The Annealing of Acetylated Potato Starch with Various Substitution Degrees
Abstract
1. Introduction
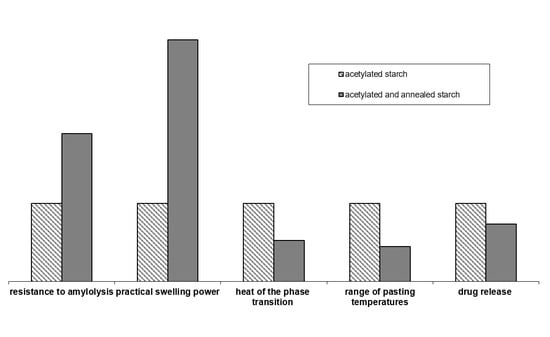
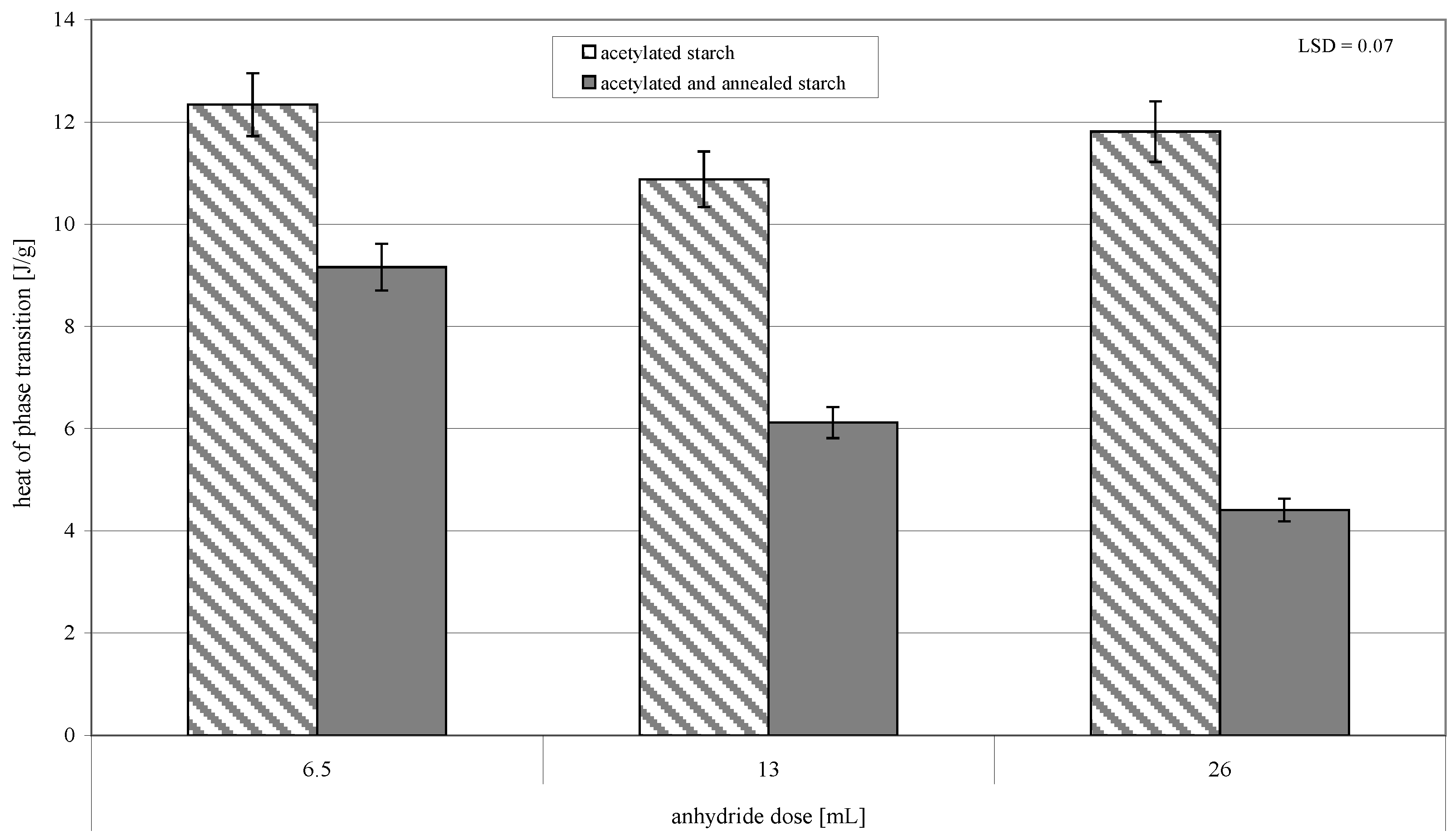
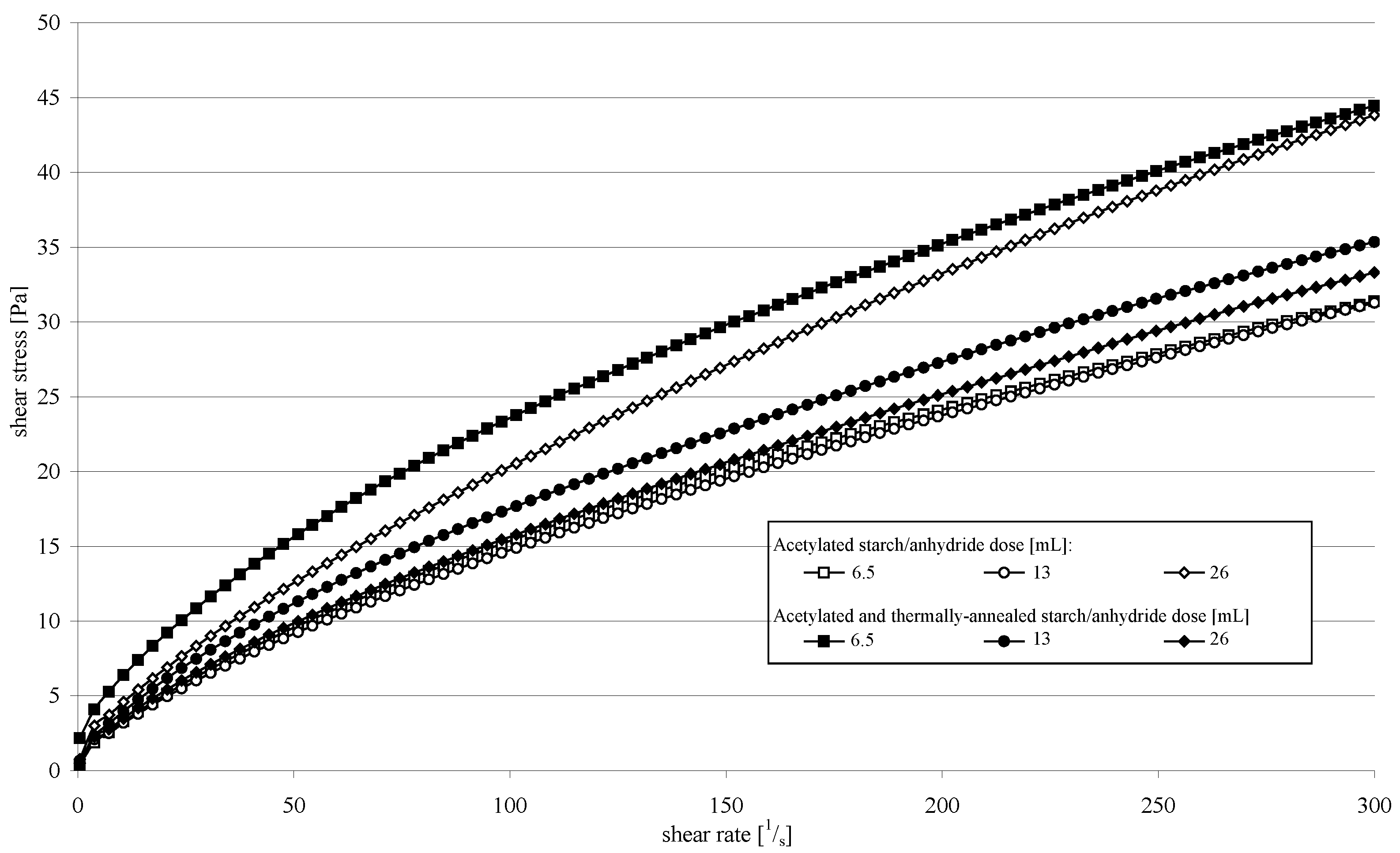
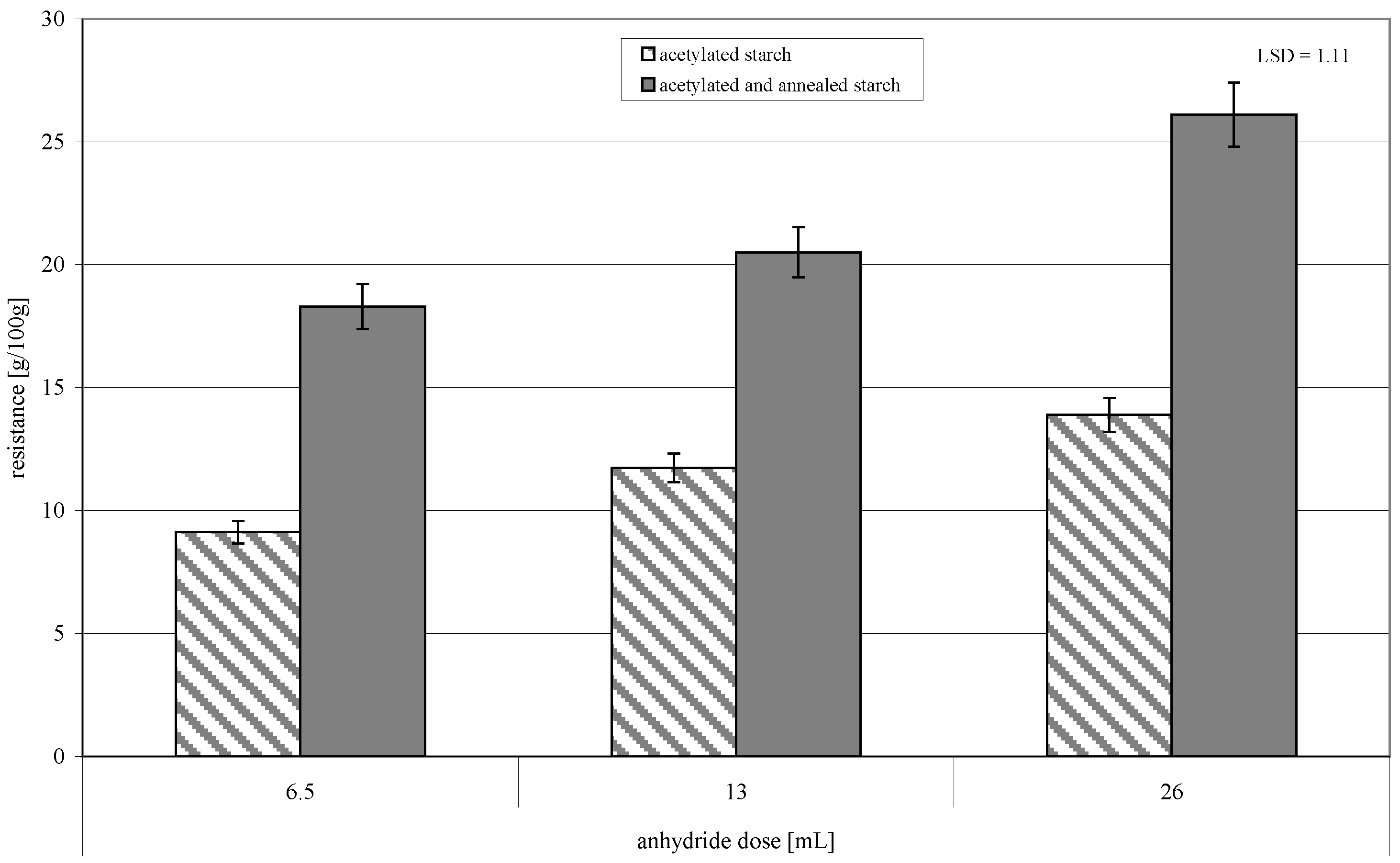
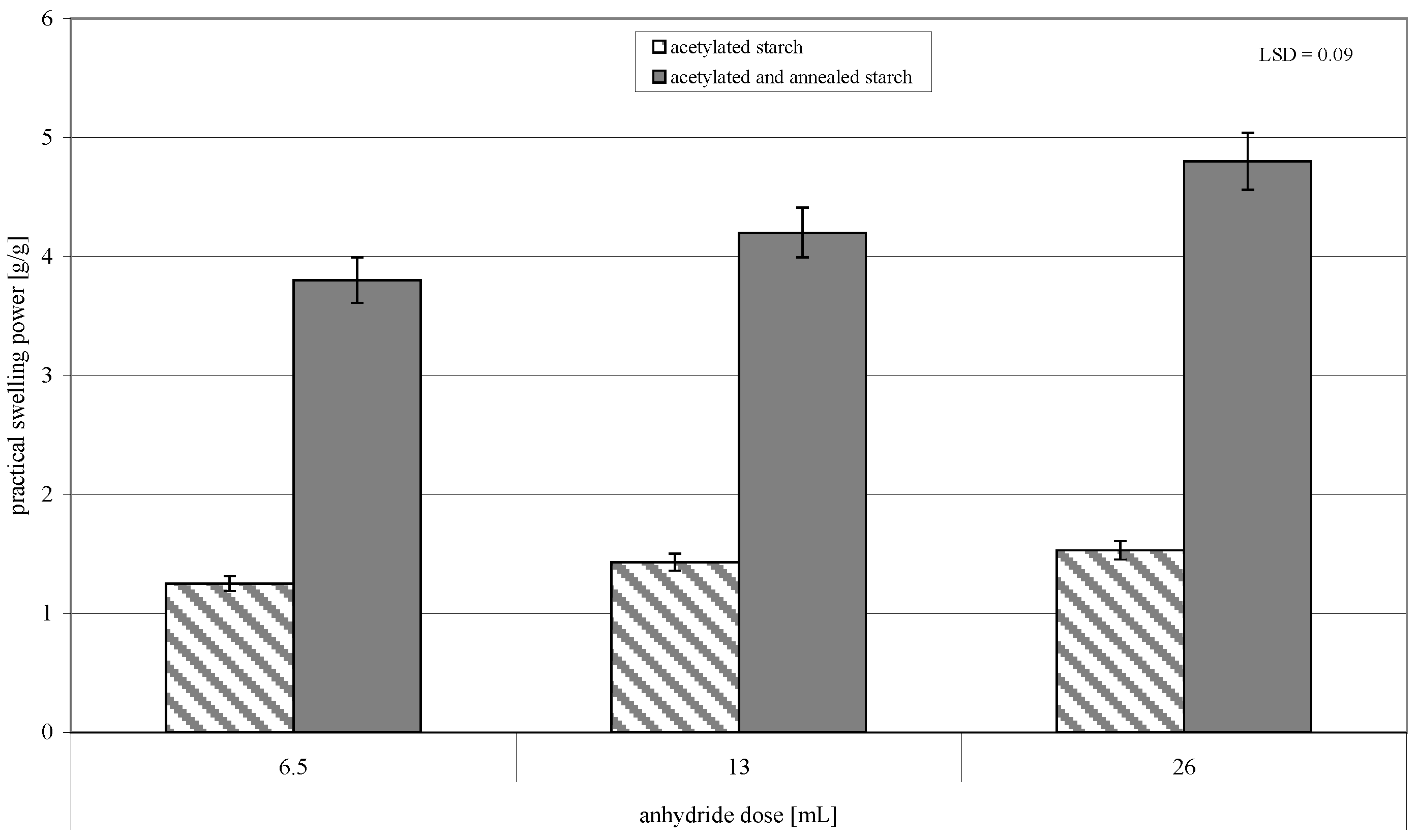
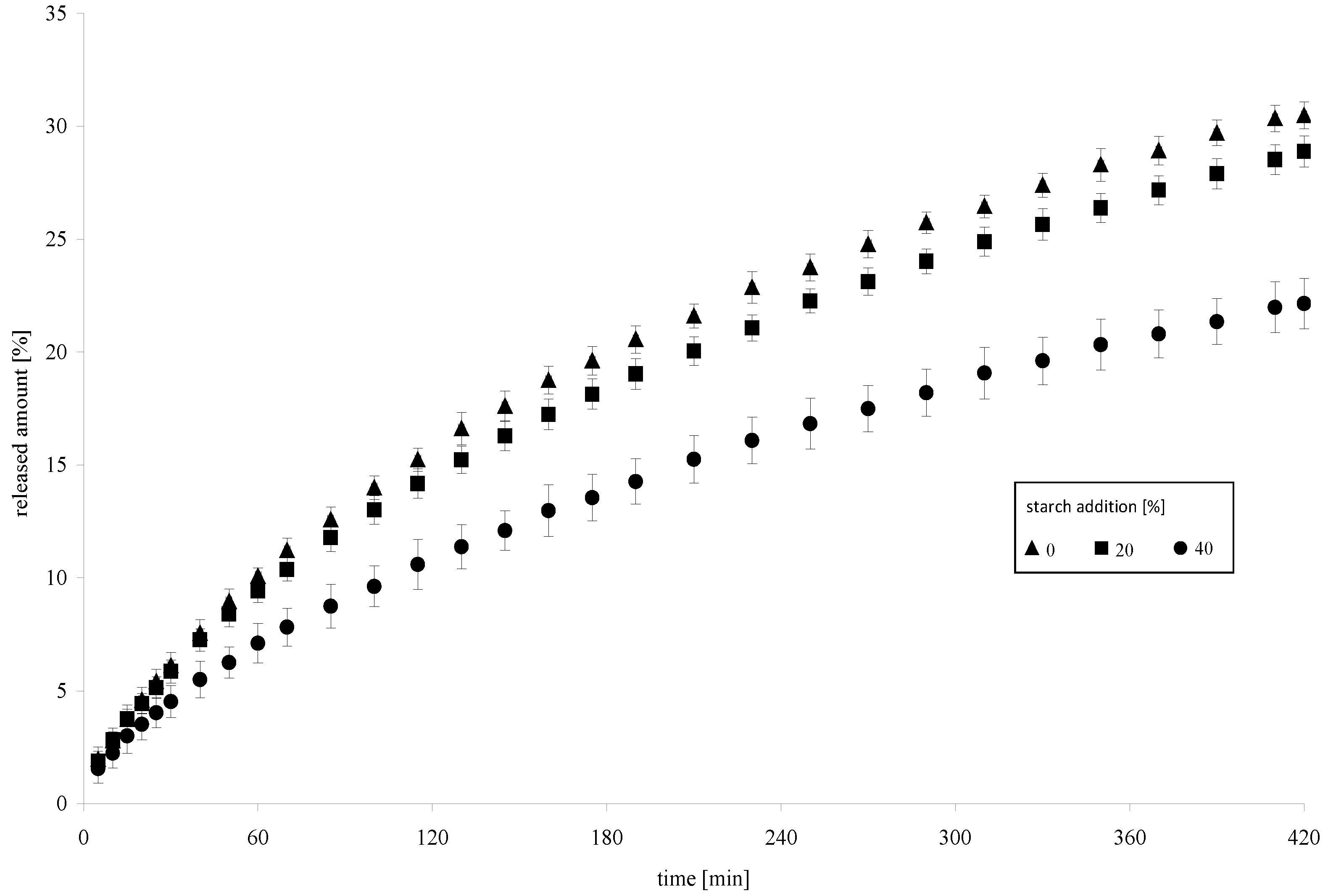
2. Results
3. Materials and Methods
3.1. Materials
3.2. Production of Modified Preparations
3.3. Determination of the Degree of Acetylation of Starch Preparations
3.4. Determination of the Characteristics of Phase Transitions of Starch Preparations with Differential Scanning Calorimetry (DSC)
3.5. Determination of the Flow Curves of Pastes Made of Starch Preparations Using a Haake Oscillating-Rotating Viscosimeter
3.6. Determination of the Resistance of Starch Preparations to Amyloglucosidase Action
3.7. Determination of Practical Swelling Power of Starch Preparations in Water Having a Temperature of 20 °C
3.8. Determination of the Dynamics of Model Drug Release from a Hydrogel with the Addition of a Modified Starch Preparation
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Din, Z.U.; Xiong, H.; Fei, P. Physical and Chemical Modification of Starches-A Review. Crit. Rev. Food Sci. Nutr. 2015, 57, 2691–2705. [Google Scholar]
- Rocha, T.S.; Felizardo, S.G.; Jane, J.; Franco, C.M.L. Effect of annealing on the semicrystalline structure of normal and waxy corn starches. Food Hydrocoll. 2012, 29, 93–99. [Google Scholar] [CrossRef]
- Piecyk, M.; Konarzewska, M.; Sitkiewicz, I. Wpływ modyfikacji hydrotermicznej typu annealing na wybrane właściwości skrobi grochu (Pisum sativum). Żywność. Nauka. Technologia. Jakość 2009, 5, 58–71. [Google Scholar]
- Zavareze, E.D.R.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Tester, R.F.; Debon, S.J.J. Annealing of starch—A review. Int. J. Biol. Macromol. 2000, 27, 1–12. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Afolabi, T.A.; Olu-Owolabi, B.I. Hydrothermal treatments of Finger millet (Eleusine coracana) starch. Food Hydrocoll. 2005, 19, 974–983. [Google Scholar] [CrossRef]
- Hormdok, R.; Noomhorm, A. Hydrothermal treatments of rice starch for improvement of rice noodle quality. LWT-Food Sci. Technol. 2007, 40, 1723–1731. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dupruel, P.; Schact, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jin, F.; Yu, J. Pea Starch Annealing: New Insights. Food Bioproc. Tech. 2012, 6, 3564–3575. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Wang, S.; Wang, S. Annealing improves paste viscosity and stability of starch. Food Hydrocoll. 2017, 62, 203–211. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Wang, Y.J. Effect of annealing on starch–palmitic acid interaction. Carbohydr. Polym. 2004, 57, 327–335. [Google Scholar] [CrossRef]
- Masina, M.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef]
- Majzoobi, M.; Sabery, B.; Farahnaky, A.; Karrila, T.T. Physicochemical properties of cross-linked-annealed wheat starch. Iran Polym. J. 2012, 21, 513–522. [Google Scholar] [CrossRef]
- Dupuis, J.H.; Liu, Q. Potato Starch: A Review of Physicochemical, Functional and Nutritional Properties. Am. J. Potato Res. 2019, 96, 127–138. [Google Scholar] [CrossRef]
- Zięba, T.; Solińska, D.; Kapelko-Żeberska, M.; Gryszkin, A.; Ačkar, Đ.; Lončarić, A.; Babić, J.; Jozinović, A. Properties of roasted starch with apple distillery wastewater. Polymers 2020, 12, 1668. [Google Scholar] [CrossRef] [PubMed]
- Bello-Pérez, L.A.; Agama-Acevedo, E.; Zamudio-Flores, P.B.; Mendez-Montealvo, G.; Rodriguez-Ambriz, S.L. Effect of low and high acetylation degree in the morphological, physicochemical and structural characteristics of barley starch. LWT-Food Sci. Technol. 2010, 43, 1434–1440. [Google Scholar] [CrossRef]
- Diop, C.I.K.; Li, H.L.; Xie, B.J.; Shi, J. Effects of acetic acid/acetic anhydride ratios on the properties of corn starch acetates. Food Chem. 2011, 126, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Kapelko, M.; Zięba, T.; Michalski, A. Effect of the production method on the properties of RS3/RS4 type resistant starch. Part 2. Effect of a degree of substitution on the selected properties of acetylated retrograded starch. Food Chem. 2012, 135, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Mbougueng, P.D.; Tenin, D.; Scher, J.; Tchiégang, C. Influence of acetylation on physicochemical, functional and thermal properties of potato and cassava starches. J. Food Eng. 2012, 108, 320–326. [Google Scholar] [CrossRef]
- Hong, J.; Zeng, X.A.; Brennan, C.S.; Brennan, M.; Han, Z. Recent Advances in Techniques for Starch Esters and the Applications: A Review. Foods 2016, 5, 50. [Google Scholar] [CrossRef]
- Chen, Z.; Schols, H.A.; Voragen, A.G.J. Differently sized granules from acetylated potato and sweet potatostarches differ in the acetyl substitution patternof their amylose populations. Carbohydr. Polym. 2004, 56, 219–226. [Google Scholar] [CrossRef]
- Das, A.M.; Singh, G.; Singh, S.; Riar, C.R. Effect of acetylation and dual modification on physico-chemical, rheological and morphological characteristics of sweet potato (Ipomoea Batatas) starch. Carbohydr. Polym. 2010, 80, 725–732. [Google Scholar] [CrossRef]
- Muhamedbegović, B.; Šubarić, D.; Babić, J.; Aćkar, D.; Jasić, M.; Keran, H.; Budimlić, A.; Matas, I. Modification of potato starch. Technologica Acta 2012, 5, 1–6. [Google Scholar]
- Punia, S. Barley starch modifications: Physical, chemical and enzymatic-A review. Int. J. Biol. Macromol. 2020, 144, 578–585. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. Resistant Starch. In Science and Technology of Fibers in Food Systems; Welti-Chanes, J., Serna-Saldívar, S., Campanella, O., Tejada-Ortigoza, V., Eds.; Springer: Cham, Swizerland, 2020; pp. 153–183. [Google Scholar] [CrossRef]
- Alimi, B.A.; Workneh, T.S.; Oyeyinka, S.A. Structural, rheological and in-vitro digestibility properties of composite corn-banana starch custard paste. LWT–Food Sci.Technol. 2017, 79, 84–91. [Google Scholar] [CrossRef]
- Kiatponglarp, W.; Tongta, S.; Rolland-Sabaté, A.; Buléon, A. Crystallization and chain reorganization of debranched rice starches in relation to resistant starch formation. Carbohydr. Polym. 2015, 122, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Trung, P.H.B.; Ngoc, L.B.B.; Hoa, P.N.; Tien, N.N.T.; Hung, P.V. Impact of heat-moisture and annealing treatments on physicochemical properties and digestibility of starches from different colored sweet potato varieties. Int. J. Biol. Macromol. 2017, 105, 1071–1078. [Google Scholar] [CrossRef]
- Xu, M.; Saleh, A.S.M.; Liu, Y.; Jing, L.; Zhao, K.; Wu, H.; Zhang, G.; Yang, S.O.; Li, W. The changes in structural, physicochemical, and digestive properties of red adzuki bean starch after repeated and continuous annealing treatments. Starch/Stärke 2018, 70, 1–9. [Google Scholar] [CrossRef]
- Jayakody, L.; Hoover, R. Effect of annealing on the molecular structure and physicochemical properties of starches from different botanical origins–A review. Carbohydr. Polym. 2008, 74, 691–703. [Google Scholar] [CrossRef]
- Simsek, S.; Ovando-Martínez, M.; Whitney, K.; Bello-Pérez, L.A. Effect of acetylation, oxidation and annealing on physicochemical properties of bean starch. Food Chem. 2012, 134, 1796–1803. [Google Scholar] [CrossRef]
- Leszczyński, W. Resistant starch–classification, structure, production. P. J. Food Nutr. Sci. 2004, 54, 37–50. [Google Scholar]
- Liu, H.; Guo, X.; Li, W.; Wang, X.; Iv, M.; Peng, Q.; Wang, M. Changes in physicochemical properties and in vitro digestibility of common buckwheat starch by heat-moisture treatment and annealing. Carbohydr. Polym. 2015, 132, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.Z.; Vanier, N.L.; Deon, V.G.; Moomand, K.; El Halal, S.L.M.; Zavareze, E.D.R.; Lim, L.T.; Dias, A.R.G. Effects of single and dual physical modifications on pinhão starch. Food Chem. 2015, 187, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ashogbon, A.O.; Akintayo, E.T. Recent trend in the physical and chemical modification of starches from different botanical sources: A review. Starch/Stärke 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Singh, N. Characteristics of acetylated starches prepared using starches separated from different rice cultivars. J. Food Eng. 2005, 70, 117–127. [Google Scholar] [CrossRef]
- Waduge, R.N.; Hoover, R.; Vasanthan, T.; Gao, J.; Li, J. Effect of annealing on the structure and physicochemical properties of barley starches of varying amylose content. Food Res. Int. 2006, 39, 59–77. [Google Scholar] [CrossRef]
- Golachowski, A. Properties of acetylated starch obtained from SO2-treated starch milk. Electron. J. Pol. Agric. Univ. 2003, 6, 1. [Google Scholar]
- Gryszkin, A.; Zięba, T.; Kapelko, M.; Buczek, A. Effect of thermal modifications of potato starch on its selected properties. Food Hydrocoll. 2014, 40, 122–127. [Google Scholar] [CrossRef]
- Balcerowiak, W. Różnicowa kalorymetria skaningowa. Materiały Trzeciej Szkoły Analizy Termicznej SAT 2002, 1, 33–48. [Google Scholar]
- Zięba, T.; Kapelko, M.; Gryszkin, A. Selected properties of potato starch subjected to multiple physical and chemical modifications. Polish J. Food Nutr. Sci. 2007, 57, 639–645. [Google Scholar]
- Kobryń, J.; Zięba, T.; Sowa, S.K.; Musiał, W. Influence of Acetylated Annealed Starch on the Release of β-Escin from the Anionic and Non-Ionic Hydrophilic Gels. Pharmaceutics 2020, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Sanjivkumar, B.; Rajkumar, D.; Mallikarjun, P.; Karankumar, B.; Rao, K.S. Development and method validation of Aesculus hippocastanum extract. Int. Res. J. Pharm. 2012, 3, 324–328. [Google Scholar]
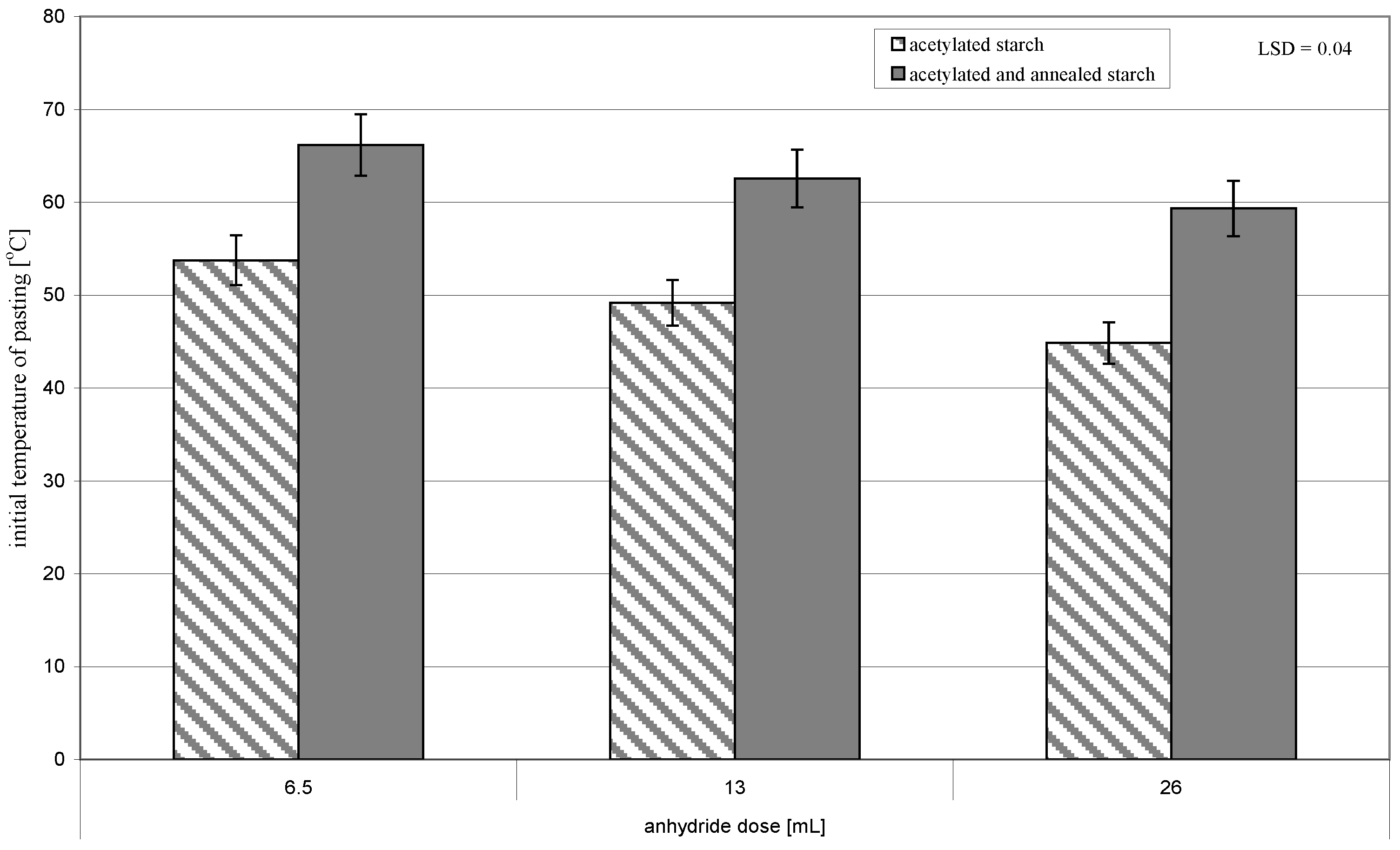
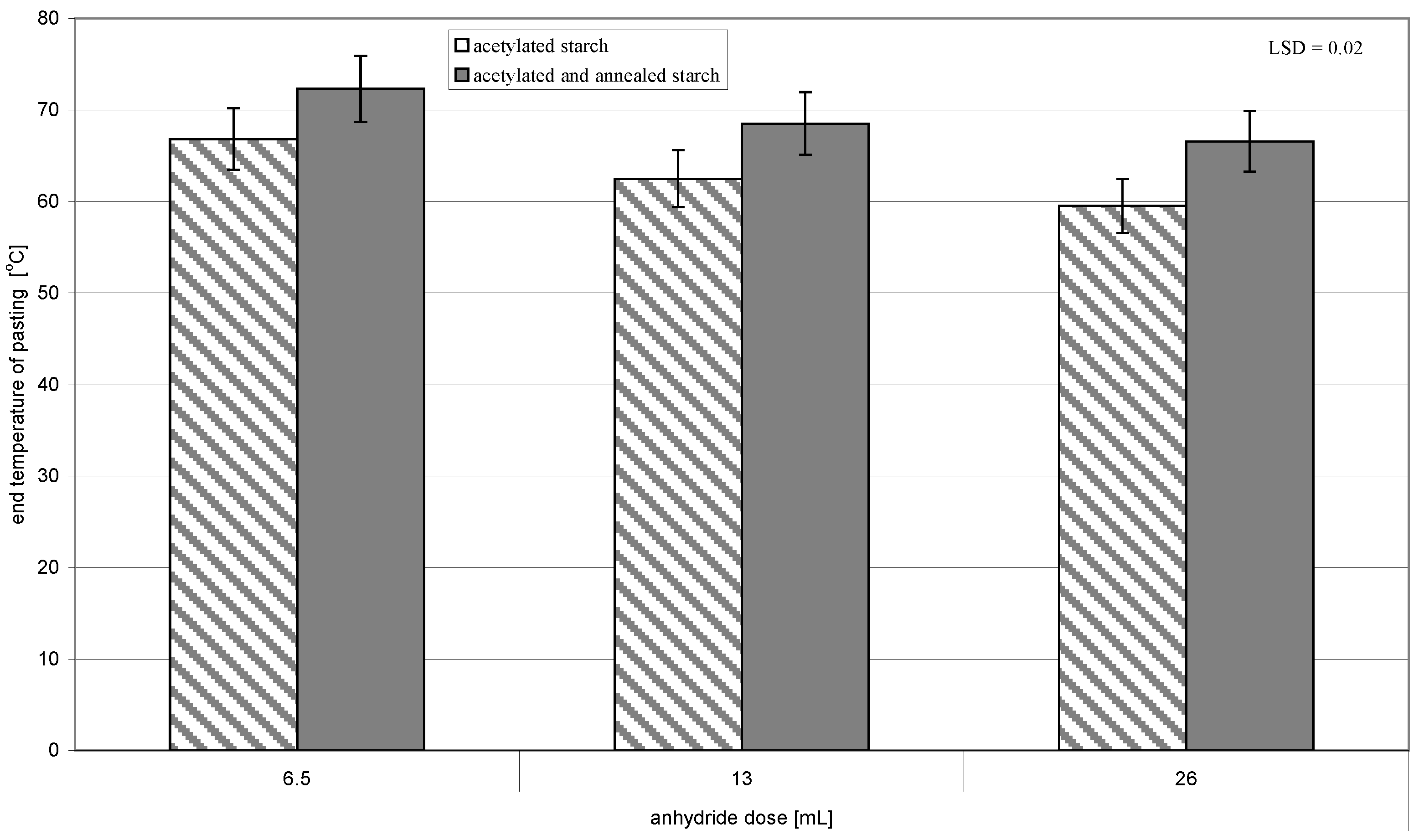
| Acetic Acid Anhydride Dose [mL] | Acetylation Degree [] | Initial Temperature of Pasting [°C] |
|---|---|---|
| 6.5 | 0.05 ± 0.001 | 53.8 ± 0.01 |
| 13 | 0.11 ± 0.001 | 49.2 ± 0.02 |
| 26 | 0.18 ± 0.001 | 44.9 ± 0.01 |
| LSD | 0.01 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zięba, T.; Wilczak, A.; Kobryń, J.; Musiał, W.; Kapelko-Żeberska, M.; Gryszkin, A.; Meisel, M. The Annealing of Acetylated Potato Starch with Various Substitution Degrees. Molecules 2021, 26, 2096. https://doi.org/10.3390/molecules26072096
Zięba T, Wilczak A, Kobryń J, Musiał W, Kapelko-Żeberska M, Gryszkin A, Meisel M. The Annealing of Acetylated Potato Starch with Various Substitution Degrees. Molecules. 2021; 26(7):2096. https://doi.org/10.3390/molecules26072096
Chicago/Turabian StyleZięba, Tomasz, Aleksandra Wilczak, Justyna Kobryń, Witold Musiał, Małgorzata Kapelko-Żeberska, Artur Gryszkin, and Marta Meisel. 2021. "The Annealing of Acetylated Potato Starch with Various Substitution Degrees" Molecules 26, no. 7: 2096. https://doi.org/10.3390/molecules26072096
APA StyleZięba, T., Wilczak, A., Kobryń, J., Musiał, W., Kapelko-Żeberska, M., Gryszkin, A., & Meisel, M. (2021). The Annealing of Acetylated Potato Starch with Various Substitution Degrees. Molecules, 26(7), 2096. https://doi.org/10.3390/molecules26072096