Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics
Abstract
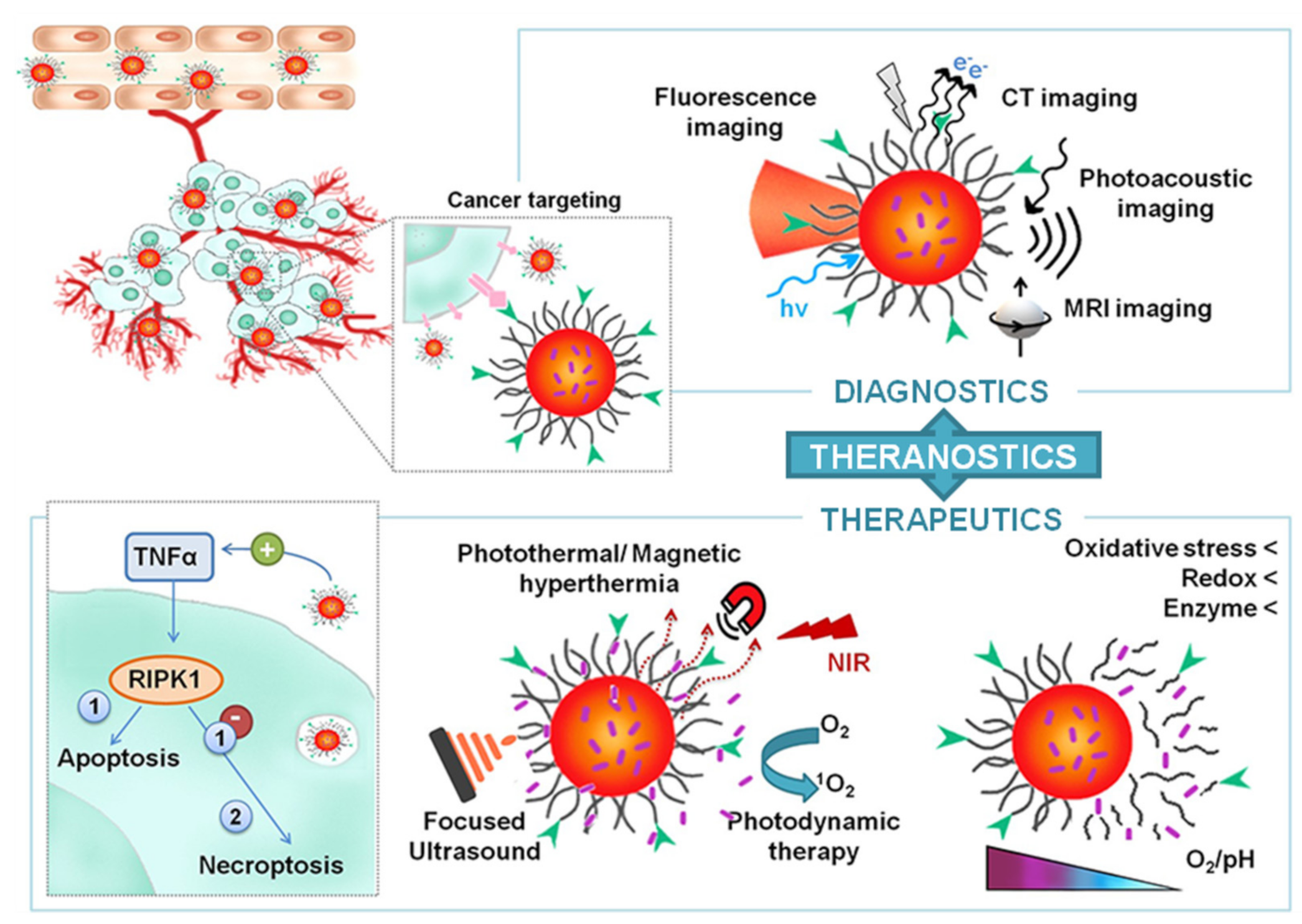
1. Introduction
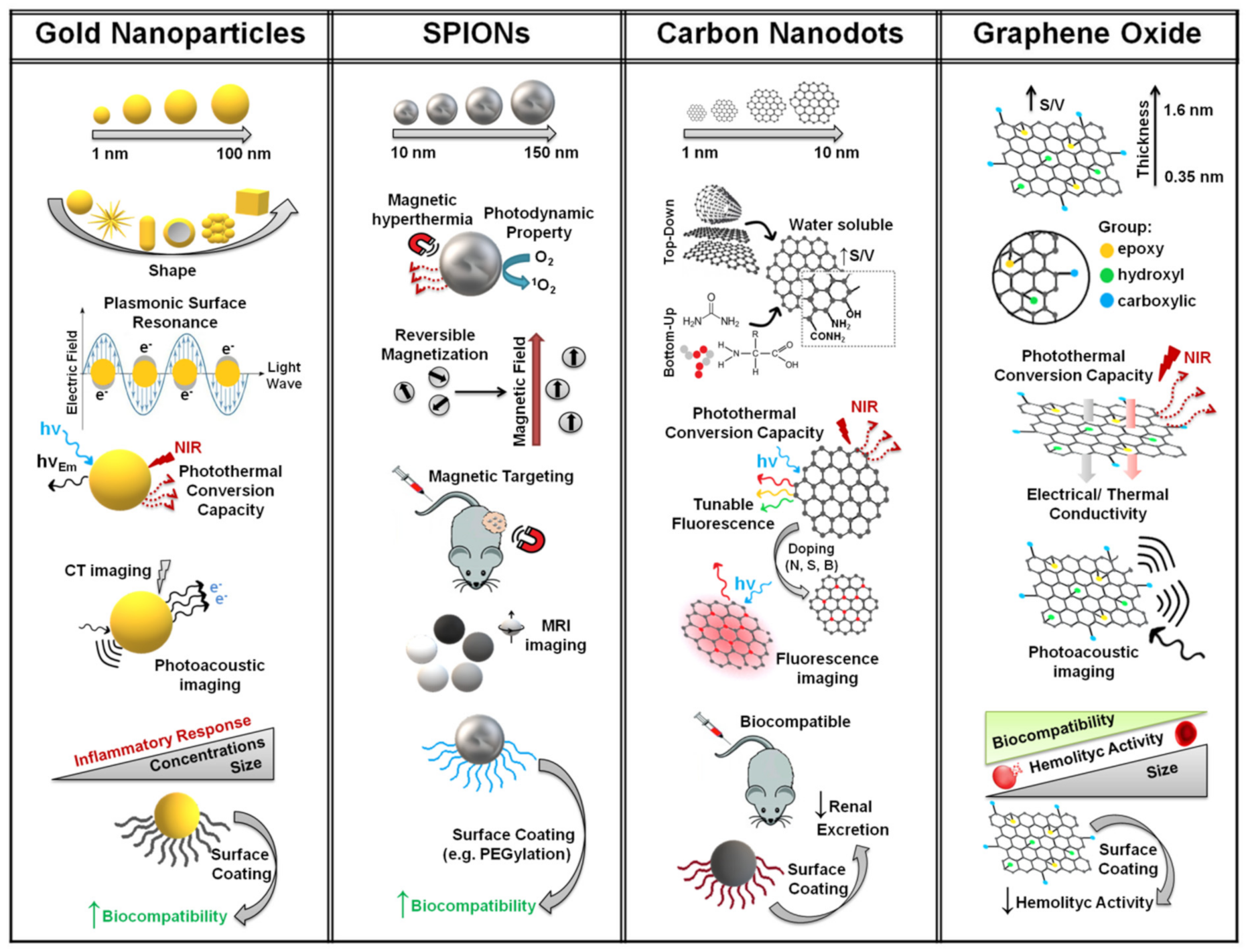
2. Synthesis and Properties of Nano-sized Particles with Theranostic Properties
2.1. Gold Nanoparticles (GNPs)
2.1.1. Bio-Imaging
2.1.2. Image-Guided Phototherapy (IG-PT)
2.2. Superparamagnetic Iron Oxide Nanoparticles (SPIONs)
2.2.1. Magnetic Targeting by Superparamagnetism
2.2.2. Magnetic Resonance Imaging (MRI)
2.2.3. Direct Magnetic Imaging and Cancer Ablation
| Nanoplatform | Functionalization | Theranostic Approach | Use | Outcome | Ref. |
|---|---|---|---|---|---|
| GNRs of 34 nm and 3.7 aspect ratio | SH coupling of MeO-PEG-SH and surface coating of photoactives | IG-PT (FRET-PT/PD) | Squamous cell carcinoma | 95% tumor reduction under the guide of NIR imaging | [88] |
| GNRs of 40 nm and 3.5 aspect ratio | SH coupling of MeO-PEG-SH | IG-PT (CT-PT) | Breast cancer | ~2-fold higher CT contrast, blood half-lives of ~17 h, ~7% ID/g tumor accumulation and total ablation of tumor mass | [89] |
| Silica-GNPs hybrids of 18 nm | NH2 surface functionalization and amide coupling with ICG | IG-PT (PA/FL-PT) | Skin cancer | Blood half-lives of ~24 h, accumulation in different organs and total remission of tumor mass under the guide of PA/FL imaging | [91] |
| SPIONs of 11 nm | Suface coating with PEI, PEG and folic acid | Magnetic targeting (MRI-DDS) | Breast cancer | MRI contrast, ~6.9-fold higher drug accumulation in tumors and total remission of tumor mass | [112] |
| SPIONs (γ-Fe2O3 and Fe3O4) of 9 nm | PEG surface coating | MRI | Solid tumors | MRI contrast ~4-fold higher than commercial counterpart | [121] |
| SPIONs-GNPs hybrids of 23 nm | Au surface deposition and folic acid coupling | IG-PT (MRI/CT-PT) | Colorectal cancer | Total remission of tumor mass under the guide of MRI, high spatial resolution MRI of the tumor | [135] |
| C3N4 CDs of 5 nm with red emission | Surface amide coupling with NH2-PEG-Biotin | IG-PT (FL/Chemio-PT) | Breast cancer | Selective accumulation in tumor cells and 98% tumor reduction under the guide of FL | [21] |
| CDs of 5 nm | Surface amide coupling with NH2-PEG-Folate | IG-PT (FL/PD-PT) | Cervical cancer | Accumulation in tumors and reduction of mouse mortality under the guide of FL | [138] |
| Cu-doped CDs of 3 nm | Non-passivated | IG-PT (FL/PD-PT) | Melanoma | Blood half-lives of ~14 d, ~8% dose accumulation in tumor, total remission of tumors under the guidance of FL imaging | [139] |
| GO of 470 nm and 1 nm in thickness | Surface amide coupling of NH2-PEG-OMe | IG-PT (FL/PD-PT) | Metastatic breast cancer | Accumulation in tumor, spleen and liver, 75% tumor inhibition under NIR exposure | [140] |
| GO-SPIONs hybrids of 174 nm | Surface functionalization with SPIONs-NH2 via amidic coupling | IG-PT (MRI/PT) | Solid tumors | Improved T2 weighted MRI contrast and high NIR photothermal potential | [141] |
| GO-SPIONs hybrids of 50 nm | In situ surface seeding of SPIONs and PEG coupling | IG-PT (PA/MRI-PT) | Metastatic breast cancer | 5% dose accumulation in tumor after 48 h, combined PA and MRI contrast, total remission of tumor mass under the guide of PA and MRI | [142] |
2.3. Carbon Nanodots (CDs)
2.3.1. Optical Properties
2.3.2. Bioimaging and Biosensing by Carbon Nanodots
2.3.3. Image-Guided Photothermal/Photodynamic Therapy (IG-PTT/PDT)
2.4. Graphene Oxide (GO)
2.4.1. Biocompatibility of Graphene Oxide: An Open Debate!
2.4.2. Biosensing
2.4.3. Image-Guided Photothermal Therapy (PTT) Using Graphene Oxide
3. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Shanbhag, P.P.; Jog, S.V.; Chogale, M.M.; Gaikwad, S.S. Theranostics for Cancer Therapy. Curr. Drug Deliv. 2013, 10, 357–362. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Mukherjee, A.; Mukherjee, S. Recent trends of the bio-inspired nanoparticles in cancer theranostics. Front. Pharmacol. 2019, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.D.; Ku, S.H.; Won, Y.Y.; Kim, S.H.; Kwon, I.C. Targeted nanotheranostics for future personalized medicine: Recent progress in cancer therapy. Theranostics 2016, 6, 1362–1377. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, J.; Yoon, J.; Chen, X. Cancer-Associated, Stimuli-Driven, Turn on Theranostics for Multimodality Imaging and Therapy. Adv. Mater. 2017, 29, 1606857. [Google Scholar] [CrossRef] [PubMed]
- Godin, B.; Tasciotti, E.; Liu, X.; Serda, R.E.; Ferrari, M. Multistage nanovectors: From concept to novel imaging contrast agents and therapeutics. Acc. Chem. Res. 2011, 44, 979–989. [Google Scholar] [CrossRef]
- Hong, H.; Wang, F.; Zhang, Y.; Graves, S.A.; Eddine, S.B.Z.; Yang, Y.; Theuer, C.P.; Nickles, R.J.; Wang, X.; Cai, W. Red fluorescent zinc oxide nanoparticle: A novel platform for cancer targeting. ACS Appl. Mater. Interfaces 2015, 7, 3373–3381. [Google Scholar] [CrossRef]
- Sudhagar, S.; Sathya, S.; Pandian, K.; Lakshmi, B.S. Targeting and sensing cancer cells with ZnO nanoprobes in vitro. Biotechnol. Lett. 2011, 33, 1891–1896. [Google Scholar] [CrossRef]
- Boca-Farcau, S.; Potara, M.; Simon, T.; Juhem, A.; Baldeck, P.; Astilean, S. Folic acid-conjugated, SERS-labeled silver nanotriangles for multimodal detection and targeted photothermal treatment on human ovarian cancer cells. Mol. Pharm. 2014, 11, 391–399. [Google Scholar] [CrossRef]
- Li, Y.; Chang, Y.; Lian, X.; Zhou, L.; Yu, Z.; Wang, H.; An, F. Silver nanoparticles for enhanced cancer theranostics: In vitro and in vivo perspectives. J. Biomed. Nanotechnol. 2018, 14, 1515–1542. [Google Scholar] [CrossRef]
- Armanetti, P.; Pocoví-Martínez, S.; Flori, A.; Avigo, C.; Cassano, D.; Menichetti, L.; Voliani, V. Dual photoacoustic/ultrasound multi-parametric imaging from passion fruit-like nano-architectures. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1787–1795. [Google Scholar] [CrossRef]
- Voliani, V.; Ricci, F.; Signore, G.; Nifosì, R.; Luin, S.; Beltram, F. Drug Delivery: Multiphoton Molecular Photorelease in Click-Chemistry-Functionalized Gold Nanoparticles (Small 23/2011). Small 2011, 7, 3270. [Google Scholar] [CrossRef]
- Puleio, R.; Licciardi, M.; Varvarà, P.; Scialabba, C.; Cassata, G.; Cicero, L.; Cavallaro, G.; Giammona, G. Effect of actively targeted copolymer coating on solid tumors eradication by gold nanorods-induced hyperthermia. Int. J. Pharm. 2020, 587. [Google Scholar] [CrossRef]
- Mauro, N.; Scialabba, C.; Puleio, R.; Varvarà, P.; Licciardi, M.; Cavallaro, G.; Giammona, G. SPIONs embedded in polyamino acid nanogels to synergistically treat tumor microenvironment and breast cancer cells. Int. J. Pharm. 2019, 555, 207–219. [Google Scholar] [CrossRef]
- Scialabba, C.; Licciardi, M.; Mauro, N.; Rocco, F.; Ceruti, M.; Giammona, G. Inulin-based polymer coated SPIONs as potential drug delivery systems for targeted cancer therapy. Eur. J. Pharm. Biopharm. 2014, 88. [Google Scholar] [CrossRef]
- Mauro, N.; Li Volsi, A.; Scialabba, C.; Licciardi, M.; Cavallaro, G.; Giammona, G. Photothermal Ablation of Cancer Cells Using Folate-Coated Gold/ Graphene Oxide Composite. Curr. Drug Deliv. 2017, 14, 433–443. [Google Scholar] [CrossRef]
- Golubewa, L.; Timoshchenko, I.; Romanov, O.; Karpicz, R.; Kulahava, T.; Rutkauskas, D.; Shuba, M.; Dementjev, A.; Svirko, Y.; Kuzhir, P. Single-walled carbon nanotubes as a photo-thermo-acoustic cancer theranostic agent: Theory and proof of the concept experiment. Sci. Rep. 2020, 10, 22174. [Google Scholar] [CrossRef]
- Augustine, S.; Singh, J.; Srivastava, M.; Sharma, M.; Das, A.; Malhotra, B.D. Recent advances in carbon based nanosystems for cancer theranostics. Biomater. Sci. 2017, 5, 901–952. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, L.; Liu, Y.; Chen, C. Applications of functionalized fullerenes in tumor theranostics. Theranostics 2012, 2, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, L.; Gao, J.; Liu, Y.; Zhang, J.; Ma, R.; Liu, R.; Zhang, Z. A fullerene-based multi-functional nanoplatform for cancer theranostic applications. Biomaterials 2014, 35, 5771–5784. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Utzeri, M.A.; Buscarino, G.; Sciortino, A.; Messina, F.; Cavallaro, G.; Giammona, G. Pressure-Dependent Tuning of Photoluminescence and Size Distribution of Carbon Nanodots for Theranostic Anticancer Applications. Materials 2020, 13, 4899. [Google Scholar] [CrossRef] [PubMed]
- Scialabba, C.; Sciortino, A.; Messina, F.; Buscarino, G.; Cannas, M.; Roscigno, G.; Condorelli, G.; Cavallaro, G.; Giammona, G.; Mauro, N. Highly Homogeneous Biotinylated Carbon Nanodots: Red-Emitting Nanoheaters as Theranostic Agents toward Precision Cancer Medicine. ACS Appl. Mater. Interfaces 2019, 11, 19854–19866. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Utzeri, M.A.; Drago, S.E.; Buscarino, G.; Cavallaro, G.; Giammona, G. Carbon nanodots as functional excipient to develop highly stable and smart PLGA nanoparticles useful in cancer theranostics. Pharmaceutics 2020, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Drago, S.E.; Cavallaro, G.; Giammona, G. Near-Infrared, Light-Triggered, On-Demand Anti-inflammatories and Antibiotics Release by Graphene Oxide/Elecrospun PCL Patch for Wound Healing. C 2019, 5, 63. [Google Scholar] [CrossRef]
- Mauro, N.; Scialabba, C.; Agnello, S.; Cavallaro, G.; Giammona, G. Folic acid-functionalized graphene oxide nanosheets via plasma etching as a platform to combine NIR anticancer phototherapy and targeted drug delivery. Mater. Sci. Eng. C 2020. [Google Scholar] [CrossRef]
- Skripka, A.; Karabanovas, V.; Jarockyte, G.; Marin, R.; Tam, V.; Cerruti, M.; Rotomskis, R.; Vetrone, F. Decoupling Theranostics with Rare Earth Doped Nanoparticles. Adv. Funct. Mater. 2019, 29, 1807105. [Google Scholar] [CrossRef]
- Yu, Z.; Eich, C.; Cruz, L.J. Recent Advances in Rare-Earth-Doped Nanoparticles for NIR-II Imaging and Cancer Theranostics. Front. Chem. 2020, 8, 496. [Google Scholar] [CrossRef]
- Krasia-Christoforou, T.; Georgiou, T.K. Polymeric theranostics: Using polymer-based systems for simultaneous imaging and therapy. J. Mater. Chem. B 2013, 1, 3002–3025. [Google Scholar] [CrossRef]
- Craparo, E.F.; Licciardi, M.; Conigliaro, A.; Palumbo, F.S.; Giammona, G.; Alessandro, R.; De Leo, G.; Cavallaro, G. Hepatocyte-targeted fluorescent nanoparticles based on a polyaspartamide for potential theranostic applications. Polymer 2015, 70, 257–270. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Liu, F.; Zhang, L.; Feng, L.; Yu, D.; Zhang, N. Theranostic polymeric micelles for the diagnosis and treatment of hepatocellular carcinoma. J. Biomed. Nanotechnol. 2015, 11, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchia, D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. Eur. J. Pharm. Biopharm. 2015, 97, 400–416. [Google Scholar] [CrossRef]
- Xia, Y.; Halas, N.J. Shape-controlled synthesis and surface plasmonic properties of metallic nanostructures. MRS Bull. 2005, 30, 338–343. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic iron oxide nanoparticles-current and prospective medical applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Yang, C.; Gao, M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Qu, Y.; He, F.; Yu, C.; Liang, X.; Liang, D.; Ma, L.; Zhang, Q.; Lv, J.; Wu, J. Advances on graphene-based nanomaterials for biomedical applications. Mater. Sci. Eng. C 2018. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lin, Q.; Chang, H. Recent Advances and Sensing Applications of Carbon Dots. Small Methods 2020, 4, 1900387. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Frontiers in carbon dots: Design, properties and applications. Mater. Chem. Front. 2019, 3, 2571–2601. [Google Scholar] [CrossRef]
- Isaacoff, B.P.; Brown, K.A. Progress in Top-Down Control of Bottom-Up Assembly. Nano Lett. 2017, 17, 6508–6510. [Google Scholar] [CrossRef]
- Jarvis, M.; Krishnan, V.; Mitragotri, S. Nanocrystals: A perspective on translational research and clinical studies. Bioeng. Transl. Med. 2019, 4, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Han, U.B.; Lee, J.S. Bottom-up synthesis of ordered metal/oxide/metal nanodots on substrates for nanoscale resistive switching memory. Sci. Rep. 2016, 6, 25537. [Google Scholar] [CrossRef] [PubMed]
- Capek, I. Polymer decorated gold nanoparticles in nanomedicine conjugates. Adv. Colloid Interface Sci. 2017, 249, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Ding, Y. Gold nanoparticle-conjugated nanomedicine: Design, construction, and structure-efficacy relationship studies. J. Mater. Chem. B 2020, 8, 4813–4830. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.A. Electromagnetics of Metals. In Plasmonics: Fundamentals and Applications; Springer: New York, NY, USA, 2007; pp. 5–19. [Google Scholar]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.H.; Ryu, N.E.; Lim, D.J.; Park, H. Gold nanoparticles for photothermal cancer therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef]
- Jain, A.; McGaughey, A.J.H. Thermal transport by phonons and electrons in aluminum, silver, and gold from first principles. Phys. Rev. B 2016, 93, 081206. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Austin, L.A.; MacKey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef]
- Goddard, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active targeting of gold nanoparticles as cancer therapeutics. Chem. Soc. Rev. 2020, 49, 8774–8789. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chang, S.S.; Lee, C.L.; Wang, C.R.C. Gold nanorods: Electrochemical synthesis and optical properties. J. Phys. Chem. B 1997, 101, 6661–6664. [Google Scholar] [CrossRef]
- Zou, C.; Yang, B.; Bin, D.; Wang, J.; Li, S.; Yang, P.; Wang, C.; Shiraishi, Y.; Du, Y. Electrochemical synthesis of gold nanoparticles decorated flower-like graphene for high sensitivity detection of nitrite. J. Colloid Interface Sci. 2017, 488, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Foss, C.A.; Hornyak, G.L.; Stockert, J.A.; Martin, C.R. Template-synthesized nanoscopic gold particles: Optical spectra and the effects of particle size and shape. J. Phys. Chem. 1994, 98, 2963–2971. [Google Scholar] [CrossRef]
- McCaffrey, R.; Long, H.; Jin, Y.; Sanders, A.; Park, W.; Zhang, W. Template synthesis of gold nanoparticles with an organic molecular cage. J. Am. Chem. Soc. 2014, 136, 1782–1785. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. J. Phys. Chem. B 2001, 105, 4065–4067. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Khoury, R.A.; Ranasinghe, J.C.; Dikkumbura, A.S.; Hamal, P.; Kumal, R.R.; Karam, T.E.; Smith, H.T.; Haber, L.H. Monitoring the Seed-Mediated Growth of Gold Nanoparticles Using in Situ Second Harmonic Generation and Extinction Spectroscopy. J. Phys. Chem. C 2018, 122, 24400–24406. [Google Scholar] [CrossRef]
- Scarabelli, L.; Sánchez-Iglesias, A.; Pérez-Juste, J.; Liz-Marzán, L.M. A “Tips and Tricks” Practical Guide to the Synthesis of Gold Nanorods. J. Phys. Chem. Lett. 2015, 6, 4270–4279. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [PubMed]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnology 2020, 18, 172. [Google Scholar] [CrossRef]
- Grönbeck, H.; Curioni, A.; Andreoni, W. Thiols and disulfides on the Au(111) surface: The headgroup-gold interaction. J. Am. Chem. Soc. 2000, 122, 3839–3842. [Google Scholar] [CrossRef]
- Gao, J.; Huang, X.; Liu, H.; Zan, F.; Ren, J. Colloidal stability of gold nanoparticles modified with thiol compounds: Bioconjugation and application in cancer cell imaging. Langmuir 2012, 28, 4464–4471. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, H. The gold-sulfur interface at the nanoscale. Nat. Chem. 2012, 4, 443–455. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, S.; Selvakannan, P.R.; Pasricha, R.; Mandale, A.B.; Sastry, M. Investigation into the interaction between surface-bound alkylamines and gold nanoparticles. Langmuir 2003, 19, 6277–6282. [Google Scholar] [CrossRef]
- Mahato, K.; Nagpal, S.; Shah, M.A.; Srivastava, A.; Maurya, P.K.; Roy, S.; Jaiswal, A.; Singh, R.; Chandra, P. Gold nanoparticle surface engineering strategies and their applications in biomedicine and diagnostics. 3 Biotech 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xie, C.; Ren, J. Nonbleaching fluorescence of gold nanoparticles and its applications in cancer cell imaging. Anal. Chem. 2008, 80, 5951–5957. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Yang, D.H.; Moon, H.J.; Lee, J.B.; Bae, M.S.; Lee, S.C.; Lee, W.J.; Sun, I.C.; Kwon, I.K. Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials 2012, 33, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.C. The contrast formation in optical microscopy. In Handbook of Biological Confocal Microscopy, 3rd ed.; Springer: New York, NY, USA, 2006; pp. 162–206. ISBN 038725921X. [Google Scholar]
- Willets, K.A.; Wilson, A.J.; Sundaresan, V.; Joshi, P.B. Super-Resolution Imaging and Plasmonics. Chem. Rev. 2017, 117, 7538–7582. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jiang, L.; Nie, Y.; Wang, J.; Li, H.; Liu, Y.; Wang, W.; Xu, G.; Luo, X. Gold Nanobipyramids as Dual-Functional Substrates for in Situ “turn On” Analyzing Intracellular Telomerase Activity Based on Target-Triggered Plasmon-Enhanced Fluorescence. ACS Appl. Mater. Interfaces 2018, 10, 26851–26858. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Chen, J.T.; Yan, X.P. Near infrared fluorescent trypsin stabilized gold nanoclusters as surface plasmon enhanced energy transfer biosensor and in vivo cancer imaging bioprobe. Anal. Chem. 2013, 85, 3238–3245. [Google Scholar] [CrossRef]
- Wu, Y.; Ali, M.R.K.; Chen, K.; Fang, N.; El-Sayed, M.A. Gold nanoparticles in biological optical imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Qian, X.; Peng, X.H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008, 26, 83–90. [Google Scholar] [CrossRef]
- Sheppard, C.J.R. Multiphoton microscopy: A personal historical review, with some future predictions. J. Biomed. Opt. 2020, 25, 014511. [Google Scholar] [CrossRef]
- Durr, N.J.; Larson, T.; Smith, D.K.; Korgel, B.A.; Sokolov, K.; Ben-Yakar, A. Two-photon luminescence imaging of cancer cells using molecularly targeted gold nanorods. Nano Lett. 2007, 7, 941–945. [Google Scholar] [CrossRef]
- Zhao, T.; Shen, X.; Li, L.; Guan, Z.; Gao, N.; Yuan, P.; Yao, S.Q.; Xu, Q.H.; Xu, G.Q. Gold nanorods as dual photo-sensitizing and imaging agents for two-photon photodynamic therapy. Nanoscale 2012, 4, 7712–7719. [Google Scholar] [CrossRef]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Mallidi, S.; Larson, T.; Tam, J.; Joshi, P.P.; Karpiouk, A.; Sokolov, K.; Emelianov, S. Multiwavelength photoacoustic imaging and plasmon resonance coupling of gold nanoparticles for selective detection of cancer. Nano Lett. 2009, 9, 2825–2831. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Kim, C.; Cobley, C.M.; Xia, Y.; Wang, L.V. Near-infrared gold nanocages as a new class of tracers for photoacoustic sentinel lymph node mapping on a rat model. Nano Lett. 2009, 9, 183–188. [Google Scholar] [CrossRef]
- Boyer, D.; Tamarat, P.; Maali, A.; Lounis, B.; Orrit, M. Photothermal imaging of nanometer-sized metal particles among scatterers. Science 2002, 297, 1160–1163. [Google Scholar] [CrossRef]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Cole, L.E.; Ross, R.D.; Tilley, J.M.; Vargo-Gogola, T.; Roeder, R.K. Gold nanoparticles as contrast agents in X-ray imaging and computed tomography. Nanomedicine 2015, 10, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Park, J.Y.; Tung, C.H.; Kim, I.H.; Choi, Y. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 2011, 5, 1086–1094. [Google Scholar] [CrossRef]
- Von Maltzahn, G.; Park, J.H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, X.; Liu, Y.; Chen, B.; Ding, X.; Zhao, N.; Xu, F. Controlled Synthesis and Surface Engineering of Janus Chitosan-Gold Nanoparticles for Photoacoustic Imaging-Guided Synergistic Gene/Photothermal Therapy. Small 2021, 17, 2006004. [Google Scholar] [CrossRef]
- Li, C.; Mei, E.; Chen, C.; Li, Y.; Nugasur, B.; Hou, L.; Ding, X.; Hu, M.; Zhang, Y.; Su, Z.; et al. Gold-Nanobipyramid-Based Nanotheranostics for Dual-Modality Imaging-Guided Phototherapy. ACS Appl. Mater. Interfaces 2020, 12, 12541–12548. [Google Scholar] [CrossRef]
- Parchur, A.K.; Sharma, G.; Jagtap, J.M.; Gogineni, V.R.; Laviolette, P.S.; Flister, M.J.; White, S.B.; Joshi, A. Vascular Interventional Radiology-Guided Photothermal Therapy of Colorectal Cancer Liver Metastasis with Theranostic Gold Nanorods. ACS Nano 2018, 12, 6597–6611. [Google Scholar] [CrossRef]
- Licciardi, M.; Li Volsi, A.; Sardo, C.; Mauro, N.; Cavallaro, G.; Giammona, G. Inulin-Ethylenediamine Coated SPIONs Magnetoplexes: A Promising Tool for Improving siRNA Delivery. Pharm. Res. 2015, 32, 3674–3687. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Sau, S.; Kesharwani, P.; Iyer, A.K. Polyvalent Folate-Dendrimer-Coated Iron Oxide Theranostic Nanoparticles for Simultaneous Magnetic Resonance Imaging and Precise Cancer Cell Targeting. Biomacromolecules 2017, 18, 1197–1209. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Toledo, V.H.; Yoshimura, T.M.; Pereira, S.T.; Castro, C.E.; Ferreira, F.F.; Ribeiro, M.S.; Haddad, P.S. Methylene blue-covered superparamagnetic iron oxide nanoparticles combined with red light as a novel platform to fight non-local bacterial infections: A proof of concept study against Escherichia coli. J. Photochem. Photobiol. B Biol. 2020, 209, 111956. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.; Naz, F.; Rahman Phull, A.; Patil-Sen, Y.; Sen, T.; Ahmed, W. Advances in multi-functional superparamagnetic iron oxide nanoparticles in magnetic fluid hyperthermia for medical applications. In Advances in Medical and Surgical Engineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 333–345. [Google Scholar]
- Sharma, S.K.; Shrivastava, N.; Rossi, F.; Tung, L.D.; Thanh, N.T.K. Nanoparticles-based magnetic and photo induced hyperthermia for cancer treatment. Nano Today 2019, 29, 100795. [Google Scholar] [CrossRef]
- Cosgrove, D. Ultrasound contrast agents: An overview. Eur. J. Radiol. 2006, 60, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Sciallero, C.; Balbi, L.; Paradossi, G.; Trucco, A. Magnetic resonance and ultrasound contrast imaging of polymer-shelled microbubbles loaded with iron oxide nanoparticles. R. Soc. Open Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Heverhagen, J.; Knopp, M.; Schmalbrock, P.; Shapiro, J.; Shiomi, M.; Moldovan, N.I.; Ferrari, M.; Lee, S.C. Localization to atherosclerotic plaque and biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI). Biomed. Microdevices 2007, 9, 719–727. [Google Scholar] [CrossRef]
- Shabestari Khiabani, S.; Farshbaf, M.; Akbarzadeh, A.; Davaran, S. Magnetic nanoparticles: Preparation methods, applications in cancer diagnosis and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2017, 45, 6–17. [Google Scholar] [CrossRef]
- Soares, P.I.P.; Romão, J.; Matos, R.; Silva, J.C.; Borges, J.P. Design and engineering of magneto-responsive devices for cancer theranostics: Nano to macro perspective. Prog. Mater. Sci. 2021, 116, 100742. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cho, S.H.; Seong, H. Multifunctional ultrasmall superparamagnetic iron oxide nanoparticles as a theranostic agent. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 892–902. [Google Scholar] [CrossRef]
- Koll, R.; Fruhner, L.S.; Heller, H.; Allgaier, J.; Pyckhout-Hintzen, W.; Kruteva, M.; Feoktystov, A.; Biehl, R.; Förster, S.; Weller, H. Creating a synthetic platform for the encapsulation of nanocrystals with covalently bound polymer shells. Nanoscale 2019, 11, 3905–3915. [Google Scholar] [CrossRef] [PubMed]
- Luque-Michel, E.; Imbuluzqueta, E.; Sebastián, V.; Blanco-Prieto, M.J. Clinical advances of nanocarrier-based cancer therapy and diagnostics. Expert Opin. Drug Deliv. 2017, 14, 75–92. [Google Scholar] [CrossRef]
- Fu, C.; Yang, R.M.; Wang, L.; Li, N.N.; Qi, M.; Xu, X.D.; Wei, X.H.; Jiang, X.Q.; Zhang, L.M. Surface functionalization of superparamagnetic nanoparticles by an acid-liable polysaccharide-based prodrug for combinatorial monitoring and chemotherapy of hepatocellular carcinoma. RSC Adv. 2017, 7, 41919–41928. [Google Scholar] [CrossRef]
- Pan, C.; Liu, Y.; Zhou, M.; Wang, W.; Shi, M.; Xing, M.; Liao, W. Theranostic pH-sensitive nanoparticles for highly efficient targeted delivery of doxorubicin for breast tumor treatment. Int. J. Nanomed. 2018, 13, 1119–1137. [Google Scholar] [CrossRef]
- Hajba, L.; Guttman, A. The use of magnetic nanoparticles in cancer theranostics: Toward handheld diagnostic devices. Biotechnol. Adv. 2016, 34, 354–361. [Google Scholar] [CrossRef]
- Schleich, N.; Po, C.; Jacobs, D.; Ucakar, B.; Gallez, B.; Danhier, F.; Préat, V. Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J. Control. Release 2014, 194, 82–91. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, K.; Zhang, B.; Zhao, Y. Superparamagnetic iron oxide nanoparticles conjugated with folic acid for dual target-specific drug delivery and MRI in cancer theranostics. Mater. Sci. Eng. C 2017, 70, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Mou, X.; Ali, Z.; Li, S.; He, N. Applications of magnetic nanoparticles in targeted drug delivery system. J. Nanosci. Nanotechnol. 2015, 15, 54–62. [Google Scholar] [CrossRef]
- Jensen, J.H.; Chandra, R. NMR relaxation in tissues with weak magnetic inhomogeneities. Magn. Reson. Med. 2000, 44, 144–156. [Google Scholar] [CrossRef]
- Xiao, Y.; Du, J. Superparamagnetic nanoparticles for biomedical applications. J. Mater. Chem. B 2020, 8, 354–367. [Google Scholar] [CrossRef]
- Pourtau, L.; Oliveira, H.; Thevenot, J.; Wan, Y.; Brisson, A.R.; Sandre, O.; Miraux, S.; Thiaudiere, E.; Lecommandoux, S. Antibody-functionalized magnetic polymersomes: In vivo targeting and imaging of bone metastases using high resolution MRI. Adv. Healthc. Mater. 2013, 2, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Song, L.; Chen, S.; Gao, J.; Zhao, P.; Du, J. A superparamagnetic polymersome with extremely high T2 relaxivity for MRI and cancer-targeted drug delivery. Biomaterials 2017, 114, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.J.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, C.; Li, S.; Khemtong, C.; Yang, S.G.; Tian, R.; Minna, J.D.; Brown, K.C.; Gao, J. A novel strategy for surface modification of superparamagnetic iron oxide nanoparticles for lung cancer imaging. J. Mater. Chem. 2009, 19, 6367–6372. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, B.; Xing, X.; Liu, F.; Cheng, Y.; Shi, D. Surface engineered antifouling optomagnetic SPIONs for bimodal targeted imaging of pancreatic cancer cells. Int. J. Nanomed. 2014, 9, 1601. [Google Scholar] [CrossRef]
- Smolensky, E.D.; Park, H.-Y.E.; Berquó, T.S.; Pierre, V.C. Surface functionalization of magnetic iron oxide nanoparticles for MRI applications—Effect of anchoring group and ligand exchange protocol. Contrast Media Mol. Imaging 2011, 6, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Harmon, B.V.; Takano, Y.S.; Winterford, C.M.; Gobé, G.C. The role of apoptosis in the response of cells and tumours to mild hyperthermia. Int. J. Radiat. Biol. 1991, 59, 489–501. [Google Scholar] [CrossRef]
- Webb, H.; Lubner, M.G.; Hinshaw, J.L. Thermal Ablation. Semin. Roentgenol. 2011, 46, 133–141. [Google Scholar] [CrossRef]
- Wu, L.; Mendoza-Garcia, A.; Li, Q.; Sun, S. Organic Phase Syntheses of Magnetic Nanoparticles and Their Applications. Chem. Rev. 2016, 116, 10473–10512. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic nanoparticles in cancer theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Gneveckow, U.; Taymoorian, K.; Thiesen, B.; Waldöfner, N.; Scholz, R.; Jung, K.; Jordan, A.; Wust, P.; Loening, S.A. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. Int. J. Hyperth. 2007, 23, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Krause, J.; Wlodarczyk, W.; Sander, B.; Vogl, T.; Felix, R. Effects of Magnetic Fluid Hyperthermia (MFH) on C3H mammary carcinoma in vivo. Int. J. Hyperth. 1997, 13, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Johannsen, M.; Thiesen, B.; Wust, P.; Jordan, A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth. 2010, 26, 790–795. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Di Corato, R.; Lartigue, L.; Marangon, I.; Guardia, P.; Silva, A.K.A.; Luciani, N.; Clément, O.; Flaud, P.; Singh, J.V.; et al. Heat-generating iron oxide nanocubes: Subtle “destructurators” of the tumoral microenvironment. ACS Nano 2014, 8, 4268–4283. [Google Scholar] [CrossRef]
- Hu, S.-H.; Liao, B.-J.; Chiang, C.-S.; Chen, P.-J.; Chen, I.-W.; Chen, S.-Y. Core-Shell Nanocapsules Stabilized by Single-Component Polymer and Nanoparticles for Magneto-Chemotherapy/Hyperthermia with Multiple Drugs. Adv. Mater. 2012, 24, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Weissleder, R. A clearer vision for in vivo imaging: Progress continues in the development of smaller, more penetrable probes for biological imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, Y.; Liu, T.; Chen, Q.; Zhou, X.; Wang, N.; Ma, M.; Cheng, Y.; Chen, H. Engineering graphene oxide with ultrasmall SPIONs and smart drug release for cancer theranostics. Chem. Commun. 2019, 55, 1963–1966. [Google Scholar] [CrossRef] [PubMed]
- Abed, Z.; Beik, J.; Laurent, S.; Eslahi, N.; Khani, T.; Davani, E.S.; Ghaznavi, H.; Shakeri-Zadeh, A. Iron oxide–gold core–shell nano-theranostic for magnetically targeted photothermal therapy under magnetic resonance imaging guidance. J. Cancer Res. Clin. Oncol. 2019, 145, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.T.; Cho, M.Y.; Choi, S. Gold hybrid nanoparticles for targeted phototherapy and cancer imaging Related content Diagnosis and therapy of macrophage cells using dextran-coated near-infrared responsivehollow-type gold nanoparticles. Nanotechnology 2010, 21, 105105. [Google Scholar] [CrossRef]
- Amini, S.M. Gold nanostructures absorption capacities of various energy forms for thermal therapy applications. J. Therm. Biol. 2019, 79, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, S.; Choi, M.-H.; Ryoo, S.-R.; Park, J.; Min, D.-H.; Kim, B.-S. Highly Biocompatible Carbon Nanodots for Simultaneous Bioimaging and Targeted Photodynamic Therapy In Vitro and In Vivo. Adv. Funct. Mater. 2014, 24, 5781–5789. [Google Scholar] [CrossRef]
- Guo, X.L.; Ding, Z.Y.; Deng, S.M.; Wen, C.C.; Shen, X.C.; Jiang, B.P.; Liang, H. A novel strategy of transition-metal doping to engineer absorption of carbon dots for near-infrared photothermal/photodynamic therapies. Carbon 2018, 134, 519–530. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, X.; Deng, L.; Yin, Z.; Tian, X.; Bhattacharyya, S.; Liu, H.; Luo, Y.; Luo, L. Graphene oxide activated by 980 nm laser for cascading two-photon photodynamic therapy and photothermal therapy against breast cancer. Appl. Mater. Today 2020, 20, 100665. [Google Scholar] [CrossRef]
- Chen, W.; Yi, P.; Zhang, Y.; Zhang, L.; Deng, Z.; Zhang, Z. Composites of aminodextran-coated Fe3O4 nanoparticles and graphene oxide for cellular magnetic resonance imaging. ACS Appl. Mater. Interfaces 2011, 3, 4085–4091. [Google Scholar] [CrossRef]
- Yang, K.; Hu, L.; Ma, X.; Ye, S.; Cheng, L.; Shi, X.; Li, C.; Li, Y.; Liu, Z. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 2012, 24, 1868–1872. [Google Scholar] [CrossRef]
- Mintz, K.J.; Bartoli, M.; Rovere, M.; Zhou, Y.; Hettiarachchi, S.D.; Paudyal, S.; Chen, J.; Domena, J.B.; Liyanage, P.Y.; Sampson, R.; et al. A deep investigation into the structure of carbon dots. Carbon 2021, 173, 433–447. [Google Scholar] [CrossRef]
- Boakye-Yiadom, K.O.; Kesse, S.; Opoku-Damoah, Y.; Filli, M.S.; Aquib, M.; Joelle, M.M.B.; Farooq, M.A.; Mavlyanova, R.; Raza, F.; Bavi, R.; et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm. 2019, 564, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Lee, S.-T. Carbon dots: Advances in nanocarbon applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Yuan, Y.; Chen, J.; Zhang, B.; Li, D.; Zhou, D.; Jing, P.; Xu, G.; Wang, Y.; Holá, K.; et al. In vivo theranostics with near-infrared-emitting carbon dots—Highly efficient photothermal therapy based on passive targeting after intravenous administration. Light Sci. Appl. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Liu, Z.X.; Yuan, Y.H. Carbon dots: Materials, synthesis, properties and approaches to long-wavelength and multicolor emission. J. Mater. Chem. B 2017, 5, 3794–3809. [Google Scholar] [CrossRef] [PubMed]
- Sciortino, A.; Cannizzo, A.; Messina, F. Carbon Nanodots: A Review—From the Current Understanding of the Fundamental Photophysics to the Full Control of the Optical Response. C 2018, 4, 67. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.S.; Zhang, P.; Zhou, Z.Y.; Gao, Q.Y.; Xiong, H.M. Solvent-Controlled Synthesis of Highly Luminescent Carbon Dots with a Wide Color Gamut and Narrowed Emission Peak Widths. Small 2018, 14. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Chang, H.; Won, S.; Kim, H.; Kwon, W. Biocompatible nitrogen-doped carbon dots: Synthesis, characterization, and application. J. Mater. Chem. B 2020, 8, 8935–8951. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, A.; Soriano, M.L.; Carrillo-Carrión, C.; Valcárcel, M. Semiconductor and carbon-based fluorescent nanodots: The need for consistency. Chem. Commun. 2016, 52, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Sciortino, A.; Gazzetto, M.; Buscarino, G.; Popescu, R.; Schneider, R.; Giammona, G.; Gerthsen, D.; Rohwer, E.J.; Mauro, N.; Feurer, T.; et al. Disentangling size effects and spectral inhomogeneity in carbon nanodots by ultrafast dynamical hole-burning. Nanoscale 2018, 10, 15317–15323. [Google Scholar] [CrossRef]
- Gazzetto, M.; Sciortino, A.; Nazari, M.; Rohwer, E.; Giammona, G.; Mauro, N.; Feurer, T.; Messina, F.; Cannizzo, A. Photocycle of Excitons in Nitrogen-Rich Carbon Nanodots: Implications for Photocatalysis and Photovoltaics. ACS Appl. Nano Mater. 2020. [Google Scholar] [CrossRef]
- Sciortino, A.; Mauro, N.; Buscarino, G.; Sciortino, L.; Popescu, R.; Schneider, R.; Giammona, G.; Gerthsen, D.; Cannas, M.; Messina, F. β-C3N4 Nanocrystals: Carbon Dots with Extraordinary Morphological, Structural, and Optical Homogeneity. Chem. Mater. 2018, 30, 1695–1700. [Google Scholar] [CrossRef]
- Sciortino, A.; Ferrante, F.; Mauro, N.; Buscarino, G.; Sciortino, L.; Giammona, G.; Cannas, M.; Duca, D.; Messina, F. Disclosing the emissive surface traps in green-emitting carbon nanodots. Carbon 2021, 173, 454–461. [Google Scholar] [CrossRef]
- Li, Y.; Bai, G.; Zeng, S.; Hao, J. Theranostic Carbon Dots with Innovative NIR-II Emission for in Vivo Renal-Excreted Optical Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11, 4737–4744. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M.L.; Iyer, G.; Koh, A.L.; Cheng, Z.; Ebenstein, Y.; Aharoni, A.; Keren, S.; Bentolila, L.A.; Li, J.; Rao, J.; et al. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small 2009. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhao, Z.; Liang, K.; Nan, F.; Li, Y.; Wang, J.; Ge, J.; Wang, P. Recent advances and prospects of carbon dots in cancer nanotheranostics. Mater. Chem. Front. 2020, 4, 449–471. [Google Scholar] [CrossRef]
- Roy, P.; Chen, P.C.; Periasamy, A.P.; Chen, Y.N.; Chang, H.T. Photoluminescent carbon nanodots: Synthesis, physicochemical properties and analytical applications. Mater. Today 2015, 18, 447–458. [Google Scholar] [CrossRef]
- Ðorđević, L.; Arcudi, F.; Prato, M. Preparation, functionalization and characterization of engineered carbon nanodots. Nat. Protoc. 2019. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chemie Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Qi, B.P.; Zhang, G.J.; Zhang, Z.L.; Pang, D.W. Photoluminescent properties of carbon nanodots. In Carbon Nanostructures; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 239–256. ISBN 9783319287805. [Google Scholar]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon dots: Synthesis, formation mechanism, fluorescence origin and sensing applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Shah, S.N.A.; Lin, L.; Zheng, Y.; Zhang, D.; Lin, J.M. Redox cycling of iron by carbon dot enhanced chemiluminescence: Mechanism of electron-hole induction in carbon dot. Phys. Chem. Chem. Phys. 2017, 19, 21604–21611. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, M.L.; Li, C.M.; Huang, C.Z. Fluorescent carbon dots functionalization. Adv. Colloid Interface Sci. 2019, 270, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, R.; Xie, Y.; Li, Y.; Chen, Y.; Cai, X. Sulphur-doped carbon dots as a highly efficient nano-photodynamic agent against oral squamous cell carcinoma. Cell Prolif. 2020, 53. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Sun, S.; Zhang, L.; Lu, Y.; Wu, A.; Cai, C.; Lin, H. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015. [Google Scholar] [CrossRef] [PubMed]
- Sciortino, L.; Sciortino, A.; Popescu, R.; Schneider, R.; Gerthsen, D.; Agnello, S.; Cannas, M.; Messina, F. Tailoring the Emission Color of Carbon Dots through Nitrogen-Induced Changes of Their Crystalline Structure. J. Phys. Chem. C 2018. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhai, Y.; Li, Z.; Zhu, P.; Mao, S.; Zhu, C.; Du, D.; Belfiore, L.A.; Tang, J.; Lin, Y. Red carbon dots: Optical property regulations and applications. Mater. Today 2019, 30, 52–79. [Google Scholar] [CrossRef]
- Tuerhong, M.; Xu, Y.; Yin, X.-B. Review on Carbon Dots and Their Applications. Chinese J. Anal. Chem. 2017, 45, 139–150. [Google Scholar] [CrossRef]
- O’Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Yan, X.; Qu, D.; Li, D.; Tao, F.F.; Sun, Z. Red Emissive Sulfur, Nitrogen Codoped Carbon Dots and Their Application in Ion Detection and Theraonostics. ACS Appl. Mater. Interfaces 2017, 9, 18549–18556. [Google Scholar] [CrossRef]
- Pan, L.; Sun, S.; Zhang, L.; Jiang, K.; Lin, H. Near-infrared emissive carbon dots for two-photon fluorescence bioimaging. Nanoscale 2016, 8, 17350–17356. [Google Scholar] [CrossRef] [PubMed]
- Lan, M.; Zhao, S.; Zhang, Z.; Yan, L.; Guo, L.; Niu, G.; Zhang, J.; Zhao, J.; Zhang, H.; Wang, P.; et al. Two-photon-excited near-infrared emissive carbon dots as multifunctional agents for fluorescence imaging and photothermal therapy. Nano Res. 2017, 10, 3113–3123. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Y.; Liu, S.; Wang, W.; Xie, Z.; Jing, X. One-Pot to Synthesize Multifunctional Carbon Dots for Near Infrared Fluorescence Imaging and Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2016. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, K.; Wu, X.; Lou, Q.; Sui, L.; Dong, L.; Yuan, K.; Shan, C. Lifetime-Engineered Carbon Nanodots for Time Division Duplexing. Adv. Sci. 2021, 8, 2003433. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, P.; Gao, G.; Gao, D.; Yang, Y.; Liu, H.; Wang, Y.; Gong, P.; Cai, L. Near-infrared-emitting two-dimensional codes based on lattice-strained core/(doped) shell quantum dots with long fluorescence lifetime. Adv. Mater. 2014, 26, 6313–6317. [Google Scholar] [CrossRef]
- Aiyer, S.; Prasad, R.; Kumar, M.; Nirvikar, K.; Jain, B.; Kushwaha, O.S. Fluorescent carbon nanodots for targeted in vitro cancer cell imaging. Appl. Mater. Today 2016, 4, 71–77. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, K.; Wu, Q.; Wu, J.; Zhang, C. Green Synthesis of Red-Emitting Carbon Nanodots as a Novel “Turn-on” Nanothermometer in Living Cells. Chem. A Eur. J. 2016, 22, 14475–14479. [Google Scholar] [CrossRef]
- Liu, K.; Song, S.; Sui, L.; Wu, S.; Jing, P.; Wang, R.; Li, Q.; Wu, G.; Zhang, Z.; Yuan, K.; et al. Efficient Red/Near-Infrared-Emissive Carbon Nanodots with Multiphoton Excited Upconversion Fluorescence. Adv. Sci. 2019, 6, 1900766. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Liao, G.; Liu, X.; Chen, Q.; Li, H.; Lu, L.; Zhao, P.; Yu, Z. iRGD-decorated red shift emissive carbon nanodots for tumor targeting fluorescence imaging. J. Colloid Interface Sci. 2018, 509, 515–521. [Google Scholar] [CrossRef]
- Ding, H.; Zhou, X.X.; Wei, J.S.; Li, X.B.; Qin, B.T.; Chen, X.B.; Xiong, H.M. Carbon dots with red/near-infrared emissions and their intrinsic merits for biomedical applications. Carbon 2020, 167, 322–344. [Google Scholar] [CrossRef]
- Li, D.; Jing, P.; Sun, L.; An, Y.; Shan, X.; Lu, X.; Zhou, D.; Han, D.; Shen, D.; Zhai, Y.; et al. Near-Infrared Excitation/Emission and Multiphoton-Induced Fluorescence of Carbon Dots. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Guo, Z.; Lin, Z.; Zhang, L.; Jiang, B.P.; Shen, X.C. Recent insights into near-infrared light-responsive carbon dots for bioimaging and cancer phototherapy. Inorg. Chem. Front. 2019, 6, 1116–1128. [Google Scholar] [CrossRef]
- Wu, L.; Cai, X.; Nelson, K.; Xing, W.; Xia, J.; Zhang, R.; Stacy, A.J.; Luderer, M.; Lanza, G.M.; Wang, L.V.; et al. A green synthesis of carbon nanoparticles from honey and their use in real-time photoacoustic imaging. Nano Res. 2013, 6, 312–325. [Google Scholar] [CrossRef]
- Xu, G.; Bao, X.; Chen, J.; Zhang, B.; Li, D.; Zhou, D.; Wang, X.; Liu, C.; Wang, Y.; Qu, S. In Vivo Tumor Photoacoustic Imaging and Photothermal Therapy Based on Supra-(Carbon Nanodots). Adv. Healthc. Mater. 2019, 8, 1800995. [Google Scholar] [CrossRef]
- Wu, F.; Su, H.; Cai, Y.; Wong, W.K.; Jiang, W.; Zhu, X. Porphyrin-Implanted Carbon Nanodots for Photoacoustic Imaging and in Vivo Breast Cancer Ablation. ACS Appl. Bio Mater. 2018, 1, 110–117. [Google Scholar] [CrossRef]
- Shi, X.; Meng, H.; Sun, Y.; Qu, L.; Lin, Y.; Li, Z.; Du, D. Far-Red to Near-Infrared Carbon Dots: Preparation and Applications in Biotechnology. Small 2019, 15, 1901507. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Q.; Li, Y.; Chen, W. Fluorescent C-dot nanocomposites as efficient photothermal agents and multi-modal imaging tracers. Mater. Chem. Front. 2017, 1, 538–541. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Zha, S.; Zhu, Y.; Wu, P.; Ehrenberg, B.; Chen, J.Y. Carbon nanodots featuring efficient FRET for two-photon photodynamic cancer therapy with a low fs laser power density. Biomaterials 2014, 35, 9372–9381. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, J.; Gooding, J.J. Strategies for chemical modification of graphene and applications of chemically modified graphene. J. Mater. Chem. 2012, 22, 12435–12452. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Feng, L.; Hong, H.; Cai, W.; Liu, Z. Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat. Protoc. 2013, 8, 2392–2403. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Peng, E.; Choo, E.S.G.; Chandrasekharan, P.; Yang, C.-T.; Ding, J.; Chuang, K.-H.; Xue, J.M. Synthesis of Manganese Ferrite/Graphene Oxide Nanocomposites for Biomedical Applications. Small 2012, 8, 3620–3630. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Kurup Sasikala, A.; Thomas, R.G.; Unnithan, A.R.; Saravanakumar, B.; Jeong, Y.Y.; Park, C.H.; Kim, C.S. Multifunctional Nanocarpets for Cancer Theranostics: Remotely Controlled Graphene Nanoheaters for Thermo-Chemosensitisation and Magnetic Resonance Imaging. Sci. Rep. 2016, 6, 20543. [Google Scholar] [CrossRef] [PubMed]
- Mauro, N.; Scialabba, C.; Cavallaro, G.; Licciardi, M.; Giammona, G. Biotin-containing reduced graphene oxide-based nanosystem as a multieffect anticancer agent: Combining hyperthermia with targeted chemotherapy. Biomacromolecules 2015, 16, 2766–2775. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, C.; Mauro, N.; Pitarresi, G.; Scialabba, C.; Palumbo, F.S.; Giammona, G. Double-Network-Structured Graphene Oxide-Containing Nanogels as Photothermal Agents for the Treatment of Colorectal Cancer. Biomacromolecules 2017, 18, 1010–1018. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Zhai, G. The effect of incubation conditions on the hemolytic properties of unmodified graphene oxide with various concentrations. RSC Adv. 2016, 6, 68322–68334. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Luo, N.; Weber, J.K.; Wang, S.; Luan, B.; Yue, H.; Xi, X.; Du, J.; Yang, Z.; Wei, W.; Zhou, R.; et al. PEGylated graphene oxide elicits strong immunological responses despite surface passivation. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity. Sci. Rep. 2020, 10, 2717. [Google Scholar] [CrossRef]
- Charmi, J.; Nosrati, H.; Mostafavi Amjad, J.; Mohammadkhani, R.; Danafar, H. Polyethylene glycol (PEG) decorated graphene oxide nanosheets for controlled release curcumin delivery. Heliyon 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Neklyudov, V.V.; Khafizov, N.R.; Sedov, I.A.; Dimiev, A.M. New insights into the solubility of graphene oxide in water and alcohols. Phys. Chem. Chem. Phys. 2017, 19, 17000–17008. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Wang, L.; Yu, D.; Dai, R.; Fu, D.; Li, W.; Guo, Z.; Cui, C.; Xu, J.; Shen, S.; Ma, K. PEGylated doxorubicin cloaked nano-graphene oxide for dual-responsive photochemical therapy. Int. J. Pharm. 2019. [Google Scholar] [CrossRef]
- Tiwari, H.; Karki, N.; Pal, M.; Basak, S.; Verma, R.K.; Bal, R.; Kandpal, N.D.; Bisht, G.; Sahoo, N.G. Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells. Colloids Surf. B Biointerfaces 2019. [Google Scholar] [CrossRef]
- Huang, C.; Wu, J.; Jiang, W.; Liu, R.; Li, Z.; Luan, Y. Amphiphilic prodrug-decorated graphene oxide as a multi-functional drug delivery system for efficient cancer therapy. Mater. Sci. Eng. C 2018. [Google Scholar] [CrossRef]
- Liu, C.C.; Zhao, J.J.; Zhang, R.; Li, H.; Chen, B.; Zhang, L.L.; Yang, H. Multifunctionalization of graphene and graphene oxide for controlled release and targeted delivery of anticancer drugs. Am. J. Transl. Res. 2017, 9, 5197–5219. [Google Scholar]
- Zhao, X.; Wei, Z.; Zhao, Z.; Miao, Y.; Qiu, Y.; Yang, W.; Jia, X.; Liu, Z.; Hou, H. Design and Development of Graphene Oxide Nanoparticle/Chitosan Hybrids Showing pH-Sensitive Surface Charge-Reversible Ability for Efficient Intracellular Doxorubicin Delivery. ACS Appl. Mater. Interfaces 2018, 10, 6608–6617. [Google Scholar] [CrossRef] [PubMed]
- Vinothini, K.; Rajendran, N.K.; Munusamy, M.A.; Alarfaj, A.A.; Rajan, M. Development of biotin molecule targeted cancer cell drug delivery of doxorubicin loaded κ-carrageenan grafted graphene oxide nanocarrier. Mater. Sci. Eng. C 2019. [Google Scholar] [CrossRef]
- Pan, Y.; Bao, H.; Sahoo, N.G.; Wu, T.; Li, L. Water-Soluble Poly(N -isopropylacrylamide)-Graphene Sheets Synthesized via Click Chemistry for Drug Delivery. Adv. Funct. Mater. 2011, 21, 2754–2763. [Google Scholar] [CrossRef]
- Tadyszak, K.; Wereszczyńska, B.; Gonet, M. CT, MR and EPR imaging of graphene oxide aerogels. Solid State Sci. 2020, 109, 106402. [Google Scholar] [CrossRef]
- Das, T.R.; Debata, S.; Madhuri, R.; Sharma, P.K. Graphene-based multifunctional magnetic nanocomposites and their multimode biomedical applications. In Complex Magnetic Nanostructures: Synthesis, Assembly and Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 359–392. ISBN 9783319520872. [Google Scholar]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Albert, E.L.; Che Abdullah, C.A.; Shiroshaki, Y. Synthesis and characterization of graphene oxide functionalized with magnetic nanoparticle via simple emulsion method. Results Phys. 2018, 11, 944–950. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, C.; Ran, W.; Meng, J.; Yin, Q.; Li, Y. Recent progress in light-triggered nanotheranostics for cancer treatment. Theranostics 2016, 6, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.M.; Huang, G.; Zhou, X.; Zou, J.; Yang, Y.; Chen, Y.F.; Men, S.K. Safety evaluation of graphene oxide-based magnetic nanocomposites as MRI contrast agents and drug delivery vehicles. RSC Adv. 2014, 4, 50464–50477. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Liao, K.H.; Lin, Y.S.; MacOsko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yang, S.T.; Liu, J.H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Abouei, E.; Hatamie, S.; Ghasemi, E. Accelerated differentiation of neural stem cells into neurons on ginseng-reduced graphene oxide sheets. Carbon 2014, 66, 395–406. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef]
- Zhou, R.; Gao, H. Cytotoxicity of graphene: Recent advances and future perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2014, 6, 452–474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xu, M.; Gao, M.; Zhang, Z.; Xu, Y.; Xia, T.; Liu, S. Graphene Oxide Induced Perturbation to Plasma Membrane and Cytoskeletal Meshwork Sensitize Cancer Cells to Chemotherapeutic Agents. ACS Nano 2017, 11, 2637–2651. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biocompatibility and biodegradability of 2D materials: Graphene and beyond. Chem. Commun. 2019, 55, 5540–5546. [Google Scholar] [CrossRef] [PubMed]
- Newman, L. The Structural Biodegradation of Graphene Oxide In Vivo. Ph.D. Thesis, University of Manchester, Manchester, UK, 2017. [Google Scholar]
- Kotchey, G.P.; Allen, B.L.; Vedala, H.; Yanamala, N.; Kapralov, A.A.; Tyurina, Y.Y.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. The enzymatic oxidation of graphene oxide. ACS Nano 2011, 5, 2098–2108. [Google Scholar] [CrossRef]
- Chen, S.L.; Chen, C.Y.; Hsieh, J.C.H.; Yu, Z.Y.; Cheng, S.J.; Hsieh, K.Y.; Yang, J.W.; Kumar, P.V.; Lin, S.F.; Chen, G.Y. Graphene oxide-based biosensors for liquid biopsies in cancer diagnosis. Nanomaterials 2019, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, Q.; Li, F.; Tang, L.; Gao, S.; Jiang, B.; Zhao, X.; Wang, L.; Fan, C. A graphene-based platform for fluorescent detection of SNPs. Analyst 2013, 138, 2678–2682. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Hu, D.; Lin, C.T.; Li, J.; Lin, Y. Aptamer/graphene oxide nanocomplex for in situ molecular probing in living cells. J. Am. Chem. Soc. 2010, 132, 9274–9276. [Google Scholar] [CrossRef]
- He, S.; Song, B.; Li, D.; Zhu, C.; Qi, W.; Wen, Y.; Wang, L.; Song, S.; Fang, H.; Fan, C. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv. Funct. Mater. 2010, 20, 453–459. [Google Scholar] [CrossRef]
- Luo, M.; Chen, X.; Zhou, G.; Xiang, X.; Chen, L.; Ji, X.; He, Z. Chemiluminescence biosensors for DNA detection using graphene oxide and a horseradish peroxidase-mimicking DNAzyme. Chem. Commun. 2012, 48, 1126–1128. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, P.; Bhirde, A.; Jin, A.; Ma, Y.; Niu, G.; Neamati, N.; Chen, X. A nanoscale graphene oxide–peptide biosensor for real-time specific biomarker detection on the cell surface. Chem. Commun. 2012, 48, 9768–9770. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Zeng, Y. Ultrasensitive microfluidic analysis of circulating exosomes using a nanostructured graphene oxide/polydopamine coating. Lab Chip 2016, 16, 3033–3042. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef] [PubMed]
- Calixto, G.; Bernegossi, J.; de Freitas, L.; Fontana, C.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef] [PubMed]
- Kalluru, P.; Vankayala, R.; Chiang, C.-S.; Hwang, K.C. Nano-graphene oxide-mediated In vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials 2016, 95, 1–10. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Parvez, K.; Nafiujjaman, M.; Revuri, V.; Khan, H.A.; Feng, X.; Lee, Y. Bioapplication of graphene oxide derivatives: Drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv. 2015, 5, 42141–42161. [Google Scholar] [CrossRef]
- Muñoz, R.; Singh, D.P.; Kumar, R.; Matsuda, A. Graphene Oxide for Drug Delivery and Cancer Therapy. In Nanostructured Polymer Composites for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–488. [Google Scholar]
- Tran, T.H.; Nguyen, H.T.; Pham, T.T.; Choi, J.Y.; Choi, H.G.; Yong, C.S.; Kim, J.O. Development of a Graphene Oxide Nanocarrier for Dual-Drug Chemo-phototherapy to Overcome Drug Resistance in Cancer. ACS Appl. Mater. Interfaces 2015, 7, 28647–28655. [Google Scholar] [CrossRef]
- Zeng, W.N.; Yu, Q.P.; Wang, D.; Liu, J.L.; Yang, Q.J.; Zhou, Z.K.; Zeng, Y.P. Mitochondria-targeting graphene oxide nanocomposites for fluorescence imaging-guided synergistic phototherapy of drug-resistant osteosarcoma. J. Nanobiotechnol. 2021, 19, 79. [Google Scholar] [CrossRef]
- Su, X.; Chan, C.; Shi, J.; Tsang, M.K.; Pan, Y.; Cheng, C.; Gerile, O.; Yang, M. A graphene quantum dot@Fe3O4@SiO2 based nanoprobe for drug delivery sensing and dual-modal fluorescence and MRI imaging in cancer cells. Biosens. Bioelectron. 2017, 92, 489–495. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, R.; Campbell, E.; Naumov, A. Multifunctional graphene oxide/iron oxide nanoparticles for magnetic targeted drug delivery dual magnetic resonance/ fluorescence imaging and cancer sensing. PLoS ONE 2019, 14, e0217072. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Yang, K.; Zhang, Y.; Engle, J.W.; Feng, L.; Yang, Y.; Nayak, T.R.; Goel, S.; Bean, J.; Theuer, C.P.; et al. In vivo targeting and imaging of tumor vasculature with radiolabeled, antibody-conjugated nanographene. ACS Nano 2012, 6, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Song, L.; Zheng, J.; Hu, D.; He, M.; Zheng, M.; Gao, G.; Gong, P.; Zhang, P.; Ma, Y.; et al. Protein-assisted fabrication of nano-reduced graphene oxide for combined invivo photoacoustic imaging and photothermal therapy. Biomaterials 2013, 34, 5236–5243. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Shim, G.; Kim, G.; Lee, S.; Lee, H.J.; Kim, Y.B.; Byun, Y.; Oh, Y.K. Image-guided synergistic photothermal therapy using photoresponsive imaging agent-loaded graphene-based nanosheets. J. Control. Release 2015, 211, 28–36. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauro, N.; Utzeri, M.A.; Varvarà, P.; Cavallaro, G. Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics. Molecules 2021, 26, 3085. https://doi.org/10.3390/molecules26113085
Mauro N, Utzeri MA, Varvarà P, Cavallaro G. Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics. Molecules. 2021; 26(11):3085. https://doi.org/10.3390/molecules26113085
Chicago/Turabian StyleMauro, Nicolò, Mara Andrea Utzeri, Paola Varvarà, and Gennara Cavallaro. 2021. "Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics" Molecules 26, no. 11: 3085. https://doi.org/10.3390/molecules26113085
APA StyleMauro, N., Utzeri, M. A., Varvarà, P., & Cavallaro, G. (2021). Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics. Molecules, 26(11), 3085. https://doi.org/10.3390/molecules26113085








