The Influence of Metabolic Inhibitors, Antibiotics, and Microgravity on Intact Cell MALDI-TOF Mass Spectra of the Cyanobacterium Synechococcus Sp. UPOC S4
Abstract
1. Introduction
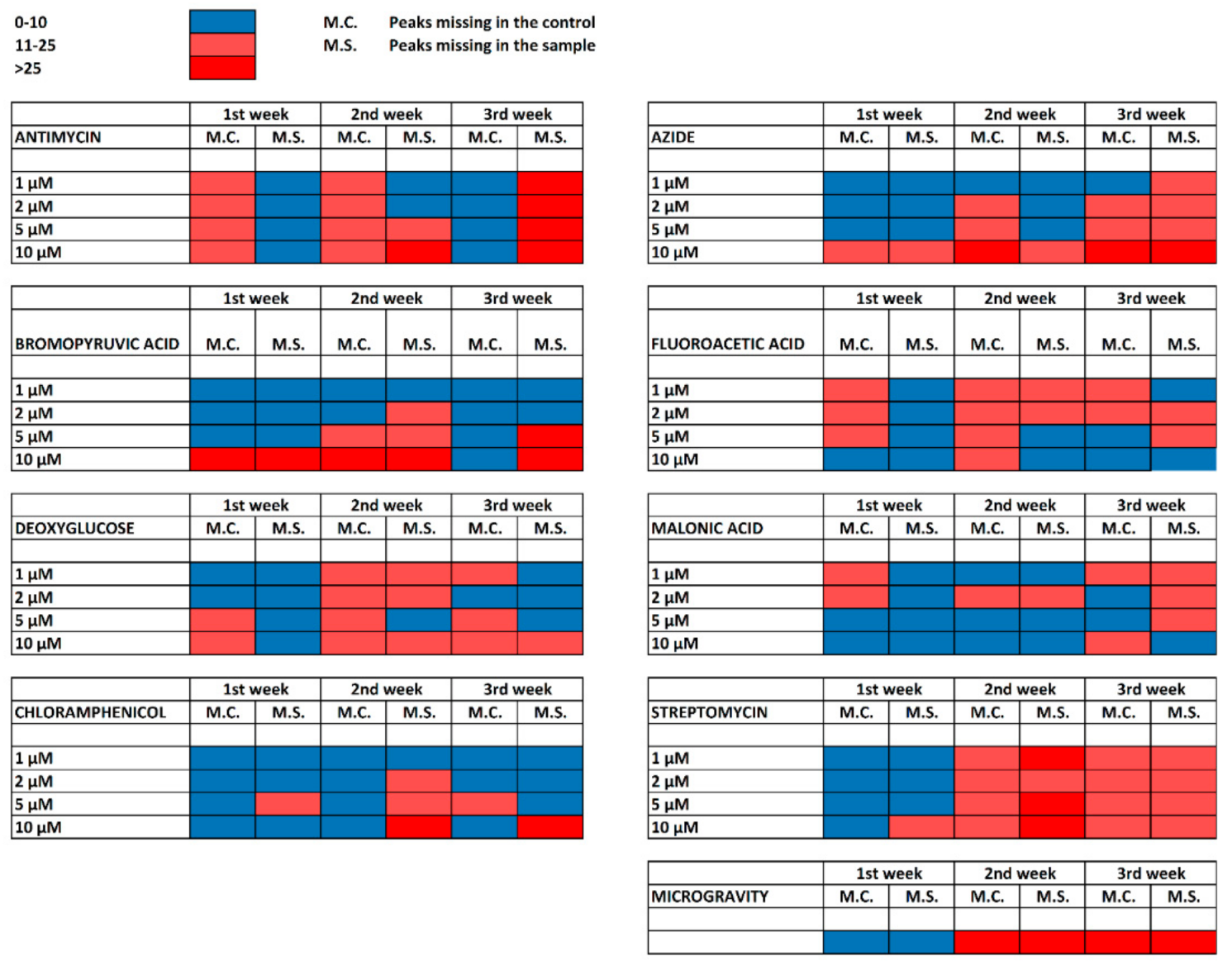
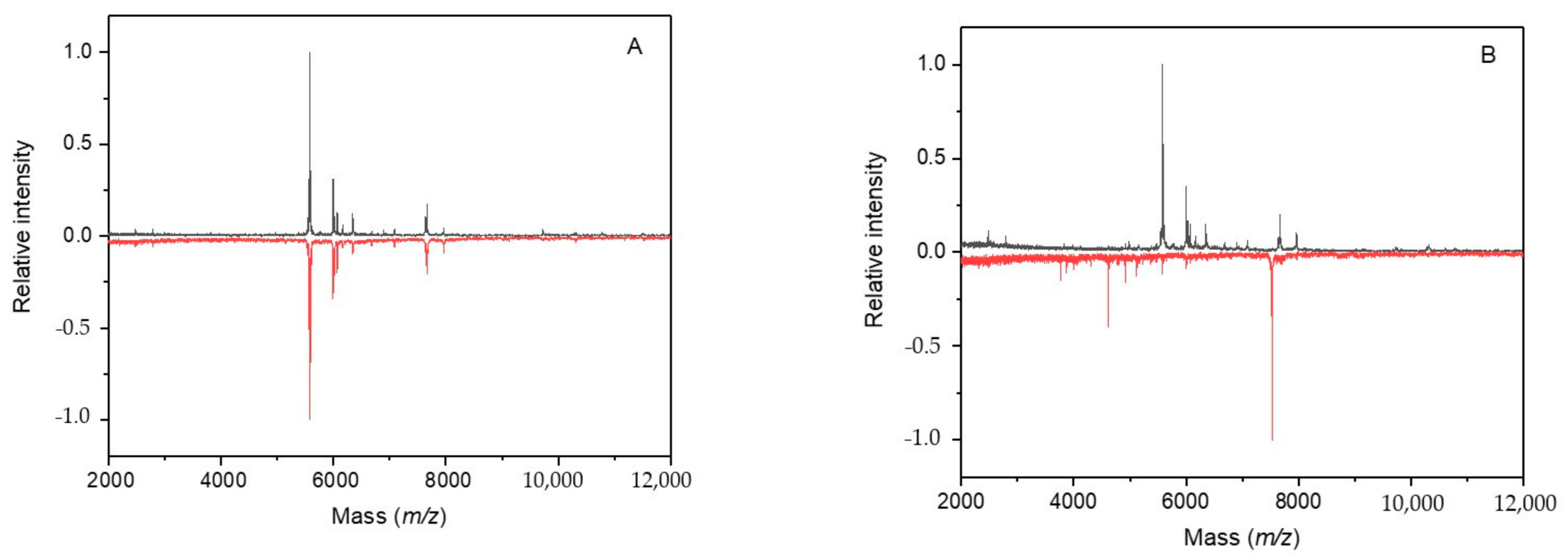
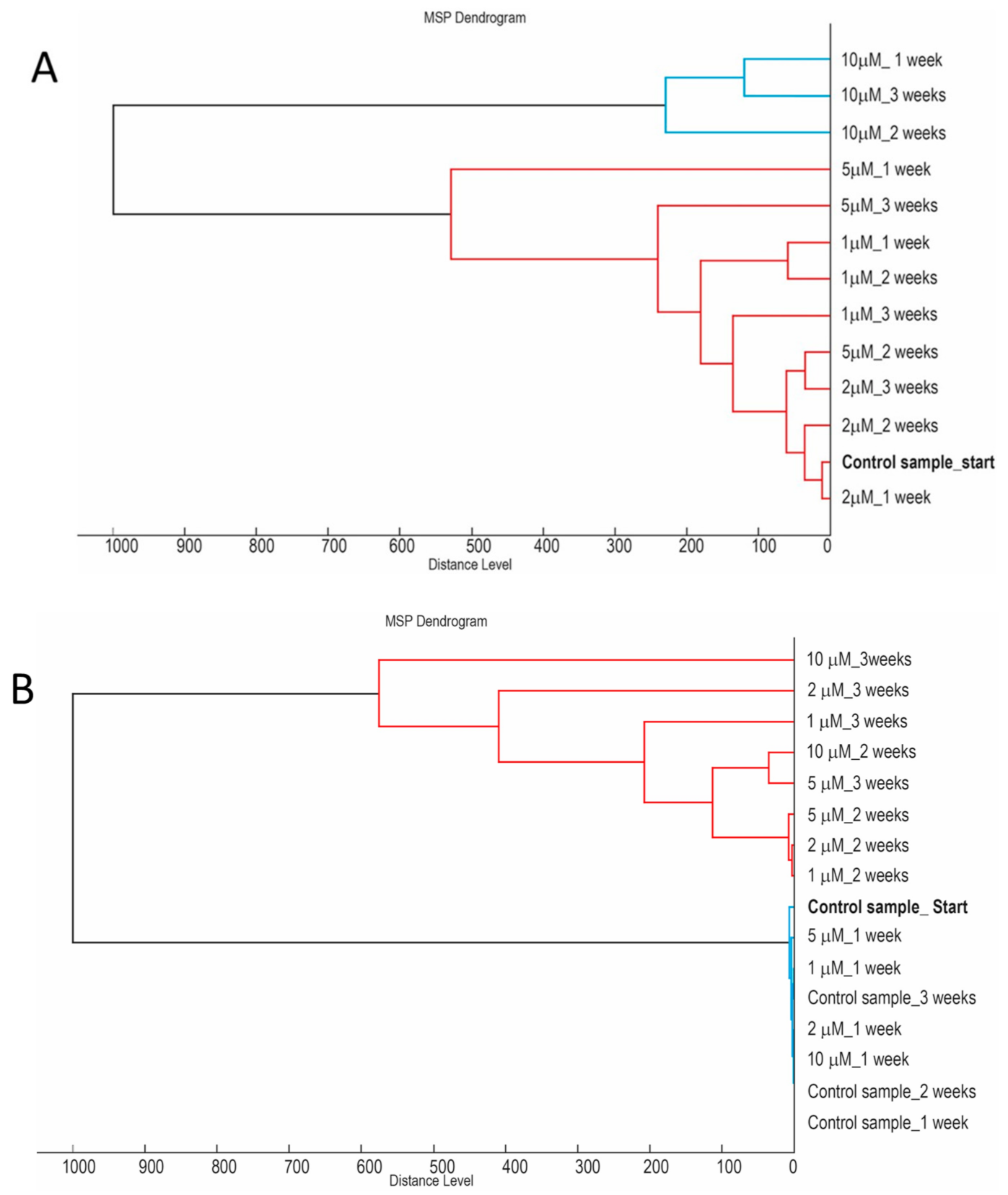
2. Results
2.1. Research Motivation and Implementation
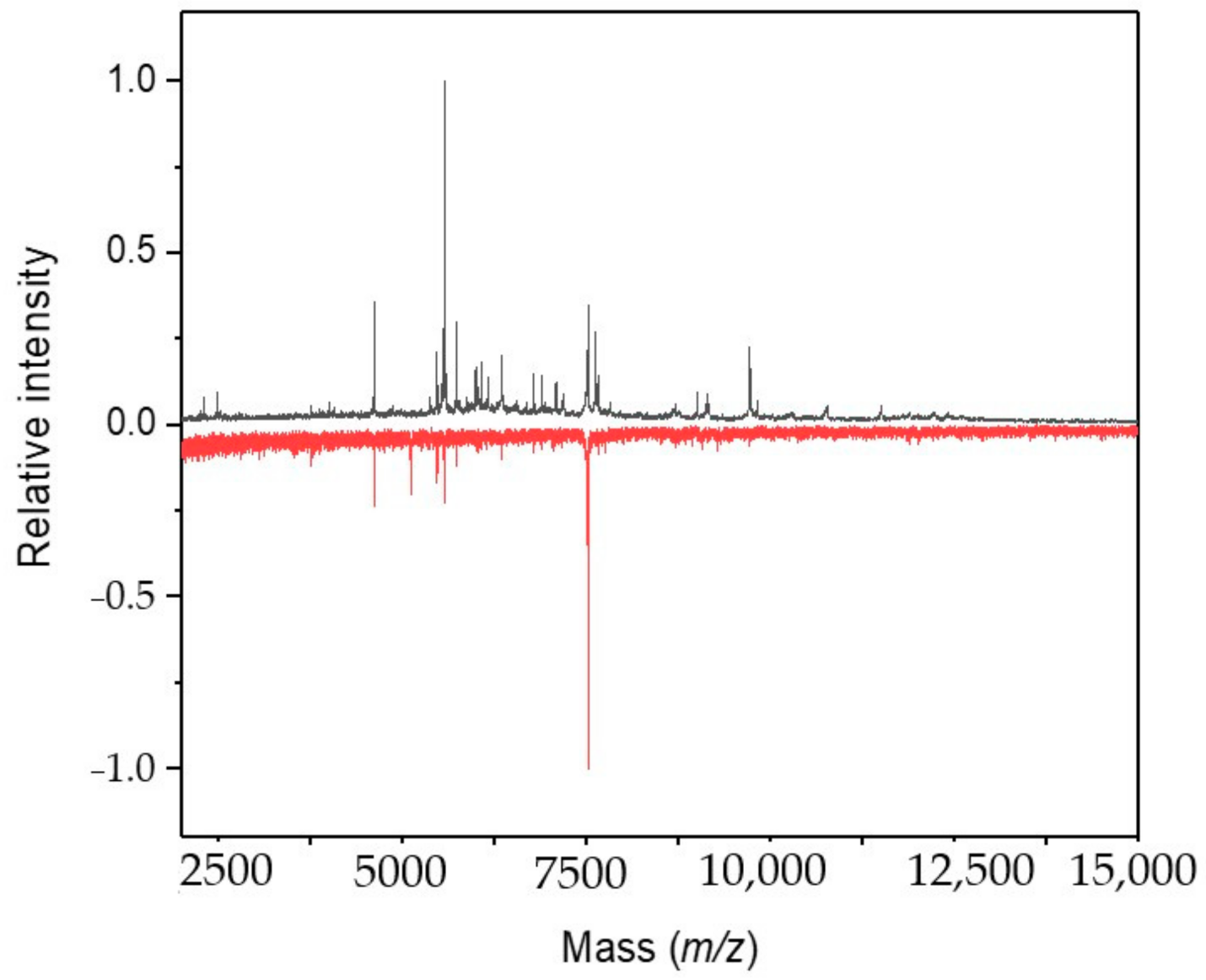
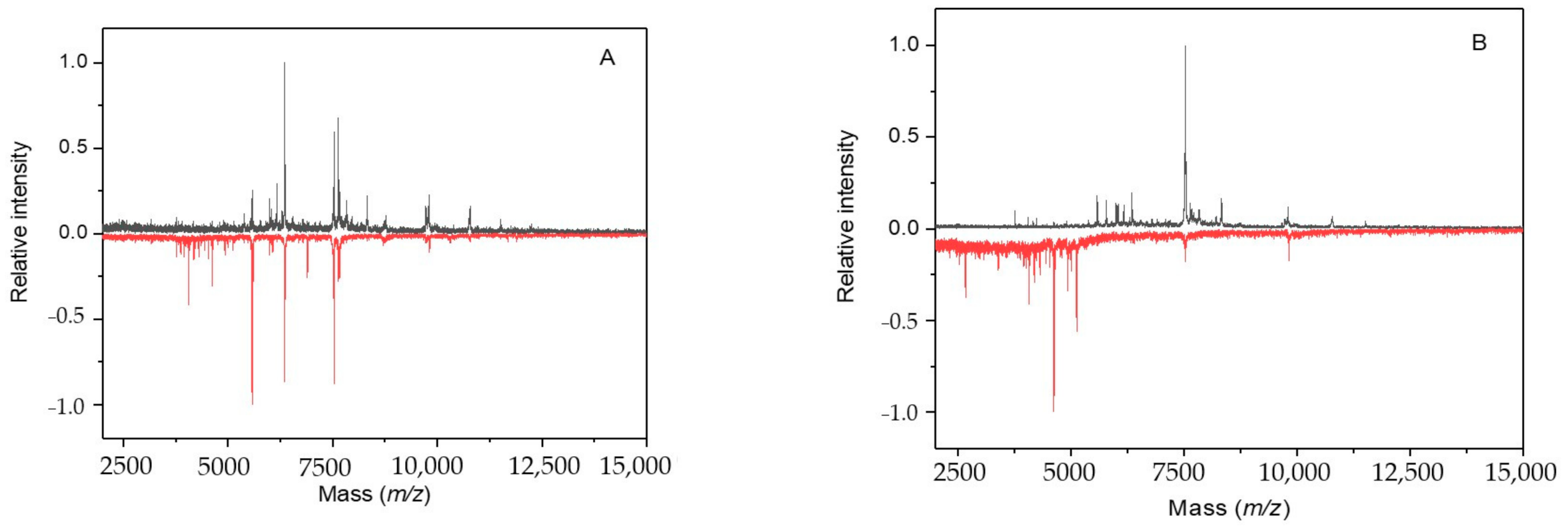
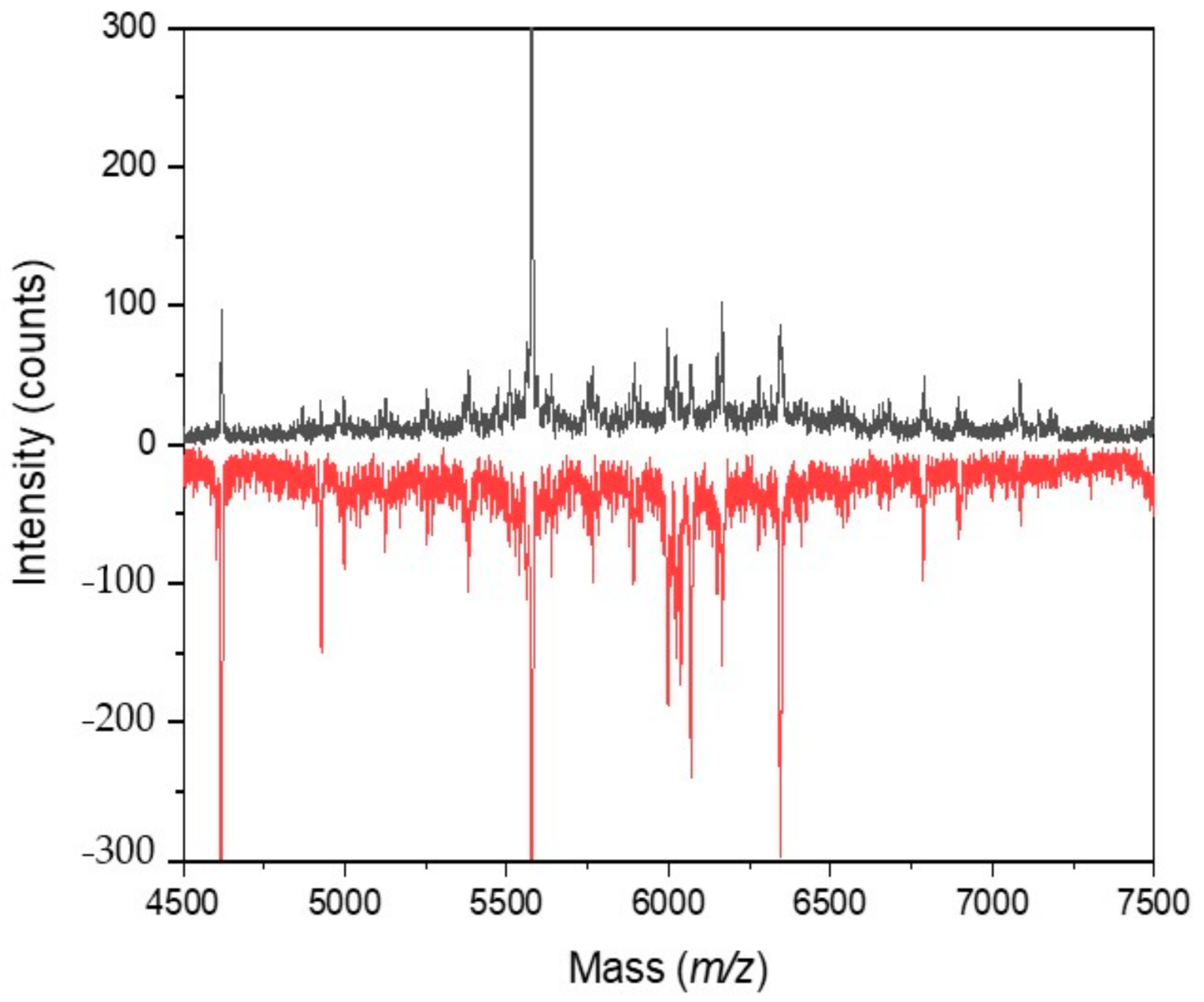
2.2. Cultivation with Antimycin
2.3. Cultivation with Sodium Azide
2.4. Cultivation with 3-Bromopyruvic Acid
2.5. Cultivation with 2-Deoxyglucose
2.6. Cultivation with 2-Fluoroacetic and Malonic Acids
2.7. Cultivation with Chloramphenicol
2.8. Cultivation with Streptomycin
2.9. Cultivation under Microgravity Conditions
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cultivation of Untreated Cyanobacterial Cells
4.3. Cultivation of Treated Cyanobacterial Cells
4.4. Mass Spectrometry
4.5. Data Processing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Palinska, K.A.; Surosz, W. Taxonomy of cyanobacteria: A contribution to consensus approach. Hydrologia 2014, 740, 1–11. [Google Scholar] [CrossRef]
- Clark, A.E.; Kaleta, E.J.; Arora, A.; Wolk, D.M. Matrix-assisted laser desorption ionization–time of flight mass apectrometry: A fundamental shift in the routine practice of clinical microbiology. Clin. Microbiol. Rev. 2013, 26, 547–603. [Google Scholar] [CrossRef] [PubMed]
- Drissner, D.; Freimoser, F.M. MALDI-TOF mass spectroscopy of yeasts and filamentous fungi for research and diagnostics in the agricultural value chain. Chem. Biol. Technol. Agric. 2017, 4, 13. [Google Scholar] [CrossRef][Green Version]
- Tadros, M.; Petrich, A. Evaluation of MALDI-TOF mass spectrometry and Sepsityper Kit™ for the direct identification of organisms from sterile body fluids in a Canadian pediatric hospital. Can. J. Infect. Dis. Med. Microbiol. 2013, 24, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.Y.; Chiang-Ni, C.; Teng, S.H. Current status of MALDI-TOF mass spectrometry in clinical microbiology. J. Food Drug Anal. 2019, 27, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.C.; Kuo, A.J.; Liu, K.L.; Wen, Y.H.; Chia, J.H.; Chang, P.Y.; Lee, M.H.; Wu, T.L.; Chang, S.C.; Lu, J.J. Routine identification of microorganisms by matrix-assisted laser desorption ionization time-of-flight mass spectrometry: Success rate, economic analysis, and clinical outcome. J. Microbiol. Immunol. Infect. 2017, 50, 662–668. [Google Scholar] [CrossRef]
- Khot, P.D.; Fisher, M.A. Novel approach for differentiating Shigella species and Escherichia coli by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 3711–3716. [Google Scholar] [CrossRef]
- Sauget, M.; Valot, B.; Bertrand, X.; Hocquet, D. Can MALDI-TOF mass spectrometry reasonably type bacteria? Trends Microbiol. 2017, 25, 447–455. [Google Scholar] [CrossRef]
- Sun, L.W.; Jiang, W.J.; Sato, H.; Kawachi, M.; Lu, X.W. Rapid classification and identification of Microcystis aeruginosa strains using MALDI–TOF MS and polygenetic analysis. PLoS ONE 2016, 11, e0156275. [Google Scholar] [CrossRef][Green Version]
- Šebela, M.; Jahodářová, E.; Raus, M.; Lenobel, R.; Hašler, P. Intact cell MALDI-TOF mass spectrometric analysis of Chroococcidiopsis cyanobacteria for taxonomic purposes and identification of marker proteins. PLoS ONE 2018, 13, e0208275. [Google Scholar] [CrossRef]
- Peng, X.; Yang, J.; Gao, Y. Proteomic analyses of changes in Synechococcus sp. PCC7942 following UV-C stress. Photochem. Photobiol. 2017, 93, 1073–1080. [Google Scholar] [CrossRef]
- Hongsthong, A.; Sirijuntarut, M.; Prommeenate, P.; Lertladaluck, K.; Porkaew, K.; Cheevadhanarak, S.; Tanticharoen, M. Proteome analysis at the subcellular level of the cyanobacterium Spirulina platensis in response to low-temperature stress conditions. FEMS Microbiol. Lett. 2008, 288, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Kurdrid, P.; Senachak, J.; Sirijuntarut, M.; Yutthanasirikul, R.; Phuengcharoen, P.; Jeamton, W.; Roytrakul, S.; Cheevadhanarak, S.; Hongsthong, A. Comparative analysis of the Spirulina platensis subcellular proteome in response to low-and high-temperature stresses: Uncovering cross-talk of signaling components. Proteome Sci. 2011, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Pandhal, J.; Ow, S.Y.; Wright, P.C.; Biggs, C.A. Comparative proteomics study of salt tolerance between a nonsequenced extremely halotolerant cyanobacterium and its mildly halotolerant relative using in vivo metabolic labeling and in vitro isobaric labeling. J. Proteome Res. 2009, 8, 818–828. [Google Scholar] [CrossRef]
- Yadav, R.K.; Thagela, P.; Tripathi, K.; Abraham, G. Physiological and proteomic analysis of salinity tolerance of the halotolerant cyanobacterium Anabaena sp. World J. Microbiol. Biotechnol. 2016, 32, 147. [Google Scholar] [CrossRef] [PubMed]
- Kurian, D.; Phadwal, K.; Mäenpää, P. Proteomic characterization of acid stress response in Synechocystis sp. PCC 6803. Proteomics 2006, 6, 3614–3624. [Google Scholar] [CrossRef]
- Zhang, L.F.; Yang, H.M.; Cui, S.X.; Hu, J.; Wang, J.; Kuang, T.Y.; Norling, B.; Huang, F. Proteomic analysis of plasma membranes of cyanobacterium Synechocystis sp. strain PCC 6803 in response to high pH stress. J. Proteome Res. 2009, 8, 2892–2902. [Google Scholar] [CrossRef]
- Mehta, A.; López-Maury, L.; Florencio, F.J. Proteomic pattern alterations of the cyanobacterium Synechocystis sp. PCC 6803 in response to cadmium, nickel and cobalt. J. Proteom. 2014, 102, 98–112. [Google Scholar] [CrossRef]
- Babele, P.K.; Kumar, J.; Chaturvedi, V. Proteomic de-regulation in cyanobacteria in response to abiotic stresses. Front. Microbiol. 2019, 10, 1315. [Google Scholar] [CrossRef]
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529. [Google Scholar] [CrossRef]
- Groendahl, S.; Fink, P. High dietary quality of non-toxic cyanobacteria for a benthic grazer and its implications for the control of cyanobacterial biofilms. BMC Ecol. 2017, 17, 20. [Google Scholar] [CrossRef]
- Quiblier, C.; Wood, S.; Echenique-Subiabre, I.; Heath, M.; Villeneuve, A.; Humbert, J.F. A review of current knowledge on toxic benthic freshwater cyanobacteria—Ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar] [CrossRef]
- Asukabe, H.; Akahori, S.; Ueno, E.; Nakayama, T.; Yamashita, R.; Arii, S.; Harada, K.; Imanishi, S.Y. Cyanobacterial classification with the toxicity using MALDI Biotyper. J. Am. Soc. Mass Spectrom. 2020, 31, 1572–1578. [Google Scholar] [CrossRef]
- Cardaci, S.; Desideri, E.; Ciriolo, M.R. Targeting aerobic glycolysis: 3-bromopyruvate as a promising anticancer drug. J. Bioenerg. Biomembr. 2012, 44, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, H.; Lu, W.; Huang, P. Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 553–560. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S.; Geschwind, J.F.H.; Kunjithapatham, R.; Buijs, M.; Vossen, J.A.; Tchernyshyov, I.; Cole, R.N.; Syed, L.H.; Rao, P.R.; Ota, S.; et al. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res. 2009, 29, 4909–4918. [Google Scholar]
- Wick, A.N.; Drury, D.R.; Nakada, H.I.; Wolfe, J.B. Localization of the primary metabolic block produced by 2-deoxyglucose. J. Biol. Chem. 1957, 224, 963–969. [Google Scholar] [CrossRef]
- Ralser, M.; Wamelink, M.M.; Struys, E.A.; Joppich, C.; Krobitsch, S.; Jakobs, C.; Lehrach, H. A catabolic block does not sufficiently explain how 2-deoxy-D-glucose inhibits cell growth. Proc. Natl. Acad. Sci. USA 2008, 105, 17807–17811. [Google Scholar] [CrossRef]
- Pajak, B.; Siwiak, E.; Sołtyka, M.; Priebe, A.; Zieliński, R.; Fokt, I.; Ziemniak, M.; Jaśkiewicz, A.; Borowski, R.; Domoradzki, T.; et al. 2-Deoxy-D-glucose and its analogs: From diagnostic to therapeutic agents. Int. J. Mol. Sci. 2020, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Goncharov, N.V.; Jenkins, R.O.; Radilov, A.S. Toxicology of fluoroacetate: A review, with possible directions for therapy research. J. App. Toxicol. 2006, 26, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Lauble, H.; Kennedy, M.C.; Emptage, M.H.; Beinert, H.; Stout, C.D. The reaction of fluorocitrate with aconitase and the crystal structure of the enzyme inhibitor complex. Proc. Natl Acad. Sci. USA 1996, 93, 13699–13703. [Google Scholar] [CrossRef]
- Potter, V.R.; Elvehjem, C.A. The effect of inhibitors on succinoxidase. J. Biol. Chem. 1937, 117, 341–349. [Google Scholar] [CrossRef]
- Kim, H.; Esser, L.; Hossain, M.B.; Xia, D.; Yu, C.A.; Rizo-Rey, J.; van der Helm, D.; Deisenhofer, J. Structure of antimycin A1, a specific electron transfer inhibitor of ubiquinol−cytochrome c oxidoreductase. J. Am. Chem. Soc. 1999, 121, 4902–4903. [Google Scholar] [CrossRef]
- Joët, T.; Cournac, L.; Horvath, E.M.; Medgyesy, P.; Peltier, G. Increased sensitivity of photosynthesis to antimycin A induced by inactivation of the chloroplast ndhB gene. Evidence for a participation of the NADH-dehydrogenase complex to cyclic electron flow around photosystem I. Plant Physiol. 2001, 125, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Montellano, P.R.; David, S.K.; Ator, M.A.; Tew, D. Mechanism-based inactivation of horseradish peroxidase by sodium azide. Formation of meso-azidoprotoporphyrin IX. Biochemistry 1988, 27, 5470–5476. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Shinzawa-Itoh, K.; Nakashima, R.; Yaono, R.; Yamashita, E.; Inoue, N.; Yao, M.; Fei, M.J.; Libeu, C.P.; Mizushima, T.; et al. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science 1998, 280, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Forti, G.; Gerola, P. Inhibition of photosynthesis by azide and cyanide and the role of oxygen in photosynthesis. Plant Physiol. 1977, 59, 859–862. [Google Scholar] [CrossRef]
- Balbi, H.J. Chloramphenicol: A review. Pediatr. Rev. 2004, 25, 284–288. [Google Scholar] [CrossRef]
- Luzzatto, L.; Apirion, D.; Schlessinger, D. Mechanism of action of streptomycin in E. coli: Interruption of the ribosome cycle at the initiation of protein synthesis. Proc. Natl. Acad. Sci. USA 1968, 60, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Demirci, H.; Murphy, F., IV; Murphy, E.; Gregory, S.T.; Dahlberg, A.E.; Jogl, G. A structural basis for streptomycin-induced misreading of the genetic code. Nat. Commun. 2013, 4, 1355. [Google Scholar] [CrossRef]
- Hoson, T.; Kamisaka, S.; Masuda, Y.; Yamashita, M. Changes in plant growth processes under microgravity conditions simulated by a three-dimensional clinostat. Bot. Mag. Tokyo 1992, 105, 53–70. [Google Scholar] [CrossRef]
- Pietsch, J.; Bauer, J.; Egli, M.; Infanger, M.; Wise, P.; Ulbrich, C.; Grimm, D. The effects of weightlessness on the human organism and mammalian cells. Curr. Mol. Med. 2011, 11, 350–364. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, A.; Parrella, A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Envion. 2005, 346, 87–98. [Google Scholar] [CrossRef]
- van der Grinten, E.; Pikkemaat, M.G.; van den Brandhof, E.J.; Stroomberg, G.J.; Kraak, M.H.S. Comparing the sensitivity of algal, cyanobacterial and bacterial bioassays to different groups of antibiotics. Chemosphere 2010, 80, 1–6. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganés, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernández-Piñas, F. Toxicity of five antibiotics and their mixtures towards photosynthetic aquatic organisms: Implications for environmental risk assessment. Water Res. 2013, 47, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Li, J.; Pan, X.; Sun, Z.; Ye, C.; Jin, G.; Fu, Z. Effects of streptomycin on growth of algae Chlorella vulgaris and Microcystis aeruginosa. Environ. Toxicol. 2012, 27, 229–237. [Google Scholar] [CrossRef]
- Brilisauer, K.; Rapp, J.; Rath, P.; Schöllhorn, A.; Bleul, L.; Weiss, E.; Stahl, M.; Grond, S.; Forchhammer, K. Cyanobacterial antimetabolite 7-deoxy-sedoheptulose blocks the shikimate pathway to inhibit the growth of prototrophic organisms. Nat. Commun. 2019, 10, 545. [Google Scholar] [CrossRef]
- Gallon, J.R.; Ul-Haque, M.I.; Chaplin, A.E. Fluoroacetate metabolism in Gloeocapsa sp. LB795 and its relationship to acetylene reduction (nitrogen fixation). J. Gen. Microbiol. 1978, 106, 329–336. [Google Scholar] [CrossRef]
- Visca, P.; Pisa, F.; Imperi, F. The antimetabolite 3-bromopyruvate selectively inhibits Staphylococcus aureus. Int. J. Antimicrob. Agents 2019, 53, 449–455. [Google Scholar] [CrossRef]
- Lichstein, H.C.; Soule, M.H. Studies of the effect of sodium azide on microbic growth and respiration I. The action of sodium azide on microbic growth. J. Bacteriol. 1944, 47, 221–230. [Google Scholar] [CrossRef]
- Oliver, D.B.; Cabelli, R.J.; Dolan, K.M.; Jarosik, G.P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc. Natl. Acad. Sci. USA 1990, 87, 8227–8231. [Google Scholar] [CrossRef]
- Hickey, C.W.; Blaise, C.; Costan, G. Microtesting appraisal of ATP and cell recovery toxicity end points after acute exposure of Selenastrum capricornutum to selected chemicals. Environ. Toxicol. Water Qual. 1991, 6, 383–403. [Google Scholar] [CrossRef]
- Hoiczyk, E.; Hansel, A. Cyanobacterial cell walls: News from an unusual prokaryotic envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar] [CrossRef]
- Amiri-Eliasi, B.; Fenselau, C. Characterization of protein biomarkers desorbed by MALDI from whole fungal cells. Anal. Chem. 2001, 73, 5228–5231. [Google Scholar] [CrossRef]
- Komenda, J.; Sobotka, R. Cyanobacterial high-light-inducible proteins—Protectors of chlorophyll–protein synthesis and assembly. Biochim. Biophys. Acta Bioenerg. 2016, 1875, 288–295. [Google Scholar] [CrossRef]
- Fei, Q.; Gao, E.B.; Liu, B.; Wei, Y.; Ning, D. A toxin-antitoxin system VapBC15 from Synechocystis sp. PCC 6803 shows distinct regulatory features. Genes 2018, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Staub, R. Ernährungphysiologish-autökologische Untersuchung an den planktonischen Blaualge Oscillatoria rubescens DC. Schweiz. Z. Hydrol. 1961, 23, 82–198. [Google Scholar]
- Raus, M.; Šebela, M. BIOSPEAN: A freeware tool for processing spectra from MALDI intact cell/spore mass spectrometry. J. Proteom. Bioinform. 2013, 6, 283–287. [Google Scholar] [CrossRef]
- Strohalm, M.; Hassman, M.; Košata, B.; Kodíček, M. mMass data miner: An open source alternative for mass spectrometric data analysis. Rapid Commun. Mass Spec. 2008, 22, 905–908. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šebela, M.; Raus, M.; Ondřej, V.; Hašler, P. The Influence of Metabolic Inhibitors, Antibiotics, and Microgravity on Intact Cell MALDI-TOF Mass Spectra of the Cyanobacterium Synechococcus Sp. UPOC S4. Molecules 2021, 26, 1683. https://doi.org/10.3390/molecules26061683
Šebela M, Raus M, Ondřej V, Hašler P. The Influence of Metabolic Inhibitors, Antibiotics, and Microgravity on Intact Cell MALDI-TOF Mass Spectra of the Cyanobacterium Synechococcus Sp. UPOC S4. Molecules. 2021; 26(6):1683. https://doi.org/10.3390/molecules26061683
Chicago/Turabian StyleŠebela, Marek, Martin Raus, Vladan Ondřej, and Petr Hašler. 2021. "The Influence of Metabolic Inhibitors, Antibiotics, and Microgravity on Intact Cell MALDI-TOF Mass Spectra of the Cyanobacterium Synechococcus Sp. UPOC S4" Molecules 26, no. 6: 1683. https://doi.org/10.3390/molecules26061683
APA StyleŠebela, M., Raus, M., Ondřej, V., & Hašler, P. (2021). The Influence of Metabolic Inhibitors, Antibiotics, and Microgravity on Intact Cell MALDI-TOF Mass Spectra of the Cyanobacterium Synechococcus Sp. UPOC S4. Molecules, 26(6), 1683. https://doi.org/10.3390/molecules26061683





