Synthesis, Molecular Docking Studies and In Silico ADMET Screening of New Heterocycles Linked Thiazole Conjugates as Potent Anti-Hepatic Cancer Agents
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Docking Study, SAR Analysis and ADMET Properties
2.3. Biological Activity
Anti-proliferative Activity
3. Conclusions
4. Experimental
4.1. Chemistry
Experimental Instrumentation
4.2. Computational Studies
4.3. Biological Activity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abdelhamid, A.O.; El-Idreesy, T.T.; Abdelriheem, N.A.; Dawoud, H.R. Green One-Pot Solvent-Free Synthesis of Pyrazolo [1, 5-a] pyrimidines, Azolo [3, 4-d] pyridiazines, and Thieno [2, 3-b] pyridines Containing Triazole Moiety. J. Heterocycl. Chem. 2016, 53, 710–718. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; Gomha, S.M.; El-Gendey, M.S.; El-Hashash, M.A.; Soliman, A.M.M. Eco-friendly one-pot synthesis of some new pyrazolo [1, 2-b] phthalazinediones with antiproliferative efficacy on human hepatic cancer cell lines. Green Chem. Lett. Rev. 2018, 11, 264–274. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; Nasr, S.M.; El-Refai, H.A.; Abdel-Aziz, M.S. A novel approach of potent antioxidant and antimicrobial agents containing coumarin moiety accompanied with cytotoxicity studies on the newly synthesized derivatives. J. Appl. Pharm. Sci. 2017, 7, 186–196. [Google Scholar]
- El-Hashash, M.A.; Sherif, S.M.; Badawy, A.A.; Rashdan, H.R. Facial synthesis of some new pyrazolopyridine, barbituric and thiobarbituric acid derivatives with antimicrobial activities. J. Adv. Res. 2014, 2, 900–913. [Google Scholar]
- Abdelhamid, A.O.; Abdel-Riheem, N.A.; El-Idreesy, T.T.; Rashdan, H.R.M. Synthesis of 5-arylazothiazoles, pyridines and thieno [2, 3-b] pyridines derivatives containing 1, 2, 3-triazole moiety. Chem. Eur. J. 2012, 3, 322–331. [Google Scholar] [CrossRef]
- Rashdan, H.R.; Roaiah, H.M.; Muhammad, Z.A.; Wietrzyk, J.; Milczarek, M.; Soliman, A.M. Design, efficient synthesis, mechanism of reaction and antiproliferative activity against cancer and normal cell lines of a novel class of fused pyrimidine derivatives. Acta Pol. Pharm. 2018, 75, 679–688. [Google Scholar]
- El-Hashash, M.A.; Sherif, S.M.; Badawy, A.A.; Rashdan, H.R. Synthesis of some new antimicrobial 5, 6, 7, 8-tetrahydro-pyrimido [4, 5-b] quinolone derivatives. Der Pharm. Chem. 2014, 6, 23–29. [Google Scholar]
- El-Hashash, M.A.; Sherif, S.M.; Badawy, A.A.; Rashdan, H.R. Synthesis of Potent Antimicrobial Pyrrole Derivatives. IJAR 2014, 2, 1022–1035. [Google Scholar]
- El-Naggar, M.; Mohamed, M.E.; Mosallam, A.M.; Salem, W.; Rashdan, H.R.; Abdelmonsef, A.H. Synthesis, Characterization, Antibacterial Activity, and Computer-Aided Design of Novel Quinazolin-2, 4-dione Derivatives as Potential Inhibitors Against Vibrio cholerae. Evol. Bioinform. 2020, 16, 1176934319897596. [Google Scholar] [CrossRef]
- Abdel-Aziem, A.; Rashdan, H.R.M.; Mohamed Ahmed, E.; Shabaan, S.N. Synthesis and cytotoxic activity of some novel benzocoumarin derivatives under solvent free conditions. Green Chem. Lett. Rev. 2019, 12, 9–18. [Google Scholar] [CrossRef]
- Rashdan, H.R.; Farag, M.M.; El-Gendey, M.S.; Mounier, M.M. Toward Rational Design of Novel Anti-Cancer Drugs Based on Targeting, Solubility, and Bioavailability Exemplified by 1, 3, 4-Thiadiazole Derivatives Synthesized Under Solvent-Free Conditions. Molecules 2019, 24, 2371. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.O.; Abdel-Riheem, N.A.; El-Idreesy, T.T.; Rashdan, H. Synthesis of some new azolotriazine, 4-arylazopyrazole and pyridine derivatives containing 1, 2, 3-triazole moiety. Int. J. 2013, 1, 729–745. [Google Scholar]
- Elnaggar, D.H.; Abdel Hafez, N.A.; Rashdan, H.R.M.; Abdelwahed, N.A.; Awad, H.M.; Ali, K.A. Synthesis, Antimicrobial and Antitumor Evaluations of a New Class of Thiazoles Substituted on the Chromene Scaffold. Mini. Rev. Med. Chem. 2019, 19, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Rashdan, H.R.M.; Abdel-Aziem, A.; El-Naggar, D.H.; Nabil, S. Synthesis and biological evaluation of some new pyridines, isoxazoles and isoxazolopyridazines bearing 1, 2, 3-triazole moiety. Acta Pol. Pharm. 2019, 2019 76, 469–482. [Google Scholar]
- Shehadi, I.A.; Rashdan, H.R.; Abdelmonsef, A.H. Homology Modeling and Virtual Screening Studies of Antigen MLAA-42 Protein: Identification of Novel Drug Candidates against Leukemia—An In Silico Approach. Comput. Math Methods Med. 2020. [Google Scholar] [CrossRef]
- El-Naggar, M.; El-All, A.S.A.; El-Naem, S.I.; Abdalla, M.M.; Rashdan, H.R. New Potent 5α-Reductase and Aromatase Inhibitors Derived from 1, 2, 3-Triazole Derivative. Molecules 2020, 25, 672. [Google Scholar] [CrossRef]
- Kashyap, S.J.; Garg, V.K.; Sharma, P.K.; Kumar, N.; Dudhe, R.; Gupta, J.K. Thiazoles: Having diverse biological activities. Med. Chem. Res. 2012, 21, 2123–2132. [Google Scholar] [CrossRef]
- Gupta, V.; Kant, V. A review on biological activity of imidazole and thiazole moieties and their derivatives. Sci. Int. 2013, 1, 253–260. [Google Scholar] [CrossRef]
- da Silva, E.B.; e Silva, D.A.O.; Oliveira, A.R.; da Silva Mendes, C.H.; dos Santos, T.A.R.; da Silva, A.C.; de Castro, M.C.A.; Ferreira, R.S.; Moreira, D.R.M.; de Oliveira Cardoso, M.V. Desing and synthesis of potent anti-Trypanosoma cruzi agents new thiazoles derivatives which induce apoptotic parasite death. Eur. J. Med. Chem. 2017, 130, 39–50. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Leonard, J.T.; Roy, K. Exploring QSAR of thiazole and thiadiazole derivatives as potent and selective human adenosine A3 receptor antagonists using FA and GFA techniques. Bioorg. Med. Chem. 2005, 13, 1159–1165. [Google Scholar] [CrossRef]
- El-Sabbagh, O.I.; Baraka, M.M.; Ibrahim, S.M.; Pannecouque, C.; Andrei, G.; Snoeck, R.; Balzarini, J.; Rashad, A.A. Synthesis and antiviral activity of new pyrazole and thiazole derivatives. Eur. J. Med. Chem. 2009, 44, 3746–3753. [Google Scholar] [CrossRef]
- Doss, M.L.; Lalitha, K. Molecular docking studies of thiazole Schiff’s bases as HIV1-protease inhibitors. J. Curr. Chem. Pharm. Sci. 2011, 1. [Google Scholar]
- Kashyap, A.; Adhikari, N.; Das, A.; Shakya, A.; Ghosh, S.K.; Singh, U.P.; Bhat, H.R. Review on Synthetic Chemistry and Antibacterial Importance of Thiazole Derivatives. Curr. Drug Discov. Technol. 2018, 15, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Morigi, R.; Locatelli, A.; Leoni, A.; Rambaldi, M. Recent patents on thiazole derivatives endowed with antitumor activity. Recent Pat Anticancer Drug Discov. 2015, 10, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Bhatt, N.; Somani, H.; Trivedi, A. Synthesis, antimicrobial and cytotoxic activities of some novel thiazole clubbed 1, 3, 4-oxadiazoles. Eur. J. Med. Chem. 2013, 67, 54–59. [Google Scholar] [CrossRef]
- Zaharia, V.; Ignat, A.; Palibroda, N.; Ngameni, B.; Kuete, V.; Fokunang, C.N.; Moungang, M.L.; Ngadjui, B.T. Synthesis of some p-toluenesulfonyl-hydrazinothiazoles and hydrazino-bis-thiazoles and their anticancer activity. Eur. J. Med. Chem. 2010, 45, 5080–5085. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Hassan, A.A.; Bräse, S.; Ibrahim, M.A.; Abd Al-Latif, E.-S.S.; Spuling, E.; Nieger, M. 1, 3, 4-Thiadiazoles and 1, 3-thiazoles from one-pot reaction of bisthioureas with 2-(bis (methylthio) methylene) malononitrile and ethyl 2-cyano-3, 3-bis (methylthio) acrylate. J. Sulphur. Chem. 2017, 38, 69–75. [Google Scholar] [CrossRef]
- Li, S.; Chen, S.; Fan, J.; Cao, Z.; Ouyang, W.; Tong, N.; Hu, X.; Hu, J.; Li, P.; Feng, Z. Anti-biofilm effect of novel thiazole acid analogs against Pseudomonas aeruginosa through IQS pathways. Eur. J. Med. Chem. 2018, 145, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Parri, M.; Chiarugi, P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 2010, 8, 23. [Google Scholar] [CrossRef]
- Dasari, T.; Kondagari, B.; Dulapalli, R.; Abdelmonsef, A.H.; Mukkera, T.; Padmarao, L.S.; Malkhed, V.; Vuruputuri, U. Design of novel lead molecules against RhoG protein as cancer target–a computational study. J. Biomol. Struct. Dyn. 2017, 35, 3119–3139. [Google Scholar] [CrossRef] [PubMed]
- Abdelmonsef, A.H. Computer-aided identification of lung cancer inhibitors through homology modeling and virtual screeningEgypt. J. Medical Hum. Genet. 2019, 20, 6. [Google Scholar] [CrossRef]
- Jung, H.; Yoon, S.R.; Lim, J.; Cho, H.J.; Lee, H.G. Dysregulation of Rho GTPases in Human Cancers. Cancers 2020, 12, 1179. [Google Scholar] [CrossRef] [PubMed]
- Ramatenki, V.; Potlapally, S.R.; Dumpati, R.K.; Vadija, R.; Vuruputuri, U. Homology modeling and virtual screening of ubiquitin conjugation enzyme E2A for designing a novel selective antagonist against cancer. J. Recept. Signal Transduct. Res. 2015, 35, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Haredi Abdelmonsef, A.; Eldeeb Mohamed, M.; El-Naggar, M.; Temairk, H.; Mohamed Mosallam, A. Novel quinazolin-2, 4-dione hybrid molecules as possible inhibitors against Malaria: Synthesis and In silico molecular docking studies. Front. Mol. Biosci. 2020, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K. Rcsb Protein Data Bank: Sustaining a Living Digital Data Resource that Enables Breakthroughs in Scientific Research and Biomedical Education. Biophys. J. 2019, 116, 329a. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Dallakyan, S.; Olson, A.J. Small-Molecule Library Screening by Docking with PyRx; Humana Press: New York, NY, USA, 2015; pp. 243–250. [Google Scholar]
- Nncube, N.B.; Ramharack, P.; Soliman, M.E. Using bioinformatics tools for the discovery of Dengue RNA-dependent RNA polymerase inhibitors. Peer J. 2018, 6, e5068. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- El-Maghraby, A.M.; Abdelmonsef, A.H. Synthesis, characterization and Insilico molecular docking studies of novel chromene derivatives as Rab23 inhibitors. Egypt J. Chem. 2020, 63, 4–5. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Banasiak, D.; Barnetson, A.R.; Odell, R.A.; Mameghan, H.; Russell, P.J. Comparison between the clonogenic, MTT, and SRB assays for determining radiosensitivity in a panel of human bladder cancer cell lines and a ureteral cell line. Radiat. Oncol. Investig. 1999, 7, 77–85. [Google Scholar] [CrossRef]
- Boeckmann, B.; Bairoch, A.; Apweiler, R.; Blatter, M.-C.; Estreicher, A.; Gasteiger, E.; Martin, M.J.; Michoud, K.; O’Donovan, C.; Phan, I. The SWISS-PROT protein knowledgebase and its supplement TrEMBL in 2003. Nucleic Acids Res. 2003, 31, 365–370. [Google Scholar] [CrossRef] [PubMed]
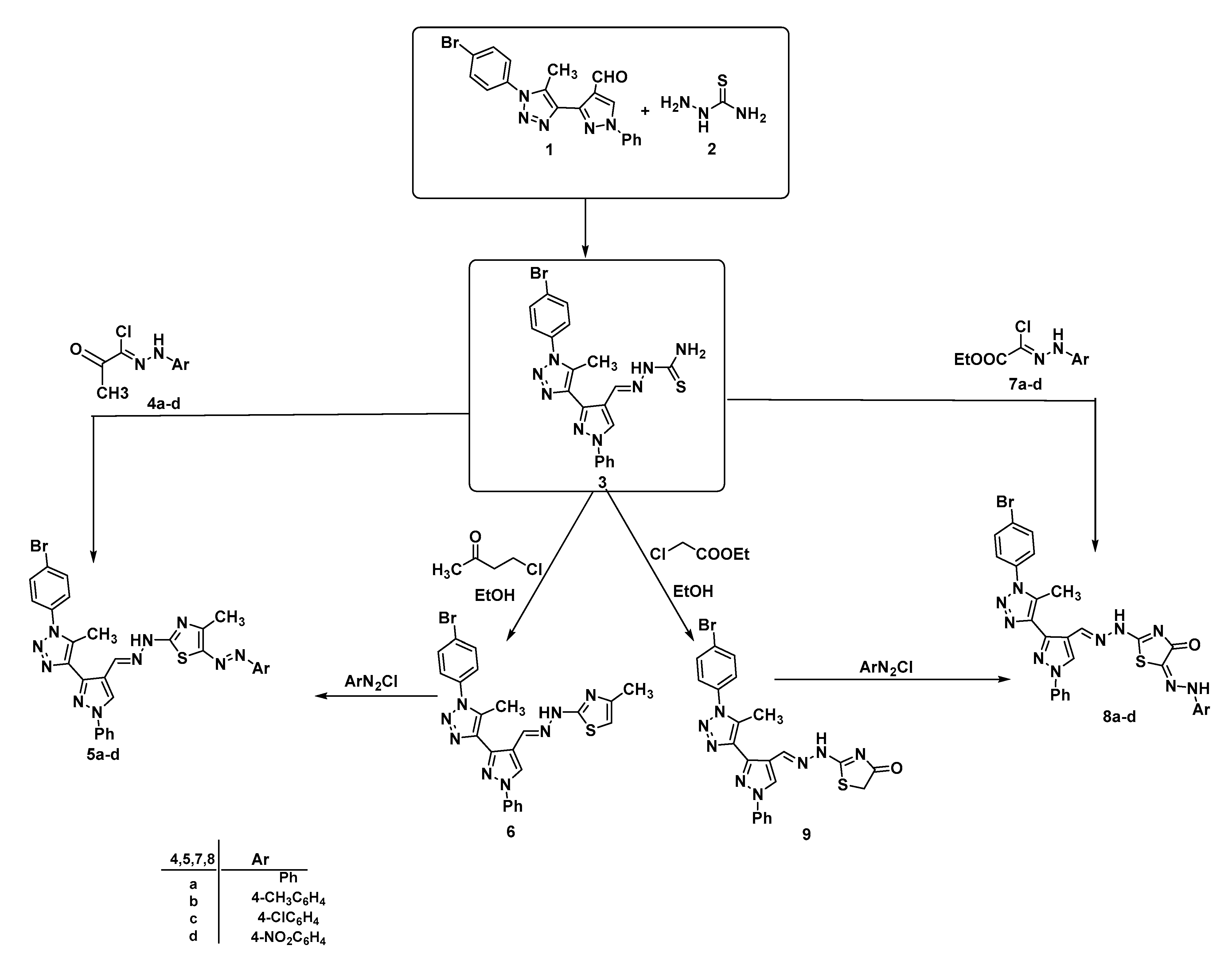
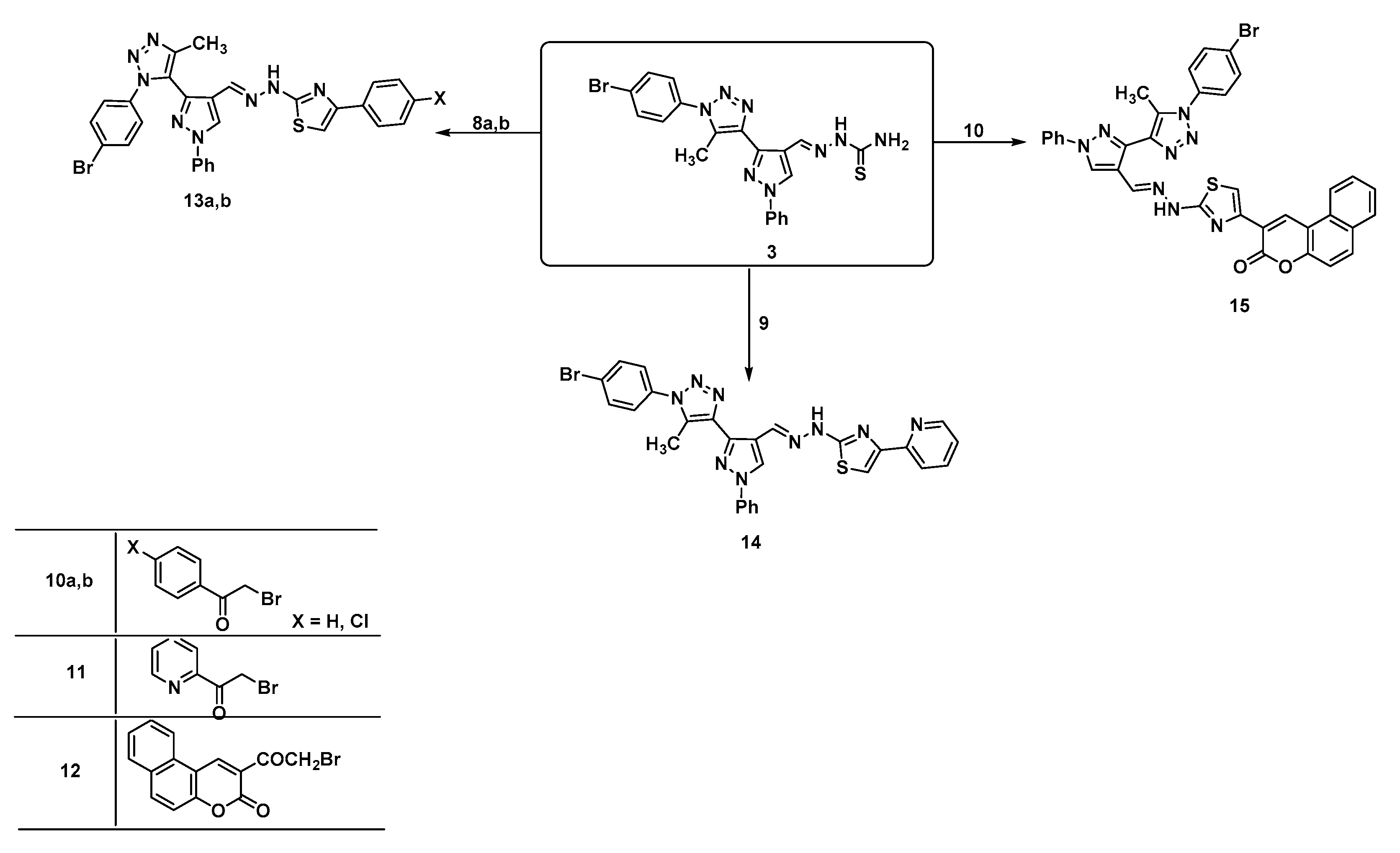
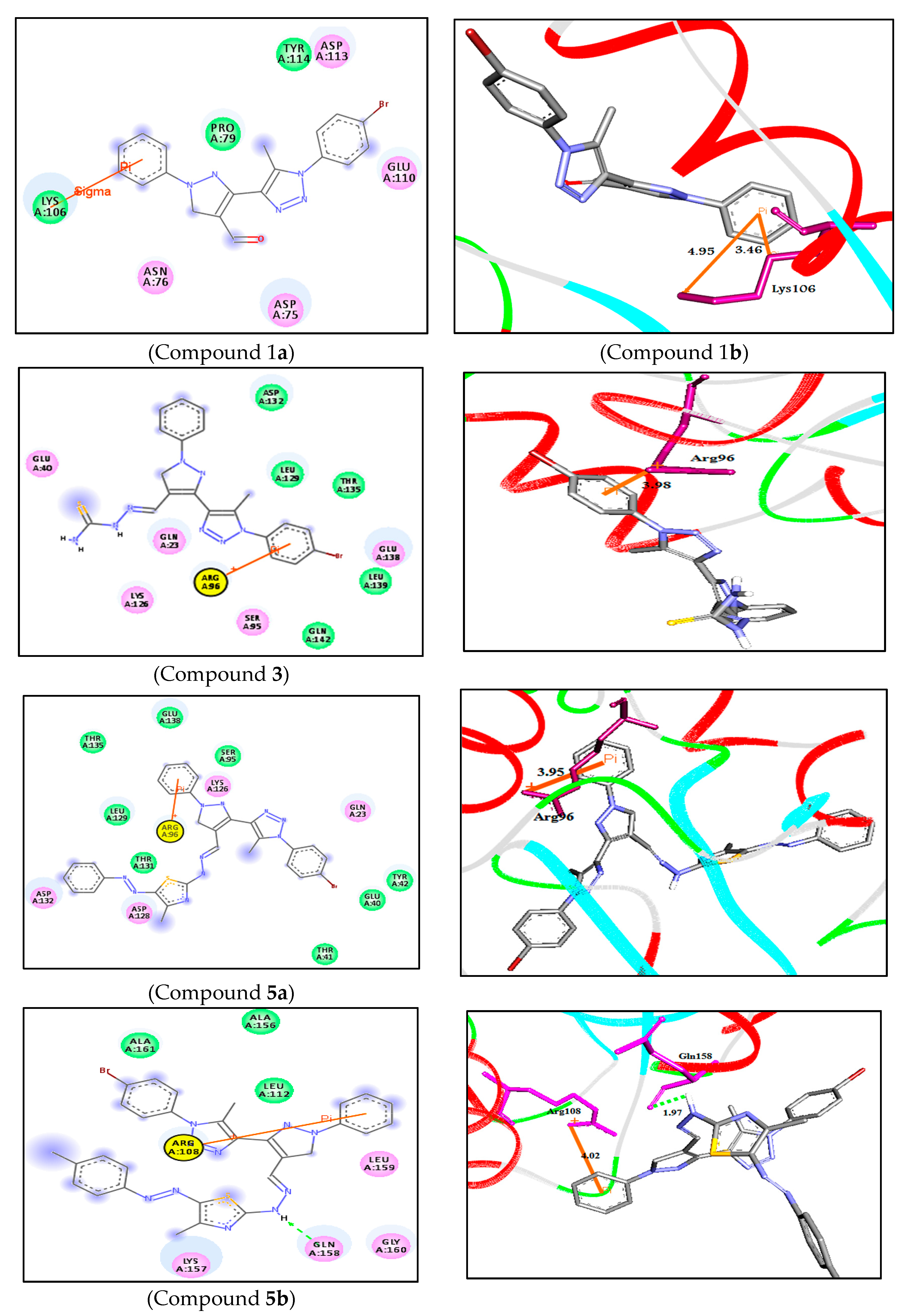
| 2D Structure | BE kcal.mol−1 | Docked Complex (Amino Acid-Ligand) Interactions | Bond Length (Å) | |
|---|---|---|---|---|
| 1 | 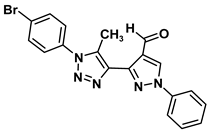 | −6.8 | Arene-cation interaction Lys106:NZ-compound 1 | 4.95 |
| Arene-sigma interaction Lys106:CG-compound 1 | 3.46 | |||
| 3 | 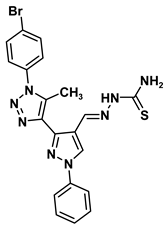 | −7.2 | Arene-cation interaction Arg96:NH1-compound 3 | 3.98 |
| 5a |  | −8.2 | Arene-cation interaction Arg96:NH1-compound 5a | 3.95 |
| 5b | 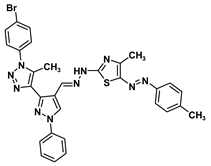 | −9.4 | H-bond interaction Gln158:O-compound 5b | 1.97 |
| Arene-cation interaction Arg108:NH1---compound 5b | 4.02 | |||
| 5c | 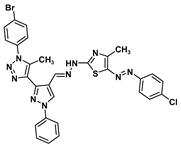 | −9.0 | H-bond interaction Gln158:N-compound 5c | 2.35 |
| H-bond interaction Leu159:N-compound 5c | 2.20 | |||
| 5d | 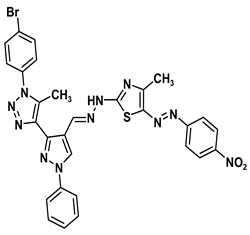 | −9.1 | H-bond interaction Gln158:N-compound 5d Leu159:N-compound 5d Gly160:N-compound 5d Ala161:O-compound 5d | 2.43 2.59 1.93 1.99 |
| Arene-cation interaction Lys157:NZ-compound 5d | 5.49 | |||
| 8a |  | −6.5 | H-bond interaction Ser64:OG-compound 8a | 2.10 |
| H-bond interaction Trp66:NE1-compound 8a | 1.96 | |||
| 8b | 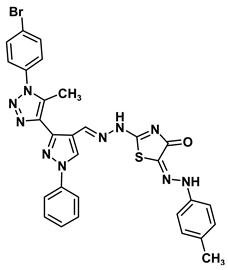 | −9.9 | H-bond interaction Gln158:N-compound 8b Leu159:N-compound 8b Gln158:O-compound 8b | 3.10 2.98 1.94 |
| 8c | 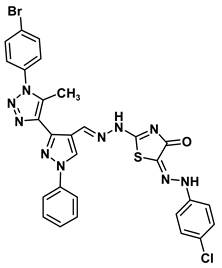 | −8.9 | H-bond interaction Arg108:NE-compound 8c Arg108:NH1-compound 8c Val120:N-compound 8c Leu121:N-compound 8c | 2.61 2.99 1.88 2.63 |
| Arene-sigma interaction Val120:CG1-compound 8c | 3.63 | |||
| 8d | 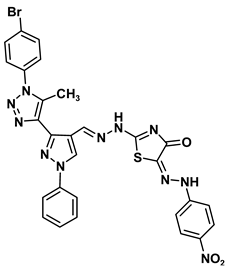 | −9.8 | H-bond interaction Gly160:N-compound 8d Gly160:N-compound 8d Gln158:O-compound 8d | 2.98 2.81 1.30 |
| 13a | 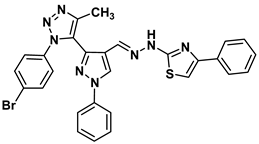 | −7.7 | H-bond interaction Ser95:OG-compound 13a Glu138:OE1-compound 13a | 2.30 2.15 |
| Arene-cation interaction Arg96:NH1---compound13a | 4.10 | |||
| 13b | 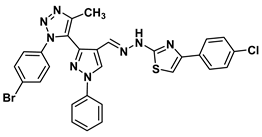 | −7.8 | H-bond interaction Asp132:OD2-compound 13b | 2.99 |
| 14 | 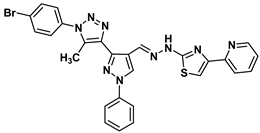 | −7.9 | H-bond interaction Ser95:OG-compound 14 | 2.95 |
| Arene-cation interaction Arg96:NH1-compound14 | 3.88 | |||
| 15 | 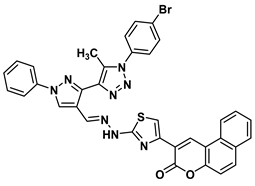 | −9.2 | Arene-cation interaction Lys15:NZ-compound 15 Lys15:NZ-compound 15 | 5.74 5.50 |
| MW (g/mol) | BBB+ | Caco2+ | HIA+ | logp | TPSA A2 | nON | nOHNH | RBs | N Violations | AMES Toxicity | Carcinogenicity | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 180–500 | −3 to 1.2 | < 25 poor > 500 great | < 25 poor >80 high | <5 | ≤ 140 | 2.0–20.0 | 0.0–6.0 | ≤ 10 | < 5 | Nontoxic | Non carcinogenic | |
| 1 | 408.26 | 0.98 | 67.17 | 98.94 | 3.15 | 65.61 | 6 | 0 | 4 | 0 | Nontoxic | Noncarcinogenic |
| 3 | 481.38 | 0.97 | 65.20 | 96.57 | 3.31 | 98.96 | 8 | 3 | 6 | 0 | Nontoxic | Noncarcinogenic |
| 5a | 623.54 | 0.98 | 82.20 | 92.98 | 6.24 | 110.56 | 10 | 1 | 8 | 2 | Nontoxic | Noncarcinogenic |
| 5b | 637.57 | 0.98 | 82.51 | 92.96 | 6.69 | 110.56 | 10 | 1 | 8 | 2 | Nontoxic | Noncarcinogenic |
| 5c | 657.99 | 0.97 | 82.77 | 92.93 | 6.92 | 110.56 | 10 | 1 | 8 | 2 | Nontoxic | Noncarcinogenic |
| 5d | 668.54 | 0.97 | 82.69 | 92.95 | 6.20 | 156.38 | 13 | 1 | 9 | 3 | Nontoxic | Noncarcinogenic |
| 8a | 625.52 | 0.98 | 83.26 | 97.32 | 4.74 | 127.29 | 11 | 2 | 8 | 1 | Nontoxic | Noncarcinogenic |
| 8b | 639.54 | 0.98 | 83.64 | 97.35 | 5.19 | 127.29 | 11 | 2 | 8 | 2 | Nontoxic | Noncarcinogenic |
| 8c | 659.96 | 0.98 | 83.67 | 97.96 | 5.42 | 127.29 | 11 | 2 | 8 | 2 | Nontoxic | Noncarcinogenic |
| 8d | 670.51 | 0.98 | 83.93 | 97.98 | 4.70 | 173.11 | 14 | 2 | 9 | 2 | Nontoxic | Noncarcinogenic |
| 13a | 581.50 | 0.97 | 79.71 | 92.98 | 5.38 | 85.83 | 8 | 1 | 7 | 2 | Nontoxic | Noncarcinogenic |
| 13b | 615.95 | 0.97 | 82.80 | 94.06 | 6.06 | 85.83 | 8 | 1 | 7 | 2 | Nontoxic | Noncarcinogenic |
| 14 | 582.49 | 0.98 | 78.83 | 92.98 | 4.23 | 98.72 | 9 | 1 | 7 | 1 | Nontoxic | Noncarcinogenic |
| 15 | 699.59 | 0.98 | 85.73 | 88.62 | 6.54 | 116.04 | 10 | 1 | 7 | 2 | Nontoxic | Noncarcinogenic |
| Comp. No | R | HepG2 IC50 ± SD [µg/mL] | BALAB/3T3 IC50 ± SD [µg/mL] | General Structure |
|---|---|---|---|---|
| Doxorubicin | 3.56 ± 0.84 | 1.86 ± 0.07 | 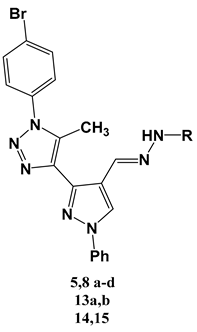 | |
| 5a |  | 35.64 ± 6.07 | Nd | |
| 5b | 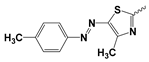 | 2.30 ± 2.72 | Nd | |
| 5c |  | 32.32 ± 6.09 | 10.09 ± 0.23 | |
| 5d |  | 36.79 ± 15.70 | Nd | |
| 8a | 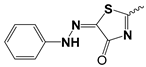 | 49.05 ± 5.37 | 16.72 ± 3.24 | |
| 8b | 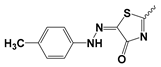 | 11.83 ± 0.29 | Nd | |
| 8c |  | 43.30 ± 14.77 | Nd | |
| 8d |  | 18.24 ± 0.08 | Nd | |
| 13a | 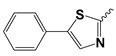 | 27.34 ± 6.14 | Nd | |
| 13b |  | 30.26 ± 3.05 | 43.23 ± 2.36 | |
| 14 | 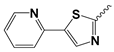 | 19.75 ± 9.37 | Nd | |
| 15 | 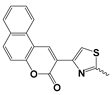 | 29.61 ± 2.74 | Nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashdan, H.R.M.; El-Naggar, M.; Abdelmonsef, A.H. Synthesis, Molecular Docking Studies and In Silico ADMET Screening of New Heterocycles Linked Thiazole Conjugates as Potent Anti-Hepatic Cancer Agents. Molecules 2021, 26, 1705. https://doi.org/10.3390/molecules26061705
Rashdan HRM, El-Naggar M, Abdelmonsef AH. Synthesis, Molecular Docking Studies and In Silico ADMET Screening of New Heterocycles Linked Thiazole Conjugates as Potent Anti-Hepatic Cancer Agents. Molecules. 2021; 26(6):1705. https://doi.org/10.3390/molecules26061705
Chicago/Turabian StyleRashdan, Huda R. M., Mohamed El-Naggar, and Aboubakr H. Abdelmonsef. 2021. "Synthesis, Molecular Docking Studies and In Silico ADMET Screening of New Heterocycles Linked Thiazole Conjugates as Potent Anti-Hepatic Cancer Agents" Molecules 26, no. 6: 1705. https://doi.org/10.3390/molecules26061705
APA StyleRashdan, H. R. M., El-Naggar, M., & Abdelmonsef, A. H. (2021). Synthesis, Molecular Docking Studies and In Silico ADMET Screening of New Heterocycles Linked Thiazole Conjugates as Potent Anti-Hepatic Cancer Agents. Molecules, 26(6), 1705. https://doi.org/10.3390/molecules26061705






