Abstract
Lauraceae species are widely represented in the Amazon, presenting a significant essential oil yield, large chemical variability, various biological applications, and high economic potential. Its taxonomic classification is difficult due to the accentuated morphological uniformity, even among taxa from a different genus. For this reason, the present work aimed to find chemical and molecular markers to discriminate Aniba species collected in the Pará State (Brazil). The chemical composition of the essential oils from Aniba canelilla, A. parviflora, A. rosaeodora, and A. terminalis were grouped by multivariate statistical analysis. The major compounds were rich in benzenoids and terpenoids such as 1-nitro-2-phenylethane (88.34–70.85%), linalool (15.2–75.3%), α-phellandrene (36.0–51.8%), and β-phellandrene (11.6–25.6%). DNA barcodes were developed using the internal transcribed spacer (ITS) nuclear region, and the matK, psbA-trnH, rbcL, and ycf1 plastid regions. The markers psbA-trnH and ITS showed the best discrimination for the species, and the phylogenic analysis in the three- (rbcL + matK + trnH − psbA and rbcL + matK + ITS) and four-locus (rbcL + matK + trnH − psbA + ITS) combination formed clades with groups strongly supported by the Bayesian inference (BI) (PP:1.00) and maximum likelihood (ML) (BS ≥ 97%). Therefore, based on statistical multivariate and phylogenetic analysis, the results showed a significant correlation between volatile chemical classes and genetic characteristics of Aniba species.
1. Introduction
Lauraceae Juss comprises the most diverse family of woody plants (except the herbaceous parasite Cassytha), with about 50 genera and approximately 2500 to 3000 species distributed throughout tropical and subtropical latitudes [1,2,3]. Lauraceae belongs to the Laurales order and systematically forms close relationships with Hernandiaceae and Monimiaceae [1,4,5].
The Aniba genus is a Lauraceae member that presents an economical and significant ecological value [6]. This genus has 48 accepted species with greater than 50% concentrated in the Brazilian Amazon [7,8]. Aniba species are represented by trees with high essential oil production in all tissues, mainly in the wood and bark. Aniba duckei Kosterm and A. rosaeodora Ducke are known as “rosewood” in the Amazon region, and these species produce an oil rich in linalool (about 85–90%) [9,10]. Brazilian rosewood oil has a characteristic aroma and is a long-established ingredient of fragrances, flavors, and food products [11]. The species Aniba parviflora (Meisn.) Mez, called “macacaporanga” or “louro rosa,” is confused with the real rosewood plants due to their morphological similarity. However, these species present very distinct aromas in wood and leaf oils because the linalool content in A. parviflora is only 40% [9,12,13]. Linalool is also detected in oils from Aniba terminalis Ducke, with amounts varying from 22.1 to 36.2% in the aerial parts and inflorescences of a specimen collected in Belém (PA, Brazil) [14].
Aniba canelilla (H.B.K.) Mez is an aromatic plant with a characteristic odor, which is easily confused with cinnamon trees. It is popularly known as “casca-preciosa” and “falsa-canela.” Its chemical composition has two main constituents, 1-nitro-2-phenylethane, which is generally found as the major component responsible for the cinnamon-like odor characteristic, and methyleugenol [15,16,17]. The A. canelilla essential oil EO displayed cardiovascular activities in normotensive rats, causing hypotension, bradycardia, and vasorelaxant effects [18,19,20,21,22]. The EO of A. rosaeodora and A. parviflora species showed antidepressant activity in rats [23], and an anesthetic potential in fish and rat species [24,25]. In folk medicine, the leaves and barks of A. parviflora are used to prepare tea and infusions, tinctures, and poultices to treat snakebite envenomation victims [26]. Additionally, the A. canelilla bark decoction is commonly used for its antispasmodic, digestive, stimulating, and carminative properties [16].
The recognition of the Lauraceae taxa, and the Aniba genus, in particular, is a difficult task due to the lack of morphological characters that can be used objectively and also due to the low level of sampling in the Amazon, which makes most species poorly represented in herbaria [27,28,29,30]. Some factors also hamper accurate identification: The use of common names for the species, which sometimes do not correspond to the scientific name; the rare collecting of fertile specimens, either for comparison in herbaria or by experts [31]. The identification methods have recently been gradually expanding to new techniques such as DNA-based identification [2,32].
DNA barcoding is designed to provide a fast, accurate, and automated identification of species using short and standardized genes as internal species markers [33]. Various molecular markers have been analyzed to develop plant DNA barcodes that can be readily sequenced and have a sufficiently high sequence divergence at the species-level [34,35]. These markers include the coding plastid regions, matK, rbcL, non-coding trnH-psbA intergenic spacer, and nuclear ribosomal internal transcribed spacer region (ITS) [36,37,38]. The DNA barcoding has been an essential tool for species identification and supplements traditional morphology-based taxonomy [33,39,40].
The identification of Aniba species by molecular markers is still unresolved. The present study aimed to explore the correlation between the volatile compositions and the genetic markers in Aniba species existing in the Amazon.
2. Results and Discussion
2.1. Chemical Composition and Multivariate Analysis
The yields and volatile compositions of the Aniba oils are displayed in Table 1. The oil yields of these species were as follows: Aniba canelilla, A. parviflora, A. rosaeodora, and A. terminalis. GC and GC-MS were used to quantify and identify the volatile constituents of Aniba oil samples. One hundred and eighteen components were identified, representing an average of 97.8% of the total percentage identified among the samples (see Table 1). A. parviflora oils showed the highest number of compounds identified, fifty-four and fifty-seven in the leaves (AP-L) and twigs (AP-T), respectively. The sample with the lowest number of compounds was A. canelilla, with fifteen compounds in the leaves (AC-L) and twenty-five in the twigs (AC-T). The species A. terminalis showed forty-two (AT-L) and forty-four (AT-T), while A. rosaeodora presented thirty-seven (AR-L) and thirty-eight (AR-T). The calculated retention index (RIC) of components of the oils were compared with the literature retention index (RIL) stored in the libraries of Mondello [41] and Adams [42]. Benzenoid compounds (0.1–91.8%), monoterpene hydrocarbons (0.0–88.9%), and oxygenated monoterpenes (1.2–81.7%) predominated in oils, followed by oxygenated sesquiterpenes (0.9–19.2%) and sesquiterpene hydrocarbons (0.6–10.9%), with minor amounts. The main constituents were 1-nitro-2-phenylethane and linalool in A. canelilla; β-phellandrene, and α- and β-pinene in A. parviflora; linalool and cis-linalool oxide in A. rosaeodora; and α-phellandrene, p-cymene, and β-phellandrene in A. terminalis.

Table 1.
Yield and volatile composition of the Aniba essential oils.
The leaf and twig oils (AC-L and AC-T, respectively) of Aniba canelilla were dominated by the benzenoid 1-nitro-2-phenylethane (88.3% and 70.90%, respectively), followed by the oxygenated monoterpene linalool, which showed higher amounts in the twigs (16.1%) in comparison to the leaves (3.9%). In the leaf and twig oils (AP-L and AP-T, respectively) of Aniba parviflora, the monoterpene hydrocarbon β-phellandrene (22.6% and 25.4%, respectively) and the oxygenated monoterpene linalool were the primary constituents, followed by α-pinene (10.6% and 4.7%), β-pinene (6.4% and 3.5%), and myrcene (3.2% and 4.1%), respectively. Significant amounts of linalool were detected in the leaves and twigs (AR-L, 67.9%; AR-T, 75.3%, respectively) of Aniba rosaeodora, followed by its oxygenation products, cis-linalool oxide (leaves, 5.4%; twigs 2.6%) and trans-linalool oxide (leaves, 4.9%; twigs 2.5%). The oils of leaves and twigs (AT-L and AT-T, respectively) of Aniba terminalis showed the monoterpene hydrocarbons α-phellandrene (51.8% and 36.0%), p-cymene (12.0%, 7.5%), and β-phellandrene (11.6%, 11.9%), as the primary components, respectively, followed by α-pinene (4.3%, 3.8%), β-pinene (1.5%, 3.6%), respectively, and myrcene (3.6%) only in the twigs (AT-T). Furthermore, in the twigs of A. terminalis, linalool (19.0%) was also identified, which was absent in its leaves.
Principal component analysis (PCA) of the Aniba oils showed that PC1 and PC2 components had explained 82.7% of the phytochemical variation among all samples, classified into four groups (Figure 1). The PC1 component explained 51.1% of the variation, displaying a positive correlation with all terpenoid classes and a negative correlation with the benzenoid compounds. The more informative contributions to group separation were observed with the oxygenated sesquiterpenoids (30.4%), benzenoids (29.7%), and sesquiterpenes hydrocarbons (29.6%). Due to the significant content of sesquiterpenoids (11.20–30.10%) in the samples, AP-T (2.43), AP-L (1.28), AR-T (0.38), and AR-L (1.00) were represented by positive scores in the PC1 component. On the other hand, the most negative scores characterized the oil samples of A. canelilla AC-L (−2.68) and AC-T (−2.12) due to the significant content of benzenoid compounds (72.3–91.8%) in oils. The PC2 component explained 31.58% of the chemical variability, and the most informative contributions to sample separation were the contents of monoterpene hydrocarbons (52.1%, negatively) and oxygenated monoterpenoids (44.4%, positively), presenting in these samples in an amount around 80%. These influences can be easily visualized in the PC2 component by the samples of A. terminalis (AT-L, −2.00; AT-T, −1.19) and A. rosaeodora (AR-L, 1.72; AR-T, 1.92), respectively.
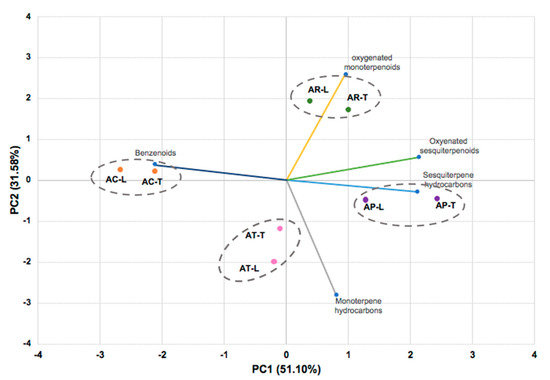
Figure 1.
The bidimensional plot of the first two components from principal component analysis (PCA) of Aniba species, based on the classes of compounds present in their essential oils: AC (A. canelilla), AP (A. parviflora), AR (A. rosaeodora), AT (A. terminalis), L (leaves), T (twigs), PC1 (first principal component), PC2 (second principal component).
Based on the dendrogram (Figure 2) resulting from the hierarchical cluster analysis (HCA), using the classes of compounds as variables, the Aniba species’ oils were arranged into two main groups, presenting a dissimilarity of 16.8%. Cluster I comprised the leaves (AC-L) and twigs (AC-T) of A. canelilla and comprised samples rich in benzenoid compounds (72.28–91.78%), especially 1-nitro-2-phenylethane, showing a dissimilarity of 99.5%. Cluster II grouped all oil samples of A. parviflora, A. terminalis, and A. rosaeodora. The oils of A. parviflora (AP-L and AP-T) showed a dissimilarity of 8.3% with the oils of A. terminalis (AT-L and AT-T), forming a subgroup that displayed a dissimilarity of only 11.39% with the oils of A. rosaeodora (AR-L and AR-T). This second group was characterized by the high content of monoterpene hydrocarbons (47.54–88.91%) as α-phellandrene (36.0–51.83%), which is present in the oils of AT-L and AT-T; as β-phellandrene (11.58–25.58%) in the oils of AP-L, AP-T, AT-L, and AT-T; and as linalool (15.23–75.30%), the primary constituent in the oil samples of AR-L, AR-T, AP-L, and AP-T.
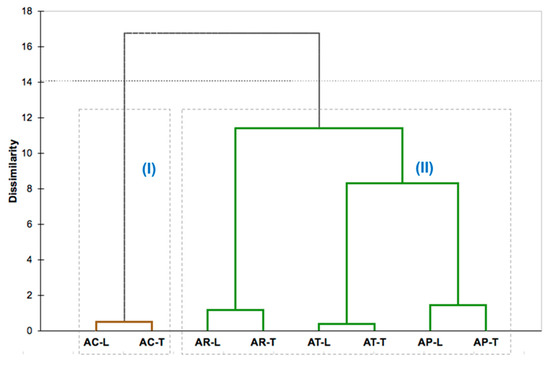
Figure 2.
Hierarchical clusters analysis (HCA) obtained by Ward linkage method to Aniba species based on compound class present in their essential oil: AC (A. canelilla), AP (A. parviflora), AR (A. rosaeodora), AT (A. terminalis), L (leaves), T (twigs).
Due to the morphological similarity, the species A. parviflora and A. rosaeodora have been confused concerning their true botanical identification. On the other hand, these species presented distinct scents, despite the significant linalool content in their oils. An olfactory analysis of EOs from A. rosaeodora and A. parviflora, performed by enantioselective gas chromatography coupled to olfactometry, showed a significant difference between these oils [12,43]. In addition, the aromas extracted from the leaves of A. parviflora and A. rosaeodora by solid-phase microextraction (SPME), monitored by electrospray ionization mass spectrometry, and followed by PCA multivariate statistical analysis, showed a high efficiency at distinguishing the samples, as well as at separating them by collecting data, indicating the influence of the maturation stage on their chemical composition [44]. Leaf oils from thirty-five trees of A. rosaeodora growing in the Pará state, Brazil, were extracted and analyzed by GC and GC-MS. Significant variations in the oil yield (1.2 to 4.2%) and linalool content (38.5–71.0%) were observed in these tree samples. On the other hand, the oil yield (0.9 to 1.3%) and linalool content (12.6 to 21.3%) determined for two trees of A. parviflora were much smaller, probably due to different botanical species, but with very similar morphology. Moreover, the hierarchical cluster analysis (HCA) showed differences in A. rosaedora and A. parviflora oil compositions. The oil of A. parviflora also showed a high content of β-phellandrene (21.1–23.6%), which is absent in the oil of A. rosaeodora [45].
2.2. DNA Barcode Analysis
According to the CBOL (Consortium for the Barcode of Life) (www.ibol.org/phase1/cbol accessed on 7 February 2021) plant working group, an ideal DNA barcode should associate conserved regions with a universal primer design, present elevated rates of PCR amplification and sequencing, and have genetic variability. This is sufficient to distinguish sequences at the species level still sufficiently conserved among individuals of the same species [36,46,47].
In the present study, the rbcL plastid DNA region exhibited a high performance, with 100% of successful reactions and specific amplifications, resulting in high-quality and straightforward sequencing for the Aniba species. The success of rbcL should be expected as it is a stable, easily amplified, and phylogenetically conserved locus [47,48,49]. Similar patterns have been reported for 133 species of Lauraceae collected in China, represented by 12 genera, including Alseodaphne, Cinnamomum, Cryptocarya, Lindera, Litsea, Machilus, and Neolitsea. The samples showed a rbcL PCR amplification and elevated sequencing rates, which were considered as barcode loci [37].
Primer universality is a critical factor unquestionably determining the reliability of barcode-based species identification [50]. The intergenic spacer psbA-trnH was highly successful in amplification and the intermediate reactions in sequencing. It is considered a robust marker that allows PCR amplification from diverse plant taxa [49] and special phylogenetic studies in Lauraceae [51,52,53,54].
The matK marker showed easy amplification of the samples, but the sequencing was not satisfactory. Many studies questioned its utility as a barcode due to low amplification, sequencing performance, and problems related to the primers universality [55]. Consequently, the literature has recommended a more significant number of primers from the matK region [49,56]. This DNA region was tested in Amazonian tree species, including Lauraceae, and the results showed a low rate of sequencing success, even after using two different pairs of primers [57].
Primers of two ITS regions were tested, but only the ITS-2 region demonstrated success in amplification and sequencing. Amplification difficulties of the ITS region were also found in the Sassafras species collected in Taiwan and 42 species from diverse genera of Lauraceae, such as Aniba, Cinnamomum, Endlicheria, Laurus, Lindera, Litsea, and Machilus, collected from the Chinese provinces of Hubei, Jiangxi, Guangdong, and Guangxi [58,59]. Despite multiple amplification attempts with varying DNA concentrations and annealing temperatures, the ycf1 marker did not amplify the tested species. The results suggested that re-development of the ycf1 barcode specific for Aniba species may be necessary.
Based on the markers used in this study, it was not possible to obtain the identification level for the species of Aniba. However, we must consider that only a few sequences of the Aniba genus are available from Genbank and BOLD, which served as a central reference for comparisons [60]. The best-match molecular identification was at the family level for psbA-trnH, matK, and ITS markers, and the genus level for rbcL.
Multiple alignments of all sequences for each region presented the most extensive length (833 bp) to rbcL and the shortest length (249 bp) to ITS (Table 2). In terms of molecular variation, matK and rbcL demonstrated more conserved sequences with the number of polymorphic sites (0 and 5, respectively) and nucleotide diversity showing low values (≥0.0034). The rbcL region is considered a benchmark locus in phylogenetic investigations by providing a taxon’s reliable placement into a plant family and genus. Therefore, it showed insufficient sequence variation to distinguish between closely related species [61].

Table 2.
Molecular characteristics of the four markers evaluated for Aniba species.
The matK region also showed surprisingly low genetic diversity when analyzing 48 species belonging to different genera of the Lauraceae family, such as Actinodaphne, Aniba, Laurus, Lindera, Litsea, Neolitsea, and Nectandra [62]. However, in a multilocus approach, rbcL and matK discriminated a total of 49% of the 100 taxa belonging to flowering plants, including species from the Asteraceae, Caryophillaceae, Fabaceae, Euphorbiaceae, Brassicaceae, and Ericaceae [63].
The barcodes ITS and psbA-trnH showed the highest number of polymorphic sites and nucleotide diversity with values of 27/0.0543 and 26/0.0336, respectively (Table 2). Studies conducted with ITS and psbA-trnH show that both loci are powerful for differentiating Apocynaceae species [64]. The psbA-trnH region has been used as a DNA barcode to authenticate several plants with similar morphological characteristics [58,65]. This region in the chloroplast genome is described as a barcode rich in simple sequence repetitions (mainly stretches of mononucleotides) and small insertions and deletions (INDELs) [47,66] that lead to species-level identification of plant taxa [67,68]. The psbA-trnH INDEL polymorphisms served as markers to identify different species of the genus Citrus and Sonneratia, belonging to the families Rutaceae and Lythraceae, respectively [67,68].
The genetic variabilities of two populations of Aniba rosaeodora in the Reserva Extrativista Tapajós-Arapiuns (RESEX) and Floresta Nacional do Tapajós (FLONA), Brazilian Government conservation units in Western Pará State, were evaluated. The results showed that the psbA-trnH region had the highest number of polymorphism sites (25 variables) in comparison to the regions psbD-trnT, trnC-rpoB, and trnS-trnG, which presented values from zero to four sites [69].
The aligned and concatenated matrices rbcL + matK + psbA − trnH, rbcL + matK + ITS, and rbcL + matK + psbA − trnH + ITS presented a total of 1590, 1368, and 1839 bp, respectively. Among the three concatenated matrices, rbcL + matK + psbA − trnH + ITS reached a greater number of polymorphic sites (58) and nucleotide diversity (0.0167). The best-fit substitution model for each gene was: HKY + F for ITS, F81 + F + I for matK and rbcL, and GTR + F for psbA − trnH. The phylogenetic relationships in Aniba species were established by the combination of rbcL + matK + ITS, rbcL + matK + psbA − trnH, and rbcL + matK + trnH − psbA + ITS. The consensus trees obtained from the Bayesian inference (BI) and maximum likelihood (ML) analyses were identical in their topologies, and the PP (posterior probabilities) and BS (bootstrap support) values (Figure 3, Figure 4 and Figure 5). The trees constructed by the ML and BI method for each individual region and their support values can be visualized in the Supplementary Material (Figures S1–S8).
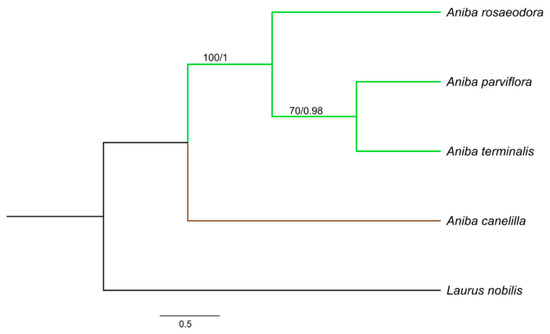
Figure 3.
Bayesian consensus tree based on rbcL + matK + trnH – psbA + internal transcribed spacer (ITS) sequences of species of Aniba and Laurus nobilis (outgroup). Bootstrap support values (≥70%)/Bayesian posterior probabilities (>0.9) are shown above the branches.
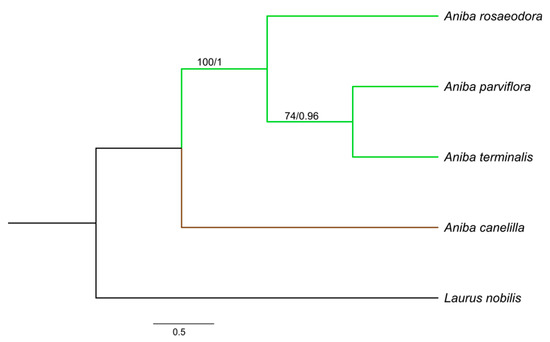
Figure 4.
Bayesian consensus tree based on rbcL + matK + ITS sequences of species of Aniba and Laurus nobilis (outgroup). Bootstrap support values (≥74%)/Bayesian posterior probabilities (>0.9) are shown above the branches.
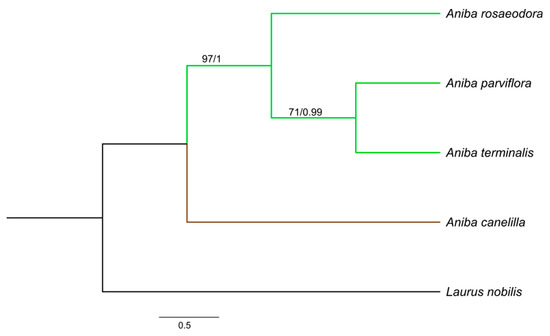
Figure 5.
Bayesian consensus tree based on rbcL + matK + trnH − psbA sequences of species of Aniba and Laurus nobilis (outgroup). Bootstrap support values (≥70%)/Bayesian posterior probabilities (>0.9) are shown above the branches.
Aniba species formed clades with groups strongly supported by Bayesian inference (BI) (PP:1.00) and maximum likelihood (ML) (BS ≥ 97%). Aniba parviflora and A. rosaeodora are botanically very similar species and, together with A. terminalis, exhibit chemical alliances due to linalool’s presence in their essential oils [11,14]. Phytochemically analyzing other secondary compounds, the presence of pseudoalkaloid anibine firmly links the species A. rosaeodora and A. parviflora, forming a complex [28,70].
In this study, A. rosaeodora is a sister to a clade composed of A. terminalis and A. parviflora. In addition, genetically and chemically, A. parviflora and A. terminalis have showed close relationships. The content of linalool detected in these species ranged from 15.23 to 21.93%, while that of A. rosaeodora was above 67%. Aniba canelilla presented a distinct clade as this species exhibits a high morphological similarity with Aniba ferrea Kubitzki. This pair of species is closely related to the exclusive presence of pyrones versus neolignans. Moreover, there are substantial quantities of eugenol-derived allylphenols [70].
The genetic and essential oil diversity was investigated with specimens of A. rosaeodora from Reserva Extrativista Tapajós-Arapiuns (RESEX), Floresta Nacional do Tapajós (FLONA), and the Municipality of Presidente Figueiredo, in the Amazonas State, Brazil. The analysis was performed with the concatenated matrix of the regions psbA-trnH, psbD-trnT, trnC-rpoB, and trnS-trnG forming two clades: (1) including collections from FLONA and the Municipality of Presidente Figueiredo, and (2) including collections from RESEX. Clade 1 showed moderate support (PP: 0.87), coinciding with the low diversity of volatile components from FLONA samples, where linalool predominated with 83.7%. By contrast, clade (2) was strongly supported by samples of RESEX (PP: 1.0), presenting monoterpene hydrocarbons (40.0%) and oxygenated monoterpenoids as most abundant (41.3%), with only 39.6% of linalool, followed by 22.8% of α-phellandrene, compound that was absent in the FLONA samples [69].
By contrast, this correlation was not observed in essential oil compositions and matK sequences of Ocotea caudata (Nees) Mez., O. cujumary Mart., and O. canaliculata (Rich.) Mez. from Caxiuanã National Forest (Amazon, Brazil). Ocotea caudata was characterized by the presence of germacrene D (19.9%) and monoterpene hydrocarbons α-pinene (9.8%) and β-pinene (9.7%). Simultaneously, O. cujumary and O. canaliculata showed a high similarity due to the amounts of β-caryophyllene (22.2% and 18.9%, respectively). However, genetically, O. cujumary was shown to be the clade’s sister (BS:70%) formed by O. caudata and O. canaliculata [71].
3. Materials and Methods
3.1. Plant Material
The specimens of Aniba parviflora (Nees) Mez, A. rosaeodora Ducke, and A. canelilla (Kunth) Mez were collected in the Campus of Universidade Federal Rural da Amazônia (UFRA), and the Aniba terminalis Ducke was sampled in the Zoobotanical Park of Museu Paraense Emilio Goeldi (MPEG), both Federal Institutions located in Belém city, Pará state, Brazil. The plant vouchers were identified and cataloged in the Herbarium João Murça Pires of Emilio Goeldi Museum, as listed in Table 3.

Table 3.
Data from Aniba species.
3.2. Essential Oil Extraction
The leaves and twigs were dried for two days at room temperature and then subjected to essential oil distillation. The samples were ground and submitted to hydrodistillation using a Clevenger-type apparatus (3 h). The oils were dried over anhydrous sodium sulfate, and the yields were calculated based on the dry weight of the plant material. The moisture content of each sample was measured using an infrared moisture balance for water loss measurement.
3.3. GC-MS Analysis
The oil samples were analyzed on a GCMS-QP2010 Ultra system (Shimadzu Corporation, Tokyo, Japan), equipped with an auto-injector (AOC-20i). The parameters of analysis were: A silica capillary column Rxi-5ms (30 m × 0.25 mm; 0.25 μm film thickness) (Restek Corporation, Bellefonte, PA, USA); injector temperature: 250 °C; oven temperature programming: 60–240 °C (3 °C/min); helium as carrier gas, adjusted to a linear velocity of 36.5 cm/s (1.0 mL/min); splitless mode injection of 1 μL of sample (oil 5 μL:hexane 500 μL); ionization by electronic impact at 70 eV; ionization source and transfer line temperatures at 200 and 250 °C, respectively. The mass spectra were obtained by automatically scanning every 0.3 s, with mass fragments in the range of 35–400 m/z. The quantitative data regarding the volatile constituents were obtained by peak-area normalization using a GC 6890 Plus Series (Agilent, Wilmington, DE, USA), coupled to a flame ionization detector (FID), operated under similar GC-MS system conditions.
The retention index was calculated for all volatile components using a homologous series of C8–C20 n-alkanes (Sigma-Aldrich, St. Louis, MI, USA), according to the linear equation of Van den Dool and Kratz [72]. The components of oils were identified by comparing their retention indices and mass spectra (molecular mass and fragmentation pattern) with data stored in the NIST [73], Mondello [41], and Adams [42] libraries.
3.4. Statistical Analysis
Each class of compound content in the leaf samples was used as a variable in multivariate analysis. First, the matrix’s data standardization was performed by subtracting the mean and dividing it by the standard deviation. For hierarchical cluster analysis (HCA) and principal component analysis (PCA), the Ward distance and a correlation matrix were applied, respectively. These analyses were performed using XLSTAT software (free trial version version 2021.1, Addinsoft, Paris, France).
3.5. Oligonucleotides Design
The primers rbcL and ITS were designed using the software Primer Blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/ accessed on 20 December 2020) based on the Aniba sequences previously deposited on GenBank (https://www.ncbi.nlm.nih.gov/ accessed on 5 December 2020). The primers of psbA-trnH, matK, and ycf1 regions were based on studies described in the literature [48,72,74,75]. The primers were synthesized by the companies Síntese Biotecnologia (ITS, psbA-trnH and rbcL; Belo Horizonte, Brazil) and Gbtoligos (ycf1 and matK, Alvorada, Brazil).
3.6. DNA Extraction, Amplification, and Sequencing
Genomic DNA was extracted from 100 mg of fresh leaves using a plant DNA isolation Kit (PureLink™ Genomic DNA, Invitrogen, Carlsbad, CA, USA), according to its specifications, and stored at −20 °C. Polymerase chain reactions (PCR) of the regions ITS, matK, psbA-trnH e rbcL, and ycf1 were performed at a volume of 50 µL containing 4.4 µL of DNA template, 0.2 pmoles of each primer, and 43.6 µL of PCR SuperMix (22 nM Tris-HCl at pH 8.4, 55 mM KCl, 1.65 mM MgCl2, 220 µM dGTP, 220 µM dATP, 220 µM dTTP, 220 µM dCTP, and 22 U/mL Taq DNA polymerase, Invitrogen, Carlsbad, CA, USA). To regions ITS, psbA-trnH, and ycf1, 0.4 µL of MgCl2 (Invitrogen, Carlsbad, CA, USA) was added in the PCR reaction, resulting in a final concentration of MgCl2 of 1.84 mM. DNA amplifications were conducted in a thermocycler (GeneAmp PCR System 9700, Foster, CA, USA), and a negative control was carried out for all PCR reactions in the absence of DNA. Amplification products were visualized in agarose gel 1.5% and purified following the GeneJET PCR Purification Kit (Life Technologies, Massachusetts, CA, USA), and then sent to the ACTgene company (Alvorada, Brazil) for DNA sequencing. Table 4 presents the sequences of the primers of each fragment and its PCR amplification conditions.

Table 4.
Primer sequences applied in DNA amplification of Aniba species and its experimental conditions.
3.7. Sequence Identity and Phylogenetic Analysis
The forward and reverse sequences of each amplified region (ITS, matK, psbA-trnH, and rbcL) were edited and aligned using the software MUSCLE algorithm [76] implemented within MEGA 7 software [77]. Sequences were compared with available sequences in the National Center for Biotechnology Information (NCBI) GenBank database (http://www.ncbi.nlm.nih.gov/ accessed on 7 December 2020), using the tool Blast N. DNA sequences generated in this study were deposited in the NCBI GenBank, and accession numbers are listed in the Supporting Information (Table 5).

Table 5.
GenBank accession numbers of Aniba species collected in the Amazon.
DNA sequences were aligned using the software MAFFT Ver 7.122 (Osaka University, Osaka, Japan) [78] and manually adjusted, when necessary, with the MEGA7 software [77]. The phylogenetic analyses were inferred from sequence variation in the three-locus (rbcL + matK + trnH−psbA and rbcL + matK + ITS) and four-locus combination (rbcL + matK + trnH − psbA + ITS). The analyses were performed in the software PhyloSuite (Github, Free Software Foundation, Inc., San Francisco, CA, USA) [79] using two different approaches: Maximum likelihood (ML) and Bayesian inference (BI) analyses using the programs IQ-Tree v.6.1 (Github, Free Software Foundation, Inc.) [80] and MrBayes v.3.2.6 (Github, Free Software Foundation, Inc.) [81], respectively. Evolutionary models were tested using the ModelFinder program implemented in IQ-TREE version 1.5.4 (Github, Free Software Foundation, Inc.) [82], based on the Akaike information criterion (AIC). For the ML and BI analyses, the dataset was partitioned by markers. For maximum likelihood analyses, the branch supports for the tree were estimated with 5000 bootstrap replicates using UFBoot (Ultrafast Boostrap Approximation) [80].
For Bayesian Inference, analyses were performed using two parallel runs and a sampling frequency set to every 10,000,000 generations. The trees were sampled every 100 generations, and the first 25% of the samples were discarded as burn-in trees. The remaining trees were used to construct a 50% majority-rule consensus tree. Laurus nobilis L. was defined as an outgroup (KM360844.1, MH552343.1, AY265392.1, and EU153959.1). The resulting trees from both analyses were output into FigTree v.1.4.4 [83], and their topologies were compared.
The median length described the genetic variability of each marker (bp) and total alignment length (bp), both discounting gaps, the number of sites with gaps, and nucleotide diversity (π), using the DnaSP v6 [84].
4. Conclusions
In this study, it was proven that the essential oils of Aniba canelilla are rich in benzenoid compounds, while A. rosaeodora, A. parviflora, and A. terminalis are rich in monoterpene hydrocarbons and oxygenated monoterpenes, with important variations in the relative quantity of major constituents. The primary classes of compounds showed a significant correlation with phylogenetic analysis. The psbA-trnH and ITS regions allowed the estimation of relatively high polymorphic sites and nucleotide diversity. The regions rbcL and matK, although very conserved, provided important information on the taxonomic levels to ordering genus and family. In general, the psbA-trnH and ITS genes were significant in terms of nucleotide differentiation, while the matK and rcbL genes indicated genetic similarity between the species studied. Based on the results, it was possible to verify that the genetic and chemical data are closely related in the studied Aniba species.
Supplementary Materials
The following are available online, Figure S1. Maximum likelihood tree based on rbcL sequences of species of Aniba and Laurus nobilis (outgroup). Bootstrap support values (67%) are shown above the branches. Figure S2. Bayesian inference tree based on rbcL sequences of species of Aniba and Laurus nobilis (outgroup). Bootstrap support values (≥0.7) are shown above the branches. Figure S3. Maximum likelihood tree based on matK sequences of species of Aniba and Laurus nobilis (outgroup). Bootstrap support values (34%) are shown above the branches. Figure S4. Bayesian inference tree based on matK sequences of species of Aniba and Laurus nobilis (outgroup). Figure S5. Maximum likelihood tree based on ITS sequences of species of Aniba and Laurus nobilis (outgroup). Bootstrap support values (≥33%) are shown above the branches. Figure S6. Bayesian inference tree based on ITS sequences of species of Aniba and Laurus nobilis (outgroup). Bayesian posterior probabilities (1) are shown above the branches. Figure S7. Maximum likelihood tree based on psbA-trnH sequences of species of Aniba and Laurus nobilis (outgroup). Bootstrap support values (≥33%) are shown above the branches. Figure S8. Bayesian inference tree based on psbA-trnH sequences of species of Aniba and Laurus nobilis (outgroup). Bayesian posterior probabilities (1) are shown above the branches.
Author Contributions
J.K.A.M.X. and L.M. conducted the experiments; J.K.A.M.X., P.L.B.F. and J.K.R.d.S. performed the statistical analysis; J.G.S.M., P.L.B.F. and E.H.A. contributed to the GC-MS analyses; A.R.R., A.F., J.K.R.d.S. and J.K.A.M.X. contributed to the phylogenetic analyses; W.N.S., J.G.S.M. and J.K.R.d.S. edited and approved the final version manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Aromatic Plant Research Center (APRC, https://aromaticplant.org/, accessed on 20 December 2020).
Data Availability Statement
Publicly available gene sequence datasets were analyzed in this study. These data can be found here: https://www.ncbi.nlm.nih.gov/genbank/, accessed on 20 December 2020; accession numbers: Aniba canelilla (MW489499, MW512551, MW512547, and MW512555), A. parviflora (MW489500, MW512552, MW512548, and MW512556), A. rosaeodora (MW489501, MW512553, MW512549, and MW512557), and A. terminalis (MW489502, MW512554, MW512550, and MW512558).
Acknowledgments
The authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Niível Superior (CAPES—Coordination for the Improvement of Higher Education Personnel) for providing a scholarship to J.K.A.M.X. and L.M.
Conflicts of Interest
The authors declare that they have no competing interests.
Sample Availability
Samples of this work are available from the authors.
References
- Angiosperm Phylogeny Group (APG) IV. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Chanderbali, A.S.; Van der Werff, H.; Renner, S.S. Phylogeny and historical biogeography of Lauraceae: Evidence from the chloroplast and nuclear genomes. Ann. Mo. Bot. Gard. 2001, 88, 104. [Google Scholar] [CrossRef]
- Rohwer, J.G. Lauraceae. In The Families and Genera of Vascular Plants: Magnoliid, Hamameliid and Caryophyliid Families; Kubitzki, K., Rohwer, J., Bittrich, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 2, pp. 336–391. [Google Scholar] [CrossRef]
- Renner, S.S. Circumscription and phylogeny of the Laurales: Evidence from molecular and morphological data. Am. J. Bot. 1999, 86, 1301–1315. [Google Scholar] [CrossRef]
- Renner, S.S.; Chanderbali, A.S. What is the relationship among Hernandiaceae, Lauraceae, and Monimiaceae, and why is this question so difficult to answer? Int. J. Plant Sci. 2000, 161, S109–S119. [Google Scholar] [CrossRef]
- Contim, L.A.S.; Carvalho, C.R.; Martins, F.A.; Freitas, D.V. Nuclear DNA content and karyotype of rosewood (Aniba rosaeodora). Genet. Mol. Biol. 2005, 28, 754–757. [Google Scholar] [CrossRef][Green Version]
- Sobral, M.; Proença, C.; Souza, M.; Mazine, F.; Lucas, E. Lista de Espécies da Flora do Brasil. 2020. Available online: http://floradobrasil.jbrj.gov.br (accessed on 2 September 2020).
- Tropicos. Lauraceae Juss. Missouri Botanical Garden. Available online: http://www.tropicos.org (accessed on 3 February 2021).
- Maia, J.G.S.; Andrade, E.H.A.; Couto, H.A.R.; Silva, A.C.M.; Marx, F.; Henke, C. Plant sources of amazon rosewood oil. Quim. Nova 2007, 30, 1906–1910. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A. Database of the Amazon aromatic plants and their essential oils. Quim. Nova 2009, 32, 595–622. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Mourão, R.H.V. Amazon rosewood (Aniba rosaeodora Ducke and A. parviflora) oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 193–201. [Google Scholar] [CrossRef]
- D’Acampora, Z.B.; Lo Presti, M.; Barata, L.E.S.; Dugo, P.; Dugo, G.; Mondello, L. Evaluation of leaf-derived extracts as an environmentally sustainable source of essential oils by using gas chromatography-mass spectrometry and enantioselective gas chromatography-olfactometry. Anal. Chem 2006, 78, 883–890. [Google Scholar] [CrossRef]
- Tranchida, P.; Souza, R.; Barata, L.; Mondello, M.; Dugo, P.; Dugo, G.; Mondello, L. Analysis of macacaporanga (Aniba parviflora) leaf essential oil by using comprehensive two-dimensional gas chromatography combined with rapid-scanning quadrupole mass spectrometry. Chromatogr. Today 2008, 1, 5–9. [Google Scholar]
- Andrade, E.H.A.; Zoghbi, M.G.B.; Maia, J.G.S. Volatiles from Aniba terminalis Ducke. J. Essent. Oil Res. 2003, 15, 81–82. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Sousa, P.J.C.; Andrade, E.H.A.; Maia, J.G.S. Antioxidant capacity and cytotoxicity of essential oil and methanol extract of Aniba canelilla (H.B.K.) Mez. J. Agric. Food Chem. 2007, 55, 9422–9426. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Zoghbi, M.G.B.; Andrade, E.H.A. Plantas Aromáticas na Amazônia e Seus Óleos Essenciais; Museu Paraense Emilio Goeldi: Belém, Brazil, 2001. [Google Scholar]
- Souza, F.J.C., Jr.; Luz-Moraes, D.; Pereira, F.S.; Barros, M.A.; Fernandes, L.M.P.; Queiroz, L.Y.; Maia, C.F.; Maia, J.G.S.; Fontes-Junior, E.A. Aniba canelilla (Kunth) Mez (Lauraceae): A review of ethnobotany, phytochemical, antioxidant, anti-inflammatory, cardiovascular, and neurological properties. Front. Pharmacol. 2020, 11, 699. [Google Scholar] [CrossRef]
- Brito, T.S.; Lima, F.J.B.; Aragão, K.S.; De Siqueira, R.J.B.; Sousa, P.J.C.; Maia, J.G.S.; Filho, J.D.; Lahlou, S.; Magalhães, P.J.C. The vasorelaxant effects of 1-nitro-2-phenylethane involve stimulation of the soluble guanylate cyclase-cGMP pathway. Biochem. Pharmacol. 2013, 85, 780–788. [Google Scholar] [CrossRef] [PubMed]
- De Siqueira, R.J.B.; Macedo, F.I.B.; Interaminense, L.F.L.; Duarte, G.P.; Magalhães, P.J.C.; Brito, T.S.; Da Silva, J.K.R.; Maia, J.G.S.; Sousa, P.J.C.; Leal-Cardoso, J.H.; et al. 1-Nitro-2-phenylethane, the main constituent of the essential oil of Aniba canelilla, elicits a vago-vagal bradycardiac and depressor reflex in normotensive rats. Eur. J. Pharmacol. 2010, 638, 90–98. [Google Scholar] [CrossRef]
- Interaminense, L.F.L.; De Siqueira, R.J.B.; Xavier, F.E.; Duarte, G.P.; Magalhães, P.J.C.; Da Silva, J.K.; Maia, J.G.S.; Sousa, P.J.C.; Leal-Cardoso, J.H.; Lahlou, S. Cardiovascular effects of 1-nitro-2-phenylethane, the main constituent of the essential oil of Aniba canelilla, in spontaneously hypertensive rats. Fundam. Clin. Pharmacol. 2011, 25, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Interaminense, L.F.L.; Ramos-Alves, F.E.; De Siqueira, R.J.B.; Xavier, F.E.; Duarte, G.P.; Magalhães, P.J.C.; Maia, J.G.S.; Sousa, P.J.C.; Lahlou, S. Vasorelaxant effects of 1-nitro-2-phenylethane, the main constituent of the essential oil of Aniba canelilla, in superior mesenteric arteries from spontaneously hypertensive rats. Eur. J. Pharm. Sci. 2013, 48, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Magalhães, P.J.C.; de Siqueira, R.J.B.; Figueiredo, A.F.; Interaminense, L.F.L.; Maia, J.G.S.; Sousa, P.J.C. Cardiovascular effects of the essential oil of Aniba canelilla bark in normotensive rats. J. Cardiovasc. Pharmacol. 2005, 46, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, É.R.Q.; Maia, C.S.F.; Fontes, E.A., Jr.; Melo, A.S.; Pinheiro, B.G.; Maia, J.G.S. Linalool-rich essential oils from the Amazon display antidepressant-type effect in rodents. J. Ethnopharmacol. 2018, 212, 43–49. [Google Scholar] [CrossRef]
- Baldisserotto, B.; Barata, L.E.S.; Silva, A.S.; Lobato, W.F.F.; Silva, L.L.; Toni, C.; Silva, L.V.F. Anesthesia of tambaqui Colossoma macropomum (Characiformes: Serrasalmidae) with the essential oils of Aniba rosaeodora and Aniba parviflora and their major compound, linalool. Neotrop. Ichthyol. 2018, 1, 16. [Google Scholar] [CrossRef]
- De Almeida, R.N.; Araújo, D.A.M.; Gonçalves, J.C.R.; Montenegro, F.C.; De Sousa, D.P.; Leite, J.R.; Mattei, R.; Benedito, M.A.C.; De Carvalho, J.G.B.; Cruz, J.S.; et al. Rosewood oil induces sedation and inhibits compound action potential in rodents. J. Ethnopharmacol. 2009, 124, 440–443. [Google Scholar] [CrossRef]
- De Moura, V.M.; Da Costa Guimarães, N.; Batista, L.T.; Freitas-de-Sousa, L.A.; De Sousa Martins, J.; De Souza, M.C.S.; de Almeida, O.P.D.; Monteiro, W.M.; De Oliveira, R.B.; Dos-Santos, M.C.; et al. Assessment of the anti-snakebite properties of extracts of Aniba fragrans Ducke (Lauraceae) used in folk medicine as complementary treatment in cases of envenomation by Bothrops atrox. J. Ethnopharmacol. 2018, 213, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.J.G. Modelling the known and unknown plant biodiversity of the Amazon Basin. J. Biogeogr. 2007, 34, 1400–1411. [Google Scholar] [CrossRef]
- Kubitzki, K.; Renner, S. Lauraceae I (Aniba and Aiouea). Flora Neotrop. 1982, 31, 1–124. [Google Scholar]
- Matta, A.; Carvalho, R.B.; Vicentini, A. Aniba inaequabilis (Lauraceae), a new species from Peru. Phytotaxa 2016, 282, 139. [Google Scholar] [CrossRef]
- Van der Werff, H. A key to the genera of Lauraceae in the new world. Ann. Mo. Bot. Gard. 1991, 78, 377. [Google Scholar] [CrossRef]
- Franciscon, C.H.; Miranda, I.S. Distribution and conservation of Aniba Aubl. (Lauraceae Jussieu) species in Brazil. Biota Neotrop. 2018, 18, 362. [Google Scholar] [CrossRef]
- Hwang, S.W.; Kobayashi, K.; Zhai, S.; Sugiyama, J. Automated identification of Lauraceae by scale-invariant feature transform. J. Wood Sci. 2018, 64, 69–77. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Gregory, T.R. The promise of DNA barcoding for taxonomy. Syst. Biol. 2005, 54, 852–859. [Google Scholar] [CrossRef]
- Kress, W.J.; Erickson, D.L. A two-locus global DNA barcode for land plants: The coding RBCL gene complements the non-coding trnH-psbA spacer region. PLoS ONE 2007, 2, e508. [Google Scholar] [CrossRef] [PubMed]
- Shneyer, V.S.; Rodionov, A.V. Plant DNA barcodes. Biol. Bull. Rev. 2019, 9, 295–300. [Google Scholar] [CrossRef]
- Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S.; van der Bank, M.; Chase, M.W.; Cowan, R.S.; Erickson, D.L.; Fazekas, A.J.; et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef]
- Liu, Z.F.; Ci, X.Q.; Li, L.; Li, H.W.; Conran, J.G.; Li, J. DNA barcoding evaluation and implications for phylogenetic relationships in Lauraceae from China. PLoS ONE 2017, 12, e0175788. [Google Scholar] [CrossRef]
- Zhang, M.; Yahara, T.; Tagane, S.; Rueangruea, S.; Suddee, S.; Moritsuka, E.; Suyama, Y. Cryptocarya kaengkrachanensis, a new species of Lauraceae from Kaeng Krachan National Park, southwest Thailand. PhytoKeys 2020, 140, 139–157. [Google Scholar] [CrossRef]
- Packer, L.; Gibbs, J.; Sheffield, C.; Hanner, R. DNA barcoding and the mediocrity of morphology. Mol. Ecol. Resour. 2009, 9, 42–50. [Google Scholar] [CrossRef]
- Wang, F.H.; Lu, J.M.; Wen, J.; Ebihara, A.; Li, D.Z. Applying DNA Barcodes to Identify Closely Related Species of Ferns: A Case Study of the Chinese Adiantum (Pteridaceae). PLoS ONE 2016, 11, e0160611. [Google Scholar] [CrossRef]
- Mondello, L. FFNSC 2: Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Sarrazin, S.; Oliveira, R.; Maia, J.; Mourão, R. Antibacterial activity of the rosewood (Aniba rosaeodora and A. parviflora) linalool-rich oils from the Amazon. Eur. J. Med. Plants 2016, 12, 1–9. [Google Scholar] [CrossRef]
- Souza, R.C.Z.; Eiras, M.M.; Cabral, E.C.; Barata, L.E.S.; Eberlin, M.N.; Catharino, R.R. The famous amazonian rosewood essential oil: Characterization and adulteration monitoring by electrospray ionization mass spectrometry fingerprinting. Anal. Lett. 2011, 44, 2417–2422. [Google Scholar] [CrossRef]
- Zoghbi, M.G.B.; Ohashi, S.T.; Salomão, R.P.; Guilhon, G.M.S.P. Chemical variability of Aniba rosaeodora oils. Glob. J. Sci. Front. Res. B Chem. 2015, 15, 780. [Google Scholar]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Kress, W.J.; Erickson, D.L.; Jones, F.A.; Swenson, N.G.; Perez, R.; Sanjur, O.; Bermingham, E. Plant DNA barcodes and a community phylogeny of a tropical forest dynamics plot in Panama. Proc. Natl. Acad. Sci. USA 2009, 106, 18621–18626. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Xu, C.; Li, C.; Sun, J.; Zuo, Y.; Shi, S.; Cheng, T.; Guo, J.; Zhou, S. Ycf1, the most promising plastid DNA barcode of land plants. Sci. Rep. 2015, 5, 8348. [Google Scholar] [CrossRef]
- Kang, Y.; Deng, Z.; Zang, R.; Long, W. DNA barcoding analysis and phylogenetic relationships of tree species in tropical cloud forests. Sci. Rep. 2017, 7, 12564. [Google Scholar] [CrossRef] [PubMed]
- Kress, W.J.; Wurdack, K.J.; Zimmer, E.A.; Weigt, L.A.; Janzen, D.H. Use of DNA barcodes to identify flowering plants. Proc. Natl. Acad. Sci. USA 2005, 102, 8369–8374. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Maya, M.E.; Barrientos-Priego, A.F.; Zelaya-Molina, L.X.; Rodríguez-de la O, J.L.; Reyes-Alemán, J.C. Phylogenetic analysis of some members of the subgenus Persea (Persea, Lauraceae). Rev. Chapingo Ser. Hortic. 2018, 24, 133–150. [Google Scholar] [CrossRef]
- Trofimov, D.; De Moraes, P.L.R.; Rohwer, J.G. Towards a phylogenetic classification of the Ocotea complex (Lauraceae): Classification principles and reinstatement of Mespilodaphne. Bot. J. Linn. Soc. 2019, 190, 25–50. [Google Scholar] [CrossRef]
- Zhao, M.-L.; Song, Y.; Ni, J.; Yao, X.; Tan, Y.-H.; Xu, Z.-F. Comparative chloroplast genomics and phylogenetics of nine Lindera species (Lauraceae). Sci. Rep. 2018, 8, 8844. [Google Scholar] [CrossRef]
- Roy, S.; Tyagi, A.; Shukla, V.; Kumar, A.; Singh, U.M.; Chaudhary, L.B.; Datt, B.; Bag, S.K.; Singh, P.K.; Nair, N.K.; et al. Universal plant DNA barcode loci may not work in complex groups: A case study with Indian Berberis species. PLoS ONE 2010, 5, e13674. [Google Scholar] [CrossRef]
- Yan, H.F.; Hao, G.; Hu, C.M.; Ge, X.J. DNA barcoding in closely related species: A case study of Primula, L. sect. Proliferae Pax (Primulaceae) in China. J. Syst. Evol. 2011, 49, 225–236. [Google Scholar] [CrossRef]
- Hollingsworth, P.M.; Graham, S.W.; Little, D.P. Choosing and using a plant DNA barcode. PLoS ONE 2011, 6, e19254. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A.; Baraloto, C.; Engel, J.; Mori, S.A.; Pétronelli, P.; Riéra, B.; Roger, A.; Thébaud, C.; Chave, J. Identification of Amazonian trees with DNA barcodes. PLoS ONE 2009, 4, e7483. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, S.L.; Song, J.Y.; Zang, S.I.; Chen, K.L. Application of deoxyribonucleic acid barcoding in Lauraceae plants. Pharmacogn. Mag. 2012, 8, 4. [Google Scholar] [CrossRef]
- Nie, Z.L.; Wen, J.; Sun, H. Phylogeny and biogeography of Sassafras (Lauraceae) disjunct between eastern Asia and eastern North America. Plant. Syst. Evol. 2007, 267, 191–203. [Google Scholar] [CrossRef]
- Tautz, D.; Arctander, P.; Minelli, A.; Thomas, R.H.; Vogler, A.P. A plea for DNA taxonomy. Trends Ecol. Evol. 2003, 18, 70–74. [Google Scholar] [CrossRef]
- Newmaster, S.G.; Fazekas, A.J.; Ragupathy, S. DNA barcoding in land plants: Evaluation of rbcL in a multigene tiered approach. Can. J. Bot. 2006, 84, 335–341. [Google Scholar] [CrossRef]
- Rohwer, J.G. Toward a phylogenetic classification of the Lauraceae: Evidence from matK sequences. Syst. Bot. 2000, 25, 60. [Google Scholar] [CrossRef]
- Giovino, A.; Martinelli, F.; Perrone, A. The technique of plant DNA barcoding: Potential application in floriculture. Caryologia. Int. J. Cytol. Cytosyst. Cytogenet. 2020, 73, 27–38. [Google Scholar] [CrossRef]
- Lv, Y.N.; Yang, C.Y.; Shi, L.C.; Zhang, Z.L.; Xu, A.S.; Zhang, L.X.; Li, X.L.; Li, H.T. Identification of medicinal plants within the Apocynaceae family using ITS2 and psbA-trnH barcodes. Chin. J. Nat. Med. 2020, 18, 594–605. [Google Scholar] [CrossRef]
- Feng, S.; Jiao, K.; Zhu, Y.; Wang, H.; Jiang, M.; Wang, H. Molecular identification of species of Physalis (Solanaceae) using a candidate DNA barcode: The chloroplast psbA-trnH intergenic region. Genome 2018, 61, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Liu, J.; Yu, J.; Wang, L.; Zhou, S. Highly variable chloroplast markers for evaluating plant phylogeny at low taxonomic levels and for DNA barcoding. PLoS ONE 2012, 7, e35071. [Google Scholar] [CrossRef]
- Mahadani, P.; Ghosh, S.K. Utility of indels for species-level identification of a biologically complex plant group: A study with intergenic spacer in Citrus. Mol. Biol. Rep. 2014, 41, 7217–7222. [Google Scholar] [CrossRef]
- Wu, F.; Li, M.; Liao, B.; Shi, X.; Xu, Y. DNA barcoding analysis and phylogenetic relation of mangroves in Guangdong Province, China. Forests 2019, 10, 56. [Google Scholar] [CrossRef]
- Amazonas, D.R.; Oliveira, C.; Barata, L.E.S.; Tepe, E.J.; Kato, M.J.; Mourão, R.H.V.; Yamaguchi, L.F. Chemical and genotypic variations in Aniba rosiodora from the Brazilian Amazon Forest. Molecules 2020, 26, 69. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, O.R.; Kubitzki, K. Chemosystematics of Aniba. Biochem. Syst. Ecol. 1981, 9, 5–12. [Google Scholar] [CrossRef]
- Da Silva, J.; da Trindade, R.; Moreira, E.; Maia, J.; Dosoky, N.; Miller, R.; Cseke, L.; Setzer, W. Chemical diversity, biological activity, and genetic aspects of three Ocotea species from the Amazon. Int. J. Mol. Sci. 2017, 18, 1081. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, A11, 463–471. [Google Scholar] [CrossRef]
- Lias, S.G.; Mikaia, A.I.; Sparkman, O.D.; Stein, S.E.; Zaikin, G. The NIST/EPA/NIH Mass Spectral Database: Simultaneous Control of Quality and Quantity. 1997. Available online: https://www.nist.gov/publications/nistepanih-mass-spectral-database-simultaneous-control-quality-and-quantity (accessed on 26 February 2021).
- Tate, J.A.; Simpson, B.B. Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Syst. Bot. 2003, 28, 723–737. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Sang, T.; Crawford, D.J.; Stuessy, T.F. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am. J. Bot. 1997, 84, 1120–1136. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree. Version 1.4.4. 2018. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 20 December 2020).
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).