Antibacterial and Photocatalytic Properties of ZnO Nanoparticles Obtained from Chemical versus Saponaria officinalis Extract-Mediated Synthesis
Abstract
1. Introduction
2. Results and Discussion
2.1. Green Synthesis and Characterization of ZnO Nanoparticles
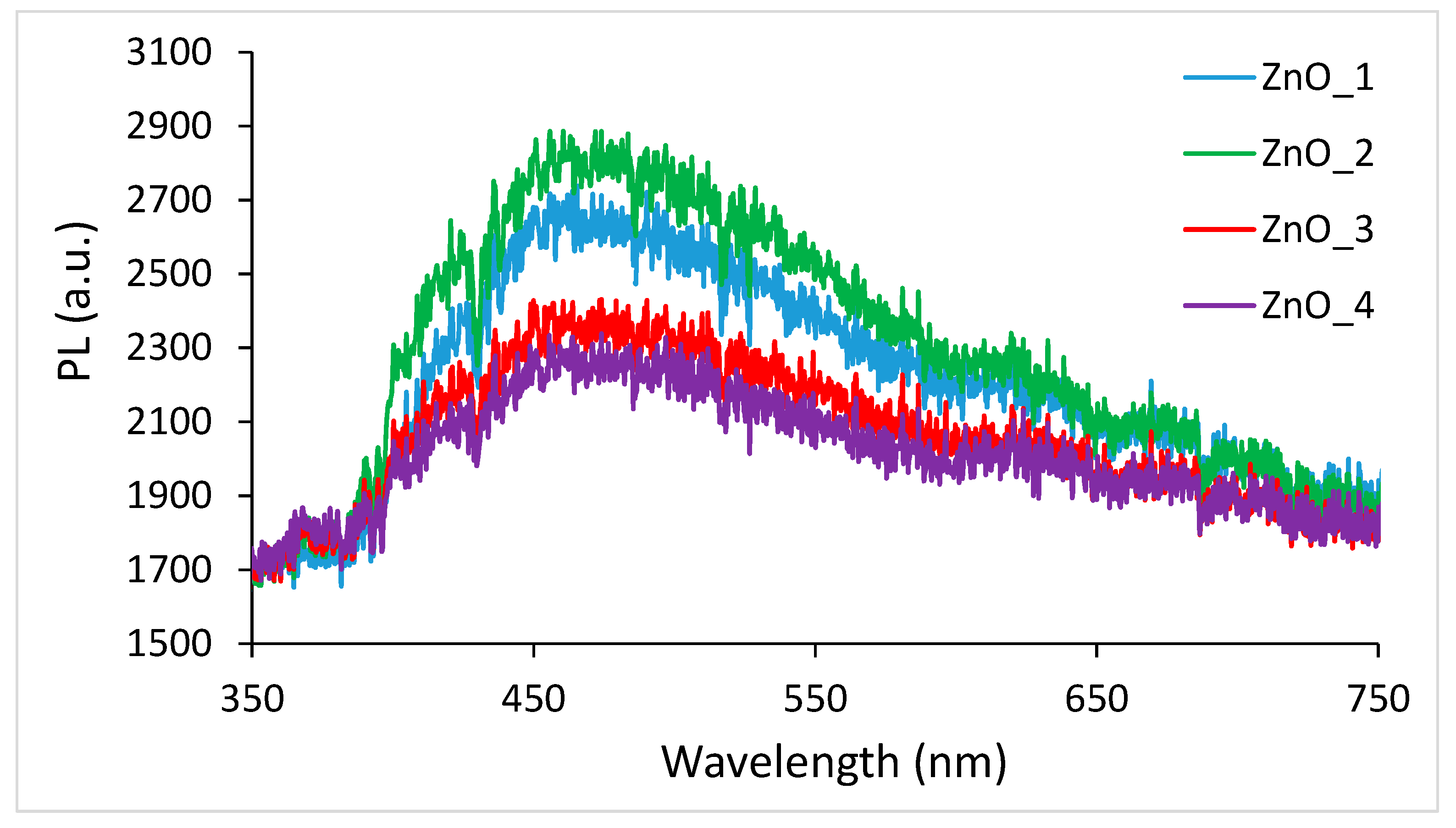
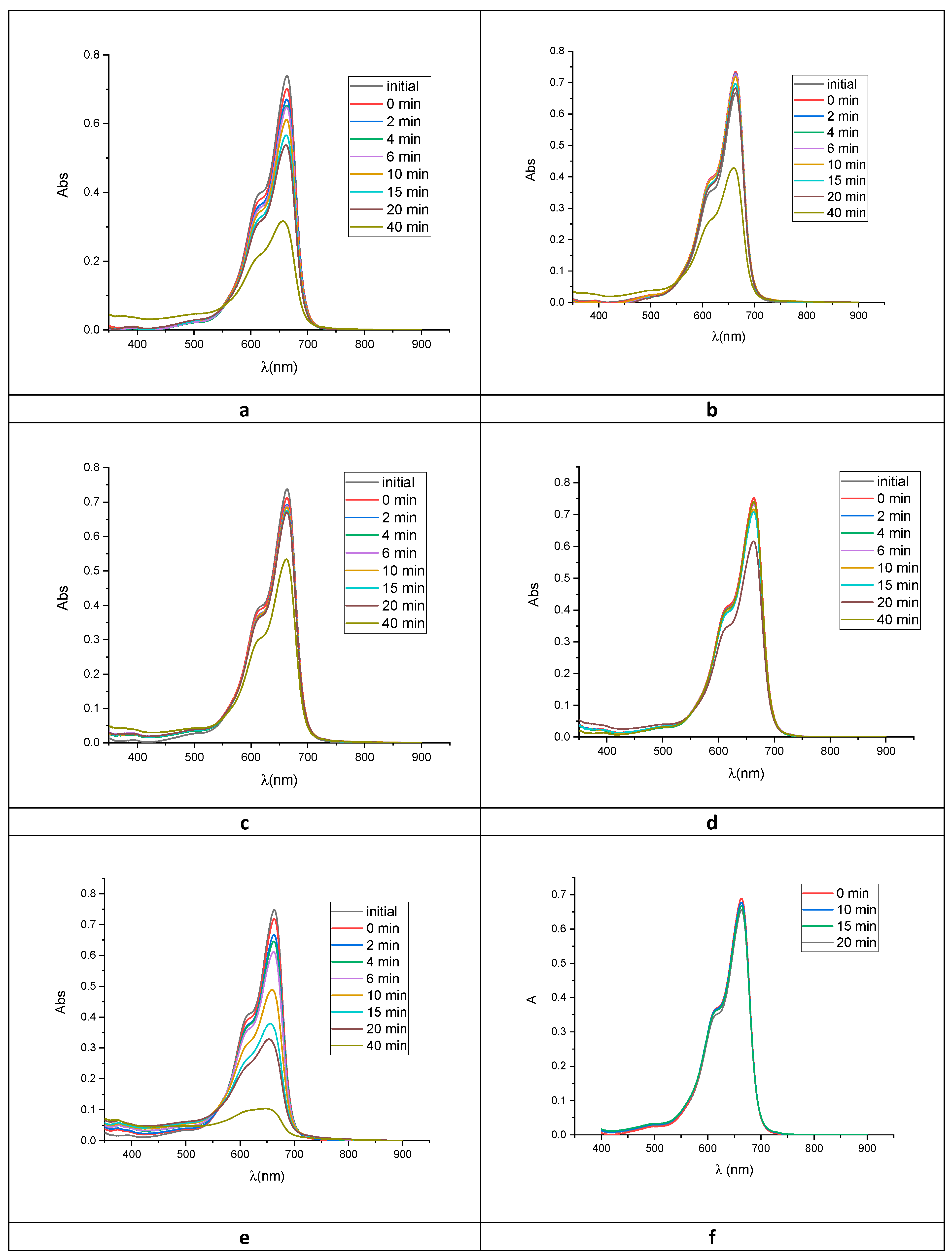
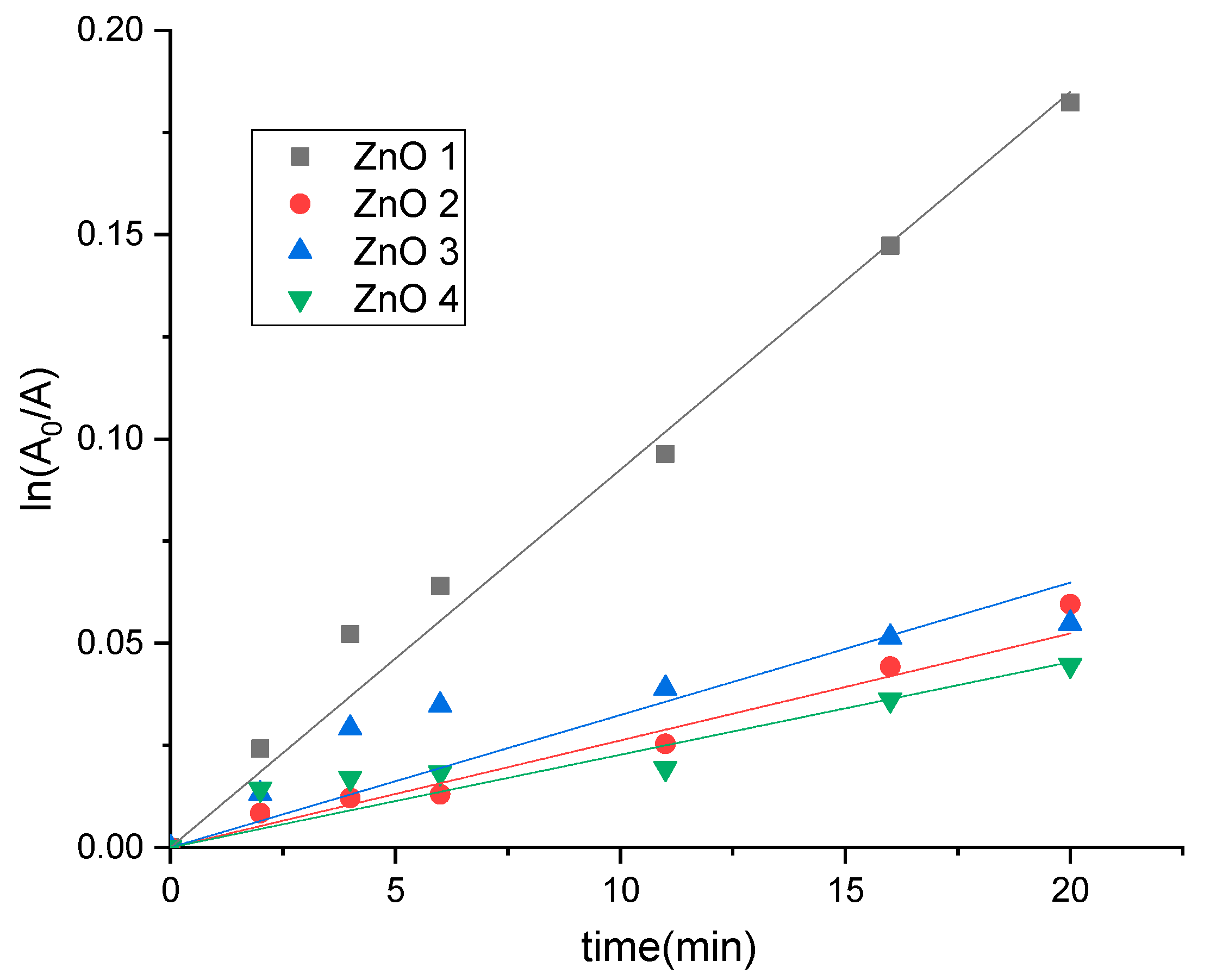
2.2. Photocatalytic Activity
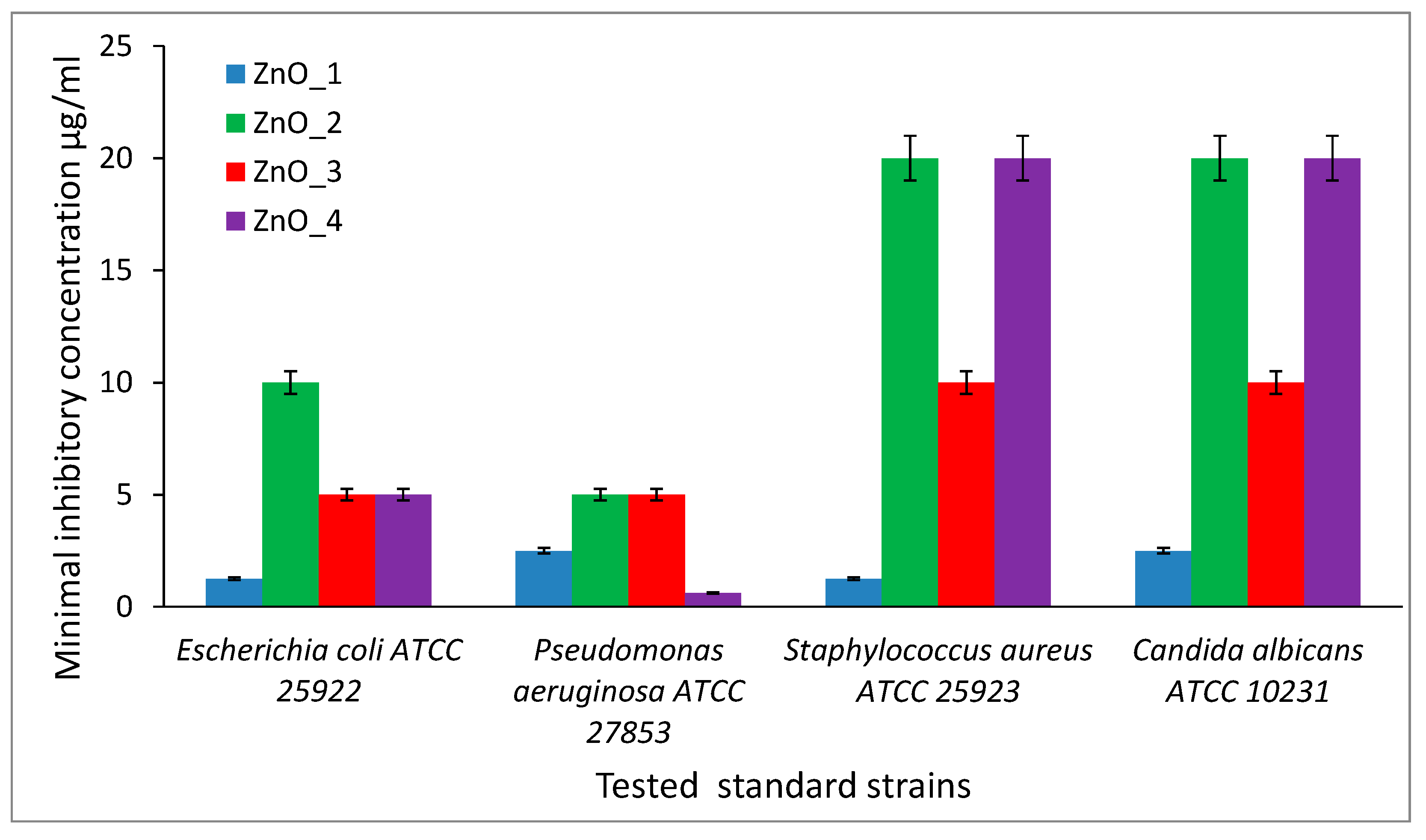
2.3. Antibacterial Activity
3. Materials and Methods
3.1. Materials
Plant Extract
3.2. ZnO Nanoparticles Synthesis
3.3. ZnO Nanoparticles Characterization
3.4. Photocatalytic Properties Assessment
3.5. Antibacterial Activity Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Li, Y.-L.; Gong, F.-L.; Xie, K.-F.; Liu, M.; Zhang, H.-L.; Fang, S.-M. Al Doped Narcissus-like ZnO for Enhanced NO2 Sensing Performance: An Experimental and DFT Investigation. Sens. Actuators B Chem. 2020, 305, 127489. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc Oxide Nanoparticles: Biological Synthesis and Biomedical Applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Wang, X.; Ahmad, M.; Sun, H. Three-Dimensional ZnO Hierarchical Nanostructures: Solution Phase Synthesis and Applications. Materials 2017, 10, 1304. [Google Scholar] [CrossRef] [PubMed]
- Baghbanzadeh, M.; Carbone, L.; Cozzoli, P.D.; Kappe, C.O. Microwave-Assisted Synthesis of Colloidal Inorganic Nanocrystals. Angew. Chem. Int. Ed. 2011, 50, 11312–11359. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Lojkowski, W. A Review of Microwave Synthesis of Zinc Oxide Nanomaterials: Reactants, Process Parameters and Morphologies. Nanomaterials 2020, 10, 1086. [Google Scholar] [CrossRef] [PubMed]
- Al-Gaashani, R.; Radiman, S.; Daud, A.R.; Tabet, N.; Al-Douri, Y. XPS and Optical Studies of Different Morphologies of ZnO Nanostructures Prepared by Microwave Methods. Ceram. Int. 2013, 39, 2283–2292. [Google Scholar] [CrossRef]
- Liang, S.; Zhu, L.; Gai, G.; Yao, Y.; Huang, J.; Ji, X.; Zhou, X.; Zhang, D.; Zhang, P. Synthesis of Morphology-Controlled ZnO Microstructures via a Microwave-Assisted Hydrothermal Method and Their Gas-Sensing Property. Ultrason. Sonochem. 2014, 21, 1335–1342. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kargozar, S.; Ghorbani, A.; Darroudi, M.; Keshavarz, M.; Baino, F.; Kim, H.-W. Biomedical Waste Management by Using Nanophotocatalysts: The Need for New Options. Materials 2020, 13, 3511. [Google Scholar] [CrossRef]
- Saravanan, R.; Karthikeyan, S.; Gupta, V.K.; Sekaran, G.; Narayanan, V.; Stephen, A. Enhanced Photocatalytic Activity of ZnO/CuO Nanocomposite for the Degradation of Textile Dye on Visible Light Illumination. Mater. Sci. Eng. C 2013, 33, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Gupta, V.K.; Narayanan, V.; Stephen, A. Comparative Study on Photocatalytic Activity of ZnO Prepared by Different Methods. J. Mol. Liq. 2013, 181, 133–141. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Siri, S.; Wadbua, P.; Thongtem, S.; Thongtem, T. Microwave-Assisted Synthesis, Photocatalysis and Antibacterial Activity of Ag Nanoparticles Supported on ZnO Flowers. J. Phys. Chem. Solids 2019, 126, 170–177. [Google Scholar] [CrossRef]
- Ahmed, G.; Hanif, M.; Khan, A.J.; Zhao, L.; Zhang, J.; Liu, Z. ZnO Flowers and Graphene Oxide Hybridization for Efficient Photocatalytic Degradation of O-Xylene in Water. Mater. Chem. Phys. 2018, 212, 479–489. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Kheirabadi, M.; Ebrahimi, M.; Moshfegh, A.Z. Design and Tailoring of One-Dimensional ZnO Nanomaterials for Photocatalytic Degradation of Organic Dyes: A Review. Res. Chem. Intermed. 2019, 45, 2197–2254. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, E.; Li, J.; Qiu, Y.; Chen, R. Rapid Ultrasonic-Microwave Assisted Synthesis of Spindle-like Ag/ZnO Nanostructures and Their Enhanced Visible-Light Photocatalytic and Antibacterial Activities. Catal. Today 2020, 339, 391–402. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Das, P.; Natan, M.; Jacobi, G.; Banin, E.; Luong, J.H.T.; Gedanken, A. Antimicrobial Activities of Zn-Doped CuO Microparticles Decorated on Polydopamine against Sensitive and Antibiotic-Resistant Bacteria. ACS Appl. Polym. Mater. 2020, 2, 5878–5888. [Google Scholar] [CrossRef]
- Seaberg, J.; Montazerian, H.; Hossen, M.N.; Bhattacharya, R.; Khademhosseini, A.; Mukherjee, P. Hybrid Nanosystems for Biomedical Applications. ACS Nano 2021, 15, 2099–2142. [Google Scholar] [CrossRef]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, K.; Cai, M. ZnO Nanomaterials: Current Advancements in Antibacterial Mechanisms and Applications. Front. Chem. 2020, 8, 580. [Google Scholar] [CrossRef]
- Untracht, Z.T.; Ozcan, A.; Santra, S.; Kang, E.H. SDS-PAGE for Monitoring the Dissolution of Zinc Oxide Bactericidal Nanoparticles (Zinkicide) in Aqueous Solutions. ACS Omega 2020, 5, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Zubair, N.; Akhtar, K. Morphology Controlled Synthesis of ZnO Nanoparticles for In-Vitro Evaluation of Antibacterial Activity. Trans. Nonferrous Met. Soc. China 2020, 30, 1605–1614. [Google Scholar] [CrossRef]
- Li, Y.; Niu, J.; Zhang, W.; Zhang, L.; Shang, E. Influence of Aqueous Media on the ROS-Mediated Toxicity of ZnO Nanoparticles toward Green Fluorescent Protein-Expressing Escherichia Coli under UV-365 Irradiation. Langmuir 2014, 30, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A Review on Enhancing the Antibacterial Activity of ZnO: Mechanisms and Microscopic Investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, J.; Zhou, Y.; Zhou, Y. Multifunctional Antibacterial Materials for the Control of Hazardous Microbes and Chemicals: A Review. ACS EST Water 2021, 1, 479–497. [Google Scholar] [CrossRef]
- Sharma, V.; Shukla, R.K.; Saxena, N.; Parmar, D.; Das, M.; Dhawan, A. DNA Damaging Potential of Zinc Oxide Nanoparticles in Human Epidermal Cells. Toxicol. Lett. 2009, 185, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Yokoi, N.; Abe, S.; Watanabe, Y. Crystal Structure Based Design of Functional Metal/Protein Hybrids. J. Inorg. Biochem. 2007, 101, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In Vitro Cytotoxicity of Oxide Nanoparticles: Comparison to Asbestos, Silica, and the Effect of Particle Solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable Synthesis of ZnO Nanoparticles and Their Morphology-Dependent Antibacterial and Optical Properties. J. Photochem. Photobiol. B 2013, 120, 66–73. [Google Scholar] [CrossRef]
- Chang, T.-H.; Lu, Y.-C.; Yang, M.-J.; Huang, J.-W.; Linda Chang, P.-F.; Hsueh, H.-Y. Multibranched Flower-like ZnO Particles from Eco-Friendly Hydrothermal Synthesis as Green Antimicrobials in Agriculture. J. Clean. Prod. 2020, 262, 121342. [Google Scholar] [CrossRef]
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A Review on Green Synthesis of Zinc Oxide Nanoparticles—An Eco-Friendly Approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Dobrucka, R.; Długaszewska, J. Biosynthesis and Antibacterial Activity of ZnO Nanoparticles Using Trifolium Pratense Flower Extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef]
- Adil, S.F.; Assal, M.E.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H.; Liz-Marzán, L.M. Biogenic Synthesis of Metallic Nanoparticles and Prospects toward Green Chemistry. Dalton Trans. 2015, 44, 9709–9717. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Venkat Kumar, S.; Rajeshkumar, S. Mechanistic Study on Antibacterial Action of Zinc Oxide Nanoparticles Synthesized Using Green Route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.U.; Khan, Z.U.H.; Ma, J.; Khan, A.U.; Sohail, M.; Chen, Y.; Yang, Y.; Pan, X. An Astragalus Membranaceus Based Eco-Friendly Biomimetic Synthesis Approach of ZnO Nanoflowers with an Excellent Antibacterial, Antioxidant and Electrochemical Sensing Effect. Mater. Sci. Eng. C 2021, 118, 111432. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, A.; Ri, H.; Khan, A.U.; Khan, U.A.; Yuan, Q. Green Synthesis of Catalytic Zinc Oxide Nano-flowers and Their Bacterial Infection Therapy. Appl. Organomet. Chem. 2020, 34, 1–11. [Google Scholar] [CrossRef]
- Rupa, E.J.; Kaliraj, L.; Abid, S.; Yang, D.-C.; Jung, S.-K. Synthesis of a Zinc Oxide Nanoflower Photocatalyst from Sea Buckthorn Fruit for Degradation of Industrial Dyes in Wastewater Treatment. Nanomaterials 2019, 9, 1692. [Google Scholar] [CrossRef]
- Irshad, S.; Salamat, A.; Anjum, A.A.; Sana, S.; Saleem, R.S.; Naheed, A.; Iqbal, A.; Yang, J. Green Tea Leaves Mediated ZnO Nanoparticles and Its Antimicrobial Activity. Cogent Chem. 2018, 4, 1469207. [Google Scholar] [CrossRef]
- Patil, B.N.; Taranath, T.C. Limonia Acidissima L. Leaf Mediated Synthesis of Silver and Zinc Oxide Nanoparticles and Their Antibacterial Activities. Microb. Pathog. 2018, 115, 227–232. [Google Scholar] [CrossRef]
- Attar, A.; Yapaoz, M.A. Biomimetic Synthesis, Characterization and Antibacterial Efficacy of ZnO and Au Nanoparticles Using Echinacea Flower Extract Precursor. Mater. Res. Express 2018, 5, 055403. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y.; Khan, M.; Adil, S.F. Green Synthesis of ZnO Hierarchical Microstructures by Cordia Myxa and Their Antibacterial Activity. Saudi J. Biol. Sci. 2019, 26, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Extract from the Roots of Saponaria Officinalis as a Potential Acaricide against Tetranychus Urticae. J. Pest Sci. 2017, 90, 683–692. [Google Scholar] [CrossRef]
- Smułek, W.; Zdarta, A.; Pacholak, A.; Zgoła-Grześkowiak, A.; Marczak, Ł.; Jarzębski, M.; Kaczorek, E. Saponaria Officinalis L. Extract: Surface Active Properties and Impact on Environmental Bacterial Strains. Colloids Surf. B Biointerfaces 2017, 150, 209–215. [Google Scholar] [CrossRef]
- Pavela, R.; Murugan, K.; Canale, A.; Benelli, G. Saponaria Officinalis-Synthesized Silver Nanocrystals as Effective Biopesticides and Oviposition Inhibitors against Tetranychus Urticae Koch. Ind. Crops Prod. 2017, 97, 338–344. [Google Scholar] [CrossRef]
- Aalami, A.H.; Mesgari, M.; Sahebkar, A. Synthesis and Characterization of Green Zinc Oxide Nanoparticles with Antiproliferative Effects through Apoptosis Induction and MicroRNA Modulation in Breast Cancer Cells. Bioinorg. Chem. Appl. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Nundy, S.; Eom, T.; Kang, J.; Suh, J.; Cho, M.; Park, J.-S.; Lee, H.-J. Flower-Shaped ZnO Nanomaterials for Low-Temperature Operations in NOX Gas Sensors. Ceram. Int. 2020, 46, 5706–5714. [Google Scholar] [CrossRef]
- Shen, Z.; Liang, P.; Wang, S.; Liu, L.; Liu, S. Green Synthesis of Carbon- and Silver-Modified Hierarchical ZnO with Excellent Solar Light Driven Photocatalytic Performance. ACS Sustain. Chem. Eng. 2015, 3, 1010–1016. [Google Scholar] [CrossRef]
- Kwok, W.M.; Djurišić, A.B.; Leung, Y.H.; Chan, W.K.; Phillips, D.L. Time-Resolved Photoluminescence Study of the Stimulated Emission in ZnO Nanoneedles. Appl. Phys. Lett. 2005, 87, 093108. [Google Scholar] [CrossRef]
- Sakata, K.; Macounová, K.M.; Nebel, R.; Krtil, P. PH Dependent ZnO Nanostructures Synthesized by Hydrothermal Approach and Surface Sensitivity of Their Photoelectrochemical Behavior. SN Appl. Sci. 2020, 2, 203. [Google Scholar] [CrossRef]
- Pholnak, C.; Sirisathitkul, C.; Harding, D.J. Characterizations of Octahedral Zinc Oxide Synthesized by Sonochemical Method. J. Phys. Chem. Solids 2011, 72, 817–823. [Google Scholar] [CrossRef]
- Sun, L.; Shao, R.; Chen, Z.; Tang, L.; Dai, Y.; Ding, J. Alkali-Dependent Synthesis of Flower-like ZnO Structures with Enhanced Photocatalytic Activity via a Facile Hydrothermal Method. Appl. Surf. Sci. 2012, 258, 5455–5461. [Google Scholar] [CrossRef]
- Wang, J.; Ma, P.; Xiang, L. Effects of NaOH on Formation of ZnO Nanorods from ε-Zn(OH)2. Mater. Lett. 2015, 141, 118–121. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, D.; Ji, Y.; Ma, X.; Xu, J.; Que, D. Low Temperature Synthesis of Flowerlike ZnO Nanostructures by Cetyltrimethylammonium Bromide-Assisted Hydrothermal Process. J. Phys. Chem. B 2004, 108, 3955–3958. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Zeng, W. Hydrothermal Synthesis of Hierarchical Flower-like ZnO Nanostructure and Its Enhanced Ethanol Gas-Sensing Properties. Appl. Surf. Sci. 2018, 427, 281–287. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Alterary, S.; Ali Abdullrahman Almoghim, R.; Awad, M.A.; Aldosari, N.S.; Fahad Alghannam, S.; Nasser Alabdan, A.; Alharbi, S.; Ali Mohammed Alateeq, B.; Abdulrahman Al Mohsen, A.; et al. Greener Synthesis of Zinc Oxide Nanoparticles: Characterization and Multifaceted Applications. Molecules 2020, 25, 4198. [Google Scholar] [CrossRef] [PubMed]
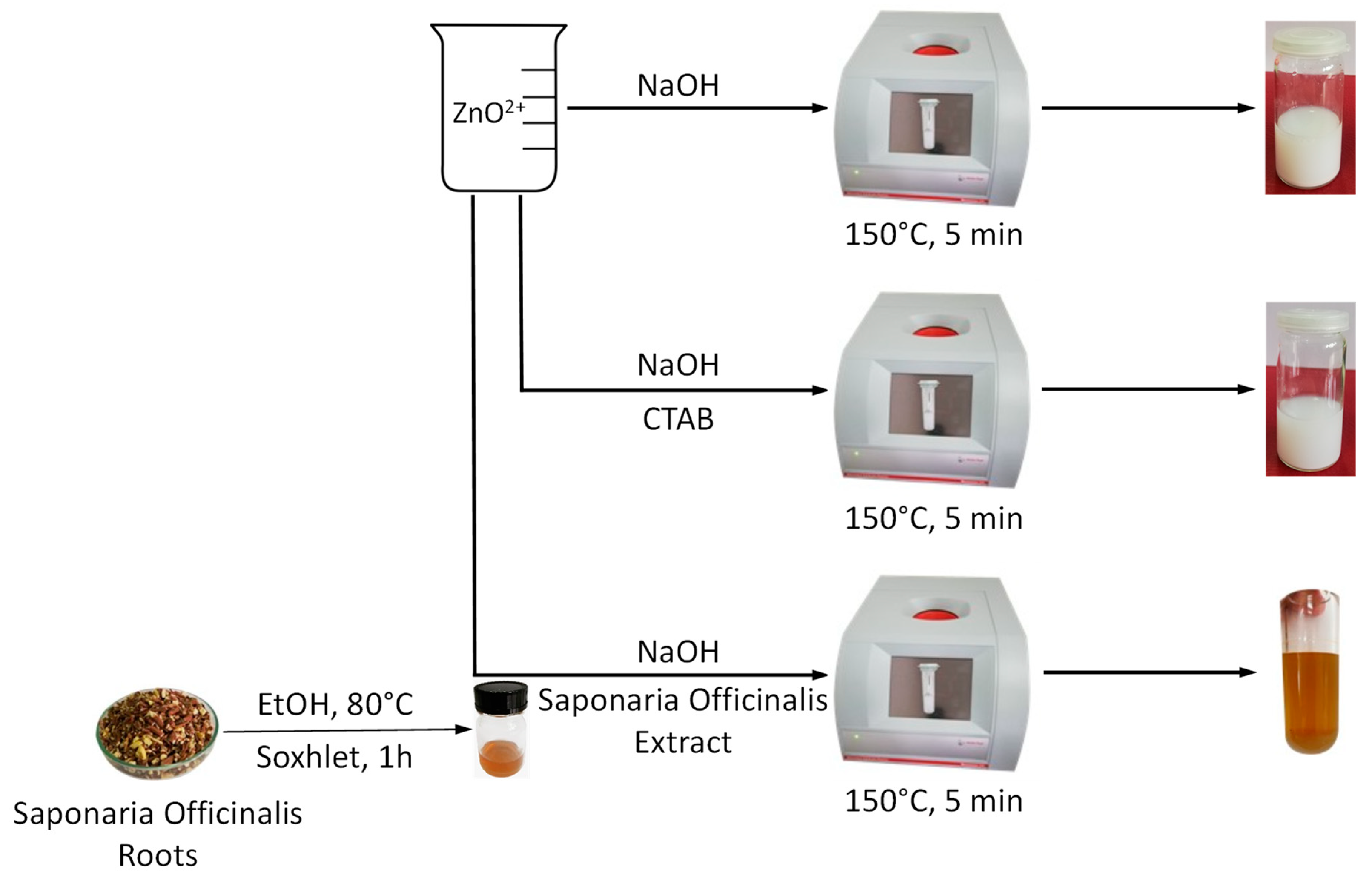
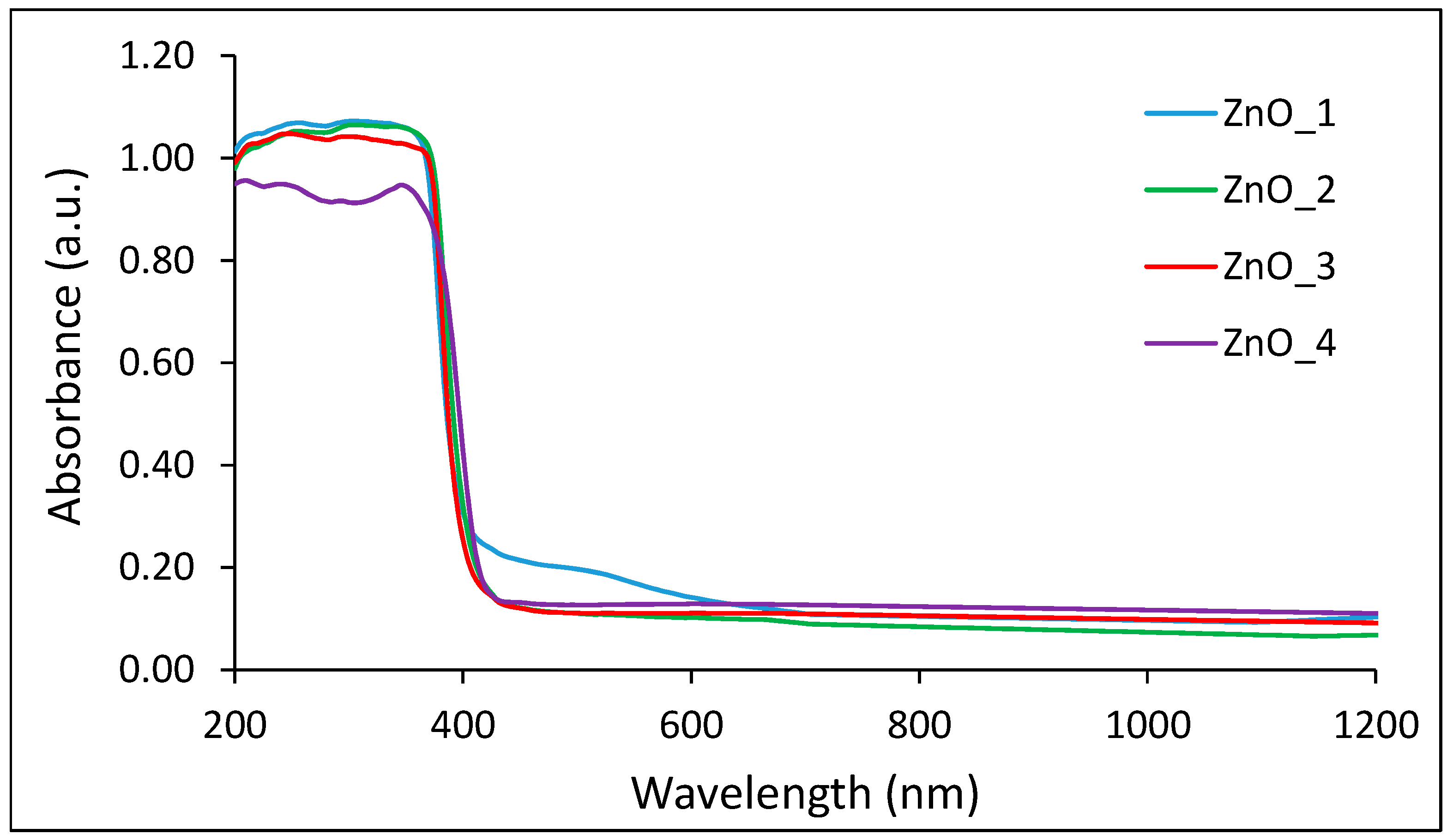



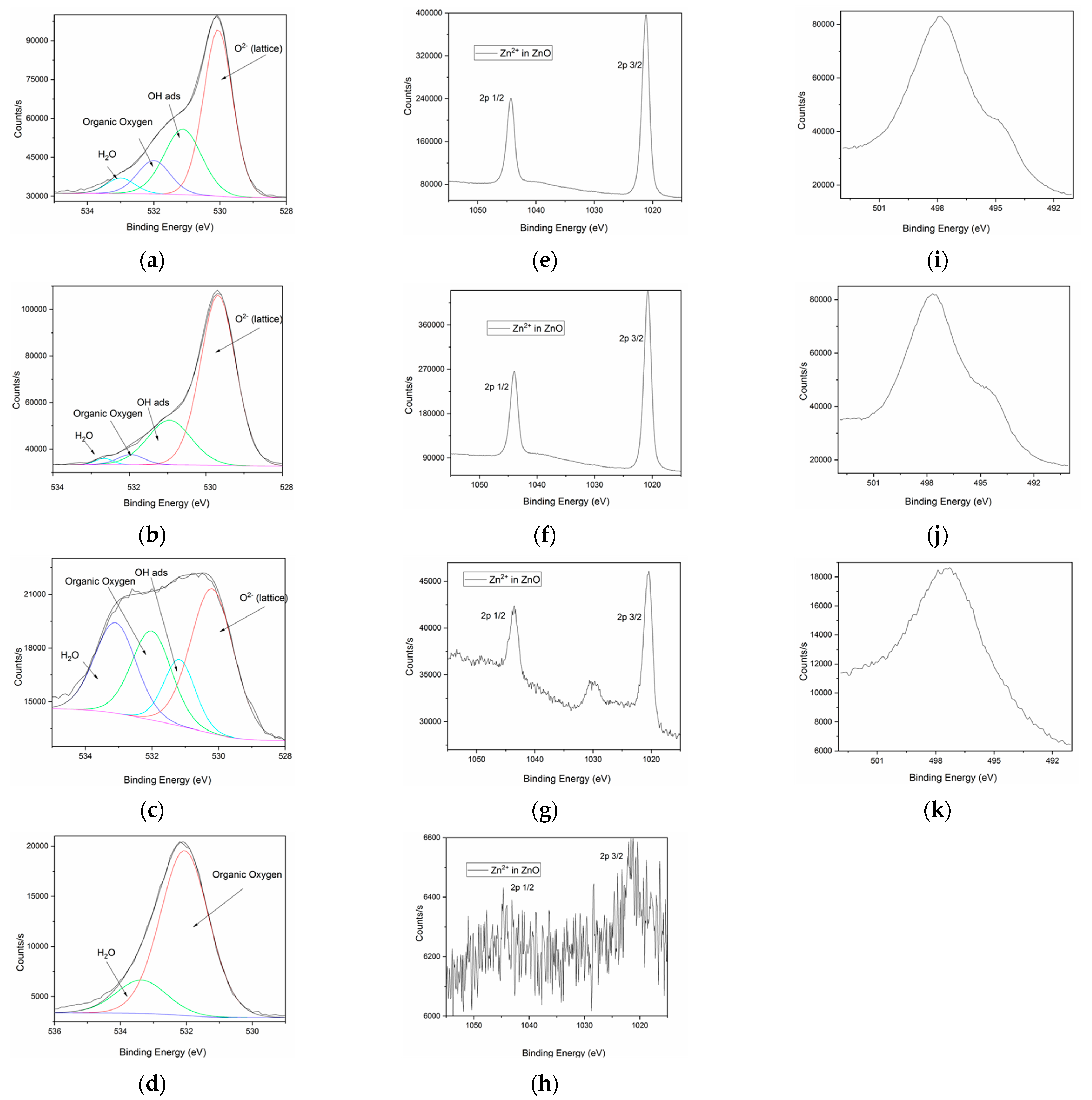
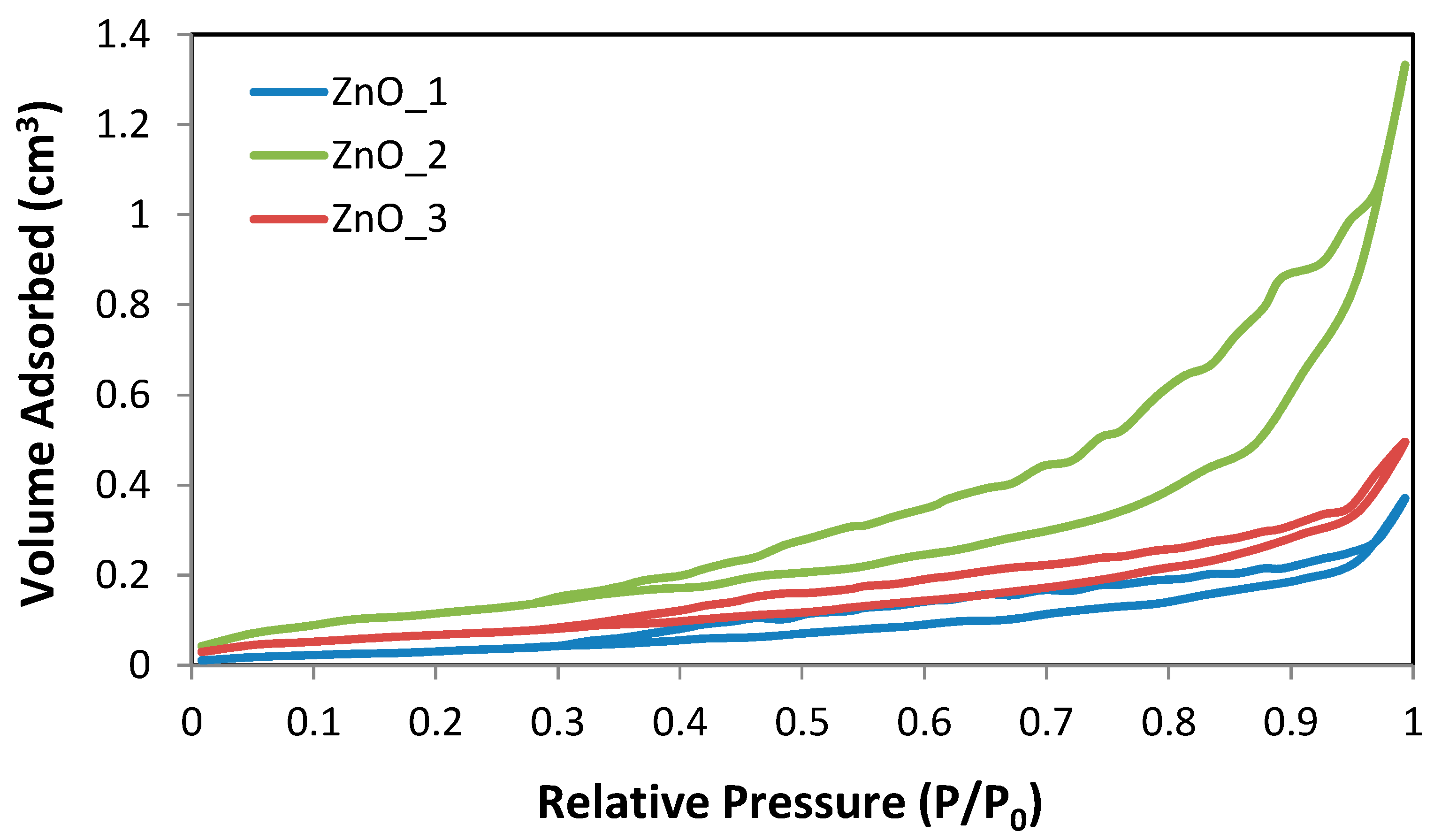

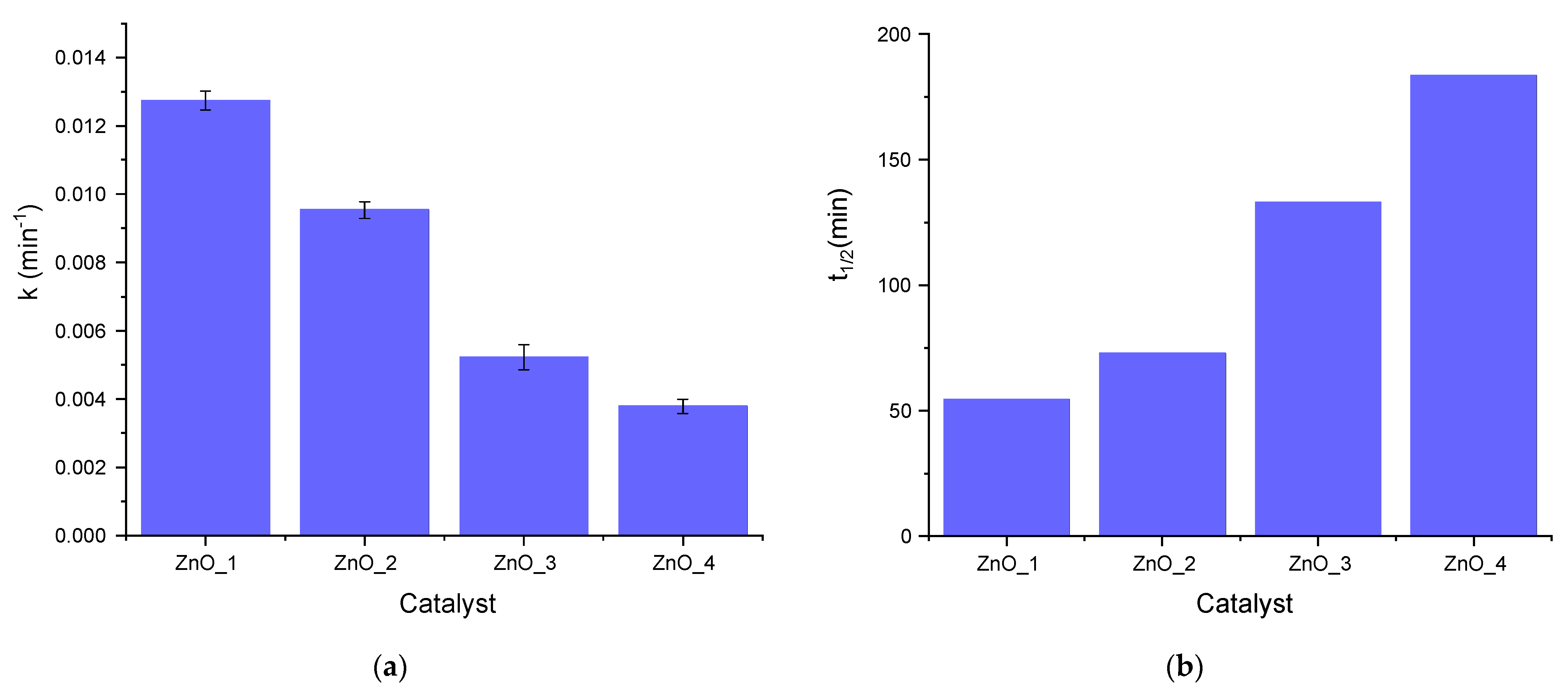
| Sample Code | Zn2+ Source | Reducing Agent | Structuring Agent |
|---|---|---|---|
| ZnO_1 | Zn(NO3)2·6H2O | NaOH | - |
| ZnO_2 | Zn(NO3)2·6H2O | NaOH | CTABr |
| ZnO_3 | Zn(NO3)2·6H2O | NaOH | S. officinalis extract |
| ZnO_4 | Zn(NO3)2·6H2O | S. officinalis extract | S. officinalis extract |
| Sample 1 | Dm (nm) | PdI | Zeta Potential (mV) | Observations |
|---|---|---|---|---|
| ZnO_1 | 1214; >5500 | 0.363 | −38.1 | Large aggregates at >5500 nm. |
| ZnO_2 | 1068 | 0.218 | −20.0 | Monomodal distribution. |
| ZnO_3 | 863 | 0.295 | −31.8 | Monomodal distribution. |
| ZnO_4 | 42; 244 | 0.358 | −16.2 | Bimodal distribution with a major population at 42 nm and aggregates at 244 nm. Few larger aggregates. |
| Sample | SBET (m2·g−1) | SBJH ads. (m2·g−1) | SBJH des. (m2·g−1) | Da (nm) | Dd (nm) | Total Pore Volume (cm3·g−1) |
|---|---|---|---|---|---|---|
| ZnO_1 | 2.292 | 3.69 | 7.005 | 3.237 | 3.301 | 0.0116 |
| ZnO_2 | 8.355 | 8.021 | 15.001 | 3.540 | 3.835 | 0.0036 |
| ZnO_3 | 3.257 | 3.011 | 4.710 | 4.294 | 3.294 | 0.0094 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tănase, M.A.; Marinescu, M.; Oancea, P.; Răducan, A.; Mihaescu, C.I.; Alexandrescu, E.; Nistor, C.L.; Jinga, L.-I.; Diţu, L.M.; Petcu, C.; et al. Antibacterial and Photocatalytic Properties of ZnO Nanoparticles Obtained from Chemical versus Saponaria officinalis Extract-Mediated Synthesis. Molecules 2021, 26, 2072. https://doi.org/10.3390/molecules26072072
Tănase MA, Marinescu M, Oancea P, Răducan A, Mihaescu CI, Alexandrescu E, Nistor CL, Jinga L-I, Diţu LM, Petcu C, et al. Antibacterial and Photocatalytic Properties of ZnO Nanoparticles Obtained from Chemical versus Saponaria officinalis Extract-Mediated Synthesis. Molecules. 2021; 26(7):2072. https://doi.org/10.3390/molecules26072072
Chicago/Turabian StyleTănase, Maria Antonia, Maria Marinescu, Petruta Oancea, Adina Răducan, Catalin Ionut Mihaescu, Elvira Alexandrescu, Cristina Lavinia Nistor, Luiza-Izabela Jinga, Lia Mara Diţu, Cristian Petcu, and et al. 2021. "Antibacterial and Photocatalytic Properties of ZnO Nanoparticles Obtained from Chemical versus Saponaria officinalis Extract-Mediated Synthesis" Molecules 26, no. 7: 2072. https://doi.org/10.3390/molecules26072072
APA StyleTănase, M. A., Marinescu, M., Oancea, P., Răducan, A., Mihaescu, C. I., Alexandrescu, E., Nistor, C. L., Jinga, L.-I., Diţu, L. M., Petcu, C., & Cinteza, L. O. (2021). Antibacterial and Photocatalytic Properties of ZnO Nanoparticles Obtained from Chemical versus Saponaria officinalis Extract-Mediated Synthesis. Molecules, 26(7), 2072. https://doi.org/10.3390/molecules26072072










