Investigation of Structural and Luminescent Properties of Sol-Gel-Derived Cr‑Substituted Mg3Al1−xCrx Layered Double Hydroxides
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Characterisation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Arrabito, G.; Bonasera, A.; Prestopino, G.; Orsini, A.; Mattoccia, A.; Martinelli, E.; Pignataro, B.; Medaglia, P.G. Layered Double Hydroxides: A Toolbox for Chemistry and Biology. Crystals 2019, 9, 361. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. A review on the recent progress, challenges and perspective of layered double hydroxides as promising photocatalysts. J. Mater. Chem. A 2016, 4, 10744–10766. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Vieira, D.E.L.; Salak, A.N.; Ferreira, M.G.S.; Katelnikovas, A.; Kareiva, A. A comparative study of co-precipitation and sol-gel synthetic approaches to fabricate cerium-substituted Mg-Al layered double hydroxides with luminescence properties. Appl. Clay Sci. 2017, 143, 175–183. [Google Scholar] [CrossRef]
- Modrogan, C.; Caprarescu, S.; Dancila, A.M.; Orbulet, O.D.; Vasile, E.; Purcar, V. Mixed oxide-layered double hydroxides materials: Synthesis, characterization and efficient application for Mn2+ removal from synthetic wastewater. Materials 2020, 13, 4089. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhao, Z.; Song, Y.; Xu, M.; Li, J.; Yao, L. A novel heat-treated humic acid/MgAl-layered double hydroxide composite for efficient removal of cadmium: Fabrication, performance and mechanisms. Appl. Clay Sci. 2020, 187, 105482. [Google Scholar] [CrossRef]
- Zhang, S.; Kano, N.; Mishima, K.; Okawa, H. Adsorption and Desorption Mechanisms of Rare Earth Elements (REEs) by Layered Double Hydroxide (LDH) Modified with Chelating Agents. Appl. Sci. 2019, 9, 4805. [Google Scholar] [CrossRef]
- Soliman, E.R.; Kotp, Y.H.; Souaya, E.R.; Guindy, K.A.; Ibrahim, R.G.M. Development the sorption behavior of nanocomposite Mg/Al LDH by chelating with different monomers. Compos. Part B Eng. 2019, 175, 107131. [Google Scholar] [CrossRef]
- Sokol, D.; Salak, A.N.; Ferreira, M.G.S.; Beganskiene, A.; Kareiva, A. Bi-substituted Mg3Al–CO3 layered double hydroxides. J. Sol-Gel Sci. Technol. 2018, 85, 221–230. [Google Scholar] [CrossRef]
- Vieira, D.E.L.; Sokol, D.; Smalenskaite, A.; Kareiva, A.; Ferreira, M.G.S.; Vieira, J.M.; Salak, A.N. Cast iron corrosion protection with chemically modified Mg-Al layered double hydroxides synthesized using a novel approach. Surf. Coat. Technol. 2019, 375, 158–163. [Google Scholar] [CrossRef]
- Valeikiene, L.; Paitian, R.; Grigoraviciute-Puroniene, I.; Ishikawa, K.; Kareiva, A. Transition metal substitution effects in sol-gel derived Mg3-xMx/Al1 (M = Mn, Co, Ni, Cu, Zn) layered double hydroxides. Mater. Chem. Phys. 2019, 237, 121863. [Google Scholar] [CrossRef]
- Szabados, M.; Adél Ádám, A.; Traj, P.; Muráth, S.; Baán, K.; Bélteky, P.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Mechanochemical and wet chemical syntheses of CaIn-layered double hydroxide and its performance in a transesterification reaction compared to those of other Ca2M(III) hydrocalumites (M: Al, Sc, V, Cr, Fe, Ga) and Mg(II)-, Ni(II)-, Co(II)- or Zn(II)-based hydrotalcites. J. Catal. 2020, 391, 282–297. [Google Scholar] [CrossRef]
- Kooli, F.; Rives, V.; Ulibarri, M.A. Preparation and Study of Decavanadate-Pillared Hydrotalcite-like Anionic Clays Containing Transition Metal Cations in the Layers. 2. Samples containing Magnesium-Chromium and Nickel-Chromium. Inorg. Chem. 1995, 34, 5122–5128. [Google Scholar] [CrossRef]
- Kooli, F.; Martín, C.; Rives, V. FT-IR Spectroscopy Study of Surface Acidity and 2-Propanol Decomposition on Mixed Oxides Obtained upon Calcination of Layered Double Hydroxides. Langmuir 1997, 13, 2303–2306. [Google Scholar] [CrossRef]
- Bouteraa, S.; Saiah, F.B.D.; Hamouda, S.; Bettahar, N. Zn-M-CO3 Layered Double Hydroxides (M=Fe, Cr, or Al): Synthesis, Characterization, and Removal of Aqueous Indigo Carmine. Bull. Chem. React. Eng. Catal. 2020, 15, 43–54. [Google Scholar] [CrossRef]
- Oktrianty, M.; Palapa, N.R.; Mohadi, R.; Lesbani, A. Effective Removal of Iron (II) from Aqueous Solution by Adsorption using Zn/Cr Layered Double Hydroxides Intercalated with Keggin Ion. J. Ecolog. Eng. 2020, 21, 63–71. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K.M. Zn–Cr layered double hydroxide: Visible light responsive photocatalyst for photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2012, 91, 73–80. [Google Scholar] [CrossRef]
- Baliarsingh, N.; Parida, K.M.; Pradhan, G.C. Effects of Co, Ni, Cu, and Zn on Photophysical and Photocatalytic Properties of Carbonate Intercalated MII/Cr LDHs for Enhanced Photodegradation of Methyl Orange. Ind. Eng. Chem. Res. 2014, 53, 3834–3841. [Google Scholar] [CrossRef]
- Tsyganok, A.I.; Inaba, M.; Tsunoda, T.; Uchida, K.; Suzuki, K.; Takehira, K.; Hayakawa, T. Rational design of Mg–Al mixed oxide-supported bimetallic catalysts for dry reforming of methane. Appl. Catal. A Gen. 2005, 292, 328–343. [Google Scholar] [CrossRef]
- Nayak, S.; Pradhan, A.C.; Parida, K.M. Topotactic Transformation of Solvated MgCr-LDH Nanosheets to Highly Efficient Porous MgO/MgCr2O4 Nanocomposite for Photocatalytic H2 Evolution. Inorg. Chem. 2018, 57, 8646–8661. [Google Scholar] [CrossRef]
- Liang, S.Y.; Ren, W.W.; Lin, W.X.; Zou, L.C.; Cui, X.P.; Chen, J.F. In-Situ Preparation of MgCr-LDH Nano-Layer on MAO Coating of Mg Alloy and Its Anti-Corrosion Mechanism. Rare Metal Mater. Eng. 2020, 49, 2830–2838. [Google Scholar]
- Gunawan, P.; Xu, R. Lanthanide-Doped Layered Double Hydroxides Intercalated with Sensitizing Anions: Efficient Energy Transfer between Host and Guest Layers. J. Phys. Chem. C 2009, 113, 17206–17214. [Google Scholar] [CrossRef]
- Domínguez, M.; Pérez-Bernal, M.E.; Ruano-Casero, R.J.; Barriga, C.; Rives, V.; Ferreira, R.A.S.; Carlos, L.D.; Rocha, J. Multiwavelength Luminescence in Lanthanide-Doped Hydrocalumite and Mayenite. Chem. Mater. 2011, 23, 1993–2004. [Google Scholar] [CrossRef]
- Posati, T.; Costantino, F.; Latterini, L.; Nocchetti, M.; Paolantoni, M.; Tarpani, L. New Insights on the Incorporation of Lanthanide Ions into Nanosized Layered Double Hydroxides. Inorg. Chem. 2012, 51, 13229–13236. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, G.; Liu, J. Tunable photoluminescence of europium-doped layered double hydroxides intercalated by coumarin-3-carboxylate. RSC Adv. 2014, 4, 7991–7997. [Google Scholar] [CrossRef]
- Gao, X.; Lei, L.; Kang, L.; Wang, Y.; Lian, Y.; Jiang, K. Synthesis, characterization and optical properties of a red organic–inorganic phosphor based on terephthalate intercalated Zn/Al/Eu layered double hydroxide. J. Alloys Compd. 2014, 585, 703–707. [Google Scholar] [CrossRef]
- Vicente, P.; Pérez-Bernal, M.E.; Ruano-Casero, R.J.; Ananias, D.; Almeida Paz, F.A.; Rocha, J.; Rives, V. Luminescence properties of lanthanide-containing layered double hydroxides. Micropor. Mesopor. Mater. 2016, 226, 209–220. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Salak, A.N.; Ferreira, M.G.S.; Skaudzius, R.; Kareiva, A. Sol-gel synthesis and characterization of hybrid inorganic-organic Tb(III)-terephthalate containing layered double hydroxides. Opt. Mater. 2018, 80, 186–196. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Salak, A.N.; Kareiva, A. Induced neodymium luminescence in sol-gel derived layered double hydroxides. Mendeleev Commun. 2018, 28, 493–494. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Pavasaryte, L.; Yang, T.C.K.; Kareiva, A. Undoped and Eu3+ Doped Magnesium-Aluminium Layered Double Hydroxides: Peculiarities of Intercalation of Organic Anions and Investigation of Luminescence Properties. Materials 2019, 12, 736. [Google Scholar] [CrossRef]
- Shirotori, M.; Nishimura, S.; Ebitani, K. Genesis of a bi-functional acid–base site on a Cr-supported layered double hydroxide catalyst surface for one-pot synthesis of furfurals from xylose with a solid acid catalyst. Catal. Sci. Technol. 2016, 6, 8200–8211. [Google Scholar] [CrossRef]
- Melo, F.; Morlanés, N. Study of the composition of ternary mixed oxides: Use of these materials on a hydrogen production process. Catal. Today 2008, 133–135, 374–382. [Google Scholar] [CrossRef]
- Rodriguez-Rivas, F.; Pastor, A.; de Miguel, G.; Cruz-Yusta, M.; Pavlovic, I.; Sánchez, L. Cr3+ substituted Zn-Al layered double hydroxides as UV–Vis light photocatalysts for NO gas removal from the urban environment. Sci. Total Environ. 2020, 706, 136009. [Google Scholar] [CrossRef]
- Correcher, V.; Garcia-Guinea, J. Cathodo- and photoluminescence emission of a natural Mg-Cr carbonate layered double hydroxide. Appl. Clay Sci. 2018, 161, 127–131. [Google Scholar] [CrossRef]
- Ishikawa, K.; Garskaite, E.; Kareiva, A. Sol–gel synthesis of calcium phosphate-based biomaterials-A review of environmentally benign, simple, and effective synthesis routes. J. Sol-Gel Sci. Technol. 2020, 94, 551–572. [Google Scholar] [CrossRef]
- Karoblis, D.; Zarkov, A.; Mazeika, K.; Baltrunas, D.; Niaura, G.; Beganskiene, A.; Kareiva, A. Sol-gel synthesis, structural, morphological and magnetic properties of BaTiO3–BiMnO3 solid solutions. Ceram. Int. 2020, 46, 16459–16464. [Google Scholar] [CrossRef]
- Grigorjevaite, J.; Janulevicius, M.; Kruopyte, A.; Ezerskyte, E.; Vargalis, R.; Sakirzanovas, S.; Katelnikovas, A. Synthesis and optical properties of efficient orange emitting GdB5O9:Sm3+ phosphors. J. Sol-Gel Sci. Technol. 2020, 94, 80–87. [Google Scholar] [CrossRef]
- Pakalniskis, A.; Marsalka, A.; Raudonis, R.; Balevicius, V.; Zarkov, A.; Skaudzius, R.; Kareiva, A. Sol-gel synthesis and study of praseodymium substitution effects in yttrium aluminium garnet Y3-xPrxAl5O12. Opt. Mater. 2021, 111, 110586. [Google Scholar] [CrossRef]
- Jitianu, M.; Zaharescu, M.; Bãlãsoiu, M.; Jitianu, A. The Sol-Gel Route in Synthesis of Cr(III)-Containing Clays. Comparison Between Mg-Cr and Ni-Cr Anionic Clays. J. Sol-Gel Sci. Technol. 2003, 26, 217–221. [Google Scholar] [CrossRef]
- Saikia, P.; Gautam, A.; Goswamee, R.L. Synthesis of nanohybrid alcogels of SiO2 and Ni–Cr/Mg–Cr–LDH: Study of their rheological and dip coating properties. RSC Adv. 2016, 6, 112092–112102. [Google Scholar] [CrossRef]
- Valeikiene, L.; Grigoraviciute-Puroniene, I.; Kareiva, A. Alkaline earth metal substitution effects in sol-gel–derived mixed metal oxides and Mg2-xMx/Al1 (M = Ca, Sr, Ba)–layered double hydroxides. J. Austral. Ceram. Soc. 2020, 56, 1531–1541. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Sen, S.; Salak, A.N.; Ferreira, M.G.S.; Skaudzius, R.; Katelnikovas, A.; Kareiva, A. Sol-Gel Synthesis and Characterization of Non-Substituted and Europium-Substituted Layered Double Hydroxides Mg3/Al1-xEux. Current Inorg. Chem. 2016, 6, 149–154. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials; Springer: Berlin, Germany, 1994; p. 232. [Google Scholar]
- Yen, W.M.; Shionoya, S.; Yamamoto, H. Fundamentals of Phosphors; CRC Press: Boca Raton, FL, USA, 2007; p. 335. [Google Scholar]
- Lahoz, F.; Martín, I.R.; Méndez-Ramos, J.; Núñez, P. Dopant distribution in a Tm3+-Yb3+ codoped silica based glass ceramic: An infrared-laser induced upconversion study. J. Chem. Phys. 2004, 120, 6180–6190. [Google Scholar] [CrossRef]
- Zhu, L.L.; Hao, C.; Wang, X.H.; Guo, Y.N. Fluffy Cotton-Like GO/Zn-Co-Ni Layered Double Hydroxides Form from a Sacrificed Template GO/ZIF-8 for High Performance Asymmetric Supercapacitors. ACS Sustain. Chem. Eng. 2020, 8, 11618–11629. [Google Scholar] [CrossRef]

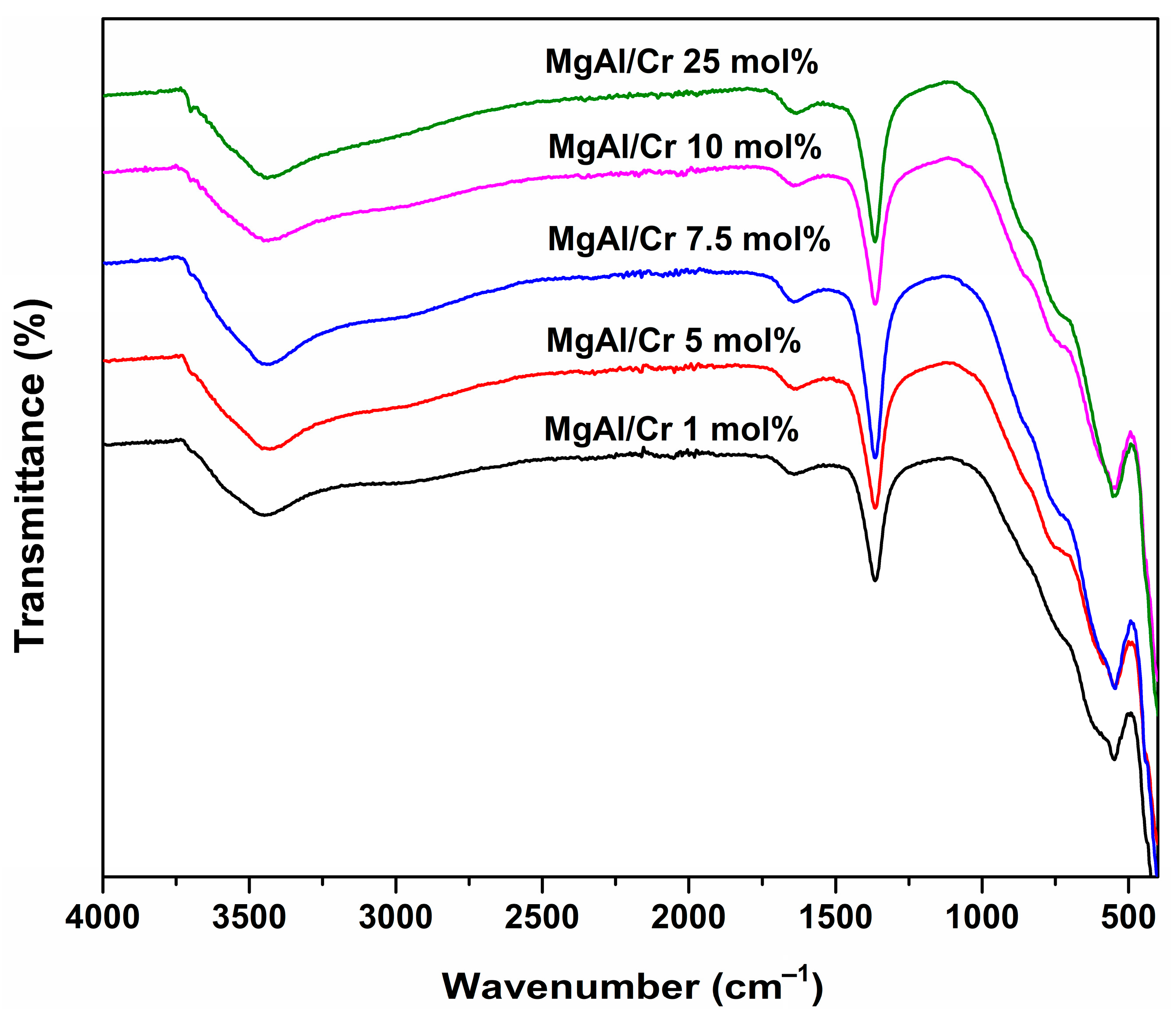
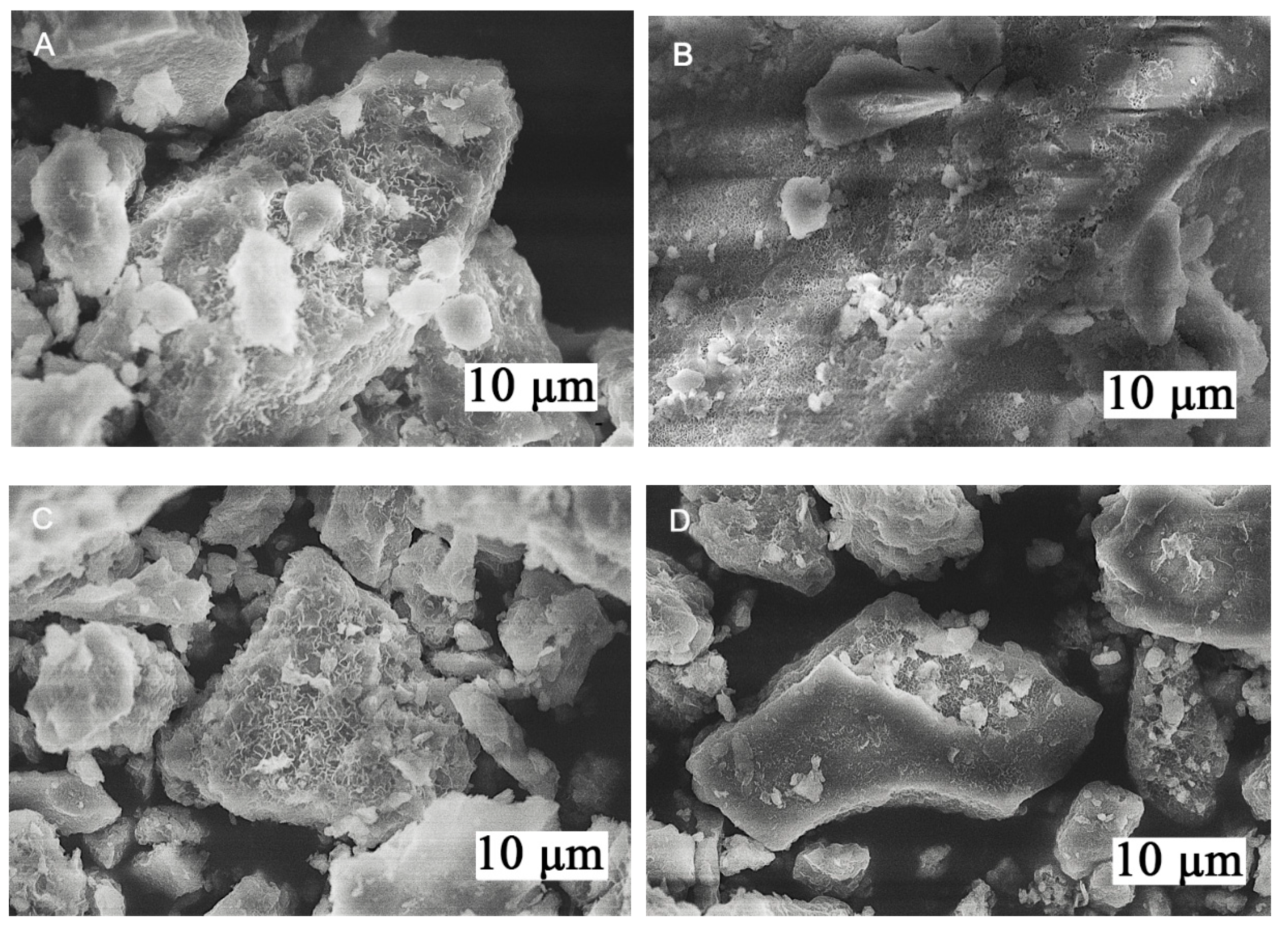
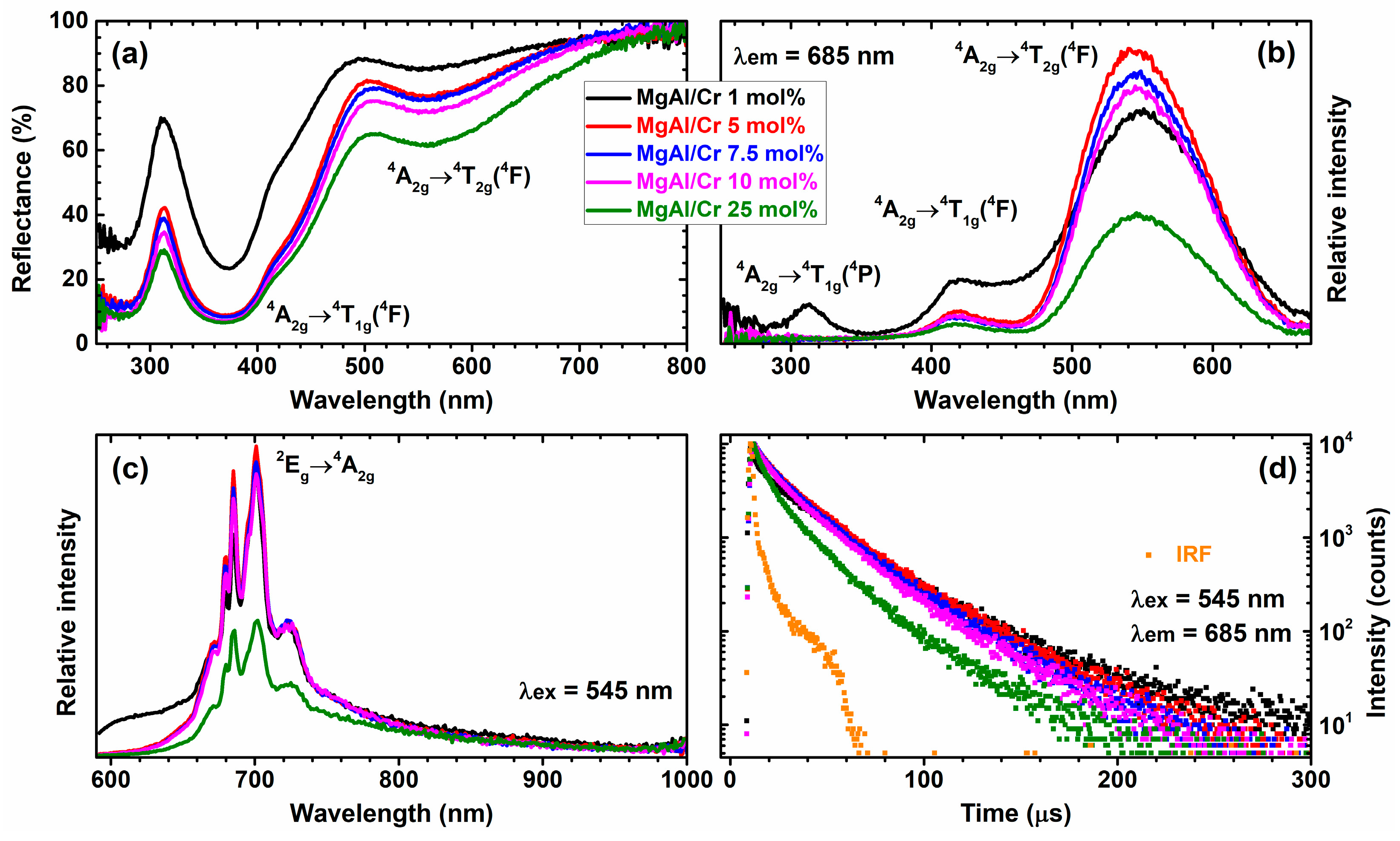
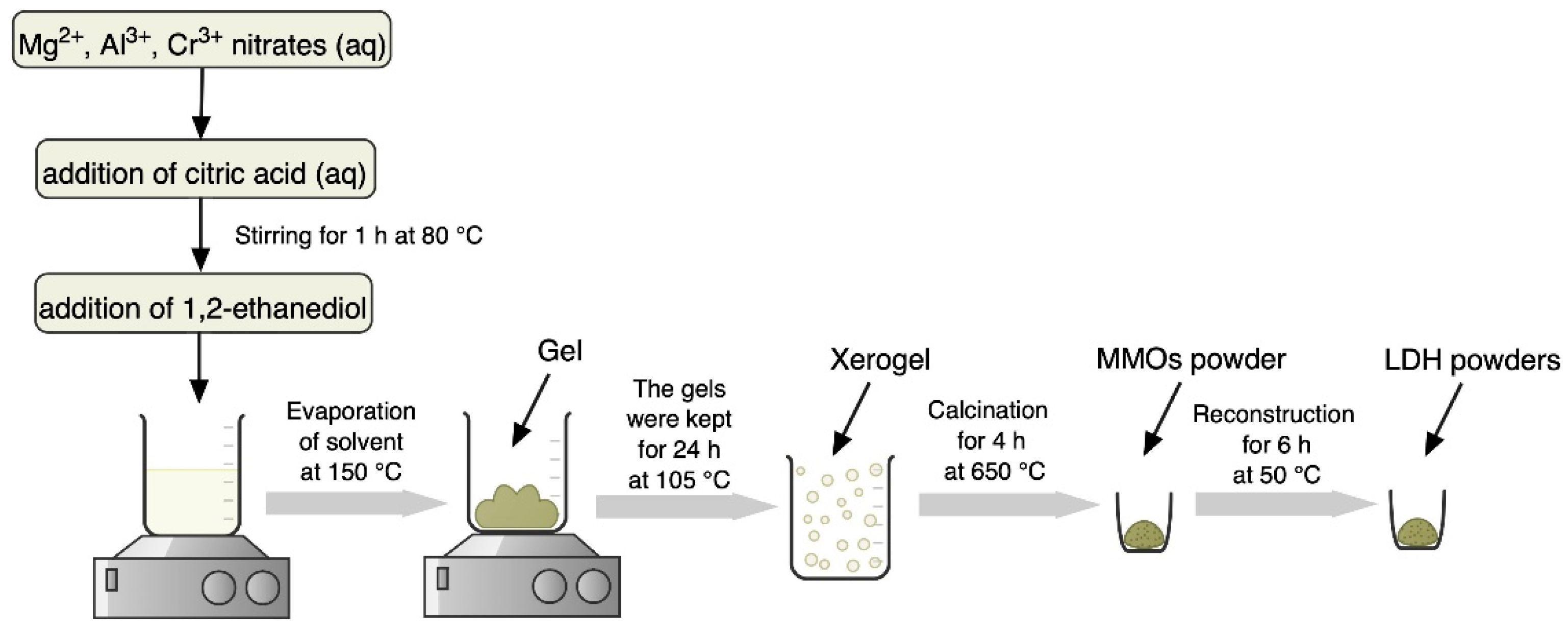
| Sample | d (003), Å | d (006), Å | d (110), Å | Lattice Parameters, Å | |
|---|---|---|---|---|---|
| a | c | ||||
| Mg-Al | 7.6033 | 3.8017 | 1.5187 | 3.0374 | 22.8100 |
| Mg-Al/Cr 1 mol% | 7.7470 | 3.8735 | 1.5242 | 3.0484 | 23.2408 |
| Mg-Al/Cr 5 mol% | 7.7748 | 3.8876 | 1.5276 | 3.0551 | 23.3248 |
| Mg-Al/Cr 7.5 mol% | 7.8028 | 3.9015 | 1.5314 | 3.0627 | 23.4094 |
| Mg-Al/Cr 10 mol% | 7.8373 | 3.9187 | 1.5367 | 3.0733 | 23.5122 |
| Mg-Al/Cr 25 mol% | 7.8774 | 3.9475 | 1.5579 | 3.1158 | 23.6586 |
| Sample | ICP-OES | EDX | ||
|---|---|---|---|---|
| n(Cr), % | n(Mg):n(Al + Cr) | n(Cr), % | n(Mg):n(Al + Cr) | |
| Mg-Al/Cr 1 mol% | 1.12 | 3:0.994 | 1.43 | 3:0.993 |
| Mg-Al/Cr 5 mol% | 5.39 | 3:0.990 | 7.22 | 3:1.06 |
| Mg-Al/Cr 7.5 mol% | 7.91 | 3:0.988 | 7.77 | 3:1.02 |
| Mg-Al/Cr 10 mol% | 10.5 | 3:0.997 | 12.9 | 3:0.875 |
| Mg-Al/Cr 25 mol% | 25.8 | 3:1.01 | 26.3 | 3:1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valeikiene, L.; Grigoraviciute-Puroniene, I.; Katelnikovas, A.; Zarkov, A.; Kareiva, A. Investigation of Structural and Luminescent Properties of Sol-Gel-Derived Cr‑Substituted Mg3Al1−xCrx Layered Double Hydroxides. Molecules 2021, 26, 1848. https://doi.org/10.3390/molecules26071848
Valeikiene L, Grigoraviciute-Puroniene I, Katelnikovas A, Zarkov A, Kareiva A. Investigation of Structural and Luminescent Properties of Sol-Gel-Derived Cr‑Substituted Mg3Al1−xCrx Layered Double Hydroxides. Molecules. 2021; 26(7):1848. https://doi.org/10.3390/molecules26071848
Chicago/Turabian StyleValeikiene, Ligita, Inga Grigoraviciute-Puroniene, Arturas Katelnikovas, Aleksej Zarkov, and Aivaras Kareiva. 2021. "Investigation of Structural and Luminescent Properties of Sol-Gel-Derived Cr‑Substituted Mg3Al1−xCrx Layered Double Hydroxides" Molecules 26, no. 7: 1848. https://doi.org/10.3390/molecules26071848
APA StyleValeikiene, L., Grigoraviciute-Puroniene, I., Katelnikovas, A., Zarkov, A., & Kareiva, A. (2021). Investigation of Structural and Luminescent Properties of Sol-Gel-Derived Cr‑Substituted Mg3Al1−xCrx Layered Double Hydroxides. Molecules, 26(7), 1848. https://doi.org/10.3390/molecules26071848









