Polymorphic Characterization, Pharmacokinetics, and Anti-Inflammatory Activity of Ginsenoside Compound K Polymorphs
Abstract
1. Introduction
2. Results
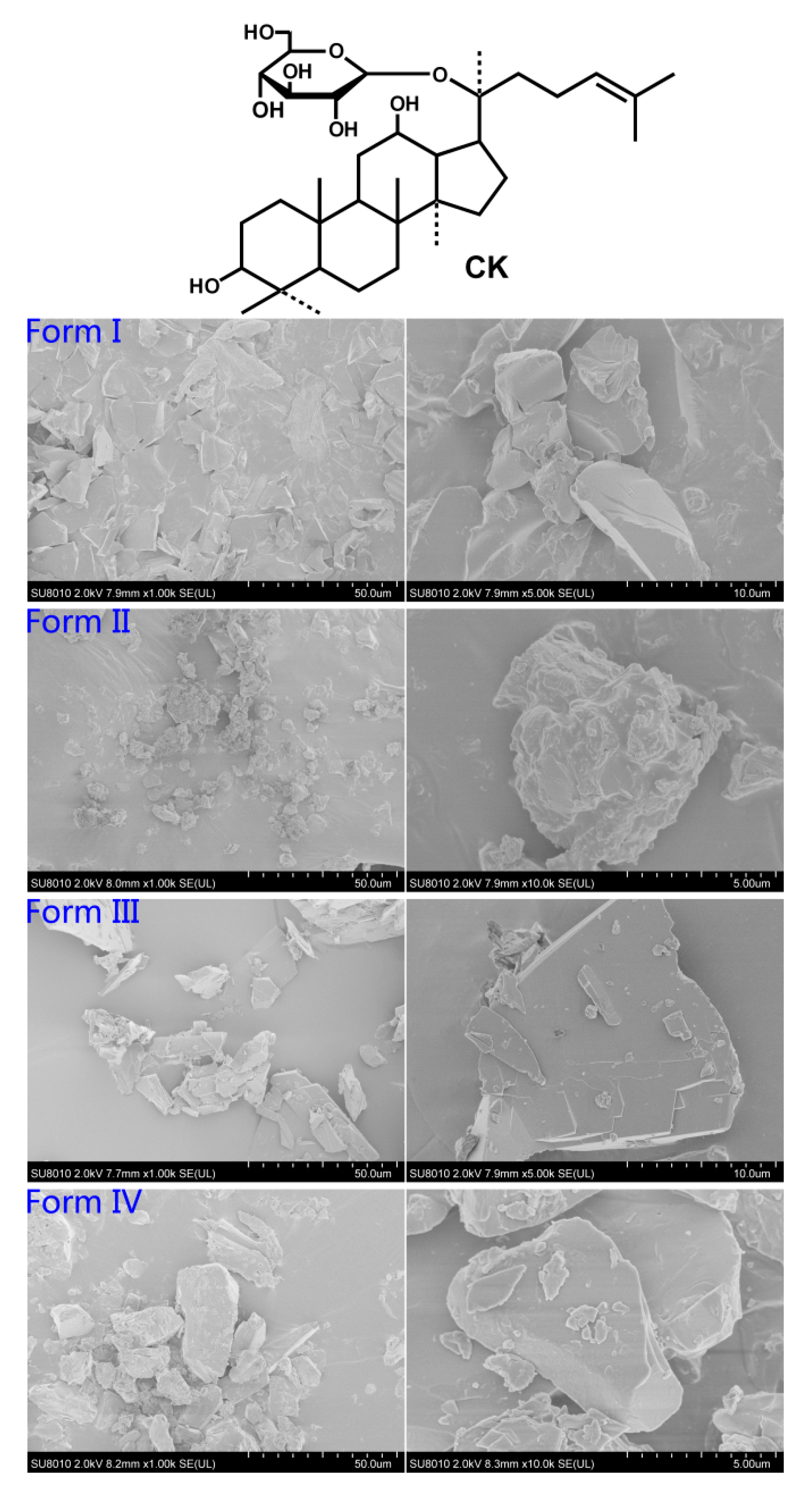
2.1. Scanning Electron Microscopy
2.2. Thermal Analysis
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Powder X-ray Diffraction (PXRD)
2.5. In Vivo Studies: Pharmacokinetic and Pharmacodynamic Profiles
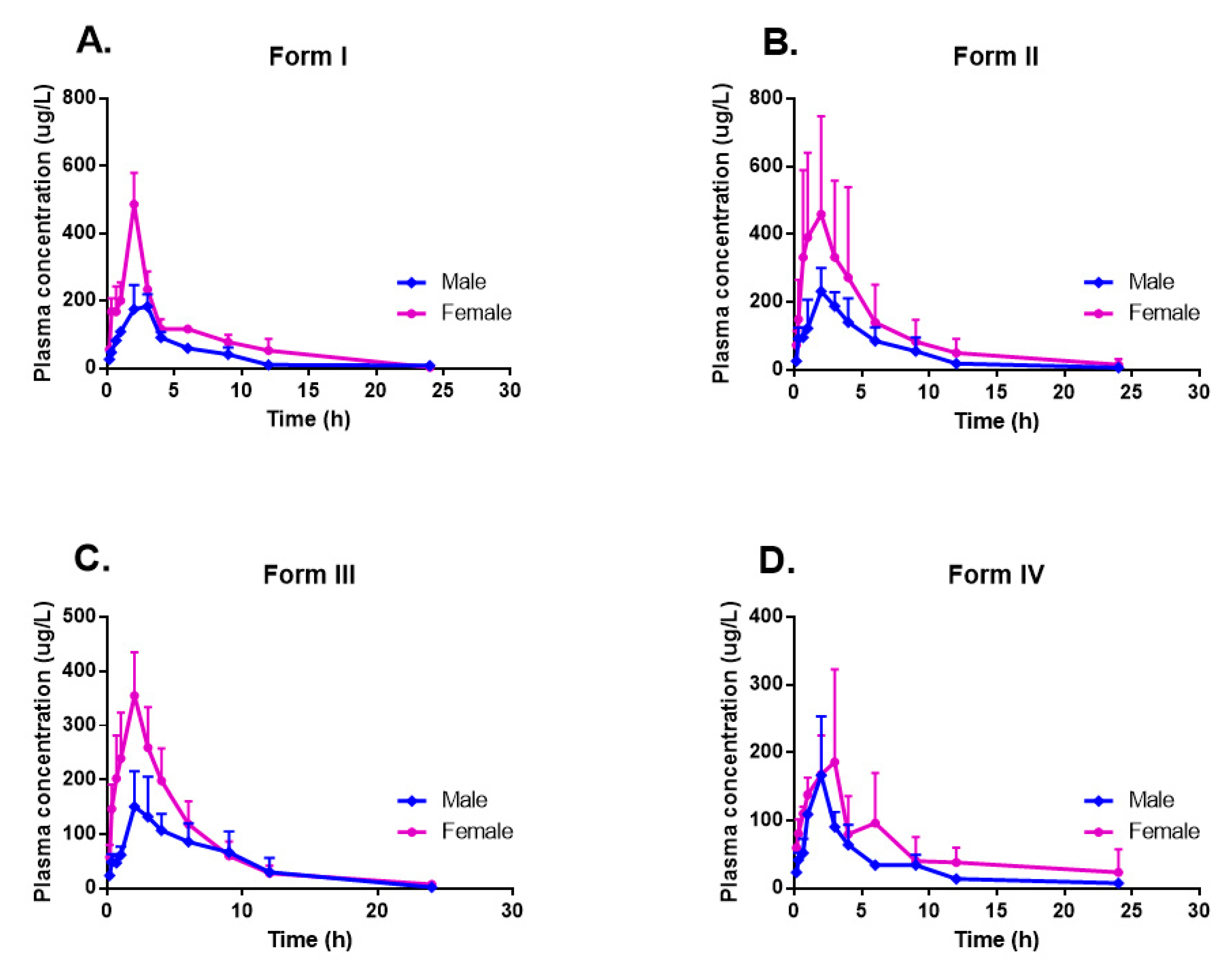
2.5.1. Crystalline Polymorphic Impacts on Pharmacokinetic Properties
2.5.2. Sex-Related Impact on Pharmacokinetic Properties
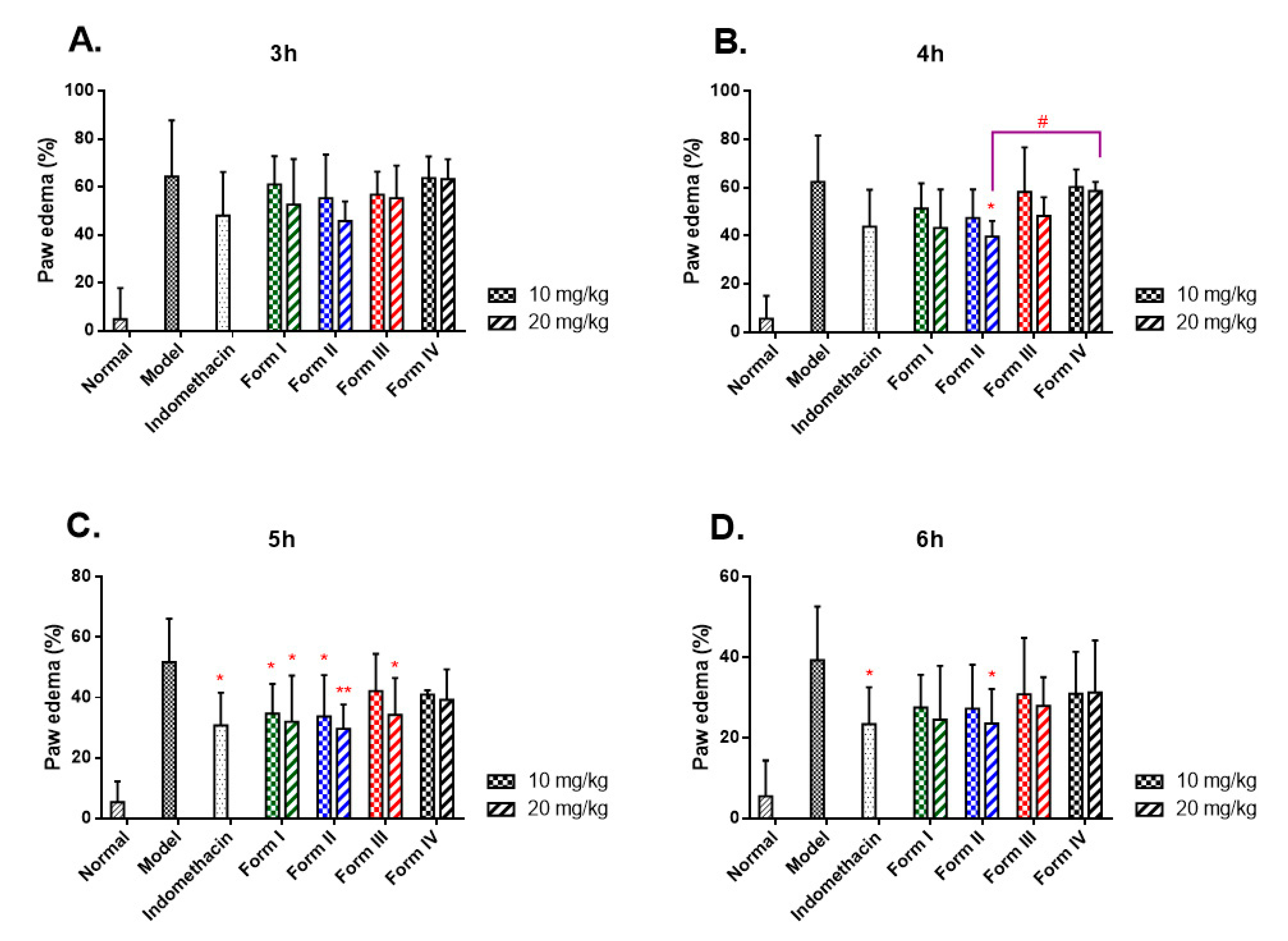
2.5.3. Crystalline Polymorphic Impacts on Anti-Inflammatory Properties
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Methods
4.3.1. Preparation of CK Polymorphs
4.3.2. Polymorphic Characterization
4.4. Pharmacokinetic Studies
4.4.1. Analytical Methods
4.4.2. Animals and Dosing
4.4.3. Sample Preparation and Analysis
4.4.4. Statistical Analysis
4.5. Anti-Inflammatory Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Park, J.D.; Rhee, D.K.; Lee, Y.H. Biological activities and chemistry of saponins from Panax ginseng C.A. Meyer. Phytochem. Rev. 2005, 4, 159–175. [Google Scholar] [CrossRef]
- Lee, G.; Nguyen, T.T.H.; Lim, T.Y.; Lim, J.; Park, B.; Lee, S.; Mok, I.-K.; Pal, K.; Lim, S.; Kim, D. Fermented wild ginseng by Rhizopus oligosporus improved l-carnitine and ginsenoside contents. Molecules 2020, 25, 2111. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Yoo, M.H.; Noh, K.H.; Oh, D.K. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl. Microbiol. Biotechnol. 2010, 87, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yan, Q.; Li, J.Y.; Zhang, X.C.; Zhou, P. Biotransformation of Panax notoginseng saponins into ginsenoside compound K production by Paecilomyces bainier sp.229. J. Appl. Microbiol. 2008, 104, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, J.Y.; Li, X.W.; Yan, Q.; Zhou, P. Development and validation of a reversed-phase HPLC method for quantitative determination of ginsenosides Rb1, Rd, F2, and compound K during the process of biotransformation of ginsenoside Rb1. J. Sep. Sci. 2008, 31, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Zhou, P.; Bai, H.; Zhou, W.; Li, J.J.; Feng, M.Q.; Hua, M.L.; Xu, J.Y. New Use of Ginsenoside Compound-K for the Prevention of Rheumatoid Arthritis. Available online: http://europepmc.org/article/PAT/WO2008034328 (accessed on 6 August 2020).
- Chen, L.L.; Zhou, L.P.; Huang, J.; Wang, Y.Q.; Yang, G.P.; Ta, Z.R.; Wang, Y.C.; Zhou, G.; Liao, J.W.; Ouyang, D.S. Single- and multiple-dose trials to determine the pharmacokinetics, safety, tolerability, and sex effect of oral ginsenoside compound K in healthy Chinese volunteers. Front. Pharmacol. 2018, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, J.Y.; Feng, M.Q.; Zhou, P. X-ray structure investigation of 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol. J. Chem. Crystallogr. 2009, 39, 99–103. [Google Scholar] [CrossRef]
- Zhou, W.; Feng, M.Q.; Li, X.W.; Yan, Q.; Zhou, C.Q.; Li, J.Y.; Zhou, P. X-ray structure investigation of 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol and antitumor effect on Lewis lung carcinoma in vivo. Chem. Biodivers. 2009, 6, 380–388. [Google Scholar] [CrossRef]
- Li, R.Y.; Dong, W.B.; He, H.N.; Yan, H.; Jiang, X.B.; Gong, J.B. Isolation, characterization and phase transformation of new ginsenoside compound k hydrate and methanol solvates. Cryst. Res. Technol. 2012, 47, 377–384. [Google Scholar] [CrossRef]
- Brog, J.P.; Chanez, C.; Crochet, A.; Fromm, K.M. Polymorphism, what it is and how to identify it: A systematic review. RSC Adv. 2013, 38, 16905–16931. [Google Scholar] [CrossRef]
- Grothe, E.; Meekes, H.; Vlieg, E.; ter Horst, J.H.; de Gelder, R. Solvates, salts, and cocrystals: A proposal for a feasible classification system. Cryst. Growth Des. 2016, 16, 3237–3243. [Google Scholar] [CrossRef]
- Khankari, R.K.; Grant, D.J.W. Pharmaceutical hydrates. Thermochimica. Acta 1995, 248, 61–79. [Google Scholar] [CrossRef]
- Vippagunta, S.R.; Brittain, H.G.; Grant, D.J.W. Crystalline solids. Adv. Drug Deliver. Rev. 2001, 48, 3–26. [Google Scholar] [CrossRef]
- Lee, A.Y.; Erdemir, D.; Myerson, A.S. Crystal polymorphism in chemical process development. Annu. Rev. Chem. Biomol. 2011, 2, 259–280. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Crystal engineering: From molecule to crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef]
- Novakovic, D.; Isomaki, A.; Pleunis, B.; Fraser-Miller, S.J.; Peltonen, L.; Laaksonen, T.; Strachan, C.J. Understanding dissolution and crystallization with imaging: A surface point of view. Mol. Pharmaceutics 2018, 15, 5361–5373. [Google Scholar] [CrossRef]
- Guillory, J.K. Generation of polymorphs, hydrates solvates and amorphous solids. In Polymorphism in Pharmaceutical Solids; Brittain, H.G., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 183–226. [Google Scholar]
- Chen, L.L.; Zhou, L.P.; Wang, Y.Q.; Yang, G.P.; Huang, J.; Tan, Z.Q.; Wang, Y.C.; Zhou, G.; Liao, J.W.; Ouyang, D.S. Food and sex-related impacts on the pharmacokinetics of a single-dose of ginsenoside compound K in healthy subjects. Front. Pharmacol. 2017, 8, 636. [Google Scholar] [CrossRef]
- Singhal, D.; Curatolo, W. Drug polymorphism and dosage form design: A practical perspective. Adv. Drug Deliv. Rev. 2004, 56, 335–347. [Google Scholar] [CrossRef]
- Peterson, M.L.; Hickey, M.B.; Zaworotko, M.J.; Almarsson, O. Expanding the scope of crystal form evaluation in pharmaceutical science. J. Pharm. Pharm. Sci. 2006, 9, 317–326. [Google Scholar]
- Yosioka, I.; Sugawara, T.; Imai, K.; Kitagawa, I. Soil bacterial hydrolysis leading to genuine aglycone. V. On ginsenosides-Rb1, Rb2 and Rc of the ginseng root saponins. Chem. Pharm. Bull. 1972, 20, 2418–2421. [Google Scholar] [CrossRef]
- Igami, K.; Ozawa, M.; Inoue, S.; Iohara, D.; Miyazaki, T.; Shinoda, M.; Anraku, M.; Hirayama, F.; Uekama, K. The formation of an inclusion complex between a metabolite of ginsenoside, compound K and γ-cyclodextrin and its dissolution characteristics. J. Pharm. Pharmacol. 2015, 68, 646–654. [Google Scholar] [CrossRef]
- Ono, M.; Tozuka, Y.; Oguchi, T.; Yamamura, S.; Yamamoto, K. Effects of dehydration temperature on water vapor adsorption and dissolution behavior of carbamazepine. Int. J. Pharm. 2002, 239, 1–12. [Google Scholar] [CrossRef]
- Rustichelli, C.; Gamberini, G.; Ferioli, V.; Gamberini, M.C.; Ficarra, R.; Tommasini, S. Solid-state study of polymorphic drugs: Carbamazepine. J. Pharm. Biomed. Anal. 2000, 23, 41–54. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.; Zhang, G.G.Z.; Grant, D.J.W.; Suryanarayanan, R. In situ dehydration of carbamazepine dihydrate: A novel technique to prepare amorphous anhydrous carbamazepine. Pharm. Dev. Technol. 2000, 5, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Calvo, E.; Munto, M.; Wurst, K.; Ventosa, N.; Masciocchi, N.; Veciana, J. Polymorphs and solvates of nicardipine hydrochloride. Selective stabilization of different diastereomeric racemates. Mol. Pharm. 2011, 8, 395–404. [Google Scholar] [CrossRef]
- Lee, P.S.; Han, J.Y.; Song, T.W.; Sung, J.H.; Kwon, O.S.; Song, S.; Chung, Y.B. Physicochemical characteristics and bioavailability of a novel intestinal metabolite of ginseng saponin (IH901) complexed with β-cyclodextrin. Int. J. Pharm. 2006, 316, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Du, G.H. Polymorphic Drugs; People’s Medical Publishing House: Beijing, China, 2009; pp. 35–36. [Google Scholar]
- Morissette, S.L.; Almarsson, Ö.; Peterson, M.L.; Remenar, J.F.; Read, M.J.; Lemmo, A.V.; Ellis, S.; Cima, M.J.; Gardner, C.R. High-throughput crystallization: Polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv. Drug Deliv. Rev. 2004, 56, 257–300. [Google Scholar] [CrossRef]
- Clayton, J.A.; Collins, F.S. Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Couzin-Frankel, J. National institutes of health needed: More females in animal and cell studies. Science 2014, 344, 679. [Google Scholar] [CrossRef]
- Gandhi, M.; Aweeka, F.; Greenblatt, R.M.; Blaschke, T.F. Sex differences in pharmacokinetics and pharmacodynamics. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Rost, D.; Kopplow, K.; Gehrke, S.; Mueller, S.; Friess, H.; Ittrich, C.; Mayer, D.; Stiehl, A. Gender-specific expression of liver organic anion transporters in rat. Eur. J. Clin. Investig. 2005, 35, 635–643. [Google Scholar] [CrossRef]
- Pastor, L.; Vettorazzi, A.; Campión, J.; Cordero, P.; López de Cerain, A. Gene expression kinetics of renal transporters induced by ochratoxin A in male and female F344 rats. Food Chem. Toxicol. 2016, 98, 169–178. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.; Jiang, R.W.; Wu, B. Hormone-related pharmacokinetic variations associated with anti-breast cancer drugs. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1085–1095. [Google Scholar] [CrossRef]
- Shore, R.; Bjorne, H.; Omoto, Y.; Siemiatkowska, A.; Gustafsson, J.A.; Lindblad, M.; Holm, L. Sex differences and effects of oestrogen in rat gastric mucosal defence. World J. Gastroenterol. 2017, 23, 426–436. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.R.; Niu, T.; Gao, S.; Yin, T.; You, M.; Jiang, Z.H.; Hu, M. Inhibition of P-glycoprotein leads to improved oral bioavailability of compound K, an anticancer metabolite of red ginseng extract produced by gut microflora. Drug Metab. Dispos. 2012, 40, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Si, M.; Wang, Y.; Liu, L.H.; Zhang, Y.F.; Zhou, A.W.; Wei, W. Ginsenoside metabolite compound K exerts anti‑inflammatory and analgesic effects via downregulating COX2. Inflammopharmacology 2019, 27, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Lee, H.J. Ginsenoside compound K: Insights into recent studies on pharmacokinetics and health-promoting activities. Biomolecules 2020, 10, 1028. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, Y.; Han, S.H.; Jeon, J.Y.; Hwang, M.; Im, Y.J.; Kim, J.H.; Lee, S.Y.; Chae, S.W.; Kim, M.G. Development and validation of an LC-MS/MS method for determination of compound K in human plasma and clinical application. J. Gin. Res. 2013, 37, 135–141. [Google Scholar] [CrossRef]

| Forms | Form I | Form II | Form III | Form IV | |
|---|---|---|---|---|---|
| Bands | Wavenumber (cm−1) | ||||
| Free O-H (methanol) | 3677(ν), 1308(δ) | ||||
| O-H stretching vibration | 3405 | 3362 | 3423 | 3385 | |
| C-H stretching vibration | 2876, 2944 | 2874, 2949 | 2876, 2948 | 2875, 2946 | |
| Free C=O (acetone) | 1710 (ν), 1250 (δ) | ||||
| C=C stretching vibration | 1637 | 1654 | 1655 | 1639 | |
| CH3, CH2 asymmetric deformation | 1388, 1454 | 1390, 1456 | 1388, 1464 | 1388, 1454 | |
| C-O stretching vibration | 990, 1037, 1076, 1118 | 991, 1013, 1035, 1076, 1111 | 989, 1025, 1077, 1095 | 990, 1031, 1078, 1111 | |
| C-C deformation | 645 | 596 | 586 | 841, 632 | |
| Forms | Form I | Form II | Form III | Form IV | |||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | |
| AUC(0–t) (ug/L·h) | 1149.8 ± 430.17 | 2202.7 ± 237.26 * | 1191.1 ± 471.90 | 2447.2 ± 1828.5 | 1171.8 ± 462.65 | 1869.6 ± 618.65 | 758.62 ± 245.16 | 1312.0 ± 471.95 | |
| AUC(0–∞) (ug/L·h) | 1188.0 ± 480.37 | 2216.0 ± 225.74 * | 1235.2 ± 474.45 | 2551.2 ± 1968.0 | 1183.5 ± 466.08 | 1896.2 ± 604.12 | 784.11 ± 219.63 | 1320.2 ± 471.22 # | |
| t1/2z (h) | 3.0 ± 0.96 | 3.0 ± 0.84 | 2.9 ± 1.1 | 3.2 ± 0.75 | 3.3 ± 0.58 | 2.8 ± 0.25 | 3.5 ± 0.47 | 3.0 ± 0.60 | |
| Tmax (h) | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.3 ± 0.58 | |
| Cmax (ug/L) | 259.4 ± 144.2 | 453.3 ± 134.8 | 232.3 ± 68.97 | 459.6 ± 289.6 | 151.2 ± 64.45 | 355.1 ± 79.98 * | 166.4 ± 87.20 | 218.8 ± 111.7 | |
| Vz/F (L/kg) | 77.1 ± 28.3 | 40.5 ± 14.2 | 74.1 ± 31.6 | 45.3 ± 20.0 | 93.6 ± 50.1 | 44.8 ± 9.37 | 136 ± 43.2 | 73.6 ± 37.7 | |
| CLz/F (L/h/kg) | 18.7 ± 7.27 | 9.09 ± 0.880 | 18.0 ± 7.11 | 10.8 ± 5.79 | 19.4 ± 9.46 | 11.2 ± 3.02 | 26.8 ± 6.91 | 16.5 ± 5.67 | |
| Fabs (%) | 3.92% | 6.84% | 4.07% | 7.87% | 3.90% | 5.85% | 2.59% | 4.07% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, Y.-Y.; Gao, X.; Niu, Y.-J.; Shi, X.-L.; Zhou, W. Polymorphic Characterization, Pharmacokinetics, and Anti-Inflammatory Activity of Ginsenoside Compound K Polymorphs. Molecules 2021, 26, 1983. https://doi.org/10.3390/molecules26071983
Kuang Y-Y, Gao X, Niu Y-J, Shi X-L, Zhou W. Polymorphic Characterization, Pharmacokinetics, and Anti-Inflammatory Activity of Ginsenoside Compound K Polymorphs. Molecules. 2021; 26(7):1983. https://doi.org/10.3390/molecules26071983
Chicago/Turabian StyleKuang, Yun-Yan, Xuan Gao, Yi-Jun Niu, Xun-Long Shi, and Wei Zhou. 2021. "Polymorphic Characterization, Pharmacokinetics, and Anti-Inflammatory Activity of Ginsenoside Compound K Polymorphs" Molecules 26, no. 7: 1983. https://doi.org/10.3390/molecules26071983
APA StyleKuang, Y.-Y., Gao, X., Niu, Y.-J., Shi, X.-L., & Zhou, W. (2021). Polymorphic Characterization, Pharmacokinetics, and Anti-Inflammatory Activity of Ginsenoside Compound K Polymorphs. Molecules, 26(7), 1983. https://doi.org/10.3390/molecules26071983







