Identification of Potential SARS-CoV-2 Main Protease and Spike Protein Inhibitors from the Genus Aloe: An In Silico Study for Drug Development
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Constituents of Aloe
2.2. Structure-Based Virtual Screening and Molecular Docking of Aloe Phytochemicals on SARS-CoV-2 Spike Glycoprotein and Main Protease
2.3. Molecular Dynamics Simulation
2.4. Drug like Properties, and Pharmacokinetic Prediction of the Ligands
3. Materials and Methods
3.1. Phytochemical Review of Genus Aloe
3.2. Molecular Docking, Data Software and Visualization
3.2.1. Preparation of Protein and Active Site Prediction
3.2.2. Preparation of Ligand
3.2.3. Docking Analysis
3.3. Molecular Dynamics Simulation
3.4. Drug Like Properties, and ADME Prediction of the Ligands
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
| No. | Name of the Compound | Main Protease (PDB ID: 6LU7) | Spike Glycoprotein (PDB ID: 6M0J) | Plant Source | Ref. | ||
|---|---|---|---|---|---|---|---|
| Score | RSMD | Score | RSMD | ||||
| I-Anthraquinones: | |||||||
| 1 | Chrysophanol | −5.4584 | 0.8734 | −5.4447 | 0.8709 | A. pulcherrima, A. dawei, A. megalacantha, A. vera | [65,66,67,68] |
| 2 | 8-O-Methylchrysophanol | −5.0678 | 0.4878 | −4.8406 | 0.9352 | A. dawei | [66] |
| 3 | Aloe-emodin | −5.3348 | 0.8782 | −6.0546 | 0.9449 | A. megalacantha, A. arborescens A. vera, A. ferox | [67,68,69,70,71,72] |
| 4 | 7-Hydroxy-aloe-emodin | −5.3660 | 1.3842 | −4.8938 | 0.9332 | A. succotrina | [73] |
| 5 | Nataloe-emodin | −5.5017 | 0.7836 | −5.2599 | 0.4924 | A. nyeriensis | [74] |
| 6 | Mono-O-methyl-nataloe-emodin | −4.8059 | 1.2584 | −4.6113 | 0.9158 | A. speciosa | [74] |
| 7 | Emodin | −4.4060 | 0.9758 | −4.8558 | 1.3623 | A. vera, A. spp | [72,75,76,77] |
| 8 | Saponarin II | −4.7520 | 1.0190 | −5.1119 | 1.3501 | A. megalacantha, A. pulcherrima, A. dawei, A. saponaria. | [65,66,67,78,79,80] |
| 9 | Saponarin I | −5.5636 | 0.8416 | −5.2625 | 1.3226 | A. megalacantha, A. pulcherrima, A. saponaria. | [65,67,78,81] |
| 10 | Saponarin III | −5.5941 | 0.9280 | −5.9907 | 0.9256 | A. megalacantha | [67] |
| 11 | Helminthosporin | −5.2698 | 0.6476 | −5.3963 | 1.3211 | A. megalacantha, A. dawei, A. Saponaria | [66,67,79] |
| 12 | 5-O-Methylziganein | −5.6054 | 0.8884 | −3.3209 | 0.8923 | A. hijazensis | [82] |
| 13 | Isoxanthorin | −4.7748 | 1.2234 | −3.9051 | 0.9124 | A. Saponaria | [79,80] |
| 14 | Deoxyerythrolaccin | −4.7968 | 1.3812 | −5.2572 | 1.2640 | A. ferox, A. Saponaria | [71] |
| 15 | Laccaic acid D Methyl ester | −6.1620 | 0.9956 | −5.3786 | 0.8172 | A. Saponaria, A. dawei | [66,78] |
| 16 | Madagascin | −4.2212 | 1.1677 | −4.0269 | 0.9379 | A. vera | [83] |
| 17 | 3-Geranyloxyemodine | −5.4161 | 1.4882 | −4.8861 | 1.1771 | A. vera | [83] |
| 18 | Aloetinic acid. | −5.6006 | 1.6111 | −2.8968 | 1.3504 | A. vera | [84] |
| 19 | Nataloe-emodin-2-O-β-D-Glucopyranoside | −5.3915 | 0.9107 | −4.4116 | 1.4211 | A. nyeriensis | [85] |
| 20 | Aloe-emodin-11-O-rhamnoside | −6.5513 | 1.1442 | −6.3233 | 1.2130 | A. vera | [72,86] |
| 21 | 1,1′,8,8′-Tetrahydroxy-3,3′-dimethyl-4,7′-bianthracene-9,9′,10 (10′H)-trione | −6.2519 | 0.8628 | −3.7287 | 1.2329 | A. Saponaria | [81] |
| 22 | Asphodelin | −5.8474 | 0.9813 | −6.1966 | 1.2760 | A. megalacantha, A. Saponaria | [67,81] |
| 23 | (1,1′,8,8′,10-Pentahydroxy-3,3′-dimethyl-10,7′-bianthracene-9,9′,10′-trione) | −5.2521 | 1.3390 | −6.4419 | 1.2682 | A. Saponaria | [81] |
| 24 | 10-(chrysophanol-7′-yl)-10-hydroxychrysophanol-9-anthrone | −6.0946 | 1.6603 | −5.7400 | 1.2765 | A. megalacantha | [67] |
| 25 | Chrysalodin | −6.3819 | 1.4365 | −5.8787 | 1.3680 | A. megalacantha | [67] |
| 26 | 10-O-Methylchrysalodin | −5.1180 | 0.9839 | −5.1616 | 1.0078 | A. megalacantha | [67] |
| 27 | Elgonica-dimer A | −6.6933 | 1.1067 | −2.5862 | 1.4349 | A. elgonica, A. vera | [87,88,89] |
| 28 | Elgonica-dimer B | −6.4416 | 1.2142 | 5.4378 | 1.6013 | A. elgonica, A. vera | [87,88,89] |
| 29 | 1,4′,5′,8,9′-Pentahydroxy-2′,6-dimethyl[2,9′-bianthracene]-9,10′(9′H,10H)-dione | −6.1595 | 1.1344 | −6.2983 | 1.4995 | A. Saponaria | [81] |
| 30 | Aloin A | −6.1758 | 1.0225 | −5.8047 | 1.3500 | A. calidophila, A. schelpei, A. vera, A. perryi, A. ghibensis, A. gilbertii, A. trigonantha | [68,69,90,91,92,93,94,95,96] |
| 31 | Aloin B | −7.2794 | 1.3710 | −6.3227 | 1.1594 | A. vera, A. perryi, A. ghibensis, A. gilbertii,A. trigonantha | [68,69,90,91,92] |
| 32 | 7-Hydroxy-8-O-methylaloin A | −6.4555 | 1.4506 | −6.1034 | 1.1466 | A. vera | [97] |
| 33 | 7-Hydroxy-8-O-methylaloin B | −3.3579 | 1.4722 | −5.1135 | 1.1263 | A. vera | [97] |
| 34 | Nataloin | −3.1203 | 0.9706 | −7.1048 | 1.2243 | A. nyeriensis | [85] |
| 35 | 6′-O-Malonylnataloin | −6.1541 | 1.3288 | −3.4142 | 1.0014 | A. ellenbeckii | [98] |
| 36 | Homonataloin A | −3.4695 | 1.0697 | −5.4443 | 1.4948 | A. lateritia, A. distans, A. cremnophila, A. citrina, A. vera | [92,99,100] |
| 37 | Homonataloin B | −4.9349 | 1.4914 | −4.9807 | 0.8487 | A. lateritia, A. excelsa, A. vera, A. perryi | [92,99,101,102] |
| 38 | 5-Hydroxyaloin A | −4.8766 | 1.1457 | −6.6159 | 1.1690 | A. nobilis, A microstigma | [99,103,104] |
| 39 | 7-Hydroxyaloin A | −3.0164 | 1.4360 | −5.5526 | 0.9072 | A. ghibensis, A. succotrina | [73,91] |
| 40 | 3′-Acetyl-5-hydroxyaloin A | −6.6767 | 1.3118 | −5.1832 | 1.2296 | A. nobilis | [104] |
| 41 | 6′-Acetylglucosyl-5-hydroxyaloin A | −5.3535 | 1.3456 | −5.4990 | 1.1070 | A. marlothii,Aloe rupestris | [105] |
| 42 | 7-Hydroxy (6′-acetylGlucosyl)-aloin | −5.2629 | 1.2352 | −6.4008 | 1.4160 | A. succotrina | [73,106] |
| 43 | 7-hydroxy (6′-acetylglucosyl)-barbloine | −4.2565 | 1.4076 | −7.2279 | 0.8115 | A. succotrina | [73] |
| 44 | 2′,6′-Diacetylglucosyl-5-hydroxyaloin A. | −6.7157 | 1.5569 | −5.8313 | 1.3241 | A. nobilis | [104] |
| 45 | 4′,6′-Diacetylglucosyl-5-hydroxyaloin A | −4.9825 | 1.7716 | −5.7746 | 1.0670 | A. nobilis | [104] |
| 46 | 8-O-Methoxy-7-hydroxyaloin A | −5.2694 | 0.8441 | −1.2609 | 1.3127 | A. vera, A. trigonantha | [92,93] |
| 47 | 4′,6′-O-diacetate-7-Hydroxyaloin A | −1.0169 | 1.1487 | −3.6664 | 1.1714 | A. succotrina | [73] |
| 48 | 4′,6′-O-diacetate-7-Hydroxyaloin B | −5.9283 | 1.8131 | −3.8189 | 1.1443 | A. succotrina | [73] |
| 49 | Aloinoside A | −5.5327 | 1.6795 | −7.3722 | 1.6129 | A. ferox, A. spp. | [92,94,95,107] |
| 50 | Aloinoside B | −4.2386 | 2.0931 | −6.1225 | 0.9157 | A. vera, A. perryi | [69,72,86,92,107,108] |
| 51 | Aloinoside C | −6.7035 | 1.3302 | −7.0975 | 1.2894 | A. spp. | [69] |
| 52 | Homonataloside B | −7.4548 | 0.9307 | −7.0966 | 1.3986 | A. spp. | [109] |
| 53 | Microdontin A | −5.3854 | 1.8687 | −3.0568 | 1.3618 | A. gilbertii, A. microdonta, A. calidophila, A. vera, A. perryi, A. schelpei | [92,94,95,96,110] |
| 54 | Microdontin B | −6.4459 | 1.4075 | −6.2021 | 1.4041 | A. vera, A. perryi, A. microdonta | [92,110] |
| 55 | Microstigmin A | −6.2212 | 1.2076 | −6.7073 | 1.1774 | A. microstigma, A. broomii | [103] |
| 56 | Desoxyaloin | −5.1323 | 1.4371 | −5.4503 | 1.1224 | A. spp. | [69] |
| 57 | 8-O-Methoxy-7-hydroxyaloin B | −4.9067 | 1.2985 | −6.6635 | 1.4981 | A. vera, A. trigonantha | [92,93] |
| 58 | 7-Hydroxyaloin B | −5.7138 | 1.6502 | −6.2245 | 0.5540 | A. ghibensis, A. vera, A. succotrina | [73,91] |
| 59 | 6′-O-Acetyl-aloin B | −6.2829 | 1.1810 | −6.2769 | 1.2832 | A. vera,A. trigonantha | [93,111] |
| 60 | 6′-O-Acetyl-aloin A | −7.0170 | 1.3290 | −6.7174 | 1.4200 | A. vera, A. trigonantha | [93,111] |
| 61 | 6′-O-Acetyl-10-hydroxyaloin B | −5.4305 | 1.3206 | −5.1675 | 1.1849 | A. claviflora | [112] |
| 62 | 10-Hydroxyaloin A | −5.5235 | 1.2517 | −5.3937 | 0.9974 | A. vera | [68,72,86,97] |
| 63 | 10-Hydroxyaloin B | −4.5207 | 1.1644 | −6.2037 | 1.4416 | A. vera,A. littoralis | [68,72,97,113] |
| 64 | Aloinoside D | −4.0281 | 1.5439 | −5.7067 | 1.6872 | A. sp. | [69] |
| 65 | Deacetyllittoraloin | −5.4766 | 0.8960 | −3.2865 | 1.2900 | A. littoralis | [114] |
| 66 | Littoraloin | −6.7095 | 1.9848 | −6.6415 | 1.4619 | A. littoralis | [114] |
| 67 | Littoraloin | −5.4568 | 1.3628 | −1.6450 | 1.3238 | A. littoralis | [113] |
| 68 | Deacetyllittoraloin | −4.6536 | 1.4897 | −3.4045 | 1.2748 | A. littoralis | [113] |
| 69 | Littoraloside | −5.1344 | 1.6895 | −5.7699 | 1.5576 | A. littoralis | [114] |
| 70 | 6,8-Dihydroxy-4-methylbenzanthrone | −4.8093 | 0.9928 | −3.6017 | 1.1112 | A. vera | [115] |
| 71 | Anthrone; Enol-form | −3.7149 | 1.7269 | −3.3277 | 1.7661 | A. vera | [116] |
| 72 | 3,4-Dihydro-3,5,7-trihydroxy-9-methyl-1(2H)-anthracenone | −5.3077 | 0.5534 | −5.5367 | 1.5708 | A. vera | [117] |
| 73 | Aloesaponol II | −5.5596 | 0.9169 | −4.0884 | 1.1997 | A. saponaria | [78,80] |
| 74 | Aloesaponol II-6-methyl ether | −5.4278 | 0.8931 | −5.6829 | 0.8923 | A. dawei | [66] |
| 75 | Aloesaponol IV | −5.3144 | 1.0041 | −5.8792 | 1.2443 | A. saponaria | [79] |
| 76 | Aloesaponol I | −6.2731 | 0.6260 | −5.5268 | 1.1253 | A. megalacantha, A. dawei, A. saponaria | [66,67,78,80] |
| 77 | Aloesaponol III | −5.2375 | 1.6252 | −2.8273 | 0.5258 | A. saponaria | [79,80] |
| 78 | 8-O-Methyl-aloesaponol III | −4.7557 | 2.6159 | −5.4051 | 0.7606 | A. saponaria | [79] |
| 79 | Aloesaponol III-8-O-β-D-Glucopyranoside | −7.1900 | 0.9527 | −4.9331 | 1.1900 | A. saponaria | [79,80] |
| 80 | Aloesaponol IV-8-O-β-D-Glucopyranoside | −6.4329 | 0.9875 | −6.1878 | 1.0722 | A. saponaria | [79] |
| 81 | O-de-Methylaloesaponol IV-4-Epimer, 4-O-β-D-glucopyranoside | −5.2621 | 1.6257 | −6.0435 | 1.6168 | A. vera | [118,119] |
| 82 | Aloesaponol IV-4-Epimer, 4-O-β-D-glucopyranoside | −5.5878 | 1.4697 | −4.4875 | 1.2372 | A. vera | [118,119] |
| 83 | Aloesaponol II-6-glucoside | −5.5097 | 1.6405 | −6.3430 | 1.7211 | A. saponaria | [78,80] |
| 84 | Aloesaponol I-6-O-β-D-Glucoside | −6.4018 | 1.0686 | −6.4720 | 0.8722 | A. saponaria | [78,80] |
| 85 | Prechrysophanol | −5.0097 | 1.3074 | −4.9503 | 0.9273 | A. graminicola | [120] |
| 86 | Aloechrysone | −5.5183 | 1.0812 | −5.2203 | 1.1941 | A. berhana | [121] |
| II-Chromones: | |||||||
| 87 | 2,7-Dihydroxy-5-methylchromone | −4.1044 | 1.5749 | −4.3380 | 2.1225 | A. arborescens | [122] |
| 88 | Altechromone A | −4.3964 | 1.3753 | −4.0177 | 2.5253 | A. vera, A. ferox | [76] |
| 89 | 7-Hydroxy-5-(hydroxymethyl)-2-methylchromone | −4.6654 | 1.0133 | −4.0034 | 1.0116 | A. vera, A. spp. | [68,69,123] |
| 90 | 5-(Hydroxymethyl)-7-methoxy-2-methylchromone | −5.0942 | 1.7087 | −4.3604 | 0.6077 | A. vera | [68] |
| 91 | Aloesone | −5.3434 | 0.8671 | −4.4459 | 1.6654 | A. spp. | [124] |
| 92 | Aloesol | −5.4824 | 1.1217 | −4.5119 | 1.3491 | A. spp. | [124] |
| 93 | Saikochromone A | −4.0246 | 1.1592 | −4.6675 | 0.8802 | A. vera | [68] |
| 94 | 2−Carboxyethenyl-5,7-dihydroxychromone | −5.4560 | 0.8633 | −4.3033 | 1.4776 | A. cremnophila | [125] |
| 95 | 5-((4E)-2′-Oxopentenyl)-2-hydroxymethylchromone | −5.2408 | 1.2458 | −4.6543 | 0.9525 | A. vera | [68] |
| 96 | 2-Acetonyl-7-hydroxy-8-(3-hydroxyacetonyl)-5-methylchromone | −6.1295 | 1.1673 | −5.6294 | 0.8828 | A. ferox | [126] |
| 97 | 5-((S)-2′-Oxo-4′-hydroxypentyl)-2-hydroxymethylchromone | −5.6533 | 0.8672 | −4.5742 | 1.0536 | A. spp., A. vera | [68,69,123] |
| 98 | 7-O-methyl-(R)-aloesinol | −5.7749 | 1.3751 | −6.3349 | 1.0728 | A. capensis, A. rubroviolacea, A. spicata | [69,123,127,128] |
| 99 | 7-O-methyl-(S)-aloesinol | −4.9862 | 1.0634 | −6.5826 | 1.3861 | A. vera | [129,130,131] |
| 100 | Aloesinol; (2′S)-form | −6.5412 | 1.3314 | −5.9231 | 0.9830 | A. vera | [129,130,131] |
| 101 | 8-C-Glucosyl-7-O-methylaloediol | −6.5616 | 1.4702 | −6.1983 | 1.3370 | A. vera | [130,131,132] |
| 102 | C-2′-Decoumaroyl-aloeresin G | −6.3203 | 2.2082 | −5.6674 | 0.6253 | A. vera, A. spp. | [69,123,132] |
| 103 | Deacetylaloesin | −5.4580 | 1.4439 | −5.6769 | 0.7237 | A. vera var. chinensis | [133] |
| 104 | Isobiflorin | −4.3917 | 1.0141 | −5.5361 | 1.3146 | A. vera | [131] |
| 105 | 8-C-Glucosyl-noreugenin | −6.0155 | 0.9498 | −5.3311 | 1.2871 | A. vera | [132] |
| 106 | Neoaloesin A | −4.1314 | 1.4402 | −5.3727 | 1.2553 | A. vera | [132,134] |
| 107 | Aloesin (Aloeresin B) | −5.1920 | 1.4721 | −6.0011 | 0.9641 | A. vera, A. monticola, A. trigonantha A. saponaria, A. arborescens | [68,92,93,132,135,136,137] |
| 108 | 7-O-Methylaloesin | −6.5211 | 1.0562 | −4.7873 | 0.7405 | A. vera, A. rupestris | [92,105] |
| 109 | 2-Acetonyl-8-(2-furoylmethyl)-7-hydroxy-5-methylchromone | −5.5785 | 1.2539 | −5.9486 | 1.4100 | A. ferox | [126] |
| 110 | 8-C-Glucosyl-(R)-aloesol | −6.9810 | 1.2199 | −6.5885 | 1.2379 | A. vera | [68,92,132,136] |
| 111 | 8-C-Glucosyl-(S)-aloesol | −5.9979 | 1.2224 | −3.0178 | 1.2511 | A. vera | [132] |
| 112 | 8-C-Glucosyl-7-methoxy-(R)-aloesol | −6.9122 | 1.5762 | −5.2251 | 0.8301 | A. vera | [132] |
| 113 | 8-C-Glucosyl-7-methoxy-(S)-aloesol | −6.2867 | 1.2856 | −4.9903 | 0.9918 | A. vera | [68,132,136] |
| 114 | 2″-O-p-Coumaroyl-(S)-aloesinol | −6.4949 | 1.1099 | −6.3880 | 1.7012 | A. nobilis | [138] |
| 115 | 2″-O-(4-methoxycinnamoyl)-(S)-aloesinol | −6.8222 | 0.9924 | −8.0574 | 1.4996 | A. nobilis | [104] |
| 116 | 2″-O-Cinnamoyl-8-C-glucosyl-7-O-methyl-aloediol A | −6.9842 | 0.9200 | −7.4491 | 1.1903 | A. vera | [131] |
| 117 | 4″-Deoxyaloeresin D | −1.9659 | 1.4660 | −7.3969 | 1.4416 | A. vera | [139] |
| 118 | Aloeresin D | −7.3865 | 1.5512 | −6.6808 | 1.3432 | A. ferox, A. vera | [69,123,132,140,141] |
| 119 | Isoaloeresin D | −5.8280 | 1.4838 | −7.5561 | 1.2248 | A. vera | [68,92,129,130,131,132,136] |
| 120 | Rabaichromone | −7.3914 | 1.9603 | −7.8715 | 1.1703 | A. rabaiensis, A. vera | [132,142] |
| 121 | Isorabaichromone | −7.5470 | 1.4108 | −7.2656 | 1.4428 | A. vera | [129,130,131,132] |
| 122 | Aloeresin J | −6.9723 | 1.9908 | −6.4352 | 1.4979 | A. vera | [132] |
| 123 | 8-[C-β-D-[2-O-(E)-cinnamoyl] glucopyranosyl]-2-[(R)-2-hydroxypropyl]7-methoxy-5-methylchromone | −7.3516 | 0.9022 | −5.8935 | 1.2315 | A. vera | [132] |
| 124 | Aloeresin K | −6.2857 | 1.5121 | −6.9020 | 1.8499 | A. vera | [111,132] |
| 125 | Aloeresin F | −7.5814 | 1.9617 | −6.6320 | 1.4268 | A. peglerae | [143,144] |
| 126 | 2″-O-E-cinnamoyl-2′-Ketone (2′R) aloesinol-7-methyl ether | −6.5227 | 1.6510 | −5.7926 | 1.2582 | A. broomii | [145] |
| 127 | 7-O-Methylaloeresin A | −7.3061 | 1.1520 | −6.8482 | 0.9873 | A. vera, A. perryi, A. marlothii | [92,105,132] |
| 128 | Aloeresin A | −7.0228 | 1.1297 | −6.1730 | 1.3563 | A. cremnophila, A. jacksonii, A. arborescens, A. vera | [92,132,146] |
| 129 | Isoaloeresin A | −7.0698 | 1.8469 | −6.9601 | 1.6099 | A. ferox | [147] |
| 130 | 6″-O-p-Coumaroylaloesin | −6.1054 | 1.3654 | −5.5547 | 1.4791 | A. vera, A. castanea | [143,144] |
| 131 | Aloeribide | −6.5988 | 1.1683 | −8.0886 | 1.1338 | A. vera, A. monticola | [86] |
| 132 | 2″-O-(3,4-dihydroxy-E-cinnamoyl) 2′-Ketone, (2′R) Aloesinol-7-methyl ether | −7.6830 | 1.3783 | −5.1857 | 0.8784 | A. broomii | [145] |
| 133 | 2″-O-Feruloylaloesin | −6.3246 | 1.4172 | −7.3571 | 1.3775 | A. arborescens | [148] |
| 134 | 2″-O-(4-hydroxy-3-methoxy-E-cinnamoyl) 2′-Ketone, (2′R)-aloesinol-7-methyl ether | −7.9510 | 1.7236 | −6.5096 | 1.2159 | A. africana | [145] |
| 135 | 2″-O-tigloyl-2′-Ketone, (2′R)-aloesinol | −7.0060 | 1.0927 | −1.4865 | 1.2366 | A. cremnophila, A. jacksonii | [125] |
| 136 | 2″,6″-bis-O-(4-hydroxy-E-cinnamoyl) 2′-Ketone, (2′R)-aloesinol | −7.6466 | 1.6059 | −6.8589 | 2.8077 | A. speciosa | [145] |
| 137 | Aloeresin E | −6.8483 | 1.7166 | −3.3935 | 1.5214 | A. peglerae | [143,144] |
| 138 | Aloeresin C | −3.1926 | 1.4036 | 4.6545 | 1.2671 | A. spp. | [140,141] |
| 139 | trans-p-coumaroyl-4′-O-Glucosyl-isoaloeresin D-I a | −7.3070 | 1.8254 | −5.2510 | 1.8902 | A. vera | [129,130,131,132] |
| 140 | cis-p-coumaroyl-4′-O-Glucosylisoaloeresin D-II a | −5.1214 | 1.9414 | −6.9804 | 1.5954 | A. vera | [129,130,131,132] |
| 141 | 8-C-(2′-O-coumaroylglucosyl)-7-hydroxy-5-methyl-chromone-2-carboxylic acid | −7.0093 | 1.2428 | −3.8621 | 1.2324 | A. vera, A. perryi | [92] |
| 142 | 9-Dihydroxyl-2′-O-(Z)-cinnamoyl-7-methoxy-aloesin | −7.6616 | 1.3432 | −6.5510 | 1.0865 | A. vera | [132] |
| 143 | 5-((S)-2′-Oxo-4′-hydroxypentyl)-2-(β-D-glucopyranosyloxy-methyl) chromone | −6.3604 | 1.6246 | −5.6622 | 0.9999 | A. vera | [68,123] |
| 144 | 8-Glucosyl-(2′-O-cinnamoyl)-7-O-methyl-aloediol A | −6.8459 | 1.3641 | −6.1606 | 1.1397 | A. vera | [132] |
| 145 | Aloeresin G | −5.6136 | 1.0175 | −7.2021 | 1.2832 | A. vera | [69,123,149] |
| 146 | Aloeresin H | −7.5738 | 1.6723 | −7.5525 | 1.2922 | A. ferox | [150] |
| 147 | Aloeresin I | −7.4396 | 2.2729 | −7.4337 | 1.7717 | A. ferox | [151] |
| 148 | Iso-aloesin | −6.5817 | 0.9104 | −5.3266 | 1.3373 | A. vera var. chinensis | [152] |
| 149 | Aloeveraside B | −7.0658 | 1.3476 | −5.6113 | 1.4352 | A. vera | [72,132] |
| 150 | Aloeveraside A | −7.1344 | 1.1636 | −4.8353 | 1.2241 | A. vera | [72,132] |
| 151 | Furoaloesone | −5.2765 | 1.3977 | −5.3016 | 1.0583 | A. ferox | [153] |
| III-Coumarins: | |||||||
| 152 | Coumarin | −4.3565 | 1.3443 | −3.5142 | 0.7049 | A. vera | [86] |
| 153 | 7-Demethylsiderin | −4.4857 | 1.2535 | −4.6641 | 1.3044 | A. vera, A. megalacantha | [72,86] |
| IV-Flavonoids: | |||||||
| 154 | Apigenin | −4.8832 | 1.1056 | −4.9431 | 1.4340 | A. vera | [154] |
| 155 | Kaempferol | −5.5622 | 0.6388 | −5.2731 | 1.3518 | A. vera | [154] |
| 156 | Quercetin | −5.5045 | 0.8816 | −5.0091 | 1.3859 | A. vera | [154] |
| 157 | Myricetin | −4.3458 | 1.1650 | −5.1872 | 1.7167 | A. vera | [154] |
| 158 | Quercitrin | −5.9830 | 1.1253 | −6.6787 | 1.2189 | A. vera | [154] |
| 159 | Rutin | −7.7285 | 1.4051 | −6.5175 | 1.7434 | A. vera | [154] |
| 160 | 3′,5′,6,7-Tetra-methyl ether, 5-O-[α-L-rhamnopyranosyl-(1→6)-β-D-galactopyranoside], 3′,4′,5,5′,6,7-Hexahydroxyflavone | −6.4680 | 1.4828 | −7.2475 | 1.4030 | A. vera | [155] |
| 161 | Catechin | −5.5232 | 0.7426 | −5.0722 | 0.8989 | A. vera | [154] |
| 162 | Epicatechin | −5.3431 | 1.3310 | −5.0829 | 1.3469 | A. vera | [154] |
| 163 | 3′,4′,6-Tri-Me ether, 5-O-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranoside], 3′,4′,5,6,7-Pentahydroxyisoflavone | 0.3390 | 0.9914 | −4.0257 | 1.8089 | A. vera | [156] |
| V-Phenolic Compounds: a-Simple phenolic compounds: | |||||||
| 164 | Pyrocatechol | −3.9037 | 0.5358 | −3.1988 | 1.0732 | A. ferox | [157] |
| 165 | Salicylaldehyde | −2.6754 | 1.0693 | −3.2178 | 1.2797 | A. vera | [72,86] |
| 166 | p-Cresol | −3.7780 | 1.1628 | −3.5110 | 1.1472 | A. vera | [72,86] |
| 167 | p-Hydroxyacetophenone | −4.0224 | 1.8629 | −3.7773 | 0.7970 | A. ferox | [157] |
| 168 | p-Hydroxybenzaldehyde | −3.1097 | 0.7939 | −3.5440 | 1.5859 | A. ferox | [157] |
| 169 | p-Anisaldehyde | −3.9164 | 1.3669 | −3.4465 | 1.1778 | A. vera | [72,86] |
| 170 | Phloretic acid | −4.3823 | 1.2905 | −4.1107 | 1.0512 | A. vera | [72,86] |
| 171 | Methyl-3-(4-hydroxyphenyl) propionate | −4.7496 | 0.8674 | −3.9136 | 0.9518 | A. vera | [72,86] |
| 172 | Coumaric acid | −4.5055 | 1.4107 | −3.6587 | 1.1690 | A. vera | [154] |
| 173 | Caffeic acid | −4.1969 | 1.2736 | −3.8859 | 1.0162 | A. vera | [154] |
| 174 | Ferulic acid | −4.3845 | 0.9040 | −4.4689 | 1.0173 | A. vera | [154] |
| 175 | Sinapic acid | −5.1021 | 1.0097 | −4.2763 | 1.1207 | A. vera | [154] |
| 176 | Orcinol | −3.9362 | 1.2430 | −3.7654 | 1.4027 | A. sp. | [69] |
| 177 | Gentisic acid | −3.8595 | 1.6010 | −3.9828 | 1.3232 | A. vera | [154] |
| 178 | Protocatechuic acid | −3.6447 | 1.5645 | −4.1049 | 1.3881 | A. vera | [154] |
| 179 | Vanillic acid | −4.0256 | 1.0470 | −4.0827 | 0.9452 | A. vera | [154] |
| 180 | Gallic acid | −4.1961 | 0.5757 | −3.5679 | 0.8059 | A. vera | [154] |
| 181 | Syringic acid | −4.1467 | 1.2874 | −4.6308 | 1.2947 | A. vera | [154] |
| 182 | 1-(2,4-Dihydroxy-6-methylphenyl) ethanone | −3.8183 | 1.3698 | −3.9336 | 0.9642 | A. vera | [72] |
| b-Phenyl-Pyran and Phenyl-pyrone derivatives: | |||||||
| 183 | Aloenin aglycone. | −4.8494 | 1.2878 | −3.8957 | 1.3574 | A. vera, A. spp. | [68,69,123,158,159] |
| 184 | 6-(2,4-Dihydroxy-6-pentylphenyl)-4-hydroxy-2H-pyran-2-one | −3.1749 | 1.0339 | −5.5428 | 1.0487 | A. arborescens | [160] |
| 185 | 6-[[2,4-Dihydroxy-6-[2-(4-hydroxyphenyl) ethenyl]-4-hydroxy-2H-pyran-2-one | −5.9742 | 1.4002 | −5.8852 | 1.4624 | A. arborescens | [160] |
| 186 | Aloenin A | −5.3723 | 1.3544 | −4.5645 | 1.5586 | A. vera, A. arborescens | [85,92,123,159] |
| 187 | 2″-O-trans-p-coumaroyl-aloenin | −7.0328 | 1.6452 | −6.1026 | 1.2183 | A. vera, A. nyeriensis, A. spicata | [159] |
| 188 | 4″,6″-Ethylidenealoenin | −6.3221 | 2.1206 | −6.8580 | 1.0640 | A. arborescens, A. hijazensis | [82] |
| 189 | Aloenin C | −4.0535 | 1.3070 | −4.7701 | 1.7252 | A. sp. | [123] |
| 190 | 10-O-β-D-glucopyranosyl-aloenin | −6.1378 | 1.0013 | −6.6976 | 1.3721 | A. sp., A. vera, A. spicata | [127,136] |
| 191 | Aloenin B | −7.0573 | 1.9426 | −4.7766 | 1.3631 | A. hijazensis, A. spicata, A. vera | [68,69,82,123,136] |
| 192 | 2,4-Dihydroxy-β-(4-hydroxyphenyl)-5-(4-methoxy-2-oxo-2H-pyran-6-yl)-6-methylbenzenepropanoic acid; Et ester | −6.0558 | 1.2415 | −6.5747 | 1.4078 | A. vera | [161] |
| 193 | 6−[[3,5-Dihydroxy-2-(1-oxohexyl) phenyl] methyl]-4-hydroxy-2H-pyran-2-one | −5.5175 | 1.3328 | −5.1989 | 1.4473 | A. arborescens | [160] |
| 194 | 6-[[3,5-Dihydroxy-2-[3-(4-hydroxyphenyl)-1-oxo-2-propenyl] phenyl] methyl]-4-hydroxy-2H-pyran-2-one | −3.9537 | 1.4667 | −6.8047 | 1.2550 | A. arborescens | [160] |
| 195 | 3,4-Dihydro-7-hydroxy-4-(4-hydroxyphenyl)-6-(4-methoxy-2-oxo-2H-pyran-6-yl)-5-methyl-2H-1-benzopyran-2-one | −6.0022 | 1.3606 | −6.4475 | 1.1932 | A. vera | [161] |
| 196 | Feralolide | −5.7980 | 1.8453 | −5.4781 | 1.4521 | A. arborescent, A. ferox, A. vera | [70,82,86,162,163] |
| 197 | 5′-O-Methylferalolide | −6.3808 | 1.3485 | −5.9990 | 1.3806 | A. vera | [164] |
| 198 | Feralolide-3′-O-β-D-Glucopyranoside | −5.6364 | 1.3200 | −4.3041 | 2.3081 | A. arborescens, A. vera | [70,165] |
| 199 | 3,3′-Bi(3,4-dihydro-6-methoxy-2H-1-benzopyran-4-ol). 3,3′-Bi(3,4-dihydro-4-hydroxy-6-methoxy-2H-1-benzopyran) | −5.3093 | 1.7270 | −5.2061 | 1.4071 | A. vera | [166] |
| c-Benzofurans: | |||||||
| 200 | 5-Hydroxy-3-methylnaphtho[2,3-c] furan-4(1H)-one | −4.3383 | 1.1118 | −4.0958 | 0.7359 | A. ferox | [71] |
| 201 | 5-Hydroxy-3-methylnaphtho[2,3-c] furan-4(9H)-one | −4.1346 | 1.3867 | −4.0957 | 0.7362 | A. ferox | [71] |
| 202 | Isoeleutherol | −4.7544 | 1.2527 | −4.5232 | 0.7749 | A. graminicola | [167] |
| 203 | Isoeleutherol glucoside | −6.0044 | 1.3381 | −4.6325 | 1.4751 | A. saponaria | [80] |
| 204 | 8-Hydroxy-1-methylnaphtho[2,3-c] furan-4,9-dione | −5.0447 | 1.3119 | −4.3271 | 0.8506 | A. ferox | [71] |
| d-Naphthalin derivatives: | |||||||
| 205 | Droserone | −4.5525 | 1.0154 | −4.1450 | 1.1420 | A. dawei | [66] |
| 206 | Droserone-5-methyl ether | −4.8881 | 1.1213 | −4.2859 | 1.2524 | A. dawei | [66] |
| 207 | Hydroxydroserone | −4.9712 | 1.0739 | −3.7656 | 1.9742 | A. dawei | [66] |
| 208 | Ancistroquinone C | −4.8894 | 1.4452 | −4.2108 | 0.5425 | A. dawei | [66] |
| 209 | 5,8-Dihydroxy-3-methoxy-2-methyl-1,4-naphthoquinone | −5.0591 | 1.0203 | −3.5906 | 0.8949 | A. dawei | [66] |
| 210 | Malvone A | −4.7003 | 0.6291 | −4.7158 | 1.0717 | A. dawei | [66] |
| 211 | 6-Hydroxy-3,5-dimethoxy-2-methyl-1,4-naphthoquinone | −4.8603 | 1.1151 | −4.2108 | 1.2529 | A. dawei | [66] |
| 212 | 1,8-Dimethoxynepodinol | −4.8625 | 1.0642 | −4.1478 | 0.9217 | A. megalacantha | [67] |
| 213 | 3-Hydroxy-1-(1,7-dihydroxy-3,6-dimethoxynaphthalen-2-yl) propan-1-one | −5.8504 | 0.7746 | −5.2376 | 1.0515 | A. vera | [168] |
| 214 | Plicataloside | −3.4118 | 1.4389 | −5.6793 | 1.2349 | A. plicatilis | [169] |
| 215 | Kenyaloside | −7.2910 | 1.6098 | −4.6954 | 1.3254 | a Kenyan A. spp. | [170] |
| 216 | Aloveroside A | −7.5802 | 1.8561 | −6.8612 | 1.5133 | A. vera, A. spp. | [69,123,171] |
| 217 | Isoeleutherin | −4.3217 | 1.3635 | −3.7234 | 0.6554 | A. graminicola | [167] |
| 218 | 1-(4-Hydroxyphenyl)-6,9-dihydroxy-7-methyl-8-acetyl-1,2-dihydro-(3H)-naphtho[2,1-b] pyran-3-one | −3.8117 | 1.5671 | −6.9288 | 0.9758 | A. ferox | [172] |
| VI-Alkaloids: | |||||||
| 219 | 4,7-Dichloroquinoline | −1.6976 | 0.2458 | −3.5415 | 1.2153 | A. hijazensis | [82] |
| 220 | N, N-Dimethyl-(+)-coniine | −1.9420 | 0.6273 | −1.7066 | 1.3831 | A. sabaea | [173] |
| 221 | γ-Coniceine | −4.2358 | 0.9461 | −3.6823 | 0.9519 | A. sp. | [173,174] |
| VII-Fatty acid derivatives: | |||||||
| 222 | 10-Hydroxyoctadecanoic acid | −6.1427 | 1.4991 | −4.4657 | 1.1518 | A. ferox | [157] |
| 223 | 10-Oxooctadecanoic acid | -6.3362 | 1.0628 | −5.8079 | 1.1759 | A. ferox | [157] |
| 224 | Methyl-26-O-feruloyl-oxyhexacosanate | −7.6053 | 1.7939 | −7.2349 | 1.2702 | A. megalacantha | [67] |
| VIII-Miscellaneous compounds: | |||||||
| 225 | Nilic acid | −4.0096 | 0.9979 | −2.3951 | 0.6370 | A. littoralis | [113] |
| 226 | N-(4-Chlorobutyl) butanamide | −4.7596 | 0.6115 | −3.9758 | 0.9418 | A. sabaea | [173] |
| 227 | 3-Furanmethanol | −3.9177 | 0.9518 | −3.4939 | 0.7289 | A. arborescens | [175] |
| 228 | 3, 6-Dioxo-3, 3a, 6, 6 a-tetrahydropyrrolo [3, 4-c] pyrrole-1, 4-dicarboxamide | −4.1012 | 1.7165 | −3.8036 | 1.0232 | A. vera | [176] |
| 229 | 1-(2,4-Dihydroxy-6-methylphenyl)-1-(4-hydroxyphenyl) ethane | −4.7735 | 2.0838 | −2.0969 | 1.0198 | A. ferox | [177] |
| 230 | Chlorogenic acid | −4.1722 | 1.3907 | −5.6761 | 1.2173 | A. vera, A. arborescens | [154] |
| 231 | Pluridone; (E)-form | −4.9712 | 1.2855 | −3.8181 | 1.0980 | A. pluridens | [178] |
| 232 | Feroxidin | −4.3149 | 1.0601 | −3.9958 | 1.0673 | A. ferox, A. arborescens | [69,70,72,86,123,179,180] |
| 233 | Feroxin A | −5.5313 | 1.4467 | −5.2869 | 1.0687 | A. spp. | [181] |
| 234 | Feroxin B | −6.6446 | 2.1091 | −6.2902 | 1.7466 | A. spp. | [181] |
| 235 | Veracylglucan A | −5.7829 | 1.2199 | −4.7653 | 0.8308 | A. vera | [182] |
| 236 | Veracylglucan B | −3.0836 | 1.0444 | −1.0368 | 1.0780 | A. vera | [182] |
| 237 | Veracylglucan C | −6.3433 | 2.2092 | −7.2512 | 1.9263 | A. vera | [182] |
References
- EI Zowalaty, M.E.; Young, S.G.; Järhult, J.D. Environmental impact of the COVID-19 pandemic–a lesson for the future. Infect. Ecol. Epidemiol. 2020, 10, 1768023. [Google Scholar] [CrossRef]
- El Zowalaty, M.E.; Järhult, J.D. From SARS to COVID-19: A previously unknown SARS-related coronavirus (SARS-CoV-2) of pandemic potential infecting humans–Call for a One Health approach. One Health 2020, 9, 100124. [Google Scholar] [CrossRef]
- Wu, F.; Wang, A.; Liu, M.; Wang, Q.; Chen, J.; Xia, S.; Ling, Y.; Zhang, Y.; Xun, J.; Lu, L.; et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. 2020. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3566211 (accessed on 15 January 2021).
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.; Netland, J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Genet. 2009, 7, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Receptor Recognition Mechanisms of Coronaviruses: A Decade of Structural Studies. J. Virol. 2015, 89, 1954–1964. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, S.; Poma, A.B.; Kolandaivel, P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 2020, 1–10. [Google Scholar] [CrossRef]
- Heald-Sargent, T.; Gallagher, T. Ready, Set, Fuse! The Coronavirus Spike Protein and Acquisition of Fusion Competence. Viruses 2012, 4, 557–580. [Google Scholar] [CrossRef]
- Moreira, R.A.; Chwastyk, M.; Baker, J.L.; Guzman, H.A.V.; Poma, A.B. Quantitative determination of mechanical stability in the novel coronavirus spike protein. Nanoscale 2020, 12, 16409–16413. [Google Scholar] [CrossRef]
- Moreira, R.A.; Guzman, H.V.; Boopathi, S.; Baker, J.L.; Poma, A.B. Characterization of Structural and Energetic Differences between Conformations of the SARS-CoV-2 Spike Protein. Materials 2020, 13, 5362. [Google Scholar] [CrossRef]
- Anand, K. Coronavirus Main Proteinase (3CLpro) Structure: Basis for Design of Anti-SARS Drugs. Science 2003, 300, 1763–1767. [Google Scholar] [CrossRef]
- Gil, C.; Ginex, T.; Maestro, I.; Nozal, V.; Barrado-Gil, L.; Cuesta-Geijo, M.Á.; Urquiza, J.; Ramírez, D.; Alonso, C.; Campillo, N.E.; et al. COVID-19: Drug Targets and Potential Treatments. J. Med. Chem. 2020, 63, 12359–12386. [Google Scholar] [CrossRef] [PubMed]
- Heimfarth, L.; Serafini, M.R.; Martins-Filho, P.R.; Quintans, J.D.S.S.; Quintans-Júnior, L.J. Drug repurposing and cytokine management in response to COVID-19: A review. Int. Immunopharmacol. 2020, 88, 106947. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Parvez, S. COVID-19: An overview of the current pharmacological interventions, vaccines, and clinical trials. Biochem. Pharmacol. 2020, 180, 114184. [Google Scholar] [CrossRef]
- Thota, S.M.; Balan, V.; Sivaramakrishnan, V. Natural products as home-based prophylactic and symptom management agents in the setting of COVID-19. Phytother. Res. 2020, 34, 3148–3167. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrient 2020, 12, 2534. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Salto, R.; Li, J.; Craik, C.; de Montellano, P.R.O. Inhibition of the HIV-1 and HIV-2 proteases by curcumin and curcumin boron complexes. Bioorg. Med. Chem. 1993, 1, 415–422. [Google Scholar] [CrossRef]
- Mehla, R.; Bivalkar-Mehla, S.; Chauhan, A. A Flavonoid, Luteolin, Cripples HIV-1 by Abrogation of Tat Function. PLoS ONE 2011, 6, e27915. [Google Scholar] [CrossRef] [PubMed]
- Ürményi, F.G.G.; Saraiva, G.D.N.; Casanova, L.M.; Matos, A.D.S.; Camargo, L.M.D.M.; Romanos, M.T.V.; Costa, S.S. Anti-HSV-1 and HSV-2 Flavonoids and a New Kaempferol Triglycoside from the Medicinal Plant Kalanchoe daigremontiana. Chem. Biodivers. 2016, 13, 1707–1714. [Google Scholar] [CrossRef]
- Behbahani, M.; Sayedipour, S.; Pourazar, A.; Shanehsazzadeh, M. In vitro anti-HIV-1 activities of kaempferol and kaempferol-7-O-glucoside isolated from Securigera securidaca. Res. Pharm. Sci. 2015, 9, 463–469. [Google Scholar]
- Mpiana, P.T.; Tshibangu, D.S.; Kilembe, J.T.; Gbolo, B.Z.; Mwanangombo, D.T.; Inkoto, C.L.; Lengbiye, E.M.; Mbadiko, C.M.; Matondo, A.; Bongo, G.N.; et al. Identification of potential inhibitors of SARS-CoV-2 main protease from Aloe vera compounds: A molecular docking study. Chem. Phys. Lett. 2020, 754, 137751. [Google Scholar] [CrossRef]
- Rezazadeh, F.; Moshaverinia, M.; Motamedifar, M.; Alyaseri, M. Assessment of Anti HSV-1 Activity of Aloe Vera Gel Extract: An In Vitro Study. J. Dent. 2016, 17, 49–54. [Google Scholar]
- Abd-Alla, H.I.; Abu-Gabal, N.S.; Hassan, A.Z.; El-Safty, M.M.; Shalaby, N.M.M. Antiviral activity of Aloe hijazensis against some haemagglutinating viruses infection and its phytoconstituents. Arch. Pharmacal Res. 2012, 35, 1347–1354. [Google Scholar] [CrossRef]
- Saoo, K.; Miki, H.; Ohmori, M.; Winters, W.D. Antiviral Activity of Aloe Extracts against Cytomegalovirus. Phytother. Res. 1996, 10, 348–350. [Google Scholar] [CrossRef]
- Huang, C.-T.; Hung, C.-Y.; Hseih, Y.-C.; Chang, C.-S.; Velu, A.B.; He, Y.-C.; Huang, Y.-L.; Chen, T.-A.; Chen, T.-C.; Lin, C.-Y.; et al. Effect of aloin on viral neuraminidase and hemagglutinin-specific T cell immunity in acute influenza. Phytomedicine 2019, 64, 152904. [Google Scholar] [CrossRef] [PubMed]
- Subbaiyan, A.; Ravichandran, K.; Singh, S.V.; Sankar, M.; Thomas, P.; Dhama, K.; Malik, Y.S.; Singh, R.K.; Chaudhuri, P. In silico Molecular Docking Analysis Targeting SARS-CoV-2 Spike Protein and Selected Herbal Constituents. J. Pure Appl. Microbiol. 2020, 14, 989–998. [Google Scholar] [CrossRef]
- Jorgensen, W.L. The Many Roles of Computation in Drug Discovery. Science 2004, 303, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- Ramajayam, R.; Tan, K.-P.; Liang, P.-H. Recent development of 3C and 3CL protease inhibitors for anti-coronavirus and anti-picornavirus drug discovery. Biochem. Soc. Trans. 2011, 39, 1371–1375. [Google Scholar] [CrossRef]
- Salman, S.; Shah, F.H.; Idrees, J.; Idrees, F.; Velagala, S.; Ali, J.; Khan, A.A. Virtual screening of immunomodulatory medicinal compounds as promising anti-SARS-COV-2 inhibitors. Future Virol. 2020, 15, 267–275. [Google Scholar] [CrossRef]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef]
- Al-Bari, M.A.A. Chloroquine analogues in drug discovery: New directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015, 70, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Shen, H.-M. Targeting the Endocytic Pathway and Autophagy Process as a Novel Therapeutic Strategy in COVID-19. Int. J. Biol. Sci. 2020, 16, 1724–1731. [Google Scholar] [CrossRef]
- Rivinoja, A.; Hassinen, A.; Kokkonen, N.; Kauppila, A.; Kellokumpu, S. Elevated Golgi pH impairs terminalN-glycosylation by inducing mislocalization of Golgi glycosyltransferases. J. Cell. Physiol. 2009, 220, 144–154. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Huang, Y.Y.; Wu, Y.; Liu, R.; Zhou, L.; Lin, Y.; Wu, D.; Zhang, L.; Liu, H.; et al. Identify potent SARS-CoV-2 main protease inhibitors via accelerated free energy perturbation-based virtual screening of existing drugs. Proc. Natl. Acad. Sci. USA 2020, 117, 27381–27387. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Libera, J.L.; Durán-Verdugo, F.; Valdés-Jiménez, A.; Núñez-Vivanco, G.; Caballero, J. LigRMSD: A web server for automatic structure matching and RMSD calculations among identical and similar compounds in protein-ligand docking. Bioinformatics 2020, 36, 2912–2914. [Google Scholar] [CrossRef]
- Totrov, M.; Abagyan, R. Flexible ligand docking to multiple receptor conformations: A practical alternative. Curr. Opin. Struct. Biol. 2008, 18, 178–184. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, M.; Masetti, M.; Bottegoni, G.; Cavalli, A. Role of Molecular Dynamics and Related Methods in Drug Discovery. J. Med. Chem. 2016, 59, 4035–4061. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, M.; Cavalli, A. Recent advances in dynamic docking for drug discovery. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2017, 7, 1320. [Google Scholar] [CrossRef]
- Souza, P.C.T.; Thallmair, S.; Conflitti, P.; Ramírez-Palacios, C.; Alessandri, R.; Raniolo, S.; Limongelli, V.; Marrink, S.J. Protein–ligand binding with the coarse-grained Martini model. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arokiyaraj, S.; Stalin, A.; Kannan, B.S.; Shin, H. Geranii Herba as a Potential Inhibitor of SARS-CoV-2 Main 3CLpro, Spike RBD, and Regulation of Unfolded Protein Response: An In Silico Approach. Antibiotics 2020, 9, 863. [Google Scholar] [CrossRef]
- Hussien, M.A.; Abdelaziz, A.E. Molecular docking suggests repurposing of brincidofovir as a potential drug targeting SARS-CoV-2 ACE2 receptor and main protease. Netw. Model. Anal. Health Inform. Bioinformatics 2020, 9, 1–18. [Google Scholar]
- Sayed, A.M.; Alhadrami, H.A.; El-Gendy, A.O.; Shamikh, Y.I.; Belbahri, L.; Hassan, H.M.; Abdelmohsen, U.R.; Rateb, M.E. Microbial natural products as potential inhibitors of SARS-CoV-2 main protease (Mpro). Microorganisms 2020, 8, 970. [Google Scholar] [CrossRef] [PubMed]
- Saurav, K.; Zhang, W.; Saha, S.; Zhang, H.; Li, S.; Zhang, Q.; Wu, Z.; Zhang, G.; Zhu, Y.; Verma, G. In silico molecular docking, preclinical evaluation of spiroindimicins A-D, lynamicin A and D isolated from deep marine sea derived Streptomyces sp. SCSIO 03032. Interdiscip. Sci. Comput. Life Sci. 2014, 6, 187–196. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Foti, R.S.; Wahlstrom, J.L. CYP2C19 Inhibition: The Impact of Substrate Probe Selection on in Vitro Inhibition Profiles. Drug Metab. Dispos. 2007, 36, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Van Booven, D.; Marsh, S.; McLeod, H.; Carrillo, M.W.; Sangkuhl, K.; Klein, T.E.; Altman, R.B. Cytochrome P450 2C9-CYP2C9. Pharm. Genom. 2010, 20, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, L.; Dahl, M.-L.; Dalén, P.; Al-Shurbaji, A. Molecular genetics of CYP2D6: Clinical relevance with focus on psychotropic drugs. Br. J. Clin. Pharmacol. 2002, 53, 111–122. [Google Scholar] [CrossRef]
- Dai, D.; Tang, J.; Rose, R.; Hodgson, E.; Bienstock, R.J.; Mohrenweiser, H.W.; A Goldstein, J. Identification of variants of CYP3A4 and characterization of their abilities to metabolize testosterone and chlorpyrifos. J. Pharmacol. Exp. Ther. 2001, 299, 825–831. [Google Scholar]
- Wessler, J.D.; Grip, L.T.; Mendell, J.; Giugliano, R.P. The P-Glycoprotein Transport System and Cardiovascular Drugs. J. Am. Coll. Cardiol. 2013, 61, 2495–2502. [Google Scholar] [CrossRef]
- Sharom, F.J. Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function. Front. Oncol. 2014, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, J. Transdermal drug delivery by passive diffusion and iontophoresis: A review. Med. Res. Rev. 1993, 13, 569–621. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Anissimov, Y.G.; Bunge, A.L.; Frasch, H.F.; Guy, R.H.; Hadgraft, J.; Kasting, G.B.; Lane, M.E.; Roberts, M.S. Mathematical models of skin permeability: An overview. Int. J. Pharm. 2011, 418, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M pro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Cozza, G.; Moro, S. Medicinal Chemistry and the Molecular Operating Environment (MOE): Application of QSAR and Molecular Docking to Drug Discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]
- MacKerell, A.D., Jr.; Bashford, D.; Bellott, M.L.D.R.; Dunbrack, R.L., Jr.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.; et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Jo, S.; Jiang, W.; Lee, H.S.; Roux, B.; Im, W. CHARMM-GUI Ligand Binder for Absolute Binding Free Energy Calculations and Its Application. J. Chem. Inf. Model. 2013, 53, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein–ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef]
- Allam, A.E.; Assaf, H.K.; Hassan, H.A.; Shimizu, K.; Elshaier, Y.A.M.M. An in silico perception for newly isolated flavonoids from peach fruit as privileged avenue for a countermeasure outbreak of COVID-19. RSC Adv. 2020, 10, 29983–29998. [Google Scholar] [CrossRef]
- Ibrahim, A.K.; Youssef, A.I.; Arafa, A.S.; Ahmed, S.A. Anti-H5N1 virus flavonoids fromCapparis sinaicaVeill. Nat. Prod. Res. 2013, 27, 2149–2153. [Google Scholar] [CrossRef] [PubMed]
- Van Vuong, Q.; Nguyen, T.T.; Li, M.S. A New Method for Navigating Optimal Direction for Pulling Ligand from Binding Pocket: Application to Ranking Binding Affinity by Steered Molecular Dynamics. J. Chem. Inf. Model. 2015, 55, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Abdissa, D.; Geleta, G.; Bacha, K.; Abdissa, N. Phytochemical investigation of Aloe pulcherrima roots and evaluation for its antibacterial and antiplasmodial activities. PLoS ONE 2017, 12, e0173882. [Google Scholar] [CrossRef] [PubMed]
- Abdissa, N.; Induli, M.; Fitzpatrick, P.; Alao, J.P.; Sunnerhagen, P.; Landberg, G.; Yenesew, A.; Erdélyi, M. Cytotoxic Quinones from the Roots of Aloe dawei. Molecules 2014, 19, 3264–3273. [Google Scholar] [CrossRef]
- Abdissa, N.; Gohlke, S.; Frese, M.; Sewald, N. Cytotoxic Compounds from Aloe megalacantha. Molecules 2017, 22, 1136. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, Y.; Ding, W.; Wu, X.; Wan, J.; Luo, H. Chemical constituents of Aloe barbadensis Miller and their inhibitory effects on phosphodiesterase-4D. Fitoterapia 2013, 91, 159–165. [Google Scholar] [CrossRef]
- Sun, Y.N.; Li, L.Y.; Li, W.; Kang, J.S.; Hwang, I.; Kim, Y.H. Chemical Components from Aloe and their Inhibition of Indoleamine 2, 3-dioxygenase. Pharmacogn. Mag. 2017, 13, 58–63. [Google Scholar]
- Kurizaki, A.; Watanabe, T.; Devkota, H.P. Chemical Constituents from the Flowers of Aloe arborescens. Nat. Prod. Commun. 2019, 14, 1934578–19844135. [Google Scholar] [CrossRef]
- Koyama, J.; Ogura, T.; Tagahara, K. Naphtho[2,3-c]furan-4,9-dione and its derivatives from Aloe ferox. Phytochemistry 1994, 37, 1147–1148. [Google Scholar] [CrossRef]
- Rehman, N.U.; Al-Riyami, S.A.; Hussain, H.; Ali, A.; Khan, A.L.; Al-Harrasi, A. Secondary metabolites from the resins of Aloe vera and Commiphora mukul mitigate lipid peroxidation. Acta Pharm. 2019, 69, 433–441. [Google Scholar] [CrossRef]
- Sigler, A.; Rauwald, H.W. Aloe Plants Accumulate Anthrone-Type Anthranoids in Inflorescence and Leaves, and Tetrahydroanthracenes in Roots. Z. Nat. C 1994, 49, 286–292. [Google Scholar] [CrossRef]
- Van Oudtshoorn, M.v.R. Chemotaxonomic investigations in asphodeleae and aloineae (liliaceae). Phytochemistry 1964, 3, 383–390. [Google Scholar] [CrossRef]
- Kambizi, L.; Sultana, N.; Afolayan, A. Bioactive Compounds Isolated fromAloe ferox.: A Plant Traditionally Used for the Treatment of Sexually Transmitted Infections in the Eastern Cape, South Africa. Pharm. Biol. 2005, 42, 636–639. [Google Scholar] [CrossRef]
- Awe, W.; Kuemmell, H. On the occurrence of aloin in Aloe vera in addition to comparative studies with the fresh juice of Cape aloe (Aloe ferox) and the dried extract prepared from it. Arch. Pharm. 1962, 295, 819–822. [Google Scholar] [CrossRef]
- Hirata, T.; Suga, T. Biologically Active Constituents of Leaves and Roots of Aloe arborescens var. natalensis. Z. Nat. C 1977, 32, 731–734. [Google Scholar] [CrossRef]
- Yagi, A.; Makino, K.; Nishioka, I. Studies on the Constituents of Aloe sapnaria HAW. I. The Structures of Tetrahydroanthracene Derivatives and the Related Anthraquinones. Chem. Pharm. Bull. 1974, 22, 1159–1166. [Google Scholar] [CrossRef]
- Yagi, A.; Makino, K.; Nishioka, I. Studies on the constituents of Aloe saponaria Haw. II. The structures of tetrahydroanthracene derivatives, aloesaponol III and -IV. Chem. Pharm. Bull. 1977, 25, 1764–1770. [Google Scholar] [CrossRef]
- Yagi, A.; Makino, K.; Nishioka, I. Studies on the constituents of Aloe saponaria Haw. III. The structures of phenol glucosides. Chem. Pharm. Bull. 1977, 25, 1771–1776. [Google Scholar] [CrossRef][Green Version]
- Yagi, A.; Makino, K.; Nishioka, I. Studies on the constituents of Aloe saponaria Haw. IV. The structures of bianthraquinoid pigments. Chem. Pharm. Bull. 1978, 26, 1111–1116. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Shaaban, M.; Shaaban, K.A.; Abu-Gabal, N.S.; Shalaby, N.M.; Laatsch, H. New bioactive compounds fromAloe hijazensis. Nat. Prod. Res. 2009, 23, 1035–1049. [Google Scholar] [CrossRef]
- Epifano, F.; Fiorito, S.; Locatelli, M.; Taddeo, V.A.; Genovese, S. Screening for novel plant sources of prenyloxyanthraquinones: Senna alexandrina Mill. and Aloe vera (L.) Burm. F. Nat. Prod. Res. 2015, 29, 180–184. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; CABI: Wallingford, England, 2018. [Google Scholar]
- Conner, J.M.; Gray, A.I.; Reynolds, T.; Waterman, P.G. Anthraquinone, anthrone and phenylpyrone components of Aloe nyeriensis var. kedongensis leaf exudate. Phytochemistry 1987, 26, 2995–2997. [Google Scholar] [CrossRef]
- Rehman, N.U.; Khan, A.; Al-Harrasi, A.; Khiat, M.; Hussain, H.; Wadood, A.; Riaz, M. Natural urease inhibitors from Aloe vera resin and Lycium shawii and their structural-activity relationship and molecular docking study. Bioorg. Chem. 2019, 88, 102955. [Google Scholar] [CrossRef]
- Conner, J.M.; Gray, A.I.; Waterman, P.G.; Reynolds, T. Novel Anthrone-Anthraquinone Dimers from Aloe elgonica. J. Nat. Prod. 1990, 53, 1362–1364. [Google Scholar] [CrossRef]
- Shin, K.H.; Woo, W.S.; Lim, S.S.; Shim, C.S.; Chung, H.S.; Kennelly, E.J.; Kinghorn, A.D. Elgonica-Dimers A and B, Two Potent Alcohol Metabolism Inhibitory Constituents of Aloe arborescens. J. Nat. Prod. 1997, 60, 1180–1182. [Google Scholar] [CrossRef]
- Saleem, R.; Faizi, S.; Hussain, S.A.; Qazi, A.; Dar, A.; Ahmad, S.I.; Qazi, M.; Akhtar, S.; Hasnain, S.N.; Siddiqui, B.S.; et al. Hypotensive Effect of Chemical Constituents fromAloe barbadensis. Planta Med. 2001, 67, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Auterhoff, H.; Graf, E.; Eurisch, G.; Alexa, M. Trennung des Aloins in Diastereomere und deren Charakterisierung. Arch. Pharm. 1980, 313, 113–120. [Google Scholar] [CrossRef]
- Tekassa, T.; Tewabe, Y.; Bisrat, D.; Hailu, A.; Asres, K. Antileishmanial activities of leaf latex and compound isolated from Aloe ghibensis Sebsebe & Friis. Ethiop. Pharm. J. 2020, 35, 51–58. [Google Scholar] [CrossRef]
- Aldayel, T.S.; Grace, M.H.; Lila, M.A.; Yahya, M.A.; Omar, U.M.; Alshammary, G. LC-MS characterization of bioactive metabolites from two Yemeni Aloe spp. with antioxidant and antidiabetic properties. Arab. J. Chem. 2020, 13, 5040–5049. [Google Scholar] [CrossRef]
- Megeressa, M.; Bisrat, D.; Mazumder, A.; Asres, K. Structural elucidation of some antimicrobial constituents from the leaf latex of Aloe trigonantha L.C. Leach. BMC Complement. Altern. Med. 2015, 15, 270. [Google Scholar] [CrossRef] [PubMed]
- Teka, T.; Kassahun, H. Characterization and Evaluation of Antioxidant Activity of Aloe schelpei Reynolds. Drug Des. Dev. Ther. 2020, ume 14, 1003–1008. [Google Scholar] [CrossRef]
- Kassahun, A.; Bisrat, D. Free radical scavenging activities of three anthrones from Aloe gilbertii reynolds leaf latex. J. Nat. Prod. Plant Resour. 2017, 7, 40–44. [Google Scholar]
- Abeje, F.; Bisrat, D.; Hailu, A.; Asres, K. Phytochemistry and Antileishmanial Activity of the Leaf Latex ofAloe calidophilaReynolds. Phytother. Res. 2014, 28, 1801–1805. [Google Scholar] [CrossRef]
- Okamura, N.; Hine, N.; Harada, S.; Fujioka, T.; Mihashi, K.; Nishi, M.; Miyahara, K.; Yagi, A. Diastereomeric C-glucosylanthrones of Aloe vera leaves. Phytochemistry 1997, 45, 1519–1522. [Google Scholar] [CrossRef]
- Grace, O.; Kokubun, T.; Veitch, N.; Simmonds, M. Characterisation of a nataloin derivative from Aloe ellenbeckii, a maculate species from east Africa. South. Afr. J. Bot. 2008, 74, 761–763. [Google Scholar] [CrossRef]
- Rauwald, H.W.; Beil, A. 5-Hydroxyaloin A in the Genus Aloe Thin Layer Chromatographic Screening and High Performance Liquid Chromatographic Determination. Z. Nat. C 1993, 48, 1–4. [Google Scholar] [CrossRef]
- Asres, K.; Girma, B.; Bisrat, D. Antimalarial evaluation of the leaf latex of Aloe citrina and its major constituent. Anc. Sci. Life 2015, 34, 142–146. [Google Scholar] [CrossRef]
- Mebe, P.P. 2′-p-Methoxycoumaroylaloeresin, A C-glucoside from Aloe excelsa. Phytochemistry 1987, 26, 2646–2647. [Google Scholar] [CrossRef]
- Beaumont, J.; Reynolds, R.; Vaughan, J.G. Homonataloin in Aloe species. Planta Med. 1984, 50, 505–508. [Google Scholar] [CrossRef]
- Dagne, E.; Bisrat, D.; Van Wyk, B.-E.; Viljoen, A.; Hellwig, V.; Steglich, W. Anthrones from Aloe microstigma. Phytochemistry 1997, 44, 1271–1274. [Google Scholar] [CrossRef]
- Lv, L.; Tian, X.-Y.; Fang, W.-S. Three new antioxidantC-glucosylanthrones fromAloe nobilis. J. Asian Nat. Prod. Res. 2010, 12, 443–447. [Google Scholar] [CrossRef]
- Bisrat, D.; Dagne, E.; Van Wyk, B.-E.; Viljoen, A. Chromones and anthrones from Aloe marlothii and Aloe rupestris. Phytochemistry 2000, 55, 949–952. [Google Scholar] [CrossRef]
- Sigler, A.; Rauwald, H.W. First proof of anthrone aglycones and diastereomeric anthrone-C-glycosyls in flowers and bracts of Aloe species. Biochem. Syst. Ecol. 1994, 22, 287–290. [Google Scholar] [CrossRef]
- Hoerhammer, L.; Wagner, H.; Bittner, G. ALOINOSIDE B, A NEW GLYCOSIDE FROM ALOE. Z. Nat. B 1964, 19, 222–226. [Google Scholar]
- Gao, J.; Zhang, G.; Dai, R.; Bi, K. Isolation of Aloinoside B and Metabolism by Rat Intestinal Bacteria. Pharm. Biol. 2005, 42, 581–587. [Google Scholar] [CrossRef]
- Viljoen, A.M.; Van Wyk, B.-E.; Van Heerden, F.R. The chemotaxonomic value of the diglucoside anthrone homonataloside B in the genus Aloe. Biochem. Syst. Ecol. 2002, 30, 35–43. [Google Scholar] [CrossRef]
- Farah, M.; Andersson, R.; Samuelsson, G. Microdontin A and B: Two New Aloin Derivatives from Aloe microdonta. Planta Med. 1992, 58, 88–93. [Google Scholar] [CrossRef]
- Zhong, J.-S.; Huang, Y.-Y.; Zhang, T.-H.; Liu, Y.-P.; Ding, W.-J.; Wu, X.-F.; Xie, Z.-Y.; Luo, H.-B.; Wan, J.-Z. Natural phosphodiesterase-4 inhibitors from the leaf skin of Aloe barbadensis Miller. Fitoterapia 2015, 100, 68–74. [Google Scholar] [CrossRef]
- Dagne, E.; Bisrat, D.; Van Wyk, B.-E.; Viljoen, A. 10-Hydroxyaloin B 6’-O-Acetate, an Oxanthrone fromAloe claviflora. J. Nat. Prod. 1998, 61, 256–257. [Google Scholar] [CrossRef]
- Dagne, E.; Van Wyk, B.-E.; Stephenson, D.; Steglich, W. Three oxanthrones from Aloe littoralis. Phytochemistry 1996, 42, 1683–1687. [Google Scholar] [CrossRef]
- Dagne, E.; Bisrat, D.; Codina, C.; Bastida, J. A C,O-diglucosylated oxanthrone from Aloe littoralis. Phytochemistry 1998, 48, 903–905. [Google Scholar] [CrossRef]
- Wang, H.M.; Shi, W.; Xu, Y.K.; Wang, P.; Chen, W.; Liu, Y.; Lu, M.J.; Pa, J.Q. Isolation and spectral study of 4-methyl-6, 8-dihydroxy-7H-benz [de] anthracen-7-one. Magn. Reson. Chem. 2003, 41, 301–303. [Google Scholar] [CrossRef]
- Asamenew, G.; Bisrat, D.; Mazumder, A.; Asres, K. In Vitro Antimicrobial and Antioxidant Activities of Anthrone and Chromone from the Latex of Aloe harlana Reynolds. Phytother. Res. 2011, 25, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Saleem, R.; Faizi, S.; Deeba, F.; Siddiqui, B.S.; Qazi, M.H. Anthrones from Aloe barbadensis. Phytochemistry 1997, 45, 1279–1282. [Google Scholar] [CrossRef]
- Yagi, A.; Shoyama, Y.; Nishioka, I. Formation of tetrahydroanthracene glucosides by callus tissue of Aloe saponaria. Phytochemistry 1983, 22, 1483–1484. [Google Scholar] [CrossRef]
- Yagi, A.; Hine, N.; Asai, M.; Nakazawa, M.; Tateyama, Y.; Okamura, N.; Fujioka, T.; Mihashi, K.; Shimomura, K. Tetrahydroanthracene glucosides in callus tissue from Aloe barbadensis leaves. Phytochemistry 1998, 47, 1267–1270. [Google Scholar] [CrossRef]
- Yenesew, A.; Ogur, J.; Duddeckt, H. (R)-Prechrysophanol from Aloe graminicola. Phytochemistry 1993, 34, 1442–1444. [Google Scholar] [CrossRef]
- Dagne, E.; Casser, I.; Steglich, W. Aloechrysone, a dihydroanthracenone from Aloe berhana. Phytochemistry 1992, 31, 1791–1793. [Google Scholar] [CrossRef]
- Abe, I.; Oguro, S.; Utsumi, Y.; Sano, Y.; Noguchi, H. Engineered Biosynthesis of Plant Polyketides: Chain Length Control in an Octaketide-Producing Plant Type III Polyketide Synthase. J. Am. Chem. Soc. 2005, 127, 12709–12716. [Google Scholar] [CrossRef]
- Sun, Y.N.; Li, W.; Yang, S.Y.; Kang, J.S.; Ma, J.Y.; Kim, Y.H. Isolation and identification of chromone and pyrone constituents from Aloe and their anti-inflammatory activities. J. Funct. Foods 2016, 21, 232–239. [Google Scholar] [CrossRef]
- Holdsworth, D. Chromones in Aloe species–Part II–Aloesone. Planta Med. 1972, 22, 54–58. [Google Scholar] [CrossRef]
- Conner, J.M.; Gray, A.I.; Reynolds, T.; Waterman, P.G. Anthrone and chromone components of Aloe cremnophila and A. jacksonii leaf exudates. Phytochemistry 1990, 29, 941–944. [Google Scholar] [CrossRef]
- Speranza, G.; Fontana, G.; Zanzola, S.; Di Meo, A. Studies on Aloe. 15.1Two New 5-Methylchromones from Cape Aloe. J. Nat. Prod. 1997, 60, 692–694. [Google Scholar] [CrossRef]
- Durı, L.; Morelli, C.; Crippa, S.; Speranza, G. 6-Phenylpyrones and 5-methylchromones from Kenya aloe. Fitoterapia 2004, 75, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Blitzke, T.; Masaoud, M.; Schmidt, J. Constituents of Aloe rubroviolacea. Fitoterapia 2001, 72, 78–79. [Google Scholar] [CrossRef]
- Okamura, N.; Hine, N.; Harada, S.; Fujioka, T.; Mihashi, K.; Yagi, A. Three chromone components from Aloe vera leaves. Phytochemistry 1996, 43, 495–498. [Google Scholar] [CrossRef]
- Okamura, N.; Hine, N.; Tateyama, Y.; Nakazawa, M.; Fujioka, T.; Mirmhi, K.; Yagi, A. Three chromones of Aloe vera leaves. Phytochemistry 1997, 45, 1511–1513. [Google Scholar] [CrossRef]
- Okamura, N.; Hine, N.; Tateyama, Y.; Nakazawa, M.; Fujioka, T.; Mihashi, K.; Yagi, A. Five chromones from Aloe Vera leaves. Phytochemistry 1998, 49, 219–223. [Google Scholar] [CrossRef]
- Kahramanoğlu, I.; Chen, C.; Chen, J.; Wan, C. Chemical Constituents, Antimicrobial Activity, and Food Preservative Characteristics of Aloe vera Gel. Agronomy 2019, 9, 831. [Google Scholar] [CrossRef]
- Yuan, A.X.; Kang, S.H.; Qin, L.; Yuan, P.; Fan, Y.J. Chemical constituents of the leaves of Chinese aloe (Aloe vera var. chinensis). Zhongcaoyao 1994, 25, 339–341. [Google Scholar]
- Park, M.; Park, J.; Shin, Y.; Kim, W.; Lee, J.; Kim, K. Neoaloesin A: A NewC-Glucofuranosyl Chromone fromAloe barbadensis. Planta Med. 1996, 62, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Haynes, L.; Holdsworth, D.; Russell, R. C-Glycosyl compounds. Part VI. Aloesin, a C-glucosylchromone from Aloe sp. J. Chem. Soc. C Org. 1970, 2581–2586. [Google Scholar] [CrossRef]
- Wu, X.-F.; Wan, J.-Z.; Luo, B.-J.; Yang, M.-R.; Ding, W.-J.; Zhong, J.-S. A novel naphthalene derivative from Aloe barbadensis. Yao xue xue bao = Acta Pharm. Sin. 2013, 48, 723–727. [Google Scholar]
- Hiruy, M.; Bisrat, D.; Mazumder, A.; Asres, K. Two chromones with antimicrobial activity from the leaf latex of Aloe monticola Reynolds. Nat. Prod. Res. 2021, 35, 1052–1056. [Google Scholar] [CrossRef]
- Lv, L.; Yang, Q.Y.; Zhao, Y.; Yao, C.S.; Sun, Y.; Yang, E.J.; Song, K.S.; Mook-Jung, I.; Fang, W.S. BACE1 (beta-secretase) inhibitory chromone glycosides from Aloe vera and Aloe nobilis. Planta Med. 2008, 74, 540–545. [Google Scholar] [CrossRef]
- Hutter, J.A.; Salman, M.; Stavinoha, W.B.; Satsangi, N.; Williams, R.F.; Streeper, R.T.; Weintraub, S.T. AntiinflammatoryC-Glucosyl Chromone fromAloe barbadensis. J. Nat. Prod. 1996, 59, 541–543. [Google Scholar] [CrossRef]
- Speranza, G.; Gramatica, P.; Dadá, G.; Manitto, P. Aloeresin C, a bitter C, O-diglucoside from Cape Aloe. Phytochemistry 1985, 24, 1571–1573. [Google Scholar] [CrossRef]
- Speranza, G.; Dada, G.; Lunazzi, L.; Gramatica, P.; Manitto, P. A C-glucosylated 5-methylchromone from Kenya aloe. Phytochemistry 1986, 25, 2219–2222. [Google Scholar] [CrossRef]
- Conner, J.M.; Gray, A.I.; Reynolds, T.; Waterman, P.G. Anthracene and chromone derivatives in the exudate of Aloe rabaiensis. Phytochemistry 1989, 28, 3551–3553. [Google Scholar] [CrossRef]
- Van Heerden, F.R.; Van Wyk, B.-E.; Viljoen, A.M. ChemInform Abstract: Aloeresins E (Ia) and F (Ib), Two Chromone Derivatives from Aloe Peglerae. ChemInform 2010, 28, 867–869. [Google Scholar] [CrossRef]
- Van Heerden, F.R.; Viljoen, A.M.; van Wyk, B.E. 6’-O-Coumaroylaloesin from Aloe castanea--a taxonomic marker for Aloe section Anguialoe. Phytochemistry 2000, 55, 117–120. [Google Scholar] [CrossRef]
- Holzapfel, C.W.; Wessels, P.L.; Van Wyk, B.-E.; Marais, W.; Portwig, M. Chromone and aloin derivatives from Aloe broomii, A. Africana and A. speciosa. Phytochemistry 1997, 45, 97–102. [Google Scholar] [CrossRef]
- Gramatica, P.; Monti, D.; Speranza, G.; Manitto, P. Aloe revisited the structure of aloeresin A. Tetrahedron Lett. 1982, 23, 2423–2424. [Google Scholar] [CrossRef]
- Speranza, G.; Martignoni, A.; Manitto, P. Iso-aloeresin A, a Minor Constituent of Cape Aloe. J. Nat. Prod. 1988, 51, 588–590. [Google Scholar] [CrossRef]
- Makino, K.; Yagi, A.; Nishioka, I. Studies on the constituents of Aloe arborescens Mill. var. natalensis Berger. II. The structures of two new aloesin esters. Chem. Pharm. Bull. 1974, 22, 1565–1570. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, D.; Si, J.; Tu, G.; Ma, L. Chemical constituents of Aloe vera. Yaoxue Xuebao 2000, 35, 120–123. [Google Scholar]
- Manitto, P.; Speranza, G.; De Tommasi, N.; Ortoleva, E.; Morelli, C.F. Aloeresin H, a new polyketide constituent of Cape aloe. Tetrahedron 2003, 59, 401–408. [Google Scholar] [CrossRef]
- Speranza, G.; Morelli, C.F.; Tubaro, A.; Altinier, G.; Durì, L.; Manitto, P. Aloeresin I, an Anti-Inflammatory 5-Methylchromone from Cape Aloe. Planta Med. 2005, 71, 79–81. [Google Scholar] [CrossRef]
- Yuan, A.X. The molecular structure of iso-aloesin isolated from the leaves of Aloe vera L. var. chinensis (Haw.) Berge. China J. Chin. Mater. Med. 1993, 18, 609–611. [Google Scholar]
- Bhaludra, C.S.S.; Bethapudi, R.R.; Murugulla, A.C.; Pullagummi, C.; Latha, T.; Venkatesh, K.; Bheemagani, A.J.; Pudutha, A.; Rani, A.R. Cultivation, phytochemical studies, biological activities and medicinal uses of Aloe ferox, grandfather of aloes an important amazing medicinal plant. Int. J. Pharmacol. 2013, 9, 405–415. [Google Scholar]
- López, A.; De Tangil, M.S.; Vega-Orellana, O.; Ramírez, A.S.; Rico, M. Phenolic Constituents, Antioxidant and Preliminary Antimycoplasmic Activities of Leaf Skin and Flowers of Aloe vera (L.) Burm. f. (syn. A. barbadensis Mill.) from the Canary Islands (Spain). Molecules 2013, 18, 4942–4954. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Sharma, D. 5, 4’-dihydroxy 6, 7, 3’, 5’-tetramethoxy flavone 5-O-alpha-L-rhamno pyranosyl 16-O-beta-D-galactopyranoside from aloe barbadensis (leaves). J. Inst. Chem. 1998, 70, 179–182. [Google Scholar]
- Sexena, V.; Chourasia, S. 7-Hydroxy, 6, 3′,4′-Trimethoxy Isoflavone-5-O-alpha-L-Rhamnopy-ranosyl[1→6]-O-beta-D-Glucopyranoside of Aloe vera. J. Inst. Chem. 2000, 72, 195–197. [Google Scholar]
- Kametani, S.; Kojima-Yuasa, A.; Kikuzaki, H.; Kennedy, D.O.; Honzawa, M.; Matsui-Yuasa, I. Chemical Constituents of Cape Aloe and Their Synergistic Growth-Inhibiting Effect on Ehrlich Ascites Tumor Cells. Biosci. Biotechnol. Biochem. 2007, 71, 1220–1229. [Google Scholar] [CrossRef]
- Makino, K.; Yagi, A.; Nishioka, I. Studies on the Constituents of Aloe arborescens MILL. var. natalensis BERGER. I. The Structure of Aloearbonaside, a Glucoside of a New Type naturally Occurring Chromene. Chem. Pharm. Bull. 1973, 21, 149–156. [Google Scholar] [CrossRef]
- Speranza, G.; Dadá, G.; Lunazzi, L.; Gramatica, P.; Manitto, P. Aloenin B, a New Diglucosylated 6-Phenyl-2-pyrone from Kenya Aloe. J. Nat. Prod. 1986, 49, 800–805. [Google Scholar] [CrossRef]
- Shi, S.-P.; Wanibuchi, K.; Morita, H.; Endo, K.; Noguchi, H.; Abe, I. Enzymatic Formation of Unnatural Novel Chalcone, Stilbene, and Benzophenone Scaffolds by Plant Type III Polyketide Synthase. Org. Lett. 2009, 11, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Wang, H.-M.; Song, Y.-L.; Nie, L.-H.; Wang, L.-F.; Liu, B.; Shen, P.-P.; Liu, Y. Isolation, structure elucidation, antioxidative and immunomodulatory properties of two novel dihydrocoumarins from Aloe vera. Bioorg. Med. Chem. Lett. 2006, 16, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Speranza, G.; Manitto, P.; Cassara’, P.; Monti, D. Feralolide, a dihydroisocoumarin from cape aloe. Phytochemistry 1993, 33, 175–178. [Google Scholar] [CrossRef]
- Rehman, N.U.; Hussain, H.; Khiat, M.; Khan, H.Y.; Abbas, G.; Green, I.R.; Al-Harrasi, A. Bioactive chemical constituents from the resin of Aloe vera. Z. Nat. B 2017, 72, 955–958. [Google Scholar] [CrossRef]
- Wang, H.M.; Shi, W.; Xu, Y.K.; Liu, Y.; Lü, M.J.; Pan, J.Q. Spectral study of a new dihydroisocoumarin. Magn. Reson. Chem. 2003, 41, 718–720. [Google Scholar] [CrossRef]
- Veitch, N.C.; Simmonds, M.S.; Blaney, W.M.; Reynolds, T. A dihydroisocoumarin glucoside from Aloe hildebrandtii. Phytochemistry 1994, 35, 1163–1166. [Google Scholar] [CrossRef]
- Saleem, R.; Faizi, S.; Deeba, F.; Siddiqui, B.S.; Qazi, M.H. A New Bisbenzopyran fromAloe barbadensisRoots. Planta Med. 1997, 63, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Dagne, E.; Yenesew, A.; Asmellash, S.; Demissew, S.; Mavi, S. Anthraquinones, pre-anthraquinones and isoeleutherol in the roots of Aloe species. Phytochemistry 1994, 35, 401–406. [Google Scholar] [CrossRef]
- Kong, W.-S.; Li, J.; Liu, X.; Mi, Q.-L.; Chen, J.-H.; Li, X.-M.; Yang, G.-Y.; Hu, Q.-F.; Li, T.; Yang, Y.-K. A new naphthalene derivative from Aloe vera and its antibacterial activity. China J. Chin. Mater. Med. 2017, 42, 3761–3763. [Google Scholar]
- Wessels, P.L.; Holzapfel, C.W.; Van Wyk, B.-E.; Marais, W. Plicataloside, an O,O-diglycosylated naphthalene derivative from Aloe plicatilis. Phytochemistry 1996, 41, 1547–1551. [Google Scholar] [CrossRef]
- Speranza, G.; Monti, D.; Crippa, S.; Cairoli, P.; Morelli, C.F.; Manitto, P. Kenyaloside, a Novel O,O,O-Triglycosylated Naphthalene Derivative from the Exudate of Kenyan Aloe Species. Nat. Prod. Commun. 2006, 1, 1085–1088. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Yao, C.-S.; Fang, W.-S. A new triglucosylated naphthalene glycoside from Aloe vera L. Fitoterapia 2010, 81, 59–62. [Google Scholar] [CrossRef]
- Speranza, G.; Di Meo, A.; Manitto, P.; Monti, D.; Fontana, G. A New Benzochromanone Derivative from Cape Aloe†. J. Agric. Food Chem. 1996, 44, 274–277. [Google Scholar] [CrossRef]
- Blitzke, T.; Porzel, A.; Masaoud, M.; Schmidt, J. A chlorinated amide and piperidine alkaloids from Aloe sabaea. Phytochemistry 2000, 55, 979–982. [Google Scholar] [CrossRef]
- Hotti, H.; Häkkinen, S.T.; Seppänen-Laakso, T.; Rischer, H. Polyketide-Derived Alkaloids and Anthraquinones in Aloe Plants and Cell Cultures. J. Plant. Biotechnol. Res. 2019, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dagne, E.; Bisrat, D.; Viljoen, A.; Van Wyk, B.-E. Chemistry of Aloe Species. Curr. Org. Chem. 2000, 4, 1055–1078. [Google Scholar] [CrossRef]
- Prasannaraja, C.; Kamalanathan, A.S.; Vijayalakshmi, M.A.; Venkataraman, K. A dipyrrole derivative from Aloe vera inhibits an anti-diabetic drug target Dipeptidyl Peptidase (DPP)-IV in vitro. Prep. Biochem. Biotechnol. 2020, 50, 511–520. [Google Scholar] [CrossRef]
- Speranza, G.; Corti, S.; Manitto, P. Isolation and Chemical Characterization of a New Constituent of Cape Aloe Having the 1,1-Diphenylethane Skeleton. J. Agric. Food Chem. 1994, 42, 2002–2006. [Google Scholar] [CrossRef]
- Confalone, P.N.; Huie, E.M.; Patel, N.G. ChemInform Abstract: The isolation, structure determination, and synthesis of pluridone, a novel insecticide from aloe pluridens. Chem. Inf. 1984, 15, 5563–5566. [Google Scholar] [CrossRef]
- Speranza, G.; Manitto, P.; Monti, D.; Lianza, F. Feroxidin, a novel 1-methyltetralin derivative isolated from cape aloe. Tetrahedron Lett. 1990, 31, 3077–3080. [Google Scholar] [CrossRef]
- Speranza, G.; Paolo, M.; Donata, P.; Diego, M. Absolute configuration of feroxidin: An experimental support to Snatzke’s helicity rules for tetralins. Chirality 1991, 3, 263–267. [Google Scholar] [CrossRef]
- Speranza, G.; Manitto, P.; Monti, D.; Pezzuto, D. Studies on Aloe, Part 10. Feroxins A and B, Two O-Glucosylated 1-Methyltetralins from Cape Aloe. J. Nat. Prod. 1992, 55, 723–729. [Google Scholar] [CrossRef]
- Esua, M.F.; Rauwald, J.-W. Novel bioactive maloyl glucans from Aloe vera gel: Isolation, structure elucidation and in vitro bioassays. Carbohydr. Res. 2006, 341, 355–364. [Google Scholar] [CrossRef] [PubMed]


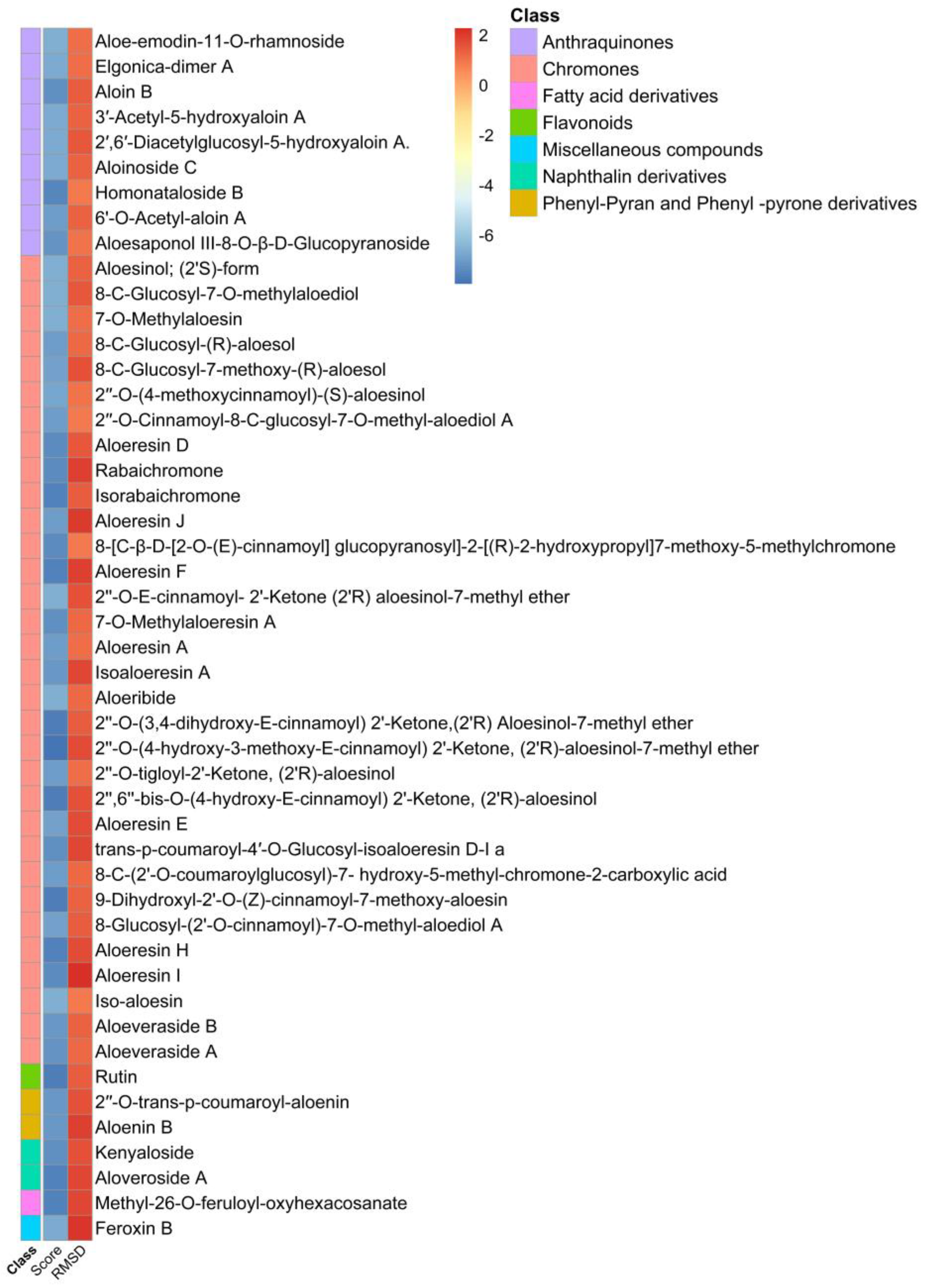
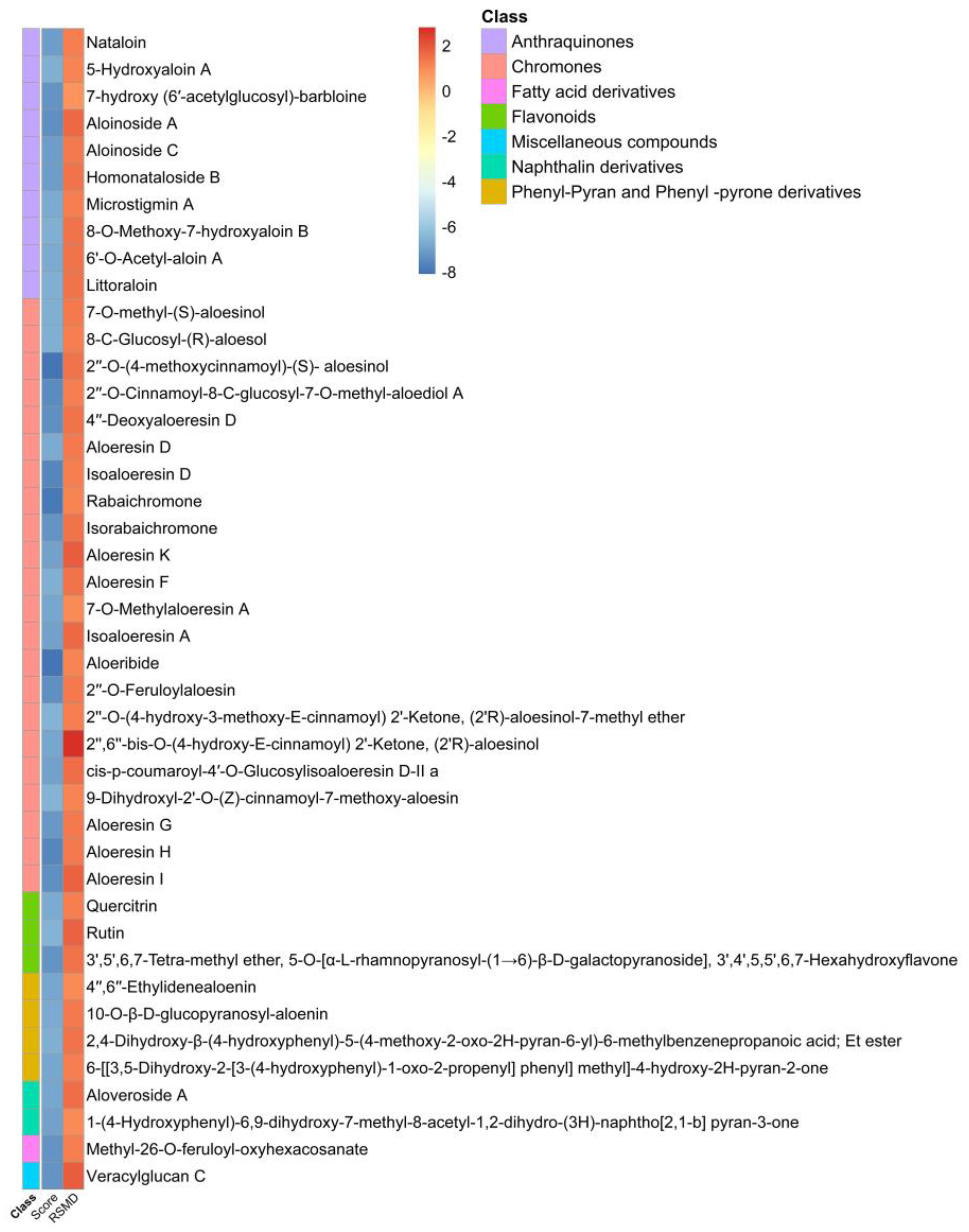
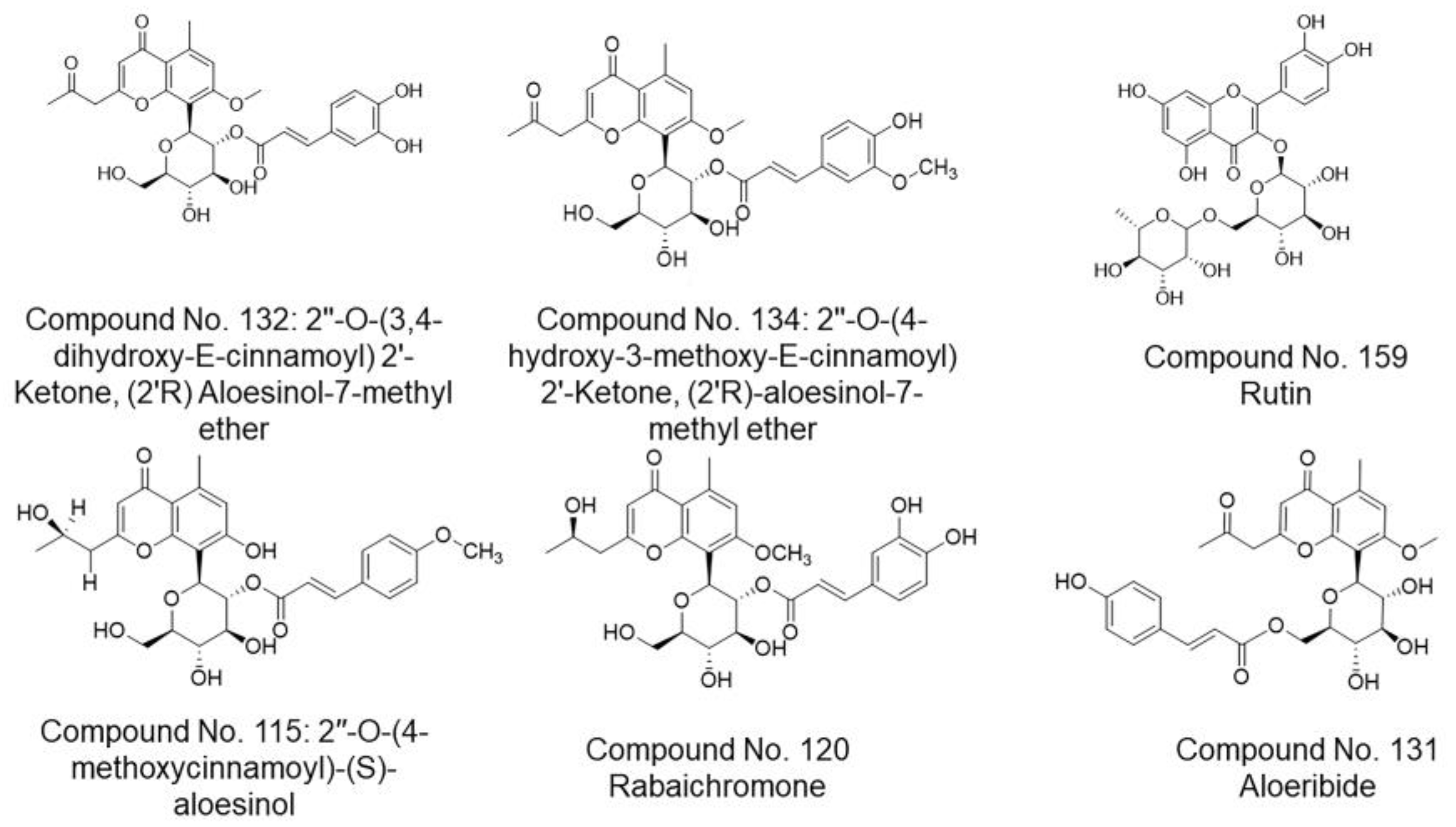



| Protein | No. | Docking Score (kcal/mol) | RSMD 1 Refine | CLogP | Receptor | Interaction | Distance | E (kcal/mol) |
|---|---|---|---|---|---|---|---|---|
| main Protease (PDB ID: 6LU7) | 132 | −7.68 | 1.37 | 0.25 | ASN142A | H-donor | 3.01 | −1.9 |
| ASN142A | H-donor | 2.81 | −2.5 | |||||
| HIS163A | H-acceptor | 3.35 | −0.7 | |||||
| GLN189A | H-acceptor | 3.03 | −1.4 | |||||
| GLU166A | pi-H | 4.42 | −0.7 | |||||
| GLN189A | pi-H | 3.62 | −0.6 | |||||
| 134 | −7.95 | 1.72 | 0.69 | ASN142A | H-donor | 3.44 | −0.6 | |
| ASN142A | H-donor | 2.78 | −2 | |||||
| HIS163A | H-acceptor | 3.29 | −1.2 | |||||
| 159 | −7.72 | 1.40 | −1.36 | THR190A | H-donor | 2.94 | −0.8 | |
| Spike Glycoprotein (PDB ID: 6M0J) | 115 | −8.05 | 1.49 | 1.21 | TRP566A | H-acceptor | 2.93 | −2.4 |
| LYS562A | H-acceptor | 3.11 | −12.2 | |||||
| LYS562A | Ionic | 3.11 | −3.8 | |||||
| VAL209 | pi-H | 4.17 | −0.6 | |||||
| VAL209 | pi-H | 4.27 | −0.6 | |||||
| 131 | −8.08 | 1.13 | 1.02 | GLN102A | H-donor | 3.01 | −1.2 | |
| ASN210A | H-acceptor | 3.31 | −0.8 | |||||
| ASP206A | pi-H | 4.26 | −0.7 | |||||
| 120 | −7.87 | 1.17 | 0.46 | ALA396A | H-donor | 2.56 | −0.5 | |
| ASP206A | H-donor | 2.78 | −3.0 | |||||
| GLU208A | H-donor | 2.77 | −1.1 |
| 115 | 120 | 131 | 132 | 134 | 159 | |
|---|---|---|---|---|---|---|
| miLogP | 2.8 | 1.84 | 2.15 | 1.66 | 1.96 | −1.06 |
| TPSA | 176.12 | 196.35 | 172.97 | 193.19 | 182.2 | 269.43 |
| natoms | 40 | 41 | 40 | 41 | 42 | 43 |
| MW | 556.56 | 572.56 | 554.55 | 570.55 | 584.57 | 610.52 |
| nON | 11 | 12 | 11 | 12 | 12 | 16 |
| nOHNH | 5 | 6 | 4 | 5 | 4 | 10 |
| nviolations | 2 | 3 | 2 | 2 | 2 | 3 |
| nrotb | 9 | 9 | 9 | 9 | 10 | 6 |
| volume | 486.85 | 494.87 | 480.99 | 489.01 | 506.53 | 496.07 |
| %ABS | 48.23 | 41.25 | 49.32 | 42.34 | 46.14 | 16.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abouelela, M.E.; Assaf, H.K.; Abdelhamid, R.A.; Elkhyat, E.S.; Sayed, A.M.; Oszako, T.; Belbahri, L.; El Zowalaty, A.E.; Abdelkader, M.S.A. Identification of Potential SARS-CoV-2 Main Protease and Spike Protein Inhibitors from the Genus Aloe: An In Silico Study for Drug Development. Molecules 2021, 26, 1767. https://doi.org/10.3390/molecules26061767
Abouelela ME, Assaf HK, Abdelhamid RA, Elkhyat ES, Sayed AM, Oszako T, Belbahri L, El Zowalaty AE, Abdelkader MSA. Identification of Potential SARS-CoV-2 Main Protease and Spike Protein Inhibitors from the Genus Aloe: An In Silico Study for Drug Development. Molecules. 2021; 26(6):1767. https://doi.org/10.3390/molecules26061767
Chicago/Turabian StyleAbouelela, Mohamed E., Hamdy K. Assaf, Reda A. Abdelhamid, Ehab S. Elkhyat, Ahmed M. Sayed, Tomasz Oszako, Lassaad Belbahri, Ahmed E. El Zowalaty, and Mohamed Salaheldin A. Abdelkader. 2021. "Identification of Potential SARS-CoV-2 Main Protease and Spike Protein Inhibitors from the Genus Aloe: An In Silico Study for Drug Development" Molecules 26, no. 6: 1767. https://doi.org/10.3390/molecules26061767
APA StyleAbouelela, M. E., Assaf, H. K., Abdelhamid, R. A., Elkhyat, E. S., Sayed, A. M., Oszako, T., Belbahri, L., El Zowalaty, A. E., & Abdelkader, M. S. A. (2021). Identification of Potential SARS-CoV-2 Main Protease and Spike Protein Inhibitors from the Genus Aloe: An In Silico Study for Drug Development. Molecules, 26(6), 1767. https://doi.org/10.3390/molecules26061767












