TiO2 as a Photocatalyst for Water Splitting—An Experimental and Theoretical Review
Abstract
1. Introduction
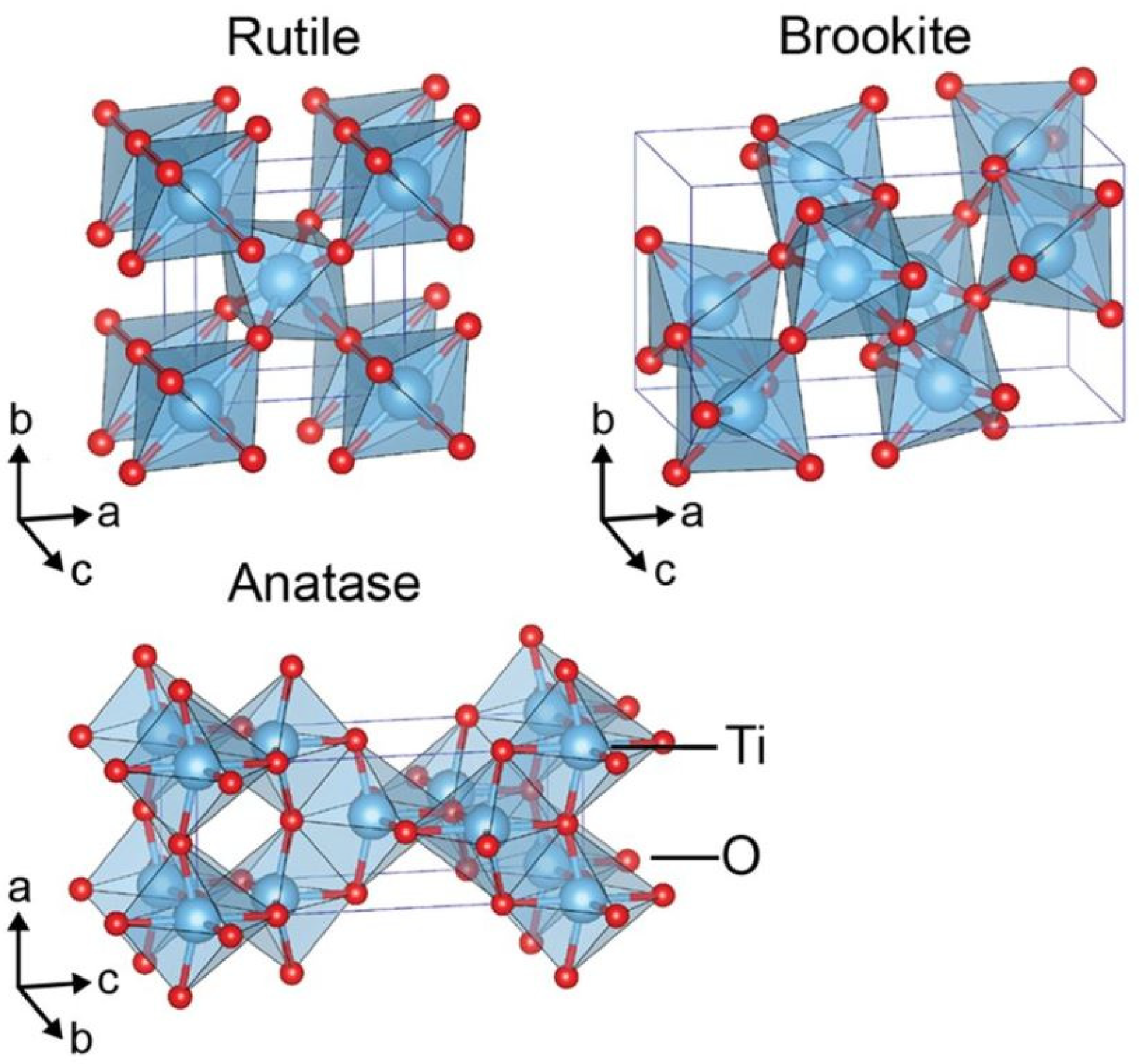
2. Solar-Driven Hydrogen Production
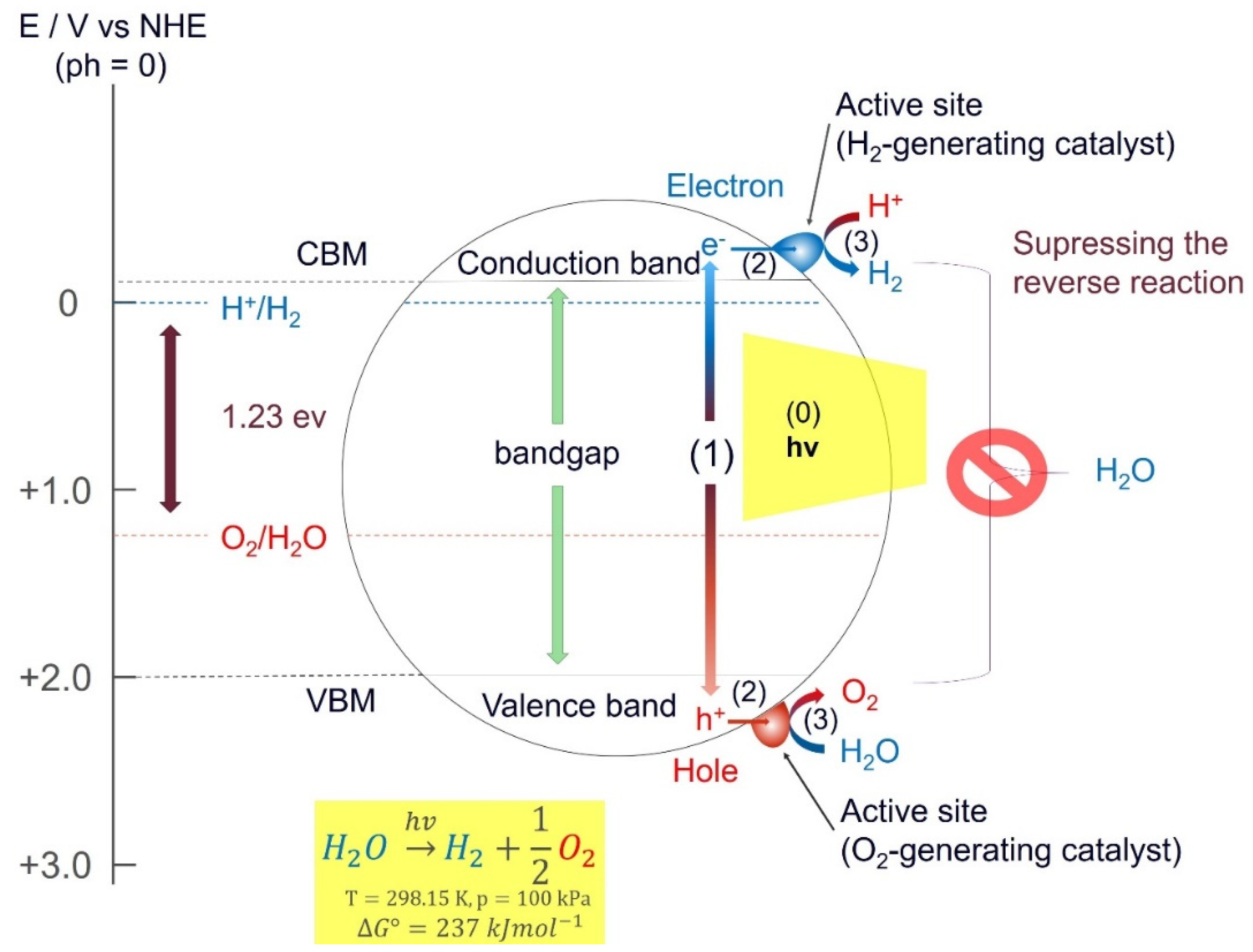
2.1. Photocatalytic Water Splitting (PWS)
2.2. Important Aspects of Photocatalytic Efficiency for Nanomaterials
2.2.1. Crystallinity
2.2.2. Dimensionality
2.2.3. Temperature and Pressure
2.2.4. Size
2.2.5. Bandgap
2.2.6. pH Dependency
2.2.7. Light
2.3. Theoretical Methods
2.4. Experimental Methods
3. Theoretical Research
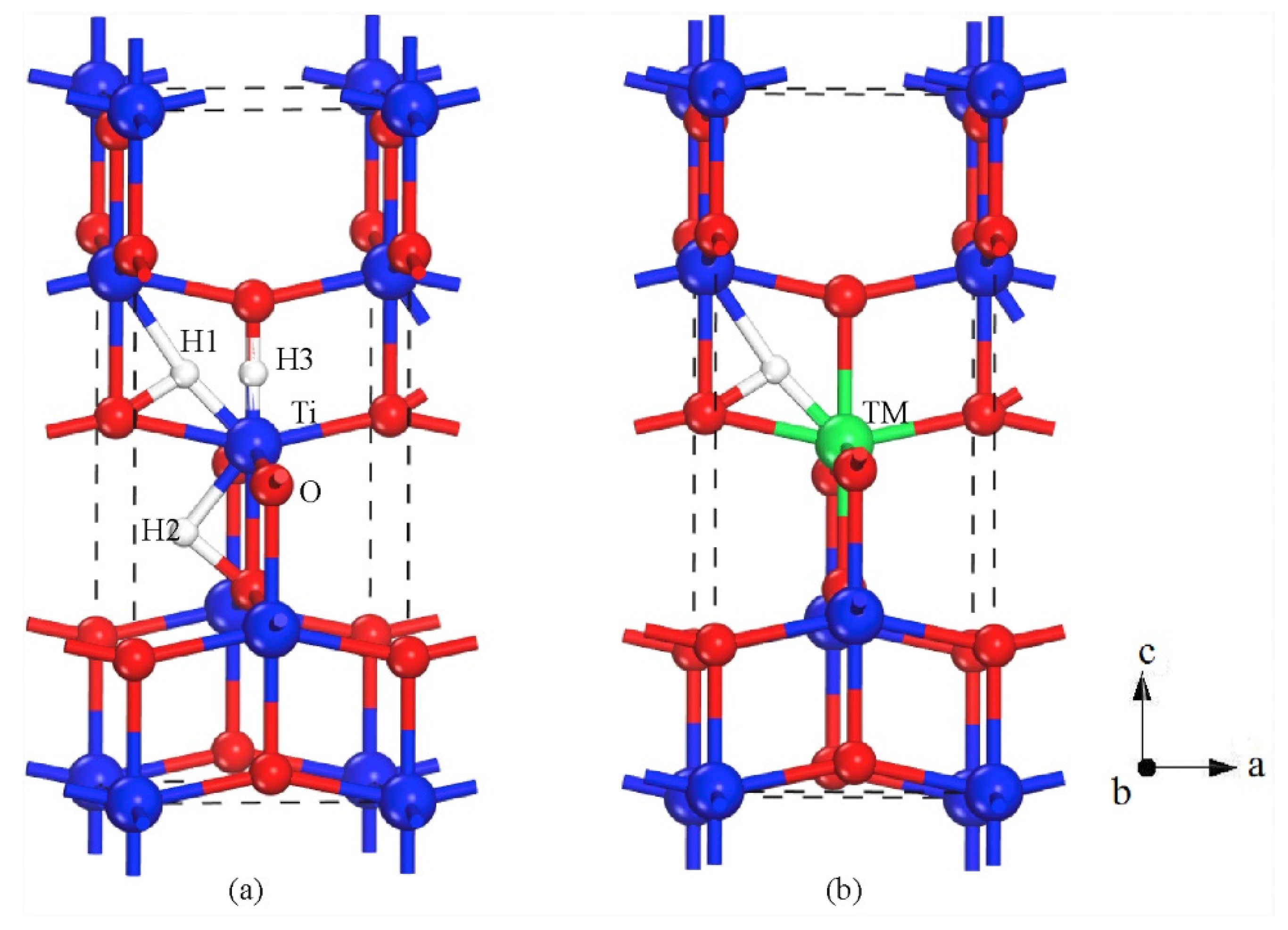
3.1. Metal Dopants
3.2. Non-Metal Dopants
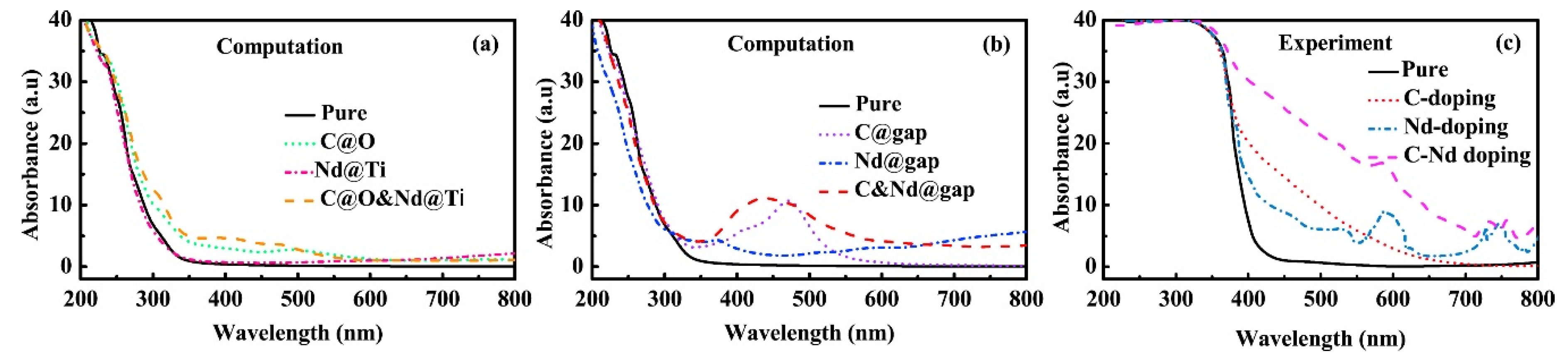
3.3. Rutile
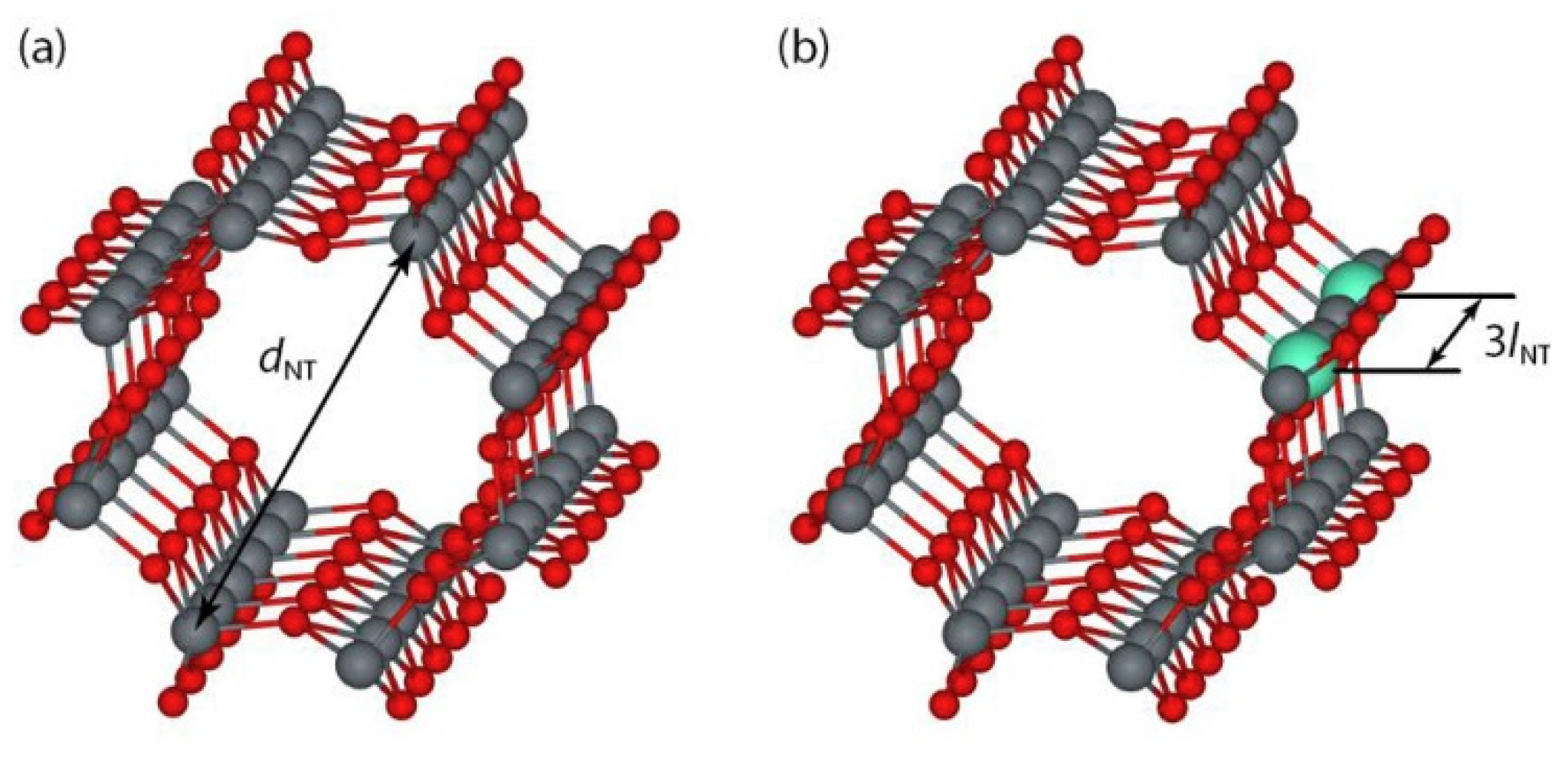
3.4. Nanotubes
3.5. Pure TiO2
3.6. Collected Data
4. Experimental Research
4.1. Metal Dopants
4.2. Non-Metal Dopants
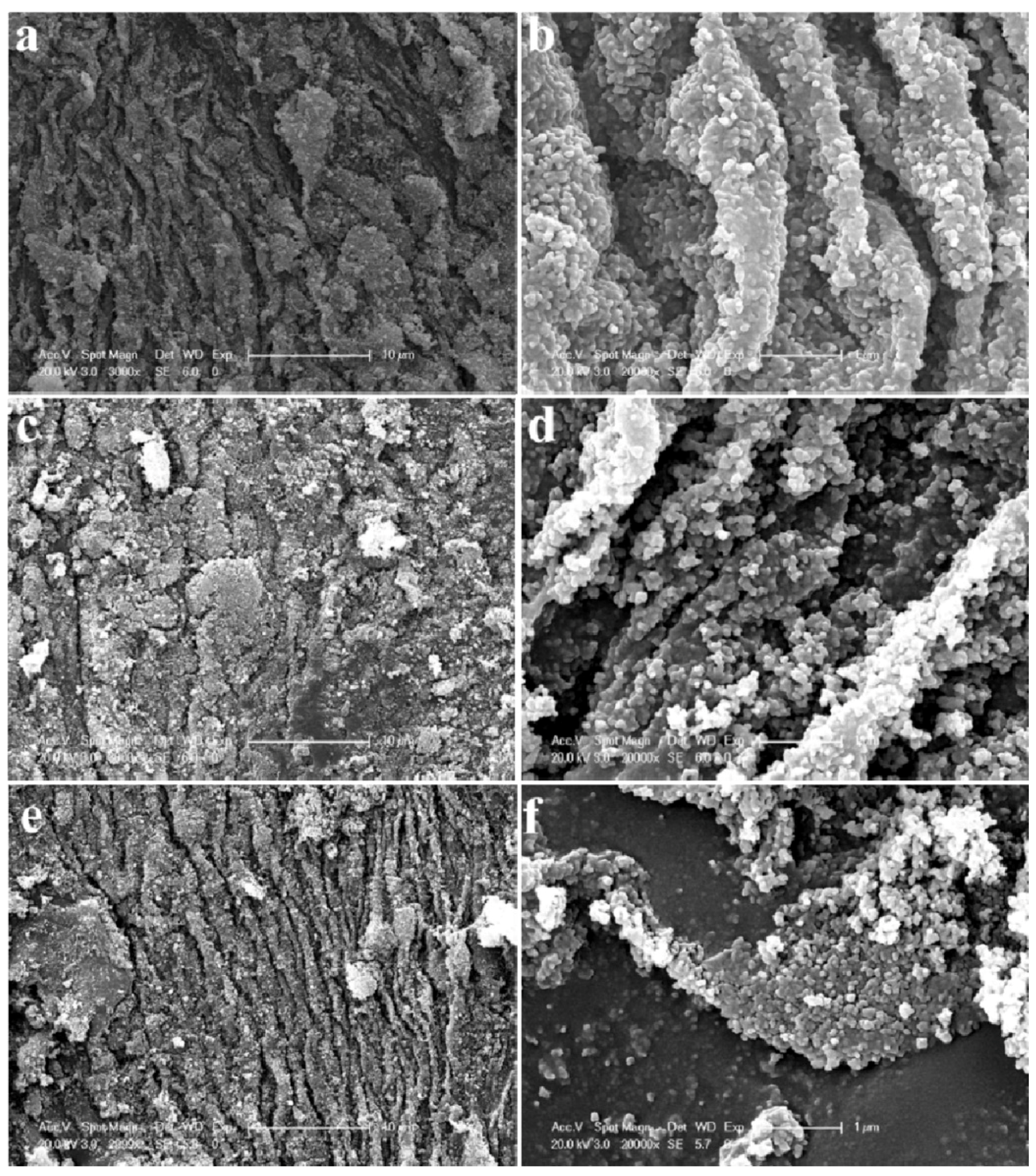
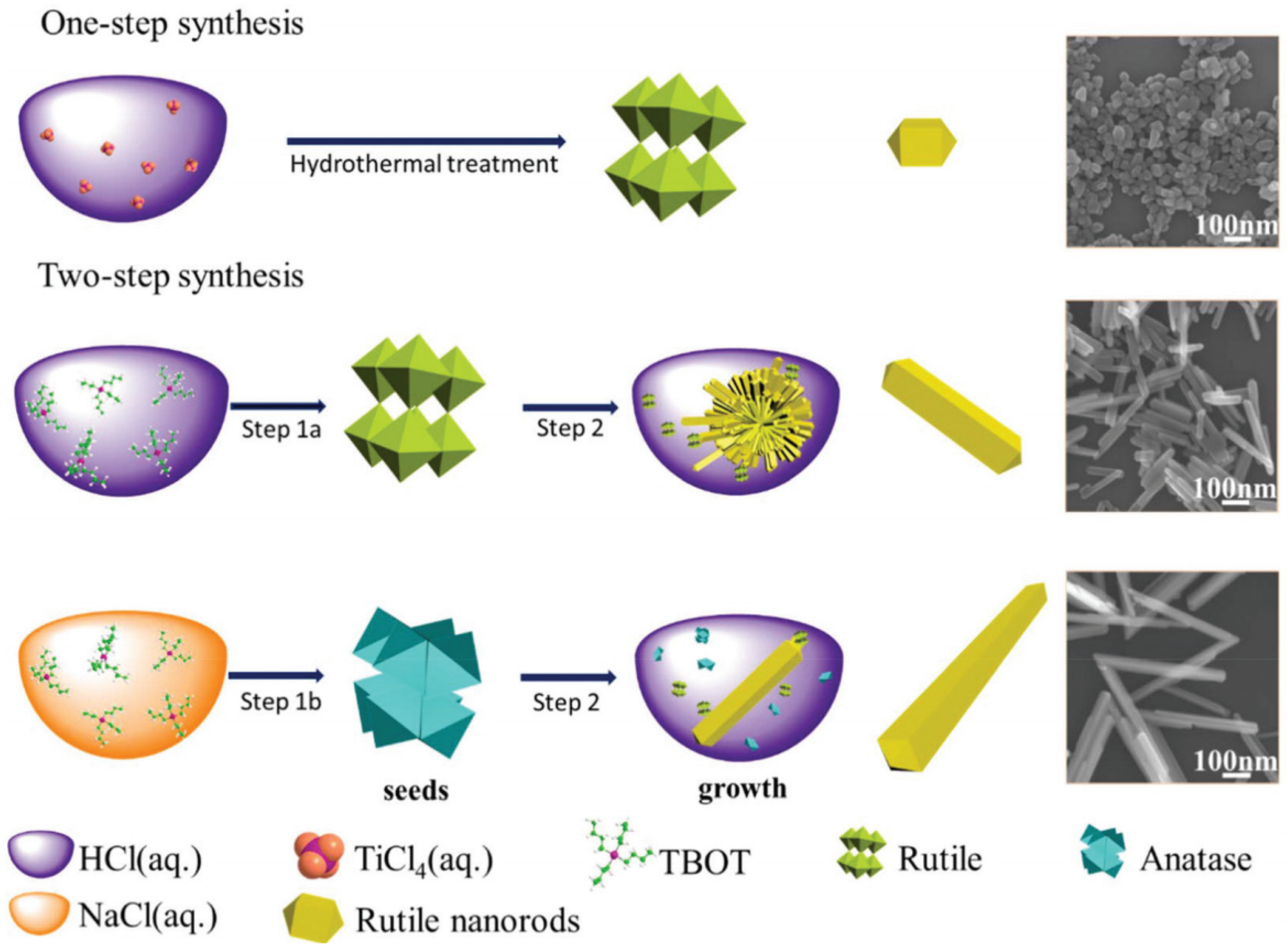
4.3. Improved Production Methods
4.4. Collected Data
| Nanomaterial | Bandgap [eV] | Photocurrent Density at 1.23 V vs. RHE [mA/cm2] | H2 Production Rate 1.5G Sunlight Bias at 1.23 vs. RHE | O2 Production Rate 1.5G Sunlight Bias at 1.23 vs. RHE | Ref. |
|---|---|---|---|---|---|
| TiO2@Fe2O3/TiO2 | 2.2 | 1.58, and 3.6 at 1.6 V vs. RHE | NA | NA | [150] |
| α-Ta2OxNy enwrapped TiO2 rutile nanorods | 2.86 | 1.32 | 244.2 mmol/m2h | 112.7 mmol/m2h | [139] |
| Ag-TiO2-NR05 | 2.64 | 0.08 and 0.10 mA/cm2 at 1.2 and 1.6 V vs. RHE | NA | NA | [129] |
| W-TiO2/BiVO4 nanorods | 2.4 | 2.5 | 41 µmol/h | NA | [144] |
| Branched multiphase TiO2 | 3.04 | 0.95 | NA | NA | [162] |
| Co3O4 quantum dots on TiO2 | 3.07 | 0.0005 | 41.8 µmol/h/g | 22.0 µmol/h/g | [130] |
| Co-Pi modified 3D TiO2/BiVO4 | NA | 4.96 at 0.63 V vs. Ag/AgCl | NA | NA | [146] |
| Co doped TiO2 nanotubes | 2.88 | 1.0 | NA | NA | [124] |
| Controllable TiO2 core shells | 2.81 | 3.88 | 49.2 µmol/cm2h | 25.2 µmol/cm2h | [165] |
| A-Fe2O3/TiO2/Si | NA | 3.5 | NA | NA | [151] |
| Al@TiO2 | NA | NA | NA | NA | [123] |
| Si-doped TiO2 nanowires | NA | NA | NA | NA | [156] |
| Three-layer (SiO2, Al2O3, and TiO2) structure with Au particles for LSPR | NA | NA | NA | NA | [168] |
| BiFeO3/TiO2 | NA | 11.25 | NA | NA | [143] |
| Graphene QDs decorated rutile TiO2 nanoflowers | NA | ~0.32 at 0.5 V vs. Ag/AgCl | NA | NA | [157] |
| Hierarchical TiO2/Fe2O3 | NA | 1.79 | NA | NA | [153] |
| CNT-GR-TiO2 | 2.79 | NA | 29 mmol/h/g | NA | [158] |
| SnO2 nanosheets with TiO2 and CdS QD | NA | 4.7 at 0V vs. Ag/AgCl | NA | NA | [135] |
| TiO2 nanotubes treated with Ar/NH3+ | NA | 1 at 1.18 V vs. RHE | NA | NA | [163] |
| TiO2 nanowire decorated with Pd | NA | 1.4 | NA | NA | [131] |
| NH2-MIL-125(Yi) on TiO2 nanorods | NA | 1.62 | NA | NA | [154] |
| Ni-doped TiO2 nanotubes | NA | 0.93 at 0 V vs. Ag/AgCl | NA | NA | [125] |
| N doped La/TiO2 | 2.96–2.99 | NA | 8.25 µmol/h/g | NA | [161] |
| TiN boosted N doped TiO2 | NA | 3.12 | NA | NA | [126] |
| CuO@TiO2 nanowires | NA | 0.56 | NA | NA | [128] |
| Pd-BaO NPs on TiO2 | NA | NA | 29.6 mmol/h/g | NA | [133] |
| S-TiO2/S-RGO | 2.15 | 3.36 at 1 V vs. Ag/AgCl | NA | NA | [160] |
| Anodized and H2 annealed TiO2 | NA | 2.5 fold TiO2 | NA | NA | [164] |
| TiO2 NPs modified with 2D MoSe2 | NA | NA | 5.12 µmol/h | NA | [138] |
| Ultrathin Ti/TiO2/BiVO4 | NA | 5.8 µa/cm2 at 0.5 V vs. Ag/AgCl | NA | NA | [145] |
| TiO2 on black Si | NA | NA | NA | NA | [166] |
| ZnO-TiO2 core-shell nanowires decorated with Au NPs | NA | 1.63 | NA | NA | [127] |
| TiO2/CdS system | 2.25 | 30 mA/cm2 (at 1 V vs. Ag/AgCl) under 1.5 AM | 1.3 mmol/cm2h | NA | [134] |
| FeOOH/TiO2/BiVO4 | NA | 3.21 | 2.36 µmol/cm2 | 1.09 µmol/cm2h | [147] |
| hematite PEC decorated with TiO2 at the grain boundaries | NA | 2.90 | NA | NA | [152] |
| the effect of annealing atmosphere on the performance of TiO2 NR | NA | 0.978 | NA | NA | [46] |
| Ti3+/Ni co-doped TiO2 nanotubes | 2.84 | 0.87 | NA | NA | [136] |
| Hydrogenated F-doped TiO2 | 3.0 | NA | 3.76 mmol/h/g | NA | [159] |
| BiVO4 deposited on TiO2 | NA | 35 µ under 100 mW/cm2 in 0.5M Na2SO4 | NA | NA | [149] |
| BiVO4 used together with TiO2 | NA | ~0.3 at 1.0 V vs. RHE | NA | NA | [148] |
| Pt/TiO2(anatase) photocatalyst | NA | NA | 7410 µmol/h/g | 5096 µmol/h/g | [132] |
| Ti3+ self-doped mesoporous black TiO2/SiO2/g-C3N4 sheets | ~2.25 | NA | NA | NA | [137] |
| Rutile TiO2 nanorods with small aspect ratio | NA | NA | 1229 µmol/h/g | 549 µmol/h/g | [167] |
| Rutile TiO2 nanorods with medium aspect ratio | NA | NA | 783 µmol/h/g | 369 µmol/h/g | [167] |
| Rutile TiO2 nanorods with large aspect ratio | NA | NA | 549 µmol/h/g | 252 µmol/h/g | [167] |
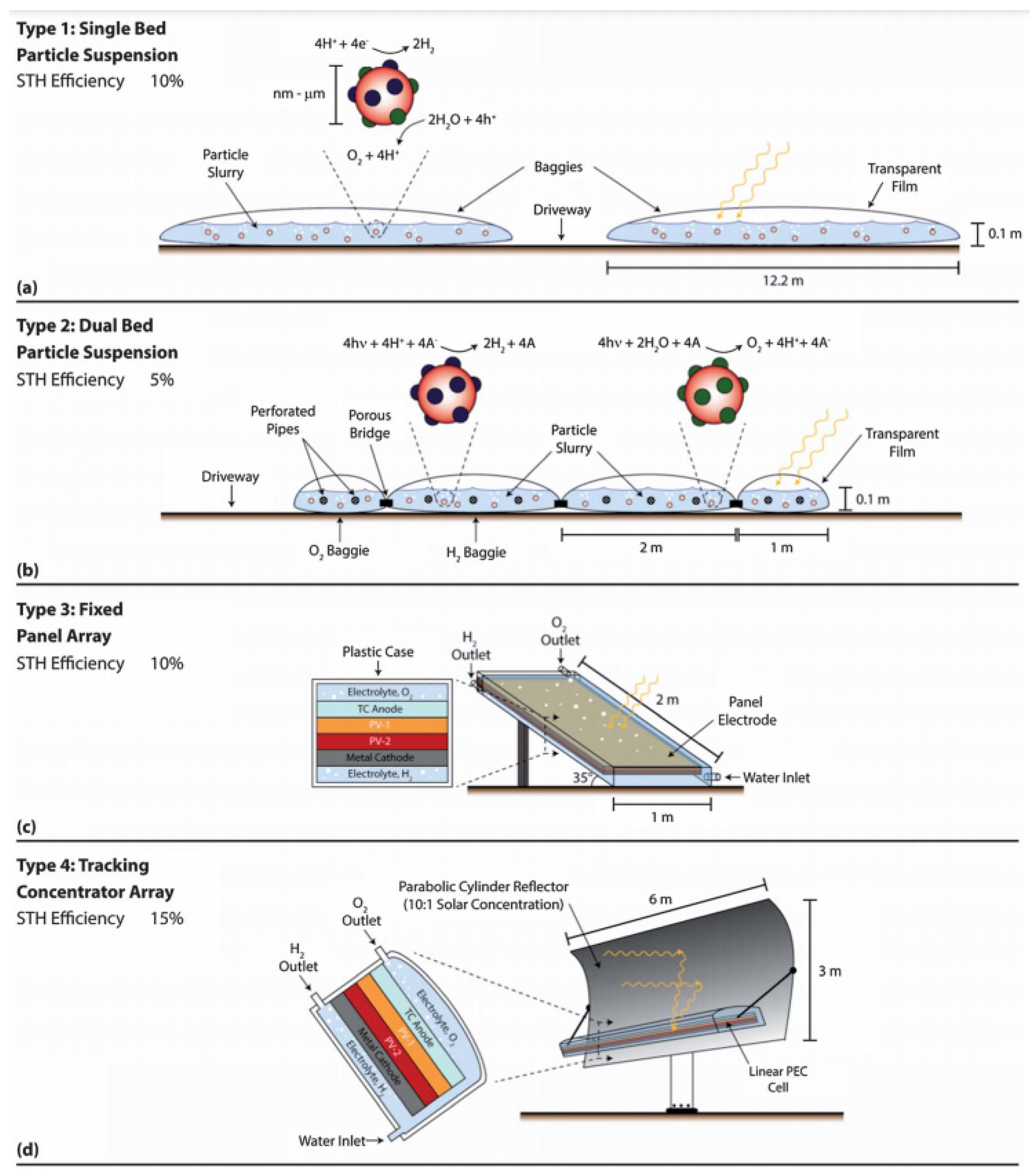
4.5. Production Facilities
5. Conclusions and Long-Term Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD/IEA. World Energy Outlook. 2016. Available online: http://www.iea.org/t&c (accessed on 14 May 2018).
- Dincer, I.; Zamfirescu, C. Sustainable Energy Systems and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2012; p. XVII. [Google Scholar] [CrossRef]
- Tong, Y.; Liang, J.; Liu, H.K.; Dou, S.X. Energy storage in Oceania. Energy Storage Mater. 2019, 20, 176–187. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting-the untamed dream: A Review of recent advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Sharma, P.; Kolhe, M.L. Review of sustainable solar hydrogen production using photon fuel on artificial leaf. Int. J. Hydrog. Energy 2017, 42, 22704–22712. [Google Scholar] [CrossRef]
- Pulido Melián, E.; González Díaz, O.; Ortega Méndez, A.; López, C.R.; Nereida Suárez, M.; Doña Rodríguez, J.M.; Navío, J.A.; Fernández Hevia, D.; Pérez Peña, J. Efficient and affordable hydrogen production by water photo-splitting using TiO2-based photocatalysts. Int. J. Hydrog. Energy 2013, 38, 2144–2155. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. Clean fuel options with hydrogen for sea transportation: A life cycle approach. Int. J. Hydrog. Energy 2018, 43, 1179–1193. [Google Scholar] [CrossRef]
- van Biert, L.; Godjevac, M.; Visser, K.; Aravind, P.V. A review of fuel cell systems for maritime applications. J. Power Sources 2016, 327, 345–364. [Google Scholar] [CrossRef]
- International Maritime Organization. Third IMO Greenhouse Gas Study 2014. Available online: http://www.imo.org/en/OurWork/Environment/PollutionPrevention/AirPollution/Pages/Greenhouse-Gas-Studies-2014.aspx (accessed on 14 May 2018).
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Shen, S.; Mao, S.S. Black TiO2 for solar hydrogen conversion. J. Mater. 2017, 3, 96–111. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Haggerty, J.E.S.; Schelhas, L.T.; Kitchaev, D.A.; Mangum, J.S.; Garten, L.M.; Sun, W.; Stone, K.H.; Perkins, J.D.; Toney, M.F.; Ceder, G.; et al. High-fraction brookite films from amorphous precursors. Sci. Rep.-Uk 2017, 7, 15232. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Singh, C.V. Amorphous TiO2 as a photocatalyst for hydrogen production: A DFT study of structural and electronic properties. Energy Proc. 2012, 29, 291–299. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.-R.; Tang, X.-Q.; Zhang, P.; Ding, Y.-H. Density functional theory studies on the structural and physical properties of Cu-doped anatase TiO2 (101) surface. Phys. E 2017, 85, 259–263. [Google Scholar] [CrossRef]
- Morgade, C.I.N.; Cabeza, G.F. First-principles study of codoping TiO2 systems capable of improving the specific surface area and the dissociation of H2O to generate H2 and O2. Comput. Mater. Sci. 2017, 127, 204–210. [Google Scholar] [CrossRef]
- Press, R.J.; Santhanam, K.S.V.; Miri, M.J.; Bailey, A.V.; Takacs, G.A. Introduction to hydrogen technology. ChemSusChem 2009, 3, 432. [Google Scholar]
- Hoang, D.L.; Chan, S.H.; Ding, O.L. Kinetic and modelling study of methane steam reforming over sulfide nickel catalyst on a gamma alumina support. Chem. Eng. J. 2005, 112, 1–11. [Google Scholar] [CrossRef]
- Ozcan, H.; Dincer, I. Thermodynamic analysis of a combined chemical looping-based trigeneration system. Energy Convers. Manag. 2014, 85, 477–487. [Google Scholar] [CrossRef]
- Steinfeld, A. Solar hydrogen production via a two-step water-splitting thermochemical cycle based on Zn/ZnO redox reactions. Int. J. Hydrog. Energy 2002, 27, 611–619. [Google Scholar] [CrossRef]
- Akkerman, I.; Janssen, M.; Rocha, J.; Wijffels, R.H. Photobiological hydrogen production: Photochemical efficiency and bioreactor design. Int. J. Hydrog. Energy 2002, 27, 1195–1208. [Google Scholar] [CrossRef]
- Liao, C.-H.; Huang, C.-W.; Wu, J.C.S. Hydrogen production from semiconductor-based photocatalysis via water splitting. Catalysts 2012, 2, 490. [Google Scholar] [CrossRef]
- Kudo, A. Photocatalysis and solar hydrogen production. Pure Appl. Chem. 2007, 79, 1917. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, H.G.; Lee, J.S. Heterojunction semiconductors: A strategy to develop efficient photocatalytic materials for visible light water splitting. Catal. Today 2012, 185, 270–277. [Google Scholar] [CrossRef]
- Moriya, Y.; Takata, T.; Domen, K. Recent progress in the development of (oxy)nitride photocatalysts for water splitting under visible-light irradiation. Coordin. Chem. Rev. 2013, 257, 1957–1969. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.-W.; Lee, K.-H.; Lee, J.S. Research Update: Strategies for efficient photoelectrochemical water splitting using metal oxide photoanodes. APL Mater. 2014, 2. [Google Scholar] [CrossRef]
- Naik, V.M.; Haddad, D.; Naik, R.; Benci, J.; Auner, G.W. Optical properties of anatase, rutile and amorphous phases of TiO2 thin films grown at room temperature by RF magnetron sputtering. Mrs Proc. 2002, 755, DD11.12. [Google Scholar] [CrossRef]
- Liu, H.; Hsu, Y.; Su, H.; Huang, R.; Hou, F.; Tu, G.; Liu, W. A Comparative study of amorphous, anatase, rutile, and mixed phase TiO2 films by mist chemical vapor deposition and ultraviolet photodetectors applications. IEEE Sens. J. 2018, 18, 4022–4029. [Google Scholar] [CrossRef]
- Furube, A.; Asahi, T.; Masuhara, H.; Yamashita, H.; Anpo, M. Charge carrier dynamics of standard TiO2 catalysts revealed by femtosecond diffuse reflectance spectroscopy. J. Phys. Chem. B 1999, 103, 3120–3127. [Google Scholar] [CrossRef]
- Ohtani, B.; Ogawa, Y.; Nishimoto, S.-I. Photocatalytic activity of amorphous−anatase mixture of Titanium(IV) oxide particles suspended in aqueous solutions. J. Phys. Chem. B 1997, 101, 3746–3752. [Google Scholar] [CrossRef]
- Stone, V.F.; Davis, R.J. Synthesis, Characterization, and Photocatalytic Activity of Titania and Niobia Mesoporous Molecular Sieves. Chem. Mater. 1998, 10, 1468–1474. [Google Scholar] [CrossRef]
- Tanaka, K.; Capule, M.F.V.; Hisanaga, T. Effect of crystallinity of TiO2 on its photocatalytic action. Chem. Phys. Lett. 1991, 187, 73–76. [Google Scholar] [CrossRef]
- Liu, N.; Albu, S.P.; Lee, K.; So, S.; Schmuki, P. Water annealing and other low temperature treatments of anodic TiO2 nanotubes: A comparison of properties and efficiencies in dye sensitized solar cells and for water splitting. Electrochim. Acta 2012, 82, 98–102. [Google Scholar] [CrossRef]
- Gong, J.; Lai, Y.; Lin, C. Electrochemically multi-anodized TiO2 nanotube arrays for enhancing hydrogen generation by photoelectrocatalytic water splitting. Electrochim. Acta 2010, 55, 4776–4782. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for photoelectrochemical water splitting—Review. Int. J. Hydrog. Energy 2018, 43, 4804–4817. [Google Scholar] [CrossRef]
- Kumar, P.; Devi, P.; Jain, R.; Shivaprasad, S.M.; Sinha, R.K.; Zhou, G.; Nötzel, R. Quantum dot activated indium gallium nitride on silicon as photoanode for solar hydrogen generation. Commun. Chem. 2019, 2, 4. [Google Scholar] [CrossRef]
- Basu, K.; Zhang, H.; Zhao, H.; Bhattacharya, S.; Navarro-Pardo, F.; Datta, P.K.; Jin, L.; Sun, S.; Vetrone, F.; Rosei, F. Highly stable photoelectrochemical cells for hydrogen production using a SnO2–TiO2/quantum dot heterostructured photoanode. Nanoscale 2018, 10, 15273–15284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, J.; Zhang, J. Quantum dots and plasmonic Ag decorated WO3 nanorod photoanodes with enhanced photoelectrochemical performances. Int. J. Hydrog. Energy 2016, 41, 20529–20535. [Google Scholar] [CrossRef]
- Beermann, N.; Vayssieres, L.; Lindquist, S.E.; Hagfeldt, A. Photoelectrochemical studies of oriented nanorod thin films of hematite. J. Electrochem. Soc. 2000, 147, 2456–2461. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Sultana, T. Photoresponse of n-TiO2 thin film and nanowire electrodes. Sol. Energy Mat. Sol. Cells 2003, 76, 211–221. [Google Scholar] [CrossRef]
- Fitch, A.; Strandwitz, N.C.; Brunschwig, B.S.; Lewis, N.S. A comparison of the behavior of single crystalline and nanowire array ZnO photoanodes. J. Phys. Chem. C 2013, 117, 2008–2015. [Google Scholar] [CrossRef]
- Zhang, J.Z. Metal oxide nanomaterials for solar hydrogen generation from photoelectrochemical water splitting. Mrs Bull. 2011, 36, 48–55. [Google Scholar] [CrossRef]
- Varghese, O.K.; Grimes, C.A. Appropriate strategies for determining the photoconversion efficiency of water photoelectrolysis cells: A review with examples using titania nanotube array photoanodes. Sol. Energy Mat. Sol. Cells 2008, 92, 374–384. [Google Scholar] [CrossRef]
- Huang, H.; Hou, X.; Xiao, J.; Zhao, L.; Huang, Q.; Chen, H.; Li, Y. Effect of annealing atmosphere on the performance of TiO2 nanorod arrays in photoelectrochemical water splitting. Catal. Today 2019, 330, 189–194. [Google Scholar] [CrossRef]
- Nie, Q.; Yang, L.; Cao, C.; Zeng, Y.; Wang, G.; Wang, C.; Lin, S. Interface optimization of ZnO nanorod/CdS quantum dots heterostructure by a facile two-step low-temperature thermal treatment for improved photoelectrochemical water splitting. Chem. Eng. J. 2017, 325, 151–159. [Google Scholar] [CrossRef]
- Pokrant, S.; Dilger, S.; Landsmann, S.; Trottmann, M. Size effects of cocatalysts in photoelectrochemical and photocatalytic water splitting. Mater. Today Energy 2017, 5, 158–163. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Vishwakarma, A.K.; Tripathi, P.; Srivastava, A.; Sinha, A.S.K.; Srivastava, O.N. Band gap engineering of Gd and Co doped BiFeO3 and their application in hydrogen production through photoelectrochemical route. Int. J. Hydrog. Energy 2017, 42, 22677–22686. [Google Scholar] [CrossRef]
- Yin, W.-J.; Tang, H.; Wei, S.-H.; Al-Jassim, M.M.; Turner, J.; Yan, Y. Band structure engineering of semiconductors for enhanced photoelectrochemical water splitting: The case of TiO2. Phys. Rev. B 2010, 82, 045106. [Google Scholar] [CrossRef]
- Wang, J.; Sun, H.; Huang, J.; Li, Q.; Yang, J. Band Structure Tuning of TiO2 for Enhanced Photoelectrochemical Water Splitting. J. Phys. Chem. C 2014, 118, 7451–7457. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; Minakata, D.; Chen, Y.C.; Crittenden, J.; Wang, P. The pH effects on H2 evolution kinetics for visible light water splitting over the Ru/(CuAg)0.15In0.3Zn1.4S2 photocatalyst. Int. J. Hydrog. Energy 2013, 38, 11727–11736. [Google Scholar] [CrossRef]
- Fekete, M.; Riedel, W.; Patti, A.F.; Spiccia, L. Photoelectrochemical water oxidation by screen printed ZnO nanoparticle films: Effect of pH on catalytic activity and stability. Nanoscale 2014, 6, 7585–7593. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y. Visible light-driven photoelectrochemical water splitting on ZnO–TiO2 heterogeneous nanotube photoanodes. J. Appl. Electrochem. 2015, 45, 557–566. [Google Scholar] [CrossRef]
- Varadhan, P.; Fu, H.-C.; Priante, D.; Retamal, J.R.D.; Zhao, C.; Ebaid, M.; Ng, T.K.; Ajia, I.; Mitra, S.; Roqan, I.S.; et al. Surface Passivation of GaN Nanowires for Enhanced Photoelectrochemical Water-Splitting. Nano Lett. 2017, 17, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mat. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys.-Condens Mat. 1994, 6, 8245–8257. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Krist.-Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. Wires Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Dovesi, R.; Pascale, F.; Civalleri, B.; Doll, K.; Harrison, N.M.; Bush, I.; D’Arco, P.; Noël, Y.; Rérat, M.; Carbonnière, P.; et al. The CRYSTAL code, 1976–2020 and beyond, a long story. J. Chem. Phys. 2020, 152, 204111. [Google Scholar] [CrossRef]
- Mortensen, J.J.; Hansen, L.B.; Jacobsen, K.W. Real-space grid implementation of the projector augmented wave method. Phys. Rev. B 2005, 71, 035109. [Google Scholar] [CrossRef]
- Kan, M.; Wang, J.Y.; Li, X.W.; Zhang, S.H.; Li, Y.W.; Kawazoe, Y.; Sun, Q.; Jena, P. Structures and Phase Transition of a MoS2 Monolayer. J. Phys. Chem. C 2014, 118, 1515–1522. [Google Scholar] [CrossRef]
- Xia, Z.; Tao, Y.; Pan, Z.; Shen, X. Enhanced photocatalytic performance and stability of 1T MoS2 transformed from 2H MoS2 via Li intercalation. Results Phys. 2019, 12, 2218–2224. [Google Scholar] [CrossRef]
- Liu, P.; Kaltak, M.; Klimeš, J.; Kresse, G. Cubic scaling GW: Towards fast quasiparticle calculations. Phys. Rev. B 2016, 94, 165109. [Google Scholar] [CrossRef]
- Ramberger, B.; Sukurma, Z.; Schäfer, T.; Kresse, G. RPA natural orbitals and their application to post-Hartree-Fock electronic structure methods. J. Chem. Phys. 2019, 151, 214106. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E. Efficient hybrid density functional calculations in solids: Assessment of the Heyd-Scuseria-Ernzerhof screened Coulomb hybrid functional. J. Chem. Phys. 2004, 121, 1187–1192. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.-T. VASPKIT: A User-friendly Interface Facilitating High-throughput Computing and Analysis Using VASP Code. arXiv 2019, arXiv:1908.08269. [Google Scholar]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Kumar, A. Different methods used for the synthesis of TiO2 based nanomaterials: A review. Am. J. Nano Res. Appl. 2018, 6, 1. [Google Scholar] [CrossRef]
- Nagaraju, G.; Tharamani, C.; Chandrappa, G.; Livage, J. Hydrothermal synthesis of amorphous MoS2nanofiber bundles via acidification of ammonium heptamolybdate tetrahydrate. Nanoscale Res. Lett. 2007, 2, 461–468. [Google Scholar] [CrossRef]
- Bai, J.; Meng, T.; Guo, D.; Wang, S.; Mao, B.; Cao, M. Co9S8@MoS2 Core–Shell Heterostructures as Trifunctional Electrocatalysts for Overall Water Splitting and Zn–Air Batteries. ACS Appl. Mater. Interfaces 2018, 10, 1678–1689. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, H.-S.; Lee, J.-H.; Kim, Y.-J.; Suh, S.-J.; Chi, C.-S.; Oh, H.-J. Fabrication of titania nanotubular film with metal nanoparticles. J. Cryst. Growth 2009, 311, 638–641. [Google Scholar] [CrossRef]
- Patil, U.M.; Kulkarni, S.B.; Deshmukh, P.R.; Salunkhe, R.R.; Lokhande, C.D. Photosensitive nanostructured TiO2 grown at room temperature by novel “bottom-up” approached CBD method. J. Alloy. Compd. 2011, 509, 6196–6199. [Google Scholar] [CrossRef]
- Wu, J.-M.; Hayakawa, S.; Tsuru, K.; Osaka, A. Nanocrystalline Titania Made from Interactions of Ti with Hydrogen Peroxide Solutions Containing Tantalum Chloride. Cryst. Growth Des. 2002, 2, 147–149. [Google Scholar] [CrossRef]
- AlHammad, M.S. Nanostructure hydroxyapatite based ceramics by sol gel method. J. Alloy. Compd. 2016, 661, 251–256. [Google Scholar] [CrossRef]
- Kluson, P.; Luskova, H.; Cajthaml, T.; Šolcová, O. Non thermal preparation of photoactive titanium (IV) oxide thin layers. Thin Solid Film. 2006, 495, 18–23. [Google Scholar] [CrossRef]
- Arami, H.; Mazloumi, M.; Khalifehzadeh, R.; Sadrnezhaad, S.K. Sonochemical preparation of TiO2 nanoparticles. Mater. Lett. 2007, 61, 4559–4561. [Google Scholar] [CrossRef]
- Amaresh, S.; Karthikeyan, K.; Jang, I.C.; Lee, Y.S. Single-step microwave mediated synthesis of the CoS2 anode material for high rate hybrid supercapacitors. J. Mater. Chem. A 2014, 2, 11099–11106. [Google Scholar] [CrossRef]
- Pan, Y.; Wen, M. Noble metals enhanced catalytic activity of anatase TiO2 for hydrogen evolution reaction. Int. J. Hydrog. Energy 2018, 43, 22055–22063. [Google Scholar] [CrossRef]
- Zhang, Y.; Kilin, D.S. Computational modeling of wet TiO2(001) anatase surfaces functionalized by transition metal doping. Int. J. Quantum Chem. 2012, 112, 3867–3873. [Google Scholar] [CrossRef]
- Zhang, S.-T.; Li, C.-M.; Yan, H.; Wei, M.; Evans, D.G.; Duan, X. Density Functional Theory Study on the Metal–Support Interaction between Ru Cluster and Anatase TiO2(101) Surface. J. Phys. Chem. C 2014, 118, 3514–3522. [Google Scholar] [CrossRef]
- Jin, C.; Dai, Y.; Wei, W.; Ma, X.; Li, M.; Huang, B. Effects of single metal atom (Pt, Pd, Rh and Ru) adsorption on the photocatalytic properties of anatase TiO2. Appl. Surf. Sci. 2017, 426, 639–646. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, Z.; Zhu, C.; Zhang, R.; Hu, X.; Zhang, X.; Zhu, H.; Lin, S.H. The electronic structure, optical absorption and photocatalytic water splitting of (Fe + Ni)-codoped TiO2: A DFT + U study. Int. J. Hydrog. Energy 2017, 42, 4966–4976. [Google Scholar] [CrossRef]
- Ghuman, K.K. Mechanistic insights into water adsorption and dissociation on amorphous -based catalysts. Sci. Technol. Adv. Mater. 2018, 19, 44–52. [Google Scholar] [CrossRef]
- Koteski, V.; Belošević-Čavor, J.; Umićević, A.; Ivanovski, V.; Toprek, D. Improving the photocatalytic properties of anatase TiO2(101) surface by co-doping with Cu and N: Ab initio study. Appl. Surf. Sci. 2017, 425, 1095–1100. [Google Scholar] [CrossRef]
- Assadi, M.H.N.; Hanaor, D.A.H. The effects of copper doping on photocatalytic activity at (101) planes of anatase TiO2: A theoretical study. Appl. Surf. Sci. 2016, 387, 682–689. [Google Scholar] [CrossRef]
- Sikam, P.; Moontragoon, P.; Sararat, C.; Karaphun, A.; Swatsitang, E.; Pinitsoontorn, S.; Thongbai, P. DFT calculation and experimental study on structural, optical and magnetic properties of Co-doped SrTiO3. Appl. Surf. Sci. 2018, 446, 92–113. [Google Scholar] [CrossRef]
- Ghuman, K.K.; Singh, C.V. Effect of doping on electronic structure and photocatalytic behavior of amorphous TiO2. J. Phys.-Condens Mat. 2013, 25, 475501. [Google Scholar] [CrossRef]
- Ren, D.; Li, H.; Cheng, X. Tailoring the electronic and optical properties of anatase TiO2 by (S, Nb) co-doping from a DFT plus U calculation. Solid State Commun. 2015, 223, 54–59. [Google Scholar] [CrossRef]
- Gao, L.; Li, Y.; Ren, J.; Wang, S.; Wang, R.; Fu, G.; Hu, Y. Passivation of defect states in anatase TiO2 hollow spheres with Mg doping: Realizing efficient photocatalytic overall water splitting. Appl. Catal. B-Environ. 2017, 202, 127–133. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, P.; Zhang, S.; Sun, Q.; Liang, E.; Jia, Y. Electronic properties of anatase TiO2 doped by lanthanides: A DFT+U study. Phys. B 2012, 407, 1038–1043. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Liu, X.; Ren, C.; Miao, X.; Li, X. Engineering of Gd/Er/Lu-triple-doped Bi2MoO6 to synergistically boost the photocatalytic performance in three different aspects: Oxidizability, light absorption and charge separation. Appl. Surf. Sci. 2019, 463, 556–565. [Google Scholar] [CrossRef]
- Shi, H.; Lin, Y.; Jiang, Z.; Su, Y.; Ding, X.; Zhang, X.; Zhu, H.; Zhang, R. Enhanced optical absorption and photocatalytic activity of anatase TiO2 through C Nd-codoped: A DFT+ U calculations. J. Phys. Chem. Solids 2017, 109, 70–77. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Wei, W.; Chen, K.; Li, H.; Wong, P.K.; Xie, J. Gentle way to build reduced titanium dioxide nanodots integrated with graphite-like carbon spheres: From DFT calculation to experimental measurement. Appl. Catal. B-Environ. 2017, 204, 283–295. [Google Scholar] [CrossRef]
- Gurkan, Y.Y.; Kasapbasi, E.; Cinar, Z. Enhanced solar photocatalytic activity of TiO2 by selenium(IV) ion-doping: Characterization and DFT modeling of the surface. Chem. Eng. J. 2013, 214, 34–44. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; He, L. The effects of Co/N dopants on the electronic, redox potential, optical, and photocatalytic water-splitting properties of TiO2: First principles calculations. Chem. Phys. Lett. 2017, 685, 108–113. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, Q.; Xia, Y.; Zhan, S.; Li, Y. Understanding the charge separation and transfer in mesoporous carbonate-doped phase-junction TiO2 nanotubes for photocatalytic hydrogen production. Appl. Catal. B-Environ. 2018, 225, 433–444. [Google Scholar] [CrossRef]
- Wu, X.; Yin, S.; Dong, Q.; Guo, C.; Kimura, T.; Matsushita, J.-I.; Sato, T. Photocatalytic properties of Nd and C Codoped TiO2 with the whole range of visible light absorption. J. Phys. Chem. C 2013, 117, 8345–8352. [Google Scholar] [CrossRef]
- Zongyan, Z.; Xiang, Z.; Juan, Y.; Qingju, L. Effects of nonmetal doping on electronic structures and optical property of anatase TiO2 from first-principles calculations. Rare Met. Mat. Eng. 2015, 44, 1568–1574. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, Y.; Wang, G.; Jiang, Z.; Zhang, R.; Zhu, C. Electronic and optical performances of (Cu, N) codoped TiO2/g-C3N4 heterostructure photocatalyst: A spin-polarized DFT + U study. Sol. Energy 2018, 162, 306–316. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, H.; Jiang, Z.; Wang, G.; Zhang, X.; Zhu, H.; Zhang, R.; Zhu, C. Enhanced optical absorption and photocatalytic H2 production activity of g-C3N4/TiO2 heterostructure by interfacial coupling: A DFT+ U study. Int. J. Hydrog. Energy 2017, 42, 9903–9913. [Google Scholar] [CrossRef]
- Atanelov, J.; Gruber, C.; Mohn, P. The electronic and magnetic structure of p -element (C,N) doped rutile-TiO2: A hybrid DFT study. Comput. Mater. Sci. 2015, 98, 42–50. [Google Scholar] [CrossRef]
- Ghuman, K.K.; Singh, C.V. A DFT + U study of (Rh, Nb)-codoped rutile TiO2. J. Phys.-Condens Mat. 2013, 25, 085501. [Google Scholar] [CrossRef]
- Lisovski, O.; Chesnokov, A.; Piskunov, S.; Bocharov, D.; Zhukovskii, Y.F.; Wessel, M.; Spohr, E. Ab initio calculations of doped TiO2 anatase (101) nanotubes for photocatalytical water splitting applications. Mat. Sci. Semicon. Proc 2016, 42, 138–141. [Google Scholar] [CrossRef]
- Lisovski, O.; Piskunov, S.; Zhukovskii, Y.F.; Ozolins, J. Ab initiomodeling of sulphur doped TiO2 nanotubular photocatalyst for water-splitting hydrogen generation. Iop Conf. Ser.-Mat. Sci. 2012, 38. [Google Scholar] [CrossRef]
- Bocharov, D.; Piskunov, S.; Zhukovskii, Y.F.; Spohr, E.; D’Yachkov, P.N. First principles modeling of 3 d -metal doped three-layer fluorite-structured TiO2(4,4) nanotube to be used for photocatalytic hydrogen production. Vacuum 2017, 146, 562–569. [Google Scholar] [CrossRef]
- D’yachkov, E.P.; Bochkov, I.A.; Zaluev, V.A.; D’yachkova, P.N. Electronic properties of titanium dioxide nanotubes doped with 4d metals. Russ. J. Inorg. Chem. 2017, 62, 1048–1050. [Google Scholar] [CrossRef]
- Lisovski, O.; Piskunov, S.; Zhukovskii, Y.F.; Bocharov, D. Quantum chemical simulations of titanium dioxide nanotubes used for photocatalytic water splitting. J. Surf. Investig. 2017, 11, 78–86. [Google Scholar] [CrossRef]
- Ma, S.; Song, W.; Liu, B.; Zhong, W.; Deng, J.; Zheng, H.; Liu, J.; Gong, X.-Q.; Zhao, Z. Facet-dependent photocatalytic performance of TiO2: A DFT study. Appl. Catal. B-Environ. 2016, 198, 1–8. [Google Scholar] [CrossRef]
- Deák, P.; Kullgren, J.; Aradi, B.; Frauenheim, T.; Kavan, L. Water splitting and the band edge positions of TiO2. Electrochim. Acta 2016, 199, 27–34. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Assadi, M.H.N.; Li, S.; Yu, A.; Sorrel, C.C. Ab Initio Study of Phase Stability in Doped TiO2. Comput. Mech. 2012, 50, 185–194. [Google Scholar] [CrossRef]
- Alghamdi, H.; Idriss, H. Study of the modes of adsorption and electronic structure of hydrogen peroxide and ethanol over TiO2 rutile (110) surface within the context of water splitting. Surf. Sci. 2017. [Google Scholar] [CrossRef]
- Tsai, C.; Chan, K.; Nørskov, J.K.; Abild-Pedersen, F. Theoretical insights into the hydrogen evolution activity of layered transition metal dichalcogenides. Surf. Sci. 2015, 640, 133–140. [Google Scholar] [CrossRef]
- Li, Z.; Shi, L.; Franklin, D.; Koul, S.; Kushima, A.; Yang, Y. Drastic enhancement of photoelectrochemical water splitting performance over plasmonic Al@TiO2 heterostructured nanocavity arrays. Nano Energy 2018, 51, 400–407. [Google Scholar] [CrossRef]
- Venturini, J.; Bonatto, F.; Guaglianoni, W.C.; Lemes, T.; Arcaro, S.; Alves, A.K.; Bergmann, C.P. Cobalt-doped titanium oxide nanotubes grown via one-step anodization for water splitting applications. Appl. Surf. Sci. 2019, 464, 351–359. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. Ni-doped TiO2 nanotubes photoanode for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 443, 321–328. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.-G.; Fan, Y.; Sun, H.; Hua, W.; Liu, H.; Wei, B. Plasmonic TiN boosting nitrogen-doped TiO2 for ultrahigh efficient photoelectrochemical oxygen evolution. Appl. Catal. B-Environ. 2019, 246, 21–29. [Google Scholar] [CrossRef]
- Park, J.; Desmukh, P.R.; Sohn, y.; Shin, W.G. ZnO-TiO2 core-shell nanowires decorated with Au nanoparticles for plasmon-enhanced photoelectrochemical water splitting. J. Alloy. Compd. 2019, 787, 1310–1319. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, X.-b.; Wu, J.; Zhu, L.-w.; Gu, L. Preparation of hierarchical CuO@TiO2 nanowire film and its application in photoelectrochemical water splitting. T. Nonferr. Metal. Soc. 2016, 26, 2094–2101. [Google Scholar] [CrossRef]
- Sawant, S.Y.; Sayed, M.S.; Han, T.H.; Karim, M.R.; Shim, J.J.; Cho, M.H. Bio-synthesis of finely distributed Ag nanoparticle-decorated TiO2 nanorods for sunlight-induced photoelectrochemical water splitting. J. Ind. Eng. Chem. 2019, 69, 48–56. [Google Scholar] [CrossRef]
- Liu, J.; Ke, J.; Li, Y.; Liu, B.J.; Wang, L.D.; Xiao, H.N.; Wang, S.B. Co3O4 quantum dots/TiO2 nanobelt hybrids for highly efficient photocatalytic overall water splitting. Appl. Catal. B-Environ. 2018, 236, 396–403. [Google Scholar] [CrossRef]
- He, J.; Wang, M.; Wu, X.; Sun, Y.; Huang, K.; Chen, H.; Gao, L.; Feng, S. Influence of controlled Pd nanoparticles decorated TiO2 nanowire arrays for efficient photoelectrochemical water splitting. J. Alloy. Compd. 2019, 785, 391–397. [Google Scholar] [CrossRef]
- Wang, L.; Cao, S.; Guo, K.; Wu, Z.; Ma, Z.; Piao, L. Simultaneous hydrogen and peroxide production by photocatalytic water splitting. Chin. J. Catal. 2019, 40, 470–475. [Google Scholar] [CrossRef]
- Hussain, E.; Majeed, I.; Nadeem, M.A.; Iqbal, A.; Chen, Y.; Choucair, M.; Jin, R.; Nadeem, M.A. Remarkable effect of BaO on photocatalytic H2 evolution from water splitting via TiO2 (P25) supported palladium nanoparticles. J. Environ. Chem. Eng. 2019, 7, 102729. [Google Scholar] [CrossRef]
- Yoo, I.-H.; Kalanur, S.S.; Seo, H. A nanoscale p–n junction photoelectrode consisting of an NiOx layer on a TiO2/CdS nanorod core-shell structure for highly efficient solar water splitting. Appl. Catal. B-Environ. 2019, 250, 200–212. [Google Scholar] [CrossRef]
- Wang, Z.W.; Li, X.L.; Tan, C.K.; Qian, C.; Grimsdale, A.C.; Tok, A.I.Y. Highly porous SnO2 nanosheet arrays sandwiched within TiO2 and CdS quantum dots for efficient photoelectrochemical water splitting. Appl. Surf. Sci. 2019, 470, 800–806. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. Facile preparation of Ti3+/Ni co-doped TiO2 nanotubes photoanode for efficient photoelectrochemical water splitting. Appl. Surf. Sci. 2019, 480, 219–228. [Google Scholar] [CrossRef]
- Hu, M.; Xing, Z.; Cao, Y.; Li, Z.; Yan, X.; Xiu, Z.; Zhao, T.; Yang, S.; Zhou, W. Ti3+ self-doped mesoporous black TiO2/SiO2/g-C3N4 sheets heterojunctions as remarkable visible-lightdriven photocatalysts. Appl. Catal. B-Environ. 2018, 226, 499–508. [Google Scholar] [CrossRef]
- Wu, L.L.; Shi, S.; Li, Q.D.; Zhang, X.Y.; Cui, X.L. TiO2 nanoparticles modified with 2D MoSe2 for enhanced photocatalytic activity on hydrogen evolution. Int. J. Hydrog. Energy 2019, 44, 720–728. [Google Scholar] [CrossRef]
- Zhang, H.W.; Ma, L.; Ming, J.T.; Liu, B.Q.; Zhao, Y.B.; Hou, Y.D.; Ding, Z.X.; Xu, C.; Zhang, Z.Z.; Long, J.L. Amorphous Ta2OxNy-enwrapped TiO2 rutile nanorods for enhanced solar photoelectrochemical water splitting. Appl. Catal. B-Environ. 2019, 243, 481–489. [Google Scholar] [CrossRef]
- Kaur, G.; Pandey, O.P.; Singh, K. Optical, structural, and mechanical properties of different valence-cation-doped bismuth vanadate oxides. Phys. Status Solidi A 2012, 209, 1231–1238. [Google Scholar] [CrossRef]
- Yang, S.Y.; Seidel, J.; Byrnes, S.J.; Shafer, P.; Yang, C.H.; Rossell, M.D.; Yu, P.; Chu, Y.H.; Scott, J.F.; Ager Iii, J.W.; et al. Above-bandgap voltages from ferroelectric photovoltaic devices. Nat. Nanotechnol. 2010, 5, 143. [Google Scholar] [CrossRef]
- Kay, A.; Cesar, I.; Grätzel, M. New Benchmark for Water Photooxidation by Nanostructured α-Fe2O3 Films. J. Am. Chem. Soc. 2006, 128, 15714–15721. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, H.; Wang, X.; Jiang, L.; Xi, J.; Du, G.; Ji, Z. Ferroelectric enhanced photoelectrochemical water splitting in BiFeO3/TiO2 composite photoanode. J. Alloy. Compd. 2019, 783, 643–651. [Google Scholar] [CrossRef]
- Jia, Y.L.; Wang, Z.H.; Ma, Y.; Liu, J.L.; Shi, W.B.; Lin, Y.H.; Hu, X.; Zhang, K. Boosting interfacial charge migration of TiO2/BiVO4 photoanode by W doping for photoelectrochemical water splitting. Electrochim. Acta. 2019, 300, 138–144. [Google Scholar] [CrossRef]
- Zhou, W.F.; Jiang, T.F.; Zhao, Y.; Xu, C.; Pei, C.G.; Xue, H.G. Ultrathin Ti/TiO2/BiVO4 nanosheet heterojunction arrays for photoelectrochemical water oxidation. J. Alloy. Compd. 2019, 777, 1152–1158. [Google Scholar] [CrossRef]
- Liu, Q.; Mo, R.; Li, X.L.; Yang, S.; Zhong, J.X.; Li, H.X. Cobalt phosphate modified 3D TiO2/BiVO4 composite inverse opals photoanode for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2019, 464, 544–551. [Google Scholar] [CrossRef]
- Yin, X.; Liu, Q.; Yang, Y.H.; Liu, Y.; Wang, K.K.; Li, Y.M.; Li, D.W.; Qiu, X.Q.; Li, W.Z.; Li, J. An efficient tandem photoelectrochemical cell composed of FeOOH/TiO2/BiVO4 and Cu2O for self-driven solar water splitting. Int. J. Hydrog. Energy 2019, 44, 594–604. [Google Scholar] [CrossRef]
- Polo, A.; Grigioni, I.; Dozzi, M.V.; Selli, E. Sensitizing effects of BiVO4 and visible light induced production of highly reductive electrons in the TiO2/BiVO4 heterojunction. Catal. Today 2018. [Google Scholar] [CrossRef]
- Radzi, A.A.S.M.; Safaei, J.; Teridi, M.A.M. Photoelectrochemical enhancement from deposition of BiVO4 photosensitizer on different thickness layer TiO2 photoanode for water splitting application. Nano-Struct. Nano-Objects 2019, 18. [Google Scholar] [CrossRef]
- Chai, X.B.; Zhang, H.F.; Pan, Q.; Bian, J.L.; Chen, Z.F.; Cheng, C.W. 3D ordered urchin-like TiO2@Fe2O3 arrays photoanode for efficient photoelectrochemical water splitting. Appl. Surf. Sci. 2019, 470, 668–676. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Zhang, L.; Zhang, P.; Dong, E.; Ma, J.; Wang, G. Design and fabrication of an α-Fe2O3 TiO2 Si 3D hierarchical photoanode for improved photoelectrochemical water splitting. J. Alloy. Compd. 2019, 773, 597–604. [Google Scholar] [CrossRef]
- Feng, F.; Li, C.; Jian, J.; Qiao, X.; Wang, H.; Jia, L. Boosting hematite photoelectrochemical water splitting by decoration of TiO2 at the grain boundaries. Chem. Eng. J. 2019, 368, 959–967. [Google Scholar] [CrossRef]
- Deng, J.; Zhuo, Q.; Lv, X. Hierarchical TiO2 Fe2O3 heterojunction photoanode for improved photoelectrochemical water oxidation. J. Electroanal. Chem. 2019, 835, 287–292. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, D.H.; Kim, J.H.; Jang, H.W.; Lee, J.H. NH2-MIL-125(Ti)/TiO2 nanorod heterojunction photoanodes for efficient photoelectrochemical water splitting. Appl. Catal. B-Environ. 2019, 244, 511–518. [Google Scholar] [CrossRef]
- Yalçın, Y.; Kılıç, M.; Çınar, Z. The Role of Non-Metal Doping in TiO2 Photocatalysis. J. Adv. Oxid. Technol. 2010, 13, 281. [Google Scholar] [CrossRef]
- Lu, Y.; Wei, Z.; Salke, N.P.; Yu, L.; Yan, H. Enhanced electron transport in rutile TiO2 nanowires via H2S-assisted incorporation of dissolved silicon for solar-driven water splitting. Appl. Catal. B-Environ. 2019, 244, 767–772. [Google Scholar] [CrossRef]
- Bayat, A.; Saievar-Iranizad, E. Graphene quantum dots decorated rutile TiO2 nanoflowers for water splitting application. J. Energy Chem. 2018, 27, 306–310. [Google Scholar] [CrossRef]
- Bellamkonda, S.; Thangavel, N.; Hafeez, H.Y.; Neppolian, B.; Rao, G.R. Highly active and stable multi-walled carbon nanotubes-graphene-TiO2 nanohybrid: An efficient non-noble metal photocatalyst for water splitting. Catal. Today 2019, 321, 120–127. [Google Scholar] [CrossRef]
- Gao, Q.; Si, F.; Zhang, S.; Fang, Y.; Chen, X.; Yang, S. Hydrogenated F-doped TiO2 for photocatalytic hydrogen evolution and pollutant degradation. Int. J. Hydrog. Energy 2019, 44, 8011–8019. [Google Scholar] [CrossRef]
- Elbakkay, M.H.; El Rouby, W.M.A.; El-Dek, S.I.; Farghali, A.A. S-TiO2/S-reduced graphene oxide for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 439, 1088–1102. [Google Scholar] [CrossRef]
- Dubnova, L.; Zvolska, M.; Edelmannova, M.; Matejova, L.; Reli, M.; Drobna, H.; Kustrowski, P.; Koci, K.; Capek, L. Photocatalytic decomposition of methanol-water solution over N-La/TiO2 photocatalysts. Appl. Surf. Sci. 2019, 469, 879–886. [Google Scholar] [CrossRef]
- Liu, X.; Cao, X.E.; Liu, Y.; Li, X.; Wang, M.; Li, M. Branched multiphase TiO2 with enhanced photoelectrochemical water splitting activity. Int. J. Hydrog. Energy 2018, 43, 21365–21373. [Google Scholar] [CrossRef]
- Xu, Y.; Ahemd, R.; Klein, D.; Cap, S.; Freedy, K.; McDonnel, S.; Zangari, G. Improving photo-oxidation activity of water by introducing Ti3+ in self-ordered TiO2 nanotube arrays treated with Ar NH3. J. Power Sour. 2019, 414, 242–249. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Q.Y.; Ahmed, R.; Hoglund, E.R.; Zangari, G. Synthesis of TiO2-based nanocomposites by anodizing and hydrogen annealing for efficient photoelectrochemical water oxidation. J. Power Sour. 2019, 410, 59–68. [Google Scholar] [CrossRef]
- Wei, N.; Liu, Y.; Feng, M.; Li, Z.X.; Chen, S.G.; Zheng, Y.B.; Wang, D.A. Controllable TiO2 core-shell phase heterojunction for efficient photoelectrochemical water splitting under solar light. Appl. Catal. B-Environ. 2019, 244, 519–528. [Google Scholar] [CrossRef]
- Alexander, F.; AlMheiri, M.; Dahal, P.; Abed, J.; Rajput, N.S.; Aubry, C.; Viegas, J.; Jouiad, M. Water splitting TiO2 composite material based on black silicon as an efficient photocatalyst. Sol. Energy Mat. Sol. Cells 2018, 180, 236–242. [Google Scholar] [CrossRef]
- Fu, B.; Wu, Z.; Cao, S.; Guo, K.; Piao, L. Effect of aspect ratios of rutile TiO2 nanorods on overall photocatalytic water splitting performance. Nanoscale 2020, 12, 4895–4902. [Google Scholar] [CrossRef] [PubMed]
- Abed, J.; AlMheiri, M.; Alexander, F.; Rajput, N.S.; Viegas, J.; Jouiad, M. Enhanced solar absorption of gold plasmon assisted TiO2-based water splitting composite. Sol. Energy Mat. Sol. Cells 2018, 180, 228–235. [Google Scholar] [CrossRef]
- Pérez-Larios, A.; Lopez, R.; Hernández-Gordillo, A.; Tzompantzi, F.; Gómez, R.; Torres-Guerra, L.M. Improved hydrogen production from water splitting using TiO2–ZnO mixed oxides photocatalysts. Fuel 2012, 100, 139–143. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Sun, H.; Zhang, K.-Q.; Lai, Y. Uniform carbon dots@TiO2 nanotube arrays with full spectrum wavelength light activation for efficient dye degradation and overall water splitting. Nanoscale 2017, 9, 16046–16058. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.; Wang, X.; Fu, X. Hydrogen production over titania-based photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef]
- Makhlouf, A.S.H.; Tiginyanu, I. Nanocoatings and Ultra-Thin Films; Woodhead Publishing: Cambridge, UK, 2011; p. 448. [Google Scholar]
- Hernández-Alonso, M.; Fresno, F.; Suárez, S.; Coronado, J. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231–1257. [Google Scholar] [CrossRef]
- Goto, Y.; Hisatomi, T.; Wang, Q.; Higashi, T.; Ishikiriyama, K.; Maeda, T.; Sakata, Y.; Okunaka, S.; Tokudome, H.; Katayama, M.; et al. A Particulate Photocatalyst Water-Splitting Panel for Large-Scale Solar Hydrogen Generation. Joule 2018, 2, 509–520. [Google Scholar] [CrossRef]
- Pinaud, B.A.; Benck, J.D.; Seitz, L.C.; Forman, A.J.; Chen, Z.; Deutsch, T.G.; James, B.D.; Baum, K.N.; Baum, G.N.; Ardo, S.; et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 2013, 6, 1983–2002. [Google Scholar] [CrossRef]
- Han, L.; Lin, M.; Haussener, S. Reliable Performance Characterization of Mediated Photocatalytic Water-Splitting Half Reactions. ChemSusChem 2017, 10, 2158–2166. [Google Scholar] [CrossRef]
- Kisch, H.; Bahnemann, D. Best Practice in Photocatalysis: Comparing Rates or Apparent Quantum Yields? J. Phys. Chem. Lett. 2015, 6, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Zhong, X.; Li, T.; Chen, M.; Feng, G. DFT Study on the Hydrogen Evolution Reaction for Different Facets of Co2P. ChemElectroChem 2019, 6, 260–267. [Google Scholar] [CrossRef]
- Skúlason, E.; Karlberg, G.S.; Rossmeisl, J.; Bligaard, T.; Greeley, J.; Jónsson, H.; Nørskov, J.K. Density functional theory calculations for the hydrogen evolution reaction in an electrochemical double layer on the Pt(111) electrode. Phys. Chem. Chem. Phys. 2007, 9, 3241–3250. [Google Scholar] [CrossRef] [PubMed]
- Parzinger, E.; Miller, B.; Blaschke, B.; Garrido, J.A.; Ager, J.W.; Holleitner, A.; Wurstbauer, U. Photocatalytic Stability of Single- and Few-Layer MoS2. ACS Nano 2015, 9, 11302–11309. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X.; Zhang, Z. Recent development on MoS2-based photocatalysis: A review. J. Photoch. Photobio. C 2018, 35, 39–55. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, C.; Zhu, J.; Qin, W.; Tao, X.; Fan, F.; Li, R.; Li, C. A Hydrogen Farm Strategy for Scalable Solar Hydrogen Production with Particulate Photocatalysts. Angew. Chem. Int. Edit. 2020, 59, 9653–9658. [Google Scholar] [CrossRef] [PubMed]

| Nanomaterial | Bandgap [eV] | Ref. |
|---|---|---|
| Ag doped TiO2 | 2.312 | [88] |
| Au doped TiO2 | 0.996 | [88] |
| Pt doped TiO2 | 0.754 | [88] |
| Pd doped TiO2 | 0.363 | [88] |
| Ru doped TiO2 | 0.176 | [88] |
| Wet TiO2 (001) | 1.8571 | [89] |
| Pt doped Wet TiO2 (001) | 1.4546 | [89] |
| Ru doped Wet TiO2 (001) | 0.1636 | [89] |
| Co doped Wet TiO2 (001) | 0.0539 | [89] |
| Ru clusters on TiO2 | NA | [90] |
| Anatase TiO2 | 3.05 | [91] |
| Pt adsorbed on TiO2 | 3.06 | [91] |
| Pd adsorbed on TiO2 | 3.05 | [91] |
| Rh adsorbed on TiO2 | 2.80 | [91] |
| Ru adsorbed on TiO2 | 3.10 | [91] |
| Anatase TiO2 | 2.98 | [92] |
| Rutile TiO2 | 2.78 | [92] |
| aTiO2 | NA | [93] |
| Cu + N co-doped TiO2 | NA | [94] |
| Cu doped anatase TiO2 (101) | NA | [95] |
| Cu doped anatase TiO2 (101) | NA | [15] |
| Co-doped SrTiO3 | 3.07 | [96] |
| N-doped aTiO2 | 2.25 | [97] |
| S-doped anatase TiO2 | 2.33 | [98] |
| Nb-doped anatase TiO2 | 2.25 | [98] |
| (S, Nb)-doped anatase TiO2 | 2.15 | [98] |
| TiO2 hollow spheres doped with Mg | NA (H2 production rate: 850 µmol/h/g. O2 production rate: 425 µmol/h/g) | [99] |
| TiO2 doped by lanthanides | NA | [100] |
| C@O-doped TiO2 | 3.019 | [102] |
| C@gap-doped TiO2 | 3.021 | [102] |
| Nd@Ti-doped TiO2 | 3.032 | [102] |
| Nd@gap-doped TiO2 | 2.353 | [102] |
| C@O&Nd@Ti-doped TiO2 | 2.372 | [102] |
| C&Nd@gap-doped TiO2 | 2.850 | [102] |
| TiO2-X | 2.6 (H2 production rate: 46.9 µmol/h/g) | [103] |
| g-CS@TiO2-X | 2.5 (H2 production rate: 255.2 µmol/h/g) | [103] |
| g-CS+TiO2-X | 2.3 (H2 production rate: 68.3 µmol/h/g) | [103] |
| Se(IV) ion doped TiO2 | 2.85 | [104] |
| N-doped TiO2 | 3.06 | [105] |
| Co-doped TiO2 | 2.92 | [105] |
| Co-1N-doped TiO2 | 2.91 | [105] |
| Co-2N-doped TiO2 | 2.90 | [105] |
| Co-3N-doped TiO2 | 2.92 | [105] |
| Mesoporous carbonate-doped phase-junction TiO2 nanotubes | 2.69-2.92 (H2 production rate: 6108 µmol/h/g) | [106] |
| B-doped TiO2 | 2.40 | [108] |
| S-doped TiO2 | 2.23 | [108] |
| C-doped TiO2 | 2.53 | [108] |
| P-doped TiO2 | 2.30 | [108] |
| N-doped TiO2 | 2.51 | [108] |
| F-doped TiO2 | 2.61 | [108] |
| Cl-doped TiO2 | 2.34 | [108] |
| N-TiO2 | 2.94 | [109] |
| Cu-TiO2 | 3.22 | [109] |
| (Cu, N)-TiO2 | 2.96 | [109] |
| TiO2/g-C3N4 | 2.34 | [109] |
| N-TiO2/g-C3N4 | 2.31 | [109] |
| Cu-TiO2/g-C3N4 | 2.23 | [109] |
| (Cu, N)-TiO2/g-C3N4 | 2.26 | [109] |
| g-C3N4/TiO2 | 2.21 | [110] |
| (C, N)-doped rutile TiO2 | 2.59 | [111] |
| Rh, Nb co-doped TiO2 | NA | [112] |
| S, N, or S+N doped TiO2 anatase (101) nanotubes | 2.78–4.32 | [113] |
| S-doped TiO2 | 2.72 | [114] |
| Sc-doped three-layer fluorite structured TiO2 | 4.00 | [115] |
| V-doped three-layer fluorite structured TiO2 | 3.95 | [115] |
| Cr-doped three-layer fluorite structured TiO2 | 3.98 | [115] |
| Mn-doped three-layer fluorite structured TiO2 | 3.66 | [115] |
| Fe-doped three-layer fluorite structured TiO2 | 3.39 | [115] |
| Co-doped three-layer fluorite structured TiO2 | 4.01 | [115] |
| Ni-doped three-layer fluorite structured TiO2 | 4.20 | [115] |
| Cu-doped three-layer fluorite structured TiO2 | 4.20 | [115] |
| Zn-doped three-layer fluorite structured TiO2 | 3.60 | [115] |
| 4d metals doped TiO2 nanotubes | 2–4 | [116] |
| Three-layer TiO2 (101) nanotubes | 3.83 | [117] |
| Six-layer TiO2 (101) nanotubes | 4.17 | [117] |
| Nine-layer TiO2 (001) nanotubes | 3.95 | [117] |
| Six-layer TiO2 (001) nanotubes | 4.15 | [117] |
| Facer dependency of TiO2 | NA | [118] |
| TiO2 | NA | [119] |
| Phase stability in TiO2 | NA | [120] |
| Rutile TiO2 | NA | [121] |
| aTiO2 | 2.70–2.85 | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a Photocatalyst for Water Splitting—An Experimental and Theoretical Review. Molecules 2021, 26, 1687. https://doi.org/10.3390/molecules26061687
Eidsvåg H, Bentouba S, Vajeeston P, Yohi S, Velauthapillai D. TiO2 as a Photocatalyst for Water Splitting—An Experimental and Theoretical Review. Molecules. 2021; 26(6):1687. https://doi.org/10.3390/molecules26061687
Chicago/Turabian StyleEidsvåg, Håkon, Said Bentouba, Ponniah Vajeeston, Shivatharsiny Yohi, and Dhayalan Velauthapillai. 2021. "TiO2 as a Photocatalyst for Water Splitting—An Experimental and Theoretical Review" Molecules 26, no. 6: 1687. https://doi.org/10.3390/molecules26061687
APA StyleEidsvåg, H., Bentouba, S., Vajeeston, P., Yohi, S., & Velauthapillai, D. (2021). TiO2 as a Photocatalyst for Water Splitting—An Experimental and Theoretical Review. Molecules, 26(6), 1687. https://doi.org/10.3390/molecules26061687









