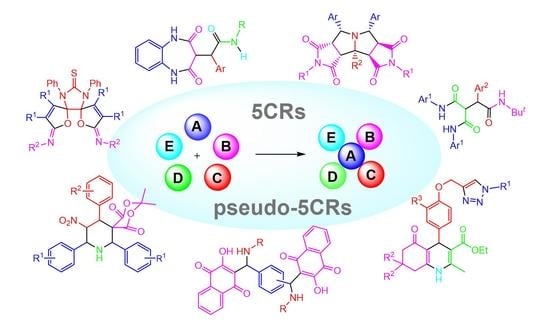
Recent Developments on Five-Component Reactions
Abstract
1. Introduction
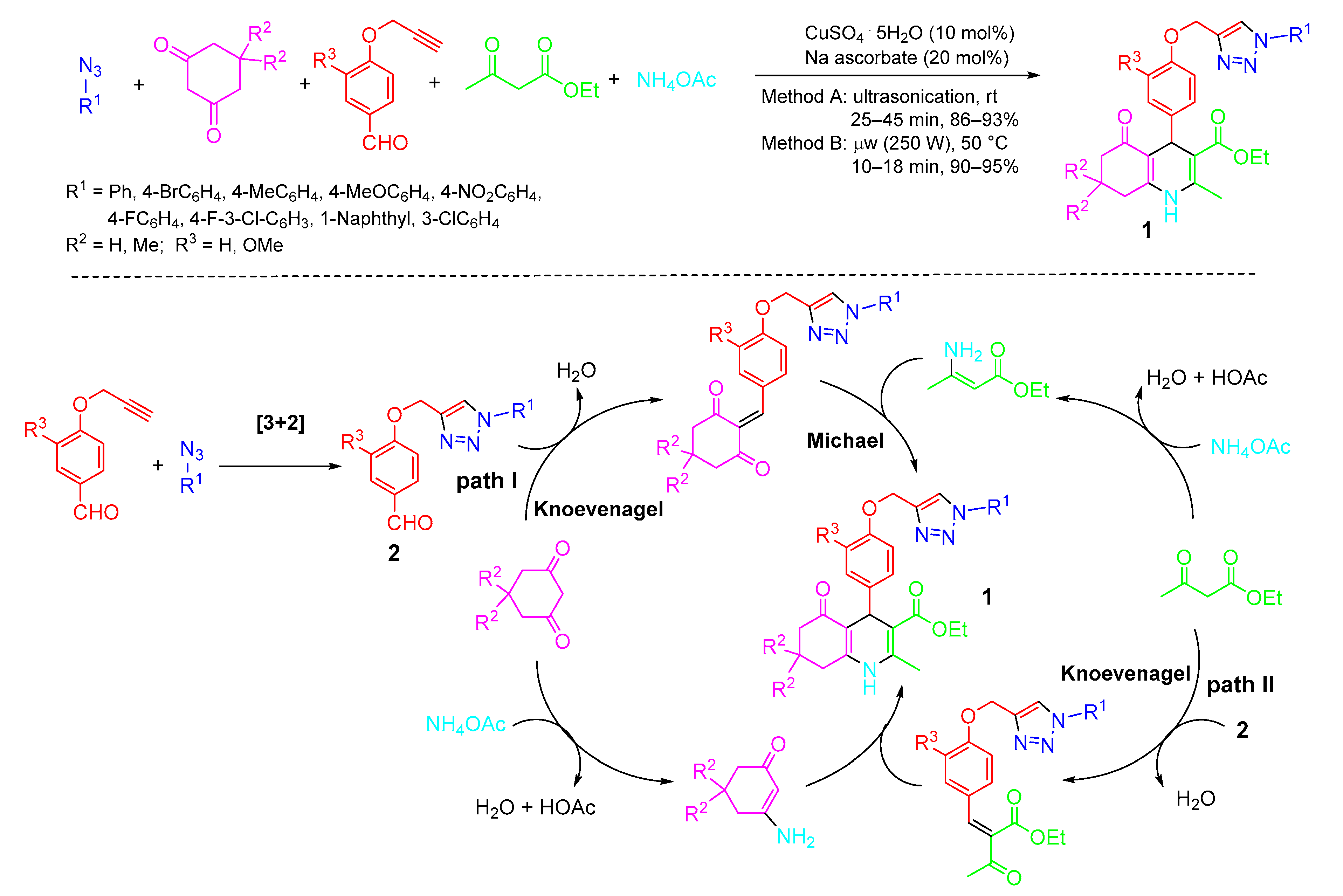
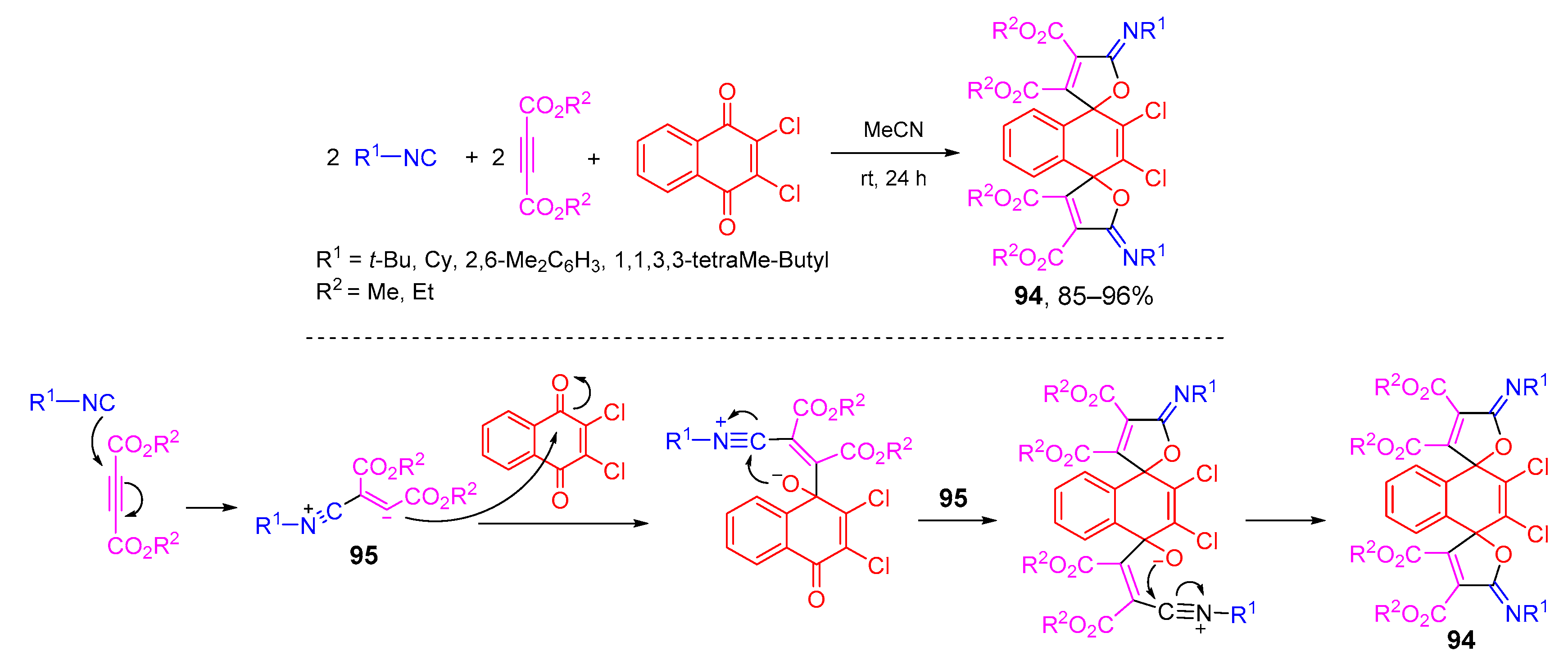
2. Type-I, 5CRs of A + B + C + D + E
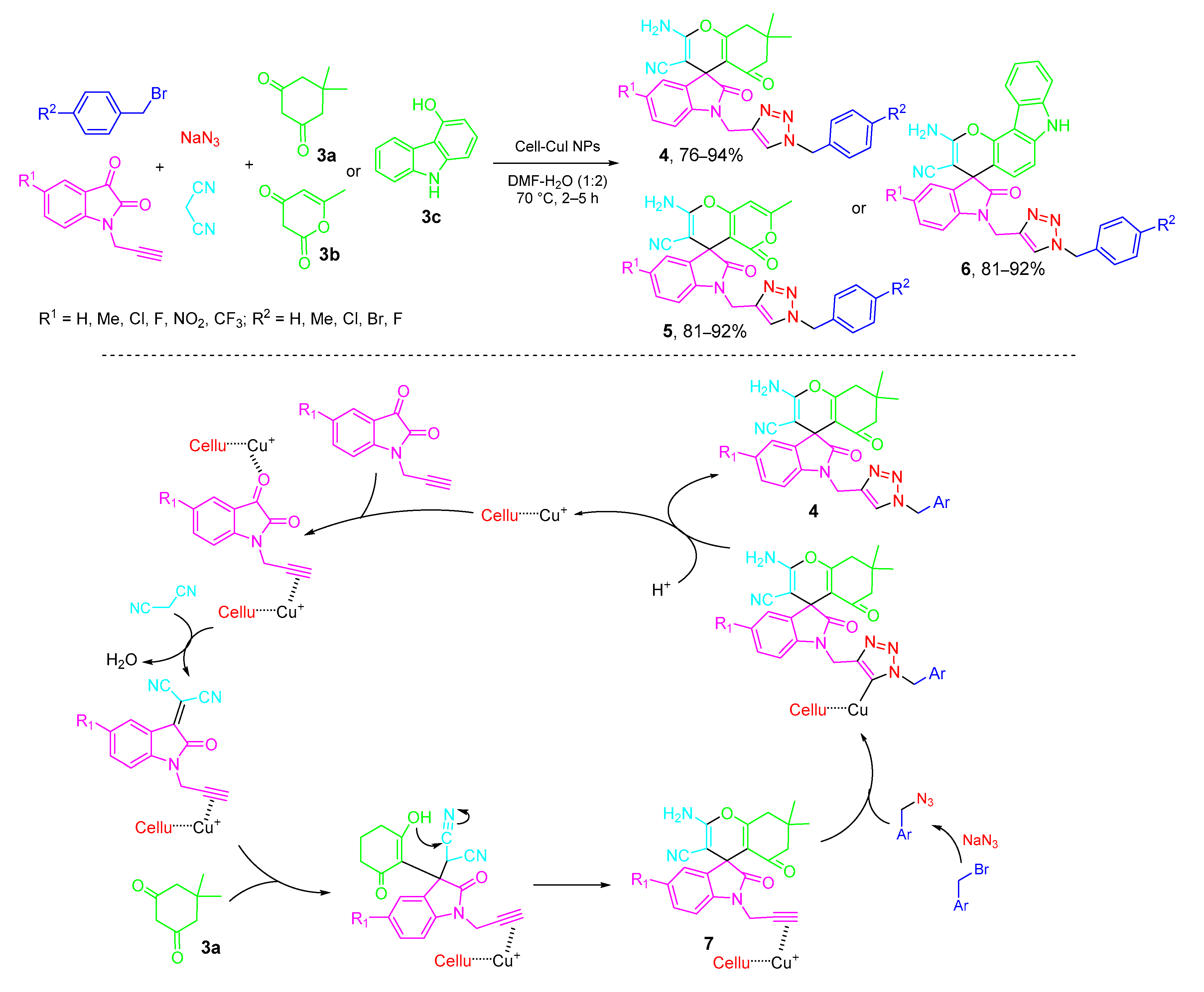
3. Type-II, Pseudo-5CRs of 2A + B + C + D
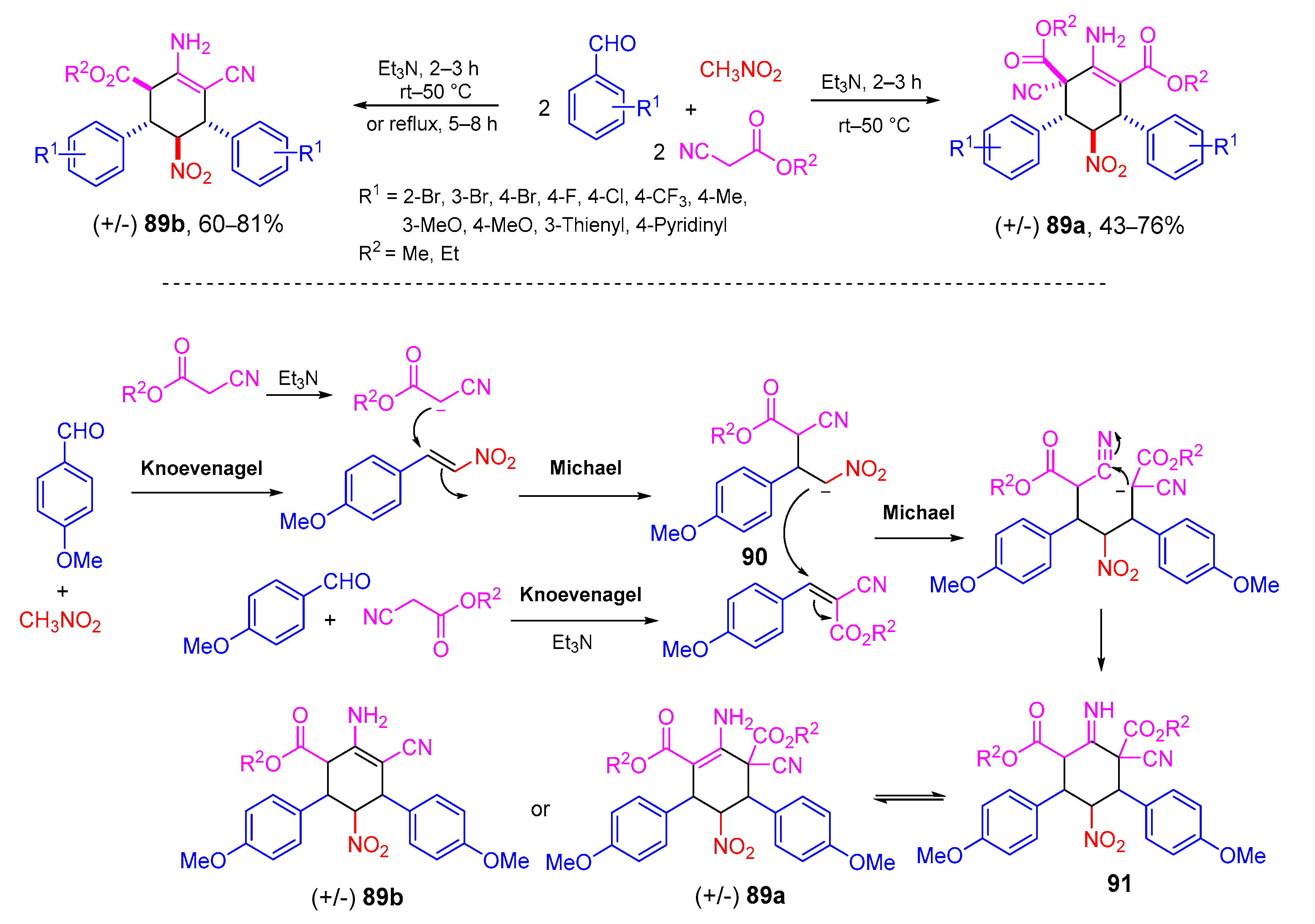
4. Type-III, Pseudo-5CRs of 2A + 2B + C
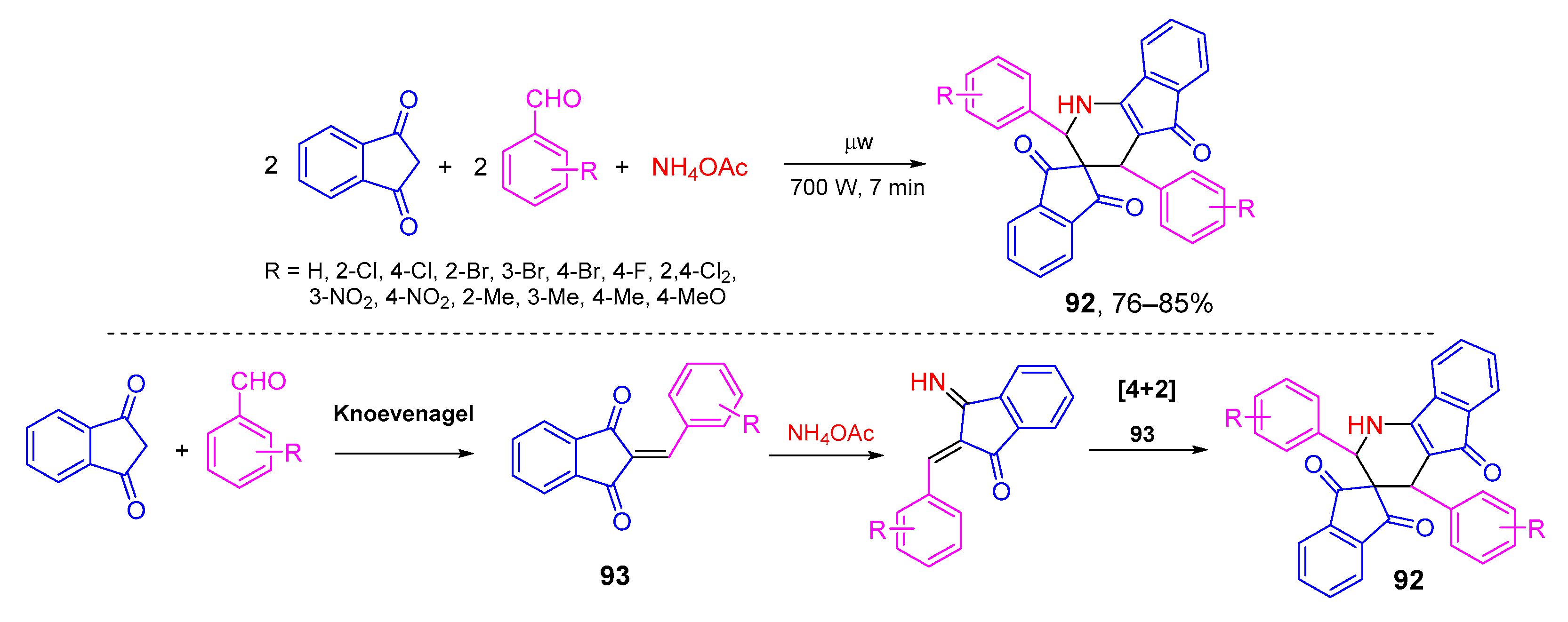
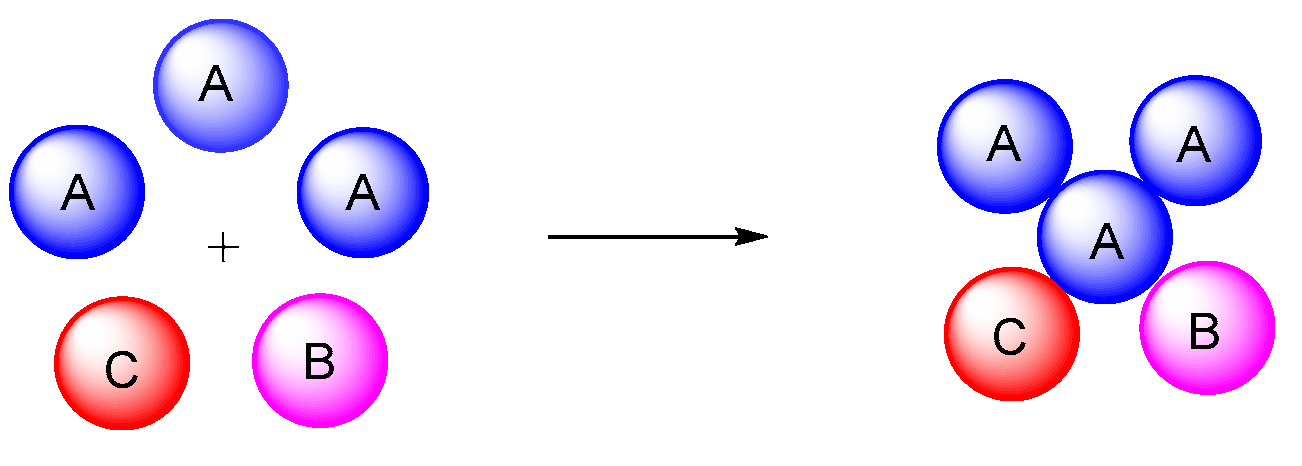
5. Type-IV, Pseudo-5CRs of 3A + B + C
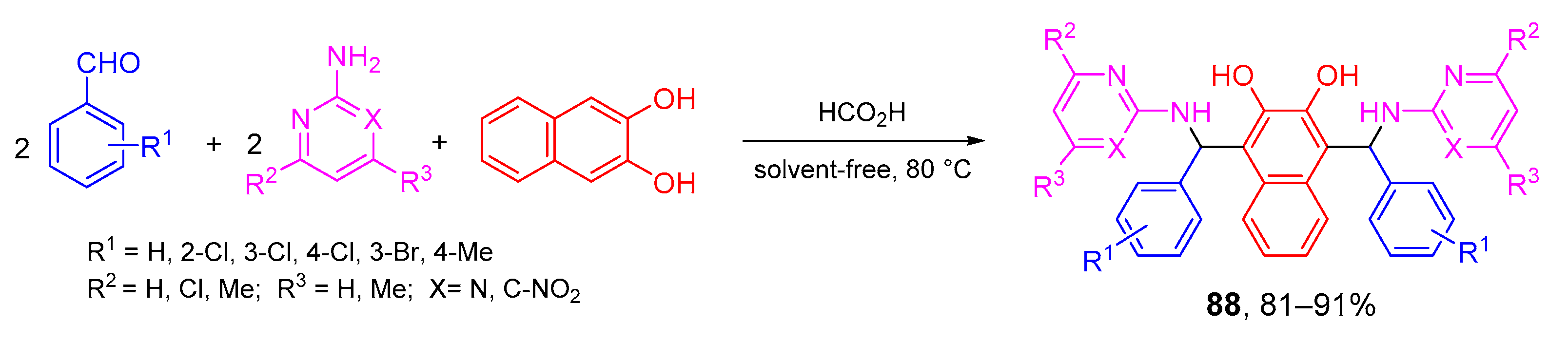
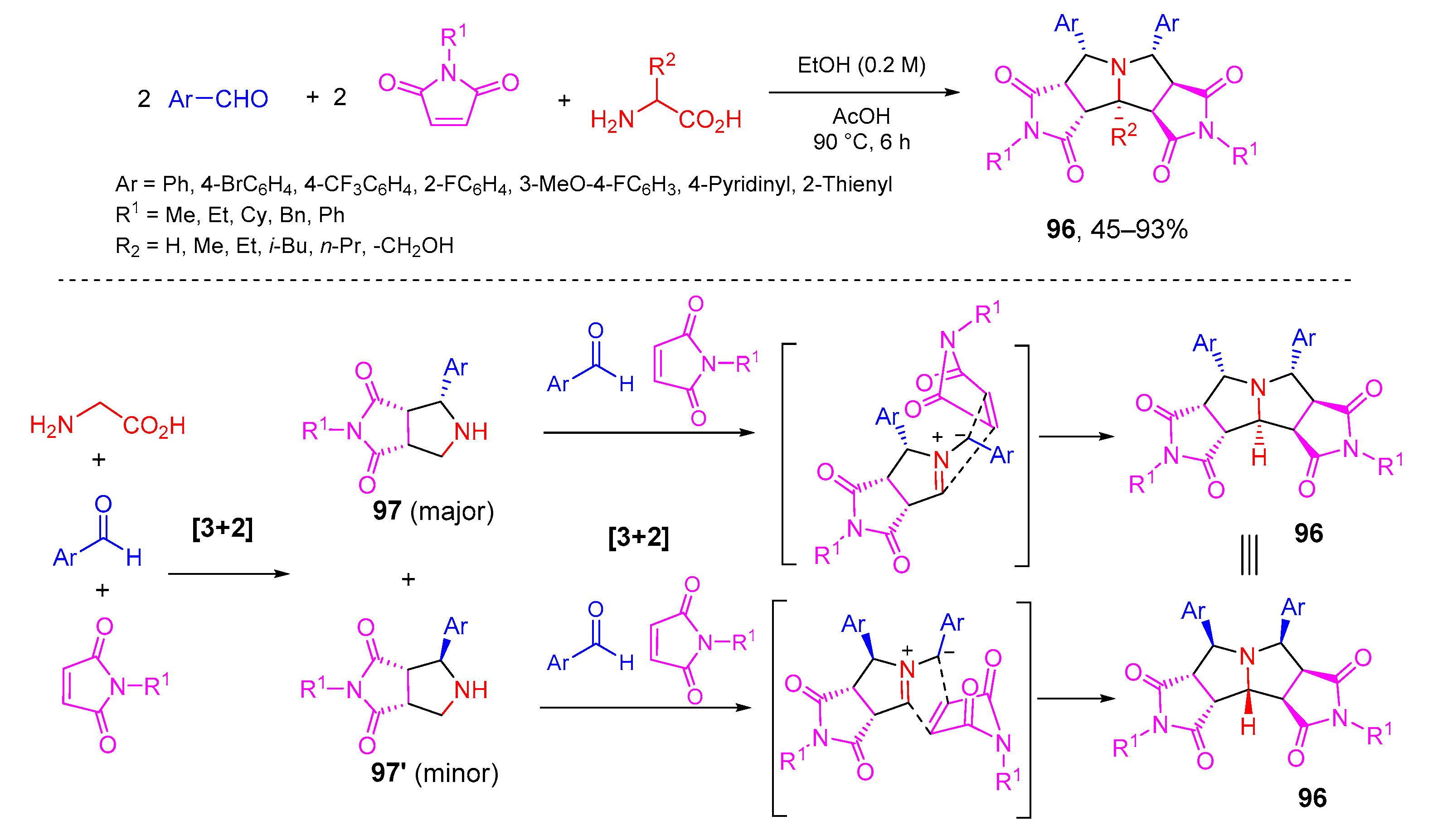
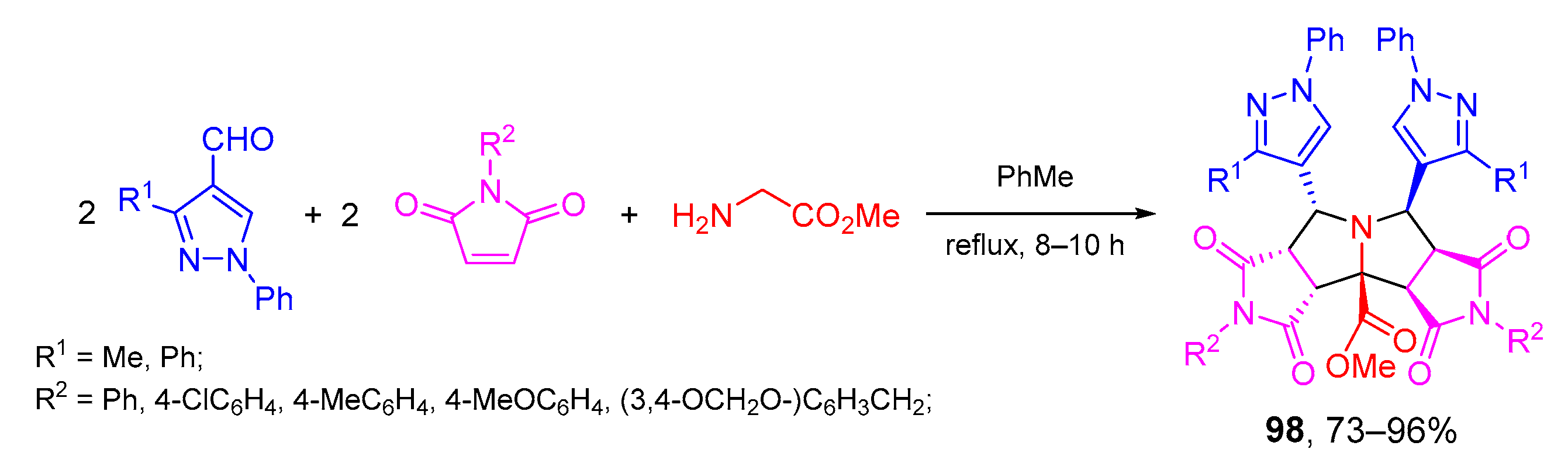
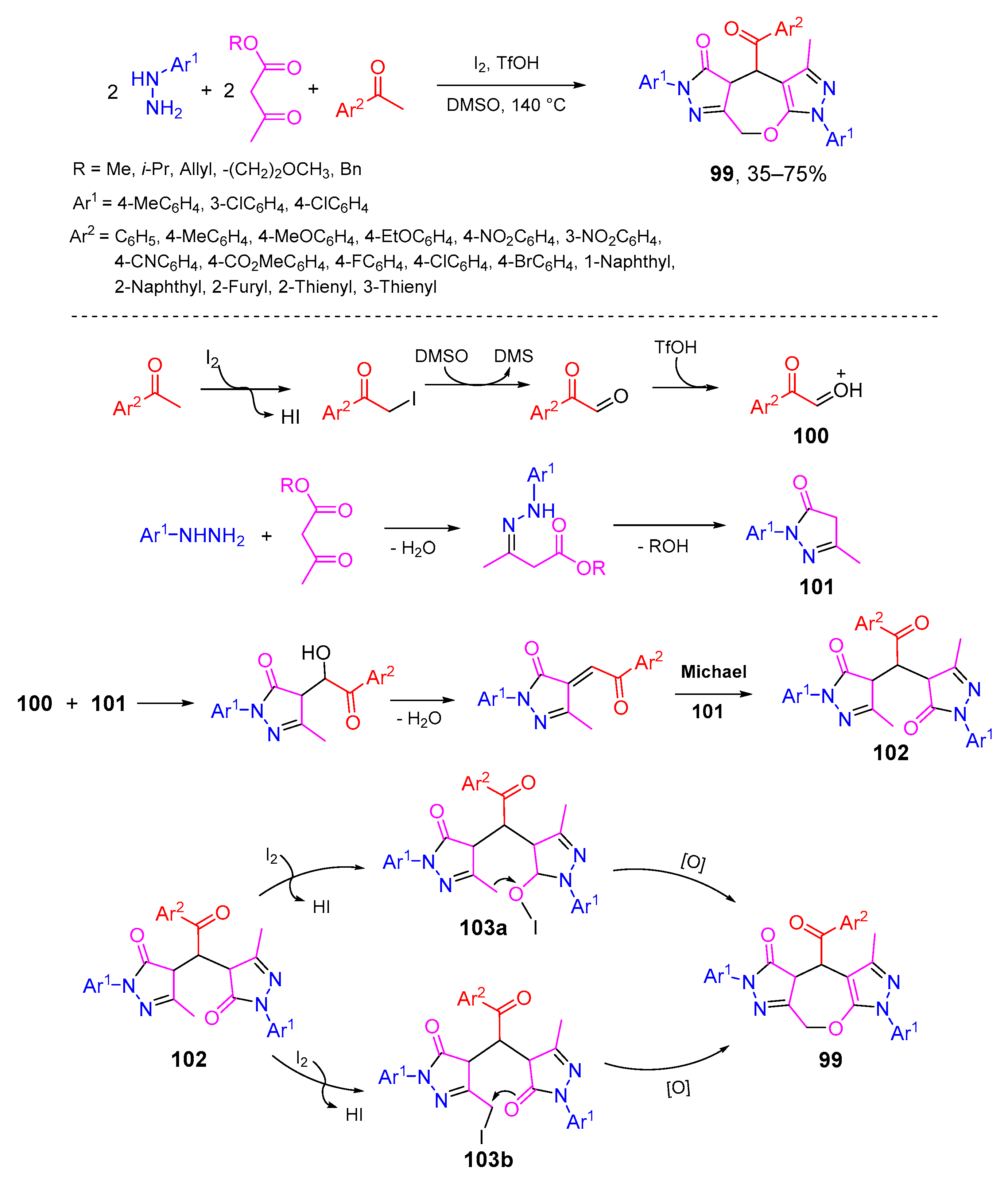
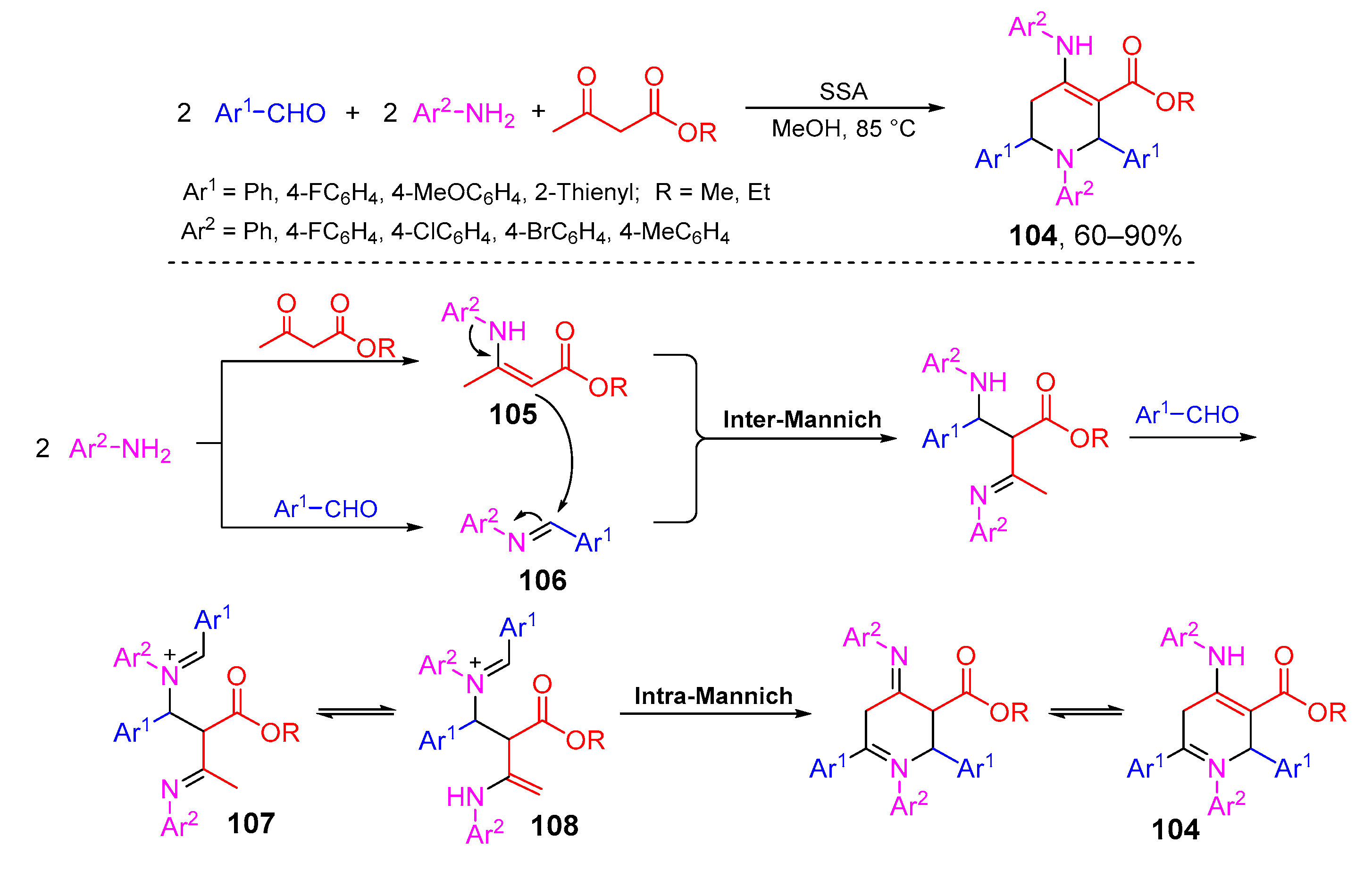
6. Type-V, Pseudo-5CRs of 3A + 2B
7. Type-VI, Pseudo-5CRs of 4A + B
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, J.; Bienaymé, H. (Eds.) Multicomponent Reactions; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Zhu, J.; Wang, Q.; Wang, M.-X. (Eds.) Multicomponent Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Herrera, R.P.; Marques-Lopez, E. (Eds.) Multicomponent Reactions—Concepts and Applications for Design and Synthesis; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Ameta, K.L.; Dandia, A. Multicomponent Reactions—Synthesis of Bioactive Heterocycles; CRC Press: London, UK, 2017. [Google Scholar]
- Clarke, P.A.; Santos, S.; Martin, W.H.C. Combining pot, atom and step economy (PASE) in organic synthesis. Synthesis of tetrahydropyran-4-ones. Green Chem. 2007, 9, 438–440. [Google Scholar] [CrossRef]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, W.-B. Pot, Atom, and Step Economy (PASE) Synthesis; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Zhang, W. One-pot Organic Reactions. In Green Synthetic Processes and Procedures; Ballini, R., Ed.; RSC: Cambridge, UK, 2019; pp. 20–38. [Google Scholar]
- Brauch, S.; van Berkel, S.S.; Westermann, B. Higher-order multicomponent reactions: Beyond four reactants. Chem. Soc. Rec. 2013, 42, 4948–4962. [Google Scholar] [CrossRef]
- Web of Science Search on “x-Component Reaction”. Available online: https://clarivate.libguides.com/webofscienceplatform/alldb (accessed on 6 February 2021).
- Nenajdenko, V.G. Access to molecular complexity. Multicomponent reactions involving five or more components. Russ. Chem. Rev. 2020, 89, 1274–1336. [Google Scholar] [CrossRef]
- Singh, H.; Sindhu, J.; Khurana, J.M.; Sharma, C.; Aneja, K.R. A facile eco-friendly one-pot five-component synthesis of novel 1,2,3-triazole-linked pentasubstituted 1,4-dihydropyridines and their biological and photophysical studies. Aust. J. Chem. 2013, 66, 1088–1096. [Google Scholar] [CrossRef]
- Chavan, P.V.; Pandit, K.S.; Desai, U.V.; Wadgaonkar, P.P.; Nawale, L.; Bhansali, S.; Sarkar, D. Click-chemistry-based multicomponent condensation approach for design and synthesis of spirochromene-tethered 1,2,3-triazoles as potential antitubercular agents. Res. Chem. Intermed. 2017, 43, 5675–5690. [Google Scholar] [CrossRef]
- Chavan, P.V.; Desai, U.V.; Wadgaonkar, P.P.; Tapase, S.R.; Kodam, K.M.; Choudhari, A.; Sarkar, D. Click chemistry based multicomponent approach in the synthesis of spirochromenocarbazole tethered 1,2,3-triazoles as potential anticancer agents. Bioorg. Chem. 2019, 85, 475–486. [Google Scholar] [CrossRef]
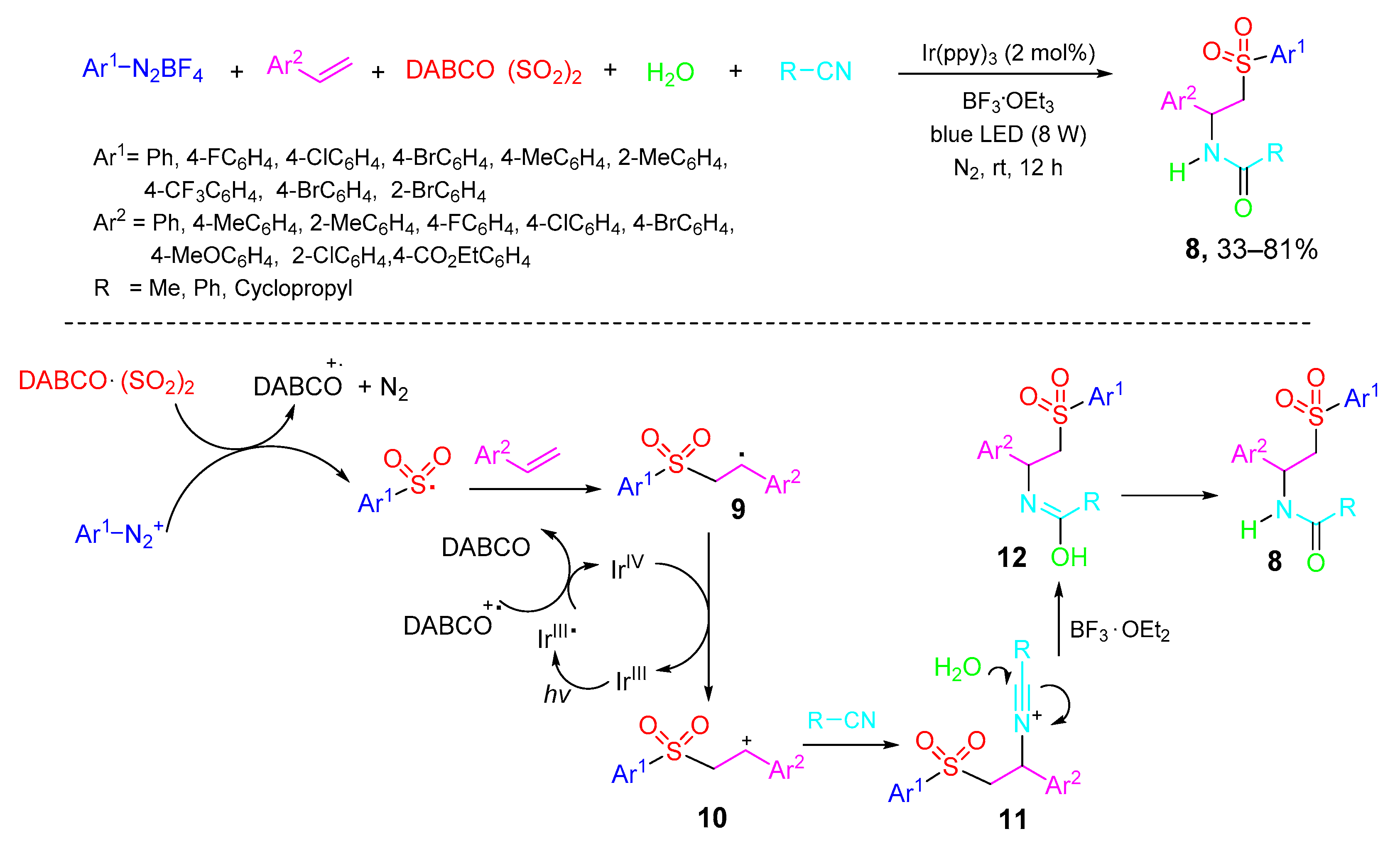
- Zong, Y.; Lang, Y.; Yang, M.; Li, X.; Fan, X.; Wu, J. Synthesis of β-sulfonyl amides through a multicomponent reaction with the insertion of sulfur dioxide under visible light irradiation. Org. Lett. 2019, 21, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
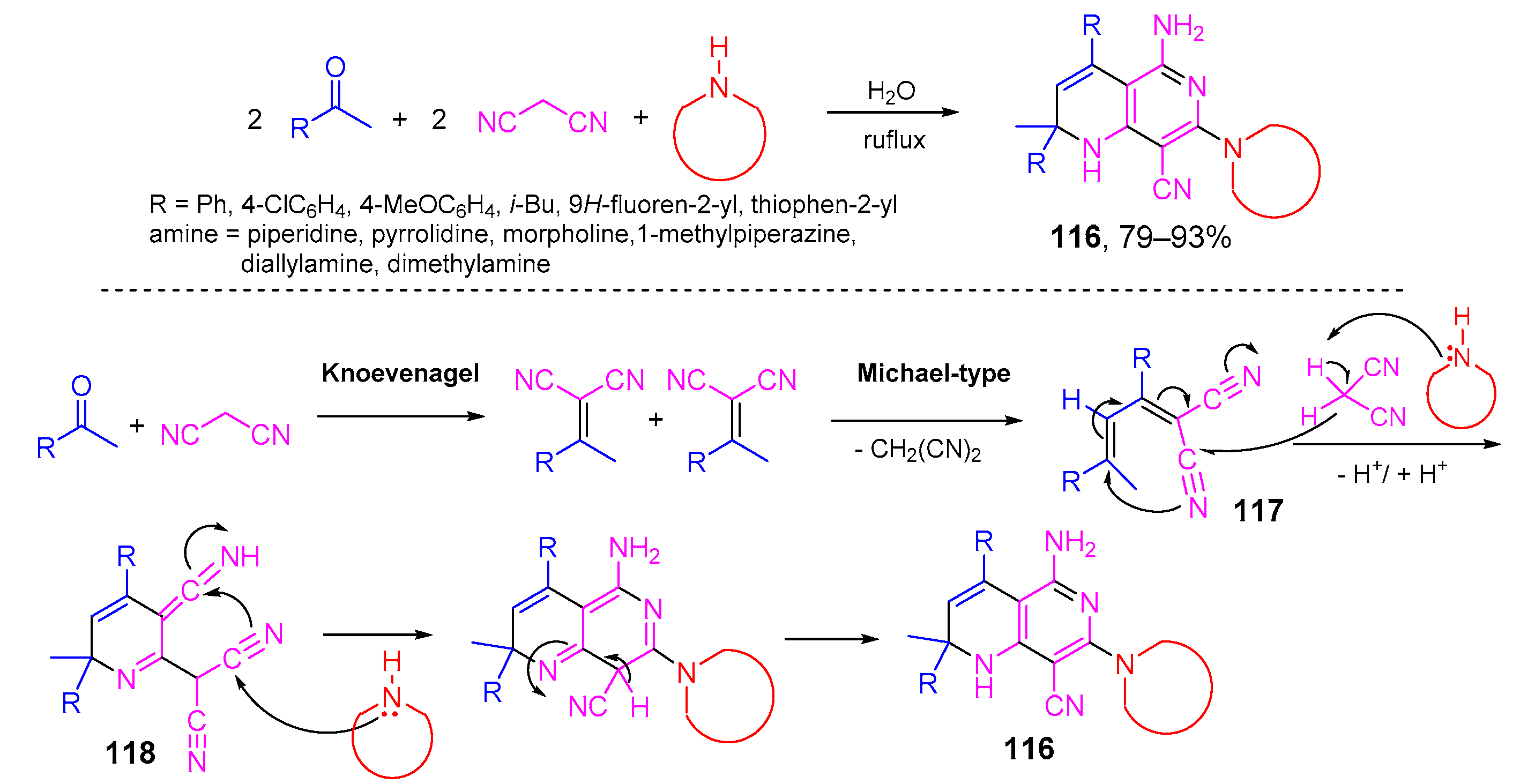
- Govindaraju, S.; Tabassum, S.; Khan, R.-R.; Pasha, M.A. Meglumine catalyzed one-pot green synthesis of novel 4,7-dihydro-1H-pyrazolo[3,4-b]pyridin-6-amines. Chin. Chem. Lett. 2017, 28, 437–441. [Google Scholar] [CrossRef]
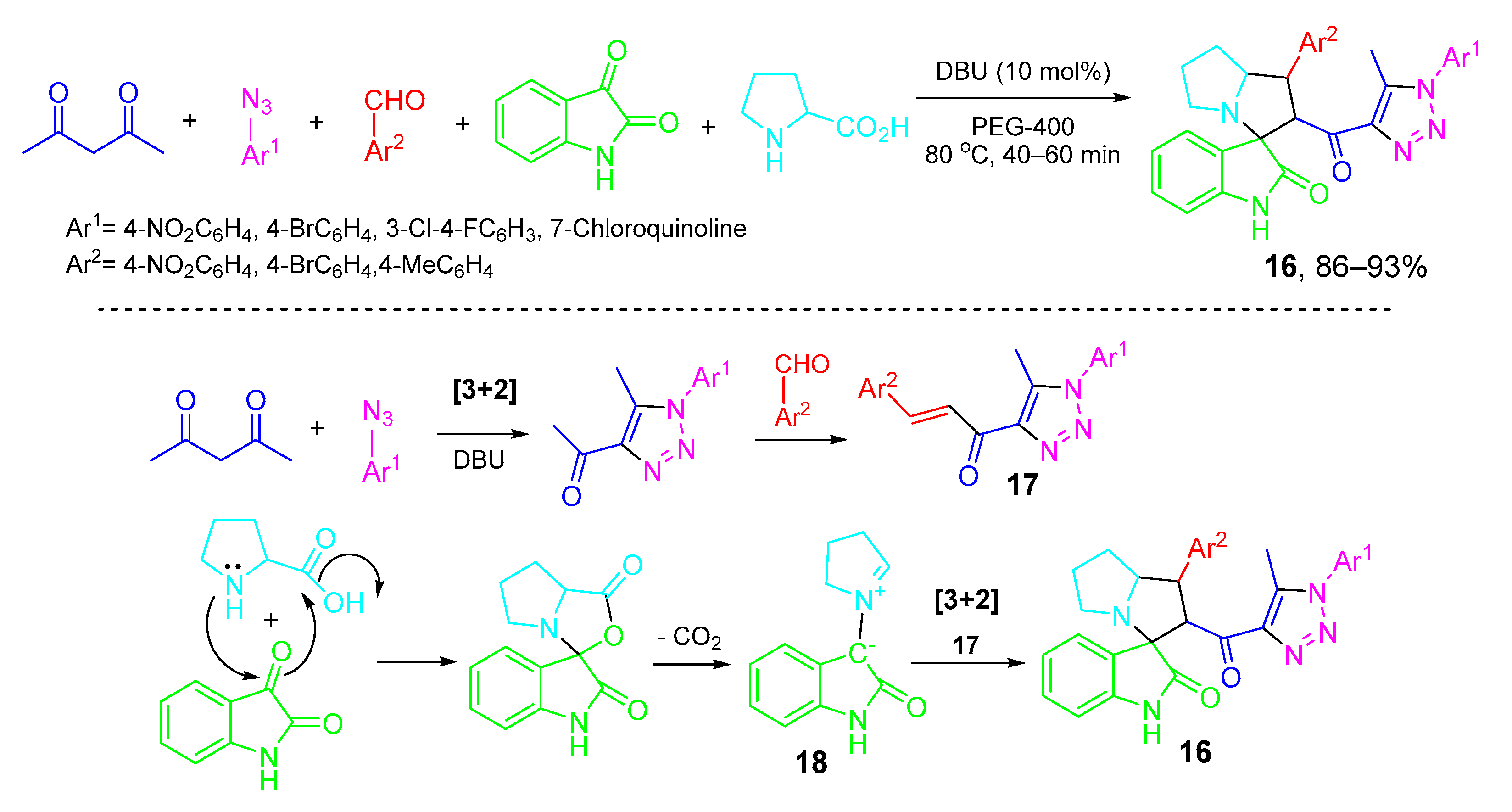
- Kumari, S.; Singh, H.; Khurana, J.M. An efficient green approach for the synthesis of novel triazolyl spirocyclic oxindole derivatives via one-pot five component protocol using DBU as catalyst in PEG-400. Tetrahedron Lett. 2016, 57, 3081–3085. [Google Scholar] [CrossRef]
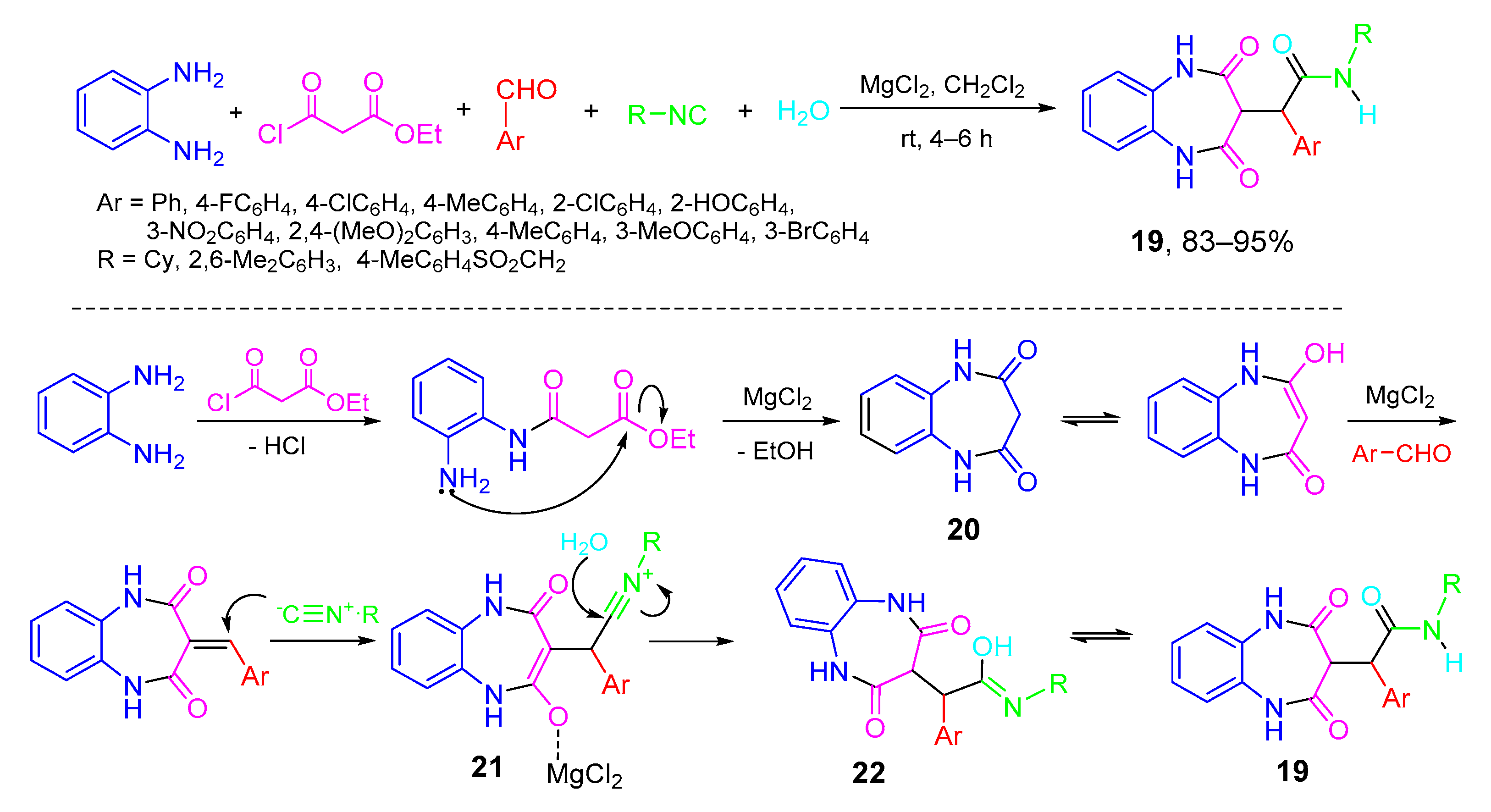
- Faraz, S.M.; Rahmati, A.; Mirkhani, V. One-pot isocyanide-based five-component reaction: Synthesis of highly functionalized N-cyclohexyl-2-(2,4-dioxo-2,3,4,5 tetrahydro-1H-benzo[b][1,5]diazepin-3-yl)-2-phenylacetamides. Synth. Commun. 2017, 47, 557–565. [Google Scholar] [CrossRef]
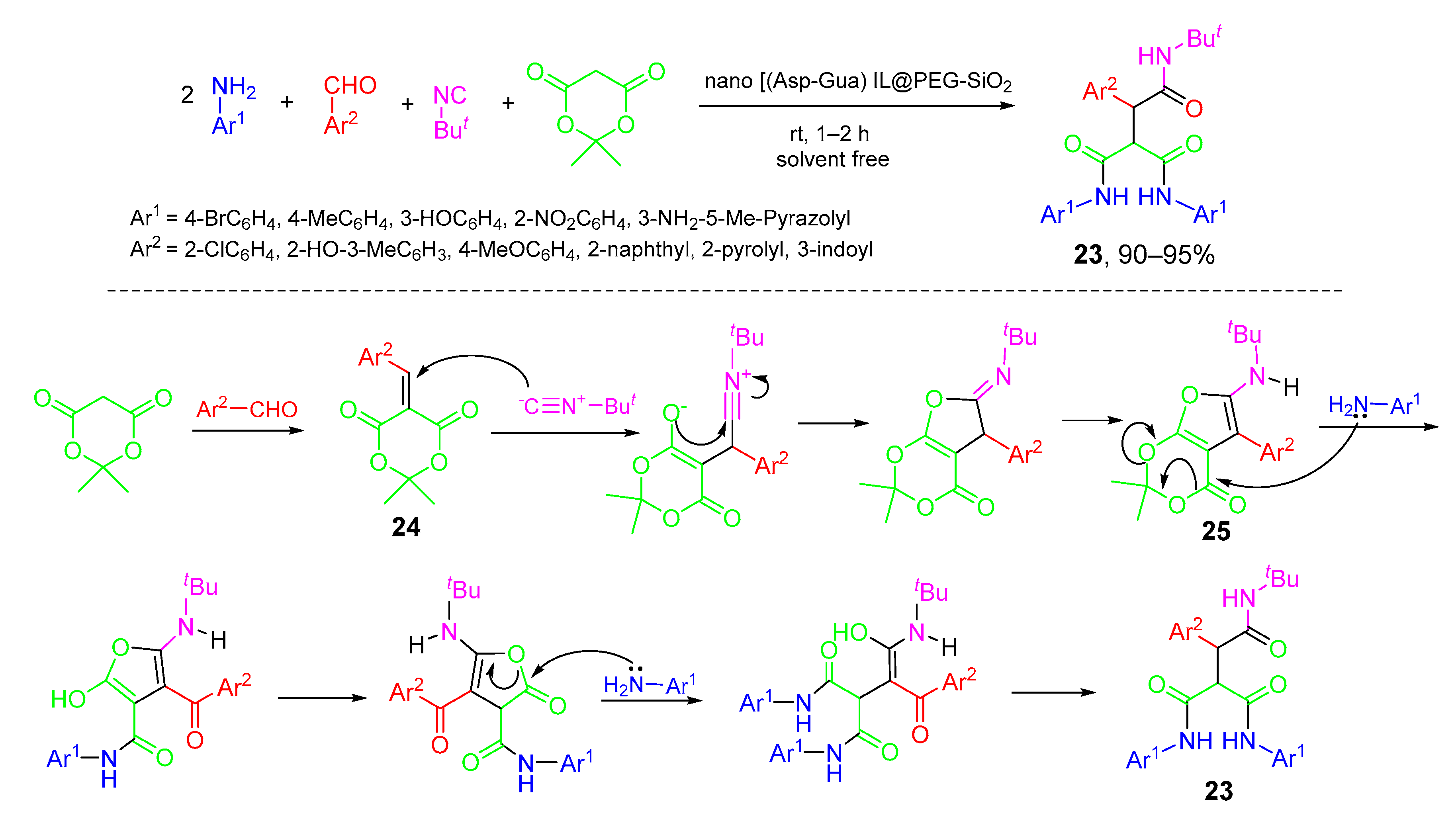
- Nikoofar, K.; Shahriyari, F. Novel bio-based core-shell organic-inorganic nanohybrid from embedding aspartic acid-guanine ionic liquid on the hydroxylated nano silica surface (nano [(Asp-Gua) IL@PEG-SiO2]): A versatile nanostructure for the synthesis of bis(2,3-dihydroquinazolin-4(1H)-one) derivatives and tricarboxamides under green media. Polyhedron 2020, 179, 114361. [Google Scholar] [CrossRef]
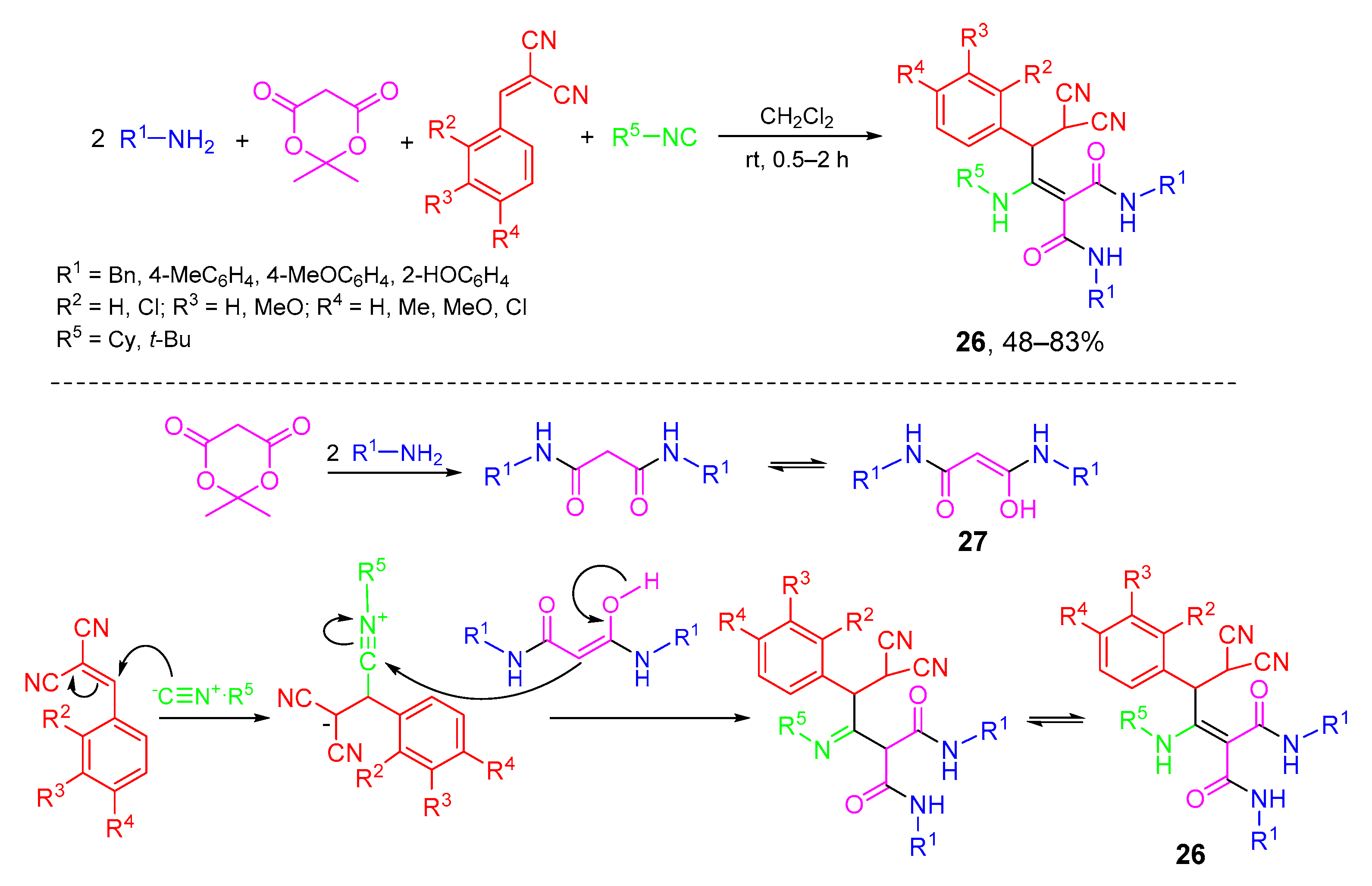
- Rahmati, A.; Kenarkoohi, T.; Ahmadi-Varzaneh, M. Synthesis of novel series of malonamides derivatives via a five-component reaction. Mol. Divers. 2013, 17, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Kenarkoohi, T.; Rahmati, A. Synthesis of malonamide pseudopeptidic compounds using a pseudo five-component reaction and evaluation of their gelation properties. J. Mol. Liq. 2019, 276, 714–720. [Google Scholar] [CrossRef]
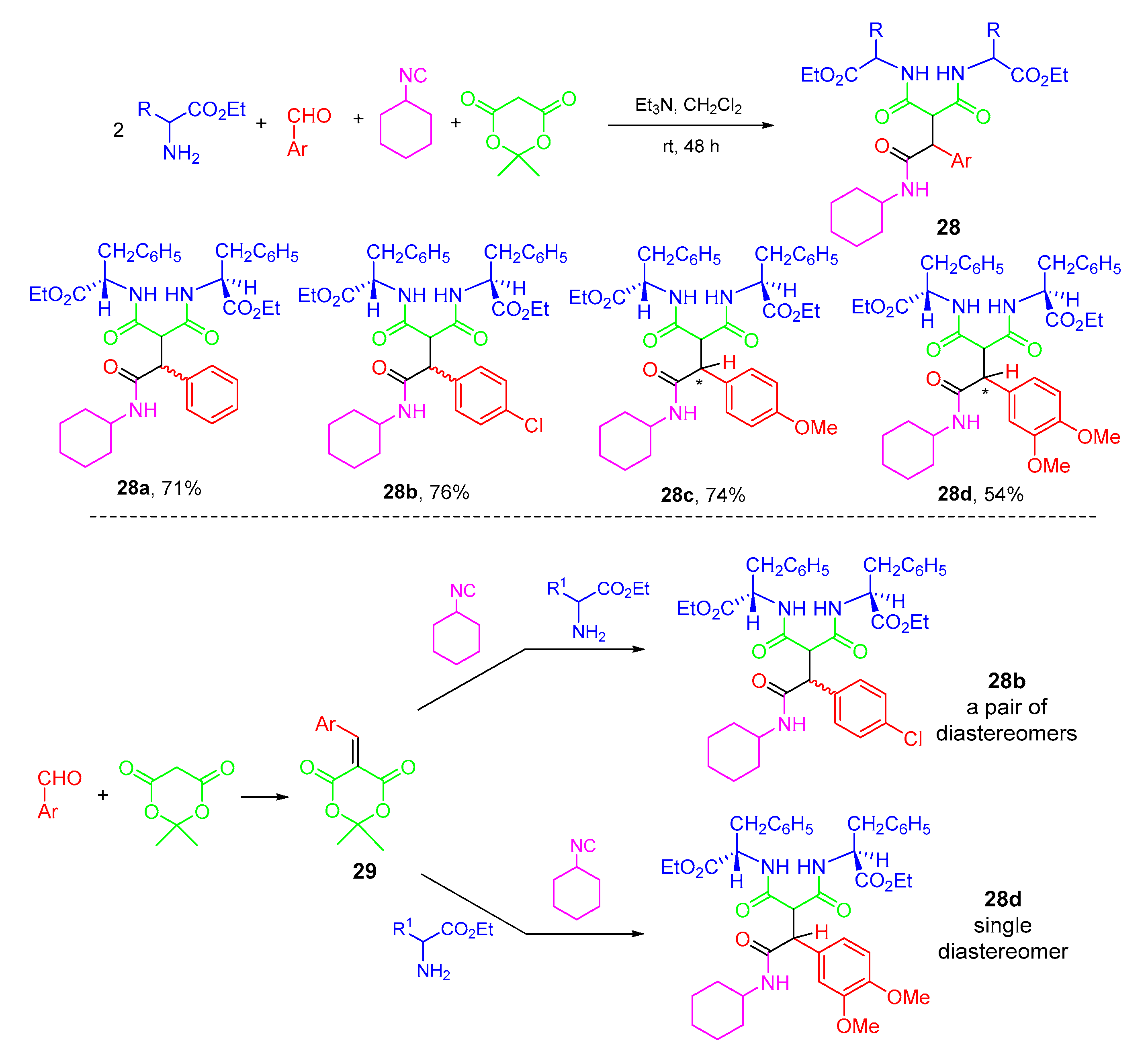
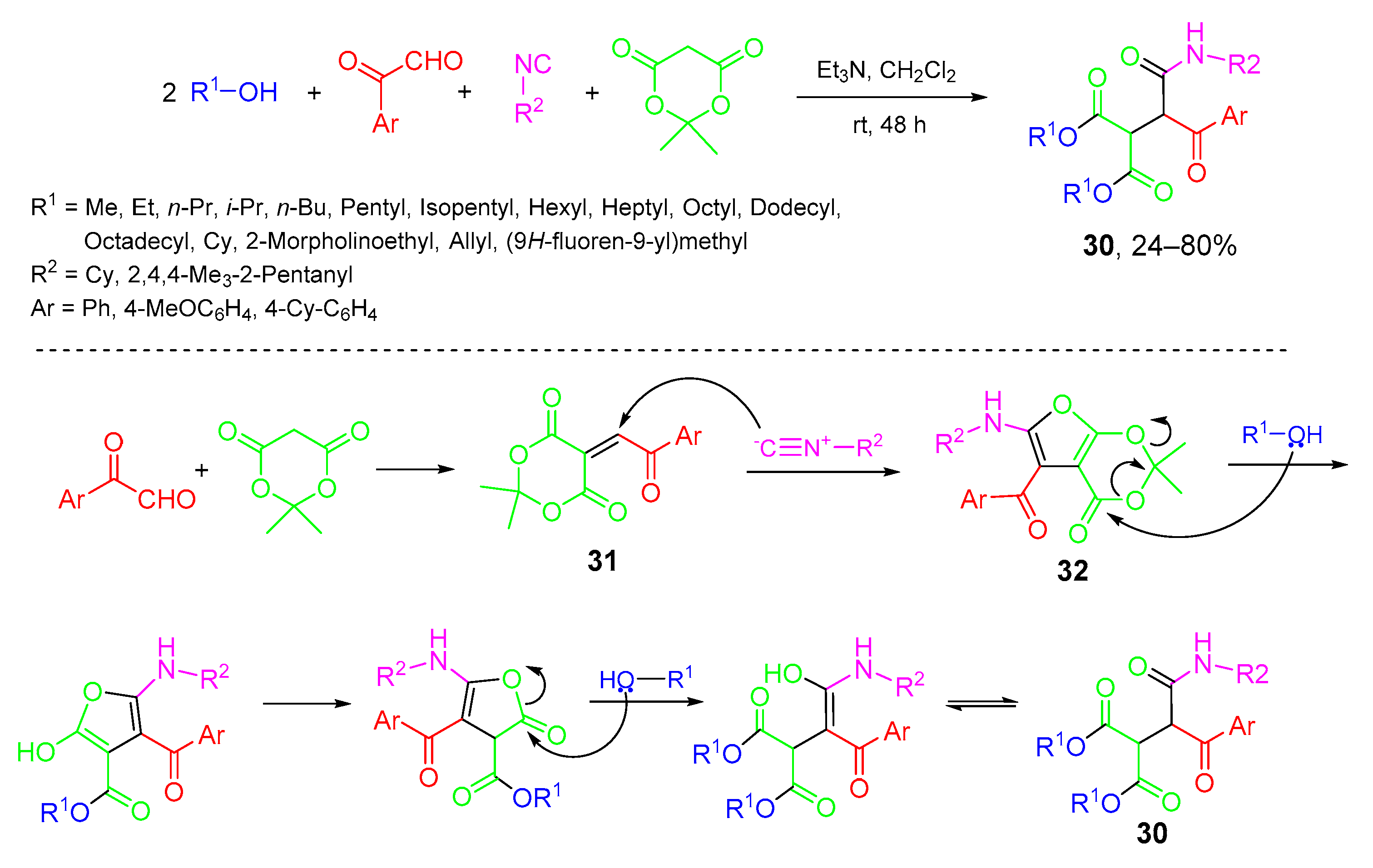
- Googol, F.; Rahmati, A. Synthesis of a new series of multifunctional dialkyl 2-(1-(alkylamino)-1,3-dioxo-3-phenylpropan-2-yl)malonates as low molecular weight supramolecular organogelators using five-component reaction. Tetrahedron 2018, 74, 240–252. [Google Scholar] [CrossRef]
- Nikbakht, A.; Ramezanpour, S.; Balalaie, S.; Rominger, F. Efficient and stereoselective synthesis of α-hydrazino tetrazoles through a pseudo five-component domino reaction. Tetrahedron 2015, 71, 6790–6795. [Google Scholar] [CrossRef]
- Amanpour, T.; Bazgir, A.; Ardekani, A.M.; Ghahremanzadeh, R. Pseudo five-component synthesis of 5-phenyldihydrospiro[diindenopyridine-indenoquinoxaline]dione derivatives via a one-pot condensation reaction. Monatsh. Chem. 2014, 145, 627–632. [Google Scholar] [CrossRef]
- Li, Y.; Xue, Z.; Ye, W.; Liu, J.; Yao, J.; Wang, C. One-pot multicomponent synthesis of highly functionalized piperidines from substituted β-nitrostyrenes, Meldrum’s acid, aromatic aldehydes, and ammonium acetate. ACS Comb. Sci. 2014, 16, 113–119. [Google Scholar] [CrossRef]
- Attorresi, C.I.; Bonifazi, E.L.; Ramirez, J.A.; Gola, G.F. One-step synthesis of N,N’-substituted 4-imidazolidinones by an isocyanide-based pseudo-five-multicomponent reaction. Org. Biomol. Chem. 2018, 16, 8944–8949. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Q.; Zhou, Z.; Liu, J.; Yang, J.; Wang, C. One-pot synthesis of polysubstituted 2-piperidinones from aromatic aldehydes, nitromethane, ammonium acetate, and dialkyl malonates. Monatsh. Chem. 2013, 144, 1031–1041. [Google Scholar] [CrossRef]
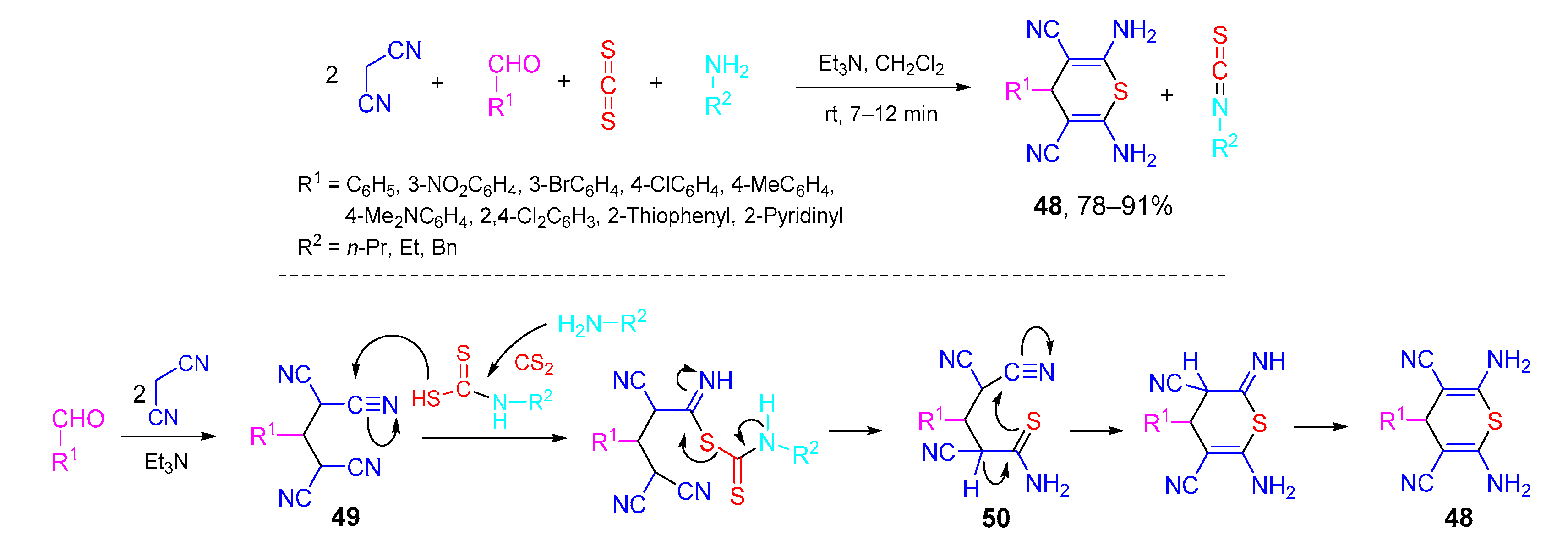
- Mobinikhaledi, A.; Bodaghifard, M.A.; Asadbegi, S. A novel four- and pseudo-five-component reaction: Unexpected efficient one-pot synthesis of 4H-thiopyran derivatives. Mol. Divers. 2016, 20, 461–468. [Google Scholar] [CrossRef]
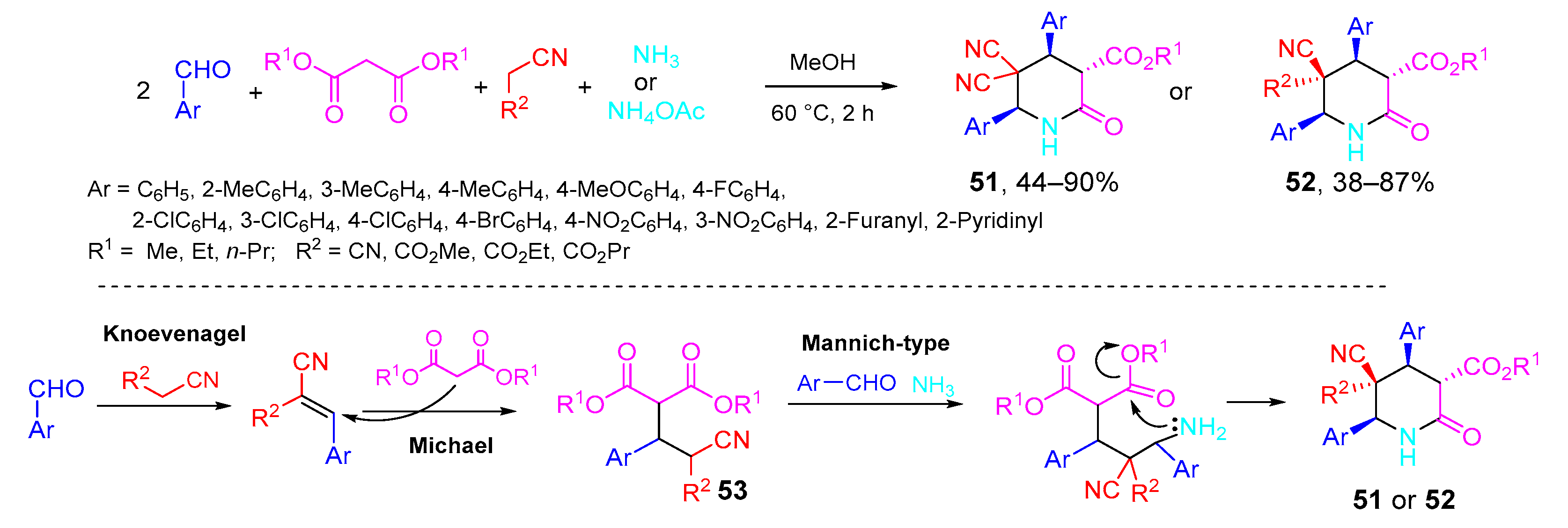
- Vereshchagin, A.N.; Karpenko, K.A.; Elinson, M.N.; Minaeva, A.P.; Goloveshkin, A.S.; Hansford, K.A.; Egorov, M.P. One-pot five-component high diastereoselective synthesis of polysubstituted 2-piperidinones from aromatic aldehydes, nitriles, dialkyl malonates and ammonium acetate. Mol. Divers. 2020, 24, 1327–1342. [Google Scholar] [CrossRef]
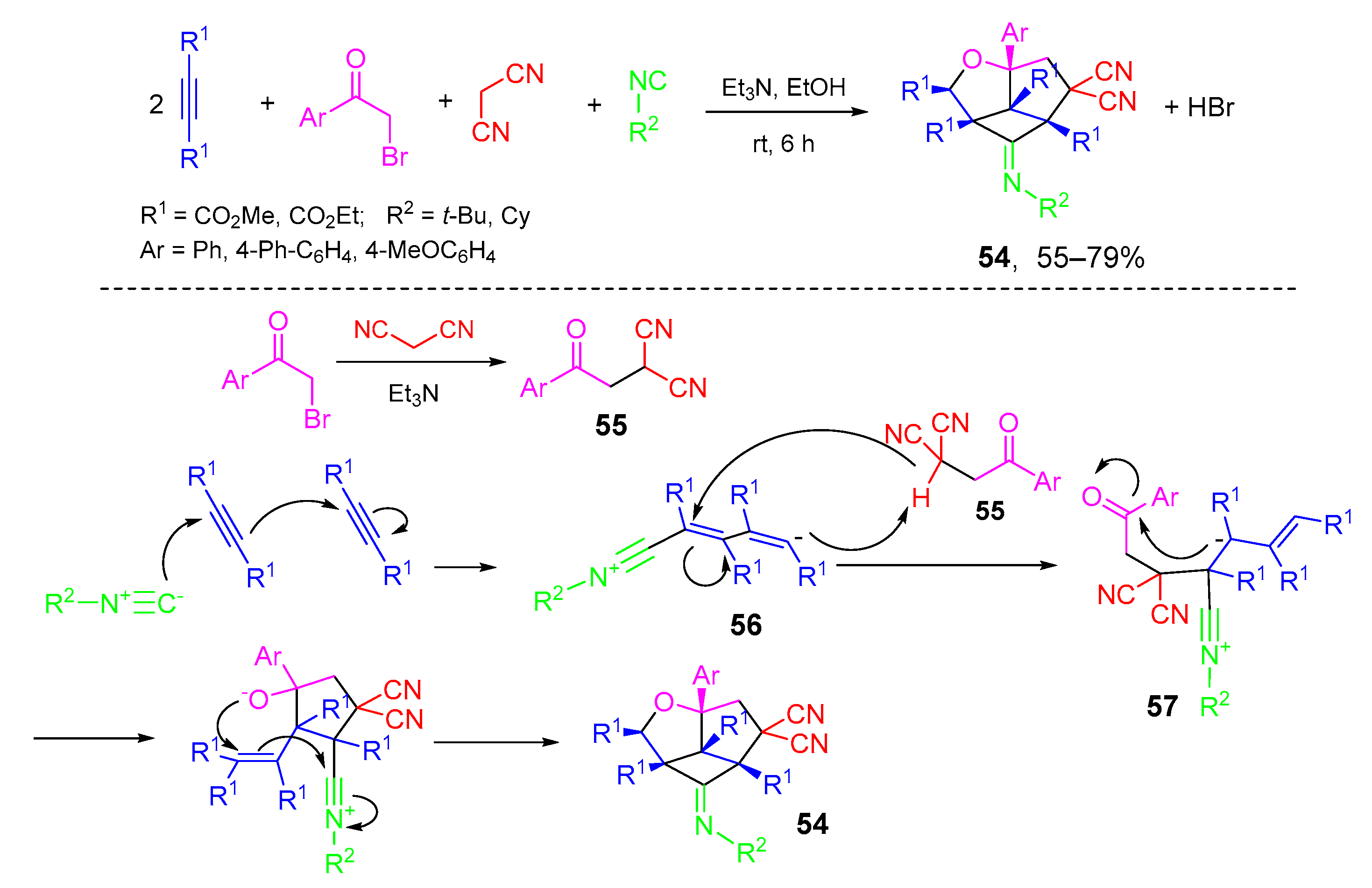
- Adib, M.; Sheikhi, E.; Haghshenas, P.; Rajai-Daryasarei, S.; Bijanzadeh, H.R.; Zhu, L.-G. A highly diastereoselective five-component synthesis of 1-(alkylimino)-5,5-dicyano-3a-aryloctahydro-3-oxacyclobuta[cd]pentalene-1a,2,5a,5b(2H,3aH)-tetracarboxylates. Tetrahedron Lett. 2014, 55, 4983–4986. [Google Scholar] [CrossRef]
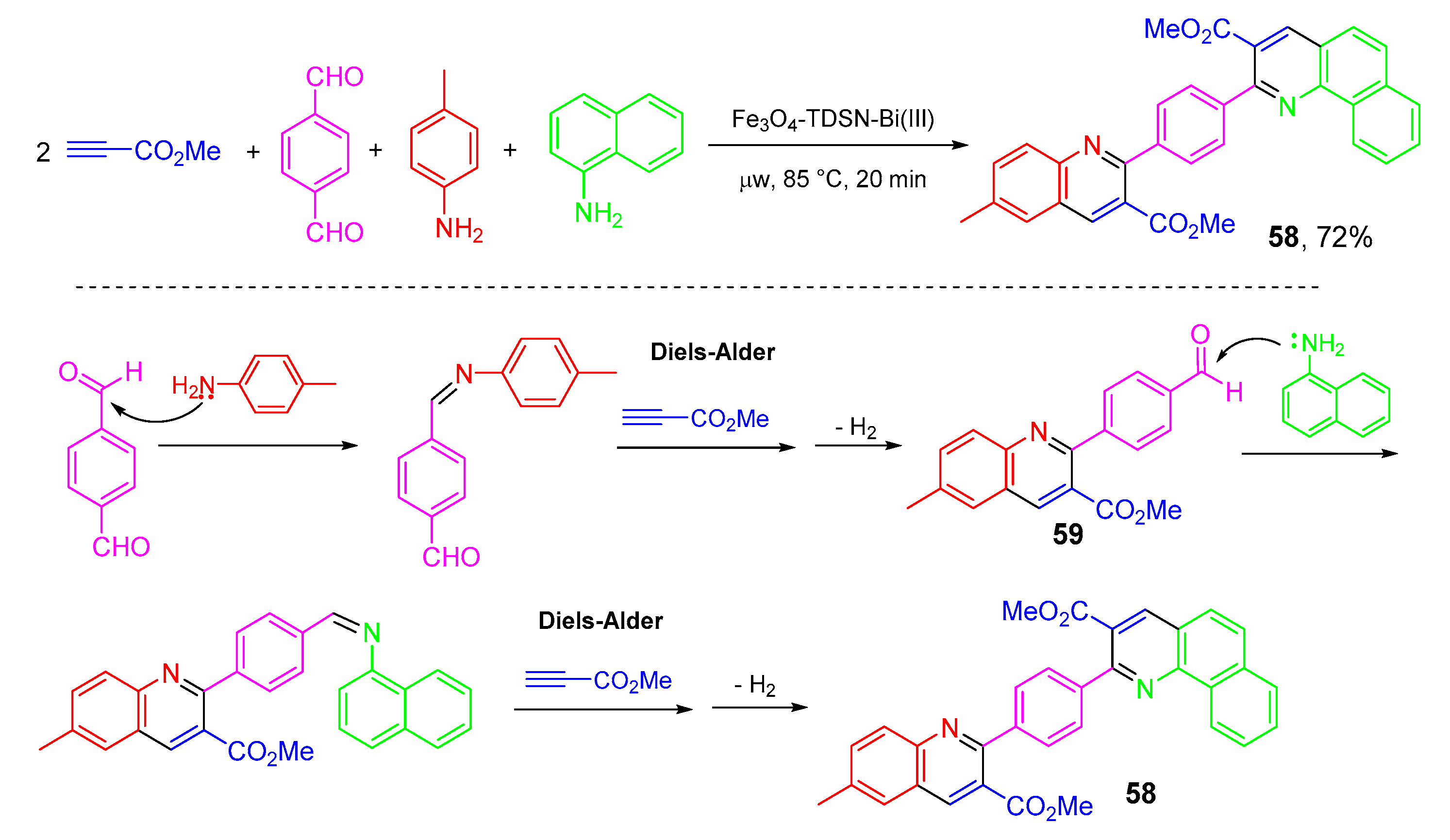
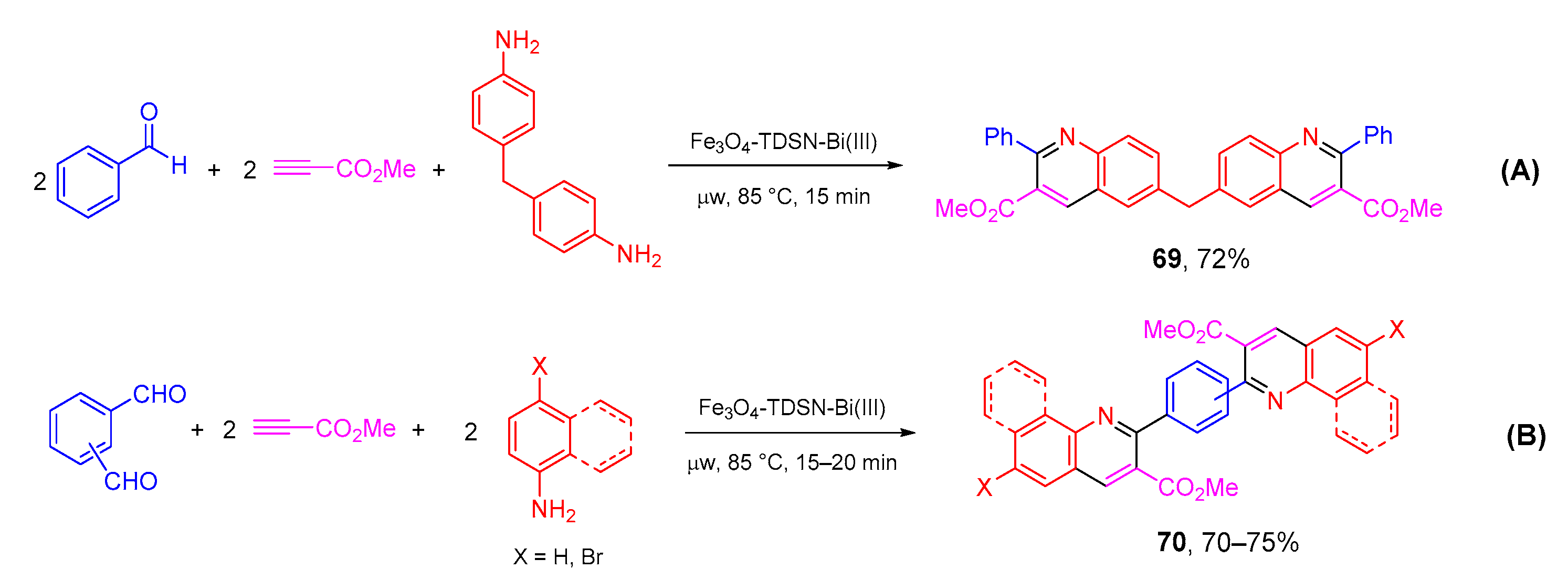
- Asadi, B.; Landarani-Isfahani, A.; Mohammadpoor-Baltork, I.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Rudbari, H.A. Microwave-assisted, regioselective one-pot synthesis of quinolines and bis -quinolines catalyzed by Bi(III) immobilized on triazine dendrimer stabilized magnetic nanoparticles. Tetrahedron Lett. 2017, 58, 71–74. [Google Scholar] [CrossRef]
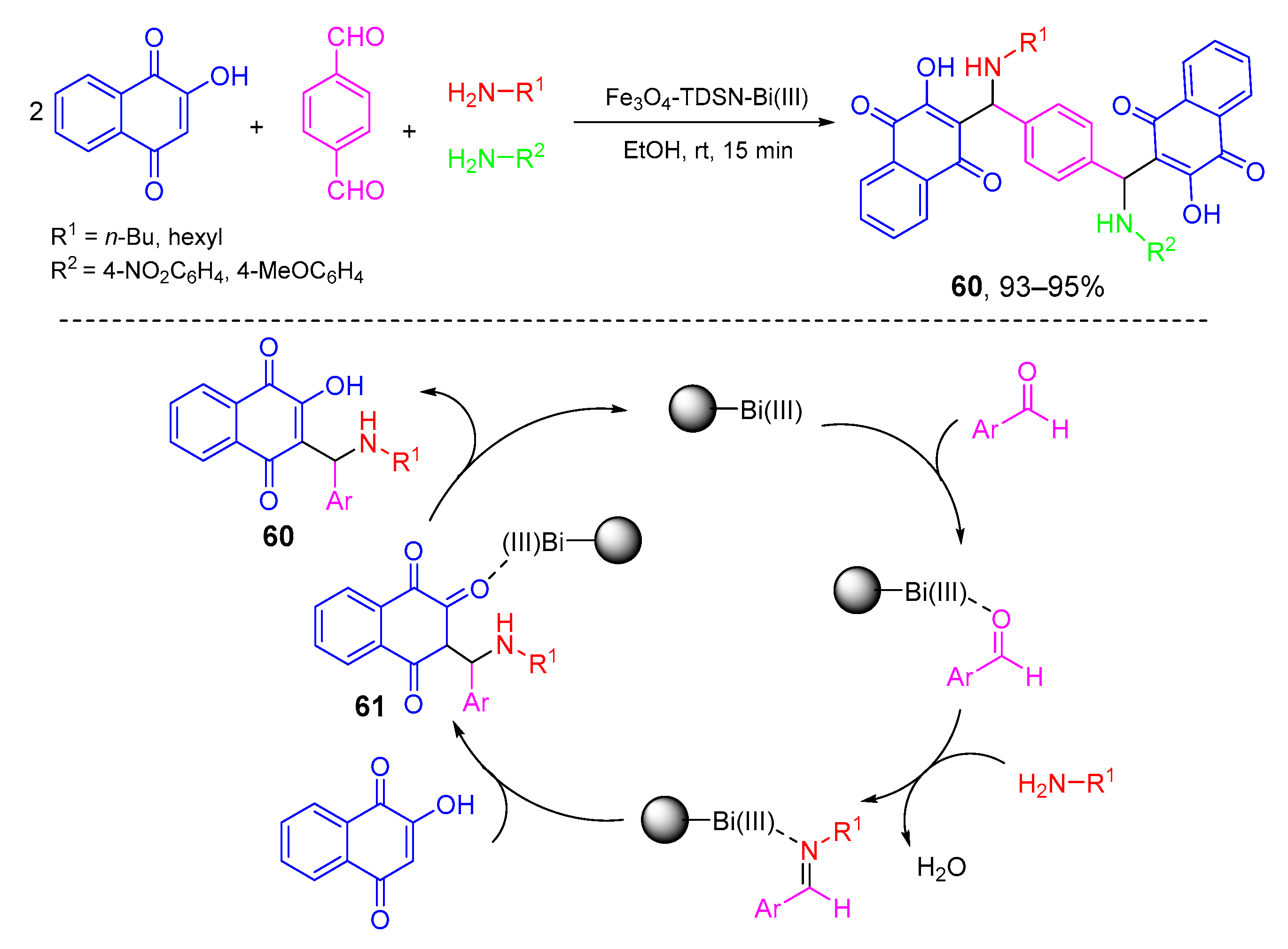
- Asadi, B.; Mohammadpoor-Baltork, I.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Landarani-Isfahani, A. Synthesis and characterization of Bi(III) immobilized on triazine dendrimer-stabilized magnetic nanoparticles: A reusable catalyst for the synthesis of aminonaphthoquinones and bis-aminonaphthoquinones. New J. Chem. 2016, 40, 6171–6184. [Google Scholar] [CrossRef]
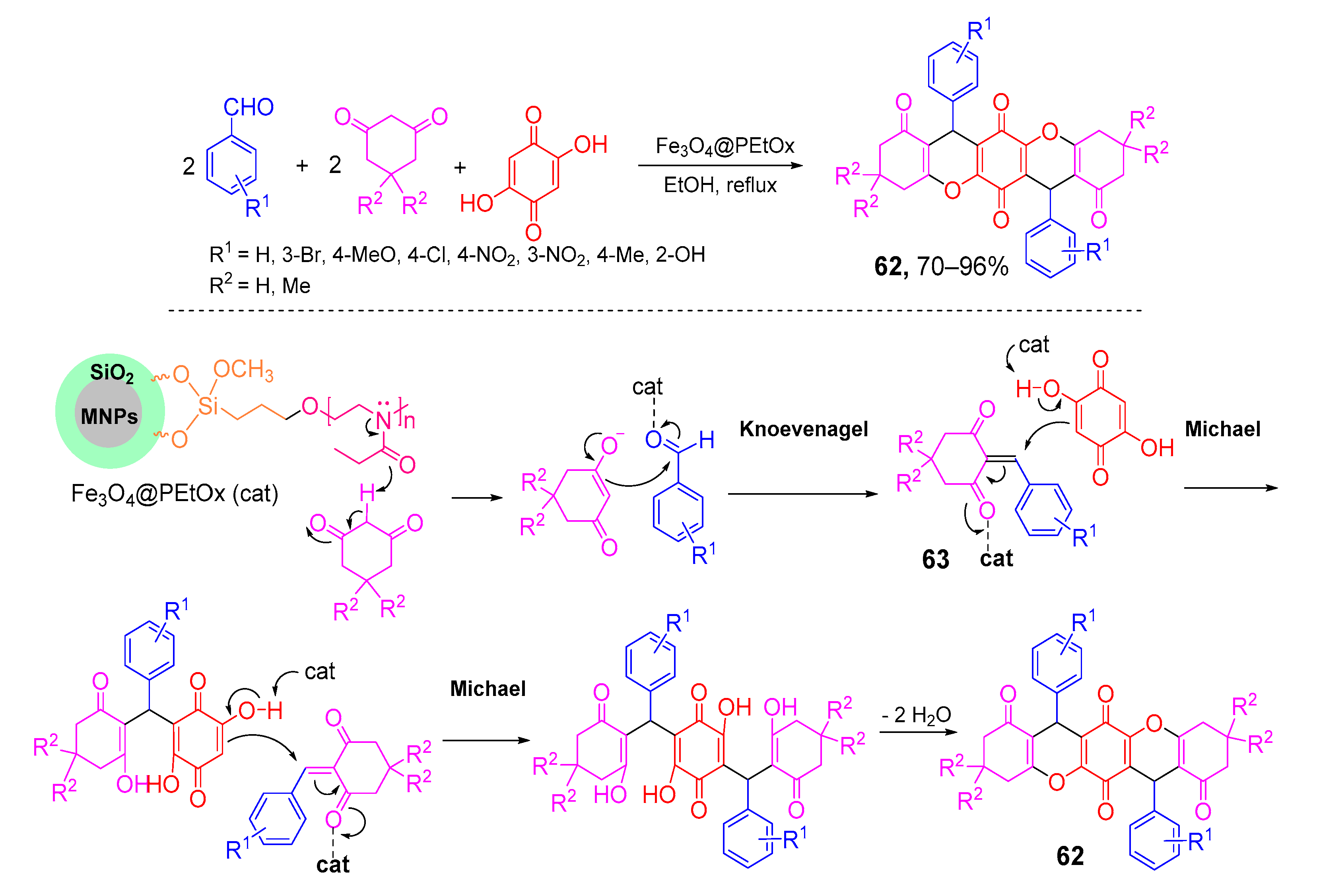
- Rahnamafar, R.; Moradi, L.; Khoobi, M. Synthesis of benzo[b]xanthene-triones and tetrahydrochromeno[2,3-b]xanthene tetraones via three- or pseudo-five-component reactions using Fe3O4@SiO2/PEtOx as a novel, magnetically recyclable, and eco-friendly nanocatalyst. J. Heterocycl. Chem. 2020, 57, 1825–1837. [Google Scholar] [CrossRef]
- Filian, H.; Kohzadian, A.; Mohammadi, M.; Ghorbani-Choghamarani, A.; Karami, A. Pd(0)-guanidine@MCM-41: A very effective catalyst for rapid production of bis (pyrazolyl)methanes. Appl. Organometal. Chem. 2020, 34, e5579. [Google Scholar] [CrossRef]
- Noruzian, F.; Olyaei, A.; Hajinasiri, R.; Sadeghpour, M. Guanidine hydrochloride catalyzed efficient one-pot pseudo five-component synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) in water. Synth. Commun. 2019, 49, 2717–2724. [Google Scholar] [CrossRef]
- Milani, J.; Maghsoodlou, M.T.; Hazeri, N.; Nassiri, M. Alpha-casein: An efficient, green, novel, and eco-friendly catalyst for one-pot multi-component synthesis of bis (pyrazol-5-ols), dihydropyrano[2,3-c]pyrazoles and spiropyranopyrazoles in an environmentally benign manner. J. Iran. Chem. Soc. 2019, 16, 1651–1664. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Khojastehbakht-Koopaei, B.; Shahbazi-Alavi, H. Pseudo five-component process for the synthesis of 4,4′-(arylmethylene)bis(3-methyl-1H-pyrazol-5-ol) derivatives using ZnAl2O4 nanoparticles in aqueous media. RSC Adv. 2014, 4, 46106–46113. [Google Scholar] [CrossRef]
- Sobhani, S.; Zarifi, F.; Skibsted, J. Immobilized lanthanum(III) triflate on graphene oxide as a new multifunctional heterogeneous catalyst for the one-pot five-component synthesis of bis(pyrazolyl)methanes. ACS Sustain. Chem. Eng. 2017, 5, 4598–4606. [Google Scholar] [CrossRef]
- Khaligh, N.G.; Mihankhah, T.; Gorjian, H.; Johan, M.R. Greener and facile synthesis of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s through a conventional heating procedure. Synth. Commun. 2020, 50, 3276–3286. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Khojastehbakht-Koopaei, B.; Zahedi, S. Copper chromite nanoparticles as an efficient and recyclable catalyst for facile synthesis of 4,4′-(arylmethanediyl)bis(3-methyl-1H-pyrazol-5-ol) derivatives. Chem. Heterocycl. Comp. 2015, 51, 34–38. [Google Scholar] [CrossRef]
- Hasaninejed, A.; Kazerooni, M.R.; Zare, A. Room-temperature, catalyst-free, one-pot pseudo-five-component synthesis of 4,4-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s under ultrasonic irradiation. ACS Sustain. Chem. Eng. 2013, 1, 679–684. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Asgari-Keirabadi, M.; Khojastehbakht-Koopaei, B.; Shahbazi-Alavi, H. Multicomponent synthesis of C-tethered bispyrazol-5-ols using CeO2 nanoparticles as an efficient and green catalyst. Res. Chem. Intermed. 2016, 42, 827–837. [Google Scholar] [CrossRef]
- Yang, C.; Liu, P.; Xu, D. A green and efficient one-pot pseudo-five-component reaction for synthesis of bis(pyrazol-5-ol) derivatives via tandem cyclocondensation-Knoevenagel-Michael reaction. ChemistrySelect 2017, 2, 1232–1236. [Google Scholar] [CrossRef]
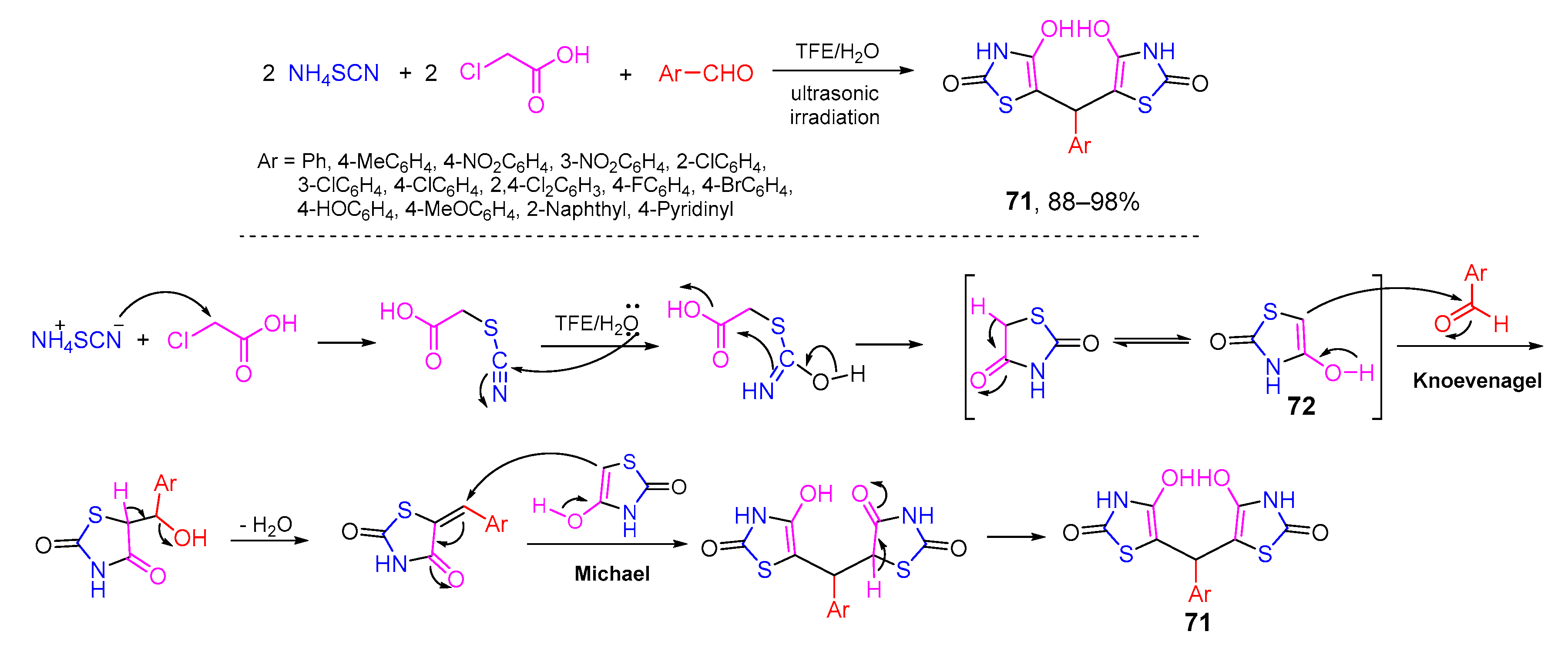
- Heravi, M.R.P.; Naghilou, M. Effective preparation of 5,5′-(arylmethylene)bis(4-hydroxythiazole-2(3H)-one) in an aqueous fluoroalcohol solvent system under ultrasound irradiation at room temperature. Chin. Chem. Lett. 2017, 28, 1039–1043. [Google Scholar] [CrossRef]
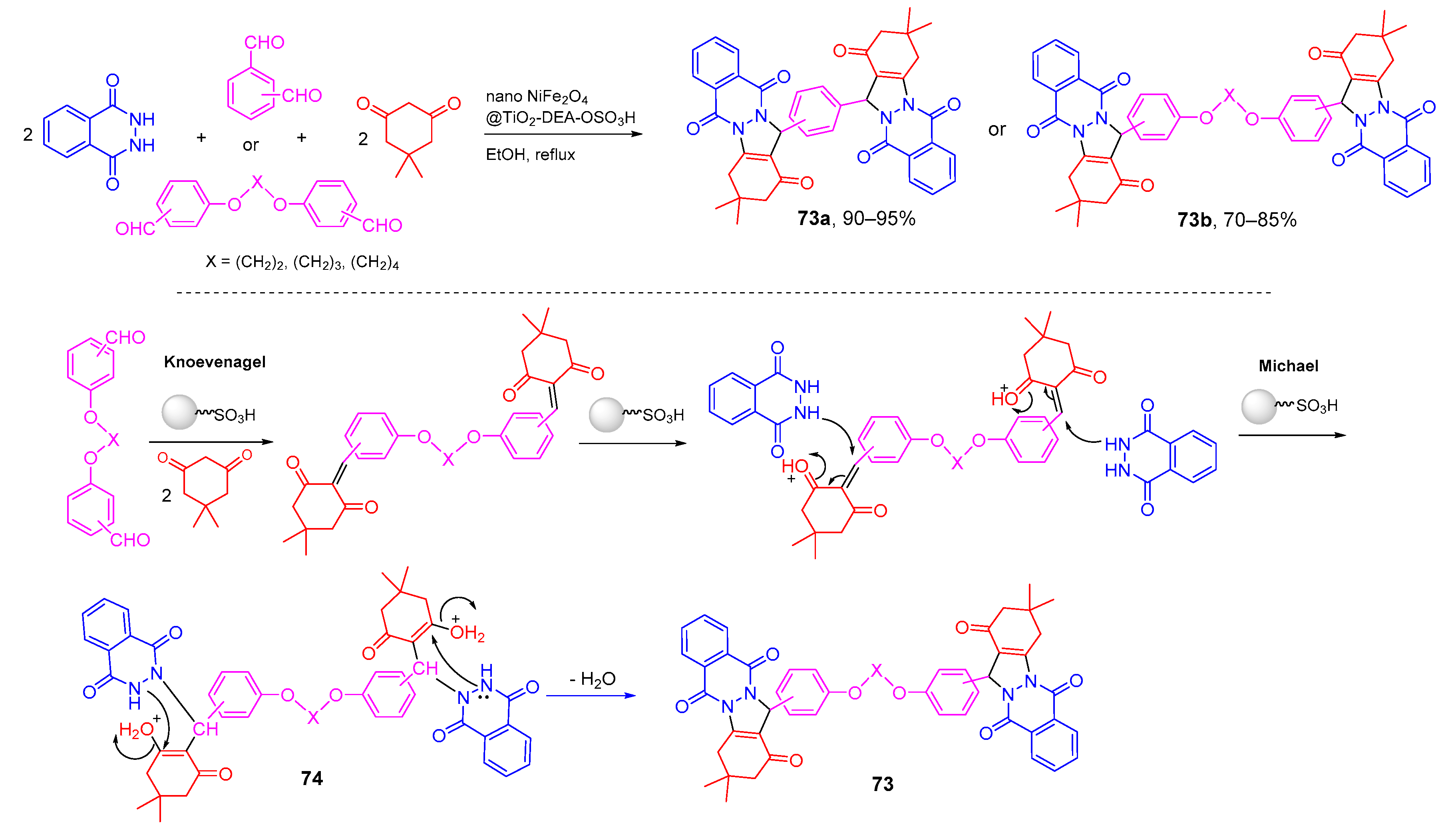
- Hamidinasab, M.; Bodaghifard, M.A.; Mobinikhaledi, A. Synthesis of new, vital and pharmacologically important bis phthalazine-triones using an efficient magnetic nanocatalyst and their HF and NBO investigation. J. Mol. Struct. 2020, 1200, 127091. [Google Scholar] [CrossRef]
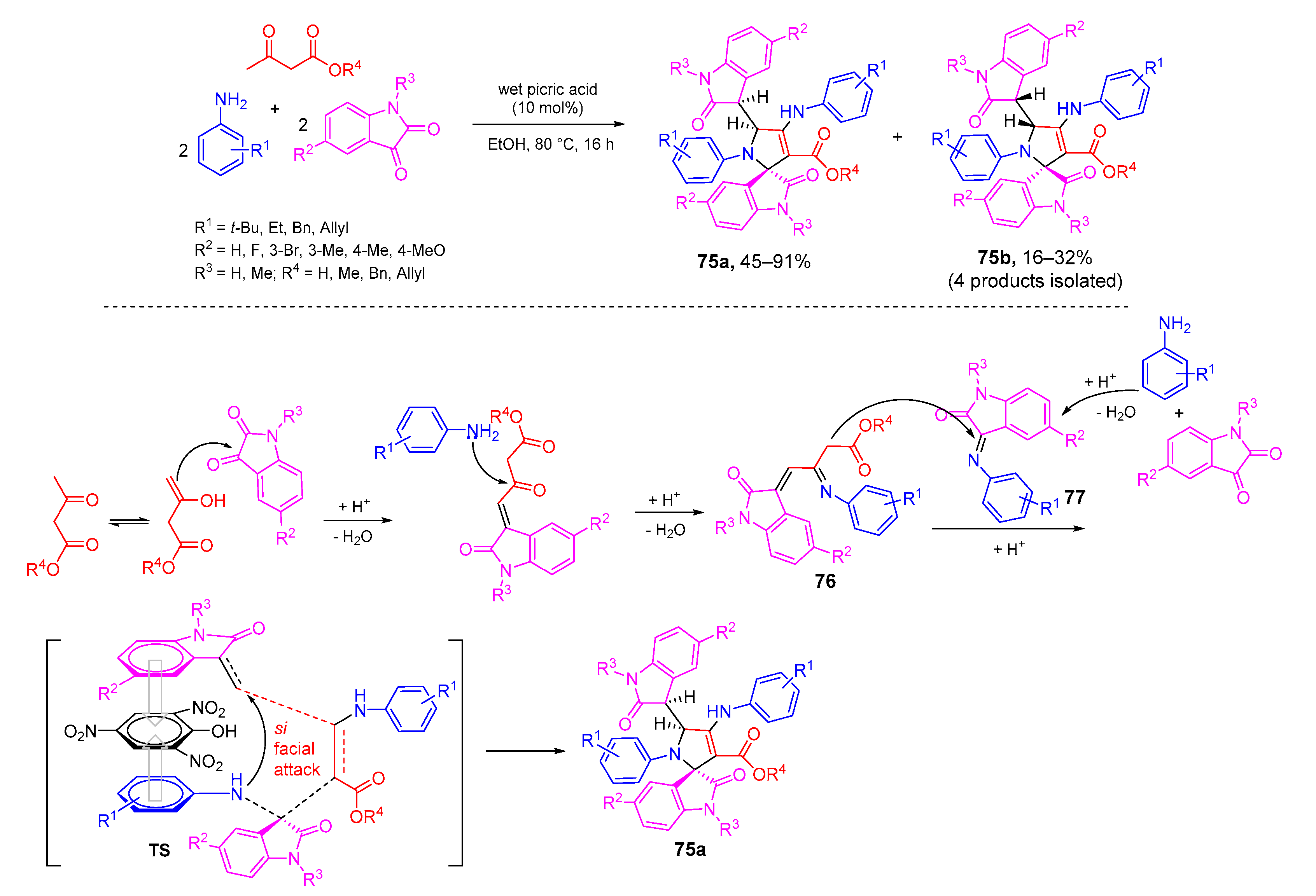
- Sengupta, A.; Maity, S.; Mondal, A.; Ghosh, P.; Rudra, S.; Mukhopadhyay, C. Pseudo five component reaction towards densely functionalized spiro[indole-3,2′-pyrrole] by picric acid, an efficient syn-diastereoselective catalyst: Insight into the diastereoselection on C(sp3)-C(sp3) axial conformation. Org. Biomol. Chem. 2019, 17, 1254–1265. [Google Scholar] [CrossRef]
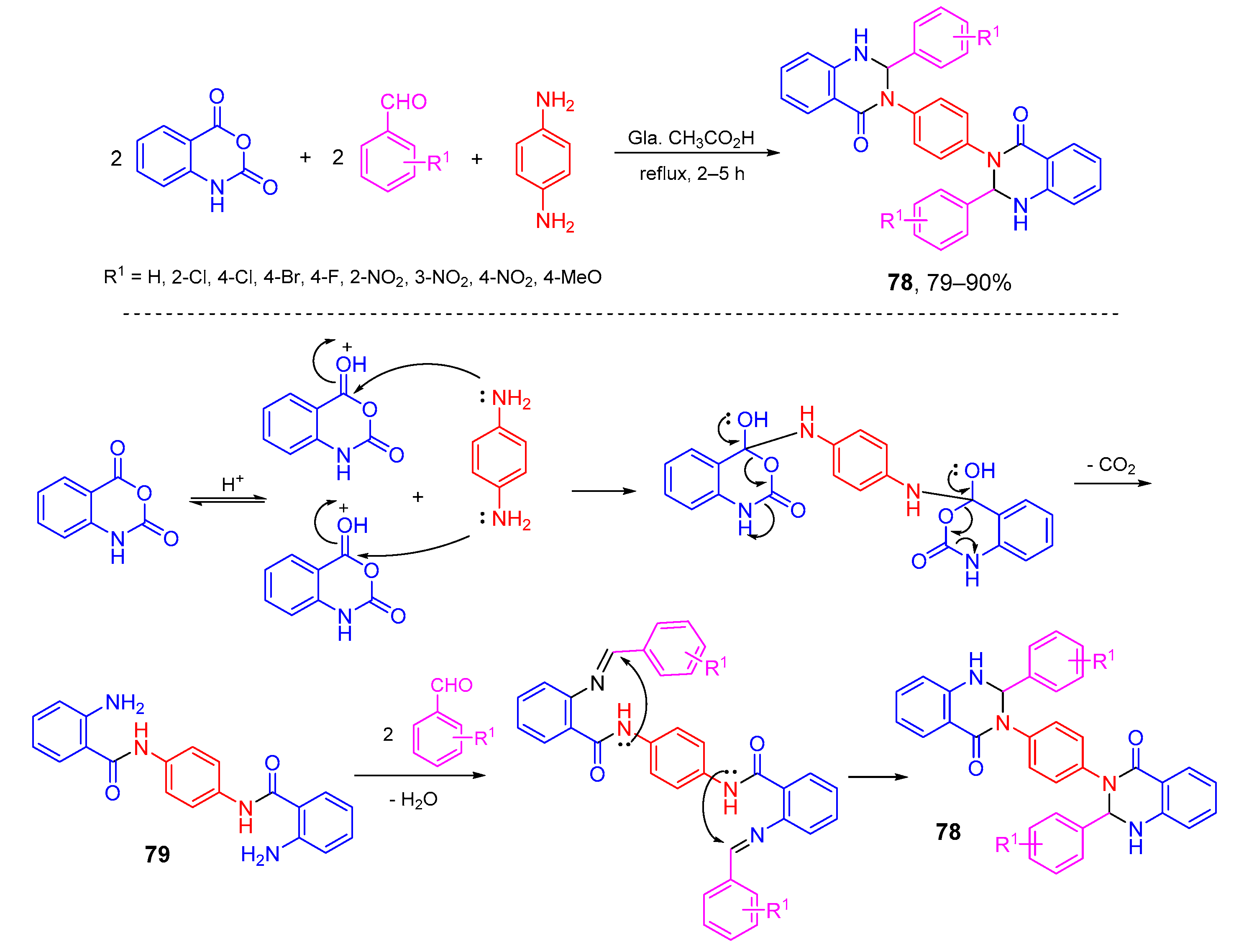
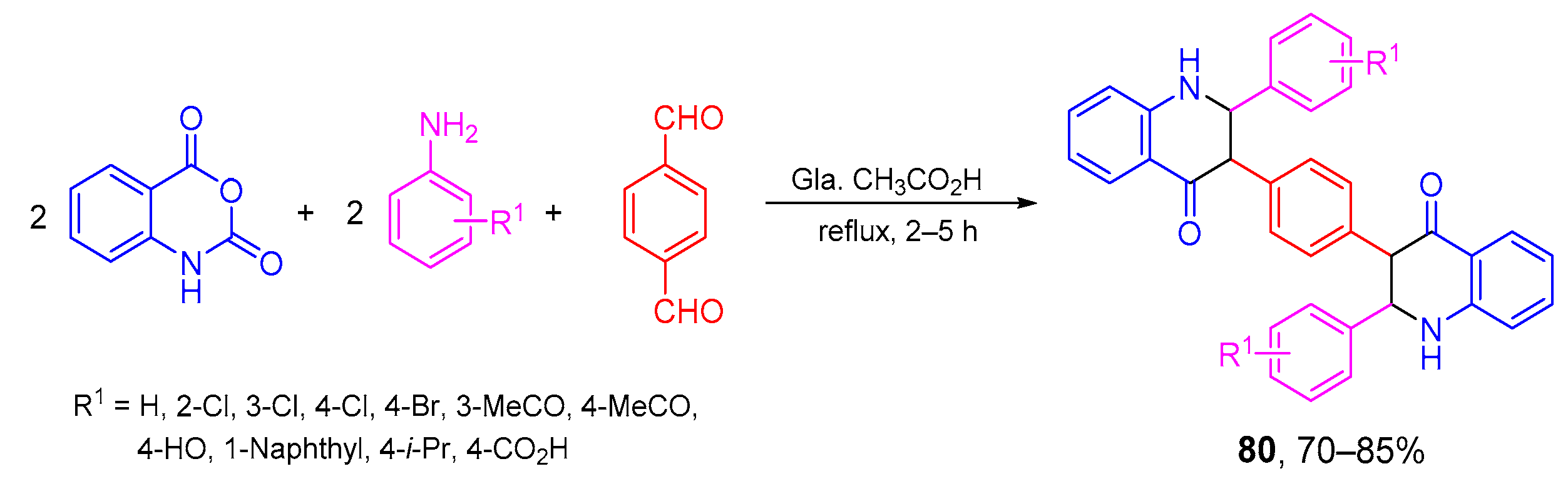
- Sivaguru, P.; Parameswaran, K.; Lalitha, A. Antioxidant, anticancer and electrochemical redox properties of new bis(2,3-dihydroquinazolin-4(1H)-one) derivatives. Mol. Divers. 2017, 21, 611–620. [Google Scholar] [CrossRef]
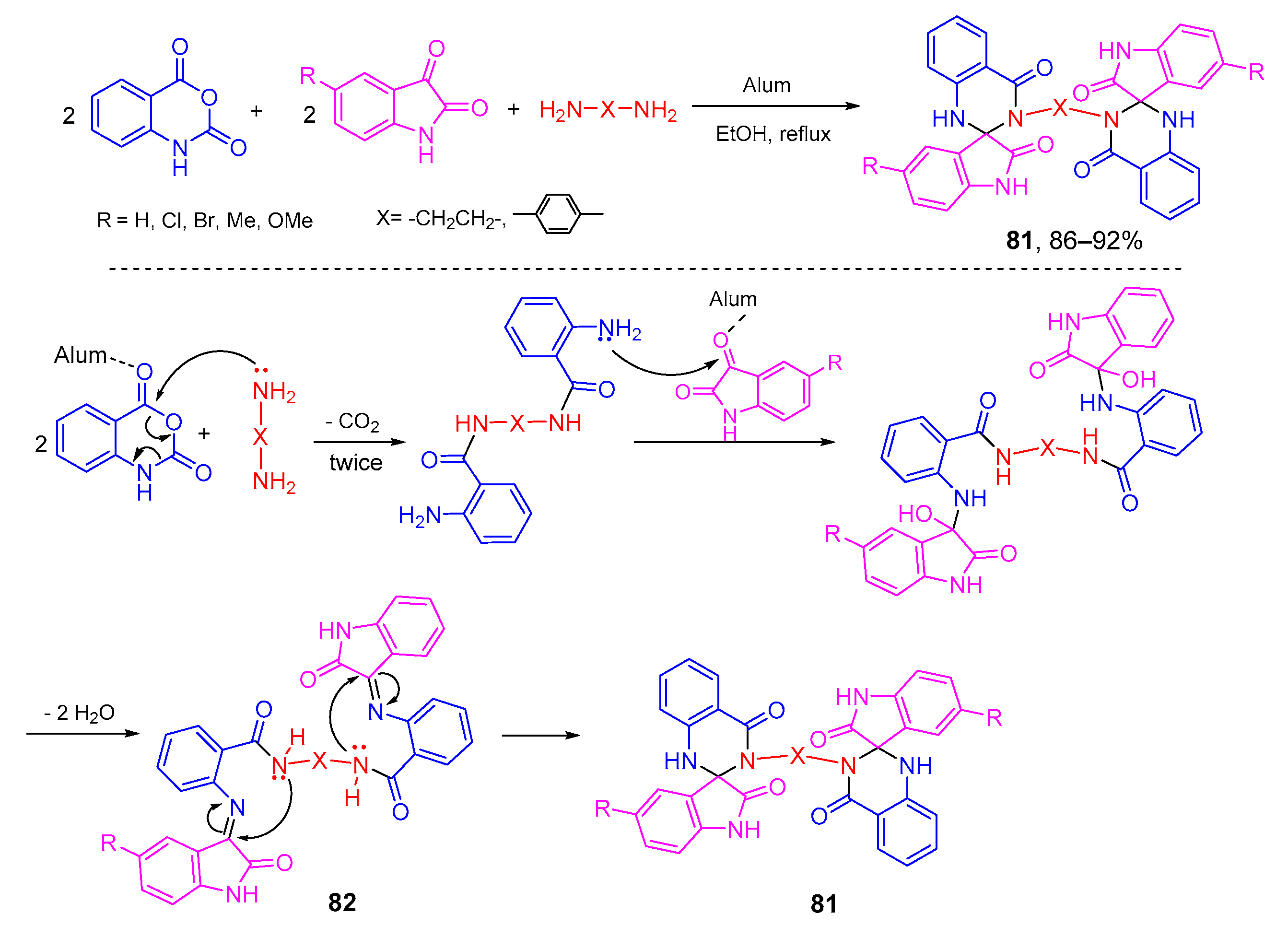
- Mohammadi, A.A.; Taheri, S.; Askari, S.; Ahdenov, R. KAl(SO4)2●12H2O (Alum): An efficient catalyst for the synthesis of novel bis[spiro(quinazoline-oxindole)] derivatives via one-pot pseudo five-component reactions. J. Heterocycl. Chem. 2015, 52, 1871–1875. [Google Scholar] [CrossRef]
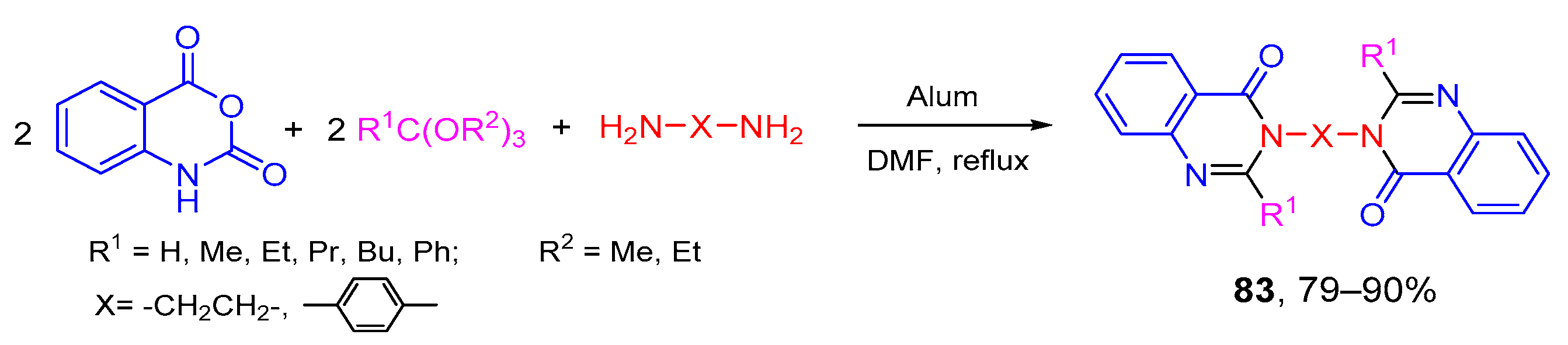
- Mohammadi, A.A.; Taheri, S.; Askari, S. One-pot pseudo five-component synthesis of some new bis(quinazolinon-4(1H)-one) derivatives. J. Heterocycl. Chem. 2017, 54, 484–488. [Google Scholar] [CrossRef]
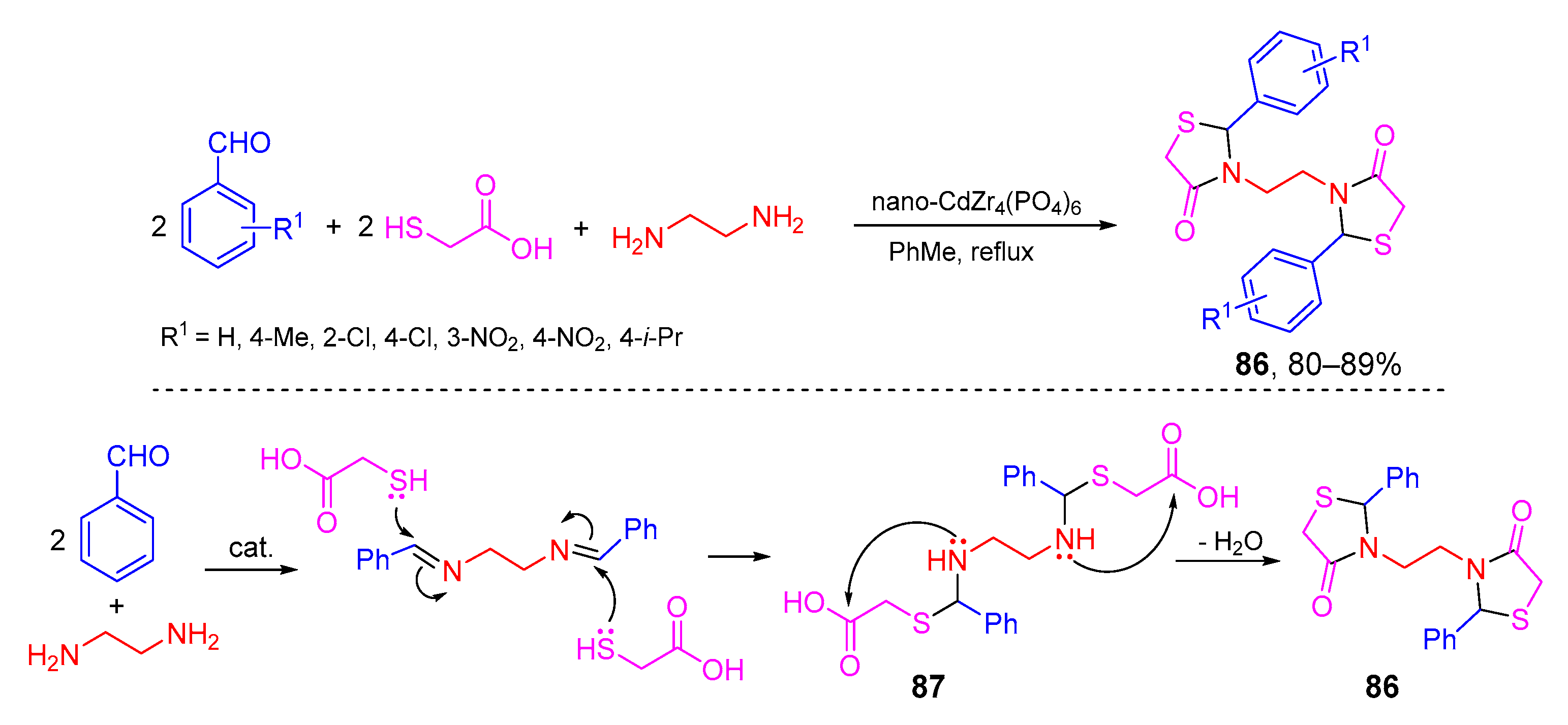
- Safaei-Ghomi, J.; Nazemzadeh, S.H.; Shahbazi-Alavi, H. Nano-CdZr4(PO4)6 as a reusable and robust catalyst for the synthesis of bis-thiazolidinones by a multicomponent reaction of aldehydes, ethylenediamine and thioglycolic acid. J. Sulfur Chem. 2017, 38, 195–205. [Google Scholar] [CrossRef]
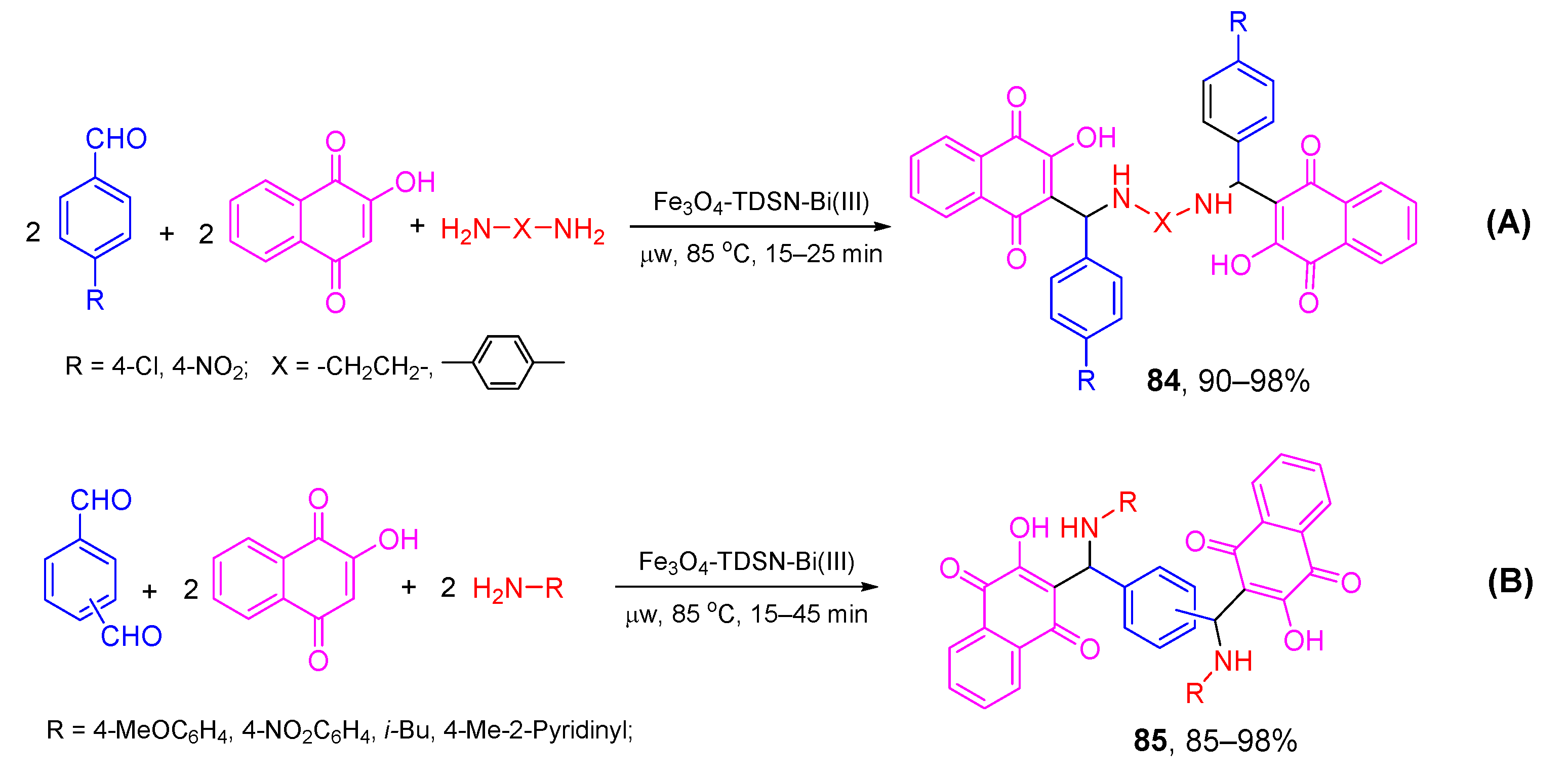
- Olyaei, A.; Abforushha, E.S.; Khoeiniha, R. Simple and efficient synthesis of novel bis-Betti bases via a one-pot pseudo-five-component reaction. Lett. Org. Chem. 2017, 14, 103–108. [Google Scholar] [CrossRef]
- Yao, J.; Zhou, L.; Tan, C.; Wang, C. One-pot synthesis of densely functionalized cyclic β-aminoesters containing four stereocenters, based on a Et3N N-promoted pseudo five-component reaction. Mol. Divers. 2015, 19, 43–53. [Google Scholar] [CrossRef]
- Bhuyan, D.; Sarmah, M.M.; Dommaraju, Y.; Prajapati, D. Microwave-promoted efficient synthesis of spiroindenotetrahydropyridine derivatives via a catalyst- and solvent-free pseudo one-pot five-component tandem Knoevenagel/aza-Diels–Alder reaction. Tetrahedron Lett. 2014, 55, 5133–5136. [Google Scholar] [CrossRef]
- Sadabad, H.R.; Bazgir, A.; Eskandari, M.; Ghahremanzadeh, R. Pseudo five-component reaction of isocyanides, dialkyl acetylenedicarboxylates, and 2,3-dichloronaphthalene-1,4-dione: A highly diastereoselective synthesis of novel dispiro[furan-2,1′-naphthalene-4′,2″-furan] derivatives. Monatsh. Chem. 2014, 145, 1851–1855. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, W.; Evans, J.; Kaur, M.; Jasinski, J.P.; Zhang, W. Double 1,3-dipolar cycloadditions of two nonstabilized azomethine ylides for polycyclic pyrrolidines. Org. Lett. 2019, 21, 2176–2179. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Song, G.; Jasinski, J.P.; Keeley, A.C.; Zhang, W. One-pot double [3+2] cycloaddition for diastereoselective synthesis of tetracyclic pyrrolidine compounds. Green Chem. 2012, 14, 3010–3012. [Google Scholar] [CrossRef]
- Quiroga, J.; Gálvez, J.; Abonia, R.; Insuasty, B.; Ortíz, A.; Cobo, J.; Nogueras, M. Highly efficient and diastereoselective synthesis of new pyrazolylpyrrolizine and pyrazolylpyrrolidine derivates by a three-component domino process. Molecules 2014, 19, 4284–4300. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, X.; Geng, X.; Wang, C.; Wu, Y.-D.; Wu, A.-X. Iodine-promoted five-component reaction using fragment assembly strategy to construct dihydrooxepines. Tetrahedron 2018, 74, 4323–4330. [Google Scholar] [CrossRef]
- Kaur, G.; Devi, M.; Kumari, A.; Devi, R.; Banerjee, B. One-pot pseudo five component synthesis of biologically relevant 1,2,6-triaryl-4-arylamino-piperidine-3-ene-3-carboxylates: A decade update. ChemistrySelect 2018, 3, 9892–9910. [Google Scholar] [CrossRef]
- Lashkari, M.; Maghsoodlou, M.T.; Hazeri, N.; Habibi-Khorassani, S.M.; Sajadikhah, S.S.; Doostmohamadi, R. Synthesis of highly functionalized piperidines via one-pot, five-component reactions in the presence of acetic acid solvent. Synth. Commun. 2013, 43, 635–644. [Google Scholar] [CrossRef]
- Zarei, M.; Sajadikhah, S.S. Green and facile synthesis of dihydropyrrol-2-ones and highly substituted piperidines using ethylenediammonium diformate (EDDF) as a reusable catalyst. Res. Chem. Intermed. 2016, 42, 7005–7016. [Google Scholar] [CrossRef]
- Sobhani-Nasab, A.; Ziarati, A.; Rahimi-Nasrabadi, M.; Ganjali, M.R.; Badiei, A. Five-component domino synthesis of tetrahydropyridines using hexagonal PbCrxFe12−xO19 as efficient magnetic nanocatalyst. Res. Chem. Intermed. 2017, 43, 6155–6165. [Google Scholar] [CrossRef]
- Asadi, B.; Landarani-Isfahani, A.; Mohammadpoor-Baltork, I.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Rudbari, H.A. Diastereoselective synthesis of symmetrical and unsymmetrical tetrahydropyridines catalyzed by Bi(III) immobilized on triazine dendrimer stabilized magnetic nanoparticles. ACS Comb. Sci. 2017, 19, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.; Roy, S.R.; Seth, K.; Kumar, A.; Chakraborti, A.K. ‘On-Water’ multicomponent reaction for the diastereoselective synthesis of functionalized tetrahydropyridines and mechanistic insight. Synthesis 2016, 8, 547–556. [Google Scholar] [CrossRef]
- Basyouni, W.M.; El-Bayouki, K.A.M.; Tohamy, W.M.; Abbas, S.Y. Silica sulfuric acid: An efficient, reusable, heterogeneous catalyst for the one-pot, five-component synthesis of highly functionalized piperidine derivatives. Synth. Commun. 2015, 45, 1073–1081. [Google Scholar] [CrossRef]
- Mokhtari, T.S.; Amrollahi, M.A.; Sheikhhosseini, E.; Sheibani, H. Isocyanide-based multi-component reactions: One-pot catalyst-free synthesis of carboxamides. ChemistrySelect 2018, 3, 12813–12815. [Google Scholar] [CrossRef]
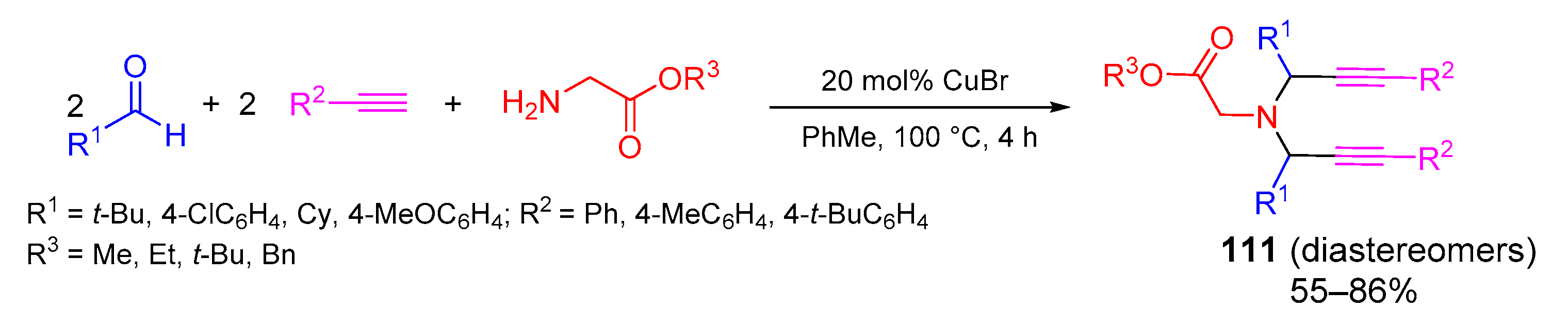
- Sharma, N.; Sharma, U.K.; Mishra, N.M.; Van der Eycken, E. Copper-catalyzed diversity-oriented three-and five-component synthesis of mono-and dipropargylic amines via coupling of alkynes, α-amino esters and aldehydes. Adv. Synth. Catal. 2014, 356, 1029–1037. [Google Scholar] [CrossRef]
- Asghari, S.; Qandalee, M.; Sarmadi, A.A. One-pot synthesis of γ-spiroiminolactones and γ-dispiroiminolactones using N,N’-disubstituted parabanic acid and thioparabanic acid derivatives. Mol. Divers. 2017, 21, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, C.; Das, P.; Butcher, R.J. An expeditious and efficient synthesis of highly functionalized [1,6]-naphthyridines under catalyst-free conditions in aqueous medium. Org. Lett. 2011, 13, 4664–4667. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, M.; Thirumalai, D.; Asharani, I.V.; Aravindan, P.G. Domino synthesis of functionalized 1,6-naphthyridines and their in vitro anti-inflammatory and anti-oxidant efficacies. RSC Adv. 2015, 5, 86330–86336. [Google Scholar] [CrossRef]
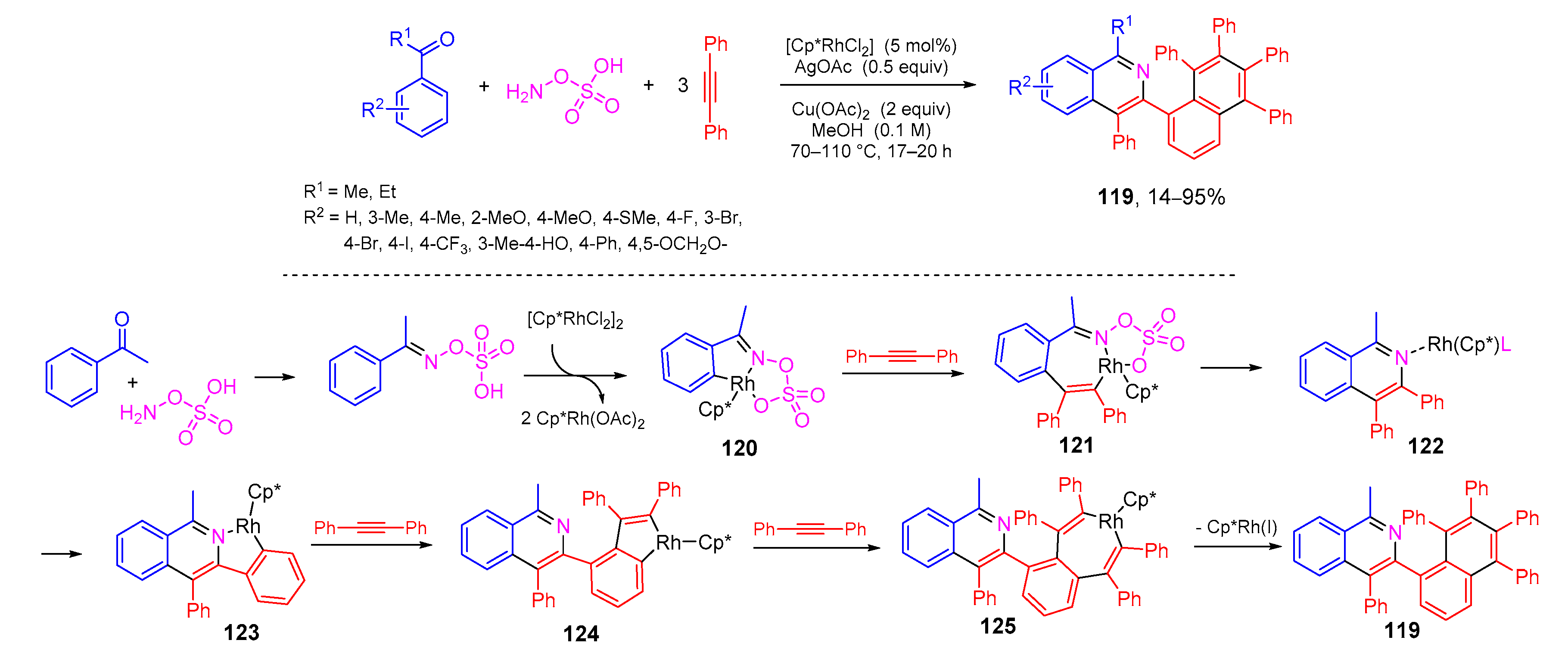
- Biswal, P.; Banjare, S.K.; Pati, B.V.; Mohanty, S.R.; Ravikumar, P.C. Rhodium-catalyzed one-pot access to N-polycyclic aromatic hydrocarbons from aryl ketones through triple C-H bond activations. J. Org. Chem. 2021, 86, 1108–1117. [Google Scholar] [CrossRef]
- Mao, K.; Dai, L.; Liu, Y.; Rong, L. An efficient five–component reaction for the synthesis of 4,4′-((2-oxoindoline-3,3-diyl)bis(methylene))bis(2-aryl-1H-pyrrolo[3,4-c]quinoline-1,3(2H)-dione) derivatives. J. Heterocycl. Chem. 2019, 56, 2111–2120. [Google Scholar] [CrossRef]
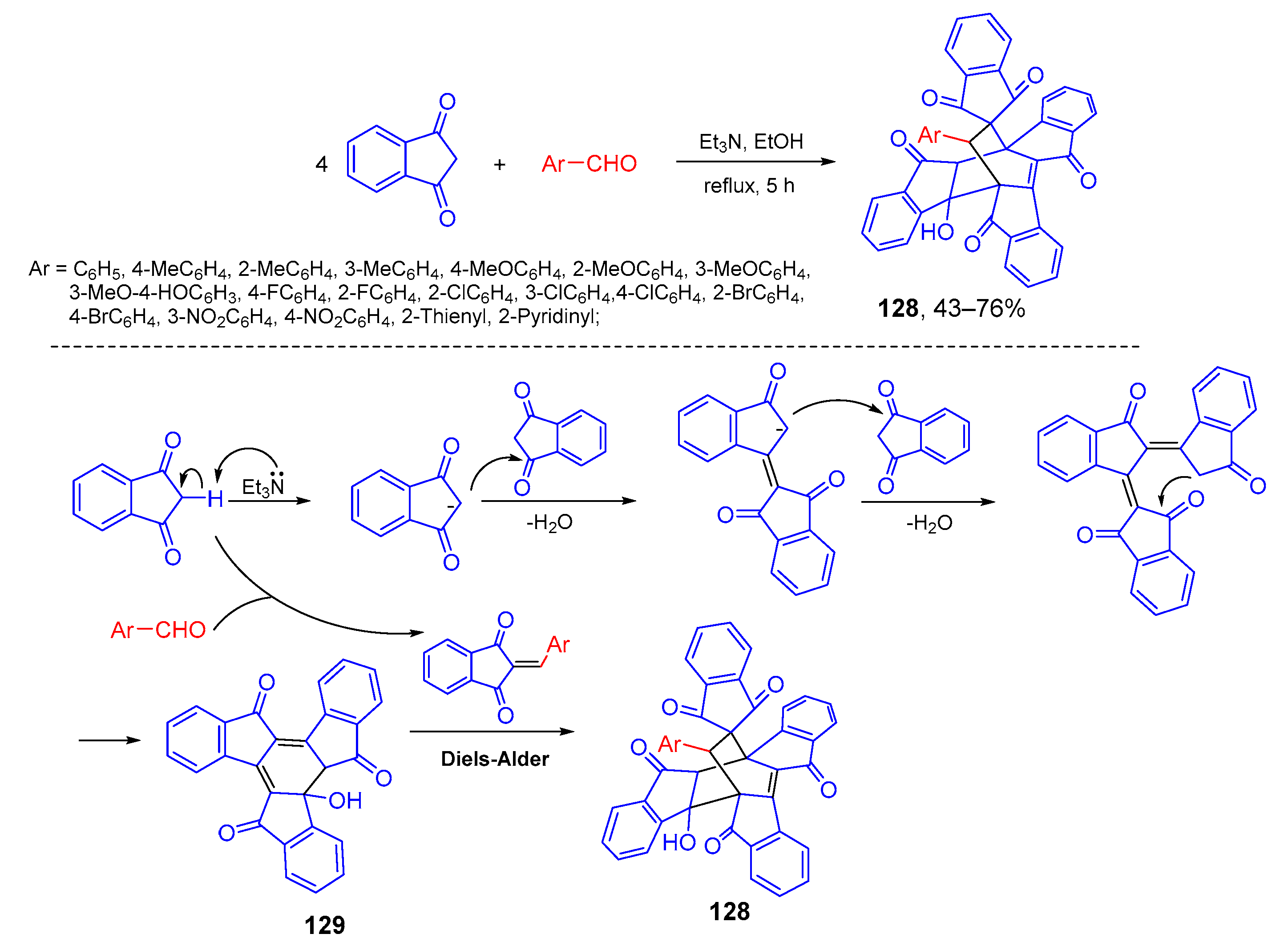
- Cao, J.; Shi, R.-G.; Sun, J.; Liu, D.; Liu, R.; Xia, X.; Wang, Y.; Yan, C.-G. Domino reaction of aromatic aldehydes and 1,3-indanediones for construction of bicyclo[2.2.2]octanes and dibenzo[b,g]indeno[1′,2′:3,4]fluoreno[1,2-d]oxonines. J. Org. Chem. 2020, 85, 2168–2179. [Google Scholar] [CrossRef]
- Zhi, S.; Ma, X.; Zhang, W. Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem. 2019, 17, 7632–7650. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Zhi, S.; Zhang, W. Recent Developments on Five-Component Reactions. Molecules 2021, 26, 1986. https://doi.org/10.3390/molecules26071986
Ma X, Zhi S, Zhang W. Recent Developments on Five-Component Reactions. Molecules. 2021; 26(7):1986. https://doi.org/10.3390/molecules26071986
Chicago/Turabian StyleMa, Xiaoming, Sanjun Zhi, and Wei Zhang. 2021. "Recent Developments on Five-Component Reactions" Molecules 26, no. 7: 1986. https://doi.org/10.3390/molecules26071986
APA StyleMa, X., Zhi, S., & Zhang, W. (2021). Recent Developments on Five-Component Reactions. Molecules, 26(7), 1986. https://doi.org/10.3390/molecules26071986