Unveiling Carbon Dioxide and Ethanol Diffusion in Carbonated Water-Ethanol Mixtures by Molecular Dynamics Simulations
Abstract
1. Introduction
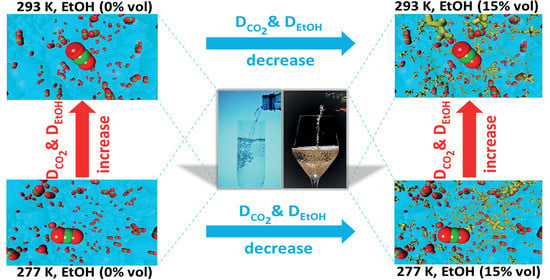
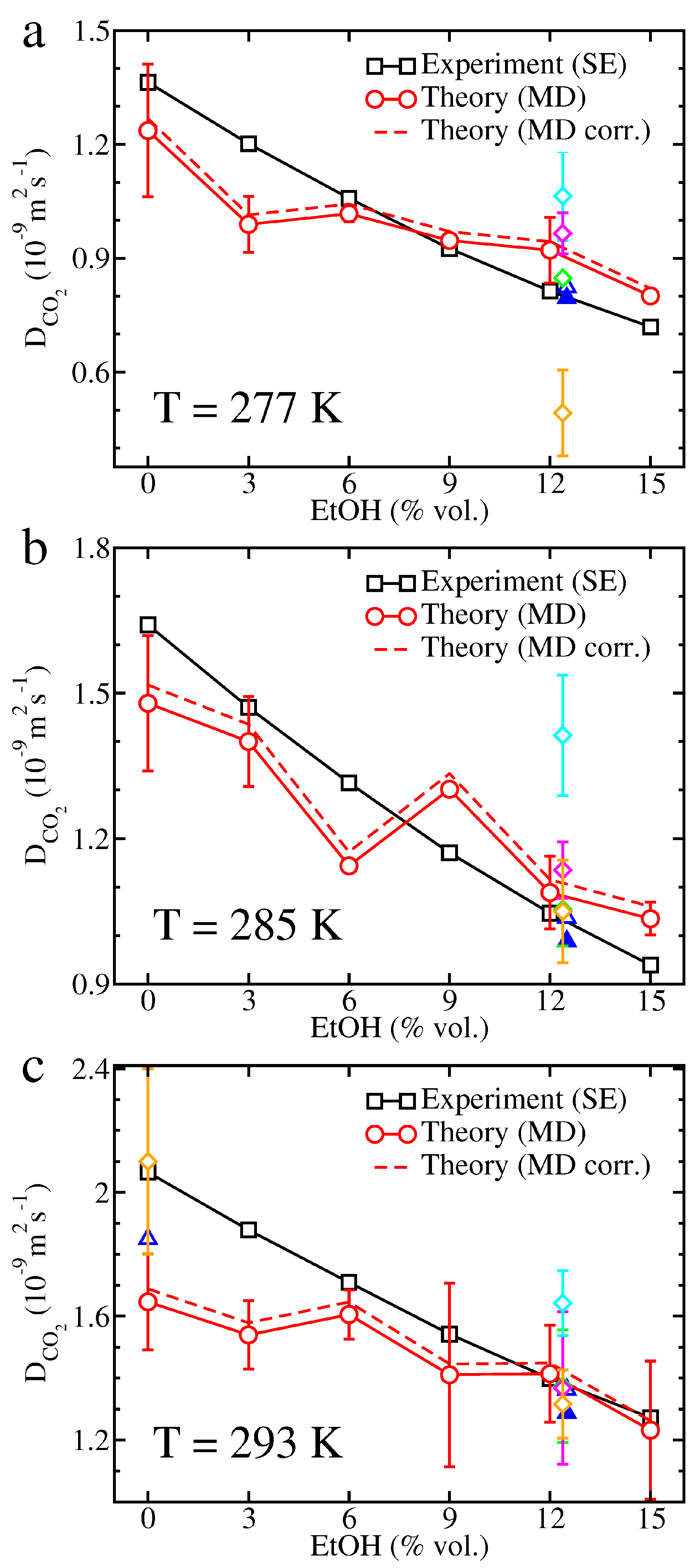
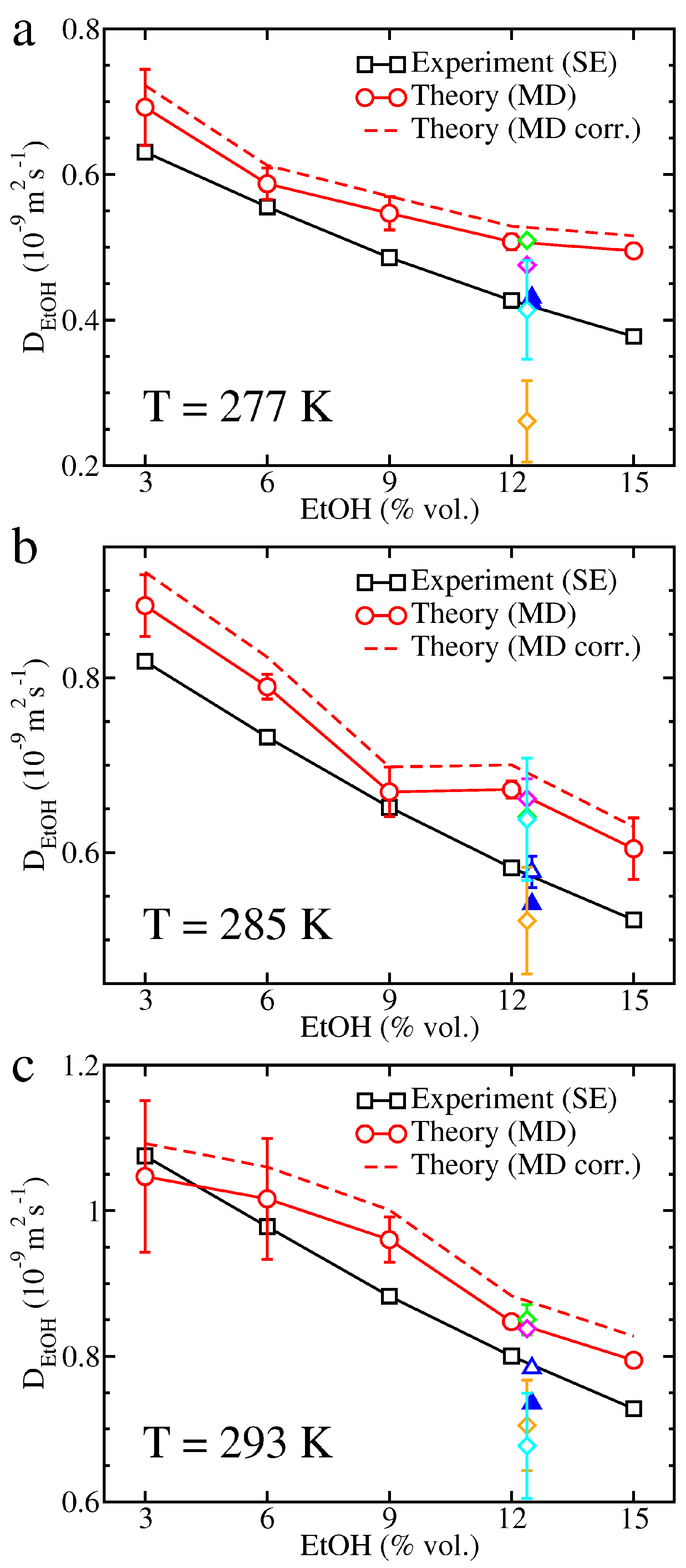
2. Results
3. Discussion
3.1. Definitions of Hydrodynamic Radii
3.2. Possible Improvements of the Theoretical Approach
4. Conclusions
5. Materials and Methods
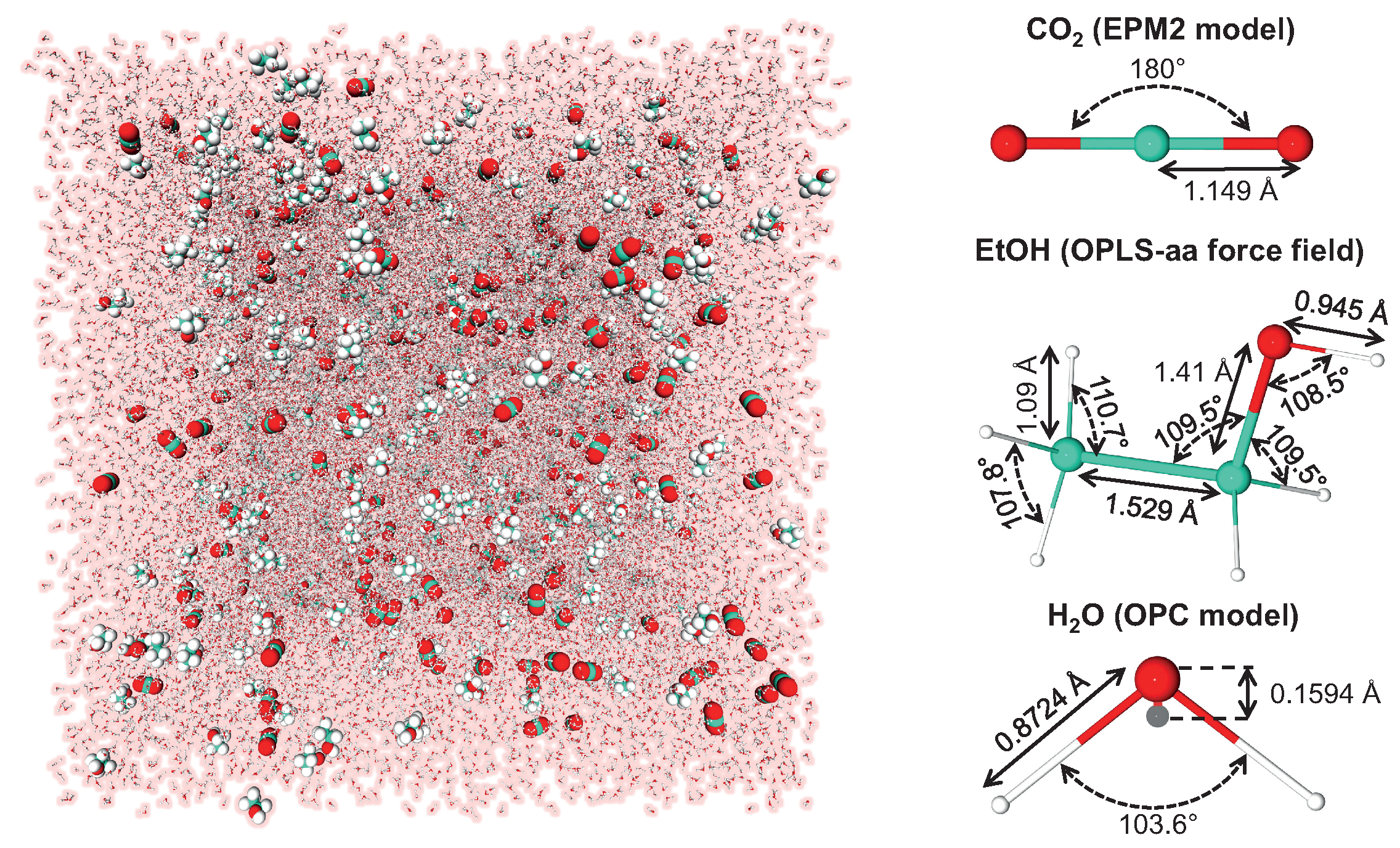
5.1. Molecular Dynamics Simulations
5.2. Theoretical Diffusion Coefficients
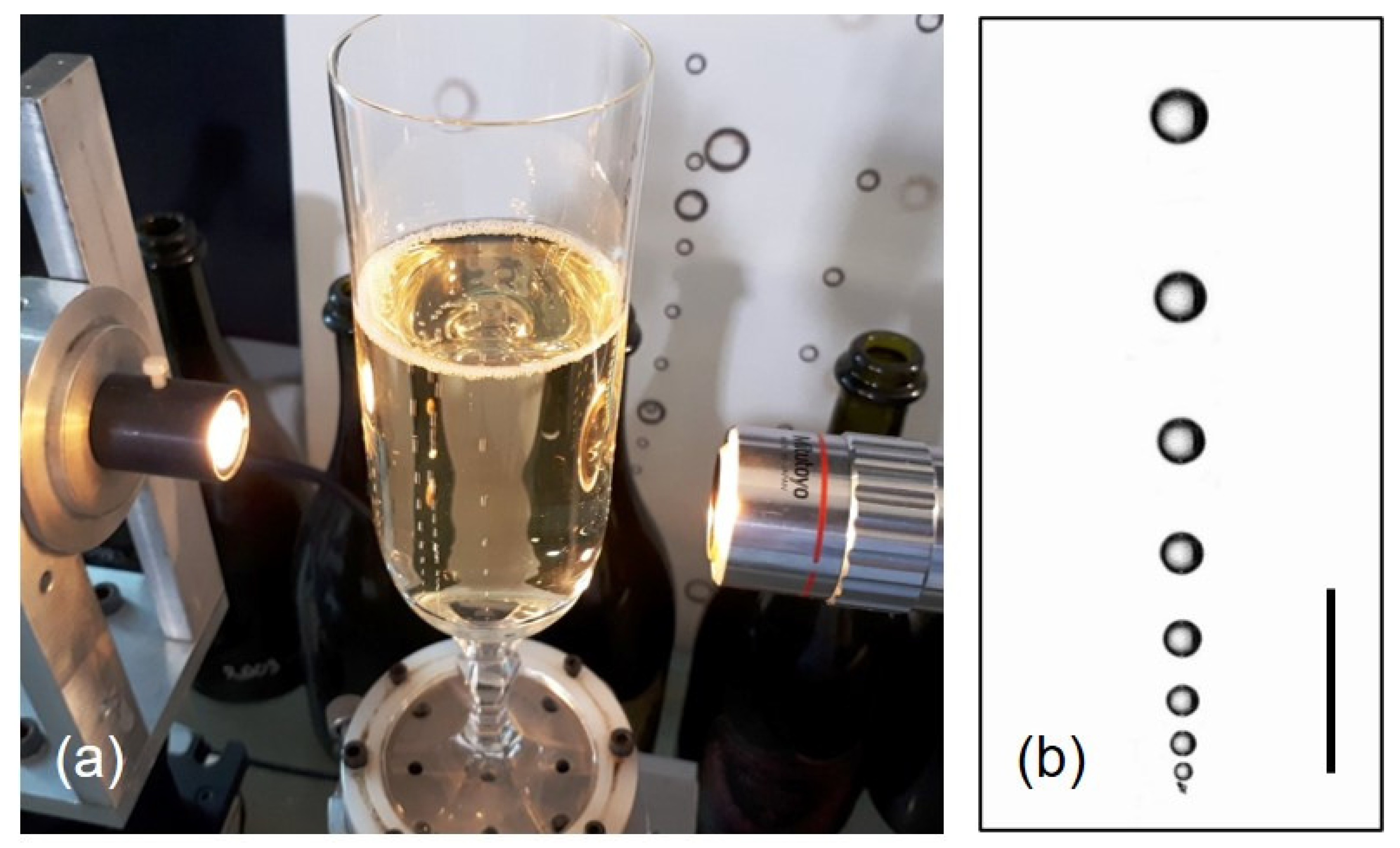
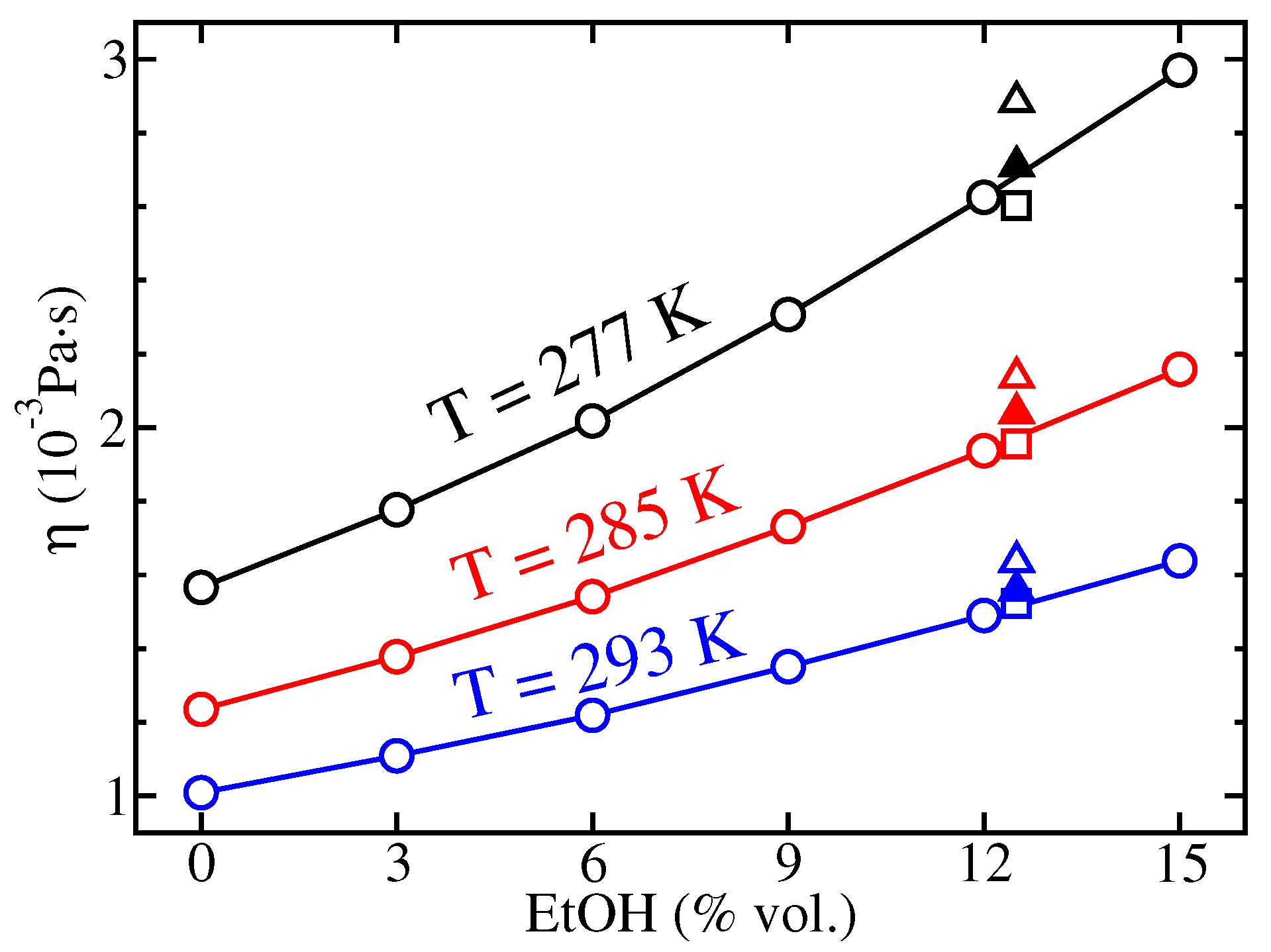
5.3. Viscometry and Experimental Diffusion Coefficients
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHARMM | Chemistry at HARvard Macromolecular Mechanics |
| EPM2 | Elementary Physical Model 2 |
| MD | Molecular Dynamics |
| MSD | Mean-Squared Displacement |
| MSM-ZD | Murthy, Singer, and McDonald—Zhang and Duan |
| NIST | National Institute of Standards and Technology |
| NMR | Nuclear Magnetic Resonance |
| OPC | Optimal Point Charge |
| OPLS-aa | Optimized Potentials for Liquid Simulations-all atom |
| RMS | Root Mean Square |
| SPC/E | Extended Simple Point Charge |
| TIP4P/2005 | Transferable Intermolecular Potential with 4 Points/2005 |
| TIP5P | Transferable Intermolecular Potential with 5 Points |
| TraPPE | Transferable Potentials for Phase Equilibria |
References
- Steen, D.; Ashurst, P.R. Carbonated Soft Drinks: Formulation and Manufacture; Wiley: Hoboken, NJ, USA, 2008; pp. 1–248. [Google Scholar]
- Denny, M. Froth! The Science of Beer; The Johns Hopkins University Press: Baltimore, MD, USA, 2009. [Google Scholar]
- Liger-Belair, G. Uncorked: The Science of Champagne; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Liger-Belair, G.; Carvajal-Pérez, D.; Cilindre, C.; Facque, J.; Brevot, M.; Litoux-Desrues, F.; Chaperon, V.; Geoffroy, R. Evidence for Moderate Losses of Dissolved CO2 during Aging on Lees of a Champagne Prestige Cuvee. J. Food Eng. 2018, 233, 40–48. [Google Scholar] [CrossRef]
- Viejo, C.G.; Torrico, D.D.; Dunshea, F.R.; Fuentes, S. Bubbles, Foam Formation, Stability and Consumer Perception of Carbonated Drinks: A Review of Current, New and Emerging Technologies for Rapid Assessment and Control. Foods 2019, 8, 596. [Google Scholar] [CrossRef]
- Vega Martinez, P.; Enriquez, O.; Rodriguez-Rodriguez, J. Some Topics on the Physics of Bubble Dynamics in Beer. Beverages 2017, 3, 38. [Google Scholar] [CrossRef]
- Smith, F.L.; Harvey, A.H. Avoid Common Pitfalls when Using Henry’s Law. Chem. Eng. Prog. 2007, 103, 33–39. [Google Scholar]
- Shafer, N.E.; Zare, R.N. Through a Beer Glass Darkly. Phys. Today 1991, 44, 48–52. [Google Scholar] [CrossRef]
- Liger-Belair, G. The Physics and Chemistry Behind the Bubbling Properties of Champagne and Sparkling Wines: A State-of-the-art Review. J. Agric. Food Chem. 2005, 53, 2788–2802. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Z. “Fizzics” of Bubble Growth in Beer and Champagne. Elements 2008, 4, 47–49. [Google Scholar] [CrossRef]
- Liger-Belair, G. How Many Bubbles in Your Glass of Bubbly? J. Phys. Chem. B 2014, 118, 3156–3163. [Google Scholar] [CrossRef]
- Liger-Belair, G.; Cilindre, C.; Gougeon, R.D.; Lucio, M.; Gebefügi, I.; Jandet, P.; Schmitt-Kopplin, P. Unraveling Different Chemical Fingerprints Between a Champagne Wine and its Aerosols. Proc. Natl. Acad. Sci. USA 2009, 106, 16545–16549. [Google Scholar] [CrossRef]
- Ghabache, E.; Liger-Belair, G.; Antkowiak, A.; Séon, T. Evaporation of Droplets in a Champagne Wine Aerosol. Sci. Rep. 2016, 6, 25148. [Google Scholar] [CrossRef]
- Séon, T.; Liger-Belair, G. Effervescence in Champagne and Sparkling Wines: From Bubble Bursting to Droplet Evaporation. Eur. Phys. J. Spec. Top. 2017, 226, 117–156. [Google Scholar] [CrossRef]
- Liger-Belair, G.; Bourget, M.; Villaume, S.; Jeandet, P.; Pron, H.; Polidori, G. On the Losses of Dissolved CO2 during Champagne Serving. J. Agric. Food Chem. 2010, 58, 8768–8775. [Google Scholar] [CrossRef] [PubMed]
- Liger-Belair, G.; Conreux, A.; Villaume, S.; Cilindre, C. Monitoring the Losses of Dissolved Carbon Dioxide from Laser-Etched Champagne Glasses. Food Res. Int. 2013, 54, 516–522. [Google Scholar] [CrossRef]
- Liger-Belair, G. Modeling the Losses of Dissolved Carbon Dioxide from Laser-Etched Champagne Glasses. J. Phys. Chem. B 2016, 120, 3724–3734. [Google Scholar] [CrossRef] [PubMed]
- Moriaux, A.L.; Vallon, R.; Parvitte, B.; Zéninari, V.; Liger-Belair, G.; Cilindre, C. Monitoring Gas-Phase CO2 in the Headspace of Champagne Glasses Through Combined Diode Laser Spectrometry and Micro Gas Chromatography. Food Chem. 2018, 264, 255–262. [Google Scholar] [CrossRef]
- Moriaux, A.L.; Vallon, R.; Lecasse, F.; Chauvin, N.; Parvitte, B.; Zéninari, V.; Liger-Belair, G.; Cilindre, C. How Does Gas-Phase CO2 Evolve in the Headspace of Champagne Glasses? J. Agric. Food Chem. 2021, 69, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, J.; Yarmolinsky, D.; von Buchholtz, L.; Oka, Y.; Sly, W.; Ryba, N.J.P.; Zuker, C.S. The Taste of Carbonation. Science 2009, 326, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, A.; Hofmann, T. Carbonic Anhydrase IV Mediates the Fizz of Carbonated Beverages. Angew. Chem. Int. Ed. 2010, 49, 2975–2977. [Google Scholar] [CrossRef]
- McMahon, K.M.; Culver, C.; Ross, C.F. The Production and Consumer Perception of Sparkling Wines of Different Carbonation Levels. J. Wine Res. 2017, 28, 123–134. [Google Scholar] [CrossRef]
- Liger-Belair, G. Effervescence in Champagne and Sparkling Wines: From Grape Harvest to Bubble Rise. Eur. Phys. J. Spec. Top. 2017, 226, 3–116. [Google Scholar] [CrossRef]
- Lee, W.T.; McKechnie, J.S.; Devereux, M.G. Bubble nucleation in Stout Beers. Phys. Rev. E 2011, 83, 051609. [Google Scholar] [CrossRef] [PubMed]
- Kuntzleman, T.S.; Johnson, R. Probing the Mechanism of Bubble Nucleation in and the Effect of Atmospheric Pressure on the Candy-Cola Soda Geyser. J. Chem. Educ. 2020, 97, 980–985. [Google Scholar] [CrossRef]
- Kuntzleman, T.S.; Sturgis, A. Effect of Temperature in Experiments Involving Carbonated Beverages. J. Chem. Educ. 2020, 97, 4033–4038. [Google Scholar] [CrossRef]
- Barker, G.S.; Jefferson, B.; Judd, S.J. The Control of Bubble Size in Carbonated Beverages. Chem. Eng. Sci. 2002, 57, 565–573. [Google Scholar] [CrossRef]
- Liger-Belair, G.; Sternenberg, F.; Brunner, S.; Robillard, B.; Cilindre, C. Bubble Dynamics in Various Commercial Sparkling Bottled Waters. J. Food Eng. 2015, 163, 60–70. [Google Scholar] [CrossRef]
- Liger-Belair, G. Carbon Dioxide in Bottled Carbonated Waters and Subsequent Bubble Nucleation under Standard Testing Condition. J. Agric. Food Chem. 2019, 67, 4560–4567. [Google Scholar] [CrossRef]
- Liger-Belair, G.; Prost, E.; Parmentier, M.; Jeandet, P.; Nuzillard, J.M. Diffusion Coefficient of CO2 Molecules as Determined by 13C NMR in Various Carbonated Beverages. J. Agric. Food Chem. 2003, 51, 7560–7563. [Google Scholar] [CrossRef]
- Cilindre, C.; Conreux, A.; Liger-Belair, G. Simultaneous Monitoring of Gaseous CO2 and Ethanol Above Champagne Glasses via Micro Gas Chromatography (μGC). J. Agric. Food Chem. 2011, 59, 7317–7323. [Google Scholar] [CrossRef]
- Perret, A.; Bonhommeau, D.A.; Liger-Belair, G.; Cours, T.; Alijah, A. CO2 Diffusion in Champagne Wines: A Molecular Dynamics Study. J. Phys. Chem. B 2014, 118, 1839–1847. [Google Scholar] [CrossRef]
- Bonhommeau, D.A.; Perret, A.; Nuzillard, J.M.; Cilindre, C.; Cours, T.; Alijah, A.; Liger-Belair, G. Unveiling the Interplay Between Diffusing CO2 and Ethanol Molecules in Champagne Wines by Classical Molecular Dynamics and 13C NMR Spectroscopy. J. Phys. Chem. Lett. 2014, 5, 4232–4237. [Google Scholar] [CrossRef]
- Ahmed Khaireh, M.; Liger-Belair, G.; Bonhommeau, D.A. Toward in Silico Prediction of CO2 Diffusion in Champagne Wines. ACS Omega 2021, in press. [Google Scholar] [CrossRef]
- Ageno, M.; Frontali, C. Viscosity Measurements of Alcohol-Water Mixtures and the Structure of Water. Proc. Natl. Acad. Sci. USA 1967, 57, 856–860. [Google Scholar] [CrossRef]
- Song, S.; Peng, C. Viscosities of Binary and Ternary Mixtures of Water, Alcohol, Acetone, and Hexane. J. Dispers. Sci. Technol. 2008, 29, 1367–1372. [Google Scholar] [CrossRef]
- Paxman, R.; Stinson, J.; Dejardin, A.; McKendry, R.A.; Hoogenboom, B.W. Using Micromechanical Resonators to Measure Rheological Properties and Alcohol Content of Model Solutions and Commercial Beverages. Sensors 2012, 12, 6497–6507. [Google Scholar] [CrossRef]
- Liger-Belair, G.; Polidori, G.; Jeandet, P. Recent Advances in the Science of Champagne Bubbles. Chem. Soc. Rev. 2008, 37, 2490–2511. [Google Scholar] [CrossRef]
- Liger-Belair, G. The Physics Behind the Fizz in Champagne and Sparkling Wines. Eur. Phys. J. Spec. Top. 2012, 201, 1–88. [Google Scholar] [CrossRef]
- Izadi, S.; Anandakrishnan, R.; Onufriev, A.V. Building Water Models: A Different Approach. J. Phys. Chem. Lett. 2014, 5, 3863–3871. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The Missing Term In Effective Pair Potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- In het Panhuis, M.; Patterson, C.H.; Lynden-Bell, R.M. A Molecular Dynamics Study of Carbon Dioxide in Water: Diffusion, Structure and Thermodynamics. Mol. Phys. 1998, 94, 963–972. [Google Scholar] [CrossRef]
- Mahoney, M.W.; Jorgensen, W.L. A five-site model for liquid water and the reproduction of the density anomaly by rigid, nonpolarizable potential functions. J. Chem. Phys. 2000, 112, 8910–8922. [Google Scholar] [CrossRef]
- Abascal, J.L.F.; Vega, C. A general purpose model for the condensed phases of water: TIP4P/2005. J. Chem. Phys. 2005, 123, 234505. [Google Scholar] [CrossRef]
- Einstein, A. The Motion of Elements Suspended in Static Liquids as Claimed in the Molecular Kinetic Theory of Heat. Ann. Phys.-Berl. 1905, 17, 549–560. [Google Scholar] [CrossRef]
- Potoff, J.J.; Siepmann, J.I. Vapor-Liquid Equilibria of Mixtures Containing Alkanes, Carbon Dioxide, and Nitrogen. AIChE J. 2001, 47, 1676–1682. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, Z. An optimized molecular potential for carbon dioxide. J. Chem. Phys. 2005, 122, 214507. [Google Scholar] [CrossRef] [PubMed]
- CO2 and EtOH Structures in Cartesian Coordinates Extracted from the NIST. Available online: https://cccbdb.nist.gov/exp1x.asp (accessed on 9 March 2021).
- Stefan, J. Über das Gleichgewicht und die Bewegung, insbesondere die Diffusion von Gasgemengen. Sitzungsber. Akad. Wiss. Wien 1871, 63, 63–124. [Google Scholar]
- Fick, A. On Liquid Diffusion. Philos. Mag 1855, 10, 30–39. [Google Scholar] [CrossRef]
- Garcia-Ratés, M.; de Hemptinne, J.C.; Avalos, J.B.; Nieto-Draghi, C. Molecular Modeling of Diffusion Coefficient and Ionic Conductivity of CO2 in Aqueous Ionic Solutions. J. Phys. Chem. B 2012, 116, 2787–2800. [Google Scholar] [CrossRef]
- Bjelkmar, P.; Larsson, P.; Cuendet, M.A.; Hess, B.; Lindahl, E. Implementation of the CHARMM Force Field in GROMACS: Analysis of Protein Stability Effects from Correction Maps, Virtual Interaction Sites, and Water Models. J. Chem. Theory Comput. 2010, 6, 459–466. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the Driving Force of Hydrophobic Assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef]
- Lv, J.; Ren, K.; Chen, Y. CO2 Diffusion in Various Carbonated Beverages: A Molecular Dynamics Study. J. Phys. Chem. B 2018, 122, 1655–1661. [Google Scholar] [CrossRef]
- Liger-Belair, G.; Topgaard, D.; Voisin, C.; Jeandet, P. Is the Wall of a Cellulose Fiber Saturated with Liquid Whether or Not Permeable with CO2 Dissolved Molecules? Application to Bubble Nucleation in Champagne Wines. Langmuir 2004, 20, 4132–4138. [Google Scholar] [CrossRef]
- Berendsen, H.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theor. Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtolad, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Harris, J.G.; Yung, K.H. Carbon Dioxide’s Liquid-Vapor Coexistence Curve and Critical Properties As Predicted by a Simple Molecular Model. J. Phys. Chem. 1995, 99, 12021–12024. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Humphrey, W. VMD: Visual molecular dynamicsi. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Tomasello, G.; Armenia, I.; Molla, G. The Protein Imager: A full-featured online molecular viewer interface with server-side HQ-rendering capabilities. Bioinformatics 2020, 36, 2909–2911. [Google Scholar] [CrossRef]
- Luty, B.A.; Davis, M.E.; Tironi, I.G.; Gunsteren, W.F.V. A Comparison of Particle-Particle, Particle-Mesh and Ewald Methods for Calculating Electrostatic Interactions in Periodic Molecular Systems. Mol. Simul. 1994, 14, 11–20. [Google Scholar] [CrossRef]
- Toukmaji, A.Y.; John, A. Board, J. Ewald summation techniques in perspective: A survey. Comput. Phys. Commun. 1996, 95, 73–92. [Google Scholar] [CrossRef]
- Abraham, M.J.; Gready, J.E. Optimization of parameters for molecular dynamics simulation using smooth particle-mesh Ewald in GROMACS 4.5. J. Comput. Chem. 2011, 32, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Parrinello, M. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182. [Google Scholar] [CrossRef]
- Vedamuthu, M.; Singh, S.; Robinson, G.W. Properties of Liquid Water: Origin of the Density Anomalies. J. Phys. Chem. 1994, 98, 2222–2230. [Google Scholar] [CrossRef]
- Vedamuthu, M.; Singh, S.; Robinson, G.W. Properties of Liquid Water. 4. The Isothermal Compressibility Minimum near 50 °C. J. Phys. Chem. 1995, 99, 9263–9267. [Google Scholar] [CrossRef]
- Taylor, R. Multicomponent Mass Transfer; Wiley Series in Chemical Engineering; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Maxwell, J.C. The Scientific Papers of James Clerk Maxwell; W. D. Niven: New York, NY, USA, 1952. [Google Scholar]
- Onsager, L. Reciprocal relations in irreversible processes. I. Phys. Rev. 1931, 37, 405–426. [Google Scholar] [CrossRef]
- Yeh, I.C.; Hummer, G. System-Size Dependence of Diffusion Coefficients and Viscosities from Molecular Dynamics Simulations with Periodic Boundary Conditions. J. Phys. Chem. B 2004, 108, 15873–15879. [Google Scholar] [CrossRef]
- Anton Paar: Rolling-Ball Viscometer Lovis 2000 M/ME. Available online: https://www.anton-paar.com/corp-en/products/details/rolling-ball-viscometer-lovis-2000-mme/ (accessed on 13 November 2020).
- Compendium of International Analysis of Methods—OIV, OIV-MA-AS312-02: R2009. Alcoholic Strength by Volume (Table of Apparent Densities of Ethanol-Water Mixtures). 2009. Available online: https://www.oiv.int/public/medias/2491/oiv-ma-as312-02.pdf (accessed on 13 November 2020).
| EtOH (% vol.) | 0 | 3 | 6 | 9 | 12 | 15 |
|---|---|---|---|---|---|---|
| 0 | 385 | 795 | 1232 | 1699 | 2199 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed Khaireh, M.; Angot, M.; Cilindre, C.; Liger-Belair, G.; Bonhommeau, D.A. Unveiling Carbon Dioxide and Ethanol Diffusion in Carbonated Water-Ethanol Mixtures by Molecular Dynamics Simulations. Molecules 2021, 26, 1711. https://doi.org/10.3390/molecules26061711
Ahmed Khaireh M, Angot M, Cilindre C, Liger-Belair G, Bonhommeau DA. Unveiling Carbon Dioxide and Ethanol Diffusion in Carbonated Water-Ethanol Mixtures by Molecular Dynamics Simulations. Molecules. 2021; 26(6):1711. https://doi.org/10.3390/molecules26061711
Chicago/Turabian StyleAhmed Khaireh, Mohamed, Marie Angot, Clara Cilindre, Gérard Liger-Belair, and David A. Bonhommeau. 2021. "Unveiling Carbon Dioxide and Ethanol Diffusion in Carbonated Water-Ethanol Mixtures by Molecular Dynamics Simulations" Molecules 26, no. 6: 1711. https://doi.org/10.3390/molecules26061711
APA StyleAhmed Khaireh, M., Angot, M., Cilindre, C., Liger-Belair, G., & Bonhommeau, D. A. (2021). Unveiling Carbon Dioxide and Ethanol Diffusion in Carbonated Water-Ethanol Mixtures by Molecular Dynamics Simulations. Molecules, 26(6), 1711. https://doi.org/10.3390/molecules26061711