Risperidone/Randomly Methylated β-Cyclodextrin Inclusion Complex—Compatibility Study with Pharmaceutical Excipients
Abstract
1. Introduction
2. Results and Discussion
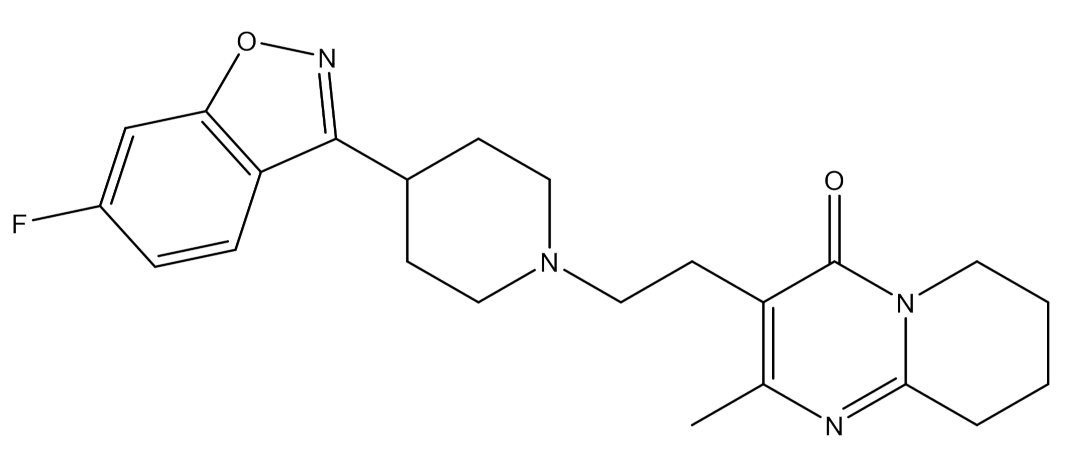
2.1. Inclusion Complex Characterization
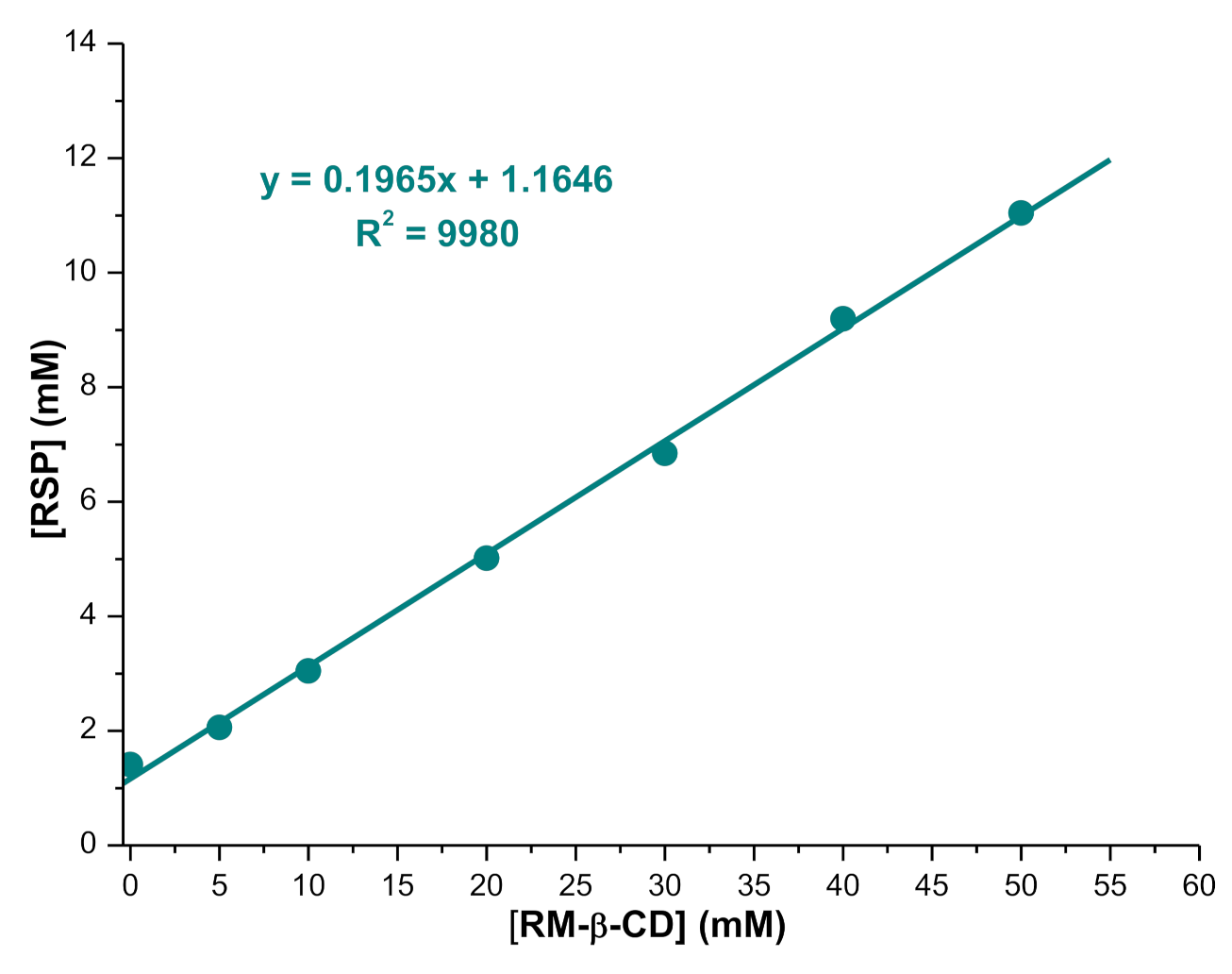
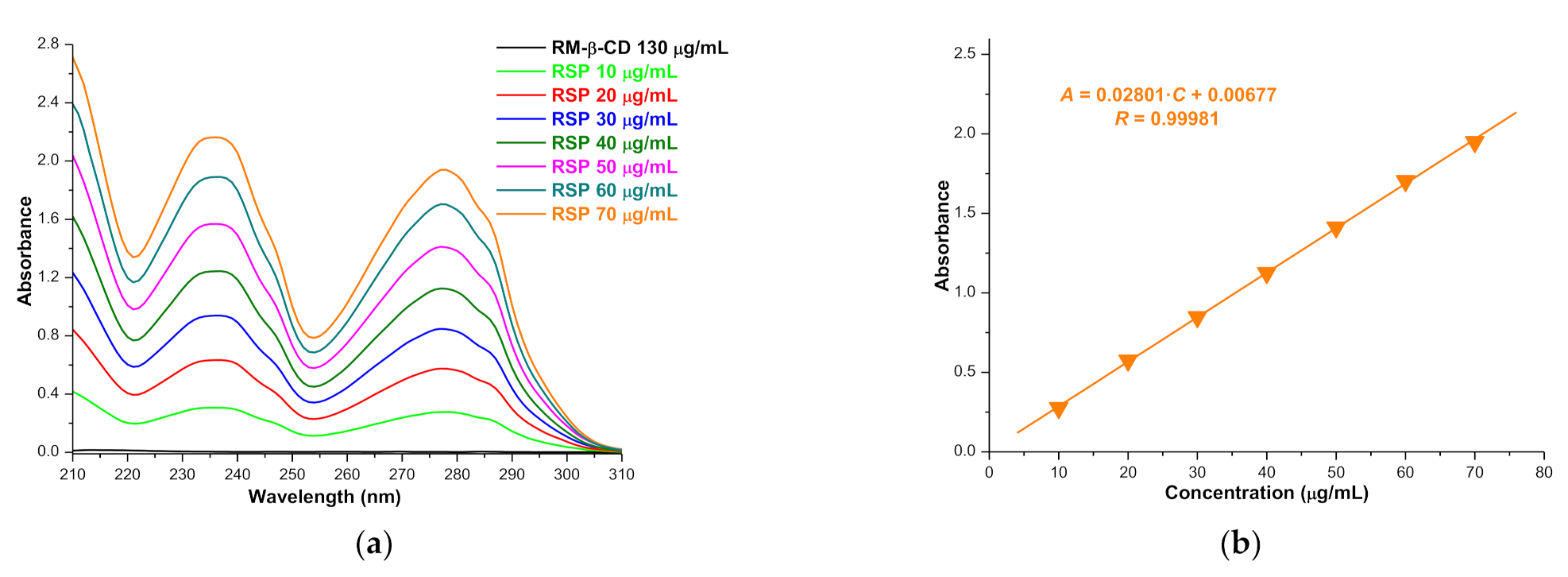
2.1.1. Phase Solubility Studies
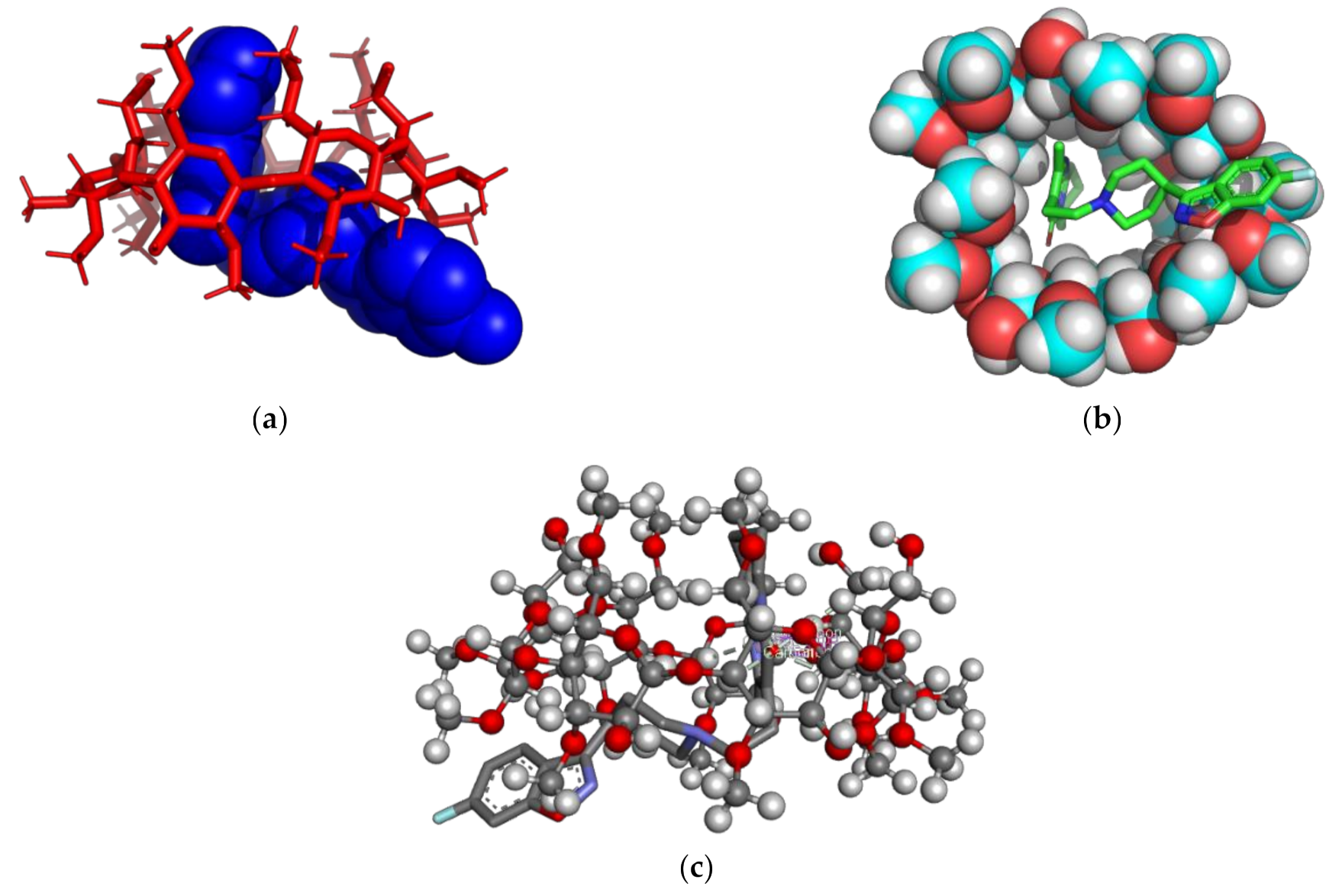
2.1.2. Molecular Modeling
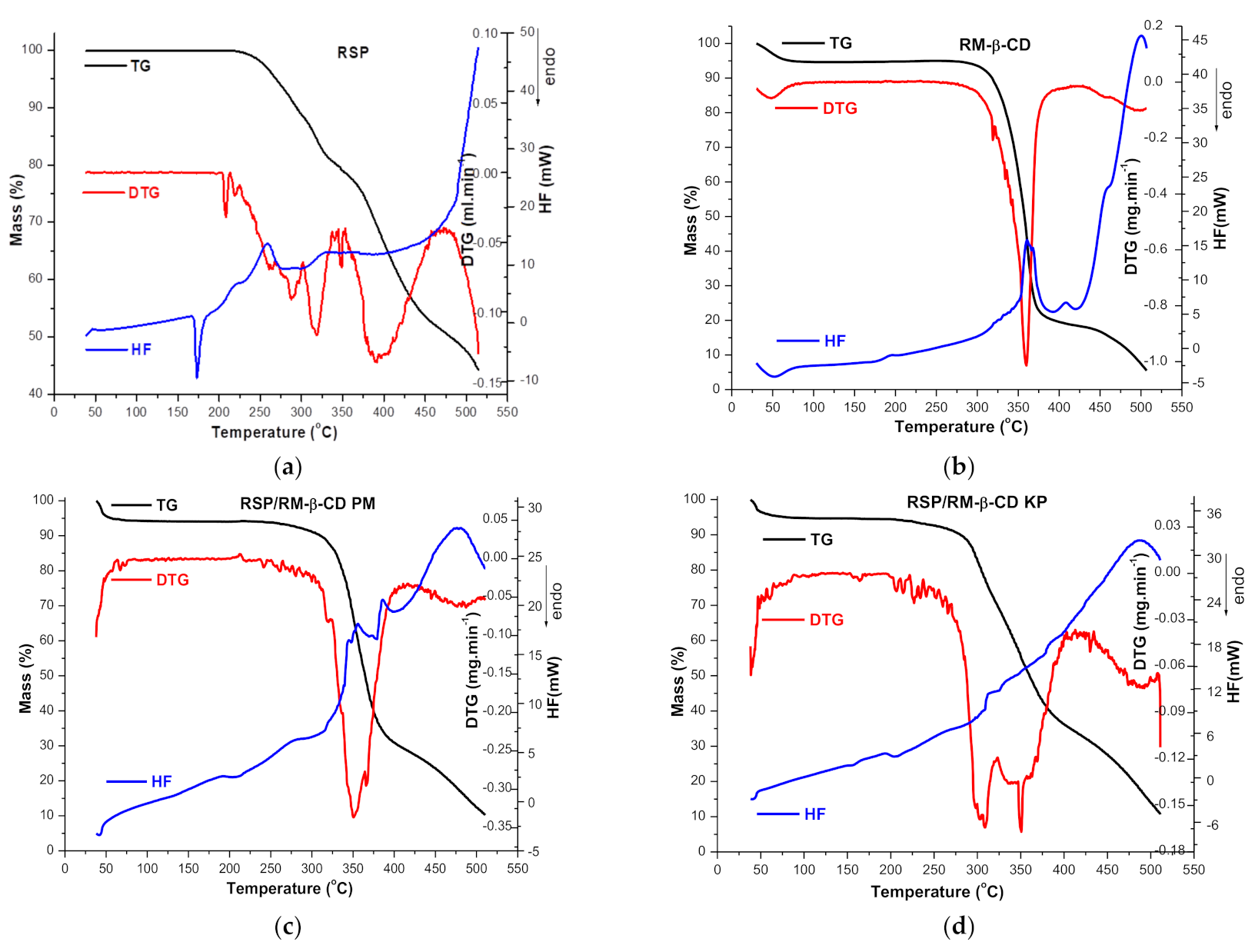
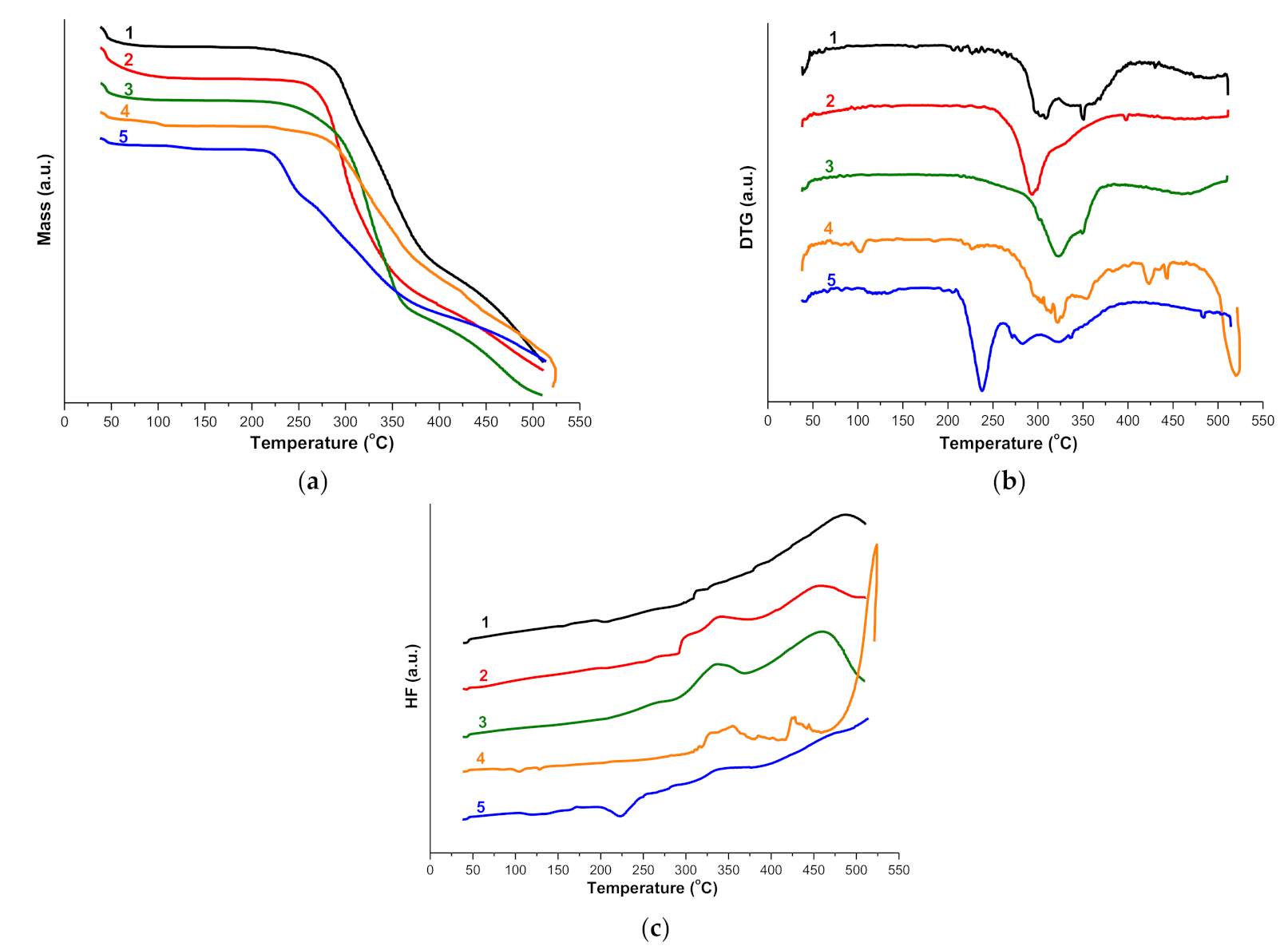
2.1.3. Thermal Analysis
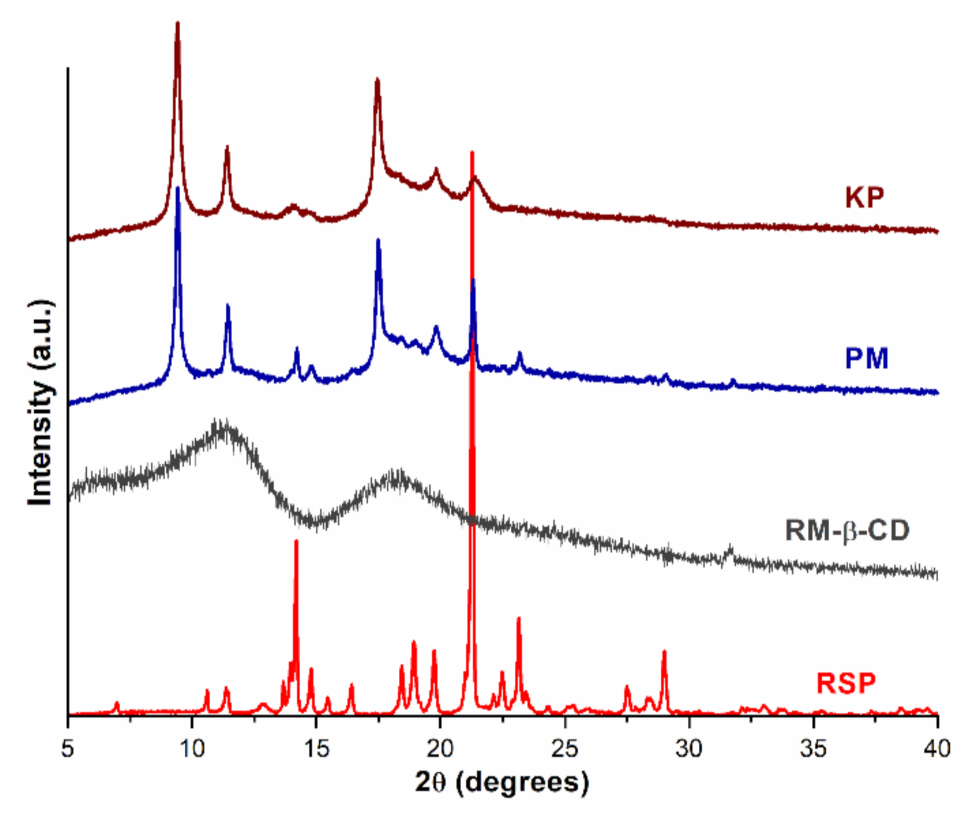
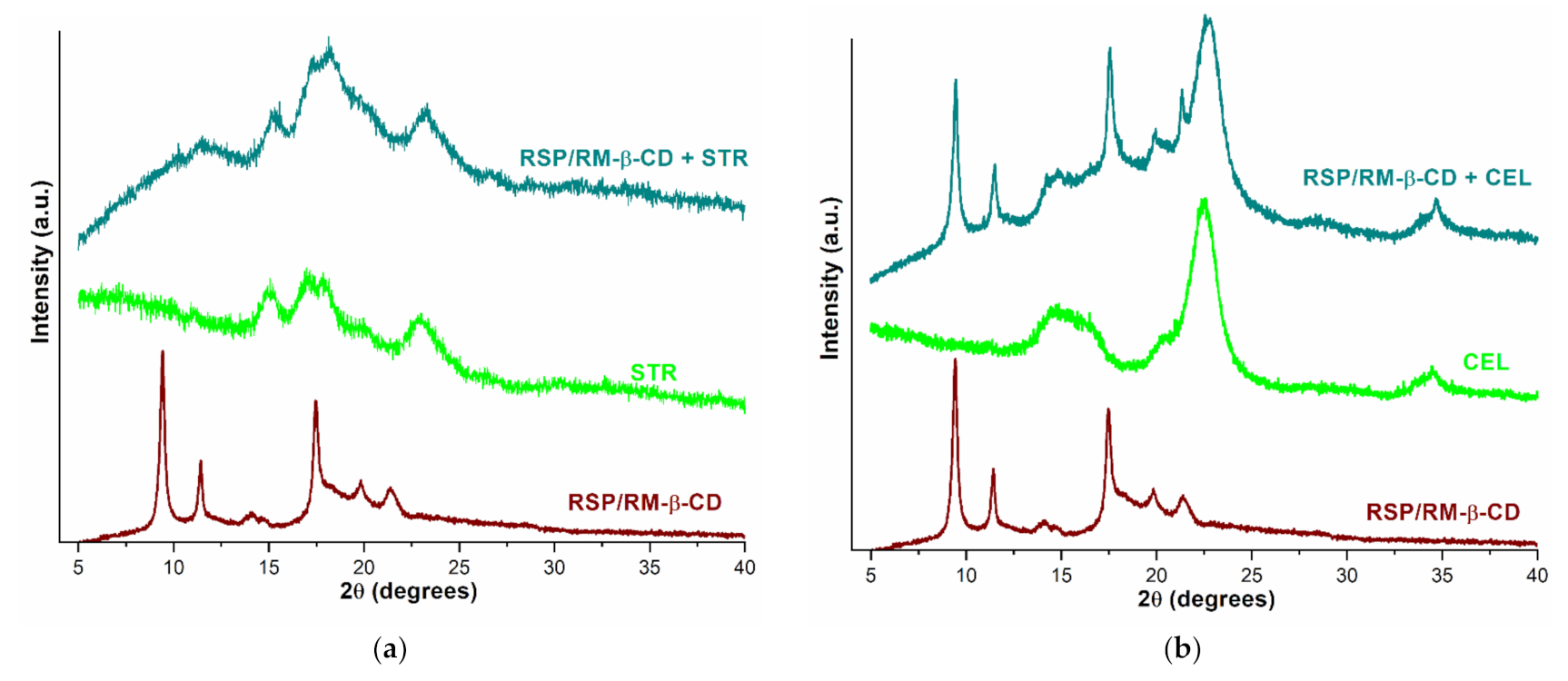
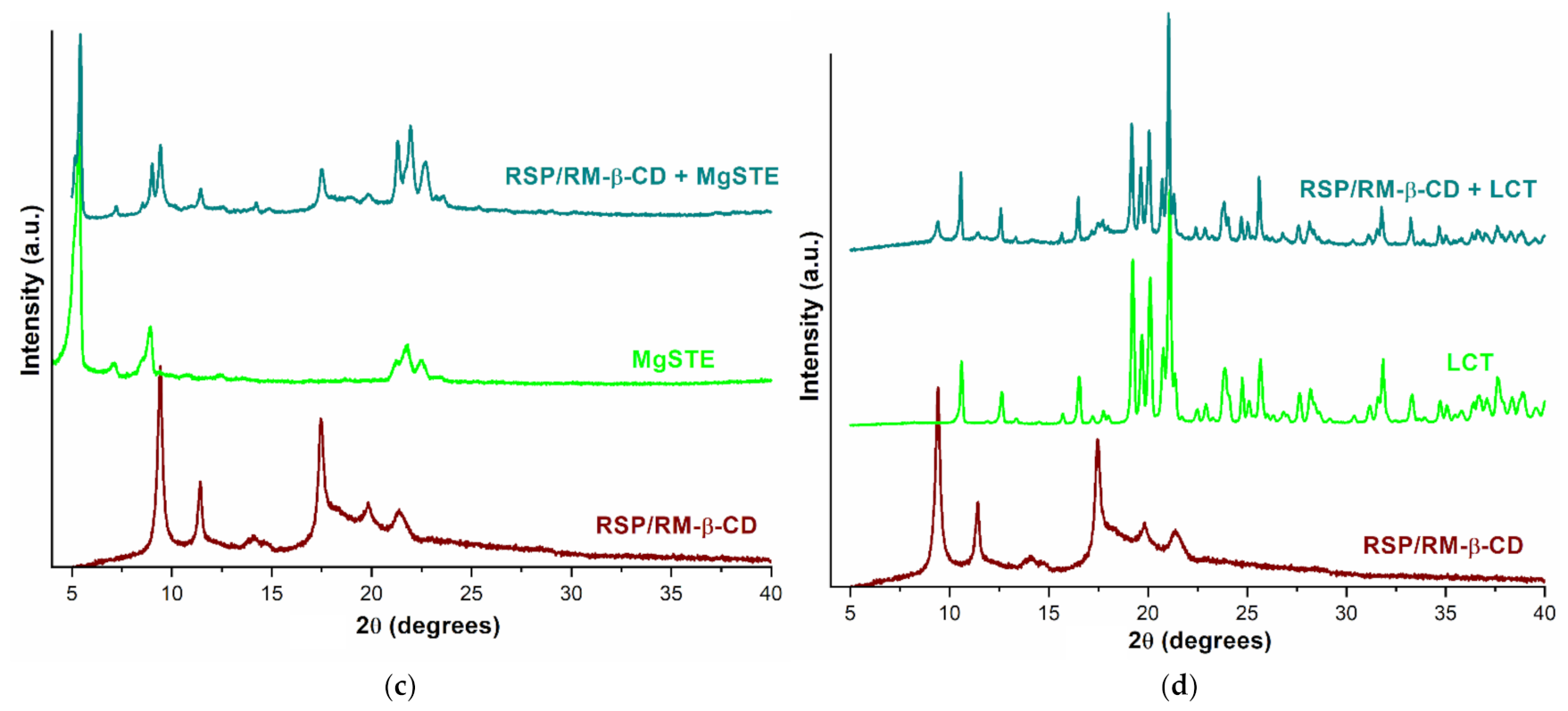
2.1.4. Powder X-ray Diffractometry
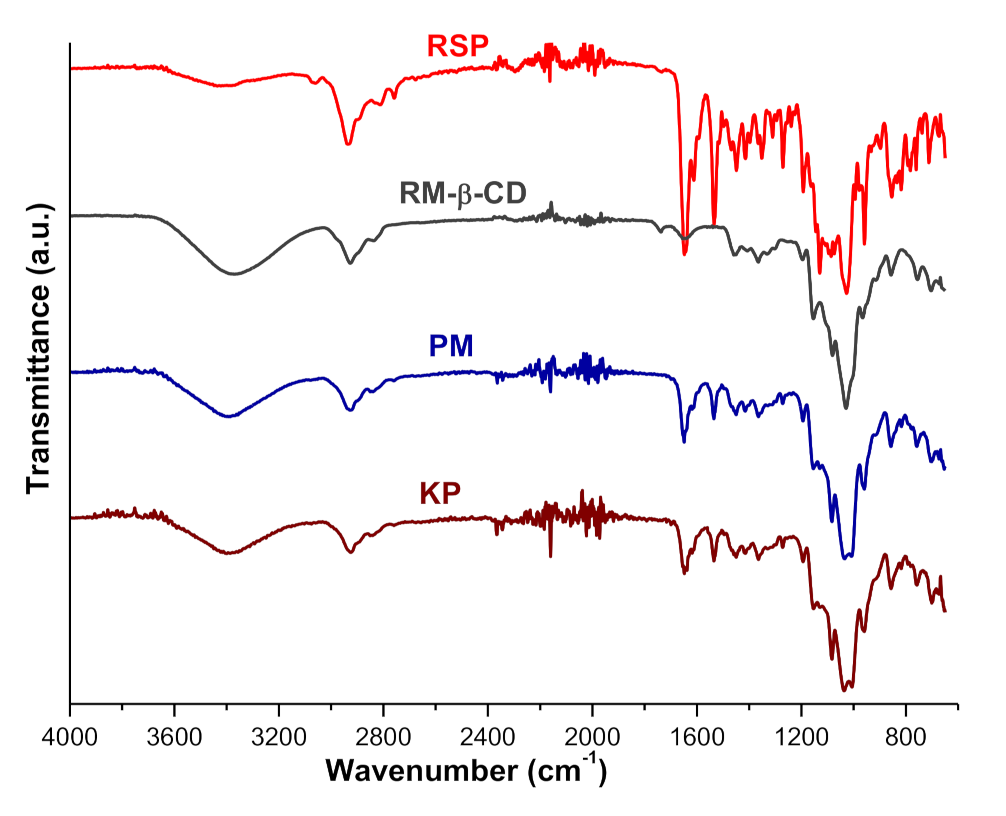
2.1.5. UATR-FTIR Spectroscopy
2.1.6. Solubility Profile of RSP/RM-β-CD Inclusion Complexes
2.2. Compatibility Studies of RSP/RM-β-CD Inclusion Complex with Excipients
2.2.1. Thermoanalytical Studies
2.2.2. UATR-FTIR Studies
2.2.3. PXRD Studies
3. Materials and Methods
3.1. Materials
3.2. Phase Solubility Studies
3.3. Molecular Docking Studies
3.4. Preparation of the Solid Inclusion Complex and Physical Mixtures with Excipients
3.5. Thermal Analysis
3.6. Powder X-ray Diffractometry
3.7. UATR-FTIR Spectroscopy
3.8. Solubility Profile of RSP/RM-β-CD Kneaded Product
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Germann, D.; Kurylo, N.; Han, F. Risperidone. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press Elsevier: Amsterdam, The Netherlands, 2012; Volume 37, pp. 313–361. ISBN 9780123972200. [Google Scholar]
- Yunusa, I.; El Helou, M.L. The Use of Risperidone in Behavioral and Psychological Symptoms of Dementia: A Review of Pharmacology, Clinical Evidence, Regulatory Approvals, and Off-Label Use. Front. Pharmacol. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Love, R.C.; Nelson, M.W. Pharmacology and clinical experience with risperidone. Expert Opin. Pharmacother. 2000, 1, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Zidan, A.S.; Khan, M.A. Risperidone solid dispersion for orally disintegrating tablet: Its formulation design and non-destructive methods of evaluation. Int. J. Pharm. 2010, 400, 49–58. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Fourmentin, S.; Fenyvesi, Éva; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Duarte, F.; Ílary, C.; Heimfarth, L.; Quintans, J.D.S.S.; Quintans-Júnior, L.J.; Júnior, V.F.D.V.; De Lima, Á.A.N. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Usacheva, T.; Kabirov, D.; Beregova, D.; Gamov, G.; Sharnin, V.; Biondi, M.; Mayol, L.; D’Aria, F.; Giancola, C. Thermodynamics of complex formation between hydroxypropyl-β-cyclodextrin and quercetin in water–ethanol solvents at T = 298.15 K. J. Therm. Anal. Calorim. 2019, 138, 417–424. [Google Scholar] [CrossRef]
- Han, D.; Han, Z.; Liu, L.; Wang, Y.; Xin, S.; Zhang, H.; Yu, Z. Han Solubility Enhancement of Myricetin by Inclusion Complexation with Heptakis-O-(2-Hydroxypropyl)-β-Cyclodextrin: A Joint Experimental and Theoretical Study. Int. J. Mol. Sci. 2020, 21, 766. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Sahakijpijarn, S.; Moon, C.; Koleng, J.; Christensen, D.; Williams, R. Development of Remdesivir as a Dry Powder for Inhalation by Thin Film Freezing. Pharmaceutics 2020, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yuan, L.; Chen, Y.; Zhou, W.; Wang, X. Studies on the Inclusion Complexes of Daidzein with β-Cyclodextrin and Derivatives. Molecules 2017, 22, 2183. [Google Scholar] [CrossRef]
- Saxena, K.; Kurian, S.; Saxena, J.; Goldberg, A.; Chen, E.; Simonetti, A. Mixed States in Early-Onset Bipolar Disorder. Psychiatr. Clin. N. Am. 2020, 43, 95–111. [Google Scholar] [CrossRef]
- Braga, S.S.; El-Saleh, F.; Lysenko, K.; Paz, F.A.A. Inclusion Compound of Efavirenz and γ-Cyclodextrin: Solid State Studies and Effect on Solubility. Molecules 2021, 26, 519. [Google Scholar] [CrossRef]
- Simsek, T.; Rasulev, B.; Mayer, C.; Simsek, S. Preparation and Characterization of Inclusion Complexes of β-Cyclodextrin and Phenolics from Wheat Bran by Combination of Experimental and Computational Techniques. Molecules 2020, 25, 4275. [Google Scholar] [CrossRef]
- He, J.; Zheng, Z.-P.; Zhu, Q.; Guo, F.; Chen, J. Encapsulation Mechanism of Oxyresveratrol by β-Cyclodextrin and Hydroxypropyl-β-Cyclodextrin and Computational Analysis. Molecules 2017, 22, 1801. [Google Scholar] [CrossRef]
- Du, F.; Pan, T.; Ji, X.; Hu, J.; Ren, T. Study on the preparation of geranyl acetone and β-cyclodextrin inclusion complex and its application in cigarette flavoring. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tănase, I.-M.; Sbârcea, L.; Ledeţi, A.; Barvinschi, P.; Cîrcioban, D.; Vlase, G.; Văruţ, R.-M.; Ledeţi, I. Compatibility studies with pharmaceutical excipients for aripiprazole–heptakis (2,6-di-O-methyl)-β-cyclodextrin supramolecular adduct. J. Therm. Anal. Calorim. 2020, 142, 1963–1976. [Google Scholar] [CrossRef]
- Tănase, I.-M.; Sbârcea, L.; Ledeți, A.; Vlase, G.; Barvinschi, P.; Văruţ, R.-M.; Dragomirescu, A.; Axente, C.; Ledeți, I. Physicochemical characterization and molecular modeling study of host–guest systems of aripiprazole and functionalized cyclodextrins. J. Therm. Anal. Calorim. 2020, 141, 1027–1039. [Google Scholar] [CrossRef]
- Sbârcea, L.; Ledeţi, I.; DrĂgan, L.; Kurunczi, L.; Fuliaş, A.; Udrescu, L. Fosinopril sodium–hydroxypropyl-β-cyclodextrin inclusion complex. J. Therm. Anal. Calorim. 2015, 120, 981–990. [Google Scholar] [CrossRef]
- Ferreira, E.B.; Júnior, W.F.D.S.; Pinheiro, J.G.D.O.; Da Fonseca, A.G.; Lemos, T.M.A.M.; Rocha, H.A.D.O.; De Azevedo, E.P.; Junior, F.J.B.M.; De Lima, Á.A.N. Characterization and Antiproliferative Activity of a Novel 2-Aminothiophene Derivative-β-Cyclodextrin Binary System. Molecules 2018, 23, 3130. [Google Scholar] [CrossRef]
- Sbârcea, L.; Tănase, I.-M.; Ledeți, A.; Cîrcioban, D.; Vlase, G.; Barvinschi, P.; Miclău, M.; Văruţ, R.-M.; Trandafirescu, C.; Ledeți, I. Encapsulation of Risperidone by Methylated β-Cyclodextrins: Physicochemical and Molecular Modeling Studies. Molecules 2020, 25, 5694. [Google Scholar] [CrossRef]
- Pires, F.Q.; Pinho, L.A.; Freire, D.O.; Silva, I.C.R.; Sa-Barreto, L.L.; Cardozo-Filho, L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Thermal analysis used to guide the production of thymol and Lippia origanoides essential oil inclusion complexes with cyclodextrin. J. Therm. Anal. Calorim. 2018, 137, 543–553. [Google Scholar] [CrossRef]
- Júnior, F.J.D.L.R.; Da Silva, K.M.A.; Brandão, D.O.; Júnior, J.V.C.; Dos Santos, J.A.B.; De Andrade, F.H.D.; Batista, R.S.D.A.; Lins, T.B.; De Sousa, D.P.; Medeiros, A.C.D.; et al. Investigation of the thermal behavior of inclusion complexes with antifungal activity. J. Therm. Anal. Calorim. 2018, 133, 641–648. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef]
- Gu, W.; Liu, Y. Characterization and stability of beta-acids/hydroxypropyl-β-cyclodextrin inclusion complex. J. Mol. Struct. 2020, 1201, 127159. [Google Scholar] [CrossRef]
- Szente, L. Highly soluble cyclodextrin derivatives: Chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 1999, 36, 17–28. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Shukla, D.; Chakraborty, S.; Singh, S.; Mishra, B. Preparation and in-vitro characterization of Risperidone-cyclodextrin inclusion complexes as a potential injectable product. DARU J. Pharm. Sci. 2009, 17, 226–235. [Google Scholar]
- Jug, M.; Kos, I.; Bećirević-Laćan, M. The pH-dependent complexation between risperidone and hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2009, 64, 163–171. [Google Scholar] [CrossRef]
- El-Barghouthi, M.I.; Masoud, N.A.; Al-Kafawein, J.K.; Zughul, M.B.; Badwan, A.A. Host–Guest Interactions of Risperidone with Natural and Modified Cyclodextrins: Phase Solubility, Thermodynamics and Molecular Modeling Studies. J. Incl. Phenom. Macrocycl. Chem. 2005, 53, 15–22. [Google Scholar] [CrossRef]
- Kacso, I.; Rus, L.M.; Martin, F.; Miclaus, M.; Filip, X.; Dan, M. Solid-state compatibility studies of Ketoconazole-Fumaric acid co-crystal with tablet excipients. J. Therm. Anal. Calorim. 2021, 143, 3499–3506. [Google Scholar] [CrossRef]
- Ledeți, I.; Romanescu, M.; Cîrcioban, D.; Ledeți, A.; Vlase, G.; Vlase, T.; Suciu, O.; Murariu, M.; Olariu, S.; Matusz, P.; et al. Stability and Compatibility Studies of Levothyroxine Sodium in Solid Binary Systems—Instrumental Screening. Pharmaceutics 2020, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Cristea, M.; Baul, B.; Ledeţi, I.; Ledeţi, A.; Vlase, G.; Vlase, T.; Karolewicz, B.; Ştefănescu, O.; Dragomirescu, A.O.; Mureşan, C.; et al. Preformulation studies for atorvastatin calcium. J. Therm. Anal. Calorim. 2019, 138, 2799–2806. [Google Scholar] [CrossRef]
- Zhang, L.; Luan, H.; Lu, W.; Wang, H. Preformulation Studies and Enabling Formulation Selection for an Insoluble Compound at Preclinical Stage—From In Vitro, In Silico to In Vivo. J. Pharm. Sci. 2020, 109, 950–958. [Google Scholar] [CrossRef]
- Jiang, W.; Yu, L.X. Modern Pharmaceutical Quality Regulations. Dev. Solid Oral Dos. Forms 2009, 885–901. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration. ICH Q8(R2) Pharmaceutical Development. Work. Qual. Des. Pharm. 2009, 8, 28. [Google Scholar]
- Higuchi, T.K.; Connors, A. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–211. [Google Scholar]
- Yin, H.; Wang, C.; Yue, J.; Deng, Y.; Jiao, S.; Zhao, Y.; Zhou, J.; Cao, T. Optimization and characterization of 1,8-cineole/hydroxypropyl-β-cyclodextrin inclusion complex and study of its release kinetics. Food Hydrocoll. 2021, 110, 106159. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, J.; Wang, Q.; Ren, L.; Zhou, J. Physicochemical properties of catechin/β-cyclodextrin inclusion complex obtained via co-precipitation. CyTA J. Food 2019, 17, 544–551. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. Newsl. Protein Crystallogr. 2002, 40, 1–8. [Google Scholar]
- Daniel, J.S.P.; Veronez, I.P.; Rodrigues, L.L.; Trevisan, M.G.; Garcia, J.S. Risperidone—Solid-state characterization and pharmaceutical compatibility using thermal and non-thermal techniques. Thermochim. Acta 2013, 568, 148–155. [Google Scholar] [CrossRef]
- Rao, K.S.; Udgirkar, D.B.; Mule, D.D. Enhancement of dissolution rate and bioavailability of Aceclofenac by Complexation with cyclodextrin. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 142–151. [Google Scholar]
- Sbârcea, L.; Ledeţi, A.; Udrescu, L.; Văruţ, R.-M.; Barvinschi, P.; Vlase, G.; Ledeţi, I. Betulonic acid—cyclodextrins inclusion complexes. J. Therm. Anal. Calorim. 2019, 138, 2787–2797. [Google Scholar] [CrossRef]
- Circioban, D.; Ledeti, I.; Suta, L.-M.; Vlase, G.; Ledeti, A.; Vlase, T.; Varut, R.; Sbarcea, L.; Trandafirescu, C.; Dehelean, C. Instrumental analysis and molecular modelling of inclusion complexes containing artesunate. J. Therm. Anal. Calorim. 2020, 142, 1951–1961. [Google Scholar] [CrossRef]
- Sbarcea, L.; Udrescu, L.; Dragan, L.; Trandafirescu, C.; Sasca, V.; Barvinschi, P.; Bojita, M. Characterization of fosinopril natrium-hydroxypropyl-β-cyclodextrin inclusion complex. Rev. Chim. 2011, 62, 349–351. [Google Scholar]
- Circioban, D.; Ledeti, A.; Vlase, G.; Coricovac, D.; Moaca, A.; Farcas, C.; Vlase, T.; Ledeti, I.; Dehelean, C. Guest–host interactions and complex formation for artemisinin with cyclodextrins: Instrumental analysis and evaluation of biological activity. J. Therm. Anal. Calorim. 2018, 134, 1375–1384. [Google Scholar] [CrossRef]
- Sbârcea, L.; Udrescu, L.; DrĂgan, L.; Trandafirescu, C.; Szabadai, Z.; Bojiţă, M. Fosinopril-cyclodextrin inclusion complexes: Phase solubility and physicochemical analysis. Die Pharm. 2011, 66. [Google Scholar]
- Circioban, D.; Ledeti, A.; Vlase, G.; Moaca, A.; Ledeti, I.; Farcaş, C.; Vlase, T.; Dehelean, C. Thermal degradation, kinetic analysis and evaluation of biological activity on human melanoma for artemisinin. J. Therm. Anal. Calorim. 2018, 134, 741–748. [Google Scholar] [CrossRef]
- Udrescu, L.; Sbârcea, L.; Fuliaș, A.; Ledeți, I.; Vlase, G.; Barvinschi, P.; Kurunczi, L. Physicochemical Analysis and Molecular Modeling of the Fosinoprilβ-Cyclodextrin Inclusion Complex. J. Spectrosc. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Koivisto, M.; Jalonen, H.; Lehto, V.-P. Effect of temperature and humidity on vegetable grade magnesium stearate. Powder Technol. 2004, 147, 79–85. [Google Scholar] [CrossRef]
- Craig, D.Q.M.; Reading, M. Thermal Analysis of Pharmaceuticals; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9781420014891. [Google Scholar]
- Segall, A.I. Preformulation: The use of FTIR in compatility studies. J. Innov. Appl. Pharm. Sci. 2019, 4, 1–6. [Google Scholar]
- da Silva, E.P.; Pereira, M.A.V.; Lima, I.P.D.B.; Lima, N.G.P.B.; Barbosa, E.G.; Aragão, C.F.S.; Gomes, A.P.B. Compatibility study between atorvastatin and excipients using DSC and FTIR. J. Therm. Anal. Calorim. 2016, 123, 933–939. [Google Scholar] [CrossRef]
- Mohamed, A.I.; Abd-Motagaly, A.M.E.; Ahmed, O.A.A.; Amin, S.; Ali, A.I.M. Investigation of Drug–Polymer Compatibility Using Chemometric-Assisted UV-Spectrophotometry. Pharmaceutics 2017, 9, 7. [Google Scholar] [CrossRef]
- Rus, L.M. Development of meloxicam oral lyophilisates: Role of thermal analysis and complementary techniques. Farmacia 2019, 67, 56–67. [Google Scholar] [CrossRef]
- Protein DATA Bank. Available online: http://www.pdb.org/pdb/home/home.do (accessed on 10 January 2021).

| Sample | Analysis of UATR-FTIR Spectral Regions (cm−1) | ||
|---|---|---|---|
| 3600–2700 | 1700–1000 | 1000–650 | |
| RSP/RM-β-CD | 3387; 2924 | 1642; 1535; 1450; 1415; 1364; 1272; 1194; 1154; 1083; 1037; 1006 | 959; 857; 758 |
| RSP/RM-β-CD + STR | 3386; 2919; 2847 | 1649; 1535; 1459; 1416; 1364; 1272; 1195; 1152; 1083;1035; 1006 | 962; 857; 758 |
| RSP/RM-β-CD + CEL | 3386; 2930 | 1649; 1535; 1458; 1416; 1364; 1272; 1194; 1155; 1083; 1034; 1009 | 956; 858; 758 |
| RSP/RM-β-CD + MgSTE | 2957; 2916; 2850 | 1639; 1572; 1541; 1465; 1418; 1365; 1272; 1192; 1154; 1083; 1038; 1007 | 961; 857; 722 |
| RSP/RM-β-CD + LCT | 3338; 2945; 2931; 2901 | 1642; 1535; 1420; 1369; 1254; 1195; 1157; 1142; 1118; 1083; 1068; 1031; 1018 | 989; 968; 877; 761 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sbârcea, L.; Tănase, I.-M.; Ledeți, A.; Cîrcioban, D.; Vlase, G.; Barvinschi, P.; Miclău, M.; Văruţ, R.-M.; Suciu, O.; Ledeți, I. Risperidone/Randomly Methylated β-Cyclodextrin Inclusion Complex—Compatibility Study with Pharmaceutical Excipients. Molecules 2021, 26, 1690. https://doi.org/10.3390/molecules26061690
Sbârcea L, Tănase I-M, Ledeți A, Cîrcioban D, Vlase G, Barvinschi P, Miclău M, Văruţ R-M, Suciu O, Ledeți I. Risperidone/Randomly Methylated β-Cyclodextrin Inclusion Complex—Compatibility Study with Pharmaceutical Excipients. Molecules. 2021; 26(6):1690. https://doi.org/10.3390/molecules26061690
Chicago/Turabian StyleSbârcea, Laura, Ionuț-Mihai Tănase, Adriana Ledeți, Denisa Cîrcioban, Gabriela Vlase, Paul Barvinschi, Marinela Miclău, Renata-Maria Văruţ, Oana Suciu, and Ionuț Ledeți. 2021. "Risperidone/Randomly Methylated β-Cyclodextrin Inclusion Complex—Compatibility Study with Pharmaceutical Excipients" Molecules 26, no. 6: 1690. https://doi.org/10.3390/molecules26061690
APA StyleSbârcea, L., Tănase, I.-M., Ledeți, A., Cîrcioban, D., Vlase, G., Barvinschi, P., Miclău, M., Văruţ, R.-M., Suciu, O., & Ledeți, I. (2021). Risperidone/Randomly Methylated β-Cyclodextrin Inclusion Complex—Compatibility Study with Pharmaceutical Excipients. Molecules, 26(6), 1690. https://doi.org/10.3390/molecules26061690











