | 5–60 µg/mL for 48 h |
| [37] |
| Thymoquinone | MCF-7, MDA-MB-231 and BT-474 cells | 48, 40 and 32 mM, 24,14 and 11 mM, 38, 18, and 21 mM for 12, 24 and 48 h |
| [38] |
| Thymoquinone | MCF7, MDA-MB-231, and BT-549 cells | 25, 50 μM for 24 h |
| [39] |
| Thymoquinone | Mouse breast cancer cell line 4T1 | 5 μM for 6 h |
| [40] |
| Thymoquinone | MDA-MB-231 and MDAMB-468 | 2.5–5 μM for 72 h |
| [41] |
| Thymoquinone | MCF-7 cells | 100 μM for 48 h |
| [42] |
| In vivo | ||||
| Thymoquinone | NCr-Foxn1nu, female mice injected with MDA-MB-231-Luc + cells | 2 mg or 4 mg/kg body weight for 4 weeks |
| [39] |
| N. sativa emulsion of oil fraction | Six-week-old female Sprague-Dawley rats | 400 mg/100 g for 3 months |
| [43] |
| Colon cancer | ||||
| In vitro | ||||
| α-hederin and thymoquinone | HT-29 cells | 6–40 μM (Alpha) and 25–150 μM (TQ) for 24, 48 and 72 h |
| [44] |
| Thymoquinone | CPT-11-R LoVo cells | 2–8 μM for 24 h |
| [45] |
| Thymoquinone | CPT-11-R LoVo cells | 2, 4, 6, and 8 μM for 24 h |
| [46] |
| Thymoquinone | Caco-2, HCT-116, LoVo, DLD-1 and HT-29 cells | 12.5–110 μM for 24 and 48 h |
| [47] |
| Thymoquinone | LoVo cells | 5–20 μmol/L for 24 h |
| [48] |
| Thymoquinone | HCT116 cells | 0.1 mL for 24 h, 48 h and 72 h |
| [49] |
| N. sativa | AOM treated male Sprague Dawley rats | 200 mg/kg for 5 weeks |
| [50] |
| Thymoquinone | 0.2 mg/kg for 5 weeks | |||
| All-trans-retinol plus | 1.2 mg/kg for 5 weeks | |||
| Selenium | 100 mg/kg for 5 weeks | |||
| DL-α-tocopherol | 10 mg/kg for 5 weeks | |||
| Thymoquinone | PGE2 treated nude mice | 0.5, 10 and 20 µmol/L/ 3 time/week for three weeks |
| [49] |
| Thymoquinone | DMH treated male Albino Wistar rats | 10 mg/kg/day |
| [51] |
| Hepatic Cancer | ||||
| In vitro | ||||
| Alcoholic extracts of N. sativa | HepG2 cells | 1000, 2500, and 5000 μg/mL for 6, 24, 48, and 72 h |
| [52] |
| Thymoquinone | HepG2 cells | 6–50 μM for 6, 12, 18 h |
| [53] |
| Thymoquinone | HepG2 cells | 20, 40,60, 80 and 100 μM for 24, 48 and 72 h |
| [54] |
| Thymol and cravacol | HepG2 cells | 25, 50, and 100 mM for 24, 48, 72 h |
| [55] |
| In vivo | ||||
| N. sativa extract | DENA induced preneoplastic stage of HCC in rats | 150, 250, 350 mg/kg/day body weight for 16 weeks |
| [56] |
| Thymoquinone | DENA induce male Wistar albino rats | 4 mg/kg/day for 7 days |
| [57] |
| N. sativa extract | DENA-induced hepatocarcinogenesis Male Wistar rats | 250 mg/kg/day for 5 days |
| [58] |
| Thymoquinone | NDEA induce male Wistar strain albino rats | 20 mg/kg body weight |
| [59] |
| Lung cancer | ||||
| In vitro | ||||
| Thymoquinone | A549 cells | 25, 50 and 100 μM For 72 h |
| [60] |
| Thymoquinone | NCI-H460 and NCI-H146 cells | 20, 40, 60, 80 and 100 μM for 24 h |
| [61] |
| Thymoquinone | A549 cells | 5, 10, 20, 40, 80, 160 μmol/L for 24, 48, or 72 h |
| [62] |
| Thymoquinone | A549 cells | 10, 25 μM for 24 h |
| [63] |
| In vivo | ||||
| Thymoquinone | MCT treated male Sprague–Dawley rats | 8 mg/kg, 12 mg/kg, 16 mg/kg/day for 2 weeks |
| [64] |
| Pancreatic cancer | ||||
| In vitro | ||||
| Thymoquinone | MUC4 expressed FG/COLO357 and CD18/HPAF cells | 10–100 μmol/L for 24 h |
| [65] |
| Thymoquinone | HS766T cells | 25, 50, 75 μM for 3, 6, 24 h |
| [66] |
|
Thymoquinone-2G, Thymoquinone-4A1 and Thymoquinone-5A1 | MiaPaCa-2, BxPC-3, AsPC-1 and HPAC | 10 μM for 72 h |
| [67] |
| ATQTHB and ATQTFB | MiaPaCa-2 and BxPC-3 cells | 5, 10, 25 μM for 72 h |
| [68] |
| Cervical cancer | ||||
| In vitro | ||||
|
Organic extracts of N. sativa (methanolic, n-hexane, and chloroform extracts) | HeLa cells | 21.1%, 30% and 42% for 24 h |
| [69] |
| N. sativa oil fraction and thymoquinone | HeLa cells | 0.03 to 2 μL/mL and 6.25 to 100 μM for 48 h |
| [70] |
| Thymoquinone | SiHa cells | to 30 μg/mL for 24, 48 and 72 h |
| [71,72] |
| Thymoquinone | SiHa and C33A cells | 10–100 μM for 22 h |
| [73] |
| Thymoquinone | SiHa and CaSki | 1, 5, 10, 20 and 40 μM for 12, 24, 36 and 48 h |
| [74] |
| Poloxin | HeLa cells | 5–25 μM for |
| [75] |
| Leukemia/Blood cancer | ||||
| In vitro | ||||
| Thymoquinone | Jurkat cells | 10 and 20 μM for 24 h |
| [76] |
| Thymoquinone | CEMss cell | 50, 25, 12.5, 6, 3 and 1.5 μg/mL for 24, 48, 72 h |
| [77] |
| Thymoquinone | Jurkat cells | 100 mM for 24h |
| [78] |
| Thymoquinone | Kasumi-1, MV4–11, THP-1 and ML-1 | 1, 10, 30 and 300 nM, 1, 3, 10, 30 and 100 μM for 24 h |
| [79] |
| Thymoquinone | Murine WEHI-3 cells | 100, 50, 25, 12.5, 6, 3 and 1.5 mg/mL for 24 h |
| [80] |
| Thymoquinone | Jurkat cells | 5–10 μM for 24 h |
| [81] |
| Thymoquinone | MDN and XG-2 cell lines | 0.5–50 μM for 0.25–48 h |
| [82] |
| Thymoquinone derivatives bound to terpene residues | Human HL-60 leukemia | 5 μM for 72 h |
| [83] |
| In vivo | ||||
| Thymoquinone | C57BL/6 mice | 15 and 30 mg/kg/2 dose/week for 3 weeks |
| [79] |
| Thymoquinone | BALB/c mice | 100 mg/mL, 50 mg/kg for 3 weeks |
| [80] |
| Urinary cancer | ||||
| In vitro | ||||
| Thymoquinone | Caki cells | 25, 50 and 75 μM for 24 h |
| [84] |
| Thymoquinone | Caki-1, Caki-2, A498 cells | 0.5–10 μM for 24, 48 h |
| [85] |
| Thymoquinone | Caki-1 cells | 1–25 μM for 24 h |
| [86] |
| Thymoquinone | 786-O and RCC 769-P cells | 0, 10, 20, 40, 60, 80 and 100 μmol/L for 24 h or 48 h |
| [87] |
| Hydroalcoholic extract of N. sativa and its fraction | ACHN cells | 50, 100, 250, 500, 750, 1000, 1250, 1500, 1750, and 2000 mg/mL for 24, 48 and 72 h |
| [88] |
| Thymoquinone | T24 and HTB-9 cells | 10 to 75 μM for 48 h |
| [89] |
| Thymoquinone | T24 and 253J bladder cancer cells | 10–40 μM for 24 h |
| [90] |
| Thymoquinone | T24 and 253J bladder cancer cells | 40–80 μmol/L for 24 h |
| [91] |
| In vivo | ||||
| Thymoquinone | male BALB/c nude mice | 1 mg/kg or 5 mg /kg for 3 times/day for 35 days |
| [86] |
| Thymoquinone | nude mice | 10 mg/kg/every 3 days for 21 days |
| [90] |
| Skin cancer | ||||
| In vitro | ||||
| Thymoquinone | A431 cells | 2–100 μM for 24 or 48 h |
| [94] |
| Thymoquinone | Melanoma MDA-MB-435 cells | 5 μM for 6 h |
| [40] |
| Thymoquinone and its conjugated derivatives | 518A2 melanoma | 3.9 μm for 72 h |
| [83] |
| Ovarian cancer | ||||
| In vitro | ||||
| Thymoquinone | Murine ID8-NGL cells | 25 μM for 24 |
| [95] |
|
Thymoquinone and its analogs | OVCAR-8 and CIS-A2780 | 10 mM for 24 h |
| [96] |
| In vivo | ||||
| Thymoquinone | ID8-NGL treated C57BL/6 mice | 20 mg/kg thrice weekly for 10 days and 30 days |
| [95,97] |
| Prostate cancer | ||||
| In vitro | ||||
| Thymoquinone | DU-145 and PC3 cells | 0.1–10 μM for 24 h |
| [98] |
| Thymoquinone | DU-145 cells | 60 μM for 72 h |
| [99] |
| Thymoquinone | LnCaP cells | 1, 5, 10, 25 and 50 μM for 24 or 48 h |
| [100] |
| Thymoquinone | C4-2B and PC-3 cells | 25–150 μmol/L for 24–48 h |
| [101] |
| Thymoquinone | PC3, LNCaP, DU145 and C42B cells | 0–20 μM for 72 h |
| [102] |
| Bone cancer | ||||
| In vitro | ||||
| Thymoquinone | SaOS-2 cells | 20, 40 and 80 μmol/l for 24 h |
| [103] |
| In vivo | ||||
| Thymoquinone | Male athymic BALB/c nu/nu mice | 6 mg/kg/day for 15 days |
| [103] |
| Thymoquinone | MDA-MB-231-lucC expressing NCr-Foxn1nu, female mice | 2 mg or 4 mg/kg for 4 weeks |
| [39] |
| Fibrous histiosarcoma | ||||
| In vivo | ||||
| N. sativa and volatile oil | DMBA induced Wistar rats | 1000 or 4000 ppm daily for 30 days |
| [104] |
| Oral cancer | ||||
| In vitro | ||||
| Thymoquinone | T28 cells and N28 cells | 5–100 μM for 24 h |
| [105] |
| Thymoquinone | SCC-4, SAS, SASVO3, OC2, and (B) S-G cells | 20, 40, and 60 μM for 24h |
| [106] |
| In vivo | ||||
| Thymoquinone | BALB/c AnN.CgFoxn nu/Crl Narl mice | 10 and 25 mg/kg body wt for 20 days |
| [106] |
| Thymoquinone | DMBA induced hamster rats | 30 mg/kg body wt for 14 weeks |
| [107] |
| Brain cancer | ||||
| In vitro | ||||
| Thymoquinone | U87 cells | 100 mM for 24 h |
| [78] |
| Thymoquinone | U87MG, U118MG, and A172 cells | 10–100 μM for 24 h |
| [108] |
| Head and neck | ||||
| In vitro | ||||
| Thymoquinone | SCC25 and CAL27 HNSCC cells | 0–80 μM for 72 h |
| [109] |
| N. sativa Extracts/Fraction/ Component/Analogs | Experimental Models | Intervention | Results | References |
|---|---|---|---|---|
| Pancreatic cancer | ||||
| In vitro | ||||
| Thymoquinone and Gemcitabine | PANC-1, AsPC-1 and BxPC-3 | 0–50 (Thymoquinone) and 0–200 μmol/L (Gemcitabine) for 48 h |
| [113] |
| TQ analogs (TQ-2G, TQ-4A1 and TQ-5A1) and Gemcitabine or Oxaliplatin | MiaPaCa-2 | 10 (Thymoquinone analogs) and 0.5 μM (Gemcitabine) or 6.0 μg/mL (Oxaliplatin) for 84 h |
| [67] |
| ATQTHB or ATQTFB analogs and Gemcitabine | MiaPaCa-2 cells | 2.5 (analog) and 0.5 μM (Gemcitabine) for 24h and 72 h |
| [68] |
| Thymoquinone and Gemcitabine | MIA PaCa-2 and PANC-1 cells | 25–36 μM for 48 h |
| [114] |
| In vivo | ||||
| Thymoquinone and Gemcitabine | BALB/c nude mice | 1.0 mg/day (Thymoquinone) and 50 mg/kg 3 times/week (Gemcitabine), |
| [113] |
| Breast cancer | ||||
| In vitro | ||||
| Thymoquinone and Gemcitabine | MCF-7 cells and T47D | 0.01 to 300 μM for 24, 48 and 72 h |
| [115] |
| Thymoquinone and Paclitaxel | 4T1 cells | 6.25, 12.5 and 25 (Thymoquinone) μM and 10 μg/mL (Paclitaxel) for 24 h |
| [116] |
| Thymoquinone and Cisplatin or Docetaxel | MDAMB-468 | 0.5–2 μM Thymoquinone for 24 h and 72 h |
| [41] |
| In vivo | ||||
| Thymoquinone and Doxorubicin | MDA-MB-231 cell xenograft nude mice | 4 mg/kg/6 days/week (Thymoquinone) and 2.5 mg/kg/once/per week |
| [35] |
| Colon cancer | ||||
| In vivo | ||||
| Thymoquinone and Vitamin D3 | azoxymethane treated rat | 35 mg/kg/day, three days/week (Thymoquinone) and 500 IU/day, 3 days/week (Vitamin D3) |
| [117] |
| Thymoquinone and 5-Fluorouracil | Azoxymethane treated male Wistar rats | 35 mg/kg/d for 3 d/week (Thymoquinone) and 12 mg/kg/d for 4 days after 6 mg/kg/day for 5-Fluorouracil |
| [118] |
| Lung cancer | ||||
| In vitro | ||||
| Thymoquinone and Cisplatin | NCI-H460 and NCI-H146 | 80 and 100 μM (Thymoquinone) and 1.25, 2.5 and 5.0 (Cisplatin) for 24, 48 and 72 h |
| [61] |
| Thymoquinone and Cisplatin | LNM35 | 10 and 50 μM (Thymoquinone) and 10 μM (Cisplatin) for 24 h |
| [119] |
| In vivo | ||||
| Thymoquinone and Cisplatin | Severe combined immunodeficiency mice | 5 and 20 mg/kg/2days for 3 weeks (Thymoquinone) and 2.5 (Cisplatin) mg/kg/week for 3 weeks |
| [61] |
| Leukemia | ||||
| In vitro | ||||
| Thymoquinone and Doxorubicin | HL-60 cells | 5 μM for 24 h |
| [120] |
| Thymoquinone and Doxorubicin | Jurkat cells | 0–30 μm for 24, 48 and 72 h |
| [121] |
| Renal Cancer | ||||
| In vivo | ||||
| N. sativa oil and Cisplatin | male Wistar rats | 2 mL/kg (Thymoquinone) and 3 mg/kg (Cisplatin) body wt for 20 days |
| [123] |
| Thymoquinone and Cisplatin | male Wistar rats | 1.5 mg/kg (Thymoquinone) and 3 mg/kg body wt (Cisplatin) for 20 days |
| |
| Ovarian cancer | ||||
| In vitro | ||||
| Thymoquinone and Cisplatin or Oxaliplatin | A2780 and A2780 cisR cells | 2.28–36.49 and 1.93- 30.83 μM (Thymoquinone) 0.26–4.09 and 1.66–26.52 μM (Cisplatin), 0.16–2.62 and 0.59–9.41 μM (Oxaliplatin) for 72 h |
| [124] |
| Thymoquinone and Cisplatin | SK-OV-3 cells | 10, 15, 20 and 25 µmol/L(Thymoquinone) and 5, 10, 15 and 20 µmol/L (Cisplatin) for 24, 48, and 72 h |
| [125] |
| Thymoquinone and Cisplatin | Murine ID8-NGL cells | 2.5, 5, 10,20, 25,50 μM (Thymoquinone) and 0.25, 0.5, 1, 2,2.5, 5 (Cisplatin) μM for 72 h |
| [95] |
| In vivo | ||||
| Thymoquinone and Cisplatin | ID8-NGL treated C57BL/6 mice | 20 mg/kg/week for three times (Thymoquinone), 2 mg/kg /weekly (Cisplatin) for 30 days |
| [95] |
| Prostate cancer | ||||
| In vitro | ||||
| Thymoquinone and Zoledronic acid | PC-3 and DU-145 cells | 55.3 and 51.0 μM (Thymoquinone) and 95.0, 52.9 μM (Zoledronic acid) for 24, 48 and 72 h |
| [126] |
| Thymoquinone and Docetaxel | DU-145 cells | 60 μM (Thymoquinone) and 0.1 and 10 nM (Docetaxel) for 24, 48 and 72 h |
| [99] |
| Oral cancer | ||||
| In vitro | ||||
| Thymoquinone and Diosgenin | Human SCC A431, Hep2 and RPMI 2650 cells | 10 µM (Thymoquinone) and 20 µM (Diosgenin) for 48 h |
| [94] |
| Thymoquinone and Cisplatin | UMSCC-14C cells | 0.01–100 μM for 24, 48 and 72 h |
| [127] |
| Head and neck cancer | ||||
| In vitro | ||||
| Thymoquinone and radiation | SCC25 and CAL27 HNSCC cell lines | 0–80 μM (Thymoquinone) and 2 Gy/min for 72 h |
| [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansary, J.; Giampieri, F.; Forbes-Hernandez, T.Y.; Regolo, L.; Quinzi, D.; Gracia Villar, S.; Garcia Villena, E.; Tutusaus Pifarre, K.; Alvarez-Suarez, J.M.; Battino, M.; et al. Nutritional Value and Preventive Role of Nigella sativa L. and Its Main Component Thymoquinone in Cancer: An Evidenced-Based Review of Preclinical and Clinical Studies. Molecules 2021, 26, 2108. https://doi.org/10.3390/molecules26082108
Ansary J, Giampieri F, Forbes-Hernandez TY, Regolo L, Quinzi D, Gracia Villar S, Garcia Villena E, Tutusaus Pifarre K, Alvarez-Suarez JM, Battino M, et al. Nutritional Value and Preventive Role of Nigella sativa L. and Its Main Component Thymoquinone in Cancer: An Evidenced-Based Review of Preclinical and Clinical Studies. Molecules. 2021; 26(8):2108. https://doi.org/10.3390/molecules26082108
Chicago/Turabian StyleAnsary, Johura, Francesca Giampieri, Tamara Y. Forbes-Hernandez, Lucia Regolo, Denise Quinzi, Santos Gracia Villar, Eduardo Garcia Villena, Kilian Tutusaus Pifarre, José M. Alvarez-Suarez, Maurizio Battino, and et al. 2021. "Nutritional Value and Preventive Role of Nigella sativa L. and Its Main Component Thymoquinone in Cancer: An Evidenced-Based Review of Preclinical and Clinical Studies" Molecules 26, no. 8: 2108. https://doi.org/10.3390/molecules26082108
APA StyleAnsary, J., Giampieri, F., Forbes-Hernandez, T. Y., Regolo, L., Quinzi, D., Gracia Villar, S., Garcia Villena, E., Tutusaus Pifarre, K., Alvarez-Suarez, J. M., Battino, M., & Cianciosi, D. (2021). Nutritional Value and Preventive Role of Nigella sativa L. and Its Main Component Thymoquinone in Cancer: An Evidenced-Based Review of Preclinical and Clinical Studies. Molecules, 26(8), 2108. https://doi.org/10.3390/molecules26082108










