Adlay Testa (Coix lachryma-jobi L. var. Ma-yuen Stapf.) Ethanolic Extract and Its Active Components Exert Anti-Proliferative Effects on Endometrial Cancer Cells via Cell Cycle Arrest
Abstract
1. Introduction
2. Results
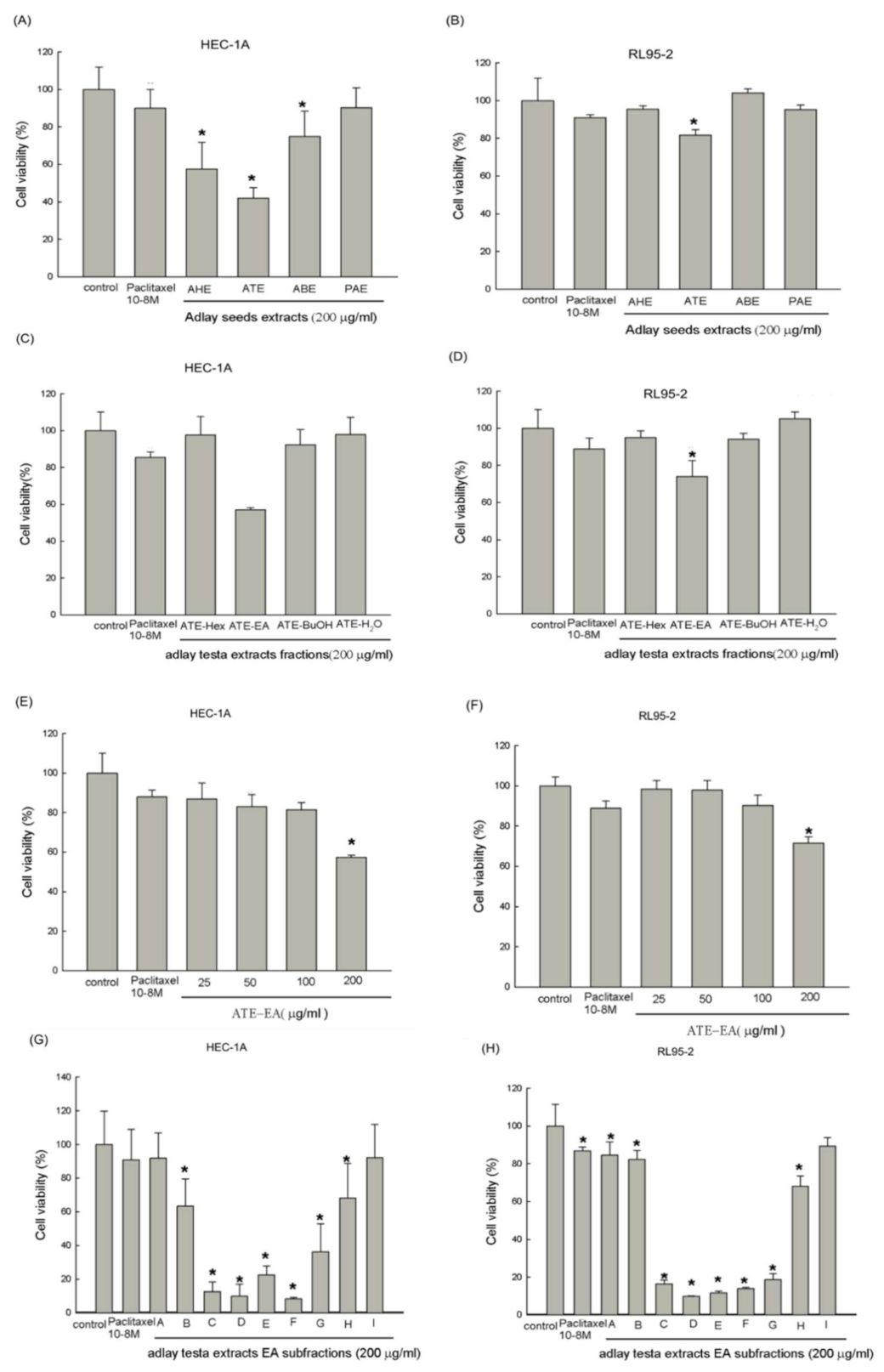
2.1. Effects of Different Parts of the Adlay on Endometrial Cancer Cell Viability
2.2. Effects of Different Fractions and Subfractions of Adlay Testa on Endometrial Cancer Cell Viability
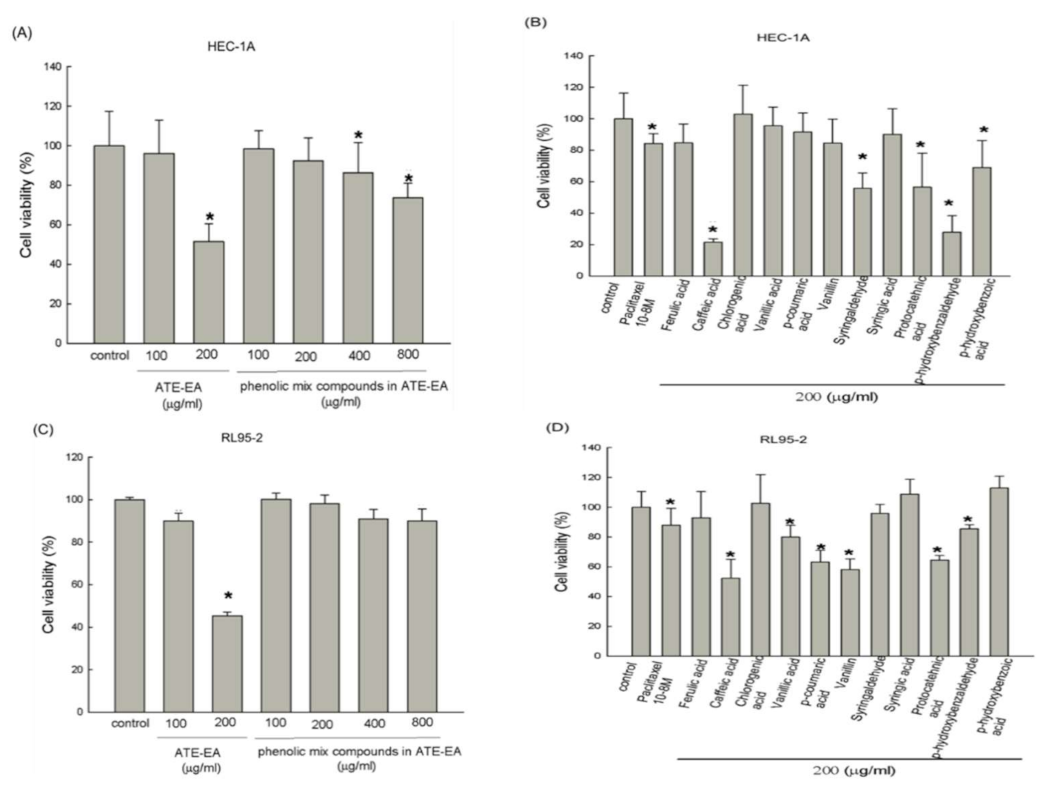
2.3. Phenolic Compounds in ATE-EA Inhibited Endometrial Cancer Cells Growth
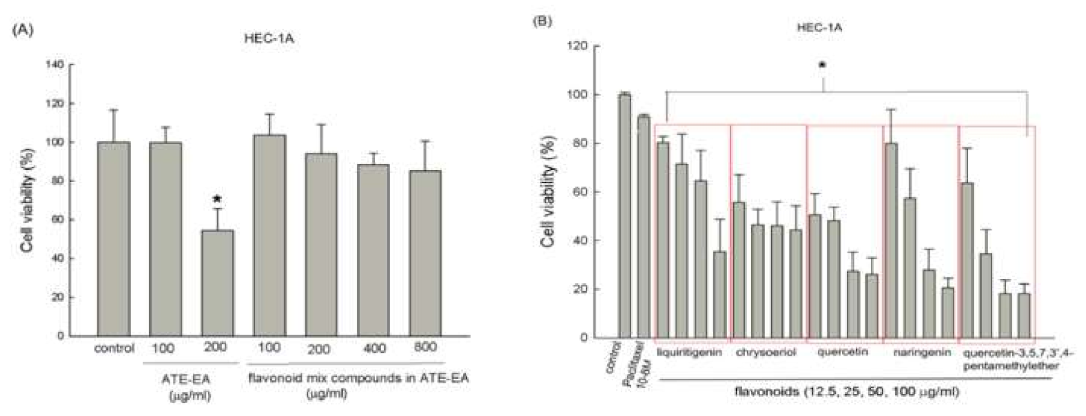
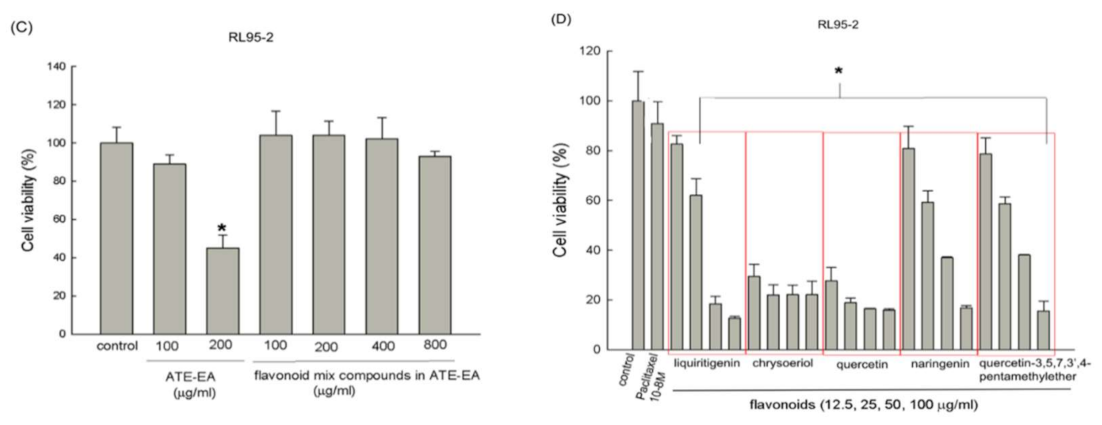
2.4. Flavonoid Compounds Inhibited Endometrial Cancer Cell Growth
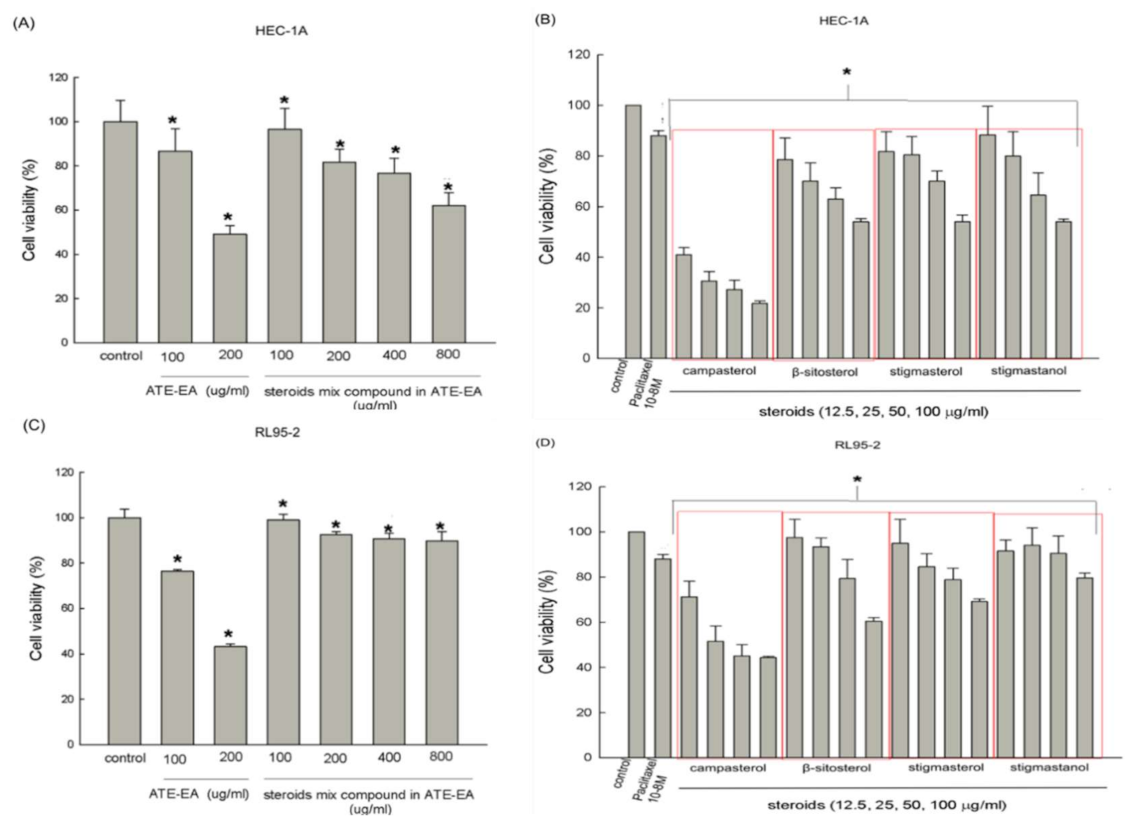
2.5. Steroids Inhibited Endometrial Cancer Cells Growth
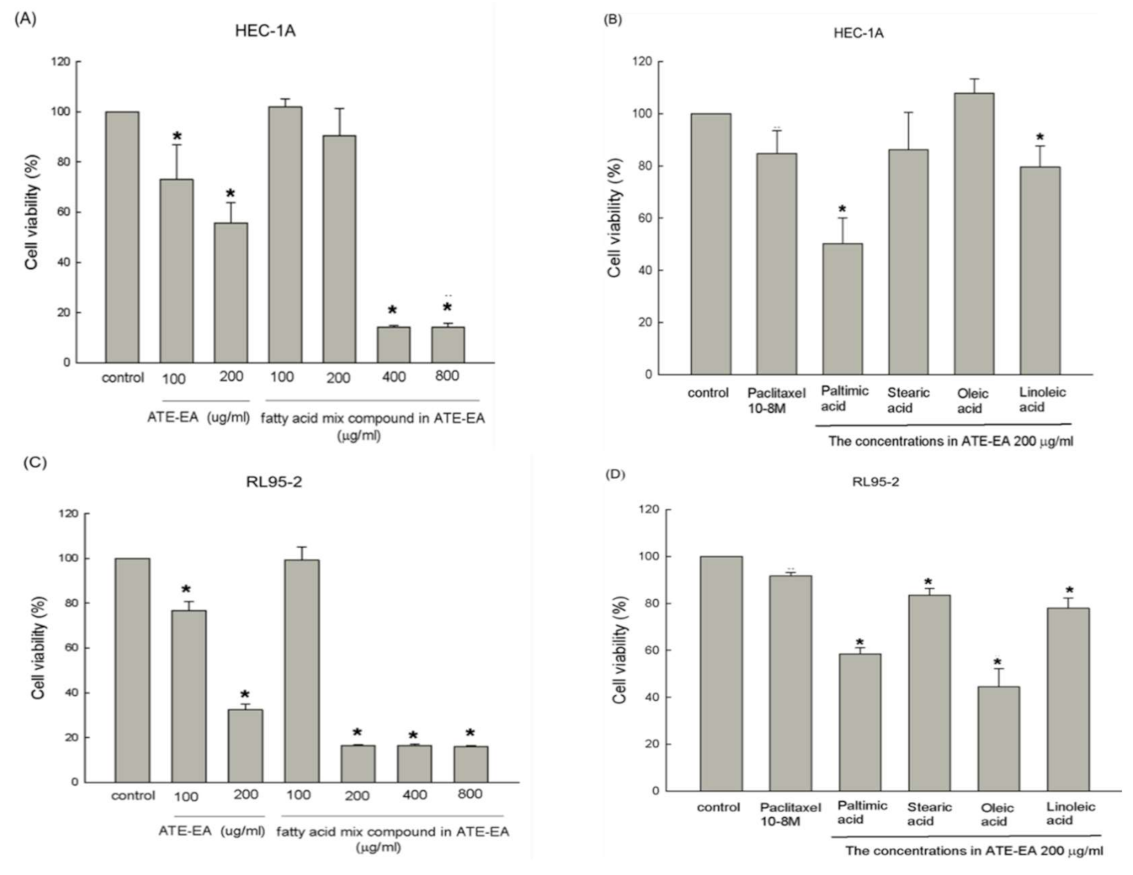
2.6. Fatty Acid Compounds Inhibited HEC-1A and RL95-2 Cells Growth
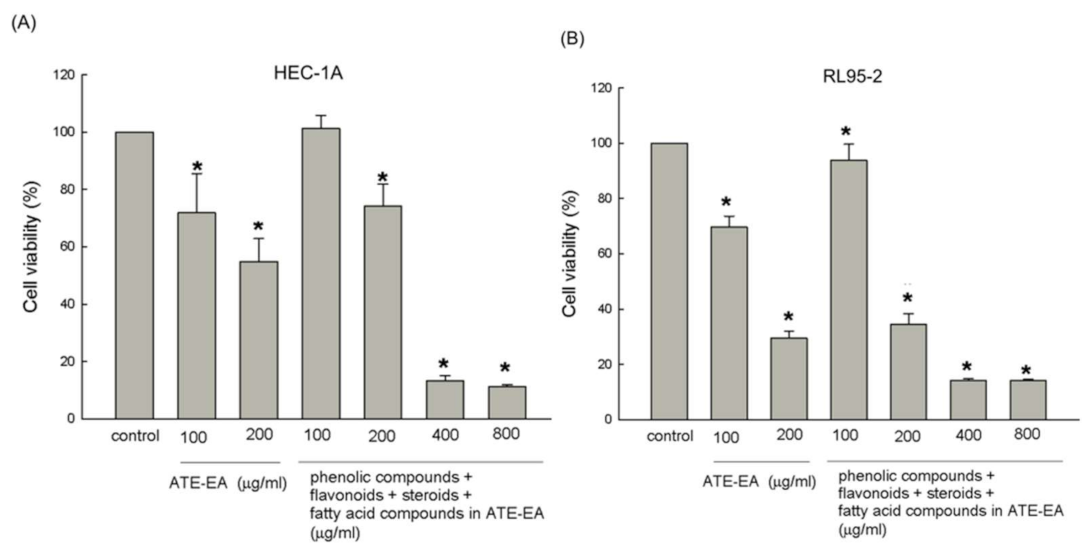
2.7. Four Mixture Compounds Inhibited HEC-1A and RL95-2 Cells Growth
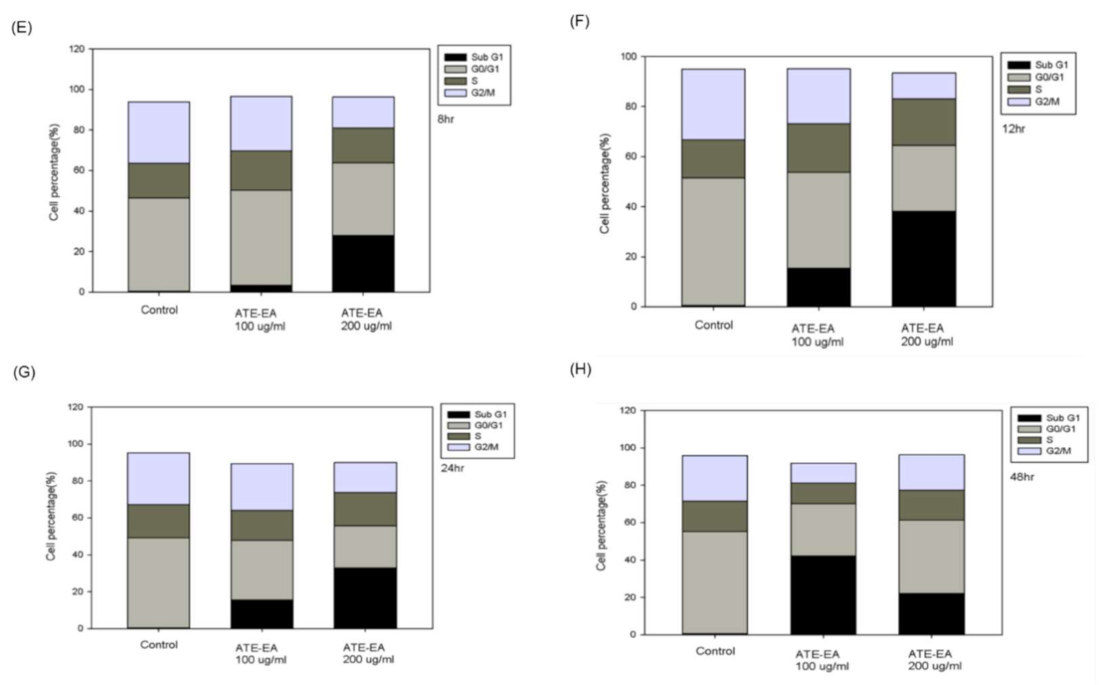
2.8. ATE-EA Induced G2/M and Sub-G1 Phase Arrest in Endometrial Cancer Cells
2.9. Identification of the Main Phenolic and Flavonoid Compounds in ATE-EA by HPLC
2.10. Identification of the Phytosterols in ATE-EA by GC/FID
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Plant Materials and Sample Preparation
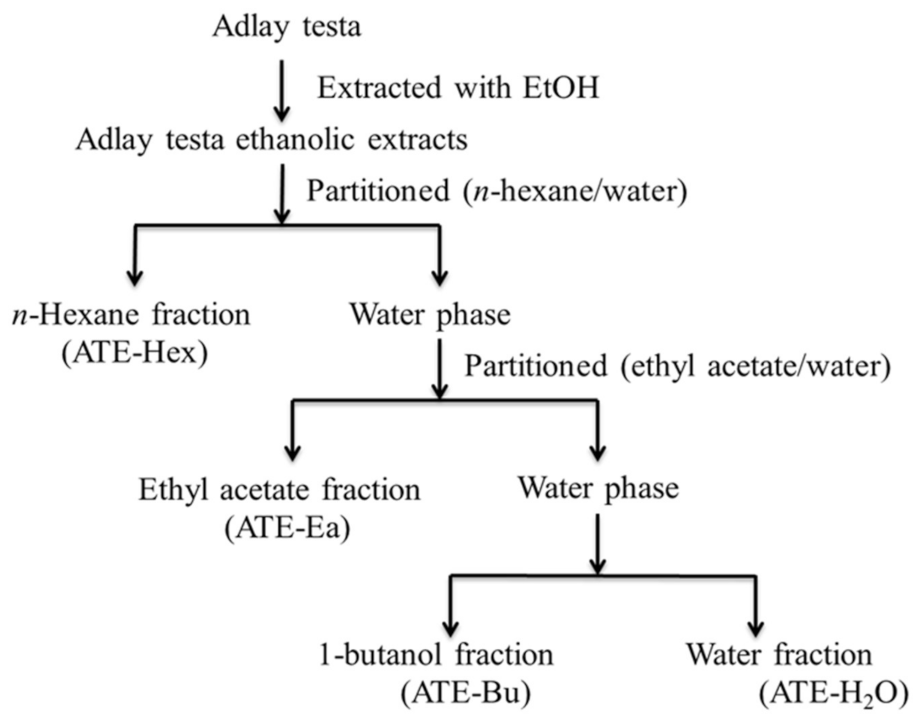
4.3. Preparation of Ethanolic Extracts and Various Fractions from Adlay Testa
4.4. Cell Lines and Culture
4.5. Cell Proliferation Analysis
4.6. Cell Cycle Analysis
4.7. HPLC Analysis
4.8. GC Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Sorosky, J.I. Endometrial cancer. Obstet. Gynecol. 2012, 120, 383–397. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Lo, W.-C.; Yang, Y.-W.; You, S.-L.; Chen, C.-J.; Lai, M.-S. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J. Formos. Med. Assoc. 2016, 115, 1076–1088. [Google Scholar] [CrossRef]
- Health TNDo. The Taiwan Cancer Registry. Cancer Registry Annual Report. Available online: http://tcr.cph.ntu.edu.tw/main.php?Page=N2# (accessed on 4 January 2021).
- Duska, L.R.; Garrett, A.; Rueda, B.R.; Haas, J.; Chang, Y.; Fuller, A.F. Endometrial cancer in women 40 years old or younger. Gynecol. Oncol. 2001, 83, 388–393. [Google Scholar] [CrossRef]
- Ben-Arie, A.; Perlman, S.; Hazan, Y.; Solomon, L.A.; Edwards, C.; Kaplan, A.L. High-risk endometrial cancer in young indigent women. Int. J. Gynecol. Cancer: Off. J. Int. Gynecol. Cancer Soc. 2004, 14, 927–930. [Google Scholar] [CrossRef]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lin, H.Y.; Cheng, F.; Chiang, W.; Kuo, Y.H. Isolation and characterization of new lactam compounds that inhibit lung and colon cancer cells from adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) bran. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2008, 46, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.G.; Pae, H.O.; Chai, K.Y.; Yun, Y.G.; Kwon, T.H.; Chung, H.T. Inhibitory effects of methanol extract of seeds of Job’s Tears (Coix lachryma-jobi L. var. ma-yuen) on nitric oxide and superoxide production in RAW 264.7 macrophages. Immunopharmacol. Immunotoxicol. 2000, 22, 545–554. [Google Scholar] [CrossRef]
- Chen, H.J.; Shih, C.K.; Hsu, H.Y.; Chiang, W. Mast cell-dependent allergic responses are inhibited by ethanolic extract of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) testa. J. Agric. Food Chem. 2010, 58, 2596–2601. [Google Scholar] [CrossRef]
- Hsia, S.M.; Tseng, Y.W.; Wang, S.W.; Kuo, Y.H.; Huang, D.W.; Wang, P.S.; Chiang, W. Effect of adlay (Coix lachryma-jobi L. var. ma-yuen Stapf.) hull extracts on testosterone release from rat Leydig cells. Phytother. Res. Ptr 2009, 23, 687–695. [Google Scholar] [CrossRef]
- Chen, L.-C.; Jiang, B.-K.; Zheng, W.-H.; Zhang, S.-Y.; Li, J.-J.; Fan, Z.-Y. Preparation, characterization and anti-diabetic activity of polysaccharides from adlay seed. Int. J. Biol. Macromol. 2019, 139, 605–613. [Google Scholar] [CrossRef]
- He, C.; Li, Z.; Liu, H.; Zhang, H.; Wang, L.; Chen, H. Chemical compositions and antioxidant activity of adlay seed (Coixlachryma-jobi L.) oil extracted from four main producing areas in China. J. Food Sci. 2020, 85, 123–131. [Google Scholar] [CrossRef]
- Kuo, C.C.; Chiang, W.; Liu, G.P.; Chien, Y.L.; Chang, J.Y.; Lee, C.K.; Lo, J.M.; Huang, S.L.; Shih, M.C.; Kuo, Y.H. 2,2’-Diphenyl-1-picrylhydrazyl radical-scavenging active components from adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) hulls. J. Agric. Food Chem. 2002, 50, 5850–5855. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Park, S.-K.; Cho, Y.-C.; Lee, Y.-S. Evaluation of phytonutrients in Adlay (Coix lacrymajobi L.) seeds. Afr. J. Biotechnol. 2012, 11, 1872–1878. [Google Scholar]
- Zhao, J.; Lyu, C.; Gao, J.; Du, L.; Shan, B.; Zhang, H.; Wang, H.-Y.; Gao, Y. Dietary fat intake and endometrial cancer risk: A dose response meta-analysis. Medicine 2016, 95, e4121. [Google Scholar] [CrossRef]
- Oyenihi, A.; Smith, C. Are polyphenol antioxidants at the root of medicinal plant anti-cancer success? J. Ethnopharmacol. 2019, 229, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Shih, C.-K.; Yen, Y.-T.; Chiang, W.; Hsia, S.-M. Adlay (Coix lachryma-jobi L. var. ma-yuen stapf.) hull extract and active compounds inhibit proliferation of primary human leiomyoma cells and protect against sexual hormone-induced mice smooth muscle hyperproliferation. Molecules 2019, 24, 1556. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Chen, M.-J.; Shieh, T.-M.; Wang, K.-L.; Wu, Y.-T.; Hsia, S.-M.; Chiang, W. Potential benefits of adlay on hyperandrogenism in human chorionic gonadotropin-treated theca cells and a rodent model of polycystic ovary syndrome. J. Funct. Foods 2014, 11, 393–406. [Google Scholar] [CrossRef]
- Chang, H.C.; Huang, Y.C.; Hung, W.C. Antiproliferative and chemopreventive effects of adlay seed on lung cancer in vitro and in vivo. J. Agric. Food Chem. 2003, 51, 3656–3660. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Hsu, C.Y.; Lin, J.H.; Kuo, Y.H.; Chiang, W.; Lin, Y.L. Antiproliferative lactams and spiroenone from adlay bran in human breast cancer cell lines. J. Agric. Food Chem. 2011, 59, 1185–1194. [Google Scholar] [CrossRef]
- Kuo, C.C.; Shih, M.C.; Kuo, Y.H.; Chiang, W. Antagonism of free-radical-induced damage of adlay seed and its antiproliferative effect in human histolytic lymphoma U937 monocytic cells. J. Agric. Food Chem. 2001, 49, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-W.; Kuo, Y.-H.; Lin, F.-Y.; Lin, Y.-L.; Chiang, W. Effect of Adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) Testa and its phenolic components on Cu2+-treated low-density lipoprotein (LDL) oxidation and lipopolysaccharide (LPS)-induced inflammation in RAW 264.7 macrophages. J. Agric. Food Chem. 2009, 57, 2259–2266. [Google Scholar] [CrossRef]
- Chen, H.-J.; Chung, C.-P.; Chiang, W.; Lin, Y.-L. Anti-inflammatory effects and chemical study of a flavonoid-enriched fraction from adlay bran. Food Chem. 2011, 126, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Supriyanto, E.; Mandal, M. Events associated with apoptotic effect of p-Coumaric acid in HCT-15 colon cancer cells. World J. Gastroenterol. 2013, 19, 7726–7734. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Huang, L.H.; Chiang, W.; Hsia, S.M. Hexane fraction of adlay (Coix lachryma-jobi L.) testa ethanolic extract inhibits human uterine sarcoma cancer cells growth and chemosensitizes human uterine sarcoma cells to doxorubicin. Phytomedicine Int. J. Phytother. Phytopharm. 2018, 47, 69–80. [Google Scholar] [CrossRef]
- Visioli, F.; De La Lastra, C.A.; Andres-Lacueva, C.; Aviram, M.; Calhau, C.; Cassano, A.; D’Archivio, M.; Faria, A.; Fave, G.; Fogliano, V.; et al. Polyphenols and human health: A prospectus. Crit. Rev. Food Sci. Nutr. 2011, 51, 524–546. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Hirun, S.; Phillips, P.A.; Chuen, T.L.; Bowyer, M.C.; Goldsmith, C.D.; Scarlett, C.J. Fruit-derived phenolic compounds and pancreatic cancer: Perspectives from Australian native fruits. J. Ethnopharmacol. 2014, 152, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Lin, B.F.; Lin, J.Y.; Kuo, C.C.; Chiang, W. Suppression of allergic reactions by dehulled adlay in association with the balance of TH1/TH2 cell responses. J. Agric. Food Chem. 2003, 51, 3763–3769. [Google Scholar] [CrossRef] [PubMed]
- Hsia, S.M.; Kuo, Y.H.; Chiang, W.; Wang, P.S. Effects of adlay hull extracts on uterine contraction and Ca2+ mobilization in the rat. Am. J. Physiology. Endocrinol. Metab. 2008, 295, E719–E726. [Google Scholar] [CrossRef]
- Wu, P.; Ma, G.; Li, N.; Deng, Q.; Yin, Y.; Huang, R. Investigation of in vitro and in vivo antioxidant activities of flavonoids rich extract from the berries of Rhodomyrtus tomentosa (Ait.) Hassk. Food Chem. 2015, 173, 194–202. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J. Flavonoids: Dietary occurrence and biochemical activity. Nutr. Res. 1998, 18, 1995–2018. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Su, A.; Zhang, Y.; Yuan, J.; Ju, X. Structural characterization of phenolic compounds and antioxidant activity of the phenolic-rich fraction from defatted adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) seed meal. Food Chem. 2016, 196, 509–517. [Google Scholar] [CrossRef]
- Hsia, S.-M.; Wu, C.-H.; Wang, K.-L.; Shieh, T.-M. P0121 Inhibitory effect of adlay hull and testa ethanolic extracts on uterine leiomyoma cell proliferation. Eur. J. Cancer 2015, 51, e25. [Google Scholar] [CrossRef]
- Fang, S.; Hao, C.; Sun, W.; Liu, Z.; Liu, S. Rapid analysis of steroidal saponin mixture using electrospray ionization mass spectrometry combined with sequential tandem mass spectrometry. Rapid Commun. Mass Spectrom. Rcm 1998, 12, 589–594. [Google Scholar] [CrossRef]
- Yang, Y.; Laval, S.; Yu, B. Chemical synthesis of saponins. Adv. Carbohydr. Chem. Biochem. 2014, 71, 137–226. [Google Scholar] [CrossRef]
- D’Uva, G.; Lauriola, M. Towards the emerging crosstalk: ERBB family and steroid hormones. Semin. Cell Dev. Biol. 2016, 50, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.B.; Fink, C.S. Phytosterols as anticancer dietary components: Evidence and mechanism of action. J. Nutr. 2000, 130, 2127–2130. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Yin, M.; Yang, R.; Zhao, W. Optimization of adlay (Coix lacryma-jobi) bran oil extraction: Variability in fatty acids profile and fatty acid synthase inhibitory activities. Biocatal. Agric. Biotechnol. 2020, 28, 101740. [Google Scholar] [CrossRef]
- Xi, X.-J.; Zhu, Y.-G.; Tong, Y.-P.; Yang, X.-L.; Tang, N.-N.; Ma, S.-M.; Li, S.; Cheng, Z. Assessment of the genetic diversity of different Job’s tears (Coix lacryma-jobi L.) accessions and the active composition and anticancer effect of its seed oil. PLoS ONE 2016, 11, e0153269. [Google Scholar] [CrossRef] [PubMed]
- Sladowski, D.; Steer, S.J.; Clothier, R.H.; Balls, M. An improved MIT assay. J. Immunol. Methods 1993, 157, 203–207. [Google Scholar] [CrossRef]
- Pozarowski, P.; Darzynkiewicz, Z. Analysis of cell cycle by flow cytometry. In Checkpoint Controls and Cancer; Springer: Berlin, Germany, 2004; pp. 301–311. [Google Scholar]
- Standards, P.f.c.o.n. Test for Edible Oils and Fats. 2019. Available online: https://www.bsmi.gov.tw/wSite/public/Data/f1573635614080.pdf (accessed on 5 January 2021).
 Sub-G1;
Sub-G1;  G0/G1;
G0/G1;  S;
S;  G2/M. ATE-EA, ethyl acetate fraction of the adlay testa ethanolic extract.
G2/M. ATE-EA, ethyl acetate fraction of the adlay testa ethanolic extract.
 Sub-G1;
Sub-G1;  G0/G1;
G0/G1;  S;
S;  G2/M. ATE-EA, ethyl acetate fraction of the adlay testa ethanolic extract.
G2/M. ATE-EA, ethyl acetate fraction of the adlay testa ethanolic extract.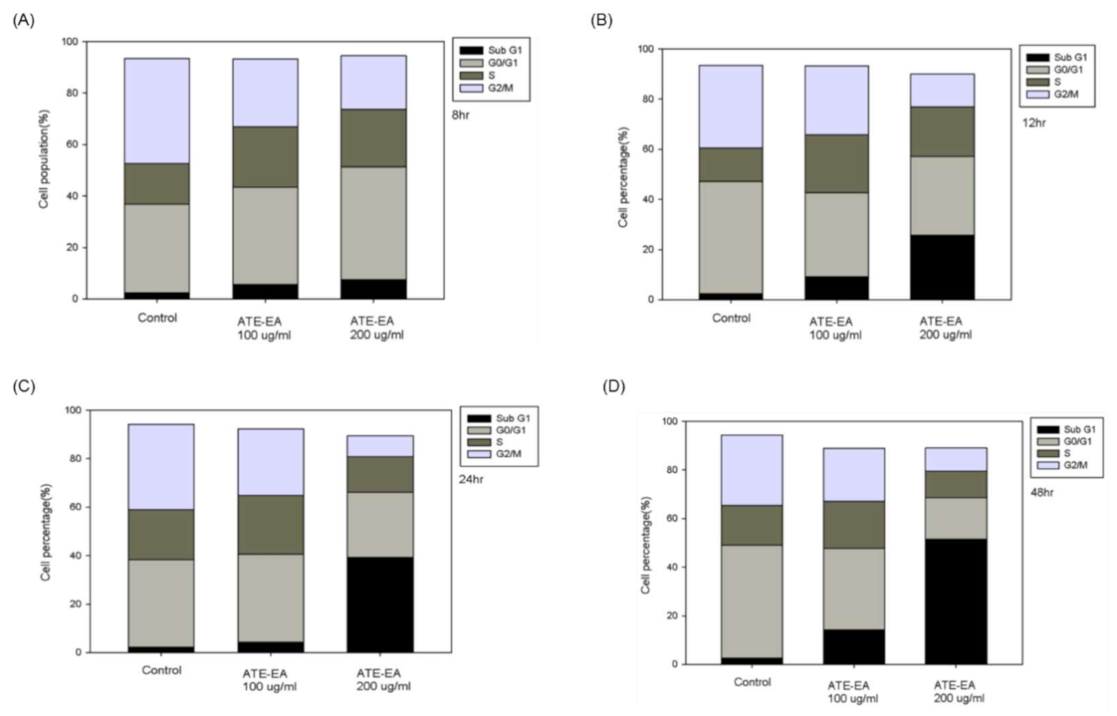
| Compound (μg/g) | ATE-EA |
|---|---|
| 280 nm | |
| Protocatechnic acid | 557.0572 |
| p-Hydroxybenzoic acid | 299.6813 |
| Chlorogenic acid | 6293.503 |
| Vanillic acid | 1836.49 |
| p-Hydroxybenzaldehyde | 213.8971 |
| Syringic acid | 585.5772 |
| Vanillin | 782.389 |
| Syringaldehyde | 1500.096 |
| 320 nm | |
| Caffeic acid | 239.356 |
| p-Coumaric acid | N.D. |
| Ferulic acid | N.D. |
| Compound (μg/g) | ATE-EA |
|---|---|
| Homoeriodictyol | N.D. |
| 5,7-dihydroxy chromone | N.D. |
| Formononetin | N.D. |
| Kaempferol | N.D. |
| Nobiletin | N.D. |
| Isoliquiritigenin | N.D. |
| Tangeretin | N.D. |
| Liquiritigenin | 7624.774 |
| Quercetin | 145.785 |
| Naringenin | 1058.989 |
| Chrysoeriol | 159.576 |
| Quercetin-3,5,7,3’,4-pentamethylether | 48.793 |
| Phytosterols | Molecular Weight | Retention Time (min) | Relative Retention Time 1 | Peak Area | Peak Area Ratio 2 | RRF | Conc (μg/g) |
|---|---|---|---|---|---|---|---|
| Cholesterol | 386 | 18.023 | 1.0000 | 28263 | |||
| Campesterol | 400 | 19.813 | 1.0993 | 14342 | 0.5074 | 0.89 | 1112.38 |
| Stigmasterol | 412 | 20.375 | 1.1305 | 13385 | 0.4736 | 0.81 | 1163.13 |
| Β-sitosterol | 414 | 21.498 | 1.1928 | 70528 | 2.4954 | 0.87 | 5696.74 |
| Stigmastanol | 416 | 21.737 | 1.2061 | 17810 | 0.6302 | 0.78 | 1711.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-J.; Chang, C.-C.; Wang, Y.-Y.; Chiang, W.-C.; Shih, Y.-H.; Shieh, T.-M.; Wang, K.-L.; Ali, M.; Hsia, S.-M. Adlay Testa (Coix lachryma-jobi L. var. Ma-yuen Stapf.) Ethanolic Extract and Its Active Components Exert Anti-Proliferative Effects on Endometrial Cancer Cells via Cell Cycle Arrest. Molecules 2021, 26, 1966. https://doi.org/10.3390/molecules26071966
Huang Y-J, Chang C-C, Wang Y-Y, Chiang W-C, Shih Y-H, Shieh T-M, Wang K-L, Ali M, Hsia S-M. Adlay Testa (Coix lachryma-jobi L. var. Ma-yuen Stapf.) Ethanolic Extract and Its Active Components Exert Anti-Proliferative Effects on Endometrial Cancer Cells via Cell Cycle Arrest. Molecules. 2021; 26(7):1966. https://doi.org/10.3390/molecules26071966
Chicago/Turabian StyleHuang, Yun-Ju, Chih-Chao Chang, Yun-Ya Wang, Wen-Chang Chiang, Yin-Hwa Shih, Tzong-Ming Shieh, Kai-Lee Wang, Mohamed Ali, and Shih-Min Hsia. 2021. "Adlay Testa (Coix lachryma-jobi L. var. Ma-yuen Stapf.) Ethanolic Extract and Its Active Components Exert Anti-Proliferative Effects on Endometrial Cancer Cells via Cell Cycle Arrest" Molecules 26, no. 7: 1966. https://doi.org/10.3390/molecules26071966
APA StyleHuang, Y.-J., Chang, C.-C., Wang, Y.-Y., Chiang, W.-C., Shih, Y.-H., Shieh, T.-M., Wang, K.-L., Ali, M., & Hsia, S.-M. (2021). Adlay Testa (Coix lachryma-jobi L. var. Ma-yuen Stapf.) Ethanolic Extract and Its Active Components Exert Anti-Proliferative Effects on Endometrial Cancer Cells via Cell Cycle Arrest. Molecules, 26(7), 1966. https://doi.org/10.3390/molecules26071966










