Solar Photooxygenations for the Manufacturing of Fine Chemicals—Technologies and Applications
Abstract
1. Introduction
2. Solar Reactors for Synthetic Applications
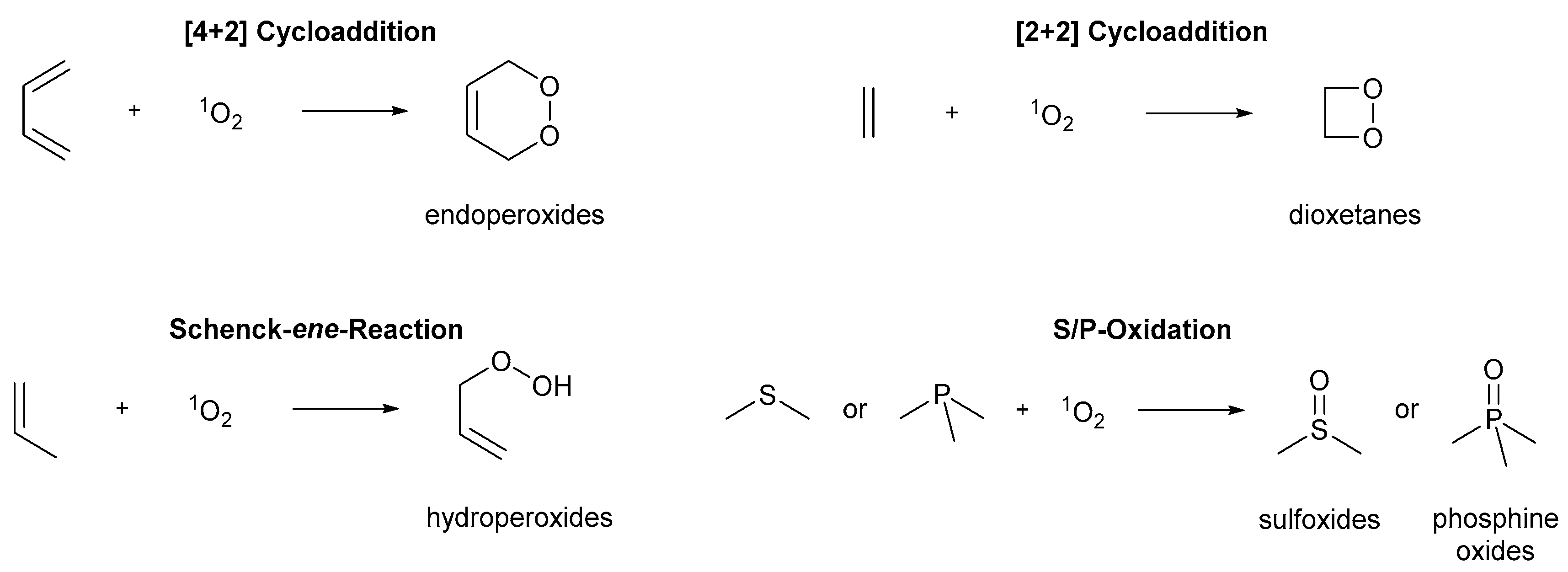
3. Photooxygenations in Organic Synthesis
4. Solar Preparative Photooxygenations
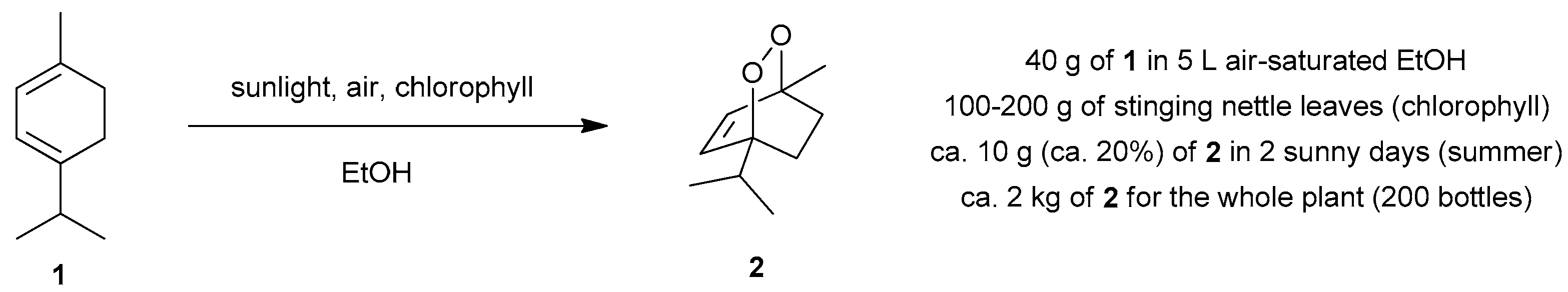
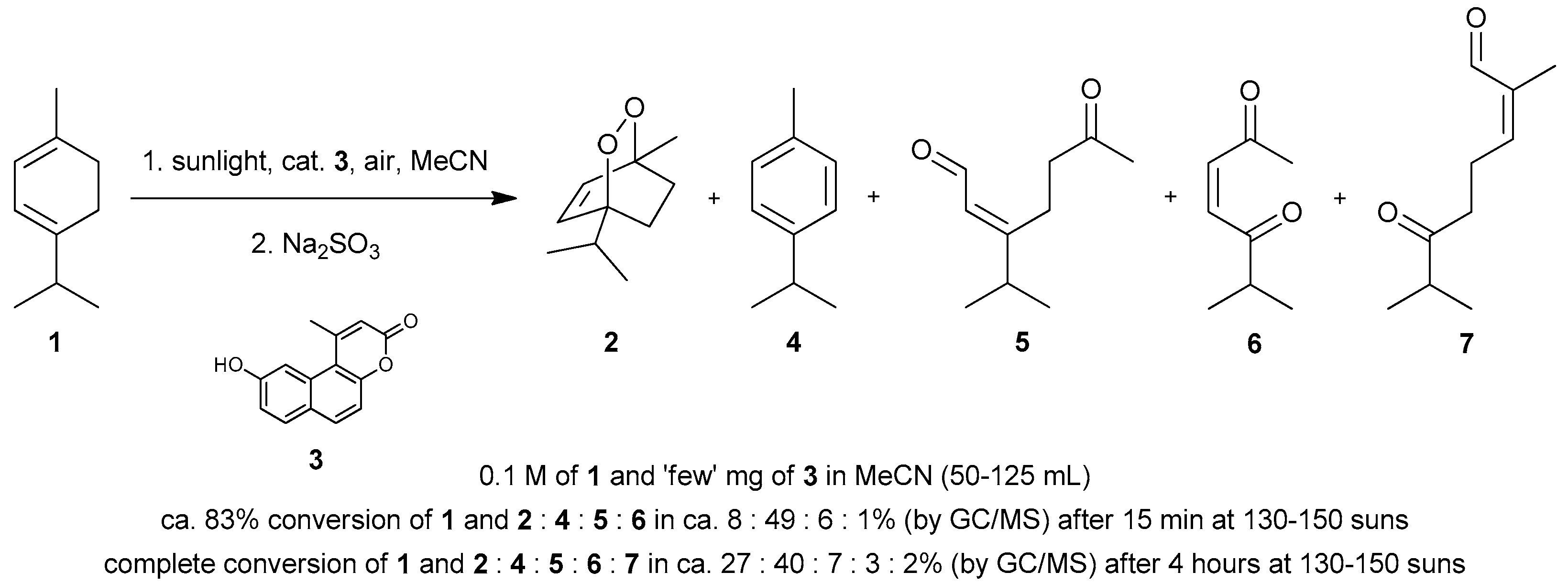
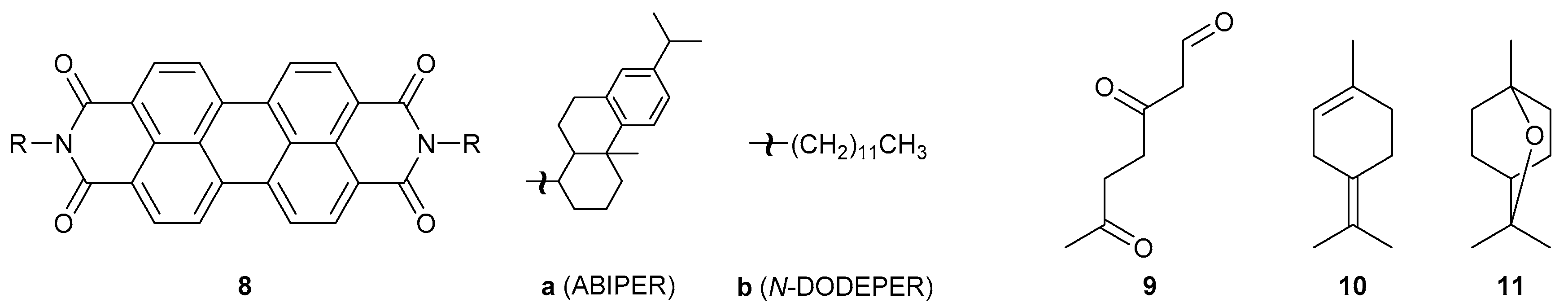
4.1. Photooxygenation of α-Terpinene and Related Reactions
4.2. Photooxygenation of Citronellol for the Production of Rose Oxide
4.3. Photooxygenations of β-Pinene for the Production of Myrtenol
4.4. Photooxygenations of α-Thujene for the Production of Trans-Sabinene Hydrate
4.5. Photooxygenations of Furfural and Furfuraldiethylacetal to 5-Hydroxy- and 5-Alkoxyfuranones
4.6. Photooxygenations of 1,5-Dihydroxynaphthalene to Juglone
4.7. Miscellaneous Solar Photooxygenations and Photooxidations
5. Limitations, Challenges and Opportunities
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bonfield, H.E.; Knauber, T.; Lévesque, F.; Moschetta, E.G.; Susanne, F.; Edwards, L.J. Photons as a 21st century reagent. Nat. Commun. 2020, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Griesbeck, A.G. The Future of Photochemistry: Just Bright. ChemPhotoChem 2018, 3, 8–9. [Google Scholar] [CrossRef]
- Pagliaro, M. Making APIs and fine chemicals with light. Chim. Oggi—Chem. Today 2017, 35, 84–85. [Google Scholar]
- Bach, T. More Chemistry with light! More light in chemistry! Angew. Chem. Int. Ed. 2015, 54, 11294–11295. [Google Scholar] [CrossRef]
- Balzani, V.; Bergamini, G.; Ceroni, P. Photochemistry and photocatalysis. Rend. Lincei 2017, 28, 125–142. [Google Scholar] [CrossRef]
- Kärkäs, M.D.; Porco, J.A., Jr.; Stephenson, C.R.J. Photochemical approaches to complex chemotypes: Applications in natural product synthesis. Chem. Rev. 2016, 116, 9683–9747. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Hoffmann, N. Studies in organic and physical photochemistry—An interdisciplinary approach. Org. Biomol. Chem. 2016, 14, 7392–7442. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef]
- Rehm, T.H. Reactor Technology Concepts for Flow Photochemistry. ChemPhotoChem 2019, 4, 235–254. [Google Scholar] [CrossRef]
- Di Filippo, M.; Bracken, C.; Baumann, M. Continuous Flow Photochemistry for the Preparation of Bioactive Molecules. Molecules 2020, 25, 356. [Google Scholar] [CrossRef]
- Politano, F.; Oksdath-Mansilla, G. Light on the Horizon: Current Research and Future Perspectives in Flow Photochemistry. Org. Process. Res. Dev. 2018, 22, 1045–1062. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Hoffmann, N.; Shvydkiv, O.; Oelgemoeller, M. From ‘Lab & Light on a Chip’ to Parallel Microflow Photochemistry. Aust. J. Chem. 2014, 67, 337–342. [Google Scholar] [CrossRef]
- Sender, M.; Ziegenbalg, D. Light Sources for Photochemical Processes—Estimation of Technological Potentials. Chem. Ing. Tech. 2017, 89, 1159–1173. [Google Scholar] [CrossRef]
- Braun, A.M.; Oller do Nascimento, C.A. Angewandte (präparative) Photochemie. Nachr. Chem. Tech. Lab. 1991, 39, 515–526. [Google Scholar]
- Braun, A.; Peschl, G.H.; Oliveros, E. Industrial photochemistry. In CRC Handbook of Organic Photochemistry and Photobiology, 3rd ed.; Griesbeck, A., Oelgemöller, M., Ghetti, F., Eds.; CRC Press: Boca Raton, FL, USA, 2012; Volume 1, Chapter 1; pp. 1–19. [Google Scholar]
- Michelin, C.; Lefebvre, C.; Hoffmann, N. Les réactions photochimiques à l’échelle industrielle. L’Act. Chim. 2019, 436, 19–27. [Google Scholar]
- Pfoertner, K.-H.; Oppenländer, T. Photochemistry. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012; pp. 1–45. [Google Scholar]
- Braun, A.M.; Jakob, L.; Oliveros, E.; Nascimento, C.A.O.D. Up-Scaling Photochemical Reactions. Adv. Photochem. 2007, 18, 235–313. [Google Scholar] [CrossRef]
- Fischer, M. Industrial Applications of Photochemical Syntheses. Angew. Chem. Int. Ed. 1978, 17, 16–26. [Google Scholar] [CrossRef]
- Mattay, J. Von der Laborsynthese zur Solarchemie. Chem. unsere Zeit 2002, 36, 98–106. [Google Scholar] [CrossRef]
- Wilkins, F.W.; Blake, D.M. Use solar energy to drive chemical processes. Chem. Eng. Prog. 1994, 90, 41–49. [Google Scholar]
- Funken, K.-H. Solare Photoreaktionen für die chemische Technik. Nachr. Chem. Tech. Lab. 1992, 40, 793–800. [Google Scholar] [CrossRef]
- Mathur, V.; Wong, E. Production of fuels and chemicals using solar photothermochemistry. Energy 1987, 12, 311–318. [Google Scholar] [CrossRef]
- Albini, A.; Dichiarante, V. The ‘belle ’epoque’ of photochemistry. Photochem. Photobiol. Sci. 2009, 8, 248–254. [Google Scholar] [CrossRef]
- Roth, H.D. Twentieth century developments in photochemistry. Brief historical sketches. Pure Appl. Chem. 2001, 73, 395–403. [Google Scholar] [CrossRef]
- Roth, H.D. The Beginnings of Organic Photochemistry. Angew. Chem. Int. Ed. 1989, 28, 1193–1207. [Google Scholar] [CrossRef]
- Ciamician, G. The photochemistry of the future. Science 1912, 36, 385–394. [Google Scholar] [CrossRef]
- Protti, S.; Fagnoni, M. The sunny side of chemistry: Green synthesis by solar light. Photochem. Photobiol. Sci. 2009, 8, 1499–1516. [Google Scholar] [CrossRef]
- Ravelli, D.; Protti, S.; Fagnoni, M. Application of Visible and Solar Light in Organic Synthesis. Lect. Notes Chem. 2016, 92, 281–342. [Google Scholar] [CrossRef]
- Fuqiang, W.; Ziming, C.; Jianyu, T.; Yuan, Y.; Yong, S.; Linhua, L. Progress in concentrated solar power technology with parabolic trough collector system: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 79, 1314–1328. [Google Scholar] [CrossRef]
- Weinstein, L.A.; Loomis, J.; Bhatia, B.S.; Bierman, D.M.; Wang, E.N.; Chen, G. Concentrating Solar Power. Chem. Rev. 2015, 115, 12797–12838. [Google Scholar] [CrossRef]
- Fernández-García, A.; Zarza, E.; Valenzuela, L.; Pérez, M. Parabolic-trough solar collectors and their applications. Renew. Sustain. Energy Rev. 2010, 14, 1695–1721. [Google Scholar] [CrossRef]
- Roeb, M.; Neises, M.; Monnerie, N.; Sattler, C.; Pitz-Paal, R. Technologies and trends in solar power and fuels. Energy Environ. Sci. 2011, 4, 2503–2511. [Google Scholar] [CrossRef]
- Funken, K.-H.; Ortner, J. Technologies for the Solar Photochemical and Photocatalytic Manufacture of Specialities and Commodities: A Review. Z. Phys. Chem. 1999, 213, 99–105. [Google Scholar] [CrossRef]
- Fendrich, M.A.; Quaranta, A.; Orlandi, M.; Bettonte, M.; Miotello, A. Solar Concentration for Wastewaters Remediation: A Review of Materials and Technologies. Appl. Sci. 2018, 9, 118. [Google Scholar] [CrossRef]
- Malato, S.; Maldonado, M.I.; Fernández-Ibáñez, P.; Oller, I.; Polo, I.; Sánchez-Moreno, R. Decontamination and disinfection of water by solar photocatalysis: The pilot plants of the Plataforma Solar de Almeria. Mater. Sci. Semicond. Process. 2016, 42, 15–23. [Google Scholar] [CrossRef]
- Tanveer, M.; Guyer, G.T. Solar assisted photo degradation of wastewater by compound parabolic collectors: Review of design and operational parameters. Renew. Sustain. Energy Rev. 2013, 24, 534–543. [Google Scholar] [CrossRef]
- Bahnemann, D. Photocatalytic water treatment: Solar energy applications. Sol. Energy 2004, 77, 445–459. [Google Scholar] [CrossRef]
- Oelgemöller, M. Solar Photochemical Synthesis: From the Beginnings of Organic Photochemistry to the Solar Manufacturing of Commodity Chemicals. Chem. Rev. 2016, 116, 9664–9682. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Cat. B Environ. 2015, 170, 90–123. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Jung, C.; Mattay, J.; Oelgemoeller, M. Green photochemistry: Production of fine chemicals with sunlight. Pure Appl. Chem. 2007, 79, 1939–1947. [Google Scholar] [CrossRef]
- Esser, P.; Pohlmann, B.; Scharf, H.-D. The Photochemical Synthesis of Fine Chemicals with Sunlight. Angew. Chem. Int. Ed. 1994, 33, 2009–2023. [Google Scholar] [CrossRef]
- Mumtaz, S.; Sattler, C.; Oelgemöller, M. Solar photochemical manufacturing of fine chemicals—historical background, modern solar technologies, recent applications and future challenges. In Chemical Processes for a Sustainable Future; Letcher, T.M., Scott, J.L., Patterson, D., Eds.; Royal Society of Chemistry: Cambridge, UK, 2015; Chapter 7; pp. 158–191. [Google Scholar]
- Coyle, E.E.; Oelgemöller, M. Solar photochemistry from the beginnings of organic photochemistry to the solar production of chem-icals. In CRC Handbook of Organic Photochemistry and Photobiology, 3rd ed.; Griesbeck, A., Oelgemöller, M., Ghetti, F., Eds.; CRC Press: Boca Raton, FL, USA, 2012; Volume 1, Chapter 10; pp. 237–247. [Google Scholar]
- Oelgemöller, M.; Jung, C.; Ortner, J.; Mattay, J.; Schiel, C.; Zimmermann, E. Green photochemistry with moderately concentrated sunlight. Spectrum 2005, 18, 28–33. [Google Scholar]
- Oelgemöller, M.; Mattay, J.; Jung, C.; Schiel, C.; Ortner, C.; Zimmermann, E. Back to the roofs—the solarchemical production of fine chemicals with sunlight. In Proceedings of the International Solar Energy Conference, ISEC 2004 Conference, Portland, OR, USA, 11–14 July 2004; American Society of Mechanical Engineers (ASME): New York, NY, USA, 2004; pp. 523–531. [Google Scholar]
- Pohlmann, B.; Scharf, H.-D.; Jarolimek, U.; Mauermann, P. Photochemical production of fine chemicals with concentrated sunlight. Sol. Energy 1997, 61, 159–168. [Google Scholar] [CrossRef]
- Fu, Q. Radiation (solar). In Encyclopedia of Atmospheric Sciences; Holton, J., Ed.; Academic Press: Amsterdam, The Netherlands, 2003; pp. 1859–1863. [Google Scholar]
- Iqbal, M. An Introduction to Solar Radiation; Academic Press: New York, NY, USA, 1983. [Google Scholar]
- Pibiri, I.B.; Piccionello, S.; Palumbo, A.; Pace, A. Photochemically produced singlet oxygen: Applications and perspectives. ChemPhotoChem 2018, 2, 535–547. [Google Scholar] [CrossRef]
- Ghogare, A.A.; Greer, A. Using Singlet Oxygen to Synthesize Natural Products and Drugs. Chem. Rev. 2016, 116, 9994–10034. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Turconi, J.; Griolet, F.; Guevel, R.; Oddon, G.; Villa, R.; Geatti, A.; Hvala, M.; Rossen, K.; Göller, R.; Burgard, A. Semisynthetic Artemisinin, the Chemical Path to Industrial Production. Org. Process. Res. Dev. 2014, 18, 417–422. [Google Scholar] [CrossRef]
- Gollnick, K. Photooxygenation and its application in industry. Chim. Ind. 1982, 64, 156–166. [Google Scholar] [CrossRef]
- Rojahn, W.; Warnecke, H.-U. Die Photosensibilisierte Sauerstoffübertragung—eine Methode zur Herstellung hochwertiger Riech-stoffe. Dragoc. Rep. 1980, 27, 159–164. [Google Scholar]
- Demuth, M. Chemie mit Sonnenlicht—auch bei Bewölkung. In Innovationspreis Ruhrgebiet; Kommunalverband Ruhrgebiet: Essen, Germany, 2000; pp. 91–94. [Google Scholar]
- Blanco, J.; Malato, S.; Fernández, P.; Vidal, A.; Morales, A.; Trincado, P.; Oliveira, J.; Minero, C.; Musci, M.; Casalle, C.; et al. Compound parabolic concentrator technology development to commercial solar detoxification applications. Sol. Energy 1999, 67, 317–330. [Google Scholar] [CrossRef]
- Ajona, J.; Vidal, A. The use of CPC collectors for detoxification of contaminated water: Design, construction and preliminary results. Sol. Energy 2000, 68, 109–120. [Google Scholar] [CrossRef]
- Jung, C.; Funken, K.-H.; Ortner, J. Prophis: Parabolic trough-facility for organic photochemical syntheses in sunlight. Photochem. Photobiol. Sci. 2005, 4, 409–411. [Google Scholar] [CrossRef]
- Funken, K.-H. Solar chemistry using concentrating solar facilities. In Proceedings of the Sixth International Summer School Solar Energy 2000, Klagenfurt, Austria, 24 July–4 August 2000; Faninger, G., Bucher, W., Wolfer, U., Eds.; Interuniversitäres Institut für interdisziplinäre Forschung und Fortbildung (IFF), Universität Klagenfurt: Klagenfurt, Austria, 2001; pp. 118–137. [Google Scholar]
- Neumann, A.; Groer, U. Experimenting with concentrated sunlight using the DLR solar furnace. Sol. Energy 1996, 58, 181–190. [Google Scholar] [CrossRef]
- Fields, C.L.; Pitts, J.R.; Hale, M.J.; Bingham, C.; Lewandowski, A.; King, D.E. Formation of fullerenes in highly concentrated solar flux. J. Phys. Chem. 1993, 97, 8701–8702. [Google Scholar] [CrossRef]
- Flamant, G.; Luxembourg, D.; Robert, J.; Laplaze, D. Optimizing fullerene synthesis in a 50 kW solar reactor. Sol. Energy 2004, 77, 73–80. [Google Scholar] [CrossRef]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef]
- Schmidt, R. Photosensitized Generation of Singlet Oxygen. Photochem. Photobiol. 2007, 82, 1161–1177. [Google Scholar] [CrossRef]
- Osterberg, P.M.; Niemeier, J.K.; Welch, C.J.; Hawkins, J.M.; Martinelli, J.R.; Johnson, T.E.; Root, T.W.; Stahl, S.S. Experimental lim-iting oxygen concentrations for nine organic solvents at temperatures and pressures relevant to aerobic oxidations in the pharma-ceutical industry. Org. Process. Res. Dev. 2015, 19, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 542–548. [Google Scholar]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef]
- Fresnadillo, D.G.; Lacombe, S. Reference photosensitizers for the production of singlet oxygen. In Singlet Oxygen: Applications in Biosciences and Nanosciences; Nonell, S., Flors, C., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2016; Volume 1, Chapter 6; pp. 105–143. [Google Scholar]
- Marin, M.L.; Santos-Juanes, L.; Arques, A.; Amat, A.M.; Miranda, M.A. Organic photocatalysts for the oxidation of pollutants and model compounds. Chem. Rev. 2012, 112, 1710–1750. [Google Scholar] [CrossRef]
- Lacombe, S.; Pigot, T. Materials for selective photo-oxygenation vs. photocatalysis: Preparation, properties and applications in en-vironmental and health fields. Catal. Sci. Technol. 2016, 6, 1571–1592. [Google Scholar] [CrossRef]
- Wahlen, J.; De Vos, D.E.; Jacobs, P.A.; Alsters, P.L. Solid Materials as Sources for Synthetically Useful Singlet Oxygen. Adv. Synth. Catal. 2004, 346, 152–164. [Google Scholar] [CrossRef]
- Malakar, P.; Deb, A.R.; Goodine, T.; Robertson, M.J.; Oelgemöller, M. Continuous-flow photooxygenations: an advantageous and sustainable oxidation methodology with a bright future. In Catalytic Aerobic Oxidations—Catalysis Series; Mejía, P., Ed.; Royal Society of Chemistry: Cambridge, UK, 2020; Chapter 7; pp. 181–251. [Google Scholar] [CrossRef]
- Nardello-Rataj, V.; Alsters, P.L.; Aubry, J.-M. Industrial Prospects for the Chemical and Photochemical Singlet Oxygenation of Organic Compounds. Liq. Phase Aerob. Oxid. Catal. Ind. Appl. Acad. Persp. 2016, 22, 369–395. [Google Scholar] [CrossRef]
- Alberti, M.N.; Orfanopoulos, M. Unravelling the mechanism of the singlet oxygen ene reaction: Recent computational and experi-mental approaches. Chem. Eur. J. 2010, 16, 9414–9421. [Google Scholar] [CrossRef] [PubMed]
- Bayer, P.; Pérez-Ruiz, R.; Von Wangelin, A.J. Stereoselective Photooxidations by the Schenck Ene Reaction. ChemPhotoChem 2018, 2, 559–570. [Google Scholar] [CrossRef]
- Khayyat, S.A.; Roselin, L.S. Recent progress in photochemical reaction on main components of some essential oils. J. Saudi Chem. Soc. 2018, 22, 855–875. [Google Scholar] [CrossRef]
- Goodine, T.; Oelgemöller, M. Corymbia citriodora: A Valuable Resource from Australian Flora for the Production of Fragrances, Repellents, and Bioactive Compounds. ChemBioEng Rev. 2020, 7, 170–192. [Google Scholar] [CrossRef]
- Schenck, O.; Ziegler, K. Die Synthese des Ascaridols. Naturwissenschaften 1944, 32, 157. [Google Scholar] [CrossRef]
- Schenck, G.O. Autoxydation von Furan und anderen Dienen (Die Synthese des Ascaridols). Ang. Chem. 1944, 57, 101–102. [Google Scholar] [CrossRef]
- Schenck, G.; Schulze-Buschoff, H. Synthetisches Askaridol, eine neue Möglichkeit der Spulwurmbehandlung. DMW—Dtsch. Med. Wochenschr. 1948, 73, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Karapire, C.; Kolancilar, H.; Oyman, Ü.; Icli, S. Fluorescence emission and photooxidation studies with 5,6- and 6,7-benzocoumarins and a 5,6-benzochromone under direct and concentrated sun light. J. Photochem. Photobiol. Chem. 2002, 153, 173–184. [Google Scholar] [CrossRef]
- Dindar, B.; Içli, S. Unusual photoreactivity of zinc oxide irradiated by concentrated sunlight. J. Photochem. Photobiol. Chem. 2001, 140, 263–268. [Google Scholar] [CrossRef]
- Karapire, C.; Kuś, M.; Turkmen, G.; Trevithick-Sutton, C.; Foote, C.; Icli, S. Photooxidation studies with perylenediimides in solution, PVC and sol–gel thin films under concentrated sun light. Sol. Energy 2005, 78, 5–17. [Google Scholar] [CrossRef]
- Avcibasi, N.; Icli, S.; Gilbert, A. Photochemical reactions of α-terpinene and acenaphthene under concentrated sunlight. Turk. J. Chem. 2003, 27, 1–7. [Google Scholar]
- Ohloff, G.; Klein, E.; Schenck, G.O. Darstellung von “Rosenoxyden” und anderen Hydropyran-Derivaten über Photohydroper-oxyde. Angew. Chem. 1961, 73, 578. [Google Scholar] [CrossRef]
- Bicas, J.L.; Dionísio, A.P.; Pastore, G.M. Bio-oxidation of Terpenes: An Approach for the Flavor Industry. Chem. Rev. 2009, 109, 4518–4531. [Google Scholar] [CrossRef]
- Pickenhagen, W.; Schatkowski, D. Verfahren zur Herstellung von Rosenoxid. DE19645922A1, 14 February 2002. [Google Scholar]
- Demuth, M.; Ritter, A. Photochemical and Thermochemical Solar Syntheses Using Flat-Bed Solar Collectors/Solar Reactors. WO99/54042A1, 28 October 1999. [Google Scholar]
- Ritterskamp, P.; Hülsdünker, A.; Heimann, F.; Goeller, F.; Uzun, D.; Ritter, A.; Demuth, M.; Kleinwächter, J.; Ortner, J.; Funken, K.-H.; et al. Synthesis of biologically active compounds with sunlight and technological developments. In Solare Chemie und Solare Materialforschung; AG-Solar NRW: Jülich, Germany, 2002; Chapter 2.2.1; pp. 1–6. ISBN 3893363068. [Google Scholar]
- Oelgemöller, M.; Jung, C.; Ortner, J.; Mattay, J.; Zimmermann, E.; Oelgemoeller, M. Green photochemistry: Solar photooxygenations with medium concentrated sunlight. Green Chem. 2004, 7, 35–38. [Google Scholar] [CrossRef]
- Ortner, J.; Faust, D.; Funken, K.-H.; Lindner, T.; Schulat, J.; Stojanoff, C.G.; Fröning, P. New developments using holographic concentration in solar photochemical reactors. J. Phys. Colloq. 1999, 9, Pr3-379–Pr3-383. [Google Scholar] [CrossRef]
- Stojanoff, C.G.; Schulat, J.; Fröning, P. Development and fabrication of holographic concentrators for solar chemistry. In Solare Chemie und Solare Materialforschung; AG-Solar NRW: Jülich, Germany, 2002; Chapter 6.4; pp. 1–10. ISBN 3893363068. [Google Scholar]
- Funken, K.-H.; Pohlmann, B.; Ortner, J.; Faust, D. Verfahren zur Oxidation ungesättigter Kohlenwasserstoffe. DE19923071A1, 21 August 2003. [Google Scholar]
- Monniere, N.; Ortner, J. Economic evaluation of the industrial photosynthesis of rose oxide via lamp or solar operated photooxidation of citronellol. J. Sol. Energy Eng. 2001, 123, 171–174. [Google Scholar] [CrossRef]
- Dincalp, H.; Icli, S. Photosynthesis of rose oxide by concentrated sunlight in the absence of singlet oxygen. J. Photochem. Photobiol. Chem. 2001, 141, 147–151. [Google Scholar] [CrossRef]
- Schenck, G.O.; Eggert, H.; Denk, W. Über die Bildung von Hydroperoxyden bei photosensibilisierten Reaktionen von O2 mit ge-eigneten Akzeptoren, insbesondere mit α- und β-Pinen. Liebigs Ann. Chem. 1953, 584, 177–198. [Google Scholar] [CrossRef]
- Bhatia, S.; McGinty, D.; Letizia, C.; Api, A. Fragrance material review on myrtenol. Food Chem. Toxicol. 2008, 46, S237–S240. [Google Scholar] [CrossRef] [PubMed]
- Mihelich, E.D.; Eickhoff, D.J. A one-pot conversion of olefins to. alpha.,. beta.-unsaturated carbonyl compounds. An easy synthesis of 2-cyclopentenone and related compounds. J. Org. Chem. 1983, 48, 4135–4137. [Google Scholar] [CrossRef]
- Jung, C.; Ortner, J.; Jarolimek, U.; Mauermann, P.; Leyen, B. Advances in photochemical production of fine chemicals with con-centrated sunlight. In Solare Chemie und Solare Materialforschung; AG-Solar NRW: Jülich, Germany, 2002; Chapter 2.2.1; pp. 1–10. ISBN 3893363068. [Google Scholar]
- Neckers, D.C. Rose Bengal. J. Photochem. Photobiol. Chem. 1989, 47, 1–29. [Google Scholar] [CrossRef]
- Esser, P. Die Anwendung von Sonnenlicht zur Photochemischen Producktion von Feinchemikalien. Ph.D. Thesis, Rheinisch-Westfälische Technische Hochschule, Aachen, Germany, 1994. [Google Scholar]
- Funken, K.-H.; Schneider, G.; Esser, E.P.; Scharf, H.-D.; Esser, P.; Wöhrle, I. The SOLARIS-experiment: Demonstration of solar-photochemical syntheses of fine chemicals. In Proceedings of the 6th International Symposium on Solar Thermal Concentrating Technologies, Mojacar, Spain, 28 September–2 October 1992; Centro de Investigaciones Energétical, Medioambientales y Tecnológias (CIEMAT): Madrid, Spain, 1992; Volume 2, pp. 1027–1037. [Google Scholar]
- Klein, E.; Rojahn, W. Die photosensibilisierte O2-Übertragung auf (+)-α-Thujen. Chem. Ber. 1965, 98, 3045–3049. [Google Scholar] [CrossRef]
- Baeckström, P.; Koutek, B.; Šaman, D.; Vrkoč, J. A convenient synthesis of trans-sabinene hydrate from (−)-3-thujol via a highly selective ene reaction of singlet oxygen. Bioorg. Med. Chem. 1996, 4, 419–421. [Google Scholar] [CrossRef]
- Scharf, H.-D.; Esser, P.; Kuhn, W.; Pelzer, R. Process for the Photooxidation of Terpene Olefins. US5620569A, 15 April 1997. [Google Scholar]
- Schenck, G.O. Über die unsensibilisierte und photosensibilisierte Autoxydation von Furanen. Liebigs Ann. Chem. 1953, 584, 156–176. [Google Scholar] [CrossRef]
- Schenck, G.O. Verfahren zur Herstellung von β-Acyl-acrylsäuren bzw. ihren Pseudoestern. DE875650C, 4 May 1953. [Google Scholar]
- Bolz, G.; Wiersdorff, W.-W. Verfahren zur Herstellung von 2-Hydroxy-2,5-dihydrofuranon-(5). DE2111119A1, 14 September 1972. [Google Scholar]
- Hoydonckx, H.E.; Van Rhijn, W.M.; De Vos, D.E.; A Jacobs, P. Furfural and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2007; Volume 16, pp. 285–313. [Google Scholar]
- Esser, P.; Pelzer, R.; Völkl, F. Verwendung substituierter Lactone als Riechstoffe. EP0761808A2, 19 May 1999. [Google Scholar]
- Strugstad, M.P.; Despotovski, S. A summary of extraction, synthesis, properties, and potential uses of juglone: A literature review. J. Ecosyst. Manag. 2012, 13, 1–16. [Google Scholar]
- Griffiths, J.; Chu, K.-Y.; Hawkins, C. Photosensitised oxidation of 1-naphthols. J. Chem. Soc. Chem. Commun. 1976, 676–677. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Healy, N.; De Oliveira, L.; Jung, C.; Mattay, J. Green photochemistry: Solar-chemical synthesis of Juglone with medium concentrated sunlight. Green Chem. 2006, 8, 831–834. [Google Scholar] [CrossRef]
- Suchard, O.; Kane, R.; Roe, B.J.; Zimmermann, E.; Jung, C.; Waske, P.A.; Mattay, J.; Oelgemöller, M. Photooxygenations of 1-naphthols: An environmentally friendly access to 1,4-naphthoquinones. Tetrahedron 2006, 62, 1467–1473. [Google Scholar] [CrossRef]
- Dincalp, H.; Icli, S. Photoinduced electron transfer-catalyzed processes of sulfoamine perylene diimide under concentrated sun light. Sol. Energy 2006, 80, 332–346. [Google Scholar] [CrossRef]
- Icli, S. Production of oxygenated rosin emulsifier by a solar photoorganic chemical method. For. Chem. Rev. 1999, 102, 7–9. [Google Scholar]
- Icli, S.; Bulut, A.; Gül, Y. Room temperature generation of dehydrogenated colophony: Solar chemical production. Turk. J. Chem. 1992, 16, 289–292. [Google Scholar]
- Erten, S.; Alp, S.; Icli, S. Photooxidation quantum yield efficiencies of naphthalene diimides under concentrated sun light in com-parisons with perylene diimides. J. Photochem. Photobiol. Chem. 2005, 175, 214–220. [Google Scholar] [CrossRef]
- Funken, K.-H.; Ortner, J.; Riffelmann, K.-J.; Sattler, C. New developments in solar photochemistry. J. Inf. Rec. 1998, 24, 61–68. [Google Scholar]
- Tirronen, E.; Salmi, T. Process development in the fine chemical industry. Chem. Eng. J. 2003, 91, 103–114. [Google Scholar] [CrossRef]
- Bruggink, A. Growth and efficiency in the (fine) chemical industry. Chim. Oggi 1998, 16, 44–47. [Google Scholar]
- Pollak, P.; Vouillamoz, R. Fine Chemicals. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000; pp. 1–17. [Google Scholar]
- Landgraf, S. Application of semiconductor light sources for investigations of photochemical reactions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 2029–2048. [Google Scholar] [CrossRef]
- Kalyani, N.T.; Dhoble, S. Organic light emitting diodes: Energy saving lighting technology—A review. Renew. Sustain. Energy Rev. 2012, 16, 2696–2723. [Google Scholar] [CrossRef]
- Monteiro, R.A.R.; Rodrigues-Silva, C.; Lopes, F.V.S.; Silva, A.M.T.; Boaventura, R.A.R.; Vilar, V.J.P. Evaluation of a solar/UV an-nular pilot scale reactor for 24 h continuous photocatalytic oxidation of n-decane. Chem. Eng. J. 2015, 280, 409–416. [Google Scholar] [CrossRef]
- Navntoft, C.; Araujo, P.; Litter, M.I.; Apella, M.C.; Fernández, D.; Puchulu, M.E.; Hidalgo, M.D.V.; Blesa, M.A. Field Tests of the Solar Water Detoxification SOLWATER Reactor in Los Pereyra, Tucumán, Argentina. J. Sol. Energy Eng. 2006, 129, 127–134. [Google Scholar] [CrossRef]
- Bolte, M.; Klaeden, K.; Beqiraj, A.; Oelgemöller, M. Photochemistry Down under—Solar chemicals from and for the Tropics. Eur. Photochem. Assoc. Newslett. 2013, 83–84, 79–83. [Google Scholar]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Horikoshi, S.; Nishimura, T.; Tsutsumi, H.; Serpone, N. Microwave Discharge Electrodeless Lamps. Part VIII: Continuous On-Site Solar Energy Remediation of Contaminated Water. Chem. Eng. Technol. 2015, 39, 102–107. [Google Scholar] [CrossRef]
- Lui, G.Y.; Roser, D.; Corkish, R.; Ashbolt, N.; Jagals, P.; Stuetz, R. Photovoltaic powered ultraviolet and visible light-emitting diodes for sustainable point-of-use disinfection of drinking waters. Sci. Total Environ. 2014, 493, 185–196. [Google Scholar] [CrossRef]
- Vassilikogiannakis, G. Singlet Oxygen and Dyes: Synthesis with Visible Light is Where the Future Lies. ChemPhotoChem 2020, 4, 385–387. [Google Scholar] [CrossRef]
- Montagnon, T.; Kalaitzakis, D.; Triantafyllakis, M.; Stratakis, M.; Vassilikogiannakis, G. Furans and singlet oxygen—why there is more to come from this powerful partnership. Chem. Commun. 2014, 50, 15480–15498. [Google Scholar] [CrossRef]
- Dinda, M.; Maiti, S.; Samanta, S.; Ghosh, P.K. Illustrations of Efficient Solar Driven Organic Reactions. Curr. Org. Synth. 2016, 13, 372–384. [Google Scholar] [CrossRef]
- Li, P.; Terrett, J.A.; Zbieg, J.R. Visible-Light Photocatalysis as an Enabling Technology for Drug Discovery: A Paradigm Shift for Chemical Reactivity. ACS Med. Chem. Lett. 2020, 11, 2120–2130. [Google Scholar] [CrossRef]
- McAtee, R.C.; McClain, E.J.; Stephenson, C.R. Illuminating Photoredox Catalysis. Trends Chem. 2019, 1, 111–125. [Google Scholar] [CrossRef]
- Douglas, J.J.; Sevrin, M.J.; Stephenson, C.R.J. Visible Light Photocatalysis: Applications and New Disconnections in the Synthesis of Pharmaceutical Agents. Org. Process. Res. Dev. 2016, 20, 1134–1147. [Google Scholar] [CrossRef]
- Nauth, A.M.; Lipp, A.; Lipp, B.; Opatz, T. Sunflow: Sunlight drives fast and green photochemical flow reactions in simple micro-capillary reactors—application to photoredox and H-atom-transfer chemistry. Eur. J. Org. Chem. 2017, 2017, 2099–2103. [Google Scholar] [CrossRef]
- Yaseen, M.A.; Mumtaz, S.; Hunter, R.L.; Wall, D.; Robertson, M.J.; Oelgemöller, M. Continuous-flow photochemical transformations of 1,4-naphthoquinones and phthalimides in a concentrating solar trough reactor. Aust. J. Chem. 2020, 73, 1149–1157. [Google Scholar] [CrossRef]
- Yaseen, M.A.; Mumtaz, S.; Hunter, R.L.; Wall, D.; Robertson, M.J.; Oelgemöller, M. Corrigendum to: Continuous-flow photochemical transformations of 1,4-naphthoquinones and phthalimides in a concentrating solar trough reactor. Aust. J. Chem. 2020, 73, 1301. [Google Scholar] [CrossRef]
- Papakonstantinou, I.; Portnoi, M.; Debije, M.G. The hidden potential of luminescent solar concentrators. Adv. Energy Mater. 2020, 11. [Google Scholar] [CrossRef]
- Poliakoff, M.; George, M.W. Manufacturing chemicals with light: Any role in the circular economy? Phil. Trans. R. Soc. A 2020, 378, #20190260. [Google Scholar] [CrossRef] [PubMed]
- Bochet, C.G. On the Sustainability of Photochemical Reactions. Chim. Int. J. Chem. 2019, 73, 720–723. [Google Scholar] [CrossRef]
- Michelin, C.; Hoffmann, N. Photocatalysis applied to organic synthesis—A green chemistry approach. Curr. Opin. Green Sustain. Chem. 2018, 10, 40–45. [Google Scholar] [CrossRef]
- Oelgemöller, M.; Oelgemoeller, M. Green Photochemical Processes and Technologies for Research & Development, Scale-up and Chemical Production. J. Chin. Chem. Soc. 2014, 61, 743–748. [Google Scholar] [CrossRef]
- Ciana, C.-L.; Bochet, C.G. Clean and Easy Photochemistry. Chim. Int. J. Chem. 2007, 61, 650–654. [Google Scholar] [CrossRef]
- Albini, A.; Fagnoni, M.; Mella, M. Environment-friendly organic synthesis. The photochemical approach. Pure Appl. Chem. 2000, 72, 1321–1326. [Google Scholar] [CrossRef]
- Ciriminna, R.; Delisi, R.; Xu, Y.-J.; Pagliaro, M. Toward the waste-free synthesis of fine chemicals with visible light. Org. Process Res. Dev. 2016, 20, 403–408. [Google Scholar] [CrossRef]
- Oelgemöller, M. The sunny side of chemistry at James Cook Uni. Chemistry in Australia, November 2014; 7. [Google Scholar]




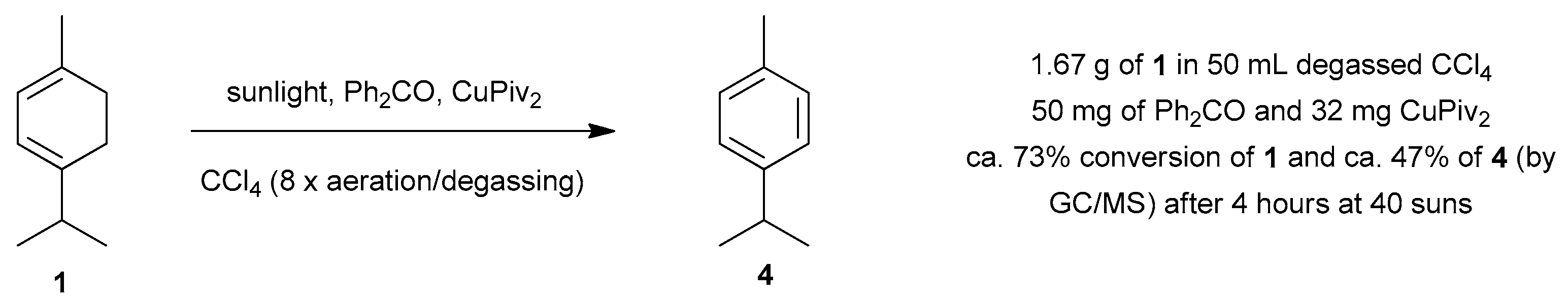


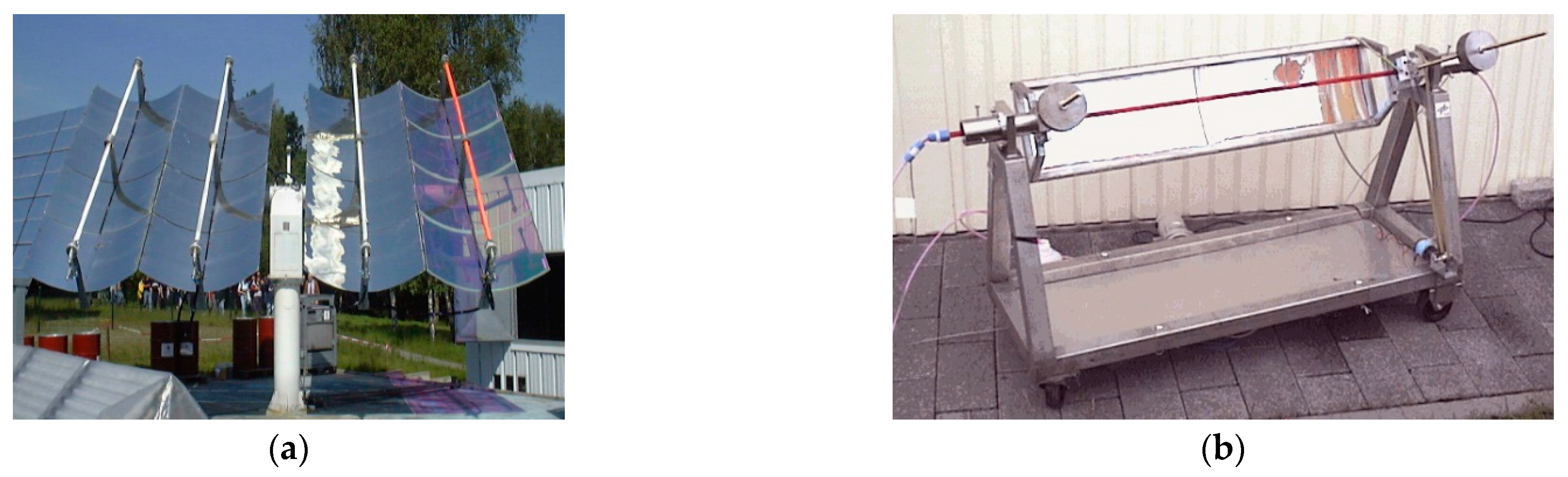


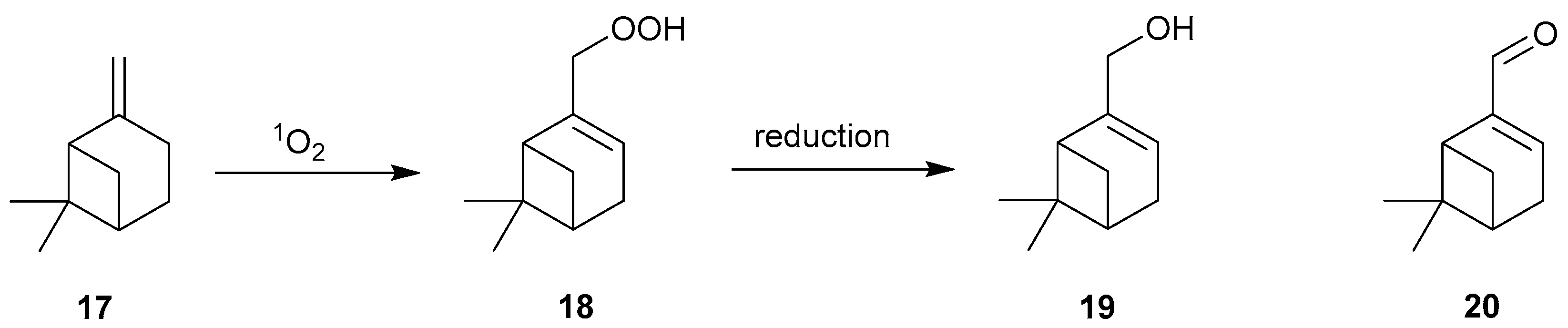

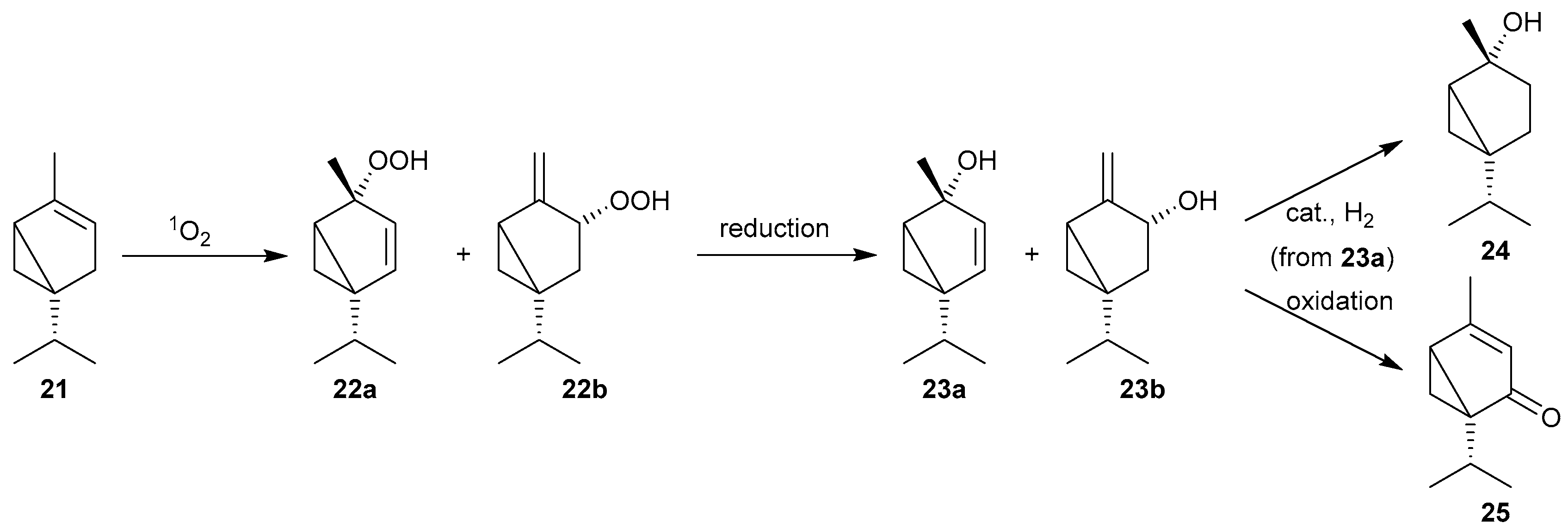





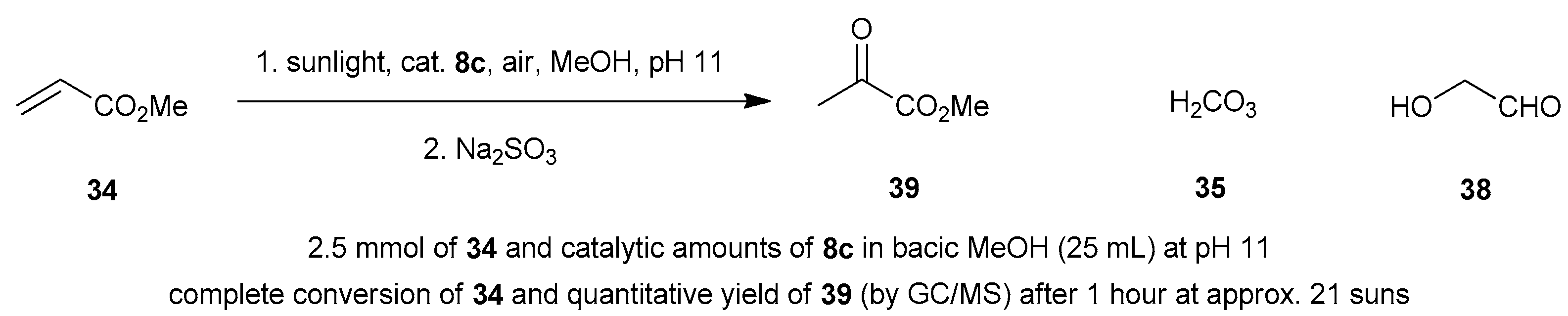
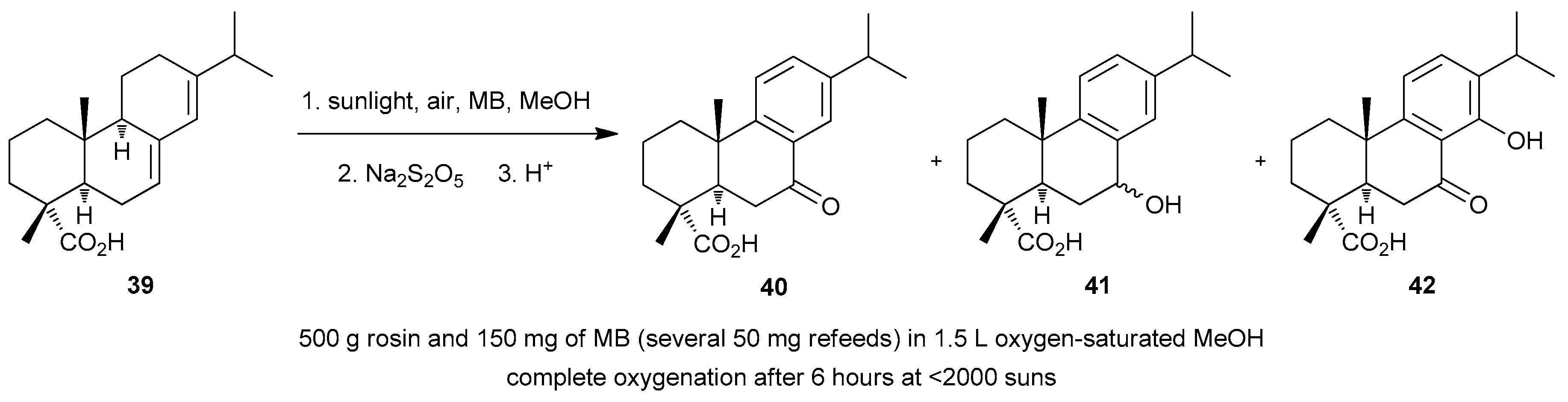


| Reaction 1 [89] | Reaction 2 [89] | Reaction 3 [90] | Reaction 4 [90] | |
|---|---|---|---|---|
| citronellol (12) | 0.5 L (2.7 mol) | 0.2 L (1.1 mol) | 0.1 L (0.55 mol) | 0.1 L (0.55 mol) |
| methanol | 400 mL | 1 L | 2 L | 2 L |
| rose bengal 1 | 5 × 6 g | 3 g + 1 g | 5 g | 5 g |
| reactor | batch (1 m2) | in series (1 m2) | batch (1 m2) | batch (1 m2) |
| operation | static | flow (2 L/h) | static | static |
| time | 3 days | 11 h | 11 h | 7 h |
| solar conditions | 50% cloudy, 50% sunny | 70% cloudy; 30% sunny | 70% cloudy; 30% sunny | 100% sunny |
| yield of 15 2 | 50% (1.36 mol) | 52% (0.57 mol) | not reported 3 | not reported 3 |
| Reaction 5 [91] | Reaction 6 [91] | Reaction 7 [94] | Reaction 8 [94] | |
|---|---|---|---|---|
| citronellol (12) | 5.8 L (31.8 mol) | 8 L (43.9 mol) | 5.8 L (31.8 mol) | 5.8 L (31.8 mol) |
| isopropanol | 40 L | 72 L | 40 L | 40 L |
| rose bengal | 20 g 1 | 36 g 1 | 2 g | 2 g |
| O2-flow | 600 L/h | 200 L/h | not reported | not reported |
| # of troughs | 1 (8 m2) | 4 (32 m2) | 1 (8 m2) 2 | 1 (8 m2) |
| time 3 | 3 h | 2¼ h | 2⅓ h | 2¼ h |
| solar conditions | largely sunny | sunny | sunny | sunny |
| conversion of 12 4 | >95% | >95% (55% 5) | >95% | >95% |
| Reactions 9 | Reactions 10 | Reactions 11 | Reactions 12 | Reactions 13 | |
|---|---|---|---|---|---|
| reactor | PROPHIS | CPC | flatbed | horizontal tubes | vertical tubes |
| CF | 32 suns | 1 sun | 1 sun | 1 sun | 1 sun |
| aperture | 32 m2 | 3 m2 | 1.5 m2 | not given | not given |
| volume | 80 L (37 L 1) | 40 L (26 L 1) | 21 L 1 | 25 L (11 L 1) | 20 L (10 L 1) |
| operation | circulation | circulation | static | circulation | circulation |
| mixing | static mixer | 7 × 180° turns | air bubbling | 9 × 180° turns | air bubbling |
| citronellol (12) | 8 L (43.9 mol) | 4 L (22 mol) | 2.1 L (11.5 mol) | 2.5 L (13.7 mol) | 2 L (11 mol) |
| isopropanol | 72 L | 36 L | 18.9 L | 22.5 L | 18 L |
| rose bengal 2 | 40 g | 20 g | 10.5 g | 12.5 g | 10 g |
| O2 source | oxygen | air | air | air | air |
| time (sunny/cloudy) 4 | 2½ h 3/not operated | 7 h/30 h | 15 h/30 h | 12 h/33 h | 13 h/33 h |
| conversion of 12 5 | >95% 3/not operated | >95%/>95% | >95%/>95% | >95%/>65% | >95%/>65% |
| Reaction 14 | Reaction 15 | Reaction 16 | Reaction 17 | |
|---|---|---|---|---|
| sensitizer | ABIPER (8a) | BUNAP (16) | ABIPER (8a) | ABIPER (8a) |
| additive | none | none | CuPiv2 | FeMyr3 |
| exposure time 1 | 2 h | 1 h | 2 h | 2 h |
| residual 12 2 | 3% | 1% | 81% | 36% |
| 14a/14b2 | 6% | 20% | 2% | 5% |
| 15 (cis/trans 3) 2 | 76%/6% | 54%/23% | 11%/5% | 53%/6% |
| Reaction 18 | Reaction 19 [100] | Reaction 20 [102] | |
|---|---|---|---|
| reactor | CPC | DLR solar furnace | SOLARIS |
| CF | 1 sun | up to 4,800 suns | 20 suns (42 suns 1) |
| volume | 40 L (26 L 2) | 5.1 L (2.03 L 2) | 70 L (30 L 2) |
| β-pinene (17) | 2 L (12.8 mol) | 0.29 L (1.84 mol) | 6.2 L (37.7 mol) |
| solvent | 38 L (iPrOH) | 3.5 L (EtOH) | 35 L (iPrOH) |
| rose bengal | not reported | 40 g 3 | 21.77 g 3 |
| exposure time | 9 days | 14 h | 3 days (ca. 27 h) |
| conversion of 17 4 | 95% | 97% | ca. 56% |
| Reaction 21 | Reaction 22 | Reaction 23 | Reaction 24 | Reaction 25 | Reaction 26 | Reaction 27 | |
|---|---|---|---|---|---|---|---|
| α-thujene (21) 1 | 2.7 kg (17.2 mol) | 5.42 kg (34.5 mol) | 5.45 kg (34.6 mol) | ~3.3 kg (20.9 mol) | 5.63 kg (35.8 mol) | 5.64 kg (35.9 mol) | 5.63 kg (35.8 mol) |
| iPrOH | 44 L | 38 L | 35 L | ~32 L | 35 L | 35 L | 35 L |
| sensitizer 2 | 5.26 g (MB) | 9.61 g (MB) | 11.11 g (MB) | 12.29 g (MB) | 12.82 g (RB) | 9.02 g (RB) | 6.95 g (RB) |
| time 3 | ~7 h (1 day) | ~11 h (2 days) | 7 h (1 day) | ~7 h (1 day) | 6 h (1 day) | 5½ h (1 day) | 4½ h (1 day) |
| total radiation 4 | 16.5 kWh (110 mol) | 37.9 kWh (271 mol) | 40.8 kWh (287 mol) | 43.0 kWh (306 mol) | 29.9 kWh (123 mol) | 27.5 kWh (113 mol) | 24.2 kWh (106 mol) |
| ηs 5 | 14% | 12% | 12% | 7% | 29% | 31% | 33% |
| conversion 6 | 88% | 97% | 96% | 97% | ~100% | 99% | 98% |
| 217 | 12% | 2% | 3% | 2% | <1% | 1% | 1% |
| 23a7 | 61% | 56% | 63% | 66% | 65% | 67% | 61% |
| 23b7 | 7% | 11% | 11% | 10% | 10% | 11% | 13% |
| selectivity 8 | 82% | 67% | 75% | 78% | 75% | 78% | 72% |
| Reaction 28 | Reaction 29 | Reaction 30 | Reaction 31 | Reaction 32 | |
|---|---|---|---|---|---|
| furane | 2.06 kg 21.5 mol (26) | 2.14 kg 22.3 mol (26) | 4.32 kg 45.0 mol (26) | 3.61 kg 37.6 mol (26) | 3.61 kg 21.2 mol (29) |
| ethanol | 35 L | 35 | 30 | 35 | 35 |
| sensitizer 1 | 6.1 g (MB) | 11.2 g (RB) | 5.1 g (MB) | 9.0 g (MB) | 6.0 g (MB) |
| additive | none | none | none | c. HCl (5 mL) | none |
| temperature | 8 °C | 20 °C | 20 °C | 20 °C | 20 °C |
| fluid flow | 35 L/min | 55 L/min | 55 L/min | 45 L/min | 45 L/min |
| time 2 | 16 h (3 days) | ~11 h (2 days) | ~12½ h (2 days) | 5½ h (1 day) | ~3¼ h (1 day) |
| total radiation 3 | 22.2 kWh 140 mol | 54.3 kWh 413 mol | 61.9 kWh 436 mol | 32.4 kWh 226 mol | 16.9 kWh 114 mol |
| ηs 4 | 15% | 5% | 10% | 16% | 18% |
| conversion 5 | 98% | 99% | ~100% | 98% | 95% |
| 27:286 | 42:1 | 133:1 | 58:1 | 98:1 | 390:1 |
| Reaction 33 | Reaction 34 | Reaction 35 | Reaction 36 | Reaction 37 | |
|---|---|---|---|---|---|
| reactor | horizontal trough | horizontal trough | horizontal trough | horizontal trough | vertical trough |
| CF | 15 suns | 15 suns | 15 suns | 15 suns | 18 suns |
| reflector | holographic | holographic | aluminum | aluminum | aluminum |
| diol 30 | 2.0 g | 1.0 g | 2.0 g | 2.0 g | 0.5 g |
| solvent | 200 mL (iPrOH) | 200 mL (iPrOH) | 250 mL (iPrOH) | 250 mL (acetone) | 100 mL (iPrOH) |
| sensitizer | 0.1 g (RB) | 0.1 g (RB) | 0.1 g (RB) | 0.1 g (RB) | 0.05 g (RB) |
| time | 8 h 1 (2 days) | 3 h 1 (2 days) | 4 h 1 (1 day) | 4 h 1 (1 day) | 2/3 h 1 (1 day) |
| conversion 2 | 83% | >95% | 93% | 99% | >95% |
| yield of 31 3 | 54% | 79% | 75% | 79% | 71% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wau, J.S.; Robertson, M.J.; Oelgemöller, M. Solar Photooxygenations for the Manufacturing of Fine Chemicals—Technologies and Applications. Molecules 2021, 26, 1685. https://doi.org/10.3390/molecules26061685
Wau JS, Robertson MJ, Oelgemöller M. Solar Photooxygenations for the Manufacturing of Fine Chemicals—Technologies and Applications. Molecules. 2021; 26(6):1685. https://doi.org/10.3390/molecules26061685
Chicago/Turabian StyleWau, Jayson S., Mark J. Robertson, and Michael Oelgemöller. 2021. "Solar Photooxygenations for the Manufacturing of Fine Chemicals—Technologies and Applications" Molecules 26, no. 6: 1685. https://doi.org/10.3390/molecules26061685
APA StyleWau, J. S., Robertson, M. J., & Oelgemöller, M. (2021). Solar Photooxygenations for the Manufacturing of Fine Chemicals—Technologies and Applications. Molecules, 26(6), 1685. https://doi.org/10.3390/molecules26061685








