Impact of Collection Volume and DNA Extraction Method on the Detection of Biomarkers and HPV DNA in First-Void Urine
Abstract
1. Introduction
2. Results
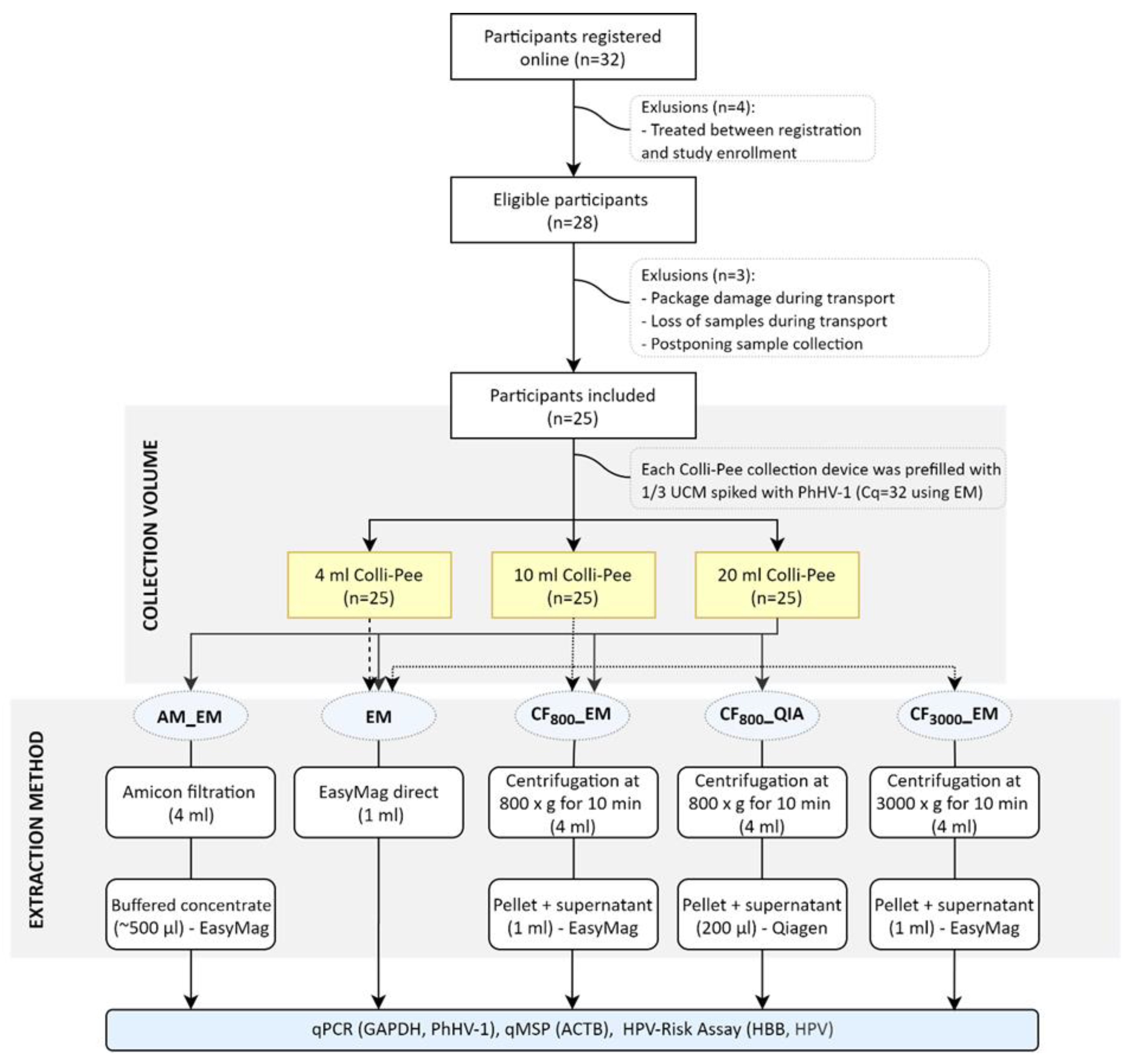
2.1. Sample Collection
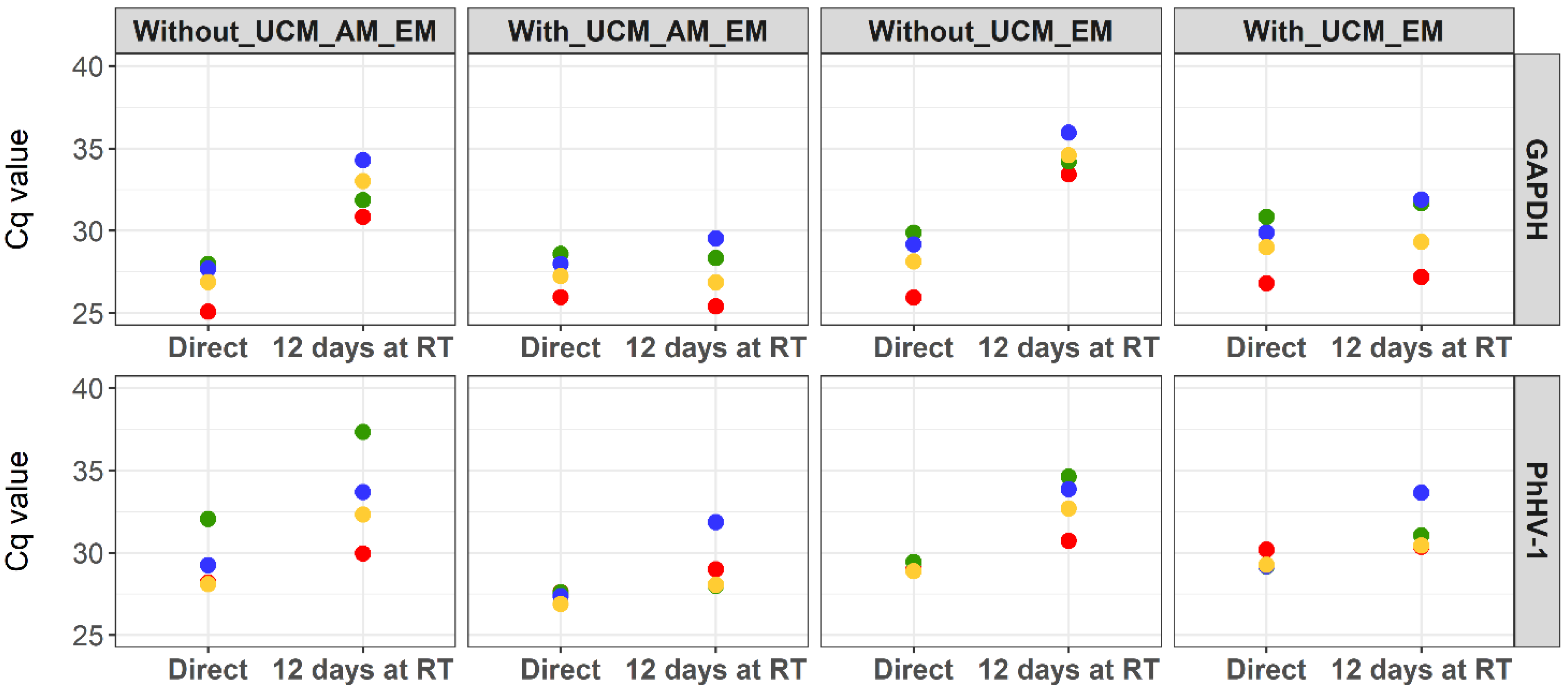
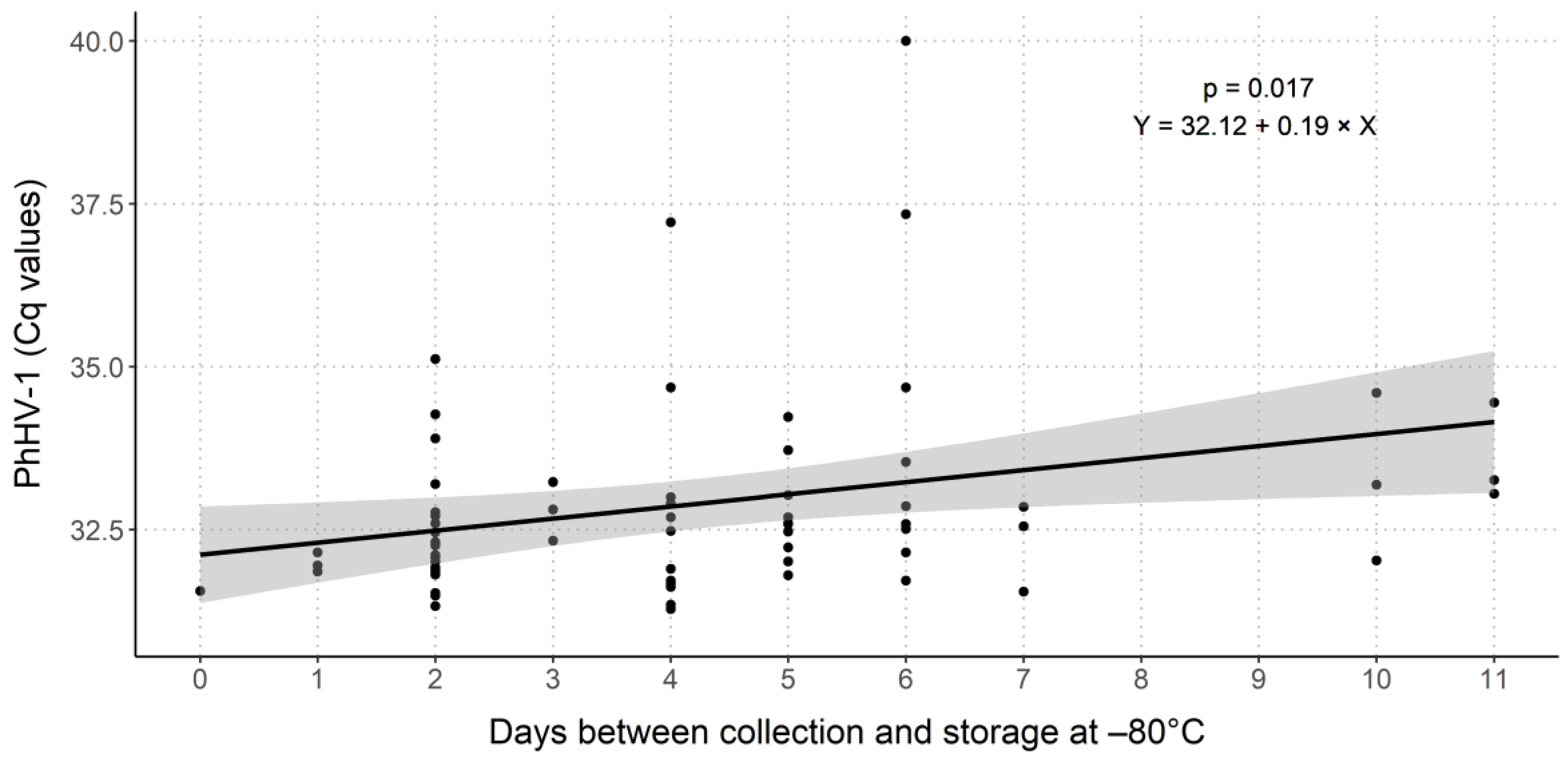
2.2. Evaluation of PhHV-1 as Internal Process Control
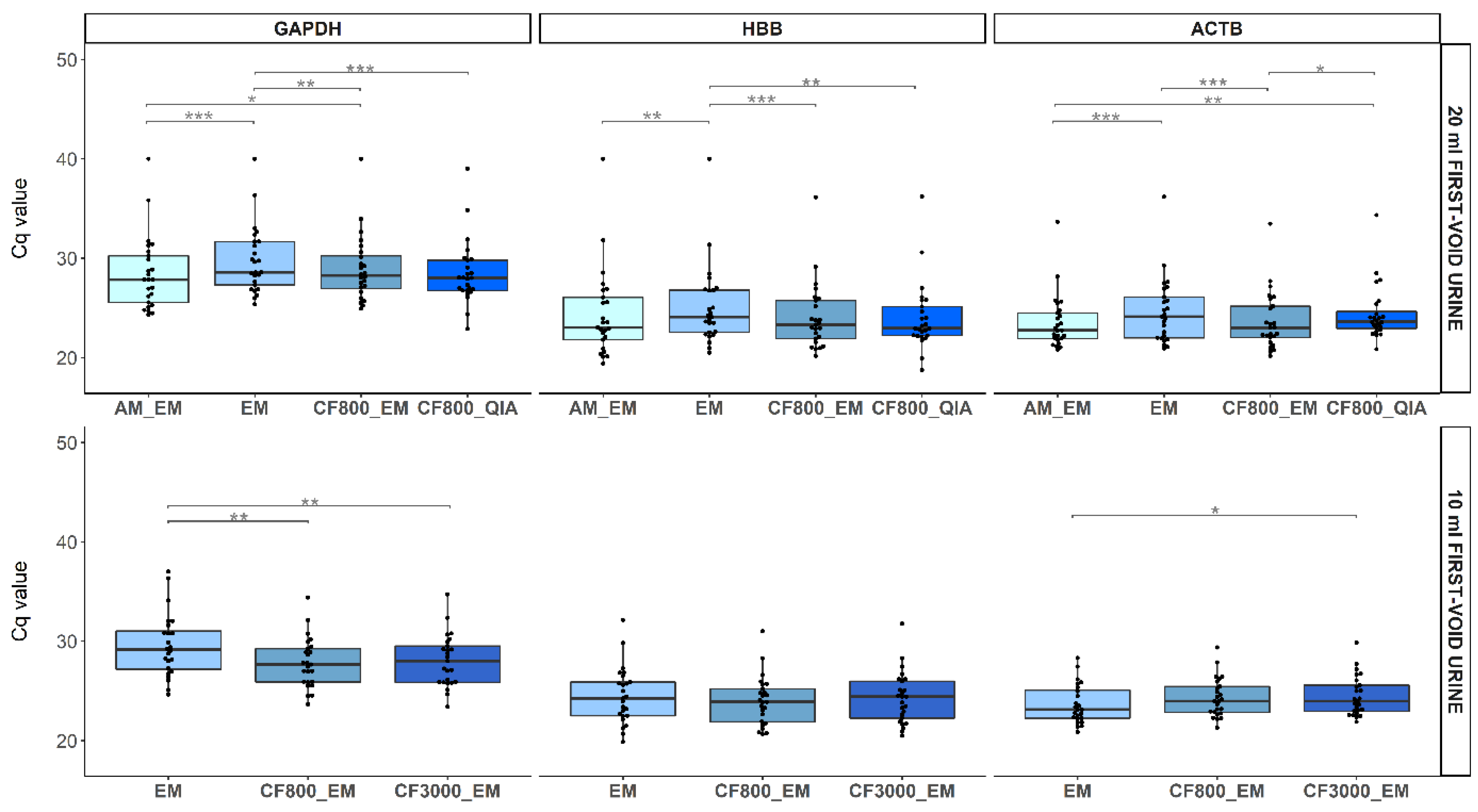
2.3. Comparison of DNA and Biomarker Yields between FV Urine Collection Volumes
2.3.1. Human Endpoints
2.3.2. Viral Endpoints
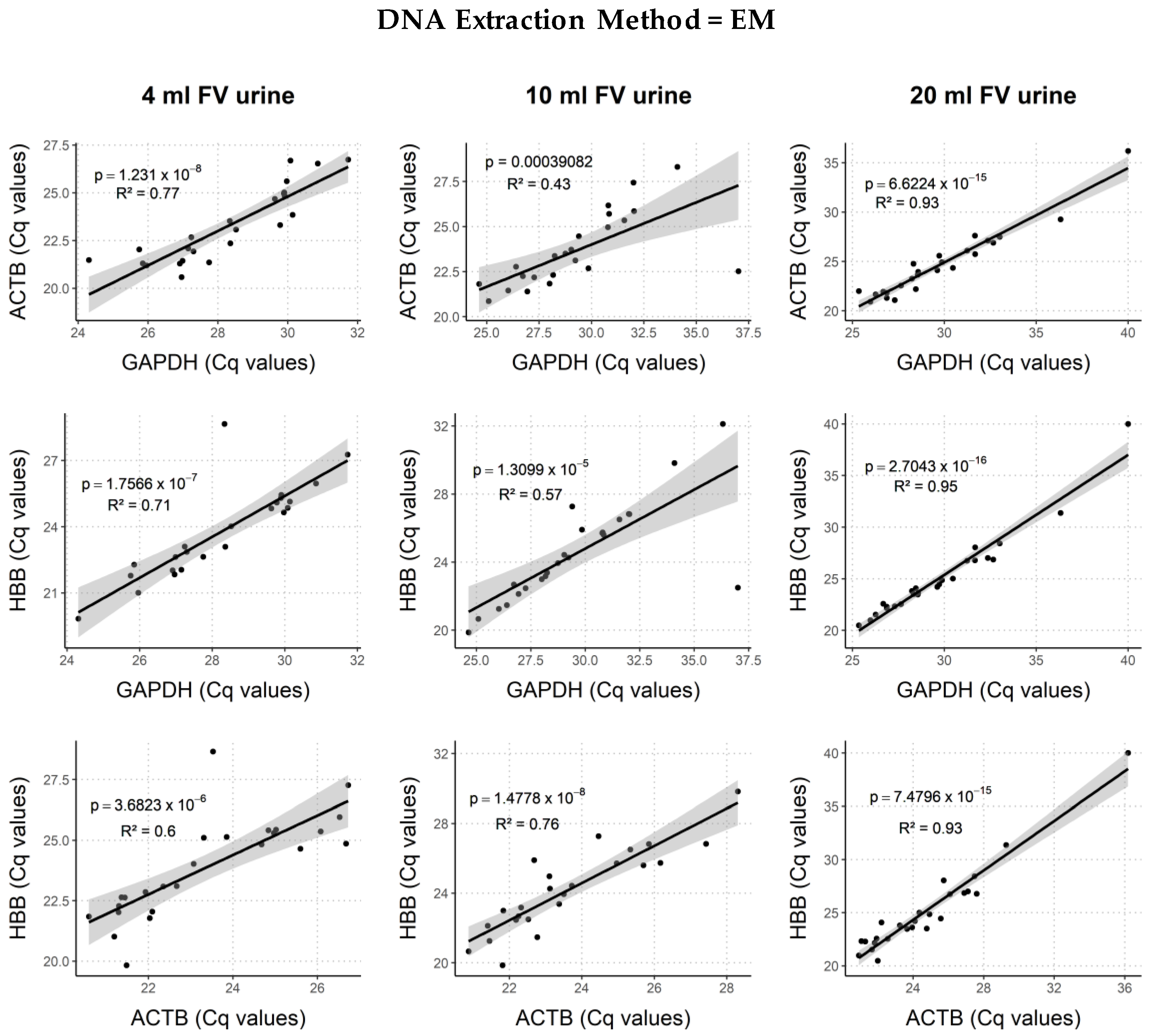
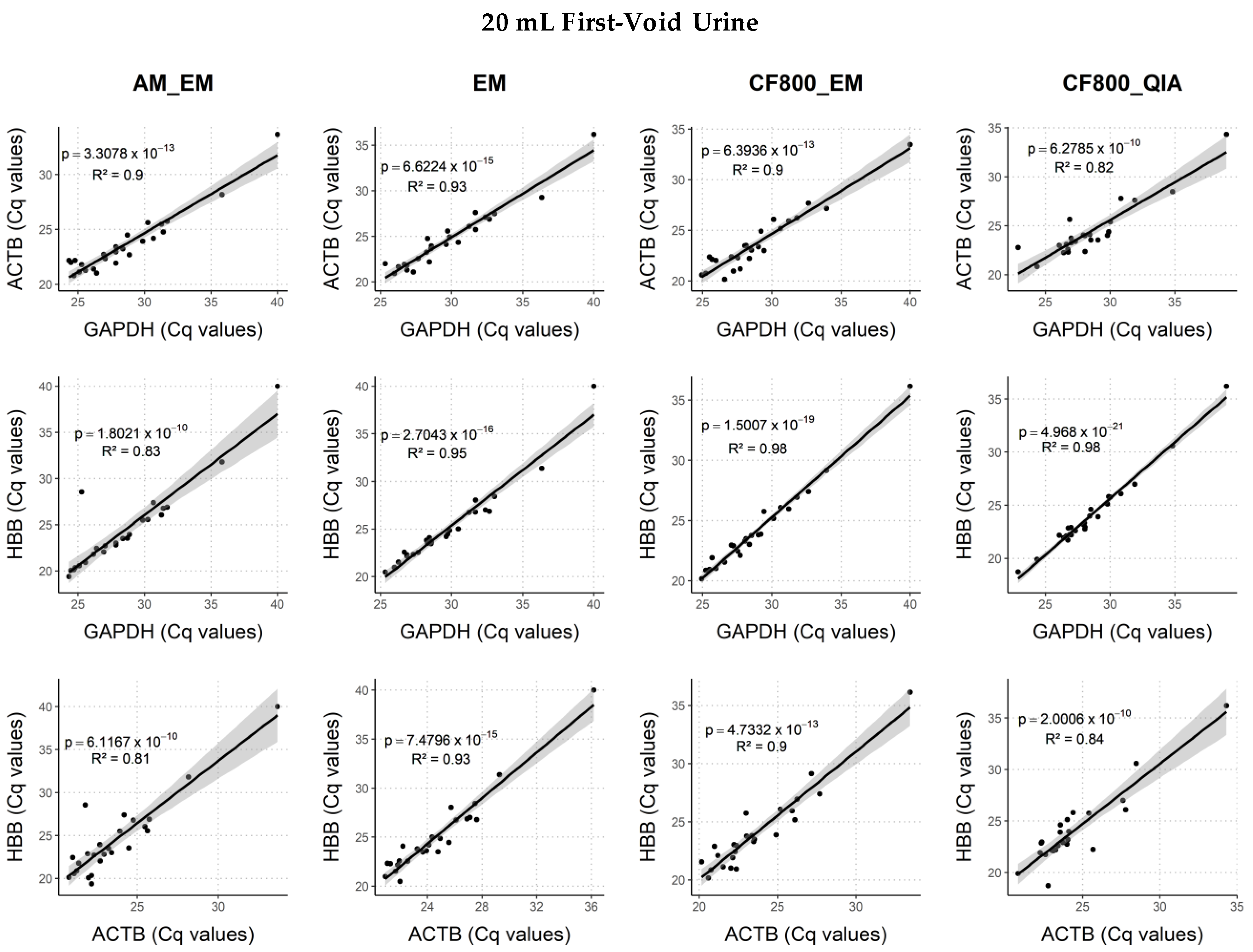
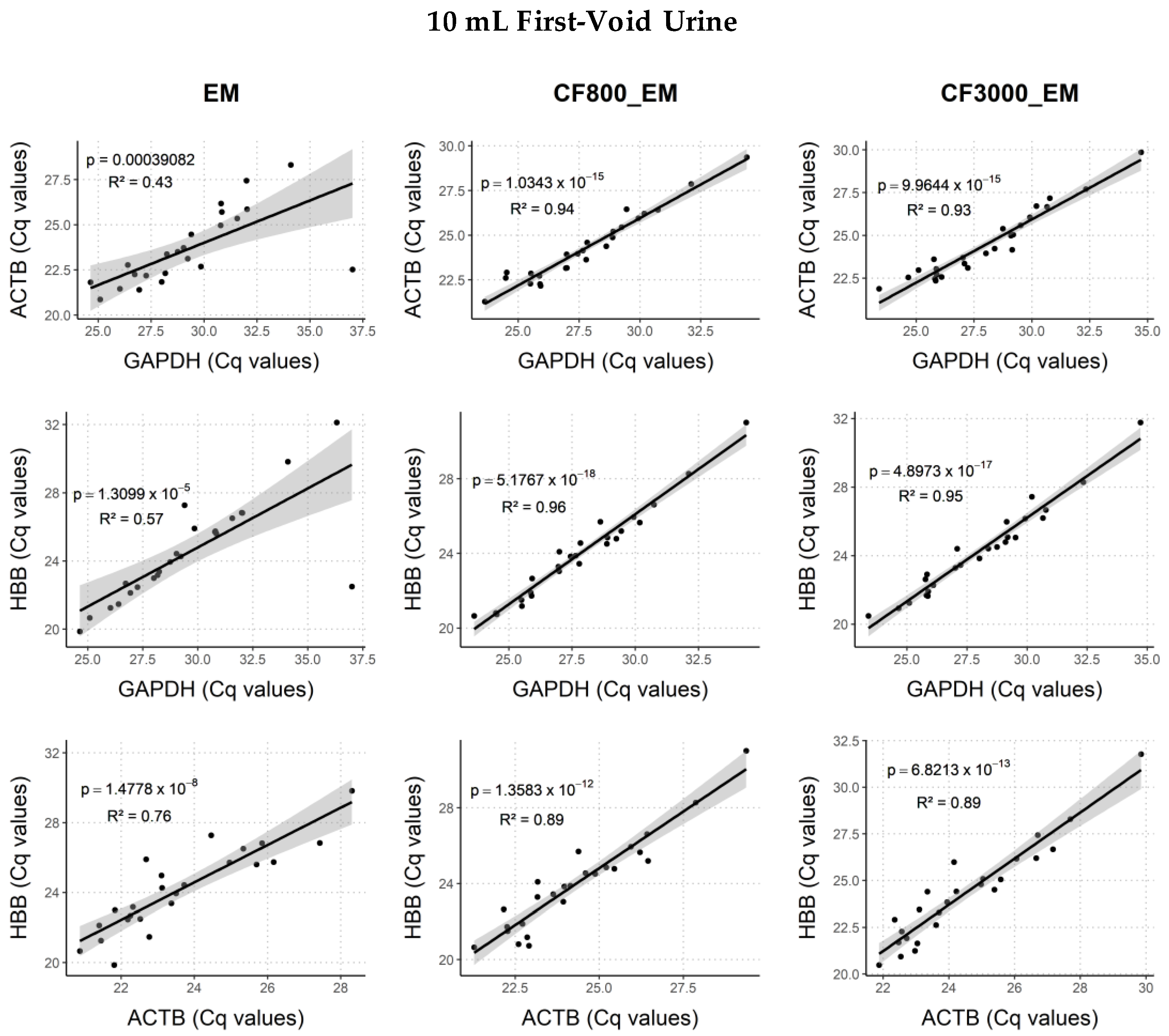
2.4. Comparison of DNA and Biomarker Yields between DNA Extraction Methods
2.4.1. Human Endpoints
2.4.2. Viral Endpoints
3. Discussion
3.1. Internal Process Control
3.2. FV Urine Collection Volume
3.3. DNA Extraction Method
3.4. Limitations of the Study
4. Materials and Methods
4.1. Identification of the Internal Control Spiking Concentration
4.2. Study Population
4.3. Sample Collection and Storage
4.4. DNA Extraction from FV Urine Samples
4.5. Semi-Quantitative PCR for GAPDH and PhHV-1
4.6. HPV-Risk Assay
4.7. Semi-Quantitative PCR on Bisulfite Converted DNA
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A

References
- Vorsters, A.; Van den Bergh, J.; Micalessi, I.; Biesmans, S.; Bogers, J.; Hens, A.; De Coster, I.; Ieven, M.; Van Damme, P. Optimization of HPV DNA detection in urine by improving collection, storage, and extraction. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2005–2014. [Google Scholar] [CrossRef]
- Vorsters, A.; Micalessi, I.; Bilcke, J.; Ieven, M.; Bogers, J.; Van Damme, P. Detection of human papillomavirus DNA in urine. A review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Snoek, B.C.; Splunter, A.P.; Bleeker, M.C.G.; Ruiten, M.C.; Heideman, D.A.M.; Rurup, W.F.; Verlaat, W.; Schotman, H.; van Gent, M.; Trommel, N.E.; et al. Cervical cancer detection by DNA methylation analysis in urine. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Van Keer, S.; Téblick, L.; Van Damme, P.; Vorsters, A. HPV DNA detection in urine samples of women: ‘An efficacious and accurate alternative to cervical samples?’. Expert Rev. Anti. Infect. Ther. 2019, 17, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Van Keer, S.; Pattyn, J.; Tjalma, W.A.A.; Van Ostade, X.; Ieven, M.; Van Damme, P.; Vorsters, A. First-void urine: A potential biomarker source for triage of high-risk human papillomavirus infected women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 216, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luttmer, R.; De Strooper, L.M.A.; Steenbergen, R.D.M.; Berkhof, J.; Snijders, P.J.F.; Heideman, D.A.M.; Meijer, C.J.L.M. Management of high-risk HPV-positive women for detection of cervical (pre)cancer. Expert Rev. Mol. Diagn. 2016, 16, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Ebisch, R.M.F.; Siebers, A.G.; Bosgraaf, R.P.; Massuger, L.F.A.G.; Bekkers, R.L.M.; Melchers, W.J.G. Triage of high-risk HPV positive women in cervical cancer screening. Expert Rev. Anticancer Ther. 2016, 16, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Ouh, Y.T.; Hong, J.H.; Min, K.J.; So, K.A.; Kim, T.J.; Paik, E.S.; Lee, J.W.; Moon, J.H.; Lee, J.K. Comparison of urine, self-collected vaginal swab, and cervical swab samples for detecting human papillomavirus (HPV) with Roche Cobas HPV, Anyplex II HPV, and RealTime HR-S HPV assay. J. Virol. Methods 2019, 269, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Burroni, E.; Bonanni, P.; Sani, C.; Lastrucci, V.; Carozzi, F. Human papillomavirus prevalence in paired urine and cervical samples in women invited for cervical cancer screening. J. Med. Virol. 2015, 87, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Cómbita, A.L.; Gheit, T.; González, P.; Puerto, D.; Murillo, R.H.; Montoya, L.; Vorsters, A.; Van Keer, S.; Van Damme, P.; Tommasino, M.; et al. Comparison between Urine and Cervical Samples for HPV DNA Detection and Typing in Young Women in Colombia. Cancer Prev. Res. (Phila.) 2016, 9, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Ducancelle, A.; Legrand, M.C.; Pivert, A.; Veillon, P.; Le Guillou-Guillemette, H.; De Brux, M.A.; Beby-Defaux, A.; Agius, G.; Hantz, S.; Alain, S.; et al. Interest of human papillomavirus DNA quantification and genotyping in paired cervical and urine samples to detect cervical lesions. Arch. Gynecol. Obstet. 2014, 290, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Dodds, J.; Zamora, J.; Khan, K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: Systematic review and meta-analysis. BMJ 2014, 349. [Google Scholar] [CrossRef] [PubMed]
- Vorsters, A.; Van Keer, S.; Biesmans, S.; Hens, A.; De Coster, I.; Goossens, H.; Ieven, M.; Van Damme, P. Long-term follow-up of HPV infection using urine and cervical quantitative HPV DNA testing. Int. J. Mol. Sci. 2016, 17, 750. [Google Scholar] [CrossRef] [PubMed]
- Van Keer, S.; Willhauck-Fleckenstein, M.; Pattyn, J.; Butt, J.; Tjalma, W.A.A.; Van Ostade, X.; Hens, N.; Van Damme, P.; Waterboer, T.; Vorsters, A. First-void urine as a non-invasive liquid biopsy source to detect vaccine-induced human papillomavirus antibodies originating from cervicovaginal secretions. J. Clin. Virol. 2019, 117, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Panicker, G.; Willhauck-Fleckenstein, M.; Van Keer, S.; Téblick, L.; Pieters, Z.; Tjalma, W.A.A.; Matheeussen, V.; Van Damme, P.; Waterboer, T.; et al. Comparison of a VLP-based and GST-L1-based multiplex immunoassay to detect vaccine-induced HPV-specific antibodies in first-void urine. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Van Keer, S.; Tjalma, W.; Matheeussen, V.; Van Damme, P.; Vorsters, A. Infection and vaccine-induced HPV-specific antibodies in cervicovaginal secretions. A review of the literature. Papillomavirus Res. 2019, 8, 100185. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, S.; Chantal Umulisa, M.; Tshomo, U.; Gheit, T.; Baussano, I.; Tenet, V.; Tshokey, T.; Gatera, M.; Ngabo, F.; Van Damme, P.; et al. Urine testing to monitor the impact of HPV vaccination in Bhutan and Rwanda. Int. J. Cancer 2016, 139, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Senkomago, V.; Des Marais, A.C.; Rahangdale, L.; Vibat, C.R.T.; Erlander, M.G.; Smith, J.S. Comparison of urine specimen collection times and testing fractions for the detection of high-risk human papillomavirus and high-grade cervical precancer. J. Clin. Virol. 2016, 74, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Rohner, E.; McGuire, F.H.; Liu, Y.; Li, Q.; Miele, K.; Desai, S.A.; Schmitt, J.W.; Knittel, A.; Nelson, J.A.E.; Edelman, C.; et al. Racial and Ethnic Differences in Acceptability of Urine and Cervico-Vaginal Sample Self-Collection for HPV-Based Cervical Cancer Screening. J. Women’s Health 2020, 29, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Sellors, J.W.; Lorincz, A.T.; Mahony, J.B.; Mielzynska, I.; Lytwyn, A.; Roth, P.; Howard, M.; Chong, S.; Daya, D.; Chapman, W.; et al. Comparison of self-collected vaginal, vulvar and urine samples with physician-collected cervical samples for human papillomavirus testing to detect high-grade squamous intraepithelial lesions. CMAJ 2000, 163, 513–518. [Google Scholar] [PubMed]
- Tamalet, C.; Le Retraite, L.; Leandri, F.X.; Heid, P.; Sancho Garnier, H.; Piana, L. Vaginal self-sampling is an adequate means of screening HR-HPV types in women not participating in regular cervical cancer screening. Clin. Microbiol. Infect. 2013, 19. [Google Scholar] [CrossRef] [PubMed]
- Racey, C.S.; Withrow, D.R.; Gesink, D. Self-collected HPV testing improves participation in cervical cancer screening: A systematic review and meta-analysis. Can. J. Public Health 2013, 104, e159–e166. [Google Scholar] [CrossRef] [PubMed]
- Verdoodt, F.; Jentschke, M.; Hillemanns, P.; Racey, C.S.; Snijders, P.J.F.; Arbyn, M. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: A systematic review and meta-analysis of randomised trials. Eur. J. Cancer 2015, 51, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Ducancelle, A.; Reiser, J.; Pivert, A.; Guillou-Guillemette, H.L.; Le Duc-Banaszuk, A.S.; Lunel-Fabiani, F. Home-based urinary HPV DNA testing in women who do not attend cervical cancer screening clinics. J. Infect. 2015, 71, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Vorsters, A.; Van Damme, P.; Clifford, G. Urine testing for HPV: Rationale for using first void. BMJ 2014, 349, 6252. [Google Scholar] [CrossRef] [PubMed]
- Gakidou, E.; Nordhagen, S.; Obermeyer, Z. Coverage of Cervical Cancer Screening in 57 Countries: Low Average Levels and Large Inequalities. PLoS Med. 2008, 5, e132. [Google Scholar] [CrossRef]
- Van Doornum, G.J.J.; Guldemeester, J.; Osterhaus, A.D.M.E.; Niesters, H.G.M. Diagnosing herpesvirus infections by real-time amplification and rapid culture. J. Clin. Microbiol. 2003, 41, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Niesters, H.G.M. Molecular and diagnostic clinical virology in real time. Clin. Microbiol. Infect. 2004, 10, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Luttmer, R.; De Strooper, L.M.A.; Dijkstra, M.G.; Berkhof, J.; Snijders, P.J.F.; Steenbergen, R.D.M.; Van Kemenade, F.J.; Rozendaal, L.; Helmerhorst, T.J.M.; Verheijen, R.H.M.; et al. FAM19A4 methylation analysis in self-samples compared with cervical scrapes for detecting cervical (pre)cancer in HPV-positive women. Br. J. Cancer 2016, 115, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Van Den Helder, R.; Van Trommel, N.E.; Van Splunter, A.P.; Lissenberg-Witte, B.I.; Bleeker, M.C.G.; Steenbergen, R.D.M. Methylation analysis in urine fractions for optimal CIN3 and cervical cancer detection. Papillomavirus Res. 2020, 9, 100193. [Google Scholar] [CrossRef] [PubMed]
- Bosschieter, J.; Bach, S.; Bijnsdorp, I.V.; Segerink, L.I.; Rurup, W.F.; Van Splunter, A.P.; Bahce, I.; Novianti, P.W.; Kazemier, G.; Van Moorselaar, R.J.A.; et al. A protocol for urine collection and storage prior to DNA methylation analysis. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Van Keer, S.; Biesmans, S.; Ieven, M.; Vanderborght, C.; Beyers, K.; Vankerckhoven, V.; Bruyndonckx, R.; Van Damme, P.; Vorsters, A. Human papillomavirus detection in urine: Effect of a first-void urine collection device and timing of collection. J. Virol. Methods 2019, 264, 23–30. [Google Scholar] [CrossRef]
- Van Keer, S.; Tjalma, W.A.A.; Pattyn, J.; Biesmans, S.; Pieters, Z.; Van Ostade, X.; Ieven, M.; Van Damme, P.; Vorsters, A. Human papillomavirus genotype and viral load agreement between paired first-void urine and clinician-collected cervical samples. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Tshomo, U.; Franceschi, S.; Tshokey, T.; Tobgay, T.; Baussano, I.; Tenet, V.; Snijders, P.J.F.; Gheit, T.; Tommasino, M.; Vorsters, A.; et al. Evaluation of the performance of Human Papillomavirus testing in paired urine and clinician-collected cervical samples among women aged over 30 years in Bhutan. Virol. J. 2017, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Wardak, S. Human Papillomavirus (HPV) and Cervical Cancer. Available online: https://www.who.int/en/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer (accessed on 5 October 2020).
- Verlaat, W.; Van Leeuwen, R.W.; Novianti, P.W.; Schuuring, E.; Meijer, C.J.L.M.; Van Der Zee, A.G.J.; Snijders, P.J.F.; Heideman, D.A.M.; Steenbergen, R.D.M.; Wisman, G.B.A. Host-cell DNA methylation patterns during high-risk HPV-induced carcinogenesis reveal a heterogeneous nature of cervical pre-cancer. Epigenetics 2018, 13, 769–778. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, L.M.A.; Berkhof, J.; Steenbergen, R.D.M.; Lissenberg-Witte, B.I.; Snijders, P.J.F.; Meijer, C.J.L.M.; Heideman, D.A.M. Cervical cancer risk in HPV-positive women after a negative FAM19A4/mir124-2 methylation test: A post hoc analysis in the POBASCAM trial with 14 year follow-up. Int. J. Cancer 2018, 143, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Schiffman, M.; Palmer, T.; Arbyn, M. Triage of HPV positive women in cervical cancer screening. J. Clin. Virol. 2016, 76, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Leeman, A.; del Pino, M.; Molijn, A.; Rodriguez, A.; Torné, A.; de Koning, M.; Ordi, J.; van Kemenade, F.; Jenkins, D.; Quint, W. HPV testing in first-void urine provides sensitivity for CIN2+ detection comparable with a smear taken by a clinician or a brush-based self-sample: Cross-sectional data from a triage population. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- BE 71030031000; Biobank Antwerpen, BBMR-ERIC; No. Access: (25), [BIORESOURCE].
- Payan, C.; Ducancelle, A.; Aboubaker, M.H.; Caer, J.; Tapia, M.; Chauvin, A.; Peyronnet, D.; Le Hen, E.; Arab, Z.; Legrand, M.C.; et al. Human papillomavirus quantification in urine and cervical samples by using the Mx4000 and LightCycler general real-time PCR systems. J. Clin. Microbiol. 2007, 45, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, A.T.; Berkhof, J.; Van Der Salm, M.L.; Van Splunter, A.P.; Geelen, T.H.; Van Kemenade, F.J.; Bleeker, M.G.B.; Heideman, D.A.M. Clinical validation of the HPV-Risk assay, a novel real-time PCR assay for detection of high-risk human papillomavirus DNA by targeting the E7 region. J. Clin. Microbiol. 2014, 52, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Verlaat, W.; Snijders, P.J.F.; Novianti, P.W.; Wilting, S.M.; De Strooper, L.M.A.; Trooskens, G.; Vandersmissen, J.; Van Criekinge, W.; Wisman, G.B.A.; Meijer, C.J.L.M.; et al. Genome-wide DNA methylation profiling reveals methylation markers associated with 3q gain for detection of cervical precancer and cancer. Clin. Cancer Res. 2017, 23, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- Verlaat, W.; Snoek, B.C.; Heideman, D.A.M.; Wilting, S.M.; Snijders, P.J.F.; Novianti, P.W.; Van Splunter, A.P.; Peeters, C.F.W.; Van Trommel, N.E.; Massuger, L.F.A.G.; et al. Identification and validation of a 3-gene methylation classifier for hpv-based cervical screening on self-samples. Clin. Cancer Res. 2018, 24, 3456–3464. [Google Scholar] [CrossRef] [PubMed]
- Snellenberg, S.; Strooper, L.M.A.D.; Hesselink, A.T.; Meijer, C.J.L.M.; Snijders, P.J.F.; Heideman, D.A.M.; Steenbergen, R.D.M. Development of a multiplex methylation-specific PCR as candidate triage test for women with an HPV-positive cervical scrape. BMC Cancer 2012, 12, 551. [Google Scholar] [CrossRef] [PubMed]
- Overmeer, R.; Henken, F.; Snijders, P.; Claassen-Kramer, D.; Berkhof, J.; Helmerhorst, T.; Heideman, D.; Wilting, S.; Murakami, Y.; Ito, A.; et al. Association between dense CADM1 promoter methylation and reduced protein expression in high-grade CIN and cervical SCC. J. Pathol. 2008, 215, 388–397. [Google Scholar] [CrossRef] [PubMed]


| HPV Cq Values | ||||||||
|---|---|---|---|---|---|---|---|---|
| AM_EM | EM | CF800_EM | CF800_QIA | CF3000_EM | ||||
| 20 mL | 4 mL | 10 mL | 20 mL | 10 mL | 20 mL | 20 mL | 10 mL | |
| ID1 | - | - | - | - | 38.78 | - | - | - |
| ID2 | 22.08 | 17.90 | 19.17 | 23.17 | 17.28 | 22.75 | 24.75 | 21.65 |
| ID3 | - | - | - | - | - | - | - | - |
| ID4 | - | - | 35.46 | - | 32.26 | - | - | 32.57 |
| ID5 | - | - | - | - | - | - | - | - |
| ID6 | 36.71 | 34.32 | - | 29.11 | - | 28.89 | 25.97 | 33.53 |
| ID7 | 29.64 | 23.58 | 24.65 | 26.25 | 23.62 | 22.81 | 24.89 | 25.70 |
| ID8 | - | - | - | - | - | - | - | - |
| ID9 | - | - | - | - | 30.83 | - | - | 37.46 |
| ID10 | - | - | - | - | - | - | - | - |
| ID12 | 22.16 | 25.09 | 24.04 | 23.73 | 20.94 | 21.66 | 22.80 | 21.12 |
| ID13 | 19.82 | 21.97 | 25.61 | 20.89 | 24.15 | 19.40 | 18.99 | 24.57 |
| ID14 | - | - | - | - | - | - | - | - |
| ID15 | 39.24 | - | 37.87 | - | - | - | - | - |
| ID16 | - | - | - | - | - | - | - | - |
| ID17 | 34.92 | 30.34 | 33.52 | 34.55 | 35.94 | 38.59 | 33.96 | 35.50 |
| ID18 | 36.19 | - | 37.00 | - | 31.25 | 36.39 | 38.16 | 31.07 |
| ID19 | 24.63 | 22.92 | 24.57 | 26.17 | 22.58 | 24.43 | 25.26 | 23.38 |
| ID21 | - | - | - | - | - | - | - | - |
| ID22 | - | - | - | - | - | - | - | - |
| ID23 | 31.59 | - | 35.11 | 35.73 | 32.98 | 38.98 | - | 36.29 |
| ID24 | - | - | - | - | - | - | - | - |
| ID25 | 29.90 | 38.90 | 27.93 | 30.65 | 30.12 | 30.67 | 33.10 | 28.37 |
| ID27 | - | - | - | - | - | - | - | - |
| ID28 | - | - | - | - | - | - | - | - |
| Percent positive (%) | 44.00 | 32.00 | 44.00 | 36.00 | 52.00 | 40.00 | 36.00 | 48.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Téblick, L.; Van Keer, S.; De Smet, A.; Van Damme, P.; Laeremans, M.; Rios Cortes, A.; Beyers, K.; Vankerckhoven, V.; Matheeussen, V.; Mandersloot, R.; et al. Impact of Collection Volume and DNA Extraction Method on the Detection of Biomarkers and HPV DNA in First-Void Urine. Molecules 2021, 26, 1989. https://doi.org/10.3390/molecules26071989
Téblick L, Van Keer S, De Smet A, Van Damme P, Laeremans M, Rios Cortes A, Beyers K, Vankerckhoven V, Matheeussen V, Mandersloot R, et al. Impact of Collection Volume and DNA Extraction Method on the Detection of Biomarkers and HPV DNA in First-Void Urine. Molecules. 2021; 26(7):1989. https://doi.org/10.3390/molecules26071989
Chicago/Turabian StyleTéblick, Laura, Severien Van Keer, Annemie De Smet, Pierre Van Damme, Michelle Laeremans, Alejandra Rios Cortes, Koen Beyers, Vanessa Vankerckhoven, Veerle Matheeussen, Renee Mandersloot, and et al. 2021. "Impact of Collection Volume and DNA Extraction Method on the Detection of Biomarkers and HPV DNA in First-Void Urine" Molecules 26, no. 7: 1989. https://doi.org/10.3390/molecules26071989
APA StyleTéblick, L., Van Keer, S., De Smet, A., Van Damme, P., Laeremans, M., Rios Cortes, A., Beyers, K., Vankerckhoven, V., Matheeussen, V., Mandersloot, R., Floore, A., Meijer, C. J. L. M., Steenbergen, R. D. M., & Vorsters, A. (2021). Impact of Collection Volume and DNA Extraction Method on the Detection of Biomarkers and HPV DNA in First-Void Urine. Molecules, 26(7), 1989. https://doi.org/10.3390/molecules26071989








